Abstract
Background
We have previously shown that maternal cow’s milk (CM) elimination results in down-regulation of CM-specific IgA antibody levels in BM, but not in serum, suggesting that an entero-mammary link may exist for food-specific antibody-secreting cells.
Objective
We sought to investigate whether food-specific IgA epitope profiles differ intra-individually between mother’s serum and BM. We also examined how infants’ food epitope-specific IgA develops in early infancy and the relationship of IgA epitope recognition with development of cow’s milk allergy (CMA).
Methods
We measured specific IgA to a series of overlapping peptides in major CM allergens (αs1-, αs2 -, β- and κ-caseins and β-lactoglobulin) in paired maternal and infant serum as well as BM samples in 31 mother-infant dyads within the first 15 postpartum months utilizing peptide microarray.
Results
There was significant discordance in epitope specificity between BM and maternal sera ranging from only 13% of sample pairs sharing at least one epitope in αs1-casein to 73% in κ-casein. Epitope-specific IgA was detectable in infants’ sera starting at less than 3 months of age. Sera of mothers with a CMA infant had increased binding of epitope-specific IgA to CM proteins compared to those with a non-CMA infant.
Conclusion & Clinical Relevance
These findings support the concept that mother’s milk has a distinct anti-food antibody repertoire when compared to the antibody repertoire of the peripheral blood. Increased binding of serum epitope-specific IgA to CM in mothers of infants with CMA may reflect inherited systemic immunogenicity of CM proteins in these families, although specific IgA in breast milk was not proportionally upregulated.
INTRODUCTION
Through the transfer of maternal antibodies, breast milk (BM) presents a unique opportunity to educate the developing infant mucosal immune system(1). Therefore, these BM-derived antibodies may have significant implications for the transfer of protection provided against mucosal pathogens as shown for postnatal mother-to-infant transfer of HIV(2) and possibly against immune-mediated diseases such as allergies. We have previously shown that high levels of BM total and specific IgA are associated with protection against cow’s milk allergy (CMA) (3), which is typically the first sign of breach in development of oral tolerance. Understanding the origins and the specificity of BM IgA may provide guidance on ways to enhance the protective properties of BM.
IgA in serum is mainly monomeric (mIgA), whereas in mucosal fluid, polymeric IgA is predominant. Mucosal IgA antibodies are produced by plasma cells in the lamina propria and are transported across epithelial cells by the polymeric immunoglobulin receptor (pIgR)(4). At apical site, IgA is released as two mIgA molecules linked by the J chain peptide and the extracellular ligand binding portion of pIgR, called the secretory component. BM is a rich source of secretory IgA (SIgA) with lesser amounts of SIgG and SIgM (5). Milk IgA is produced by mammary gland B cells that have migrated from the mother’s intestine via the “enteromammary link” (6, 7). This is suggested by animal studies, in which B cells from Peyer’s patches and mesenteric lymph nodes were shown to migrate specifically to the lactating mammary gland (8–11). This is controlled by hormones antepartum(12), and by the mucosal vascular addressin MadCAM-1, which interacts with the gut homing receptor α4β7 integrin (13) and mucosa-associated CCL28/CCR10 link (14). Consistent with this, in a rabbit model oral and inhaled RSV resulted in RSV-IgA production in milk, bronchial and enteral secretions, whereas systemic immunization did not.(15) Studies in women in late pregnancy (16) showed an increase in plasma cells in milk, but not in saliva or serum, specific to the non-pathogenic E. coli strain used for oral immunization. Thereby the antibody specificity of BM reflects the antigenic stimulation encountered e.g. by the maternal gut. Antibodies in breast milk may also partly originate from the systemic compartment as suggested by high levels of dimeric IgA detected in breast milk after systemic passive immunization with broadly neutralizing HIV antibodies (17). For food-specific IgA, the presence of an enteromammary link was suggested by our previous work, which showed that a strict maternal diet eliminating CM was associated with lower levels of CM-specific IgA in BM than a regular diet containing CM (3). This implies that the food antigenic stimulation encountered by the maternal gut acutely directs the antibody specificity of BM, similar to what has been shown to be the case for IgA responses to microbes that are constantly modified to reflect the microbiota present in the gut lumen.(18)
Pioneering work has suggested that IgA production is associated with oral tolerance (19) and transient IgA deficiency in infancy seen in serum and saliva has been documented in atopy (20–23). In CMA, low serum IgA levels to β-lactoglobulin at diagnosis have been shown to be associated with persistence of CMA (24). Increases in serum specific IgA and IgA2 have been associated with development of natural tolerance in egg allergy (23). Antigen-specific serum IgA has been shown to increase in subcutaneous immunotherapy with aeroallergens (25), in oral immunotherapy (OIT) to egg (26) and in successful CM OIT(27). Simultaneously, CM allergen binding by serum IgA has been shown to increase over time in persisting CMA (28). There is some evidence to suggest that IgA2 may reflect changes in local environments as indicated by increases in TGF-β from nasal biopsies that correlate with serum IgA2 levels and by salivary specific IgA that has been shown to be induced with peanut sublingual immunotherapy.(29) Interestingly, serum IgA may play a role in preventing food-induced reactions, as seen in mouse models of egg allergy,(30) and in vitro experiments of IgE blocking activity.(31)
In light of the relative delay in maturation of the total IgA levels in early childhood, and the possible importance of serum IgA in infants prone to developing food allergy, we were interested in how infants’ epitope-specific IgA develops in early infancy. We also investigated whether food-specific IgA epitope profiles differ intra-individually between mother’s serum and BM, which would suggest different homing of food-specific antibody-secreting cells (ASC) to systemic and mammary immune compartments. Our clinical material consisted of banked mothers’ and infants’ sera and BM samples from dyads recruited for an allergy birth cohort.
METHODS
Subjects
We utilized stored BM and paired maternal and infant serum samples from a prospective birth cohort, designed to assess the development of the infant immune system and association between immunologic factors in human milk and development of food allergies in breastfed infants. Results for total and CM-specific IgA in BM of this cohort have been previously published.(32) (3) In brief, mothers volunteered to the study and were recruited before or shortly after birth from two different populations of differing risk for food allergy: 1) those who were atopic themselves by report and/or had a child with food allergy diagnosed by elimination and open food challenge or other allergic diseases assessed by the investigator (KMJ), and 2) those who had no atopic diseases or first-degree relatives with atopic diseases. They were followed prospectively with clinical follow-up visits occurring at 0–2 weeks, 1, 3, 6, 12 and 18 months to assess for any signs or symptoms suggestive of food allergies. Infants from two groups of differing risks for atopy were recruited: those with an increased risk of food allergy defined either as presence of an older sibling with food allergy and those with low risk as defined by having only non-atopic first degree relatives. Overall, 24 of the mother-infant pairs were in the group with increased risk of food allergy and 7 in the group with low risk. All infants were born full-term and had no other chronic diseases. They had diets appropriate for their age. A total of 31 mother-infant pairs had matched infant and maternal serum and milk samples available. In a given mother, BM and serum samples used in the analysis were collected at the same time point, between 5 days and 5 months of lactation (mean 54, IQR 31–67.5 days). Paired infant serum samples were collected between 1 and 18 months of age and the earliest available sample was used for the analyses (mean 8.5, IQR 4.2–12.6 months). During the follow-up, 9 infants developed IgE-mediated CMA as evidenced by presence of CM-specific IgE by skin prick testing and/or serum-specific IgE and an immediate-type reaction (hives, maculopapular rash, swelling, vomiting, and abdominal pain) during an open CM challenge, and 7 developed non-IgE-mediated CMA, diagnosed by delayed-type reactions (eczema exacerbation, diarrhea) during CM challenge and lack of CM-specific IgE. Fifteen infants did not develop CMA.
The initial cohort study was approved by the ethics committees of the Skin and Allergy Hospital of the Helsinki University Central Hospital and the City of Helsinki. Written informed consent was obtained from the mothers. Internal Review Board of the Icahn School of Medicine at Mount Sinai, New York, NY (HS# 11-01838) approved the use of clinical data and stored and frozen historical samples for the additional antibody assays.
Samples
BM and serum samples were collected on each clinical follow-up visit if possible. BM samples were collected in the morning and processed immediately. Samples were centrifuged (400 g, 15 min), fat was removed, and supernatant collected, frozen and stored at −80°C. After thawing, samples were centrifuged at 17 000 g, 10 min at 4°C and the fatty layer was removed. The samples from mothers who had mastitis during the preceding 4 weeks were excluded. The serum samples were collected by venipuncture, frozen, and stored at −80°C.
Measurement of epitope-specific IgA antibodies
Samples were analyzed using a peptide microarray of αs1-, αs2- β- and κ-casein and β-lactoglobulin, which are the most common CM allergens. Each protein represented as a set of 20 amino acid peptides with offset of 3 amino acids (i.e. overlapping by 17 amino acids) covering the entire polypeptide (50–65 peptides/protein). IgA binding was detected using fluor-labeled anti-IgA and quantitated by a microarray scanner. Background binding was measured from blank spots. Raw fluorescence intensity from three replicate spots was converted to a Z-score as previously described.(33) Briefly, a Z- score is defined as IgA binding measured in relation to background binding: (sample intensity – median blank intensity) divided by median absolute deviation of blank intensity. In addition to individual peptides Z-scores, total Z-score for a protein is expressed as a sum of all peptide Z-scores for that protein. Data for the same CM antigen epitopes for specific serum IgE, IgG4 and IgA have been previously published (24, 34).
Statistical analysis
Statistical comparison of immunoglobulin levels was by a Wilcoxon rank sum test for two groups or by a Kruskal-Wallis test for three groups. Nonparametric methods were used because of non-normality of the measurements. Statistical significance for contingency tables was assessed using chi-square tests (or Fisher’s exact test if any cell had less than five counts). On the box plots the median is represented by a horizontal line within the box representing the 25th to 75th percentile, and whiskers show the 5th to 95th percentile.
For comparisons between individuals and between groups, peptide Z-scores ≥3 were considered significant. Each individual sample was filtered for noise and spurious binding by rejecting Z-scores that did not have a neighboring signal that was also significant. Statistical significance testing was performed using R software version 3.2.3 (35).
For comparing epitope recognition between paired BM and mothers’ serum samples, peptide series were grouped to epitopes by calculating Jaccard distance between each pair of neighboring peptides, and grouping together peptides with a distance < 0.4. An epitope was then determined to be positive for binding if any of its constituent peptides were positive (See Table 3). Binding agreement between paired samples was then assessed using Cohen’s Kappa for inter-rater agreement, with the two sample types, mother’s milk and serum representing raters. Cohen’s Kappa measures agreement between two categorical variables emphasizing agreement beyond that obtained by random chance. It’s values range between 1 and −1 such that 1 indicates perfect agreement between the two raters, 0 indicates agreement that is no better than what would be achieved by random chance, and negative values indicate exclusivity.
RESULTS
IgA to κ, α s2- and β-casein are present in the majority of BM and serum samples
When IgA response to each protein was measured as a sum of its individual peptides, κ-casein was the most immunogenic CM protein followed by αs2- and β-casein. These proteins elicited IgA responses in 90% of the BM samples and 61–77% of the infant and maternal serum samples (Table 1). In contrast, β-lactoglobulin and αs1-casein induced specific IgA responses in only 13–19% of the infant serum samples and in about 30% of mothers’ serum and BM samples (Table 1). In general, BM samples had IgA antibodies to the most numerous CM proteins and infant serum had IgA to the least numerous CM proteins. However, up to 87% of infant sera had IgA against at least one CM protein.
Table 1.
Number of samples with IgA binding to at least one cow’s milk peptide per protein. A total of 31 samples of each type were analyzed.
| α s1-casein | α s2-casein | β-casein | κ-casein | β-lactoglobulin | |
|---|---|---|---|---|---|
| Breast Milk | 8 (26%) | 19 (61%) | 19 (61%) | 28 (90%) | 10 (32%) |
| Mothers’ Sera | 10 (32%) | 22 (71%) | 18 (58%) | 24 (77%) | 9 (29%) |
| Infants’ Sera | 6 (19%) | 21 (68%) | 13 (42%) | 19 (61%) | 4 (13%) |
The same IgA-binding epitopes are generally immunogenic in BM, mothers’ and infants’ sera
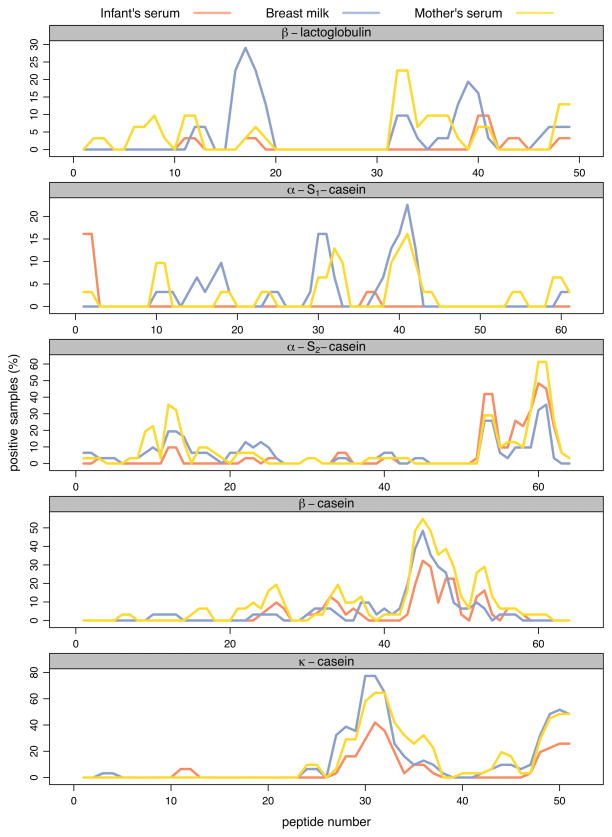
IgA binding areas in most CM proteins coincided between BM and serum samples, although β-lactoglobulin had distinct patterns of recognition that varied between BM and serum (Fig. 1). This distinction was most noticeable in recognition of β-lactoglobulin epitopes 1 and 3 that were only recognized by milk samples but not by serum samples (8/1/1 and 5/1/1 positive in milk only/serum only/both, respectively) (Fig 2 and Table S1). On the contrary, epitope 2 on β-lactoglobulin was predominantly recognized by serum samples, and only by a small number of BM samples (2/6/1 positive in milk only/serum only/both).
Figure 1.
Frequency of IgA binding to individual cow’s milk protein peptides. Peptide binding frequency for each protein is plotted as a percentage of positive (Z-score > 3) samples. The three different sample types (or compartments) are shown as separate lines.
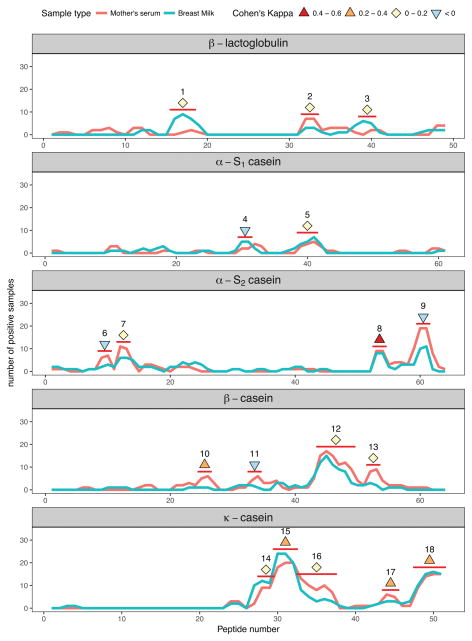
Figure 2.
Concordance of milk protein epitope recognition by IgA in paired mother’s serum and breast milk samples. The frequency of peptide recognition is plotted for each protein. Peptide epitopes are marked with a red bar and determined as described in Materials and Methods section. Cohen’s Kappa coefficient for paired milk and serum samples is shown for each epitope as a symbol. Cohen’s Kappa varies between 1 and −1. The maximum value of 1 signifies complete concordance between the two sample types whereas 0 signifies complete independence, i.e. coincidence is not greater than what would be expected by random chance.
IgA specificity is distinct between paired samples of mother’s milk and serum and infant’s serum
Because the same epitopes were generally immunogenic between BM and sera in the whole cohort, we were next interested in comparing epitope recognition patterns within a mother infant-pair. When IgA peptide binding profiles were compared between mother’s milk and serum, it was noted that there was significant discordance. Although some epitopes were recognized by IgA antibodies in the same individual’s milk and serum samples, many were bound by BM IgA or by mother’s serum IgA but not by both (Fig. 2, Table S1). The percentage of mothers with both milk and serum IgA recognizing the same epitope varied depending on the protein, ranging from 13% in αs1-casein to 73% in κ-casein (Table 2). The most immunogenic epitopes in α s1-, β- and κ-casein, epitopes 8, 10, 15 and 18 were recognized by IgA in both mother’s BM and serum (kappa 0.23–0.43, see table S1), whereas most epitopes were recognized by BM or serum IgA, but not both (kappa < 0.2).
Table 2.
Coincidence of CM epitope recognition by IgA in BM and serum
| Protein | Number of mothers (total = 31) | Ratio | |
|---|---|---|---|
| BM and serum IgA recognize one or more of the same epitope | BM or serum IgA recognize any epitope | ||
| β-lactoglobulin | 3 | 15 | 20% |
| αs1-casein | 2 | 16 | 13% |
| αs2-casein | 11 | 27 | 41% |
| β-casein | 11 | 26 | 42% |
| κ-casein | 22 | 30 | 73% |
IgA-binding epitopes in mothers’ and infants’ sera were also discordant (Fig S1b) as were epitopes between breast milk and infant serum (Fig S1c). Only a minority of epitopes were recognized by both infant’s and mother’s sera within the dyad.
Infants who develop CMA have mothers with elevated serum CM-specific IgA binding
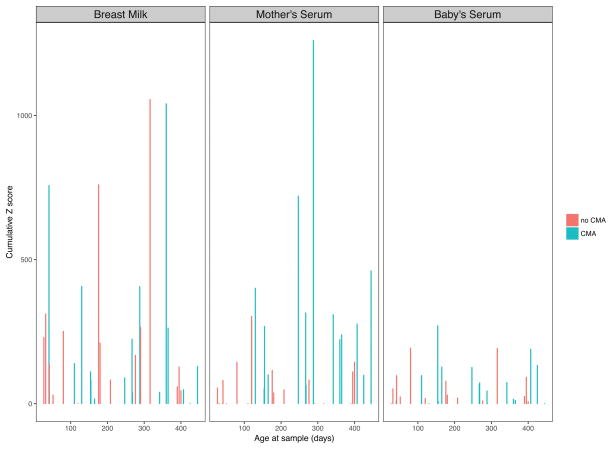
In the next step, we sought to assess the impact of the infant’s age on the production of CM allergen-specific IgA in all three sample types (Fig. 3). We noted that in infant sera, IgA reactivity was present even in the earliest (1 month) samples, and varied throughout the entire range of ages with no significant correlation with age (data not shown). Stratifying the data by infants’ future CMA status did not affect the distribution or age correlation of composite IgA score (data not shown). We did not detect a statistically significant difference in specific IgA levels between dyads in infants with IgE-mediated and non-IgE-mediated CMA and thus data from all CMA were treated as one group. There was no significant correlation between infant’s age and IgA reactivity in BM or mother’s serum (data not shown). However, we noticed that IgA from sera of mothers with a CMA infant appeared to have more IgA epitope binding.
Figure 3.
Comparison of IgA binding to cow’s milk proteins in mother-infant dyads with CMA and in those with no CMA in BM, mother’s serum and infant’s serum. Combined peptide Z-scores for each protein were summed and are displayed for each sample. Samples are labeled on x-axis by infant’s age and sorted in increasing order of age.
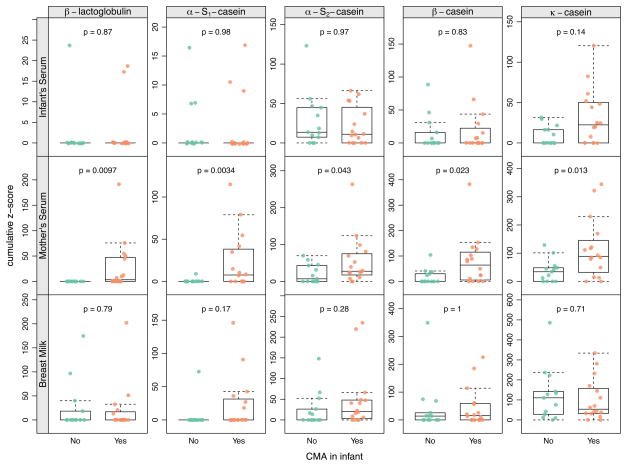
We then compared Z-score sums for individual proteins between mothers with an infant that develops CMA and those that did not (Fig. 4) and noted that mothers with CMA infants had significantly elevated levels of IgA to all of the CM proteins (p < 0.05). No difference was seen in their BM or infant serum CM-epitope-specific IgA levels between CMA and non-CMA dyads.
Figure 4.
Difference of IgA binding between dyads with and without CMA. Results are shown separately for cow’s milk protein and sample type. Boxes indicate the 25th and 75th percentile with a horizontal line at median. Whiskers indicate 5th and 95th percentile. P-values for group-wise comparisons are calculated using the Wilcoxon rank-sum test.
DISCUSSION
In the present study we have shown that epitope specificity of BM IgA significantly differed from that of the paired maternal serum for each assessed CM allergen. This suggests that distinct pools of food-specific IgA ASCs home to mucosal and systemic immune systems. It is possible that these mammary gland ASCs represent B cells that originate in the gut, similar to what has been described for the entero-mammary link of pathogen-specific IgA ASCs (13). This could be further assessed by comparing IgA epitope-specificity in BM to that of the salivary IgA from the sublingual compartment, which has recently been suggested as a novel noninvasive proxy for intestinal immune induction.(36) Alternatively, this could result from distinct epitopes being presented to mucosal and systemic T and B cells. We also found that epitope-specific IgA was detectable in infants’ sera as early as 3 months of age. Lastly, sera of mothers with a CMA infant had increased binding of epitope-specific IgA to CM proteins compared to those with a non-CMA infant.
In serum, there is a relative delay in the development of total IgA seen until about 2 years of life, and there is a dramatic increase in the number of peripheral blood CD27+IgA+ memory B cells, consistent with T cell-dependent development within the first 18 months of life (37). In the current study, we showed that epitope-specific IgA was detectable in infants’ sera starting at a relatively young age of less than 3 months. Plasma cells producing IgA are only generated after birth to provide SIgA to the lumen and therefore infants’ mucosal IgA production is considered compromised at least for the first month of life (38–40). Normally, Peyer’s patch, mesenteric lymph node and mucosal lamina propria B-cells convert IgM to IgA via class switch recombination (CSR).(41) Two pathways for producing IgA, T-cell dependent (TD) and T-cell independent (TI) pathways, both utilize plasma B-cell CSR but involve different upstream factors.(38) Therefore, delayed TI responses may contribute to the delayed induction of intestinal IgA during early infancy. Given breast milk IgA does not cross the infant gut barrier, the early serum specific IgA production reflects the infant’s own mucosal production of IgA to antigens such as cow’s milk, which are among the first foreign proteins introduced to the infant gut. We did not have samples to assess the development of mucosal IgA, nor did we have samples prior to one month of age to assess when epitope-specific IgA responses were initially seen.
Interestingly, we also show that mothers of CMA infants had significantly increased binding of serum IgA, but not breast milk IgA specific to CM epitopes, compared to mothers with a non-CMA infant. However, utilizing conventional ELISA, we and others have shown that milk from mothers of CMA infants have lower levels of total and CM-specific IgA than those with healthy infants. The significant difference between these methods is that ELISA detects both sequential and conformational epitopes (32, 42) whereas the current method detects sequential epitopes only. In our previous study, we found that maternal diets eliminating CM are associated with lower specific IgA levels in BM.(3) In light of the findings of the current study, another possibility is that the low BM IgA production is due to an inability to produce mucosal IgA antibodies, with simultaneous upregulation of systemic antibody responses. Although the mothers in the present study were tolerating CM, their systemic IgA responses were increased similarly to what has been reported in persistent CMA (28). Because avoidance of CM was seen in most mothers with CMA infants, the present study was too small in number to dissect out at the epitope level whether low BM IgA in mothers with a CMA infant is due to an inability to produce mucosal IgA or due to lack of stimulation by CM exposure. Nevertheless, strategies to increase production of mucosal (breast milk) IgA production may aid in prevention of food allergies.
Other limitations of the study include limited amounts of infant serum available to examine production of other immunoglobulin classes, including IgA1 vs predominantly mucosally-derived IgA2, as well as systemically prevalent IgG, which would have been of interest. Furthermore, we investigated IgA binding only to linear, not conformational epitopes, which certainly play a major role in CMA (43). The strengths include the access to paired mother-infant samples at a young age, prior to the development of established CMA.
The function of BM IgA antibodies is not completely understood.(44, 45) By reinforcing the epithelial barrier, SIgA inhibits inappropriate immune activation by microorganisms and antigens in the lumen of the intestinal and respiratory tracts.(46) This immune exclusion could mediate tolerance by providing protection against excessive and uncontrolled antigen influx. Our previous data using cell culture transcytosis assays support the view that BM plays a role in immune exclusion preventing excess antigen uptake in enterocytes.(3) Alternatively, BM antibodies could favor focused antigen uptake, e.g. via M cells as immune complexes, which could target the antigen for presentation favoring tolerance development. This is suggested by studies of Rey and Corthesy (47) who showed that SIgA was targeted to Peyer’s patch dendritic and T cells after transport by intestinal M cells, via a so-far unknown IgA receptor as described by Mantis.(48) This possibility is supported by our findings of facilitated peanut uptake to Peyer’s patches in the presence of peanut-specific antibodies in mice.(49) Lastly, it is known that BM antibodies play a role in gut homeostasis by shaping the gut microbiota.(50)
In summary, we show that the mammary gland IgA antibody repertoire to CM proteins is distinct from the systemic immune compartment, which may be due to specific homing of mucosa-associated ASCs to mammary glands. Mothers who have an infant with CMA show increased systemic immunogenicity with CM proteins as denoted by epitope-specific IgA in their serum but not in their BM, which may play a role in development of CMA. The regulation of the BM IgA repertoire and levels of antibodies are an important part of the mucosal immune system in the mammary gland, and may have implications to the specific immune imprinting of the infant’s immune system by BM. Strategies to increase mucosal (breast milk) IgA production may aid in prevention of food allergies.
Supplementary Material
Coincidence of peptide recognition in A) breast milk and mother’s serum, B) mother’s and infant’s serum and C) infant’s serum and breast milk. Heatmaps show individual sample pairs as horizontal rows. In each heatmap samples are grouped by the infant’s CMA status. IgA binding is coded such that binding to only one sample type is labeled in pink or blue and coincidence of binding in both sample types is labeled in purple.
Table 3.
Details of the peptide epitopes
| Epitope | Protein | Peptide numbers | Common sequence in peptides |
|---|---|---|---|
| 1 | β-lactoglobulin | 16–18 | EGNEWKQLLIELDG |
| 2 | β-lactoglobulin | 32–33 | PEASNEMCFLLYKKYDT |
| 3 | β-lactoglobulin | 39–40 | ELAEDDVEPTRVLCQCA |
| 4 | α-S1 casein | 30–31 | PVKYKKLRLLQELYGLY |
| 5 | α-S1 casein | 39–41 | PEKQQAHIGEKMSH |
| 6 | α-S2 casein | 9–10 | VVEKCFTSCLNEKSPNI |
| 7 | α-S2 casein | 12–13 | SYEEENANRVVEKCFTS |
| 8 | α-S2 casein | 53–54 | LAFKQYRQSIKKLFNLR |
| 9 | α-S2 casein | 60–61 | KPQIWPKMAKQHQYVTK |
| 10 | β-casein | 25–26 | VEPQLFPPVVVPTQTLP |
| 11 | β-casein | 33–34 | VPYKPFPMEKHKPAMAE |
| 12 | β-casein | 44–49 | PQHPQ |
| 13 | β-casein | 52–53 | PVPLVKSQSLSLVSQPP |
| 14 | κ-casein | 28–29 | PHPHRAMTTPQAQCSKA |
| 15 | κ-casein | 30–32 | AMFSLHPHPHRAMT |
| 16 | κ-casein | 33–37 | KDQNKKPP |
| 17 | κ-casein | 44–45 | SDELTAVTSEVAETTPT |
| 18 | κ-casein | 48–51 | TNIEPPSEIVE |
Acknowledgments
The authors thank Marina Goldman or her technical help and Mayte Suarez-Farinas, PhD for her advice on statistical analysis.
Funding: The project described was supported by Grant Number K08 AI091655 (K.M. Järvinen) from the National Institute of Allergy and Infectious Diseases. H.A. Sampson is supported in part by grant AI66738 and AI66738 from NIAID, and the Food Allergy Research & Education organization. Cecilia Berin is supported in part by NIH grants AI093577, AI124062, and AI118644. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. E.M. Savilahti was supported for this project by the Foundation for Pediatric Research (Finland).
ABBREVIATIONS
- BLG
β-lactoglobulin
- BM
breast milk
- CM
cow’s milk
- CMA
cow’s milk allergy
- OFC
oral food challenge
- SIgA
secretory IgA
Footnotes
DISCLOSURE
KMJ is a consultant for DBV Technologies and Merck and received royalties from Up-To-Date. HAS is employed 60% as a faculty member at the Icahn School of Medicine at Mount Sinai and 40% as CSO at DBV Technologies; has served on Advisory Boards at Allertein Therapeutics, LLC, Genentech/Novartis, Sanofi, Inc., and the DANONE Institute; and has received royalties from Elsevier and UpToDate. AES, EMS and MCB have no disclosures.
REFRENCES
- 1.Torow N, Marsland BJ, Hornef MW, Gollwitzer ES. Neonatal mucosal immunology. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.81. [DOI] [PubMed] [Google Scholar]
- 2.Pollara J, McGuire E, Fouda GG, Rountree W, Eudailey J, Overman RG, et al. Association of HIV-1 Envelope-Specific Breast Milk IgA Responses with Reduced Risk of Postnatal Mother-to-Child Transmission of HIV-1. J Virol. 2015;89(19):9952–61. doi: 10.1128/JVI.01560-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarvinen KM, Westfall JE, Seppo MS, James AK, Tsuang AJ, Feustel PJ, et al. Role of maternal elimination diets and human milk IgA in the development of cow’s milk allergy in the infants. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2014;44(1):69–78. doi: 10.1111/cea.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaetzel CS. Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host-microbial mutualism. Immunology letters. 2014;162(2 Pt A):10–21. doi: 10.1016/j.imlet.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koenig A, de Albuquerque Diniz EM, Barbosa SF, Vaz FA. Immunologic factors in human milk: the effects of gestational age and pasteurization. Journal of human lactation : official journal of International Lactation Consultant Association. 2005;21(4):439–43. doi: 10.1177/0890334405280652. [DOI] [PubMed] [Google Scholar]
- 6.Hanson LA, Korotkova M. The role of breastfeeding in prevention of neonatal infection. Seminars in neonatology : SN. 2002;7(4):275–81. doi: 10.1016/s1084-2756(02)90124-7. [DOI] [PubMed] [Google Scholar]
- 7.Nathavitharana KA, Catty D, McNeish AS. IgA antibodies in human milk: epidemiological markers of previous infections? Arch Dis Child Fetal Neonatal Ed. 1994;71(3):F192–7. doi: 10.1136/fn.71.3.f192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roux ME, McWilliams M, Phillips-Quagliata JM, Weisz-Carrington P, Lamm ME. Origin of IgA-secreting plasma cells in the mammary gland. The Journal of experimental medicine. 1977;146(5):1311–22. doi: 10.1084/jem.146.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roux ME, McWilliams M, Phillips-Quagliata JM, Lamm ME. Differentiation pathway of Peyer’s patch precursors of IgA plasma cells in the secretory immune system. Cellular immunology. 1981;61(1):141–53. doi: 10.1016/0008-8749(81)90361-0. [DOI] [PubMed] [Google Scholar]
- 10.Hanson LA, Ahlstedt S, Carlsson B, Fallstrom SP, Kaijser B, Lindblad BS, et al. New knowledge in human milk immunoglobulin. Acta Paediatrica Scandinavica. 1978;67(5):577–82. doi: 10.1111/j.1651-2227.1978.tb17805.x. [DOI] [PubMed] [Google Scholar]
- 11.Dahlgren UI, Ahlstedt S, Hanson LA. The localization of the antibody response in milk or bile depends on the nature of the antigen. Journal of immunology (Baltimore, Md: 1950) 1987;138(5):1397–402. [PubMed] [Google Scholar]
- 12.Weisz-Carrington P, Roux ME, McWilliams M, JMPH-Q, Lamm ME. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. Journal of immunology. 1979;123(4):1705–8. [PubMed] [Google Scholar]
- 13.Tanneau GM, Hibrand-Saint Oyant L, Chevaleyre CC, Salmon HP. Differential recruitment of T- and IgA B-lymphocytes in the developing mammary gland in relation to homing receptors and vascular addressins. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1999;47(12):1581–92. doi: 10.1177/002215549904701210. [DOI] [PubMed] [Google Scholar]
- 14.Morteau O, Gerard C, Lu B, Ghiran S, Rits M, Fujiwara Y, et al. An indispensable role for the chemokine receptor CCR10 in IgA antibody-secreting cell accumulation. Journal of immunology (Baltimore, Md: 1950) 2008;181(9):6309–15. doi: 10.4049/jimmunol.181.9.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peri BA, Theodore CM, Losonsky GA, Fishaut JM, Rothberg RM, Ogra PL. Antibody content of rabbit milk and serum following inhalation or ingestion of respiratory syncytial virus and bovine serum albumin. Clinical and experimental immunology. 1982;48(1):91–101. [PMC free article] [PubMed] [Google Scholar]
- 16.Goldblum RM, Ahlstedt S, Carlsson B, Hanson LA, Jodal U, Lidin-Janson G, et al. Antibody-forming cells in human colostrum after oral immunisation. Nature. 1975;257(5529):797–8. doi: 10.1038/257797a0. [DOI] [PubMed] [Google Scholar]
- 17.Fouda GG, Eudailey J, Kunz EL, Amos JD, Liebl BE, Himes J, et al. Systemic administration of an HIV-1 broadly neutralizing dimeric IgA yields mucosal secretory IgA and virus neutralization. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science (New York, NY) 2010;328(5986):1705–9. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Challacombe SJ, Tomasi TB., Jr Systemic tolerance and secretory immunity after oral immunization. J Exp Med. 1980;152(6):1459–72. doi: 10.1084/jem.152.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor B, Norman AP, Orgel HA, Stokes CR, Turner MW, Soothill JF. Transient IgA deficiency and pathogenesis of infantile atopy. Lancet. 1973;2(7821):111–3. doi: 10.1016/s0140-6736(73)93060-2. [DOI] [PubMed] [Google Scholar]
- 21.van Asperen PP, Gleeson M, Kemp AS, Cripps AW, Geraghty SB, Mellis CM, et al. The relationship between atopy and salivary IgA deficiency in infancy. Clin Exp Immunol. 1985;62(3):753–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Bottcher MF, Haggstrom P, Bjorksten B, Jenmalm MC. Total and allergen-specific immunoglobulin A levels in saliva in relation to the development of allergy in infants up to 2 years of age. Clin Exp Allergy. 2002;32(9):1293–8. doi: 10.1046/j.1365-2222.2002.01470.x. [DOI] [PubMed] [Google Scholar]
- 23.Konstantinou GN, Nowak-Wegrzyn A, Bencharitiwong R, Bardina L, Sicherer SH, Sampson HA. Egg-white-specific IgA and IgA2 antibodies in egg-allergic children: is there a role in tolerance induction? Pediatr Allergy Immunol. 2014;25(1):64–70. doi: 10.1111/pai.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savilahti EM, Rantanen V, Lin JS, Karinen S, Saarinen KM, Goldis M, et al. Early recovery from cow’s milk allergy is associated with decreasing IgE and increasing IgG4 binding to cow’s milk epitopes. The Journal of allergy and clinical immunology. 2010;125(6):1315–21. e9. doi: 10.1016/j.jaci.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pilette C, Nouri-Aria KT, Jacobson MR, Wilcock LK, Detry B, Walker SM, et al. Grass pollen immunotherapy induces an allergen-specific IgA2 antibody response associated with mucosal TGF-beta expression. Journal of immunology (Baltimore, Md: 1950) 2007;178(7):4658–66. doi: 10.4049/jimmunol.178.7.4658. [DOI] [PubMed] [Google Scholar]
- 26.Wright BL, Kulis M, Orgel KA, Burks AW, Dawson P, Henning AK, et al. Component-resolved analysis of IgA, IgE, and IgG4 during egg OIT identifies markers associated with sustained unresponsiveness. Allergy. 2016 doi: 10.1111/all.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savilahti EM, Kuitunen M, Valori M, Rantanen V, Bardina L, Gimenez G, et al. Use of IgE and IgG4 epitope binding to predict the outcome of oral immunotherapy in cow’s milk allergy. Pediatr Allergy Immunol. 2014;25(3):227–35. doi: 10.1111/pai.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savilahti EM, Saarinen KM, Savilahti E. Specific antibodies to cow’s milk proteins in infants: effect of early feeding and diagnosis of cow’s milk allergy. Eur J Nutr. 2010;49(8):501–4. doi: 10.1007/s00394-010-0109-8. [DOI] [PubMed] [Google Scholar]
- 29.Kulis M, Saba K, Kim EH, Bird JA, Kamilaris N, Vickery BP, et al. Increased peanut-specific IgA levels in saliva correlate with food challenge outcomes after peanut sublingual immunotherapy. J Allergy Clin Immunol. 2012;129(4):1159–62. doi: 10.1016/j.jaci.2011.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strait RT, Mahler A, Hogan S, Khodoun M, Shibuya A, Finkelman FD. Ingested allergens must be absorbed systemically to induce systemic anaphylaxis. J Allergy Clin Immunol. 2011;127(4):982–9. e1. doi: 10.1016/j.jaci.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dodev TS, Bowen H, Shamji MH, Bax HJ, Beavil AJ, McDonnell JM, et al. Inhibition of allergen-dependent IgE activity by antibodies of the same specificity but different class. Allergy. 2015;70(6):720–4. doi: 10.1111/all.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jarvinen KM, Laine ST, Jarvenpaa AL, Suomalainen HK. Does low IgA in human milk predispose the infant to development of cow’s milk allergy? Pediatric research. 2000;48(4):457–62. doi: 10.1203/00006450-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Lin J, Bardina L, Shreffler WG. Microarrayed allergen molecules for diagnostics of allergy. Methods in molecular biology (Clifton, NJ) 2009;524:259–72. doi: 10.1007/978-1-59745-450-6_19. [DOI] [PubMed] [Google Scholar]
- 34.Jarvinen KM, Suarez-Farinas M, Savilahti E, Sampson HA, Berin MC. Immune factors in breast milk related to infant milk allergy are independent of maternal atopy. The Journal of allergy and clinical immunology. 2014 doi: 10.1016/j.jaci.2014.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Team RDC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2016. [Google Scholar]
- 36.Aase A, Sommerfelt H, Petersen LB, Bolstad M, Cox RJ, Langeland N, et al. Salivary IgA from the sublingual compartment as a novel noninvasive proxy for intestinal immune induction. Mucosal Immunol. 2016;9(4):884–93. doi: 10.1038/mi.2015.107. [DOI] [PubMed] [Google Scholar]
- 37.van Gent R, van Tilburg CM, Nibbelke EE, Otto SA, Gaiser JF, Janssens-Korpela PL, et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin Immunol. 2009;133(1):95–107. doi: 10.1016/j.clim.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Gustafson CE, Higbee D, Yeckes AR, Wilson CC, De Zoeten EF, Jedlicka P, et al. Limited expression of APRIL and its receptors prior to intestinal IgA plasma cell development during human infancy. Mucosal Immunol. 2014;7(3):467–77. doi: 10.1038/mi.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sloper KS, Brook CG, Kingston D, Pearson JR, Shiner M. Eczema and atopy in early childhood: low IgA plasma cell counts in the jejunal mucosa. Arch Dis Child. 1981;56(12):939–42. doi: 10.1136/adc.56.12.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perkkio M, Savilahti E. Time of appearance of immunoglobulin-containing cells in the mucosa of the neonatal intestine. Pediatr Res. 1980;14(8):953–5. doi: 10.1203/00006450-198008000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Lin M, Du L, Brandtzaeg P, Pan-Hammarstrom Q. IgA subclass switch recombination in human mucosal and systemic immune compartments. Mucosal Immunol. 2014;7(3):511–20. doi: 10.1038/mi.2013.68. [DOI] [PubMed] [Google Scholar]
- 42.Savilahti E, Tainio VM, Salmenpera L, Arjomaa P, Kallio M, Perheentupa J, et al. Low colostral IgA associated with cow’s milk allergy. Acta Paediatrica Scandinavica. 1991;80(12):1207–13. doi: 10.1111/j.1651-2227.1991.tb11810.x. [DOI] [PubMed] [Google Scholar]
- 43.Jarvinen KM, Beyer K, Vila L, Chatchatee P, Busse PJ, Sampson HA. B-cell epitopes as a screening instrument for persistent cow’s milk allergy. The Journal of allergy and clinical immunology. 2002;110(2):293–7. doi: 10.1067/mai.2002.126080. [DOI] [PubMed] [Google Scholar]
- 44.Brandtzaeg P. Update on mucosal immunoglobulin A in gastrointestinal disease. Current opinion in gastroenterology. 2010;26(6):554–63. doi: 10.1097/MOG.0b013e32833dccf8. [DOI] [PubMed] [Google Scholar]
- 45.Brandtzaeg P. Food allergy: separating the science from the mythology. Nature reviewsGastroenterology & hepatology. 2010;7(7):380–400. doi: 10.1038/nrgastro.2010.80. [DOI] [PubMed] [Google Scholar]
- 46.Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nature reviewsImmunology. 2011;12(1):9–23. doi: 10.1038/nri3112. [DOI] [PubMed] [Google Scholar]
- 47.Rey J, Garin N, Spertini F, Corthesy B. Targeting of secretory IgA to Peyer’s patch dendritic and T cells after transport by intestinal M cells. Journal of immunology (Baltimore, Md: 1950) 2004;172(5):3026–33. doi: 10.4049/jimmunol.172.5.3026. [DOI] [PubMed] [Google Scholar]
- 48.Mantis NJ, Cheung MC, Chintalacharuvu KR, Rey J, Corthesy B, Neutra MR. Selective adherence of IgA to murine Peyer’s patch M cells: evidence for a novel IgA receptor. Journal of immunology (Baltimore, Md: 1950) 2002;169(4):1844–51. doi: 10.4049/jimmunol.169.4.1844. [DOI] [PubMed] [Google Scholar]
- 49.Jarvinen KM, Westfall J, De Jesus M, Mantis NJ, Carroll JA, Metzger DW, et al. Role of Maternal Dietary Peanut Exposure in Development of Food Allergy and Oral Tolerance. PLoS One. 2015;10(12):e0143855. doi: 10.1371/journal.pone.0143855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogier EW, Frantz AL, Bruno ME, Wedlund L, Cohen DA, Stromberg AJ, et al. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(8):3074–9. doi: 10.1073/pnas.1315792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coincidence of peptide recognition in A) breast milk and mother’s serum, B) mother’s and infant’s serum and C) infant’s serum and breast milk. Heatmaps show individual sample pairs as horizontal rows. In each heatmap samples are grouped by the infant’s CMA status. IgA binding is coded such that binding to only one sample type is labeled in pink or blue and coincidence of binding in both sample types is labeled in purple.