Abstract
Debilitating heart conditions, notably dilated and hypertrophic cardiomyopathies (CM), are associated with point mutations in metavinculin, a larger isoform of the essential cytoskeletal protein vinculin. Metavinculin is co-expressed with vinculin at sub-stoichiometric ratios in cardiac tissues. Cardiomyopathy mutations in the metavinculin tail domain (MVt) occur within the extra 68-residue insert that differentiates it from the vinculin tail domain (Vt). Vt binds actin filaments (F-actin) and promotes vinculin dimerization to bundle F-actin into thick fibers. While MVt binds to F-actin in a similar manner to Vt, MVt is incapable of F-actin bundling and inhibits Vt-mediated F-actin bundling. We performed F-actin co-sedimentation and negative-stain EM experiments to dissect the coordinated roles of metavinculin and vinculin in actin fiber assembly and the effects of three known metavinculin CM mutations. These CM mutants were found to weakly induce the formation of disordered F-actin assemblies. Notably, they fail to inhibit Vt mediated F-actin bundling, and instead promote formation of large assemblies embedded with linear bundles. Computational models of MVt bound to F-actin suggest that MVt undergoes a conformational change licensing the formation of a protruding sub-domain incorporating the insert, which sterically prevents dimerization and bundling of F-actin by Vt. Sub-domain formation is destabilized by CM mutations, disrupting this inhibitory mechanism. These findings provide new mechanistic insights into the ability of metavinculin to tune actin organization by vinculin and suggest that dysregulation of this process by CM mutants could underlie their malfunction in disease.
Keywords: Vinculin, metavinculin, actin, cardiomyopathy, heart
Graphical Abstract

Introduction
Vinculin is an essential cytoskeletal protein that localizes to focal adhesions (FAs) and adherens junctions1; 2. At these sites of adhesion, vinculin acts as a scaffold to link transmembrane receptors to actin filaments, thereby playing a crucial role in cell adhesion, motility, and force transmission between cells and the cell-matrix interface. Without vinculin, defects in heart and nerve formation are observed, and mouse embryos do not survive past E103. Additionally, lack of vinculin in cells leads to rounded morphology, increased motility3; 4, and resistance to apoptosis and anoikis5.
Vinculin is a 117 kD protein that functions as a molecular scaffold. It is comprised of a large 90 kD head domain (Vh), a flexible proline-rich linker, and a 22 kD tail domain (Vt)6. Vh interacts with talin at FAs, α-catenin at cell-cell junctions, and α-actinin7; 8; 9. The proline-rich linker that connects Vh to Vt can bind to VASP, vinexin, CAP/ponsin, and the Arp2/3 complex10; 11; 12; 13. Vt directly binds to filamentous actin (F-actin)14, phosphatidylinositol (4,5) bisphosphate (PIP2)15 and Raver116. Autoinhibitory interactions between Vh and Vt retain vinculin in its closed inactive state which obscures ligand binding6. Although mechanisms of activation are not fully understood, it is currently believed that engagement of talin or catenin to Vh in conjunction with binding of additional ligands such as actin2; 7; 17, post-translational modifications18 and/or force19; 20; 21; 22, promotes activation and exposure of multiple ligand binding sites.
While vinculin is ubiquitously expressed, metavinculin, a larger splice isoform, is selectively expressed in smooth and cardiac muscle cells23; 24; 25. Metavinculin expression is tightly controlled at substoichiometric levels relative to vinculin (9–42%), and correlates with contractile needs of muscle cells26; 27. Reduced metavinculin expression level is associated with cardiomyopathy (CM) and disorganized intercalated disc structures28, suggesting that metavinculin is necessary for the maintenance of smooth muscle actin-membrane adhesion sites and in force generation and transmission through its interaction with the actin cytoskeleton.
While the tail domain of vinculin possesses an N-terminal strap (NtS) followed by a 5-helix bundle fold and a C-terminal hairpin (CtHP)6, metavinculin contains an additional exon that encodes a 68-amino acid insert between helix 1 (H1) and helix 2 (H2) within the tail domain (MVt)23. MVt also adopts a 5-helix bundle fold similar to Vt; however, the sequence that makes up H1 and NtS of Vt is displaced by homologous H1’ and NtS’ sequences contained within the 68 amino acid insert29. Similar to vinculin, metavinculin can directly associate with F-actin29; 30; 31; however, unlike Vt, MVt does not bundle filamentous actin in vitro30; 31; 32. Point mutations identified within the 68-residue insert cause altered actin organization and heart disease, notably dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM)28; 32; 33. The A934V and ΔL954 metavinculin mutations are associated with DCM32, whereas the R975W mutation has been identified in patients with both DCM and HCM33. Both DCM and HCM are diseases of myocardium that prevent normal blood flow within the heart resulting from disruption in force transmission, consistent with the prevailing notion that metavinculin-actin interactions play a key role in force transmission.
We and others have previously shown that binding of F-actin to Vt causes a conformational change in Vt that promotes dimerization and actin filament bundling34; 35. However, the structure of the actin-induced Vt dimer is currently unknown. As H1 is sensitive to proteolysis upon addition of F-actin36, and is not observed in our cryo-EM reconstruction of the Vt-actin complex31, H1 likely partitions away from the helix bundle upon engagement with filamentous actin to promote vinculin dimerization. Similarly, electron density for metavinculin H1’ is lacking in the cryo-EM reconstruction of the metavinculin-actin complex31. Notably, the presence of displaced H1 appears to interfere with the ability of metavinculin to bundle F-actin, as its deletion promotes actin filament bundling29.
While vinculin and metavinculin show similar modes of binding to F-actin, metavinculin is unable to bundle actin filaments. However, metavinculin mutants associated with DCM and HCM are able to form higher order actin assemblies in vitro32. As metavinculin is co-expressed with vinculin in smooth muscle and cardiac tissues23; 25; 26; 27, it is likely that vinculin and metavinculin coordinately regulate the architecture of F-actin networks. In fact, we and others have previously observed that the presence of metavinculin at sub-stoichiometric ratios impairs vinculin mediated F-actin bundling31; 36, suggesting that MVt may negatively regulate Vt-mediated actin bundling.
To better understand the coordinated role of metavinculin and vinculin in F-actin fiber assembly and the consequences of cardiomyopathy-related mutations, we conducted a series of actin cosedimentation and negative stain EM experiments utilizing MVt and Vt constructs. Consistent with our previous findings31, MVt is unable to induce actin bundling, whereas the presence of sub-stoichiometric amounts MVt relative to Vt inhibits the assembly of actin filaments into parallel bundles. In contrast to wild-type (WT) MVt, and consistent with previous fluorescence microscopy data32, MVt CM mutants alone weakly induce the formation of disordered higher-order F-actin assemblies. Strikingly, in the more physiological scenario of Vt-MVt mixtures, CM mutants lose the ability to inhibit Vt-mediated bundles, instead promote the formation of very large assemblies which are morphologically consistent with aggregations of Vt-mediated bundles. To investigate the molecular basis for MVt’s negative regulation of Vt-mediated actin bundling, we performed discrete molecular dynamics (DMD) simulations. Actin binding to vinculin promotes release of H1 from the tail domain helix bundle, exposing an interface in vinculin that promotes dimerization31. However, in the case of metavinculin, our simulations suggest that the insert and displaced H1 form a new structural element, a protruding sub-domain, with H1’ released from the helix bundle upon actin binding. This additional MVt sub-domain, generated upon actin engagement, could sterically block actin assembly into parallel F-actin bundles. Our DMD simulations also indicate that CM mutations within the C-terminus of metavinculin destabilize formation of this protruding actin-induced sub-domain, likely exposing residues which can mediate the formation of disordered assemblies. In summary, our results provide new mechanistic insights into the coordinated activities of vinculin and metavinculin in controlling F-actin network architecture, and suggest that regulation of vinculin-mediated actin bundling activity by metavinculin, constitutes one key function that is compromised by the CM mutants.
Results
Wild-type metavinculin tail domain does not induce F-actin bundling
The F-actin binding sites of vinculin and metavinculin are located in their tail domains, Vt and MVt, respectively, but these binding sites are occluded in the context of the full-length proteins due to autoinhibitory interactions between the head and tail domains. As the tail domains are functional in isolation, we have used recombinantly expressed Vt and MVt protein constructs in this study (shown by schematics in Figure 1A) to probe the effects of MVt WT and MVt CM mutations on the formation of actin assemblies in the absence and presence of Vt. The Vt domain encompasses 188 C-terminal residues of vinculin and consists of five α-helices (H1–H5) flanked on both sides by flexible regions, an N-terminal strap (NtS) and a C-terminal hairpin (CtHP). MVt contains an extra 68-residue insert between helices H1 and H2 of the corresponding sequence of Vt. Part of the insert displaces the 37 N-terminal residues comprising H1 (PDB ID 3MYI) and the NtS, with H1’ and NtS’29. Here we investigate single point mutations specific to the metavinculin insert region, namely A934V, ΔL954 and R975W, associated with cardiomyopathies and atherosclerosis32; 37. We have also utilized a construct we designate as MVtp, which includes the additional 21-residue proline-rich linker preceding the NtS of MVt.
Figure 1:
Metavinculin tail (MVt) domain exhibits similar F-actin binding affinity but dramatically reduced bundling (crosslinking) of the filaments relative to the vinculin tail (Vt) domain. (A) Schematic representation of Vt and MVt constructs used in this study. Vt consists of five α-helices (H1–H5) flanked by an N-terminal strap (NtS) and a C-terminal hairpin (CtHP). MVt contains a 68-residue insert between helices H1 and H2 that replaces the original H1 and NtS by H1’ and NtS’. MVtp includes the 21-residue proline-rich linker preceding the NtS of MVt. Mutations within the metavinculin insert region, namely A934V, ΔL954 and R975W, have been identified in cardiomyopathies. (B) X-ray crystal structures of Vt (PDB ID 1TR2) and MVt (PDB ID 3MYI) as well as the cryo-EM reconstruction of Vt-actin (EMD-6446) and MVt-actin (EMD-6447) interfaces. Vt and MVt are comprised of a highly similar 5-helix bundle fold in the absence of actin, where H1’ of MVt swaps with H1 of Vt. Upon actin engagement, regions N-terminal of H2 are not detectable in the cryo-EM reconstructions. (C) Negative stain EM images of actin filaments. Micrographs are acquired at the same magnification (scale bar = 500 nm, shown in the left panel). Crosslinking or bundling of actin filaments by Vt generates thick fibers. In contrast, MVt and MVtp do not promote actin filament bundling.
The core regions of both Vt and MVt comprise a compactly folded H2–H5 bundle. In the absence of actin, the H1–H5 helices are observable in X-ray crystal structures of full-length vinculin and Vt, and correspondingly, H1’-H5 are resolved in crystal structures of full-length metavinculin and MVt29. However, the regions preceding the H2–H5 bundle in both Vt and MVt were not detectable in our previously published cryo-EM reconstructions of these domains bound to F-actin31. We reported a twisting structural rearrangement of H2–H5 concomitant with actin binding that displaces H1/H1’ from the helical bundle by remodeling its hydrophobic core, which we inferred renders these regions disordered and/or dynamic. Vt and MVt both engage F-actin in an indistinguishable manner at subnanometer resolution through their compactly folded H2H5 regions31, which are identical in sequence (Figure 1B).
Before investigating the effects of CM mutants, we first validated our wild-type Vt and MVt constructs to recapitulate previously reported binding and bundling activities30; 31; 32; 36, and also verified F-actin bundling inhibition by both MVt and MVtp proteins. We acquired negative stain EM images of actin filaments in the absence and presence of wild-type Vt, MVt and MVtp (Figure 1C). The actin-alone sample showed single, linear actin filaments, whereas Vt induced crosslinking of filaments into parallel bundles as expected, resulting in the formation of thick fibers. When either MVt or MVtp was added to F-actin instead of Vt, F-actin bundling was dramatically reduced, with few observable thick fibers. This is consistent with previous reports by our group and others that MVt does not induce large linear actin bundles like Vt30; 31; 32; 36, indicating that the MVt insert region prohibits actin-induced MVt dimerization and negatively regulates F-actin bundling. Additionally, inclusion of the proline-rich linker, as in MVtp, has a minor, albeit significant effect on its regulatory activity.
MVt cardiomyopathy mutants induce higher-order F-actin assemblies
Having validated our constructs and confirmed similar behavior of MVt and MVtp by negative stain EM (Figure 1C), we next used F-actin co-sedimentation assays to examine the F-actin binding and aggregation activities of MVtp constructs featuring the cardiomyopathy mutations A934V, ΔL954 and R975W. First, we compared the F-actin binding of MVtp wild-type and cardiomyopathy mutants relative to Vt. Samples containing actin (10 or 20 μM) plus individual tail domains (10 μM) were subjected to high-speed centrifugation (see methods for experimental details). Under these conditions, the supernatant (S) contains unbound tail domain, while the pellet (P) contains F-actin and bound protein. The amount of tail domain present in each fraction was analyzed by SDS-PAGE (Figure 2A) and quantified using ImageJ38. WT Vt, MVt and MVtp, as well as all three MVtp CM mutants, exhibited similar binding affinity to F-actin (Figure 2B). From these data, we conclude that MVt mutations do not impair F-actin binding. This is consistent with the known actin binding interface, comprising the compactly folded H2–H5 region, which is not predicted to be impacted by any of the cardiomyopathy mutations31.
Figure 2:
Metavinculin tail domain wild-type (WT) and cardiomyopathy (CM) mutants exhibit similar F-actin binding but differ in the ability to form higher order actin assemblies. (A) Representative SDS-PAGE results obtained from high speed F-actin co-sedimentation (100K g, 30 min) assays in the presence of Vt, MVt or MVtp WT and CM proteins (S - supernatant, P - pellet). (B) Quantification of protein fractions present in pellets representing individual sub-populations of Vt, MVt or MVtp constructs bound to F-actin. Error bars represent standard deviation (SD) (n=2, 2 replicates for each n). (C) Representative SDS-PAGE results obtained from low speed F-actin co-sedimentation (12K g, 15 min) assays in the presence of Vt, MVt or MVtp WT and CM proteins. (D) Quantification of actin fractions in pellets representing sub-populations of F-actin present in bundles or in higher-order assemblies induced by Vt, MVt or MVtp constructs. Error bars represent SD (n=2, 5 replicates for each n). Vt and MVt or MVtp WT (labeled as MVt, MVtp), as well as all MVtp CM mutants (labeled as A934V, ΔL954 and R975W), bind actin filaments similarly. However, while Vt drives almost the entire population of actin filaments into bundles or higher-order assemblies (~95%), MVt or MVtp WT display little assembly formation (~15–20%). In contrast, increased F-actin assembly formation is observed for MVtp CM mutants (~30–40%) compared to MVtp WT. Statistical significances in panels B (with respect to Vt) and D (with respect to MVtp) are indicated by; non-significant (n.s.), p < 0.05 (*), p < 0.01 (**).
We next employed low-speed centrifugation assays to assess the F-actin crosslinking activities of this same panel of constructs (see methods for experimental details). Under these conditions, only large cross-linked assemblies of F-actin and bound protein are pelleted, while individual actin filaments are retained in the supernatant. The fraction of actin present in the pellet was quantified as described above to determine the sub-populations of F-actin involved in higher-order assemblies (Figure 2D). It should be noted that while this is often referred to as a “bundling” assay, the pellets from low-speed centrifugation may contain both thick fibers representing canonically bundled actin filaments, as in the case with Vt (Figure 1C), but also other sufficiently large structures (ordered or disordered) that pellet under these conditions. These assays were conducted in parallel with negative stain EM, to visualize the actin assemblies formed in the presence of Vt and MVt proteins. For isolated F-actin, little actin was found in the pellet (~5%), but upon addition of Vt almost all of the F-actin was found in the pellet (~95%), consistent with the negative-stain EM data (Figure 1C). Also consistent with the negative-stain EM results (Figure 1C), the amount of actin found in the pellet was dramatically reduced when MVt was added (~26%) and to an even lower amount when MVtp was added (~13%). Interestingly, an increase in the amounts of pelleted actin were found for the MVtp CM mutants; A934V (~29%), ΔL954 (~41%) and R975W (37%).
Quantitative negative-stain EM assay probes F-actin assemblies
To visualize and quantify differences in actin filament organization in the presence of Vt and MVt WT, as well as MVt CM mutants, we developed a quantitative negative-stain transmission electron microscopy (TEM) assay (Figure 3, Supplementary Figure 1). The quantitative power of traditional negative-stain EM imaging of F-actin assemblies is limited by selection bias, as choosing which part of the grid to image is dependent on input of an experienced user. Additionally, the frequently irregular structure of these assemblies, compounded by their inherently variable contrast after negative staining, is refractory to image analysis methodology which depends on classification or averaging.
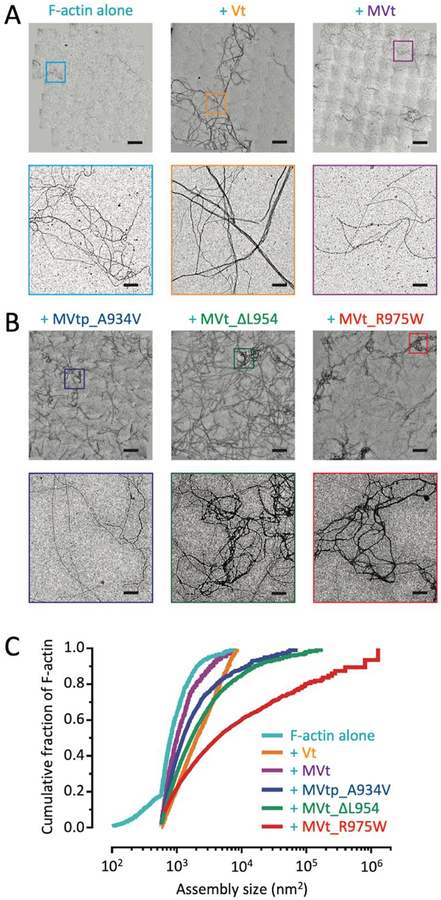
Figure 3:
Metavinculin cardiomyopathy mutants promote formation of disordered, mesh-like F-actin assemblies. (A) Stitched negative-stain EM images of F-actin alone and in the presence of wild-type Vt and MVt proteins (bars = 10 μm). Zoomed views of the boxed regions are shown in the bottom panel (bars = 1 μm). (B) Stitched negative-stain EM images of F-actin in the presence of MVt or MVtp proteins featuring cardiomyopathy mutations (bars = 10 μm). Zoomed views of the boxed regions are shown in the bottom panel (bars = 1 μm). (C) Cumulative plots of F-actin assemblies from the indicated conditions. Pair-wise comparisons show all distributions to be significantly different (KS test, p < 0.01). N ≥ 10 fields and n ≥ 764 regions were quantified for each condition. F-actin, 0.5 μM; Vt/MVt/MVtp constructs, 2.0 μM.
To overcome selection bias, we targeted grid squares at low magnification where their contents were invisible. We then imaged the entirety of each targeted square at a magnification sufficient to resolve individual actin filaments, tiling it with overlapping fields of view which were subsequently stitched together (Figure 3A). We next implemented an image analysis procedure to detect and quantify the size of higher-order assemblies which is insensitive to their variable internal structure. Images were thresholded (the only step requiring user input due to stain variability) and binarized, then automatically segmented into continuous regions using the “Analyze Particles” procedure in ImageJ38. To analyze these data, we plot the cumulative fraction of F-actin + bound proteins detected in all regions versus region size (Figure 3C, Supplementary Figure 1), which we find to be a sensitive metric of higher-order assembly state that is robust against variations in background noise between different datasets (see Methods for details).
MVt cardiomyopathy mutants form mesh-like actin assemblies
To validate this assay, we first examined the known differential F-actin assembly properties of Vt, which strongly bundles actin, in comparison to MVt WT which does not induce assemblies as previously established30; 31; 32; 36, and here re-validated through qualitative negative-stain EM and quantitative low-speed co-sedimentation assays (Figure 1C, Figure 2C–D). As expected, although other assembly states are present as a minor fraction, we find the presence of Vt primarily induces the formation of linear actin bundles (Figure 3A) and correspondingly shifts the distribution towards larger assemblies versus actin alone (Figure 3C). While the presence of MVt WT did produce a slight but significant shift relative to actin alone, the effects were modest, with substantially fewer large assemblies than for Vt (Figure 3C). Consistently, no obvious bundles and few other assemblies were visible in the images, which were qualitatively similar in appearance to F-actin alone (Figure 3A).
We next examined the effects of MVt cardiomyopathy mutants. These experiments, which require small amounts of material, were initiated mostly with the tail domain constructs (i.e., MVt) and later we confirmed that inclusion of the proline-rich linker (i.e., MVtp) does not adversely affect metavinculin’s regulatory role (Figure 1C, Figure 2C–D). Thus, we investigated R975W and ΔL954 in the MVt background and MVtp A934V in our negative stain EM experiments. As our studies establish MVt and MVtp show similar activity profiles (Figures 1 and 2), we believe experiments between mutants in these backgrounds can safely be compared.
Consistent with low-speed co-sedimentation assays (Figure 2C–D), we observed a significant increase in assemblies in the presence of cardiomyopathy mutants MVtp A934V, MVt ΔL954, and MVt R975W, with R975W having the most dramatic effect (Figure 3C), in accordance with the severity of disease caused by this mutation in patients32; 33. Examination of the images shows primarily an irregular, mesh-like organization of actin filaments (Figure 3B), unlike the majority species present as linear bundles formed in the presence of Vt (Figure 1C, Figure 3A).
MVt cardiomyopathy mutants fail to inhibit Vt-induced higher-order actin assemblies
Metavinculin is co-expressed with vinculin at sub-stoichiometric levels in smooth muscle and cardiac tissues25; 26; 27. We and others have previously reported that the presence of sub-stoichiometric MVt impairs Vt mediated F-actin bundling31; 36. Hence, metavinculin likely acts as a negative regulator of vinculin-mediated F-actin bundling. Here, we employed low-speed pelleting assays to probe the effects of MVtp cardiomyopathy mutants in comparison to MVtp wild-type, on Vt-induced F-actin assemblies. Three sets of actin co-sedimentation data were acquired, with 20 μM actin and Vt:MVtp at 5:5 μM, 10:10 μM and 10:5 μM (Figure 4). Supernatant and pellet fractions were analyzed by SDS-PAGE (Figure 4A–C) and quantified (Figure 4D) as described above. In the presence of MVtp WT, a proportionate reduction of Vt-induced F-actin assemblies was observed; as ~47–52% F-actin was found in the pellet for Vt:MVtp at 1:1 and ~74% F-actin was found in the pellet for Vt:MVtp at 2:1; as opposed to 95% F-actin in the pellet for Vt alone. Interestingly, for all 3 MVtp CM mutants, at both 1:1 and 1:2 ratios with Vt, almost all of F-actin was found in the pellet fractions; ~87–95% for A934V, ~81–90% for ΔL954, and ~84–89% for R975W. Thus, in a dramatic contrast to the MVtp WT, the amount of F-actin existing in the pellets representing higher-order actin assemblies, was reduced only marginally by the MVtp CM mutants. These results indicate that unlike wild-type MVt or MVtp, MVtp cardiomyopathy mutants are unable to negatively regulate higher order actin assemblies in the presence of Vt.
Figure 4:
Metavinculin tail cardiomyopathy mutants fail to inhibit vinculin tail induced F-actin bundling. (A-C) Representative SDS-PAGE analysis of low speed F-actin co-sedimentation (12K g, 15 min) assays incubated with Vt in the presence of MVtp WT protein (labeled as MVtp) or MVtp CM mutants (labeled as A934V, ΔL954, R975W) at indicated concentrations (S - supernatant, P - pellet). (D) Quantification of the actin fraction present in pellets representing higher-order F-actin assemblies that include F-actin bundles in case of Vt. Error bars represent standard deviation (SD) (n=2, 5 replicates for each n). At both 1:1 and 2:1 ratio with Vt, MVtp WT proportionately reduces the amounts of higher-order F-actin assemblies, but in all cases MVtp CM mutants fail to inhibit such Vt-induced higher order F-actin assemblies. Statistical significances in panel D are indicated by; non-significant (n.s.), p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****), p < 0.00001 (*****) with respect to Vt.
MVt cardiomyopathy mutants coalesce Vt-mediated bundles into large higher-order actin assemblies
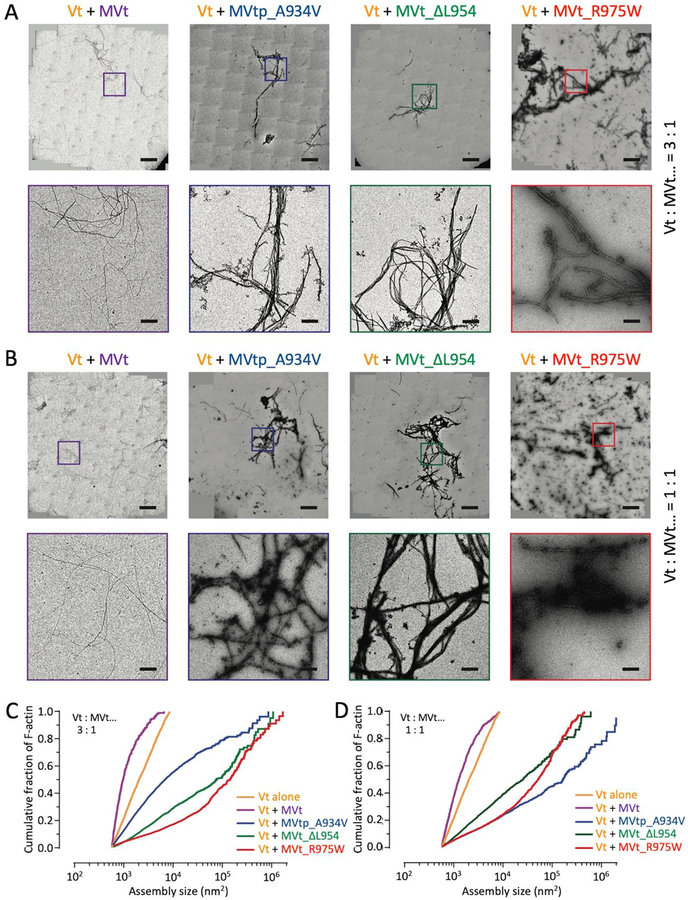
We next employed negative-stain EM to visualize the morphology and quantify the size distributions of higher order assemblies formed in the presence of both Vt and MVt or MVtp wild-type and cardiomyopathy mutants. Consistent with previous reports30; 32; 36 and co-sedimentation assays (Figure 4), we find that the presence of MVt WT inhibits the bundling activity of Vt at both a sub-stoichiometric 1:3 ratio (Figure 5A,C) and stoichiometric 1:1 ratio (Figure 5B,D). An examination of the images suggests a reduction in large parallel bundles (Figure 5A,B). However, we find that all MVt or MVtp CM mutants dramatically increase the size of F-actin assemblies formed in the presence of Vt, at both ratios (Figure 5A–D). These results are consistent with our co-sedimentation assays demonstrating almost the entire population of actin pelleting in the presence of Vt + MVtp CM mutants as with Vt alone (Figure 4), but additionally suggest that even larger assemblies are formed than in the presence of Vt alone.
Figure 5:
MVt cardiomyopathy mutants aggregate Vt-induced actin bundles. (A) Stitched negative-stain EM images of F-actin in the presence of Vt + WT or CM mutant MVt(p) at Vt to MVt(p) ratio of 3:1 (bars = 10 μm). Zoomed views of the boxed regions are shown in the bottom panel (bars = 1 μm). F-actin 0.5 μM, Vt 3.75 μM and MVt(p) 1.25 μM. (B) Stitched negative-stain EM images of F-actin in the presence of Vt + WT or CM mutant MVt(p) at Vt to MVt(p) ratio of 1:1 (bars = 10 μm). Zoomed views of the boxed regions are shown in the bottom panel (bars = 1 μm). F-actin 0.5 μM, Vt 2.5 μM and MVt(p) 2.5 μM. (C) Cumulative plots of F-actin assemblies in the presence of Vt to MVt(p) at ratio of 3:1. (D) Cumulative plots of F-actin assemblies in the presence of Vt to MVt(p) at ratio of 1:1. Pair-wise comparisons show all distributions to be significantly different (KS test, p < 0.0001) in panels C and D. N > 10 fields and n > 655 regions were quantified for each condition in panel C and N > 10 fields and n > 2241 regions were quantified for each condition in panel D. Vt alone data are replotted from Figure 3.
The morphology of these assemblies is consistent with a combined effect between what we observe for the MVt or MVtp CM mutants alone, which primarily form disordered meshes (Figure 3B) and Vt-mediated bundling. Linear bundles are clearly visible (Figure 5A–B); however, these are frequently coated with large, amorphous aggregates (Figure 5A–B), which are rarely observed in the presence of Vt alone, MVt alone, or Vt + MVt WT (Figure 3A–B). This observation is consistent with MVt or MVtp CM mutants driving the coalescence and aggregation of Vt-induced actin bundles rather than inhibiting their formation, as is the case for MVt WT.
The MVt-specific insert region forms an additional sub-domain upon actin binding
In the cryo-EM reconstruction determined for MVt and F-actin complex31, density for the N-terminus containing the H1’ or displaced H1 and straps was not observable. This is likely due to dynamics or disorder associated with these regions. To gain structural insight into rearrangements associated with MVt NT, CT and the insert upon actin binding, we employed discrete molecular dynamics simulations39. Our model system comprises a single MVt including residues 896–1134 bound to an actin homodimer (F-actin) using the deposited model based on the cryo-EM reconstruction as the starting point (PDB ID 3JBK) for MVt residues 985–1115 and actin. The simulations were performed using replica exchange for 2 million steps (see Methods for details). One hundred minimal energy structures were selected and clustered.
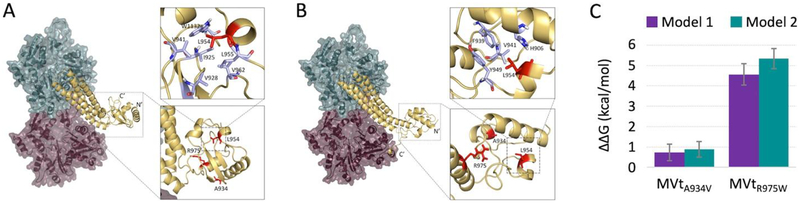
We identified two clusters (Figure 6), which differ in N- and C-termini conformations. While structures from the first cluster have tightly intertwined N- and C-termini (Figure 6A), structures from the second cluster show the C-terminus interacting with the surface of F-actin (Figure 6B). The common feature of both clusters is formation of a new additional structural sub-domain protruding outwards from F-actin. We further explored stability of the protruding sub-domains in both clusters. We subjected structures representing centroids of these clusters to DMD simulations. For each structure, we ran 10 independent simulations at two different temperatures (T1=0.5 kcal/(mol kB) and T2=0.55 kcal/(mol kB)). In further support of the protruding MVt sub-domain that is formed upon F-actin engagement, the insert-dependent protruding structural element did not unfold and appeared stable throughout the simulations (Supplemental Video 1,2). We argue that this sub-domain mediates unique biological functions of MVt relative to Vt, such as the inability to produce F-actin bundles30; 31; 32 and the ability to suppress Vt-mediated F-actin bundling31; 36. We also propose that formation of this higher order structure may sterically occlude formation of tightly-packed parallel bundles. As the CM mutants perturb the ability of MVt to inhibit bundling, we hypothesize that CM mutations within the insert impair formation of the protruding globular structure formed at the N-terminus.
Figure 6:
Actin binding to MVt may induce a higher order structural element that prevents F-actin bundling. Discrete molecular dynamics simulations identify two distinct MVt-actin clusters. In contrast to Vt, actin binding to MVt induces a protruding sub-domain. Representative MVt and F-actin models show that additional structure is formed between N-terminus, insert and C-terminus in the first cluster (A) and between N-terminus and insert in the second cluster (B). Metavinculin tail residues mutated in cardiomyopathies (A934, L954, R975) are colored red. Point mutations A934V and R975W cause an increase in ΔΔG (D) and thus destabilize the folded sub-domain. These findings suggest that MVt cardiomyopathy mutants fail to antagonize Vt-induced actin bundling due to destabilization of the additional folded structure.
To test this hypothesis, we assessed the effect of these mutants on the stability of the globule for each of the two clusters40. We find that A934V and R975W are indeed destabilizing to formation of the actin-induced MVt higher order structure observed in both clusters. For the first model ΔΔGA934V=0.73±0.41 and ΔΔG-R975W=4.56±0.52, and for the second model ΔΔG-A934V=0.88±0.39 and ΔΔG-R975W=5.34±0.49. We are unable to calculate ΔΔG for the ΔL954 since backbone is altered by the deletion40. However, we note that in each of the models, L954 forms an extensive network of hydrophobic contacts (zoomed in regions, Figure 6A–B) and its deletion would be expected to cause disruptions of these networks. Interestingly, correlating with the severity of CM mutations32; 33, the R975W exhibits a much higher ΔΔG.
Discussion
Debilitating heart conditions resulting from cardiomyopathies are a major health issue. According to Centers for Disease Control and Prevention (CDC), as many as 1 in 500 adults may have this condition41; 42. Both dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM) have been shown to be associated with inherited and sporadic mutations in genes encoding the cardiac tissue-specific vinculin isoform, metavinculin. Here we have investigated the effects of disease-associated point mutations that occur within the 68-residue metavinculin insert that differentiates the actin-binding tail domains of vinculin (Vt) and metavinculin (MVt). We and others have previously shown that MVt lacks the actin bundling activity of Vt, and furthermore that MVt can negatively-regulate the formation of Vt-induced bundles at physiologically relevant sub-stoichiometric ratios26; 27. It is interesting to note that, while vinculin is ubiquitously expressed in all tissue types, metavinculin is co-expressed only in cardiac and smooth muscle cells. Both of these cell types exhibit a high degree of contractility for proper functioning, with increased metavinculin expression corresponding to the contractile load on the tissue26. Cardiomyocytes, in particular, undergo rapid contraction and expansion during beating for regular heart function. It is plausible that if only vinculin is present, the heart muscle would become stiff due to a large network of thick F-actin fibers and would not retain the necessary contractile properties. Co-expression of metavinculin may thus reduce vinculin-mediated actin bundling so that cardiac cells remain flexible and functional, acting as a molecular rheostat. The main finding of our study, that metavinculin CM mutants are dysfunctional in this regard, make the strong prediction that cardiomyocytes expressing them should stiffen, and experiments are ongoing to test this hypothesis.
In this paper, we dissect the biophysical mechanisms underlying the metavinculin-vinculin interplay in F-actin fiber formation, and the dysregulation of this process by disease-associated point mutations in metavinculin. We previously reported that the N-terminal helices in both Vt (H1) and MVt (H1’) are released from the H2–H4 helical bundle upon actin engagement, and that Vt H1 residue M898, which is buried in the bundle’s hydrophobic core in the pre-bound conformation, is important for actin bundling by Vt31. This led us to speculate that the H1 could mediate bundling contacts between Vt molecules after actin binding (Figure 7A), and furthermore, that released H1’ and NtS’ plus upstream disordered sequence in MVt could be important for its inhibitory activity by unknown mechanisms. As metavinculin CM-associated point mutants are located within the insert composing H1’ and NtS’, but distal from the direct actin-binding H2–H4 regions, we hypothesized these lesions would compromise MVt’s regulation of Vt-mediated actin bundling without impacting actin binding. The results we present here are broadly consistent with this model. Using co-sedimentation assays, we find that all of the CM mutants we examined have unimpaired actin binding affinity. However, both co-sedimentation assays and negative stain EM experiments clearly demonstrate that all of the mutants have a strikingly similar defect in regulating Vt’s actin bundling activity. These results strongly indicate that the H1’-NtS’ region is required for metavinculin’s regulation of vinculin, and furthermore motivate us to speculate that defects in this process may underlie the pathophysiology of metavinculin CM mutants in vivo, a hypothesis which will guide future cell biological and animal studies.
Figure 7:
Model for inhibition of Vt-induced F-actin bundle by wild-type MVt (labeled as MVt(WT)) but failure of that by MVt cardiomyopathy mutants (labeled as MVt(CM)). (A) Release of H1 upon F-actin engagement enables Vt dimerization thus resulting in parallel F-actin bundle formation. (B) An additional protruding structural sub-domain formed by the insert and displaced H1 at the N-terminus of MVt(WT) blocks homo- or hetero-dimer formation with Vt, thus preventing F-actin bundling. (C) The protruding sub-domain is destabilized by the cardiomyopathy related mutations in MVt(CM), resulting in disordered F-actin assemblies due to alternative interactions with another subunit of MVt(CM) (left) or a subunit of Vt (right).
Mechanistically, our studies lead us to propose a steric occlusion model for metavinculin’s ability to negatively regulate vinculin-mediated actin bundling in sub-stoichiometric amounts, orchestrated by differential folding of the MVt domain upon actin binding. Our simulation studies suggest that the MVt specific-insert coordinates the folding of a protruding globular sub-domain upon actin-binding-induced release from the H2–H4 helical bundle. While the detailed structure of the Vtdimer that promotes 3D actin bundles is unknown and remains an important subject for future studies, electron tomographic studies of Vt-induced 2D F-actin arrays on lipid monolayers suggested that filaments are very tightly apposed when cross-linked by Vt, with intimate contacts between Vt molecules mediating the interface43. We thus propose that the MVt sub-domain acts a steric block, and prevents actin filaments from coming close enough together for Vt molecules to bind (Figure 7B). We note that this simple model readily explains the ability of sub-stoichiometric amounts of MVt to inhibit Vt bundling, as it does not require any specific molecular interactions between MVt and Vt to, e.g., occupy a binding interface required for dimerization on Vt.
Consistent with this model, our computational studies suggest MVt CM mutants destabilize sub-domain formation, thereby removing the steric block to Vt dimerization and bundle formation (a loss of function). Furthermore, the R975W substitution had the greatest impact on ΔΔG of sub-domain formation (Figure 6C) consistent with the enhanced severity of this lesion in vivo32; 33 relative to the other the CM mutations we tested32. However, the sub-domain blockage model can only partially explain the behavior we observed for the CM mutants. All of the mutants we tested also have a gain-of-function effect on their own, inducing increased formation of higher-order F-actin assemblies (Figure 3) with a disordered, mesh-like morphology. Furthermore, our studies suggest that the CM mutants drive the coalescence of Vt induced bundles into aberrantly large assemblies (Figure 5), which could also play a role in their pathophysiology in vivo. We speculate that CM mutations stimulate aggregation through the MVt insert region, which becomes exposed due to defects in sub-domain or globule folding, an effect which would be enhanced by high local concentrations within F-actin assemblies (Figure 7C). Overall, our results suggest that small-molecules which enhance the folding and stability of the predicted MVt globule may have therapeutic potential for patients with CM mutations in the metavinculin insert, abrogating both loss- and gain-of-function effects, and the findings we have presented will motivate future efforts in this direction.
While our studies have focused on Vt and MVt interactions with F-actin, vinculin is a scaffold protein that interacts with a number of proteins and inositol phospholipids (e.g., phosphatidylinositol 4, 5-bisphosphate). Hence, the metavinculin insert may alter association with other ligands that bind to the tail domain. Experiments are in progress to investigate whether metavinculin coordinately regulates membrane association of vinculin through direct binding to phosphatidylinositol 4, 5-bisphosphate or additional ligand interactions with the tail and proline-rich domain. Modulation of these additional interactions could affect localization, formation of structural assemblies and mechanotransduction properties.
Materials & Methods
Protein Expression and Purification
Vinculin tail (Vt) containing residues 879–1066 of the chicken sequence was cloned into pQlinkH vector (Addgene, Cambridge, MA). Metavinculin tail (MVt) containing residues 879–1134 and metavinculin tail plus proline-rich linker (MVtp) containing residues 858–1134 of the human sequence were cloned in 2HR-T vector (Addgene, Cambridge, MA). Plasmids for the MVt and MVtp cardiomyopathy mutants, namely A934V, ΔL954 and R975W, were prepared using appropriate primers (IDT, Skokie, IL) and QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) and verified by DNA sequencing (Genewiz, South Plainfield, NJ). All of the Vt and MVt/MVtp vectors contained an N-terminal TEV cleavable hexa-histidine tag. Vectors were transformed into Escherichia coli strain BL21(DE3) and cells were first grown at 37 °C to an optical density of 0.6–0.8 (600 nm). Protein expression was then initiated by addition of isopropyl-D-1-thiogalactopyranoside (0.5 mM for Vt, 1 mM for MVt/MVtp). Cells were then grown at 18 °C overnight and harvested by centrifugation (4.5k rpm, 30 min). Cell pellets were resuspended in lysis buffer (20 mM Tris, 150 mM NaCl, 5 mM imidazole, 2 mM β-mercaptoethanol, pH 7.5 for Vt and 50 mM Tris, 200 mM NaCl, 10 mM imidazole, 2 mM β-mercaptoethanol, pH 8.0 for MVt/MVtp). Cells were lysed by sonication. Vt and MVt/MVtp proteins remained in the soluble fractions that were separated from the particulate fractions by centrifugation (15k rpm, 45 min). Proteins were purified by affinity separation using Ni-NTA-agarose beads (Qiagen). Proteins were bound to the beads through His-tag. Wash buffer (20 mM Tris, 150 mM NaCl, 60 mM imidazole, 2 mM β-mercaptoethanol, pH 7.5 for Vt and 50 mM Tris, 200 mM NaCl, 25 mM imidazole, 2 mM β-mercaptoethanol, pH 8.0 for MVt/MVtp) was run through the column before eluting target proteins using elution buffer (20 mM Tris, 150 mM NaCl, 500 mM imidazole, 2 mM β-mercaptoethanol, pH 7.5 for Vt and 50 mM Tris, 200 mM NaCl, 250 mM imidazole, 2 mM β-mercaptoethanol, pH 8.0 for MVt/MVtp). For His-tag removal, eluted volume was dialyzed into TEV cleavage buffer (20 mM Tris, 150 mM NaCl, 50 mM imidazole, 2 mM β-mercaptoethanol, pH 7.5 for Vt and 50 mM Tris, 200 mM NaCl, 20 mM imidazole, 2 mM β-mercaptoethanol, pH 8.0 for MVt/MVtp) overnight at 4 °C in presence of TEV. Vt and MVt/MVtp proteins were collected by re-running the dialyzed/cleaved volumes over Ni-NTA-agarose beads. Size exclusion chromatography by S100 column (GE, Pittsburg, PA) was used to obtain the highest level of purity in gel filtration buffer (10 mM Tris, 200 mM KCl, 10 mM imidazole, 2.5 mM MgCl2, 1 mM EGTA, 2 mM DTT, pH 7.5). Purified proteins were concentrated between 200 to 500 μM by centrifugation, aliquoted and snap frozen using liquid nitrogen. Protein stocks were stored at −80 °C.
Actin co-sedimentation
The actin binding and bundling (cross-linking) properties of individual Vt and MVt/MVtp WT & CM proteins as well as their mixtures were investigated using an adapted actin co-sedimentation assay previously reported44. Monomeric actin (G-actin), purified from rabbit muscle acetone powder (Pel-Freez Biologicals, Rogers, AR), was stored at −80 °C in storage buffer (50 mM imidazole, 100 mM NaCl, 10 mM MgCl2, 10 mM EGTA, 0.5 mM DTT, 0.2 mM ATP, pH 7.0). Polymerization to filamentous actin (F-actin) was done by diluting and incubating G-actin at 100 μM concentration in actin polymerization buffer (10 mM Tris, 200 mM KCl, 10 mM imidazole, 2.5 mM MgCl2, 1 mM EGTA, 2 mM DTT, pH 7.5) at room temperature for 30 min. The actin concentrations reported in this work were based on G-actin concentration, since the heterogeneity of F-actin polymers made it difficult to quantify F-actin concentrations. Vt and MVt/MVtp variants were also diluted by actin polymerization buffer to prepare 100 μM stocks. To assess actin binding, 100 ul samples were prepared containing 10 μM Vt/MVt/MVtp variants and 10 or 20 μM actin. The samples were incubated at room temperature for 1 hr and then centrifuged at 100,000 RCF for 30 min. To assess actin bundling, 100 ul samples were prepared containing 10–20 μM Vt/MVt/MVtp variants and 10 μM actin. The samples were incubated at room temperature for 1 hr and then centrifuged at 12,000 RCF for 15 min. For both binding and bundling co-sedimentation, the supernatant and pellet were separated, resuspended to equal volumes, and analyzed by 15% SDS-PAGE. Actin binding properties were calculated by determining the fractions of Vt/MVt/MVtp variants present in pellets using the densities of the pellet and supernatant bands. Actin bundling properties were calculated by determining the fractions of actin present in pellets using the densities of the pellet and supernatant bands. Densitometry was performed using ImageJ38. Statistical significances (p values) of the measurements were determined using the Microsoft Excel t-Test function.
Negative staining and TEM
For the experiments displayed in Figure 1, an aliquot of actin (1 μM) without or with Vt, MVt, or MVtp (10 μM) was incubated in actin polymerization buffer (10 mM Tris, 200 mM KCl, 10 mM imidazole, 2.5 mM MgCl2, 1 mM EGTA, 2 mM DTT, pH 7.5) for 15 minutes and absorbed directly onto glow-discharged carbon-coated 400 mesh copper grids for 3 minutes, and then stained with 2% (w/v) uranyl acetate in water. Transmission electron microscopy (TEM) images were obtained using a FEI Tecnai 12 electron microscope at 80 kV and captured on a Gatan First Light CCD camera using Gatan Digital Micrograph software (Gatan, Pleasanton, CA).
For the experiments displayed in Figures 3 and 5, F-actin and the indicated Vt ± MVt constructs were mixed in KMEI (50 mM KCl, 1 mM MgCl2, 1 mM ethylene glycol bis(b-aminoethyl ether) N,N’-tetraacetic acid (EGTA), 10 mM imidazole, 1 mM dithiothreitol (DTT), pH 7.0) and incubated at room temperature for 15 min. Sample (4 μl) was then applied to a glow-discharged continuous carbon grid (Ted Pella) and incubated for 60 s. After incubation, the grid was washed with three 100 μl drops of 1 % uranyl acetate, then blotted to dryness. Images were acquired with the SerialEM package45 on a Tecnai F20 operating at 120 kV with a Gatan Ultrascan 4000 CCD camera. Tiled images with 20% overlap were acquired at 7,800 magnification, 3 mm underfocus, and four-fold camera binning, corresponding to a calibrated pixel size of 5.7 nm at the specimen level. Stitched images were assembled with the “blendmont” program from the IMOD software package46.
F-actin assembly quantification
Images were thresholded and binarized using ImageJ38, then segmented into contiguous regions of pixels using the built-in “Analyze Particles” plugin, including regions 100–500,000 pixels in size and with a circularity of 0–0.3. This procedure does not always capture every region that an expert user would designate to contain F-actin in every image (e.g. areas of Supplementary Figure 1A, right, which are not outlined in yellow). However, we find its performance superior to both manual segmentation of the images, which requires user decisions on region boundaries and the minimum size of regions, as well as a sliding-box quantification (a measure of local density), which is extremely sensitive to noise introduced by slight differences in thresholding (data not shown).
Size measurements of regions were pooled from all images for a given condition, then divided into 10,000 equally-sized bins per dataset and plotted via a normalized cumulative histogram (Supplementary figure 1B). Data were binned and cumulative sums calculated with a python script (available at www.github.com/alushinlab/FactinAssemblyQuant) using the function “binned_statistic” implemented in SciPy (www.scipy.org). Plots were generated and statistical tests were conducted with GraphPad Prism.
MD simulation
Modeling was performed using Discrete Molecular Dynamics (DMD) package39; 40; 47. The initial structure was obtained by extending missing N- and C-termini of MVt (PDB ID: 3JBK)31 with PYMOL built-in tool to the range of 896–1134 residues. The initial structure was relaxed at temperature T=0.5 with high heat exchange coefficient Cex=10 for 10,000 steps. The temperature unit is kcal/(mol kB). The relaxation was followed by replica exchange simulations with 10 replicas (T=0.330, 0.360, 0.390, 0.420, 0.450, 0.480, 0.510, 0.540, 0.570, 0.600), Cex=0.1 for 2 million steps. Replicas were exchanged every 1000 steps. To preserve contacts between MVt and actin, we applied harmonic constraints to the N, CA, C backbone atoms of selected residues (R1044, I1045, N1048, R1055, T1058, I1059, Q1062, I1065, Q1086, E1089, M1090, H1093, N1094, E1104, R1107, E1108, A1111, I1114). These constraints restrict atoms to move within 2A around initial positions. All atoms within actin were considered static and were not allowed to move. 100 lowest energy structures were selected and clustered based on pairwise Root Mean Square Distance between structures. Two clusters were identified.
Structures representing centroid of two clusters were subject to DMD simulations at two constant temperatures T1=0.5 kcal/(mol kB) and T2=0.55 kcal/(mol kB). For each temperature and for each structure, 5 independent simulations were run for 1 million steps with Cex=0.1. To preserve contacts between MVt and actin, we applied harmonic constraints to N, CA and C backbone atoms of selected MVt residues (as described above). All atoms within actin were considered static.
Supplementary Material
Supplementary figure 1: Quantification of actin assemblies from negative stain images.
Supplemental video 1: A single DMD trajectory of the structure representing centroid of the first cluster (Figure 6A). The trajectory is obtained at T=0.5 kcal/(mol kB).
Supplemental video 2: A single DMD trajectory of the structure representing centroid of the second cluster (Figure 6B). The trajectory is obtained at T=0.5 kcal/(mol kB).
Highlights.
The cardiac tissue-specific splice isoform of vinculin, metavinculin, negatively regulates vinculin-mediated higher order F-actin assembly.
Metavinculin cardiomyopathy mutants promote disordered F-actin assembly.
Computational modeling suggests that actin binding to metavinculin induces a conformational change within the metavinculin-specific insert region and its displaced N-terminus, to form a protruding sub-domain, that prevents dimerization and Vt-induced actin bundling.
Cardiomyopathy mutations are predicted to destabilize the protruding sub-domain thereby leading to the loss of metavinculin’s regulatory role.
Acknowledgements
We gratefully acknowledge Jenny Hinshaw (NIDDK) for shared use of her F20 TEM and Lucas Axiotakis Jr. for preliminary efforts in quantification of EM data. This work was supported by the NIH (RO1GM115597, PI: S.C.), (7DP5OD017885, PI: G.M.A.), (F31HL131429, PI: H.L.), (GM31819 and ES013773, PI: J.G.) and start-up funds from the Rockefeller University (PI: G.M.A).
References
- 1.Parsons JT, Horwitz AR & Schwartz MA (2010). Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nature reviews. Molecular cell biology 11, 633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler WH, Liddington RC & Critchley DR (2006). The structure and regulation of vinculin. Trends in cell biology 16, 453–60. [DOI] [PubMed] [Google Scholar]
- 3.Xu W, Baribault H & Adamson ED (1998). Vinculin knockout results in heart and brain defects during embryonic development. Development 125, 327–37. [DOI] [PubMed] [Google Scholar]
- 4.Coll JL, Ben-Ze’ev A, Ezzell RM, Rodriguez Fernandez JL, Baribault H, Oshima RG & Adamson ED (1995). Targeted disruption of vinculin genes in F9 and embryonic stem cells changes cell morphology, adhesion, and locomotion. Proceedings of the National Academy of Sciences of the United States of America 92, 9161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subauste MC, Pertz O, Adamson ED, Turner CE, Junger S & Hahn KM (2004). Vinculin modulation of paxillin-FAK interactions regulates ERK to control survival and motility. The Journal of cell biology 165, 371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakolitsa C, Cohen DM, Bankston LA, Bobkov AA, Cadwell GW, Jennings L, Critchley DR, Craig SW & Liddington RC (2004). Structural basis for vinculin activation at sites of cell adhesion. Nature 430, 583–6. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RP & Craig SW (1994). An intramolecular association between the head and tail domains of vinculin modulates talin binding. The Journal of biological chemistry 269, 12611–9. [PubMed] [Google Scholar]
- 8.Weiss EE, Kroemker M, Rudiger AH, Jockusch BM & Rudiger M (1998). Vinculin is part of the cadherin-catenin junctional complex: complex formation between alpha-catenin and vinculin. The Journal of cell biology 141, 755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroemker M, Rudiger AH, Jockusch BM & Rudiger M (1994). Intramolecular interactions in vinculin control alpha-actinin binding to the vinculin head. FEBS letters 355, 259–62. [DOI] [PubMed] [Google Scholar]
- 10.Brindle NP, Holt MR, Davies JE, Price CJ & Critchley DR (1996). The focal-adhesion vasodilator-stimulated phosphoprotein (VASP) binds to the proline-rich domain in vinculin. The Biochemical journal 318 (Pt 3), 753–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeMali KA, Barlow CA & Burridge K (2002). Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. The Journal of cell biology 159, 881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kioka N, Sakata S, Kawauchi T, Amachi T, Akiyama SK, Okazaki K, Yaen C, Yamada KM & Aota S (1999). Vinexin: a novel vinculin-binding protein with multiple SH3 domains enhances actin cytoskeletal organization. The Journal of cell biology 144, 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandai K, Nakanishi H, Satoh A, Takahashi K, Satoh K, Nishioka H, Mizoguchi A & Takai Y (1999). Ponsin/SH3P12: an l-afadin- and vinculin-binding protein localized at cell-cell and cell-matrix adherens junctions. The Journal of cell biology 144, 1001–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huttelmaier S, Bubeck P, Rudiger M & Jockusch BM (1997). Characterization of two F-actin-binding and oligomerization sites in the cell-contact protein vinculin. European journal of biochemistry 247, 1136–42. [DOI] [PubMed] [Google Scholar]
- 15.Johnson RP, Niggli V, Durrer P & Craig SW (1998). A conserved motif in the tail domain of vinculin mediates association with and insertion into acidic phospholipid bilayers. Biochemistry 37, 10211–22. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Rangarajan ES, Yogesha SD & Izard T (2009). Raver1 interactions with vinculin and RNA suggest a feed-forward pathway in directing mRNA to focal adhesions. Structure 17, 833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler J, Lunsdorf H & Jockusch BM (1996). The ultrastructure of chicken gizzard vinculin as visualized by high-resolution electron microscopy. Journal of structural biology 116, 270–7. [DOI] [PubMed] [Google Scholar]
- 18.Golji J, Wendorff T & Mofrad MR (2012). Phosphorylation primes vinculin for activation. Biophysical journal 102, 2022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galbraith CG, Yamada KM & Sheetz MP (2002). The relationship between force and focal complex development. The Journal of cell biology 159, 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannone G, Jiang G, Sutton DH, Critchley DR & Sheetz MP (2003). Talin1 is critical for force-dependent reinforcement of initial integrin-cytoskeleton bonds but not tyrosine kinase activation. The Journal of cell biology 163, 409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B & Bershadsky AD (2001). Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. The Journal of cell biology 153, 1175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T & Schwartz MA (2010). Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byrne BJ, Kaczorowski YJ, Coutu MD & Craig SW (1992). Chicken vinculin and meta-vinculin are derived from a single gene by alternative splicing of a 207-base pair exon unique to meta-vinculin. The Journal of biological chemistry 267, 12845–50. [PubMed] [Google Scholar]
- 24.Burridge K & Feramisco JR (1980). Microinjection and localization of a 130K protein in living fibroblasts: a relationship to actin and fibronectin. Cell 19, 587–95. [DOI] [PubMed] [Google Scholar]
- 25.Feramisco JR, Smart JE, Burridge K, Helfman DM & Thomas GP (1982). Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. The Journal of biological chemistry 257, 11024–31. [PubMed] [Google Scholar]
- 26.Belkin AM, Ornatsky OI, Kabakov AE, Glukhova MA & Koteliansky VE (1988). Diversity of vinculin/meta-vinculin in human tissues and cultivated cells. Expression of muscle specific variants of vinculin in human aorta smooth muscle cells. The Journal of biological chemistry 263, 6631–5. [PubMed] [Google Scholar]
- 27.Witt S, Zieseniss A, Fock U, Jockusch BM & Illenberger S (2004). Comparative biochemical analysis suggests that vinculin and metavinculin cooperate in muscular adhesion sites. The Journal of biological chemistry 279, 31533–43. [DOI] [PubMed] [Google Scholar]
- 28.Maeda M, Holder E, Lowes B, Valent S & Bies RD (1997). Dilated cardiomyopathy associated with deficiency of the cytoskeletal protein metavinculin. Circulation 95, 17–20. [DOI] [PubMed] [Google Scholar]
- 29.Rangarajan ES, Lee JH, Yogesha SD & Izard T (2010). A helix replacement mechanism directs metavinculin functions. PloS one 5, e10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janssen ME, Liu H, Volkmann N & Hanein D (2012). The C-terminal tail domain of metavinculin, vinculin’s splice variant, severs actin filaments. The Journal of cell biology 197, 585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim LY, Thompson PM, Lee HT, Pershad M, Campbell SL & Alushin GM (2016). The Structural Basis of Actin Organization by Vinculin and Metavinculin. Journal of molecular biology 428, 10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson TM, Illenberger S, Kishimoto NY, Huttelmaier S, Keating MT & Jockusch BM (2002). Metavinculin mutations alter actin interaction in dilated cardiomyopathy. Circulation 105, 431–7. [DOI] [PubMed] [Google Scholar]
- 33.Vasile VC, Will ML, Ommen SR, Edwards WD, Olson TM & Ackerman MJ (2006). Identification of a metavinculin missense mutation, R975W, associated with both hypertrophic and dilated cardiomyopathy. Molecular genetics and metabolism 87, 169–74. [DOI] [PubMed] [Google Scholar]
- 34.Shen K, Tolbert CE, Guilluy C, Swaminathan VS, Berginski ME, Burridge K, Superfine R & Campbell SL (2011). The vinculin C-terminal hairpin mediates F-actin bundle formation, focal adhesion, and cell mechanical properties. The Journal of biological chemistry 286, 45103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson RP & Craig SW (2000). Actin activates a cryptic dimerization potential of the vinculin tail domain. The Journal of biological chemistry 275, 95–105. [DOI] [PubMed] [Google Scholar]
- 36.Oztug Durer ZA, McGillivary RM, Kang H, Elam WA, Vizcarra CL, Hanein D, De La Cruz EM, Reisler E & Quinlan ME (2015). Metavinculin Tunes the Flexibility and the Architecture of Vinculin-Induced Bundles of Actin Filaments. Journal of molecular biology 427, 2782–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer T, Brink U, Unterberg C, Stohr S, Kreuzer H & Buchwald AB (1994). Expression of meta-vinculin in human coronary arteriosclerosis is related to the histological grade of plaque formation. Atherosclerosis 111, 111–9. [DOI] [PubMed] [Google Scholar]
- 38.Schneider CA, Rasband WS & Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nature methods 9, 671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding F, Tsao D, Nie H & Dokholyan NV (2008). Ab initio folding of proteins with all-atom discrete molecular dynamics. Structure 16, 1010–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin S, Ding F & Dokholyan NV (2007). Eris: an automated estimator of protein stability. Nature methods 4, 466–7. [DOI] [PubMed] [Google Scholar]
- 41.Maron BJ, Doerer JJ, Haas TS, Tierney DM & Mueller FO (2009). Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation 119, 1085–92. [DOI] [PubMed] [Google Scholar]
- 42.Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH 3rd, Spirito P, Ten Cate FJ & Wigle ED (2003). American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. Journal of the American College of Cardiology 42, 1687–713. [DOI] [PubMed] [Google Scholar]
- 43.Janssen ME, Kim E, Liu H, Fujimoto LM, Bobkov A, Volkmann N & Hanein D (2006). Three-dimensional structure of vinculin bound to actin filaments. Molecular cell 21, 271–81. [DOI] [PubMed] [Google Scholar]
- 44.Dixon RD, Arneman DK, Rachlin AS, Sundaresan NR, Costello MJ, Campbell SL & Otey CA (2008). Palladin is an actin cross-linking protein that uses immunoglobulin-like domains to bind filamentous actin. The Journal of biological chemistry 283, 6222–31. [DOI] [PubMed] [Google Scholar]
- 45.Mastronarde DN (2005). Automated electron microscope tomography using robust prediction of specimen movements. Journal of structural biology 152, 36–51. [DOI] [PubMed] [Google Scholar]
- 46.Kremer JR, Mastronarde DN & McIntosh JR (1996). Computer visualization of three-dimensional image data using IMOD. Journal of structural biology 116, 71–6. [DOI] [PubMed] [Google Scholar]
- 47.Proctor EA, Ding F, & Dokholyan NV (2011). Discrete Molecular Dynamics. Wiley I.R.C. Mol. Sci 1, 80–92. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Quantification of actin assemblies from negative stain images.
Supplemental video 1: A single DMD trajectory of the structure representing centroid of the first cluster (Figure 6A). The trajectory is obtained at T=0.5 kcal/(mol kB).
Supplemental video 2: A single DMD trajectory of the structure representing centroid of the second cluster (Figure 6B). The trajectory is obtained at T=0.5 kcal/(mol kB).