Abstract
Objectives
Antimicrobial resistance to gonorrhoea is a threat to global health security. There have been concerns expressed that countries with high rates of disease have poor surveillance. The objectives of the study were: to determine the antimicrobial resistance patterns of N. gonorrhoeae clinical isolates to antimicrobial agents in patients with HIV or high risk of HIV acquisition; to compare the concordance of disc diffusion and agar dilution as methods for determining antimicrobial resistance to N. gonorrhoeae, and to describe methodological challenges to carrying out AMR testing.
Methods
The study was conducted at an HIV outpatient service for at-risk populations and an outreach clinic for commercial sex workers in Kampala. Patients were offered a sexually transmitted infection screen using a PCR-based assay. Samples positive for gonorrhoea were cultured. Antimicrobial susceptibility testing was performed using disc diffusion and isolates were sent to a reference laboratory for agar dilution direct susceptibility testing.
Results
Five hundred and seventy five patients were screened. There were 33 (5.7%) patients with gonorrhoea by PCR. Of the 16 viable N. gonorrhoeae isolates, 100% were resistant to ciprofloxacin and tetracycline by disk diffusion; and 31% exhibited reduced susceptibility to ceftriaxone and cefixime. By agar dilution, 100% of isolates were resistant to ciprofloxacin and all isolates were susceptible to ceftriaxone and cefixime.
Conclusions
One hundred percent resistance to ciprofloxacin was identified. There was concordance between disk diffusion and agar dilution for ciprofloxacin and tetracycline resistance and a significant discordance for third generation cephalosporins. More than half the women with gonorrhoea were asymptomatic, and represent a potential reservoir for ongoing transmission. Antimicrobial resistance testing of N.gonorrhoeae isolates is needed to ensure optimal treatment and prevention of antibiotic resistance progression.
Keywords: Gonorrhoea, sexually transmitted infections, antimicrobial resistance, HIV
INTRODUCTION
Antimicrobial resistance (AMR) is a global threat. The World Health Organization (WHO) warns that a growing number of infections “are becoming harder, and sometimes impossible, to treat as antibiotics become less effective”.1
Gonorrhoea is common, with 78 million new cases a year worldwide.2 Penicillin has been the antibiotic of choice for the treatment of gonorrhoea since the 1940s. By 1970, resistance to penicillin and tetracycline was discovered in Asia and became widespread by the 1980s, guidelines were revised and quinolones became the drug of choice. By the mid-1990s reports emerged of quinolone resistance in Southeast Asia, with widespread resistance reported by 2007. The pattern has followed the same general rule, with each new class of antibiotic introduced to treat gonorrhoea resistance develops and persists.3
One of the WHO key priority global action plans is to control the spread and impact of AMR of Neisseria gonorrhoeae by strengthening surveillance particularly in countries with a high burden of gonococcal infections, other sexually transmitted infections and HIV.4 The WHO Global Gonococcal Antimicrobial Surveillance Programme (WHO GASP) is a collaborative global network of regional and sub-regional reference laboratories started in 1990 to monitor global AMR worldwide.5 Sub-Saharan Africa, an area with a high burden of gonococcal infections has limited routine surveillance for AMR; only two of the forty seven WHO African member states provided national GASP data on 30 or more isolates in 2013.3 In a recent WHO report by Wi et al5 there were only three African member states that participated in reporting data on Neisseria gonorrhoeae antimicrobial susceptibility to extended-spectrum cephalosporins, azithromycin or ciprofloxacin between 2009–2014, with only two country reports in 2014. The syndromic approach in the management of STIs in the African region in addition to limited laboratory capacity and capability has led to poor and varied methodologies.7
Current WHO (2016) guidance recommends dual therapy with a third generation cephalosporin plus the macrolide azithromycin over single therapy as the preferred option for the treatment of gonococcal infections8 following reports from countries of reduced susceptibility and treatment failures to third generation cephalosporins.9,10
Diagnosis of STIs in most resource-limited settings is based on the syndromic approach for symptomatic patients. In Uganda, syndromic management of STIs is provided in a variety of settings: the public sector, private providers, complementary health practitioners and traditional healers. The 2012 Ugandan clinical guidelines recommend a single dose of cefixime 400mg as the drug of choice to treat gonorrhoea in STI-associated syndromes.11 Despite these national recommendations, ciprofloxacin is still commonly used to treat presumed gonococcal disease, as it is readily available at no cost in many treatment centres. Cefixime 200mg is provided by the government and is available at hospitals but is liable to stock-outs, therefore a number of patients are given a private prescription. Cefixime 400mg is approximately US$ 1.30. In addition, due to the diverse settings in which treatment is provided, there is a marked difference in the levels of education of practitioners, with varied knowledge of the most current guidelines and the most up to date data on AMR.
As part of a study to investigate the prevalence of chlamydia and gonorrhoea in specific participants belonging to key populations in adults attending an HIV and an outreach clinic in Kampala Uganda our primary objectives were:
-
1)
to determine the antimicrobial resistance pattern of N. gonorrhoeae clinical isolates to antimicrobial agents,
-
2)
to compare the concordance of disc diffusion and agar dilution as methods for determining antimicrobial resistance to N. gonorrhoeae,
-
3)
to describe methodological challenges to carrying out AMR testing.
METHODS
Study design and participants
This was a cross-sectional study conducted at 2 sites; the Adult Infectious Disease Clinic (AIDC) based at the Infectious Disease Institute (IDI) which cares for approximately 7,000 people living with HIV at Mulago National Tertiary Referral Hospital Kampala, Uganda, and an outreach clinic for commercial sex workers (CSWs) in Katanga, a densely-populated informal settlement also in Kampala.
From 18th March 2015 to the 1st August 2015, participants aged 14 years and above attending the following clinics: HIV discordant couples; young persons; pregnant women and most at risk populations (MARPs) at IDI were eligible to be recruited as ‘asymptomatic’ participants. At the AIDC, MARPs are defined by the Ministry of Health as populations which account for high HIV prevalence rates: fisher folk, CSWs and their partners, men who have sex with men, and men in uniformed services. In addition, other groups deemed to be at high risk for HIV are included: mobile men with money (e.g. motorcycle and truck drivers), and bar workers. All participants that attended IDI for routine HIV care with genital symptoms were eligible to take part in the study as ‘symptomatic’ participants. Asymptomatic and symptomatic CSWs attending the outreach clinic in Katanga regardless of HIV status were also recruited.
Procedures
Study staff obtained written consent from all participants prior to any study procedures. Sample collection was varied dependent on symptom status and history of GC contact as per British Association of Sexual Health and HIV (BASHH) guidelines. Asymptomatic participants provided approximately 30mls of first void urine and/or a self-collected vaginal swab for nucleic acid amplification testing (NAAT) for gonorrhoea and chlamydia. Symptomatic participants underwent a genital examination; males provided approximately 30mls of first void urine and a cervical specimen was obtained from women. Male participants with urethral discharge and participants with a history of contact with gonorrhoea had a culture performed at the same visit. Samples were collected with a dacron swab (endocervical for women; urethral for men) and were inoculated immediately onto chocolate agar and a microscope slide. These were transported at ambient temperature in a sample carrier box to the translational research laboratory at Makerere University within ten minutes of inoculation.
Laboratory procedures
Nucleic acid amplification testing
Urine, vaginal, and endocervical samples were tested for N. gonorrhoeae and Chlamydia trachomatis by PCR assay (BD Probetec™ ET CT/NG test; BD Diagnostics, Sparks, MD, USA). All tests were carried out according to the manufacturer’s instructions. Gonorrhoea was confirmed if NAAT was positive for N. gonorrhoeae.
Culture and biochemical tests
The initial inoculum on the agar plate was spread by streaking along the surface of the agar media, using a sterile disposable loop. The plates were incubated at 37°C in 5% CO2 until growth or for a maximum of 48 hours. They were reviewed after 24 hours; small translucent colonies suspected to be N. gonorrhoeae were sub-cultured on chocolate agar to obtain pure cultures. From the pure cultures a gram stain was performed. Presence of gram-negative diplococci on a gram-stained indicated a presumptive diagnosis of N. gonorrhoeae. Gram-negative diplococci cultures that tested positive for both oxidase and superoxal (catalase) tests were identified as N. gonorrhoeae.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed on 24-hour pure cultures identified to be N. gonorrhoeae. Using the Kirby-Bauer disk diffusion method colonies from pure cultures were streaked over the entire agar surface using a sterile disposable loop. The plates were then allowed to sit at room temperature for up to 2 minutes. Using sterile forceps, appropriate antimicrobial impregnated disks were placed on the agar surface one at a time, leaving a considerable distance between each disk. The disks used were penicillin G (10 U), tetracycline (30 μg), cefuroxime (30 μg), cefoxitin (30 μg), ciprofloxacin (5 μg), ceftriaxone (30 μg), ceftazidime (30 μg) and cefixime (5 μg), (Becton-Dickinson). The plates were then incubated at 37°C under 5% CO2 and observed for 18–24 hours. Zones of inhibition around each disk were measured in millimetres using a ruler. These were compared against reference standards provided by the Clinical and Laboratory Standards Institute guidelines (CLSI) 2012 to determine susceptibility.12
Viable N. gonorrhoeae isolates were sent to a national reference laboratory supervised by one of the authors (SR), located at Beth Israel Deaconess Medical Center, Boston, MA. Standard procedures and methods for organism transport were used. Once received, the isolates were sub-cultured twice on chocolate agar before being used for antimicrobial susceptibility testing, and all organism identifications were confirmed using the API NH test kits (bioMérieux, Durham, NC), following standard laboratory guidelines for organism identification. For antimicrobial susceptibility testing (AST), using the agar dilution method, the following antimicrobial agents were used for testing: penicillin; tetracycline; ciprofloxacin; ceftriaxone; cefixime; gentamicin; azithromycin. Gonorrhoea agar plates, supplemented with IsoVitalex, containing defined ranges of each of these antimicrobial agents were prepared following standard procedures for agar dilution testing. For inoculum preparation, colonies of the N. gonorrhoeae isolates grown on chocolate agar plates (20 to 24 h of incubation) were suspended in Mueller-Hinton broth to prepare a solution adjusted to a 0.5 McFarland standard density. The agar plates were then inoculated with 1 to 2 μl of each suspension using an inoculum-replicating apparatus (Steers replicator). The agar plates were incubated at 35°C with 5% CO2 for 20 to 24 h, after which the minimum inhibitory concentration (MIC) for each tested isolate and antimicrobial agent was determined. All participants with presumed or confirmed gonorrhoea were treated with cefixime 400mg single dose and advised to return in two weeks for a test of cure with a NAAT.
Statistical analysis
It was estimated that 27 isolates would be obtained based on the following assumptions: 600 STI screens would be performed; 30 cases of gonorrhoea would be identified assuming a prevalence of 5%; 27 isolates would be obtained assuming 90% of the isolates would be viable. Data was analysed using SPSS version 22, descriptive statistics (for proportions, means with SD, and medians with IQR) were obtained to summarize the data. Chi-square test was employed to compare difference between proportions.
Written approval was obtained to conduct the study from Makerere University School of Public Health Research and Ethics Committee (IRB no HREC 275), Uganda National Council for Science and Technology (HS 1743), and Johns Hopkins University School of Medicine internal review board (IRB00061162).
RESULTS
Five hundred and seventy five participants were recruited during the study period: 518 at AIDC and 57 CSWs at the outreach clinic. Of these, 396 (68.8%) were women; 485 (84.2%) were HIV-positive and 454 (78.9%) reported no symptoms. By NAAT, 33 (5.7%) of participants were positive for N. gonorrhoeae and 16 (2.8%) positive for C. trachomatis. Of those positive for N. gonorrhoeae, 25 (75.6%) were women, the median age was 24 years (IQR 21–30 years), 29 (87.8%) were HIV-positive, see Table 1 for baseline characteristics of those diagnosed with gonorrhoea. Patients with symptoms accounted for 60.6% (20) of infections: 36.4% (12) in women and 24.2% (8) in men. Of these 20 patients, 5 had received treatment with ciprofloxacin in the previous 3 months for STI-related symptoms. Patients who reported no symptoms accounted for just over a third (36.4%) of infections. All asymptomatic gonococcal infections were in women. Women were also more likely to have a concurrent STI 16% (4) versus none in men.
Table 1:
Characteristics of patients diagnosed with gonorrhoea
| Characteristics | Males (n=8) | Females (n=25) |
|---|---|---|
| Age, Median (IQR) | 34.5 (25.5–41.5) | 23 (21–26) |
| Symptom status, n (%) | ||
| Asymptomatic | 0 (0.0) | 13 (52.0) |
| Symptomatic | 8 (100.0) | 12 (48.0) |
| Previously diagnosed with an STI, n (%) | ||
| Yes | 3 (37.5) | 8 (32.0) |
| No | 5 (62.5) | 17 (68.0) |
| Concurrent STI, n (%) | ||
| Chlamydia | 0 (0.00) | 2 (8.0) |
| Syphilis & genital ulcer disease | 0 (0.00) | 1 (4.0) |
| Genital ulcer disease | 0 (0.00) | 1 (4.0) |
| Trichomonas | 0 (0.00) | 1 (4.0) |
| HIV status, n (%) | ||
| Positive | 8 (100.0) | 21 (84.0) |
| Negative | 0 (0.0) | 4 (16.0) |
|
Reporting more than one current partner in the past year, n (%) |
||
| Yes | 3 (37.5) | 8 (32.0) |
| No | 5 (62.5) | 17 (68.0) |
n, number; IQR, interquartile range; STI, sexually transmitted infections; HIV, human immunodeficiency virus
Of the 33 participants with a positive NAAT, we were able to obtain cultures from 31 participants (1 never attended; 1 was unable to attend the clinic and was treated in the community). Of the 31 participants positive by NAAT, 16 participants had N. gonorrhoea isolated in culture (Figure 1). The 16 isolates were from 9 women and 7 men, of which 15/16 were HIV-positive.
Figure 1:
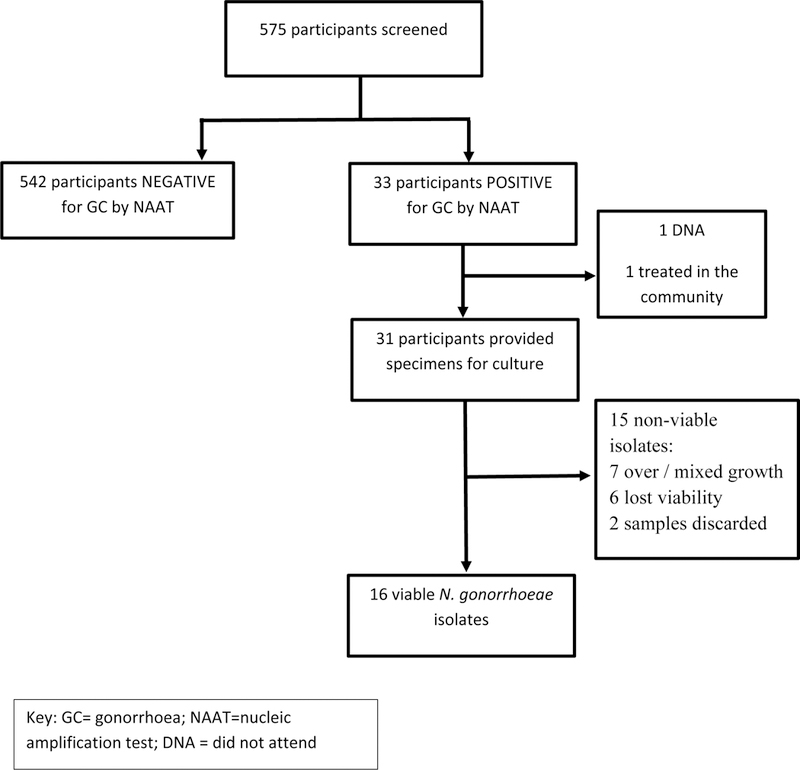
Flow diagram showing number of viable isolates following positive gonorrhoea NAAT.
AST was performed on these 16 isolates. Table 2 shows the antimicrobial susceptibility by disk diffusion and agar dilution methods. All isolates were resistant to ciprofloxacin and tetracycline by both methods. All isolates were susceptible to cefixime and ceftriaxone by agar dilution in contrast to 5 (31%) which exhibited reduced susceptibility to both drugs by disk diffusion. We have only agar dilution results for azithromycin and gentamicin. There are no CLSI breakpoints for azithromycin or gentamicin: using the CDC cut-off of >2 mg/L for resistance to azithromycin, all isolates were susceptible to azithromycin; and using the Australian Gonococcal Surveillance Programme suggested break-point of >16 mg/L for resistance to gentamicin, there was no resistance. Table 3 demonstrates the resistance / reduced susceptibility according to sex and testing method. Patients that attended for treatment 32 out of 33 received cefixime 400mg as a single dose, with 30 (85.7%) attending for follow-up with ‘test of cure’, 3 were still positive on NAAT and from the patients’ history this was due to re-infection.
Table 2:
Antimicrobials susceptibility test results on 16 isolates by both Agar dilution and Disk-diffusion method
| MIC (μg/ml)/ ZD (mm) values |
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | N (%) Resistant/ reduced susceptibility |
P-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Penicillin | MIC | 4 | 1 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 1 | 4 | 1 | 4 | 4 | 4 | 13 (81) | 0.2 |
| ZD | 6 | 18 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 17 | 6 | 18 | 6 | 6 | 6 | 16 (100) | ||
| Tetracycline | MIC | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 (100) | 1.0 |
| ZD | 8 | 13 | 16 | 10 | 12 | 10 | 12 | 12 | 12 | 12 | 9 | 11 | 12 | 13 | 11 | 12 | 16 (100) | ||
| Gentamicin | MIC | 4 | 8 | 8 | 4 | 8 | 4 | 8 | 4 | 8 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 0 (0) | n/a |
| ZD | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Cefixime | MIC | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0 (0) | 0.017 |
| ZD | 15 | 31 | 31 | 31 | 35 | 31 | 21 | 31 | 32 | 32 | 15 | 26 | 31 | 25 | 26 | 35 | 5 (31) | ||
| Ceftriaxone | MIC | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0 (0) | 0.017 |
| ZD | 35 | 35 | 30 | 32 | 40 | 36 | 40 | 35 | 31 | 40 | 28 | 35 | 35 | 15 | 35 | 35 | 5 (31) | ||
| Ciprofloxacin | MIC | 4 | 2 | 2 | 4 | 2 | 2 | 4 | 2 | 2 | 2 | 4 | 2 | 2 | 1 | 2 | 4 | 16 (100) | 1.0 |
| ZD | 8 | 15 | 12 | 12 | 8 | 13 | 16 | 10 | 14 | 13 | 9 | 13 | 15 | 14 | 9 | 9 | 16 (100) | ||
| Azithromycin | MIC | 0.06 | 0.06 | 0.25 | 0.125 | 0.125 | 0.03 | 0.06 | 0.125 | 0.06 | 0.125 | 0.125 | 0.03 | 0.03 | 0.03 | 0.03 | 0.06 | 0 (0) | n/a |
| ZD | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| key: | ||||
|---|---|---|---|---|
| Antimicrobial | Interpretive criteria | |||
| Zone diameter (ZD) breakpoint (mm) | MIC breakpoint (μg/ml) | |||
| Susceptible | Resistant | Susceptible | Resistant | |
| Penicillin | ≥47 | ≤26 | ≤0.06 | ≥2 |
| Tetracycline | ≥38 | ≤30 | ≤0.25 | ≥2 |
| Gentamicin* | - | - | <8 | >16 |
| Cefixime | ≥31 | -- | ≤0.25 | - |
| Ceftriaxone | ≥35 | - | ≤0.125 | - |
| Ciprofloxacin | ≥41 | ≤27 | ≤0.06 | ≥1 |
| Azithromycin* | - | ≤30 | <2 | ≥2 |
There are no CLSI breakpoints for gentamicin and azithromycin
Table 3:
Number of isolates with resistance/ reduced susceptibility by testing method and sex
| Agar Dilution | Disk diffusion | |||||
|---|---|---|---|---|---|---|
| Male (N=7) |
Female (N=9) |
Total | Male (N=7) |
Female (N=9) |
Total | |
| Penicillin | 7 | 6 | 13 | 7 | 9 | 16 |
| Tetracycline | 7 | 9 | 16 | 7 | 9 | 16 |
| Gentamicin | 0 | 0 | 0 | ND | ND | - |
| Cefixime | 0 | 0 | 0 | 3 | 2 | 15 |
| Ceftriaxone | 0 | 0 | 0 | 3 | 2 | 15 |
| Ciprofloxacin | 7 | 9 | 16 | 7 | 9 | 16 |
| Azithromycin | 0 | 0 | 0 | ND | ND | - |
DISCUSSION
In this cohort of participants at high risk for HIV or HIV-positive in Uganda, 100% of the gonococcal isolates were ciprofloxacin resistance, a higher prevalence than seen in previous studies done in Uganda. Vandepitte et al13 reported rates of ciprofloxacin resistance of 83.1% among CSWs in Kampala, and Amito et al14 found a prevalence of 23.1% amongst men and women attending an outpatient department at Lacor hospital in northern Uganda. There is limited data on antimicrobial surveillance for gonorrhoea in HIV-positive individuals. A study in Thailand done among HIV-positive individuals with a gonorrhoea prevalence of 1.3% had similar antimicrobial resistance results to our study. They identified high rates of antimicrobial resistance to penicillin (86.1%), tetracycline (95.1%) and ciprofloxacin (90.2%) among their cohort of HIV-positive participants with 100% susceptibility to ceftriaxone and cefotaxime.15
A Ugandan study looking at antibiotic prescription rates in hospitalised patients found that ciprofloxacin was the fourth most commonly prescribed systemic antibiotic16. Of great concern, this same study found that ceftriaxone was the first most commonly prescribed antibiotic. Treatment decisions to prescribe a systemic antibiotic are mainly based on unconfirmed diagnoses. There are limited alternatives (spectinomycin and gentamicin) to the third generation cephalosporins in the treatment of gonorrhoea, with three drugs in various stages of development: solithromycin (a new-generation macrolide) has completed phase III clinical trials; and both zoliflodacin (a DNA gyrase inhibitor) and gepotidacin (a novel triazaacenaphythylene antibacterial compound) have completed phase II clinical trials).17 The utility of these drugs once licenced would probably be cost-prohibitive in resource limited settings.
There was concordance between disc diffusion and agar dilution for ciprofloxacin and tetracycline and a clear and significant discordance (p=0.017) with susceptibility to third generation cephalosporins. Disc diffusion, though a simple and inexpensive method (approximately US$ 25) is limited in that it does not provide MICs, lacks automation, reading the results can be subjective and it is difficult to apply to fastidious or slow-growing bacteria. Studies have demonstrated good concordance for agar dilution and the Etest technique for susceptibility testing for ceftriaxone and ciprofloxacin 18. The Etest (BioMerieux, France) method is a simple and quick method which provides a less laborious MIC alternative to agar dilution. However, each strip costs approximately US$ 5 dollars. There is a study by Liao et el19 which compared the use of chocolate agar and GC specific agar with agar dilution, disc diffusion and E-test methods. They concluded that the use of chocolate agar with disc diffusion was associated with a 5.5% false resistance rate compared to GC agar, and this could may be why we had the discrepancy with the third generation cephalosporins.
Overwhelmingly all but one of the 15 failed cultures were from women – seven of which demonstrated overgrowth with vaginal flora as a non-selective medium (chocolate agar) was used which less fastidious organism can grow. Six of the cultures were reported as non-viable, again these were all in women, four symptomatic and two asymptomatic. The causes for this are potentially dead organisms, storage and use of non-selective medium. All symptomatic male N. gonorrhoea isolates cultured on chocolate agar. In resource-limited settings, symptomatic males are a reasonable patient group to target for surveillance due ease of sample collection, higher yield of positive culture and low cost.
Importantly, more than half the women with gonorrhoea reported no symptoms. With the current syndromic approach to the management of STIs, these women would not be diagnosed and treated and would continue to be a reservoir for ongoing infections and development of AMR if treated with antibiotics for other conditions. There is a need for rapid, cost-effective, simple diagnostic tests for gonorrhoea in asymptomatic women.
Limitations
The unavailability of gonorrhoea-specific culture media led to a reduced number of isolates from cervical specimens due to overgrowth by other organisms. Gonorrhoea specific agar though more costly is more cost-effective as the need to serially sub-culture in order to obtain a pure culture is reduced.
Disc diffusion overcalled reduced susceptibility to third generation cephalosporins. Our results support the WHO GASP recommendation that, decreased susceptibility or resistance to extended spectrum cephalosporins identified with disc diffusion methods should be verified with quantitative MIC determination as we did with the national reference laboratory located at Beth Israel Deaconess Medical Center, Boston, MA.
Conclusion
We found significant gonococcal AMR in key populations with HIV who are at high risk of transmitting both infections. The results of our study demonstrate that AMR surveillance for N. gonorrhoeae and other organisms is urgently needed in sub-Saharan Africa to provide current emerging patterns of resistance to inform treatment guidelines using syndromic diagnosis. Methods that provide quick, accurate, inexpensive tests to confirm the diagnosis of gonorrhoea as well as guide antibiotic choices will be important as multi-drug resistance has become pervasive.
Acknowledgements
Professor Jonathan Zenilman for his advice and technical input, in addition he was instrumental in the establishment of the microbiology laboratory at the Infectious Disease Institute; Beckton Dickinson for donating the chlamydia and gonorrhoea testing kits.
Funding This work was supported by grants from: Foundation for the National Institutes of Health, [grant number 5U54EB007958 to Professor Charlotte Gaydos and Dr Yukari Manabe]; the Sacharuna Foundation and the Johns Hopkins Center for Innovative Medicine. The funders had no role in data collection, management, analysis, and interpretation of the data; and preparation, review or approval of the manuscript for publication
Footnotes
Ethics approval Written approval to conduct the study was obtained from Makerere University School of Public Health Research and Ethics Committee (IRB no HREC 275), Uganda National Council for Science and Technology (HS 1743), and Johns Hopkins University School of Medicine internal review board (IRB00061162).
Competing interests None declared.
REFERENCES:
- 1.WHO Media Centre. Antimcicrobial resistance. Fact sheet; updated October 2017. Available at: http://www.who.int/mediacentre/factsheets/fs194/en/. Accessed October 20, 2017. [Google Scholar]
- 2.WHO Media Centre. Sexually Transmitted Infections (STIs). Fact sheet; updated November 2016. Available at: http://www.who.int/mediacentre/factsheets/fs110/en/#. Accessed January 2, 2017. [Google Scholar]
- 3.World Health Organization 2014. Antimicrobial resistance: global report on surveillance. Available at http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1. Accessed January 2, 2017.
- 4.World Health Organization 2012, Department of Reproductive Health and Research. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. Available at http://www.who.int/reproductivehealth/publications/rtis/9789241503501/en/. Accessed September 10, 2017 [Google Scholar]
- 5.Wi T, Lahra MM, Ndowa F et al. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med 2017, 14(7). Available at 10.1371/journal.pmed.1002344. Accessed June 30, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization 1990. Global surveillance network for gonococcal antimicrobial susceptibility. Available at: http://apps.who.int/medicinedocs/documents/s16348e/s16348e.pdf. Accessed September 10, 2017
- 7.Ndowa FJ, Francis JM, Machiha A, et al. Gonococcal antimicrobial resistance: perspectives from the African region. Sex Transm Infect 2013;89 (4):11–15 [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization 2016. WHO Guidelines for the treatment of Neisseria gonorrhoeae. Available at: http://apps.who.int/iris/bitstream/10665/246114/1/9789241549691-eng.pdf. Accessed December 7, 2016 [PubMed]
- 9.Unemo M, Golparian D, Stary A et al. First Neisseria gonorrhoeae strain with resistance to cefixime causing gonorrhoea treatment failure in Austria, 2011. Euro Surveill 2011;16 (43):19998. [PubMed] [Google Scholar]
- 10.Fifer H, Natarajan U, Jones L et al. Failure of Dual Antimicrobial Therapy in Treatment of Gonorrhea. N Engl J Med 2016;374(25):2504–2506 [DOI] [PubMed] [Google Scholar]
- 11.Ministry of Health. Uganda Clinical Guidelines 2012. Available at: http://apps.who.int/medicinedocs/documents/s21741en/s21741en.pdf. Accessed January 2, 2017
- 12.Clinical and Laboratory Standards Institute: Performance standards for standards for antimicrobial susceptibility testing, twenty second information supplement. CLSI document M100-S22. vol.32;2012 [Google Scholar]
- 13.Vandepitte J, Hughes P, Matovu G et al. High prevalence of ciprofloxacin-resistant gonorrhea among female sex workers in Kampala, Uganda (2008–2009). Sex Transm Dis 2014;41(4):233–237 [DOI] [PubMed] [Google Scholar]
- 14.Amito FP, Otim F, Okongo F et al. The prevalence and antibiotics susceptibility pattern of Neisseria gonorrhoeae in patients attending OPD clinics at St. Mary’s Hospital Lacor Uganda. J Prev Med Hyg 2012;53(4):186–189. [PubMed] [Google Scholar]
- 15.Srifeung S, Roongpisuthipong A, Asavapiriyanont S et al. Prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae in HIV-Seropositive Patients and Gonococcal Antimicrobial Susceptibility: an Update in Thailand. Jpn J Infect Dis 2009;62(6):467–470. [PubMed] [Google Scholar]
- 16.Kiguba R, Karamagi C, Bird SM. Extensive antibiotic prescription rate among hospitalized patients in Uganda: but with frequent missed-dose days. J Antimicrob Chemother 2016;71(6):1697–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howell M, Future Science group. Gonorrhoea- the current antibiotic pipeline and the need for new drugs (Infectious Disease Hub Web site; ). Available at: https://www,id-hub.com > 2017/07/07. Accessed October 2, 2017. [Google Scholar]
- 18.Liu H, Taylor TH Jr, Pettus K et al. Assessment of Etest as an alternative to agar dilution for antimicrobial susceptibility of Neisseria gonorrhoeae. J Clin Microbiol 2014;52(5):1435–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao CH, Lai CC, Hsu MS et al. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates determined by the agar dilution, disk diffusion and Etest methods: comparison of results using GC agar and chocolate agar. Int J Antimicrob Agents 2010: 35(5):457–60 [DOI] [PubMed] [Google Scholar]


