INTRODUCTION
Human milk (HM) is not only considered the ideal nutrition source for term infants during the first six months of life by providing nutrients needed for growth and development 1, but it also provides antimicrobial and immunomodulatory factors providing defense against infections 2, 3. Thereby, breastfeeding provides continued exposure to the mother’s immune system during the crucial window of first few months of life, when the infant’s immune system is constantly developing. However, the immunomodulatory make up of HM has not been well-characterized and demonstrates a great deal of variability between mothers, which implies that the benefit of breastfeeding to the breastfed infants may differ between dyads. This can have an impact on the degree to which breast milk can provide benefit in individual mother-infant pairs (Figure 1).
Figure 1.
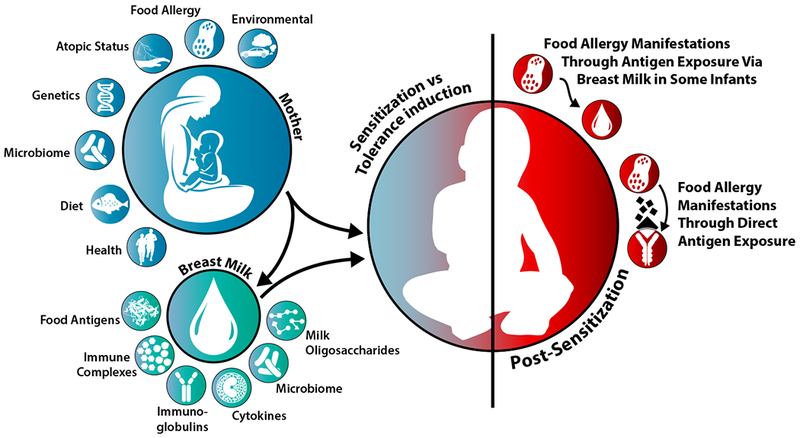
Maternal and breast milk factors that influence the development of the neonatal immune system.
Breastfeeding is currently recommended as primary prevention for allergic diseases, including food allergy (FA) 4–6. A systematic review concluded that there is an protective effect of exclusive breastfeeding against cow’s milk allergy (CMA) in early childhood among high-risk infants 7. However, more recent studies did not find an association between FA and breastfeeding, although concluded that the evidence on FA had high heterogeneity and low quality 8, insufficient to draw conclusions about breastfeeding impact on FA 9. The most recent population-based study from Australia, reported no association between breastfeeding and length of breastfeeding with FA at 1 year (likely underpowered for CMA) 10. Unfortunately, to date the majority of studies have either lacked adequate statistical power to assess the impact of breastfeeding on FA, or have not evaluated this association at all (or only assessed food sensitization) due to methodologic problems. Randomized controlled trials are lacking for ethical reasons and the definitions of breastfeeding and allergic outcomes varies greatly between existing studies.
The only randomized trial on the topic, the Promotion of Breastfeeding Intervention Trial (PROBIT) from Belarus, designed to promote breastfeeding duration and exclusivity of breastfeeding documented a reduction in gastrointestinal infections and eczema in the 12 months in the intervention group11. The 6-year followup in this cohort found that this protective effect was no longer observable; however FA was not assessed 12. Some of the discrepancy between studies may be explained by differences in HM composition, which has not been accounted for in the broad definition of breastfeeding. Breastfeeding in general may protect against wheezing and eczema by protecting against early life (viral) infections 8, but protection against FA might require more defined composition of immune factors from HM. Also, the mechanisms by which HM affords protection are largely unknown. Gene-environment interaction have also been shown to impact protection. The association between breastfeeding and food sensitization modified by single nucleotide polymorphisms (SNPs) in the interleukin (IL)-12 receptor β1, Toll-like receptor (TLR)-9, and thymic stromal lymphopoietin (TSLP) genes: for example, breastfeeding increases the risk of food sensitization in children carrying the GG genotype but decreases the risk in children carrying the GT/TT genotype of the IL-12Rβ1 13.
Breast milk originates from secretory cells within the lactating breast. The majority of HM components including lactose, milk lipids, and a variety of milk proteins are produced in the lactating cells 14. Beyond the major macronutrient components, HM also contains human milk oligosaccharides (HMOs), immune cells, cytokines, chemokines and hormones, immunoglobulins, critical growth factors, active enzymes including peroxidases and lysozymes, lactoferrin, and additional secretory components, soluble CD14, TLR2 and tumor necrosis factor (TNF) receptor, along with maternal diet-derived food antigens, and its own microbiome made up of bacteria and viruses 15, 16. Previous studies have evaluated many of these HM components and their relationship with allergy development in offspring. This review discusses most recent (last 10+ years) and selected key older studies found in PubMed assessing relationship between breastfeeding and FA in infants and young children, and summarizes existing animal and human studies that have evaluated the association of human milk immunologically active components with FA in offspring, and their impact on infant gut microbiome. This includes allergens, immunoglobulins, immune complexes, cytokines, chemokines, growth factors and HMOs, and excludes enzymes, lactoferrin, sCD14, sTLR2 and sTNFR, where no data is available in association with FA.
BREAST MILK AND INFANT GUT MICROBIOME
Some of the impact of breastfeeding on infant immune system may be due to its impact on infant gut microbiome. In addition to prenatal environment, delivery mode, antibiotic treatment, and diet, the infant microbiome composition is significantly influenced by breastfeeding 17, 18. Among breastfed infants, microbiome diversity is initially lower compared to formula fed infants, given that HM creates an intestinal environment that gives selective advantage to highly adapted intestinal microbiota, by providing HMOs. The dominant microbes of breastfed infants (Bifidobacteria, Lactobacilli, Enterobacteriaceae) are surpassed by Clostridium and Bacteroides species after cessation of breastfeeding and introduction of complementary foods 19–22. Cohort studies including the WHEALS study found that breastfeeding is one of the most influential factors in the development of the infant microbiome 18. In humans, breastfeeding is a major source of beneficial bacterial species 23, indicating the essential role of this food source in determining the microbiota in early life that may impact the later development of allergic diseases, including FA. Kourosh et al. showed that children with IgE-mediated FA had significantly different microbial composition, particularly among Clostridia species, compared with controls subjects 24 Fieten et al. 25, in a study conducted in children with atopic dermatitis, found fewer of two Bifidobacterium species (B. breve, B. adolescentis) and in addition to lower numbers of F. prausnitzii, and A. muciniphila in the fecal microbiome of children with concomitant FA compared to those without FA. Finally, Fazlollahi et al found a higher alpha-diversity of microbial flora in egg-allergic children compared to non-FA controls 26. These studies and a recent review 27 highlight that alterations in gut microbiome may be a key factor in the development of FA.
The impact of microbiome on the infant immune system is likely due to the short-chain fatty acids (SCFAs) including propionate, butyrate, and acetate that are a byproduct of microbial metabolism, and have anti-inflammatory functions 28. A recent study found that SCFAs were altered in children who are atopic 28. Similarly, in a mouse model, researchers found that higher levels the SCFAs acetate and butyrate may inhibit the development of FA by inducing tolerance via CD103+ dendritic cells 29. In studies of term infants, while overall SCFA levels are higher in formula-fed compared to breastfed infants, the highest levels of acetate are observed in exclusively breastfed infants 28, 30. Taken together, these studies support the hypothesis that one mechanism of FA protection conferred via breastfeeding may be through the influence of HM on the infant gut microbiome that leads to reduction in pro-inflammatory processes.
INSIGHTS FROM ANIMAL MODELS
Animal models of FA have been developed to explore the mechanisms involved in the development of diseases to normally harmless food allergens 31. These models of FA have been utilized to dissect how maternal factors influence the susceptibility of offspring to allergies. This section focuses on the role of breast milk factors in the development of experimental FA in offspring (Table 1).
Table 1.
Studies assessing the association between maternal allergen exposure and tolerance in offspring.
| Study | Mouse strain | Allergen | Maternal sensitization (route, timing) | Maternal allergen exposure (route, timing) | Cross-fostering by sensitized mothers | Offspring outcome | Maternal impact on offspring allergies | Comments |
|---|---|---|---|---|---|---|---|---|
| Verhasselt 2008 32 | BALB/c | OVA | n/a | aerosol, lactating | Yes | Asthma | Protection | Protection is milk TGFβ dependent |
| Mosconi 2010 33 | BALB/c | OVA | i.p., preconceptual | aerosol, lactating | Yes | Asthma | Protection | Protection is milk IC dependent |
| Turfkruyer 2016 42 | BALB/c | OVA | n/a | Oral, lactating | No | Asthma | Protection | Inefficient neonatal tolerance is due to vitamin A deficiency |
| Hamada 2003 34 | BALB/c | OVA | i.p., preconceptual | aerosol, preconceptual and pregnancy | No | Asthma | Asthma exaggeration | Lack of maternal allergen exposure during lactating |
| Macchiaverni 201435 | BALB/c | Der p | i.n. preconceptual | i.n., lactating | Yes | Asthma | Sensitization | |
| Jarvinen 2015 36 | C3H/HeJ | Peanut | Ad libitum, preconceptual | Ad libitum, pregnancy and lactating | No | FA | No impact | |
| Bernard 201437 | BALB/c | Peanut | n/a | n/a | No | FA | Protection | Human milk containing peanut and peanut-IC prevents sensitization in mice |
| Lopez-Exposito 200938 | C3H/HeJ | Peanut | Oral, preconceptual | Oral, pregnancy and lactating | No | FA | Protection | |
| Ohsaki 201839 | BALB/c | OVA, peanut | Epicutaneous, preconceptual | Epicutaneous, pregnancy and lactating | Yes | FA | Protection | Milk IC-offspring FcRn axis induces neonatal tolerance |
| Rekima 201743 | BALB/c | OVA | i.p., preconceptual | Oral, lactating | Yes | FA | Protection | Milk allergen and TGFβ supplementation protect offspring against FA |
n/a, not applicable; i.p., intraperitoneal; i.n., intranasal; OVA, ovalbumin; Der p, Dermatophagoides pteronissinus; TGFβ, transforming factor-β; IC, immune complex; FcRn, neonatal crystallizable fragment receptor; FA, food allergy;
Allergen, immunoglobulins (Igs), and immune complexes (IC)
The concept of tolerance induction by allergen in breast milk has been shown in experimental asthma 32. Aerosol allergen (ovalbumin; OVA) exposure of wild-type (WT) mothers during breastfeeding resulted in an allergen transfer to offspring through milk and the induction of allergen-specific, transforming growth factor (TGF)-β-dependent tolerance towards allergic airway inflammation. Naive offspring adopted and nursed by pre-conceptually sensitized WT mothers with OVA and alum adjuvant intraperitoneally (i.p.) exposed to OVA aerosols during breastfeeding received maternal IC (allergen and allergen-specific immunoglobulin (Ig) G) via milk and exhibited a long-lasting allergen-specific protection from asthma 33. In contrast, earlier studies have indicated maternal transmission of asthma susceptibility to offspring 34 from WT mothers pre-conceptually immunized with OVA/alum and exposed to OVA aerosol during pregnancy. As OVA-sensitized mothers were not exposed to OVA during breastfeeding, these data imply that allergen transfer via milk is critical for successful tolerance induction in offspring. Respiratory allergen from house dust mite is present in HM and primes for allergic sensitization in experimental asthma 35, suggesting that maternal exposure to environmental allergen reflected in milk may facilitate allergic sensitization, rather than protection in offspring. In conclusion, these asthma studies imply that milk-borne allergen, IC, and TGF-β may induce tolerance in offspring against asthma, while insufficient milk-borne allergen exposure or specific environmental allergen exposure of offspring may promote asthma.
Although the mechanisms of experimental asthma may not simply be applicable to experimental food allergy, these observations led to the hypothesis that allergens, Igs, and IC in milk might facilitate the induction of tolerance to food allergens in neonates. On the subject of FA, we have demonstrated that pre-conceptional peanut exposure of C3H/HeJ mothers (a substrain of C3H mice that lacks a functional toll-like receptor 4 that recognizes lipopolysaccharide) resulted in the generation of varying levels of maternal Igs in serum and breast milk. However, maternal peanut exposure preconceptionally or during pregnancy and breastfeeding had no impact on offspring’s rates of peanut allergy assessed by oral and i.p. peanut challenge in a model where peanut allergy was elicited by oral immunization with peanut and cholera toxin (CT) 36. These findings support the recommendations of no dietary restrictions for pregnant and breastfeeding mothers. Bernard and colleagues found that HM containing peanut allergens induced partial oral tolerance in peanut-sensitized young mice 37, supporting a beneficial role of milk-born allergen in decreasing disease susceptibility in offspring. A stronger immunization of C3H/HeJ mothers with oral peanut/CT and peanut exposure during pregnancy and breastfeeding indeed reduced peanut allergy risk in offspring38. Offspring of preconceptually peanut/CT sensitized mothers exhibited IgG1-mediated anaphylaxis in response to oral peanut challenge (first exposure). Interestingly, when sensitized mothers received peanut/CT also during pregnancy and breastfeeding, offspring were protected from anaphylaxis following first peanut challenge as well as active peanut/CT sensitization and oral peanut challenge. These results suggest that maternal immune responses during pregnancy and breastfeeding are critical in the reduction of disease susceptibility in offspring.
We have recently delineated for the first time the mechanisms of neonatal tolerance induction against FA, mediated by maternal IgG-IC and neonatal crystallizable fragment receptor (FcRn) 39. Maternal allergen sensitization through epicutaneous route 40 prevented food anaphylaxis and allergen-specific IgE in offspring following epicutaneous sensitization and oral challenge of offspring with allergen. This protection was mediated by FcRn-dependent transfer of maternal allergen-IgG-IC via breast milk and induction of allergen-specific Treg cells in offspring. Neonatal tolerance was induced in offspring of naive mothers that were fostered immediately after birth and nursed by OVA-sensitized mothers. The induction of tolerance was also observed in offspring of naïve mothers supplemented with IgG-IC during breastfeeding. Induction of oral tolerance in offspring required FcRn-dependent antigen presentation by CD11c+ dendritic cells. Human breast milk containing OVA-IgG-IC induced tolerance in humanized FcRn mice, providing a particularly important evidence for the potential clinical relevance of our findings in humans. Jointly, these findings from our mouse model demonstrate that interactions of offspring FcRn and maternal IgG-IC are key components of the induction of Treg cell responses and regulation of food-specific tolerance in the neonatal period. This study provided experimental evidence for the key role played by maternal allergen-specific Igs in milk in establishing effective tolerance that prevents FA in offspring. These effects extend well past the previously described roles of maternal antibodies and FcRn in the provision of passive immunity. Additionally, such food-specific IgG antibodies are also induced during oral immunotherapy in humans and have been shown to act through FcγRIIb to suppress IgE-mediated hypersensitivity 41. Strategies that favor maternal IgG responses might prove useful in the prevention of FA in offspring.
Vitamin A and TGF-β
In humans, increased risk of allergy during early childhood indicates insufficient immune regulation in this period of life. Also in mice, neonates (i.e. first week) are refractory to oral tolerance elicited by maternal allergen transfer via milk due to a physiological vitamin A deficiency 42. Unsensitized BALB/c WT mothers were fed OVA in the first, second, and third week of or throughout breastfeeding and tolerance induction in offspring to OVA-induced airway inflammation was assessed. Oral tolerance induction was fully efficient only starting third week of life. Insufficient tolerance in one-week-old neonates was associated with a reduction in gut barrier, retinaldehyde dehydrogenase expression by mesenteric lymph node CD103+ neonatal dendritic cells, and serum retinol levels as compared to those in adult mice. Vitamin A supplementation rescued neonatal defects and resulted in sufficient tolerance induction in one-week-old mice. Oral tolerance was dependent on interferon (IFN)-γ. These results may suggest vitamin A supplementation together with maternal allergen transfer via milk as possible interventions for allergy prevention in the neonatal stage of life.
To assess the duration of oral tolerance induced by breast milk towards FA, BALB/c WT mothers were pre-conceptually immunized i.p. with OVA/alum then fed OVA during breastfeeding while nursing offspring from unsensitized mothers 43. Breastfeeding by OVA-mothers resulted in a decrease in frequencies of allergic diarrhea and serum levels of mast cell proteinase-1 following repeated oral OVA challenges in 6-week-old, but not in 13-week-old, offspring. Supplementation with TGF-β after weaning till 12 weeks prolonged protection against diarrhea and improved gut barrier in 13-week-old mice breastfed by OVA-mothers. Although the precise mechanisms responsible for allergic diarrhea are not fully understood, expansion of intestinal mast cells is a common phenomenon in food allergic patients 44, 45 and has been associated with disease severity in experimental FA models 46, 47 Breastfeeding by allergen-sensitized mothers together with offspring TGF-β supplementation may provide long-lasting prevention of allergic diarrhea and intestinal mast cell expansion.
Food-specific IgG and food allergen-immune complexes are present in sera and breast milk of healthy human subjects 48–53. Allergen-sensitized female mice in these studies above are not necessary allergic or atopic but were used to investigate the role of maternal allergen and allergen-specific immunoglobulins in milk in influencing offspring allergies. Analysis of how atopic status of mothers may influence tolerance induction or food allergy in offspring in humans will be an important future issue to be addressed. One of the critical advantages of using mouse models to study the impact of breast milk on the development of FA in offspring is that sensitization or tolerance can be induced to specific allergens under controlled environmental conditions within defined genetic backgrounds at specific timing, which is not possible in human subjects. This aspect of mouse models allows extensive and precise investigations into the mechanisms involved in maternal-fetal interaction during breastfeeding period, such as identification of possible triggers of food sensitization, as well as pathways involved in the induction of tolerance towards FA. Increasing animal studies underscore the critical role of breast milk in the induction of tolerance towards FA in offspring.
HUMAN MILK IMMUNOMODULATORY COMPONENTS AND FOOD ALLERGY
Dietary allergen and maternal diet
Debate over whether the presence of food antigens in HM results in sensitization or tolerance in infants is ongoing. The detection of specific dietary antigens in HM following maternal consumption has been well established for a wide variety of food proteins, including ovalbumin 54, 55, β-lactoglobulin 56, 57, gliadin 58, and peanut 37, 59–61. Although maternal ingestion of dietary allergens can induce symptoms in some sensitized infants 62, this is certainly not always the case. Furthermore their role in the initial sensitization to foods remains unclear, and the currently available evidence does not support restriction of the maternal diet during pregnancy or lactation 5.
In an effort to account for variation in maternal dietary patterns, several studies have evaluated the relationship between intake of allergenic foods during lactation and FA outcomes in offspring 63, 64,65, 66,67,68–71,72 (Table 2). Studies evaluating peanut consumption during lactation reported mixed findings; two reported no association 63, 64, two studies found reduced risk of peanut sensitization or FA 65, 66, and one study found that maternal consumption during lactation increased the infant’s risk of peanut allergy 67. One study evaluating maternal peanut and tree nut consumption during the peri-pregnancy period found that higher levels of consumption in non-allergic mothers resulted in protection from peanut and tree nut allergy in the infant 72. This study did not report the independent effect of consumption during lactation, however. Studies evaluating cow’s milk, egg, and other allergens found no association between maternal diet during lactation and risk of allergy or sensitization 68–71. A number of methodological considerations may explain the discrepant findings between studies. Definitions of exposure (estimates of maternal consumption during lactation, along with breastfeeding intensity and duration) and outcome (oral food challenge diagnosed FA, self-report FA, sensitization on skin prick test or allergen specific IgE) are not utilized systematically between studies, making associations difficult to compare directly. Many of these studies have relatively small sample sizes, relying on small numbers of events (FA detection) for statistical analysis. Several of the studies found no association between maternal diet during lactation and FA outcomes, however very small numbers of subjects in these studies experienced these outcomes 68, 69, generating significant concerns about the risk of type II error. Another important factor to consider is that the high concordance between maternal diet during pregnancy and lactation further complicates evaluating these associations separately 64,73, 74, however statistical control for maternal diet during pregnancy is likely necessary for obtaining an unconfounded effect estimate of the association of interest. If we assume that maternal diet during pregnancy is associated with FA risk in the offspring, and that maternal diet in pregnancy is also tightly associated with maternal diet during lactation, then this variable meets the classical definition of a confounder, and should be adjusted for in statistical models (Figure 2). Randomized controlled trials would be an optimal tool for eliminating these statistical concerns. Given the large variability in findings reported and numerous methodological concerns, observational studies that evaluate maternal diet during lactation (and do not consider the composition of HM) thus far have not been able to conclusively determine the role played by maternal dietary antigens in HM in the development or prevention of food allergy.
Table 2.
Recent studies evaluating maternal diet including or excluding allergenic foods during lactation (+/− pregnancy) and FA/sensitization in offspring
| Citation, location | Number of subjects§ | General population or high risk for atopy | Exposure | Outcome | Adequate statistical adjustment† | Study Design | Impact of intake during pregnancy | Impact of intake during lactation | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Peanut | |||||||||
| Du Toit 200866, UK and Israel | 176 | General population | Infant and maternal consumption vs no consumption between countries (Israel vs UK) (grams and frequency monthly) | FA (self-report followed by SPT, IgE, or OFC) | No | Retrospective | No association | Protective | Early infant exposure to peanut showed strongest protective effect |
| Fox 2009 63, UK UK |
443 | 2 control groups; a general population group and a high risk (egg allergy) group | Maternal consumption vs no consumption (g/week) | FA (≥95% predictive value on SPT, IgE or OFC) | Yes | Case-control | No association | No association | Maternal effect disappeared when adjusted for household peanut exposure |
| Des Roches 2010 67, Canada Canada |
403 | General population | Maternal consumption (frequency) | FA (clinical history plus positive SPT or IgE) | Yes | Case-Control | Increased risk | Increased risk | |
| Sicherer 2010 64, USA | 503 | High risk | Maternal consumption (≥2 times per week vs < 2 times per week) | High level sensitization (IgE ≥ 5 kU/L) | Yes | Retrospective | Increased risk | No association | |
| Frazier 2014 72, USA | 8205 | General population | Maternal consumption (servings per week) of peanuts and tree nuts during peripregnancy period (exposure captured via survey completed closest to index birth) | Physician reviewed self-report FA diagnosis | Prospective cohort | Protective | Did not evaluate lactation and pregnancy separately. Greatest benefit seen in mothers without peanut/tree nut allergy | ||
| Pitt 2017 65, Canada Canada |
342 | High risk | Maternal consumption while lactating (ever vs never) | Sensitization (positive SPT) | Yes | Prospective cohort (nested) | Not assessed | Protective# | |
| Cow’s milk, egg and others | |||||||||
| Herrmann 199668, Germany Germany |
99 -120 | High risk | Maternal unrestricted vs restricted diet (cow’s milk and egg) during pregnancy and lactation, vs lactation only | Sensitization (IgE ≥0.35kU/L) | No | Non-randomized comparison | No association | No association | |
| Hattevig 199969, Sweden Sweden |
115 | High risk | Maternal unrestricted diet during lactation vs restricted diet (cow’s milk, egg, fish) | Sensitization (SPT and IgE ≥0.35PRU/ml) | Yes | Non-randomized comparison | Not assessed | No association | |
| Nwaru 201170, Finland (DIPP Nutrition study, 1998 – 2000) | 1018 | High risk for type 1 diabetes (all with HLA-DQB1) | Maternal consumption of foods (z-scores) during lactation (milk and egg) | Cow’s milk, egg and wheat sensitization (IgE ≥0.35kU/L) | Yes | Prospective cohort | Not assessed | No association | |
| Tuokkola 201671, Finland (DIPP Nutrition study, 1997-2004 | 2820 | High risk for type 1 diabetes (all with HLA-DQB1) | Maternal consumption of milk during pregnancy and lactation (quartiles – 1st vs 2nd and 3rd, 4th vs 2nd and 3rd) | Cow’s milk allergy‡ (physician diagnosis or self-report) | Yes | Prospective cohort | Protective | No association | Using the same cohort as above study does |
Abbreviations: FA - FA, SPT - skin prick test, IgE - immunoglobulin E, OFC - oral food challenge, NS = not significant
N included in lactation specific analyses
Includes (at minimum) adjustment for atopic status of family and/or subject if different between comparison groups
Among group with maternal peanut consumption during lactation and introduction to infants before 12 months, no adjusted effect estimate reported for peanut consumption during lactation without interaction with direct ingestion because this was highly significant (p = 0.003).
Registry-obtained physician diagnosis used to justify cost of non-cow’s milk formula, or self-report if breastfed or diagnosed at > 1 year of life
DIPP Study - The Finnish Type 1 Diabetes Prediction and Prevention Study
Figure 2.
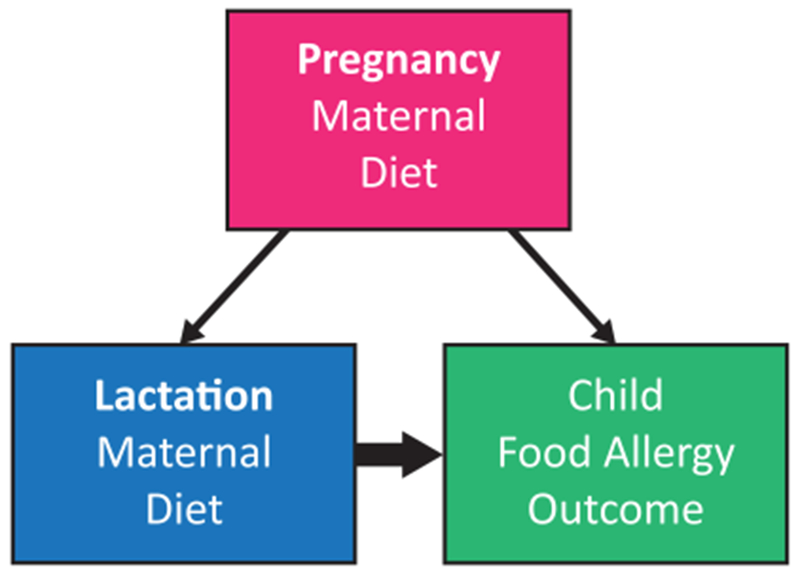
In order to estimate the effect of maternal diet during lactation on FA outcomes in the offspring (bold arrow), it is necessary to control for the effect of maternal diet during pregnancy. Without statistically controlling for this variable given its association to both the outcome (child FA) and the exposure (maternal diet during lactation), the resulting effect estimate would not reflect the true association of interest.
One of the major limitations of studies that do not measure dietary antigens and rely on maternal dietary intake as a proxy measure, is potentially significant misclassification of a child’s exposure to these antigens via HM. Several reports have found large inter-individual variation in food allergens in HM, and have demonstrated that a non-trivial proportion of women had undetectable levels of food allergens following consumption of these foods. Following cow’s milk consumption, 15% - 47% of subjects had no detectable β-lactoglobulin in HM 56, 57. Over 25% of mothers in egg consumption arms of a randomized trial demonstrated no detectable OVA in their milk 54, and in a randomized cross over trial, 24% of mothers never demonstrated detectable OVA in their milk55. Following peanut consumption, 52% of women had no detectable peanut proteins 61, and 72% had no detectable Ara h2, the most potent allergenic peanut protein, in breast milk 59. Beyond the presence or absence of detectable antigen, it is important to note that for women with detectable levels of food antigens in their HM, the timing of the onset of detection, the peak concentration, and the duration throughout which the food protein is detectable also varied greatly from subject to subject. The sources of variability in these factors (timing, concentration and duration of detection) are likely multifactorial, and have been shown to be unrelated to mammary epithelial permeability 54, suggesting that perhaps the variability may be in part due to differences in maternal intestinal permeability to these antigens.
Maternal dietary intake has been shown to impact infant immune markers that likely alter the risk of FA development. Metcalfe found that high dose egg exposure in the mother resulted in higher levels of specific IgG4, a marker of exposure and possible immune tolerance in breastfed infants, compared to mothers in low or no egg arms of the trial 54. Similarly, our study found that mothers who restricted cow’s milk intake during lactation had infants with lower levels of specific IgG4 75. These data suggest that the effect of dietary antigens in HM on immune markers of exposure in offspring have demonstrated benefit among subjects with detectable levels of food antigen.
Taken together, findings from existing epidemiological studies evaluating the relationship between maternal diet during lactation and FA outcomes in offspring are limited in their explanatory power due to the inability to control for wide inter-individual variation concentration of food antigens in HM. Importantly, the maternal diet during lactation influences HM composition in ways that antigen concentration along with food-specific immunoglobulins in humans 75. This may be confounded by maternal diet during pregnancy, which itself may be protective 76. Additional large-scale studies that carefully evaluate the concentration of dietary antigens in HM and the associated risk of allergic disease will be needed to clearly elucidate these complex relationships.
Immunoglobulin A (IgA)
IgA is the fifth most prevalent component in HM, with smaller amounts of IgG and IgM in HM 77. Present mostly in secretory form, secretory IgA (SIgA) controls gut microbiota and intestinal homeostasis 78. Plasma cells in the lamina propria of the gut produce mucosal IgAs, which are transported by the polymeric immunoglobulin receptor (pIgR) across the epithelium 79. B cells originating from the maternal gut migrate to the mammary tissue via the “enteromammary link”, and secrete specific IgA that enters HM 80. This process is regulated by the mucosal vascular addressin MadCAM-1, a protein that interacts with both the mucosa-associated CCR10 81 and the gut-targeting receptor α4β7 integrin 82. Given the interaction between these components, it is hypothesized that that the specificity of IgA in a mother’s milk is a reflection of the antigenic exposure her immune system experiences to dietary proteins in her gut.
Consistent with prior reports 83, 84, in a prospective birth cohort oversampled for factors that confer high risk of FA development, we found that increased levels of both total IgA 85 and cow’s milk-specific IgA 75 in HM were associated with protection against CMA. Maternal exposures dictated by geography, microbial burden, contact with animals (specifically farm animals and cats), are likely a major determinant the levels and specificity of the IgA observed in HM 77. We also showed that a restriction of cow’s milk from the maternal diet was associated with reduced levels of cow’s milk-specific IgA levels in HM when compared to mothers with unrestricted diets 75, implying that the dietary antigenic exposure of the maternal immune system via the gastrointestinal tract shifts the antibody specificity observed in HM. This could be the reason why maternal diets restricting highly allergenic foods may not be advantageous in prevention of FA. We also showed the IgA in HM had different specificity to cow’s milk antigens than the IgA in serum, indicating that distinct antibody-producing lymphocytes likely contribute to HM and serum IgA, thereby providing further evidence of the “enteromammary link” 86. This evidence suggests that maternal diet influences the specific IgA composition of HM. An active connection between immune tissues in the maternal gut and the mammary gland results in a HM-specific IgA profile influenced by the mother’s dietary exposures.
While the precise function of HM IgA remains unclear, it is believed to provide passive protection until infant production of IgA commences after birth 87. During lactation, IgAs also influence the microbiome composition of the infant, conferring protection to colitis in adulthood 78. In summary, the IgA found in HM is shaped by many factors, including maternal exposures that vary based on diet, geographical location, exposure to microbes, among others. These immunoglobulins are likely an important factor in the prevention of CMA in the infant. There are no studies assessing the impact of immunoglobulins or antibodies in other FA.
Cytokines, chemokines and growth factors
HM contains numerous immunomodulating cytokines that stimulate or suppress immune cells 77. Whereas some are found in relatively low concentrations in HM, others, such as TGF-β is found in larger quantities 88. Inter-individual variation in HM cytokine levels may stem from differences in microbial exposures; TGF-β, IFN-γ, and IL-10 levels vary based on maternal country of origin and country of residence 77.
TGF-β is a key regulatory cytokine involved in the inhibition of Th1 and Th2 pathways, and thus far, is the most well studied soluble factor in HM. Considering all three isoforms, TGF-β is the predominant cytokine in HM, the most common isoform being TGFβ2 77. TGF-β is involved in the induction of production of specific IgA and intestinal epithelial cell maturation 89. A 2010 review by Oddy and Rosales included 12 studies conducted in humans, and reported that 8 of the studies found that higher levels of TGF-β1 or TGFβ2 resulted in lower risk of atopic outcomes in the first years of life 90, however the results from other studies differed. A summary of association between TGF-β and the development of FA can be found in Table 3. Although three studies did not find an association between TGFβ1 (and in one also TGFβ2) levels in milk and FA, two studies have found higher levels being associated with lower risk of FA. Among the latter two studies, the first study of FA showed that mothers of infants with IgE-mediated CMA had lower TGFβ1 concentrations in colostrum than mothers of infants with non-IgE-mediated CMA, and that the concentration of TGFβ1 in healthy controls fell between these two groups 91 (Table 3). The second study found a protective association between a group of several pro-inflammatory and regulatory cytokines (including TGF-β1) in HM and CMA 88. Based on these studies, the role of TGF-β levels on FA development has not been conclusively determined, and may differ among different types of FA. Additional studies specifically evaluating FA are needed to better understand the influence of TGF-β on this process.
Table 3.
Studies assessing the association between cytokine levels in HM and FA in infants.
| Study | Number of subjects | Duration of followup | Cytokines assessed | FA |
|---|---|---|---|---|
| Saarinen 1999 91, Finland | 6209 | 12 months | TGFβ-1, TGFβ-2 | Higher TGFβ-1 levels in colostrum are associated with infants who develop IgE-mediated cow’s milk allergy versus non-IgE-mediated cow’s milk allergy; healthy controls were found in between |
| Bottcher 2003 92, Sweden | 53 | 2 years | IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, IL-16, IFN-γ, TGFβ-1, TGFβ-2, RANTES, eotaxin | No significant association |
| Snijders 2006 93, Netherlands | 315 | 2 years | IL-12 or TGFβ-1 (IL-10 undetectable) | No significant association |
| Kuitunen 2012 94, Finland | 364 (colostrum) 321 (BM) | 2 years | IL-10, TGFβ-1 | No significant association |
| Järvinen 2015 88, Finland | 145 | 2 years | IL-1α, IL-1β, IL-6,
IL-10 PDGF-BB, CCL27, VEGF, TSLP, CCL11, CXCL10, and CXCL11, CCL22, TGFβ-1, TNF-a and -b, CCL1, CCL17, IL-31, eotaxin 3, CXCL9, IL-5, GM-CSF, and IL-12p70 |
Higher levels of IL-1β, IL-6, IL-10, and TGFβ-1 in HM showed association with cow’s milk tolerance |
| Munblit 2017 95, United Kingdom, Russia and Italy | 398 | 6 months | IL-2, IL-4, IL-5, IL-10, IFNγ, IL-12, IL-13, HGF, TGFβ-1, TGFβ-2, TGFβ-3 | Higher IL-13 levels associated with protection, otherwise no significant association |
| Berdi 2018 96, France | 263 | 5 years | Eotaxin, CX3CL1, CXCL1, IL-8, CXCL10, CCL7, CCL22, CCL2, CCL3, CCL4, RANTES, IFNα2, IFNγ, IL-10, IL-12p40, IL-12p70, IL-15, IL-17A, IL-1RA, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-13, sCD40L, TNFα, TNFβ, EGF, FGF-2, G-CSF, Flt3L, GM-CSF, PDGF-AA, PDGF-BB, TGFα, VEGF | Higher levels of CXCL10, TNFβ and IL-2 associated with protection against FA |
New and ongoing studies evaluating the impact of additional cytokines and chemokines present in HM the development of atopic conditions has shown variable results77. Several authors report an absence or very low concentrations of several of these bioactive substances including IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IFN-γ, CCL5, CXCL8, CXCL10, and TNF-α. These studies have shown no relationship between these compounds and atopy development 77. Table 3 summarizes the current literature evaluating the relationships between cytokines and FA development 72, 88,91–96. Our study showed that protection against CMA was associated with the levels of IL-1β, IL-6, IL-10, and TGF-α1 in HM 88. It is not possible to determine from this study whether these chemokines and cytokines are biomarkers of the mechanism that confers protection from CMA, or if they are directly related to the process of protection. However, given that these factors promote differentiation of Th17 cells, the production of IgA, and crosstalk between the RORγt+ innate lymphoid cell (ILC)-3 population and gut macrophages 88, it is plausible that these chemical factors we detected in HM samples are directly involved in promoting protective immunomodulation. Another study reported that increased IL-13 concentration in both colostrum and mature HM were associated with lower risk of FA and eczema, respectively by parent report. These authors found no significant association between levels of IL-2, IL-4, IL-5, IL-10, IL-12 and IFN-γ with either of these conditions or wheeze 95. Finally, a recent study using the case-cohort design among the EDEN birth cohort showed a positive association between the concentration of IL-2, CXCL10, and TNF-β and reported FA in childhood 96. Higher concentrations of IL-1β and IL-17 were found in mothers who reported increased physical activity 77. Overall, maternal atopic status was not systematically associated with cytokine levels in HM 88. Overall, these studies demonstrated the strongest association between high levels of TGF-β and perhaps some other regulatory and inflammatory cytokines and decreased FA risk, but data are few and to some extent conflicting.
Human milk oligosaccharides (HMOs)
HMOs are abundant sugar chains found exclusively in HM. During the period of exclusive breastfeeding, the infant’s gut microbiome feeds primarily off of these HMOs, which promotes the growth of Bifidobacteria and Bacteroides species, leading to their predominance in the gut microbiome of exclusively breastfed infants 97. The infant in unable to digest these HMOs, which originate as lactose molecules that are modified by a series of glycosyltransferases, enzymes which are encoded by the genes responsible for the Lewis blood groups and secretor status. Two fucosyltransferases FUT2 (secretor gene) and FUT3 (Lewis gene) are responsible for the addition of fucose. Mothers express a unique set of enzymes and synthesize their own variety of HMOs based on which allelic variants they express. Resulting from this genetic variation, HM demonstrates significant inter-individual heterogeneity in HMO composition, both in terms of the oligosaccharides present and their relative abundance. This variation indicates that breastfed infants are exposed only to certain HMOs in their mother’s milk. 15-25% (varying by ethnic background) of mothers lack a functional lack a functional FUT2 enzyme (FUT2−/−) 98, 99 and are labeled non-secretors, given that they lack all HMOs containing alpha-2 linked fucose 100. Infants who receive HM from non-secretor mothers have microbiota with later establishment of bifidobacteria predominance 101. The study by Sprenger et al. found that infants fed by non-secretor mothers who were born by cesarean section had an increased risk of developing IgE-associated atopic dermatitis 100. This study did not evaluate the risk of FA development, nor did it consider the concentration of individual HMOs. In our study, infants fed HM containing low concentrations of Lacto-N-fucopentaose (LNFP) III had a higher risk of developing CMA compared to infants who received HM with higher levels of this oligosaccharide 102. Similarly, in a report from the Canadian Healthy Infant Longitudinal Development (CHILD) study, authors evaluated the association between HMO profiles and food sensitization at 12 months, and found that the presence of specific HMOs conferred reduced risk of food sensitization in the infant 103. Taken together, these findings suggest that specific HMO profiles may result in protection from the development FA, and that this protection may be conferred via their impact on infant gut microbiome.
The protection observed in these studies may be due to the effect of HMOs on gut microbiome or direct effects on immune cells. Certain HMOs have demonstrated anti-inflammatory characteristics, and promote the maturation of the gastrointestinal immune system. Other HMOs inhibit intestinal cell growth, while others interact with dendritic cells via the lectin receptor DC-SIGN (Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbng Non-integrin) 77. Additional studies with a focus on the specific HMO composition of HM will be required to more clearly elucidate the role of these compounds in mechanisms of atopic disease development and prevention.
CONCLUSIONS AND RECOMMENDATIONS FOR FUTURE RESEARCH
The composition of immunologically active substances and significant interpersonal variation of HM has yet to be fully described. Past epidemiologic studies have often failed to account for these variables, and have historically operationalized HM exposure crudely, and in ways that may obscure associations of interest. Additionally, the outcome definitions for FA also vary from study to study, with some authors utilizing gold standard oral food challenge, others relying on clinical diagnosis, and still others reporting associations with evidence of sensitization. These challenges, in combination with a number of factors that have historically not be accounted for including wide variation in biologically active components in HM, differences in maternal diet, microbial exposures, and geographical differences, it is of no surprise that findings have been inconsistent in the literature thus far. Due to ethical considerations given the established benefits of breastfeeding, large scale randomized trials with assignment to infant diet type are lacking, which makes simultaneously controlling for all of these factors very challenging. To date, most studies have lacked the statistical power to properly evaluate the relationship between breastfeeding and FA, or have avoided FA as an outcome altogether due to these challenges.
Improved understanding of maternal-offspring interactions from animal models, in parallel with human studies will contribute to the development of novel strategies through breastfeeding to prevent and treat FA. The challenges with animal studies include its limited applicability to human systems. In rodents, FcRn functions in the neonatal period, transporting maternally derived IgG in milk across the epithelial barrier in the gut, whereas in humans FcRn transports robustly maternal IgG to the fetus across placenta 104. Furthermore, the composition of breast milk differs between these species; as an example milk oligosaccharides are much more complex in human than in mouse. Also, the ratio of IgA to IgG differs in human and murine milk. These and other differences may limit the ability to fully translate findings in animal studies to those mechanisms that are biologically similar, although the strengths of animal models include being able to answer specific questions that are difficult or unethical to address in human subjects.
The studies reviewed highlight the complex, variable composition of HM, and the impact that differences in HM characteristics may have on FA development or prevention. The biologically active components of HM may either directly impact FA risk, or perhaps indirectly influence this process through impact on gut microbiota or other still unknown mechanisms. Dietary allergens in HM in combination with immunoglobulins may provide a tolerogenic environment, explaining the reduced risk seen in some studies. Based on all of the information available to date, it is clear that it is critical to consider the combination of each of these factors, and that the impact of this complex system considered together is likely greater than the sum of each individual part. In order to enhance our understanding of this system, future studies must evaluate the networks of bioactive components in HM, their impacts on the development of the infant immune system and microbiome as a system, and recognize that this field is still “in its infancy”. Clarification of the role of these HM components may lead to key targets for the primary prevention and potential treatment of FA. Large prospective cohort studies that include careful characterization of exposure to these key HM components, as well as careful outcome consideration would provide critical evidence regarding mechanisms and targetable factors in the development of atopic diseases.
KEY MESSAGES.
Breastfeeding is recommended to prevent the development of allergic diseases, however studies have not adequately assessed the role of human milk in food allergy.
Potential benefits against food allergy may differ between infants given differences in immunomodulatory composition of human milk between mothers - differences not captured in epidemiologic studies.
The protection against allergy development provided by human milk may be due to impact on the infant gut microbiome or via direct effects on immune system.
High levels, ratios or combinations of certain human milk immune factors (IgA, cytokines, oligosaccharides) are associated with reduced risk of food allergy in the infant; it remains uncertain whether these are directly protective, or biomarkers of transferred protection. The role of food antigens in human milk in initial sensitization or tolerance induction is unclear.
Animal studies highlight potential mechanisms of protection provided by antigens, TGF-β, and immunocomplexes, yet their relevance is poorly understood in humans.
Studies evaluating the impact of breastfeeding and human milk composition on food allergy are needed.
Acknowledgments
Funding: KMJ was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number U01AI131344 and National Center for Advancing Translational Sciences under Award Number R21002516. HM is a trainee in the Medical Scientist Training Program funded by 5T32 GM007356. MKO was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI142872, and the Food Allergy Research & Education, Inc., the HOPE APFED/ARTrust™ Pilot Grant, the William F. Milton Fund, the Harvard Catalyst Clinical and Translational Research Center, (NCATS grant #8UL 1TR000170), and the Boston Children’s Hospital Pediatric Associates Award (MKO).
Abbreviations:
- CMA
cow’s milk allergy
- CT
cholera toxin
- FA
food allergy
- FcRn
neonatal crystallizable fragment receptor
- HM
human milk
- HMO
human milk oligosaccharide
- IC
immune complex
- Ig
immunoglobulin
- IL
interleukin
- ILC
innate lymphoid cell
- INF
interferon
- i.p.
intraperitoneal
- OVA
ovalbumin
- SCFA
short-chain fatty acid
- SNP
single nucleotide polymorphism
- TGF-β
transforming growth factor-β
- TLR
Toll-like receptor
- TSLP
thymic stromal lymphopoietin
- TNF
tumor necrosis factor
- Treg
regulatory T cell
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
REFERENCES:
- 1.Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–490. [DOI] [PubMed] [Google Scholar]
- 2.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012:CD003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachrach VR SEaBL. Breastfeeding and the risk of hospitalization for respiratory disease in infancy: a meta-analysis. Arch Pediatr Adolesc Med 2003:237–243. [DOI] [PubMed] [Google Scholar]
- 4.Fleischer DM, Spergel JM, Assa’ad AH, Pongracic JA. Primary prevention of allergic disease through nutritional interventions. The journal of allergy and clinical immunology.In practice. 2013;1:29–36. [DOI] [PubMed] [Google Scholar]
- 5.Muraro A, Halken S, Arshad SH, et al. EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy. 2014;69:590–601. [DOI] [PubMed] [Google Scholar]
- 6.Greer FR, Sicherer SH, Burks AW, American Academy of Pediatrics Committee on N, American Academy of Pediatrics Section on A, Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121:183–191. [DOI] [PubMed] [Google Scholar]
- 7.van Odijk J, Kull I, Borres MP, et al. Breastfeeding and allergic disease: a multidisciplinary review of the literature (1966-2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy. 2003;58:833–843. [DOI] [PubMed] [Google Scholar]
- 8.Lodge CJ, Tan DJ, Lau MX, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr 2015;104:38–53. [DOI] [PubMed] [Google Scholar]
- 9.de Silva D, Geromi M, Halken S, et al. Primary prevention of food allergy in children and adults: systematic review. Allergy. 2014;69:581–589. [DOI] [PubMed] [Google Scholar]
- 10.Goldsmith AJ, Koplin JJ, Lowe AJ, et al. Formula and breast feeding in infant food allergy: A population-based study. J Paediatr Child Health. 2016;52:377–384. [DOI] [PubMed] [Google Scholar]
- 11.Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413–420. [DOI] [PubMed] [Google Scholar]
- 12.Kramer MS, Matush L, Vanilovich I, et al. Effect of prolonged and exclusive breast feeding on risk of allergy and asthma: cluster randomised trial. BMJ. 2007;335:815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong X, Wang G, Liu X, et al. Gene polymorphisms, breast-feeding, and development of food sensitization in early childhood. J Allergy Clin Immunol 2011;128:374–381 e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao X, McMahon RJ, Woo JG, Davidson BS, Morrow AL, Zhang Q. Temporal changes in milk proteomes reveal developing milk functions. J Proteome Res 2012;11:3897–3907. [DOI] [PubMed] [Google Scholar]
- 15.Andreas NJ, Kampmann B, Mehring Le-Doare K. Human breast milk: A review on its composition and bioactivity. Early Hum Dev 2015;91:629–635. [DOI] [PubMed] [Google Scholar]
- 16.Järvinen KM, Bergmann, KE., & Bergmann, R. Breast—Always Best? Allergy, Immunity and Tolerance in Early Childhood: The First Steps of the Atopic March. 2015:235. [Google Scholar]
- 17.Azad MB, Konya T, Maughan H, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;185:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin AM, Sitarik AR, Havstad SL, et al. Joint effects of pregnancy, sociocultural, and environmental factors on early life gut microbiome structure and diversity. Sci Rep 2016;6:31775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergstrom A, Skov TH, Bahl MI, et al. Establishment of intestinal microbiota during early life: a longitudinal, explorative study of a large cohort of Danish infants. Appl Environ Microbiol 2014;80:2889–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backhed F, Roswall J, Peng Y, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell host & microbe. 2015;17:690–703. [DOI] [PubMed] [Google Scholar]
- 21.Voreades N, Kozil A, Weir TL. Diet and the development of the human intestinal microbiome. Front Microbiol. 2014;5:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subramanian S, Huq S, Yatsunenko T, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510:417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pannaraj PS, Li F, Cerini C, et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr 2017;171:647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kourosh A, Luna RA, Balderas M, et al. Fecal microbiome signatures are different in food-allergic children compared to siblings and healthy children. Pediatr Allergy Immunol 2018;29:545–554. [DOI] [PubMed] [Google Scholar]
- 25.Fieten KB, Totte JEE, Levin E, et al. Fecal Microbiome and Food Allergy in Pediatric Atopic Dermatitis: A Cross-Sectional Pilot Study. Int Arch Allergy Immunol 2018;175:77–84. [DOI] [PubMed] [Google Scholar]
- 26.Fazlollahi M, Chun Y, Grishin A, et al. Early-life gut microbiome and egg allergy. Allergy. 2018;73:1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunyavanich S Food allergy: could the gut microbiota hold the key? Nat Rev Gastroenterol Hepatol 2019;16:201–202. [DOI] [PubMed] [Google Scholar]
- 28.Kumari M, Kozyrskyj AL. Gut microbial metabolism defines host metabolism: an emerging perspective in obesity and allergic inflammation. Obes Rev 2017;18:18–31. [DOI] [PubMed] [Google Scholar]
- 29.Tan J, McKenzie C, Vuillermin PJ, et al. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep 2016;15:2809–2824. [DOI] [PubMed] [Google Scholar]
- 30.Bridgman SL, Azad MB, Field CJ, et al. Fecal Short-Chain Fatty Acid Variations by Breastfeeding Status in Infants at 4 Months: Differences in Relative versus Absolute Concentrations. Front Nutr 2017;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oyoshi MK, Oettgen HC, Chatila TA, Geha RS, Bryce PJ. Food allergy: Insights into etiology, prevention, and treatment provided by murine models. J Allergy Clin Immunol 2014;133:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verhasselt V, Milcent V, Cazareth J, et al. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nature medicine. 2008;14:170–175. [DOI] [PubMed] [Google Scholar]
- 33.Mosconi E, Rekima A, Seitz-Polski B, et al. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal immunology. 2010;3:461–474. [DOI] [PubMed] [Google Scholar]
- 34.Hamada K, Suzaki Y, Goldman A, et al. Allergen-independent maternal transmission of asthma susceptibility. Journal of immunology. 2003;170:1683–1689. [DOI] [PubMed] [Google Scholar]
- 35.Macchiaverni P, Rekima A, Turfkruyer M, et al. Respiratory allergen from house dust mite is present in human milk and primes for allergic sensitization in a mouse model of asthma. Allergy. 2014;69:395–398. [DOI] [PubMed] [Google Scholar]
- 36.Jarvinen KM, Westfall J, De Jesus M, et al. Role of Maternal Dietary Peanut Exposure in Development of Food Allergy and Oral Tolerance. PLoS One. 2015;10:e0143855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernard H, Ah-Leung S, Drumare MF, et al. Peanut allergens are rapidly transferred in human breast milk and can prevent sensitization in mice. Allergy. 2014;69:888–897. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Exposito I, Song Y, Jarvinen KM, Srivastava K, Li XM. Maternal peanut exposure during pregnancy and lactation reduces peanut allergy risk in offspring. The Journal of allergy and clinical immunology. 2009;124:1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohsaki A, Venturelli N, Buccigrosso TM, et al. Maternal IgG immune complexes induce food allergen-specific tolerance in offspring. J Exp Med 2018;215:91–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartnikas LM, Gurish MF, Burton OT, et al. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J Allergy Clin Immunol 2013;131:451–460 e451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burton OT, Logsdon SL, Zhou JS, et al. Oral immunotherapy induces IgG antibodies that act through FcgammaRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol 2014;134:1310–1317 e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turfkruyer M, Rekima A, Macchiaverni P, et al. Oral tolerance is inefficient in neonatal mice due to a physiological vitamin A deficiency. Mucosal Immunol 2016;9:479–491. [DOI] [PubMed] [Google Scholar]
- 43.Rekima A, Macchiaverni P, Turfkruyer M, et al. Long-term reduction in food allergy susceptibility in mice by combining breastfeeding-induced tolerance and TGF-beta-enriched formula after weaning. Clin Exp Allergy. 2017;47:565–576. [DOI] [PubMed] [Google Scholar]
- 44.Hogan SP, Wang YH, Strait R, Finkelman FD. Food-induced anaphylaxis: mast cells as modulators of anaphylactic severity. Semin Immunopathol 2012;34:643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caffarelli C, Romanini E, Caruana P, Street ME, de’ Angelis G. Clinical food hypersensitivity: the relevance of duodenal immunoglobulin E-positive cells. Pediatr Res 1998;44:485–490. [DOI] [PubMed] [Google Scholar]
- 46.Brandt EB, Strait RT, Hershko D, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest 2003;112:1666–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burton OT, Darling AR, Zhou JS, et al. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal Immunol 2013;6:740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Husby S, Oxelius VA, Teisner B, Jensenius JC, Svehag SE. Humoral immunity to dietary antigens in healthy adults. Occurrence, isotype and IgG subclass distribution of serum antibodies to protein antigens. Int. Arch. Allergy Appl. Immunol 1985;77:416–422. [DOI] [PubMed] [Google Scholar]
- 49.Bernard H, Ah-Leung S, Drumare MF, et al. Peanut allergens are rapidly transferred in human breast milk and can prevent sensitization in mice. Allergy. 2014;69:888–897. [DOI] [PubMed] [Google Scholar]
- 50.Hirose J, Ito S, Hirata N, Kido S, Kitabatake N, Narita H. Occurrence of the major food allergen, ovomucoid, in human breast milk as an immune complex. Biosci. Biotechnol. Biochem 2001;65:1438–1440. [DOI] [PubMed] [Google Scholar]
- 51.Rumbo M, Chirdo FG, Anon MC, Fossati CA. Detection and characterization of antibodies specific to food antigens (gliadin, ovalbumin and beta-lactoglobulin) in human serum, saliva, colostrum and milk. Clin. Exp. Immunol 1998;112:453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hochwallner H, Alm J, Lupinek C, et al. Transmission of allergen-specific IgG and IgE from maternal blood into breast milk visualized with microarray technology. J. Allergy Clin. Immunol 2014;134:1213–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwarz A, Panetta V, Cappella A, et al. IgG and IgG4 to 91 allergenic molecules in early childhood by route of exposure and current and future IgE sensitization: Results from the Multicentre Allergy Study birth cohort. J. Allergy Clin. Immunol 2016;138:1426–1433 e1412. [DOI] [PubMed] [Google Scholar]
- 54.Metcalfe JR, Marsh JA, D’Vaz N, et al. Effects of maternal dietary egg intake during early lactation on human milk ovalbumin concentration: a randomized controlled trial. Clin Exp Allergy. 2016;46:1605–1613. [DOI] [PubMed] [Google Scholar]
- 55.Palmer DJ, Gold MS, Makrides M. Effect of cooked and raw egg consumption on ovalbumin content of human milk: a randomized, double-blind, cross-over trial. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2005;35:173–178. [DOI] [PubMed] [Google Scholar]
- 56.Sorva R, Makinen-Kiljunen S, Juntunen-Backman K. Beta-lactoglobulin secretion in human milk varies widely after cow’s milk ingestion in mothers of infants with cow’s milk allergy. J Allergy Clin Immunol 1994;93:787–792. [DOI] [PubMed] [Google Scholar]
- 57.Jarvinen KM, Makinen-Kiljunen S, Suomalainen H. Cow’s milk challenge through human milk evokes immune responses in infants with cow’s milk allergy. J Pediatr 1999;135:506–512. [DOI] [PubMed] [Google Scholar]
- 58.Chirdo FG, Rumbo M, Anon MC, Fossati CA. Presence of high levels of non-degraded gliadin in breast milk from healthy mothers. Scand J Gastroenterol 1998;33:1186–1192. [DOI] [PubMed] [Google Scholar]
- 59.Schocker F, Baumert J, Kull S, Petersen A, Becker WM, Jappe U. Prospective investigation on the transfer of Ara h 2, the most potent peanut allergen, in human breast milk. Pediatr Allergy Immunol 2016;27:348–355. [DOI] [PubMed] [Google Scholar]
- 60.Schocker F, Scharf A, Kull S, Jappe U. Detection of the Peanut Allergens Ara h 2 and Ara h 6 in Human Breast Milk: Development of 2 Sensitive and Specific Sandwich ELISA Assays. Int Arch Allergy Immunol 2017;174:17–25. [DOI] [PubMed] [Google Scholar]
- 61.Vadas P, Wai Y, Burks W, Perelman B. Detection of peanut allergens in breast milk of lactating women. JAMA. 2001;285:1746–1748. [DOI] [PubMed] [Google Scholar]
- 62.Jarvinen KM, Makinen-Kiljunen S, Suomalainen H. Cow’s milk challenge through human milk evokes immune responses in infants with cow’s milk allergy. The Journal of pediatrics. 1999;135:506–512. [DOI] [PubMed] [Google Scholar]
- 63.Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. The Journal of allergy and clinical immunology. 2009;123:417–423. [DOI] [PubMed] [Google Scholar]
- 64.Sicherer SH, Wood RA, Stablein D, et al. Maternal consumption of peanut during pregnancy is associated with peanut sensitization in atopic infants. The Journal of allergy and clinical immunology. 2010;126:1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pitt TJ, Becker AB, Chan-Yeung M, et al. Reduced risk of peanut sensitization following exposure through breast-feeding and early peanut introduction. The Journal of allergy and clinical immunology. 2018;141:620–625.e621. [DOI] [PubMed] [Google Scholar]
- 66.Du Toit G, Katz Y, Sasieni P, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. The Journal of allergy and clinical immunology. 2008;122:984–991. [DOI] [PubMed] [Google Scholar]
- 67.DesRoches A, Infante-Rivard C, Paradis L, Paradis J, Haddad E. Peanut allergy: is maternal transmission of antigens during pregnancy and breastfeeding a risk factor? Journal of investigational allergology & clinical immunology. 2010;20:289–294. [PubMed] [Google Scholar]
- 68.Herrmann ME, Dannemann A, Gruters A, et al. Prospective study of the atopy preventive effect of maternal avoidance of milk and eggs during pregnancy and lactation. Eur J Pediatr 1996;155:770–774. [DOI] [PubMed] [Google Scholar]
- 69.Hattevig G, Sigurs N, Kjellman B. Effects of maternal dietary avoidance during lactation on allergy in children at 10 years of age. Acta Paediatr 1999;88:7–12. [PubMed] [Google Scholar]
- 70.Nwaru BI, Erkkola M, Ahonen S, et al. Maternal diet during lactation and allergic sensitization in the offspring at age of 5. Pediatr Allergy Immunol 2011;22:334–341. [DOI] [PubMed] [Google Scholar]
- 71.Tuokkola J, Luukkainen P, Tapanainen H, et al. Maternal diet during pregnancy and lactation and cow’s milk allergy in offspring. Eur J Clin Nutr 2016;70:554–559. [DOI] [PubMed] [Google Scholar]
- 72.Frazier AL, Camargo CA Jr., Malspeis S, Willett WC, Young MC. Prospective study of peripregnancy consumption of peanuts or tree nuts by mothers and the risk of peanut or tree nut allergy in their offspring. JAMA pediatrics. 2014;168:156–162. [DOI] [PubMed] [Google Scholar]
- 73.Des Roches A, Bégin P, Infante-Rivard C, Paradis J, Paradis L. Peanut allergy and the impact of maternal consumption during pregnancy and breast-feeding. Journal of Allergy and Clinical Immunology. 2011;128:248–249. [DOI] [PubMed] [Google Scholar]
- 74.Sicherer SH, Wood RA, Stablein D, et al. Reply. Journal of Allergy and Clinical Immunology. 2011;128:249–250. [Google Scholar]
- 75.Jarvinen KM, Westfall JE, Seppo MS, et al. Role of maternal elimination diets and human milk IgA in the development of cow’s milk allergy in the infants. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2014;44:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bunyavanich S, Rifas-Shiman SL, Platts-Mills TA, et al. Peanut, milk, and wheat intake during pregnancy is associated with reduced allergy and asthma in children. The Journal of allergy and clinical immunology. 2014;133:1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rajani PS, Seppo AE, Jarvinen KM. Immunologically Active Components in Human Milk and Development of Atopic Disease, With Emphasis on Food Allergy, in the Pediatric Population. Front Pediatr 2018;6:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rogier EW, Frantz AL, Bruno ME, et al. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3074–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaetzel CS. Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host-microbial mutualism. Immunol Lett 2014;162:10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanson LA, Korotkova M. The role of breastfeeding in prevention of neonatal infection. Seminars in neonatology: SN. 2002;7:275–281. [DOI] [PubMed] [Google Scholar]
- 81.Morteau O, Gerard C, Lu B, et al. An indispensable role for the chemokine receptor CCR10 in IgA antibody-secreting cell accumulation. J Immunol 2008;181:6309–6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tanneau GM, Hibrand-Saint Oyant L, Chevaleyre CC, Salmon HP. Differential recruitment of T- and IgA B-lymphocytes in the developing mammary gland in relation to homing receptors and vascular addressins. J Histochem Cytochem 1999;47:1581–1592. [DOI] [PubMed] [Google Scholar]
- 83.Machtinger S, Moss R. Cow’s milk allergy in breast-fed infants: the role of allergen and maternal secretory IgA antibody. J Allergy Clin Immunol 1986;77:341–347. [DOI] [PubMed] [Google Scholar]
- 84.Savilahti E, Tainio VM, Salmenpera L, et al. Low colostral IgA associated with cow’s milk allergy. Acta Paediatr Scand 1991;80:1207–1213. [DOI] [PubMed] [Google Scholar]
- 85.Jarvinen KM, Laine ST, Jarvenpaa AL, Suomalainen HK. Does low IgA in human milk predispose the infant to development of cow’s milk allergy? Pediatr Res 2000;48:457–462. [DOI] [PubMed] [Google Scholar]
- 86.Seppo AE, Savilahti EM, Berin MC, Sampson HA, Jarvinen KM. Breast milk IgA to foods has different epitope specificity than serum IgA-Evidence for entero-mammary link for food-specific IgA? Clin Exp Allergy. 2017;47:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nature reviews.Immunology. 2011;12:9–23. [DOI] [PubMed] [Google Scholar]
- 88.Jarvinen KM, Suarez-Farinas M, Savilahti E, Sampson HA, Berin MC. Immune factors in breast milk related to infant milk allergy are independent of maternal atopy. The Journal of allergy and clinical immunology. 2015;135:1390–1393. e1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rautava S, Lu L, Nanthakumar NN, Dubert-Ferrandon A, Walker WA. TGF-beta2 induces maturation of immature human intestinal epithelial cells and inhibits inflammatory cytokine responses induced via the NF-kappaB pathway. J Pediatr Gastroenterol Nutr 2012;54:630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oddy WH, Rosales F. A systematic review of the importance of milk TGF-beta on immunological outcomes in the infant and young child. Pediatr Allergy Immunol 2010;21:47–59. [DOI] [PubMed] [Google Scholar]
- 91.Saarinen KM, Vaarala O, Klemetti P, Savilahti E. Transforming growth factor-beta1 in mothers’ colostrum and immune responses to cows’ milk proteins in infants with cows’ milk allergy. J Allergy Clin Immunol 1999;104:1093–1098. [DOI] [PubMed] [Google Scholar]
- 92.Bottcher MF, Jenmalm MC, Bjorksten B. Cytokine, chemokine and secretory IgA levels in human milk in relation to atopic disease and IgA production in infants. Pediatr Allergy Immunol 2003;14:35–41. [DOI] [PubMed] [Google Scholar]
- 93.Snijders BE, Damoiseaux JG, Penders J, et al. Cytokines and soluble CD14 in breast milk in relation with atopic manifestations in mother and infant (KOALA Study). Clin Exp Allergy. 2006;36:1609–1615. [DOI] [PubMed] [Google Scholar]
- 94.Kuitunen M, Kukkonen AK, Savilahti E. Impact of maternal allergy and use of probiotics during pregnancy on breast milk cytokines and food antibodies and development of allergy in children until 5 years. Int Arch Allergy Immunol 2012;159:162–170. [DOI] [PubMed] [Google Scholar]
- 95.Munblit D, Treneva M, Peroni DG, et al. Immune Components in Human Milk Are Associated with Early Infant Immunological Health Outcomes: A Prospective Three-Country Analysis. Nutrients. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Berdi M, de Lauzon-Guillain B, Forhan A, et al. Immune components of early breastmilk: association with maternal factors and with reported food allergy in childhood. Pediatr Allergy Immunol 2018. [DOI] [PubMed] [Google Scholar]
- 97.Yu ZT, Chen C, Newburg DS. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology. 2013;23:1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Castanys-Munoz E, Martin MJ, Prieto PA. 2′-fucosyllactose: an abundant, genetically determined soluble glycan present in human milk. Nutr Rev 2013;71:773–789. [DOI] [PubMed] [Google Scholar]
- 99.Erney RM, Malone WT, Skelding MB, et al. Variability of human milk neutral oligosaccharides in a diverse population. J Pediatr Gastroenterol Nutr 2000;30:181–192. [DOI] [PubMed] [Google Scholar]
- 100.Sprenger N, Odenwald H, Kukkonen AK, Kuitunen M, Savilahti E, Kunz C. FUT2-dependent breast milk oligosaccharides and allergy at 2 and 5 years of age in infants with high hereditary allergy risk. Eur J Nutr 2016. [DOI] [PubMed] [Google Scholar]
- 101.Lewis ZT, Totten SM, Smilowitz JT, et al. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome. 2015;3:13-015-0071-z eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seppo AE, Autran CA, Bode L, Jarvinen KM. Human milk oligosaccharides and development of cow’s milk allergy in infants. J Allergy Clin Immunol 2017;139:708–711 e705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miliku K, Robertson B, Sharma AK, et al. Human milk oligosaccharide profiles and food sensitization among infants in the CHILD Study. Allergy. 2018. [DOI] [PubMed] [Google Scholar]
- 104.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007;7:715–725. [DOI] [PubMed] [Google Scholar]


