Summary
Signals emanating from the B cell receptor (BCR) promote proliferation and survival in diverse forms of B cell lymphoma. Precision medicine strategies targeting the BCR pathway have been generally effective in treating lymphoma, but often fail to produce durable responses in diffuse large B cell lymphoma (DLBCL), a common and aggressive cancer. New insights into DLBCL biology garnered from genomic analyses and functional proteogenomic studies have identified novel modes of BCR signaling in this disease. Herein, we describe the distinct roles of antigen-dependent and antigen-independent BCR signaling in different subtypes of DLBCL. We highlight mechanisms by which the BCR cooperates with TLR9 and mutant isoforms of MYD88 to drive sustained NF-κB activity in the activated B cell-like (ABC) subtype of DLBCL. Finally, we discuss progress in detecting and targeting oncogenic BCR signaling to improve the survival of patients with lymphoma.
Keywords: lymphoma, B cell receptor, cancer, signal transduction
1. Introduction
Lymphomas comprise the 7th most frequently diagnosed form of human cancer (1). No less than six dozen forms of human lymphoma with distinct clinical presentations and prognoses have been described (2). These comprise aggressive non-Hodgkin lymphomas, including diffuse large B cell lymphoma (DLBCL) and Burkitt lymphoma (BL), and more indolent lymphomas, including follicular lymphoma (FL), marginal zone lymphoma (MGZL) and others. Most non-Hodgkin lymphomas are the malignant counterpart of mature B lymphocytes at various stages of differentiation and activation, with only a minority (~10%) derived from T lymphocytes (2). This pronounced skewing toward the B cell lineage is driven, in part, by the generation and expression of the B cell receptor (BCR). The BCR is of central importance to the biology of normal B cells, but the genetic processes of DNA rearrangement and somatic hypermutation that generate the BCR pose a risk of chromosomal translocations and oncogenic mutations. In addition to these genetic insults, intensive research over the past decade has revealed that various signals originating from the BCR can sustain malignant B cell growth and survival. These insights have enabled the clinical application of therapeutic agents targeting kinases that signal downstream of the BCR. Targeted precision medicine agents have been a game-changer in the treatment of lymphoma, particularly when used in combination with other cancer therapies. Nonetheless, more work is needed to fully realize the therapeutic potential of targeting BCR signaling in aggressive lymphomas.
The application of functional proteogenomic technologies to BCR signaling in aggressive lymphomas has recently uncovered novel modes of signal transduction that are distinct from those reported thus far in normal B cells. In the activated B cell-like (ABC) subgroup of DLBCL, the BCR can directly collaborate with TLR9 and MYD88 to promote lymphoma growth and survival. In this review, we will summarize the varied modes of BCR signaling in lymphoma, with particular focus on BCR signaling in aggressive lymphomas. We will further explore how these insights from the laboratory can be optimally translated to the clinic. Particular emphasis will be given to how our new understanding of pathogenic BCR signaling mechanisms can be exploited to identify patients who will benefit from BCR pathway inhibitors and to devise strategies to overcome resistance to these therapeutic agents.
2. B cell receptor signaling
2.1. BCR expression
Every B cell expresses a unique BCR. In essence, the BCR is a membrane bound antibody that can transduce signals intracellularly by virtue of its non-covalent association with a CD79A-CD79B heterodimer. The antibody portion of the BCR is composed of two immunoglobulin heavy (IgH) and two immunoglobulin light (IgL) chains. The shuffling of gene segments that encode the IgH and IgL chains generates BCRs that can recognize a tremendous diversity of antigens, both foreign and self. The IgH locus on chromosome 14 is composed of numerous variable (V), diversity (D), joining (J) and constant (C) gene segments. In the process of V(D)J recombination, recombination activating genes (RAG)-1 and RAG-2 combine the V, D and J segments to form an intact V region. Assembled V regions contain three hypervariable complementarity-determining regions (CDRs) that recognize antigen and are surrounded by more conserved framework (FR) regions that contribute to the three-dimensional structure of the V region. In addition to the diversity inherent in joining random V, D and J gene segments together, V(D)J recombination results in nucleotide loss and addition focused in the CDR3 region, adding substantial variation to the final amino acid sequence. To form a complete antibody, each IgH must pair with either and Igκ or Igλ light chain. Each light chain locus contains VL and JL gene segments that are rearranged by the same machinery that rearranges and diversifies VH gene segments. In naïve B cells, the assembled VH region is spliced to either the Cμ or Cδ constant region segments to generate IgM and IgD, respectively, and the assembled VL regions are spliced to the CL regions in their respective loci. Antigen exposure induces a germinal center reaction in secondary lymphoid organs in which B cells undergo a second DNA rearrangement process known as immunoglobulin heavy chain class switch recombination (CSR), generating IgG, IgA or IgE.
2.1. BCR signaling mechanisms
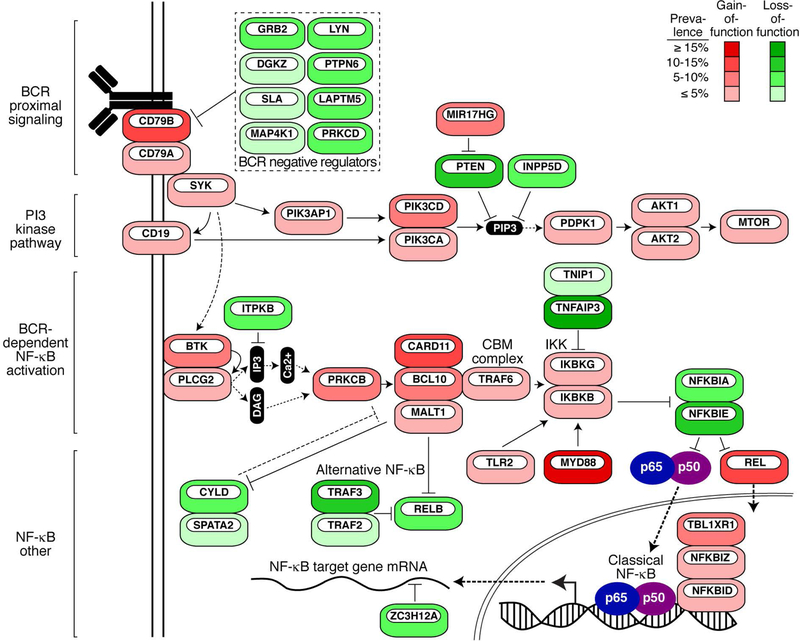
The antibody portion of the BCR associates with a heterodimer of CD79A and CD79B to form a complete BCR on the cell surface, which binds antigen and initiates signal transduction (Fig. 1). Signaling is primarily mediated through the single Immunoreceptor Tyrosine-based Activation Motif (ITAM) motifs present in the cytoplasmic tail of both CD79A and CD79B (3). Each ITAM contains two tyrosine residues surrounded by a conserved YxxL/Ix(6–8)YxxL/I sequence. The BCR transmits low-level ‘tonic’ signals even in the absence of antigen, and this mode of signaling sustains the survival of all mature B cells. In contrast, antigen engagement induces aggregation of the BCR on the plasma membrane, leading to rapid phosphorylation of CD79A and CD79B ITAM tyrosines by Src-family kinases (SFKs), including LYN, FYN and BLK (4). Phosphorylated ITAMs can then recruit SYK kinase by virtue of its tandem Src homology 2 (SH2) domains that can bind dually phosphorylated ITAMs, ultimately resulting in SYK activation (5). This active BCR signalosome recruits a host of adaptor proteins, including BLNK and LAT2, and additional kinases, including Bruton’s tyrosine kinase (BTK), phosphatidylinositol 3-kinase (PI3K) and AKT. Subsequently, antigen-stimulated BCR activation engages multiple signaling cascades to alter the proliferation, survival and differentiation of B cells (reviewed in detail in ref. (6)). Calcium signaling and consequent NF-κB activation is a dominant downstream pathway utilized by B cells to maintain their viability. Many of the kinases and adapters that mediate BCR signaling in normal B cells are altered genetically in lymphomas, leading to dysregulated, constitutive oncogenic signaling (Fig. 1)
Figure 1.
A simplified schematic of BCR signaling in lymphoma depicting genes frequently targeted for gain-of-function (red) or loss-of-function (green) genetic alterations. Percentages are estimate of frequency in all DLBCL subtypes. Frequencies include mutation and copy number alterations. Data are derived from ref. (37).
2.3. The germinal center reaction
Foreign antigens can induce potent B and T cell activation, spurring B cell migration to the germinal center (GC), where these B cells hone the affinity of their BCR to antigen. The GC is a specialized immune structure in secondary lymphoid organs – including lymph nodes, tonsils and spleen – where B cells interact with T follicular helper (TFH) cells and follicular dendritic cells (FDCs) (reviewed in ref. (7)). Within the GC light zone, B cells (also known as centrocytes,) acquire antigen from FDCs and present it to TFH cells (8). These B cells upregulate MYC and enter the GC dark zone (referred to as centroblasts), where they rapidly proliferate and undergo somatic hypermutation (SHM) (9). SHM targets the V regions of IgH and IgL by activation-induced cytidine deaminase (AID), which catalyzes the deamination of cytosine to uracil. Deaminated bases often result in somatic mutations by a variety of mechanisms. Centroblasts exit the dark zone to return to the light zone, where only cells expressing the highest affinity BCRs can compete for pro-survival signals from TFH cells. Positively selected cells can again enter the dark zone and, following many iterative rounds of affinity selection, the B cells with high affinity BCRs receive signals to terminally differentiate into antibody secreting plasma cells or long-lived circulating memory B cells. The GC reaction also promotes AID-dependent CSR, which generates two double-stranded breaks in the IgH locus at switch regions upstream of the IgM constant region and one of the downstream constant regions, leading to deletion of the intervening DNA and repair by non-homologous end joining. CSR can also occur outside of the confines of the GC in some instances (10). Whereas signaling by IgM BCRs promotes B cell proliferation, signaling by non-IgM BCRs instead promotes plasmacytic differentiation (11).
3. Diffuse Large B Cell Lymphoma
An appreciation of the cell-of-origin for cancer subtypes is important to understanding their underlying biology since the malignant cell inherits an epigenetic state that can influence the response to treatment. Categorization of lymphoma subtypes has evolved rapidly with the advent of various genomic technologies. DLBCL, the most common form of human lymphoma, is a good case in point. DLBCL was originally defined by histologic morphology, essentially as a diffuse proliferation of large, malignant B cells (12). By these criteria DLBCL was a single diagnostic entity, but this simplicity was inconsistent with differences in clinical presentation and response to chemotherapies. In an effort to make sense of this heterogeneity, gene expression profiling (GEP) studies were performed on a large cohort of DLBCL patient samples (13). Unsupervised clustering of these GEP data revealed two dominant molecular subgroups of DLBCL present at roughly equal frequencies. The germinal center B cell-like (GCB) subgroup is marked by expression of genes commonly found in germinal center B cells (13, 14). By contrast, the activated B cell-like (ABC) subgroup expresses genes characteristic of blood B cells that have been acutely stimulated through the BCR, notably including many NF-κB target genes (13, 14). When treated with the current standard immuno-chemotherapy (Rituximab plus the CHOP regimen; R-CHOP), DLBLB patients with ABC tumors have a substantially lower overall survival than those with GCB tumors (13, 15, 16).
The gene expression subgroups of DLBCL differ profoundly in both regulatory mechanisms and oncogenic pathways. The viability of ABC DLBCL cells is strongly dependent on constitutive NF-κB activation, which is the hallmark of this subgroup (17) and likely contributes to its resistance to immuno-chemotherapy. The ABC subgroup resembles the plasmablast, which is a rare B cell that marks the transition between a GC B cell and an antibody-secreting plasma cell (14). Chronic NF-κB activity drives expression of the transcription factor IRF4, which is essential in ABC DLBCL (18), and is highly expressed in normal and neoplastic plasma cells (19). In fact, IRF4 expression alone is sufficient to drive plasma cell differentiation (20, 21), but full terminal differentiation is frequently suppressed in ABC DLBCL by genetic inactivation of PRDM1 (encoding BLIMP-1) (22) and by two other repressors of IRF4, SPIB and BCL6, which are overexpressed by copy number amplification and translocation, respectively (23, 24). By definition, GCB DLBCL expresses genes that are characteristic of normal GC cells (13, 14), specifically those expressed by centrocytes in the GC light zone (8). Genetic inactivation of several chromatin modifiers promotes the GC differentiation of mature B cells, including KMT2D (MLL2), CREBBP, and EP300 (25–28). The histone lysine N-methyltransferase EZH2 maintains the GC phenotype by repressing gene expression as part of the polycomb repressive complex (29, 30), and recurrent EZH2 mutations in GCB DLBCL create mutant isoforms that promote tri-methylation of lysine 27 of the histone H3 tail (31). In addition to these two defined molecular subgroups, roughly 15–20% of DLBCL cases fall between the gene expression extremes of GCB to ABC and are declared “Unclassified”. Their nature was elusive prior to the deep genetic analysis of DLBCL described below.
3.1. Genetic subtypes of DLBCL
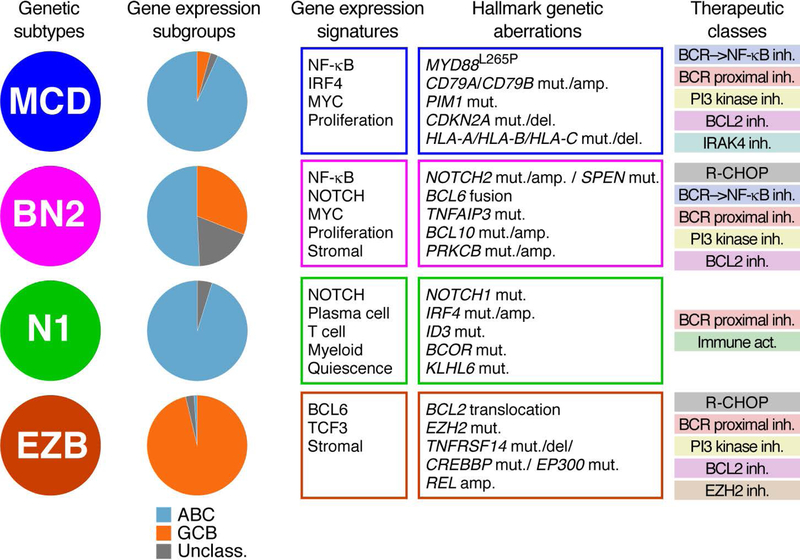
Since the discovery of the ABC and GCB subgroups, a variety of genomic studies have revealed that they differ strikingly in the frequency of particular genetic aberrations (23, 24, 32–36). These observations suggested that a normal B cell may be transformed into a malignant DLBCL by more than one evolutionary path, each of which entails the stepwise acquisition of a constellation of genetic aberrations and results in phenotypic differences discernable by gene expression profiling. An integrated approach involving whole exome sequencing, deep-amplicon DNA sequencing, array comparative genomic hybridization and RNA-seq was used to discover four genetic subtypes of DLBCL (Fig. 2) (37).
Figure 2.
Comparison of DLBCL subtypes. Relationships between genetic subtypes and gene expression subgroups (left), the gene expression signatures and associated genetic alterations (middle), as well as potential therapeutic drug classes that may have activity within these subtypes (right). Abbreviations used: inh.: drugs that inhibit the indicated targets; Immune act.: Immune activation by immune checkpoint blockade.
The analytic approach to discover DLBCL genetic subtypes began with the observation that certain genetic abnormalities co-occur in the same DLBCL tumors more often than expected by chance. For example, mutations affecting the BCR subunit CD79B and a particular oncogenic MYD88 mutation, L265P, frequently co-occur in tumors that are the most ABC-like based on gene expression profiling, and these mutations are highly enriched in the “MCD” genetic subtype. Conversely, the many GCB-like tumors frequently had both EZH2 mutations and BCL2 translocations, and these genetic aberrations are characteristic of the “EZB” subtype. NOTCH2 mutations often co-occur with BCL6 translocations, leading to the discovery of the “BN2” subtype. BN2 tumors were enriched among “Unclassified” DLBCLs, providing the first indication that tumors with this indeterminant gene expression phenotype might be genetically distinct. Finally, the “N1” genetic subtype is defined by NOTCH1 mutations that are present in a subset of ABC tumors that lack NOTCH2 mutations. An automated algorithm termed GenClass was devised to iteratively re-assort DLBCL tumors into one of these four genetic subtypes in order to maximize an overall genetic distinctiveness statistic. Using this method, roughly half of the DLBCL tumors were genetically assigned while the remainder of tumors were declared “Other” (37). Additional, less prevalent, genetic subtypes may exist among these unassigned tumors. Ongoing efforts aim to use Bayesian methods to assign a probability that a given tumor belongs to a specific genetic subtype. Such probabilistic methods are analytically distinct from GenClass, which is essentially a clustering procedure, and are likely to classify a higher fraction of DLBCL tumors into one of the genetic subtypes.
Each genetic subtype is characterized by scores of genes that were either mutated and/or altered in copy number (Fig. 2). Additional validation that these genetic subtypes represent biologically distinct malignancies was provided by: (1) significant differences in gene expression signatures of B cell differentiation, oncogenic pathways, and the tumor microenvironment; (2) differences in overall survival following treatment with R-CHOP chemotherapy. Of note, the ABC gene expression subgroup was comprised of tumors belonging to the MCD, BN2, and N1 genetic subtypes, and R-CHOP therapy was not equally effective in these ABC subsets, with the MCD and N1 having an having inferior prognosis and the BN2 subtype having a more favorable prognosis that was similar to that of GCB DLBCLs. Of note, a separate study using entirely distinct analytical methods reported equivalent genetic subtypes of DLBCL that also differed in survival following R-CHOP therapy, demonstrating that the genetic subtypes are a consistent feature of multiple DLBCL cohorts (38).
4. Chronic active BCR signaling
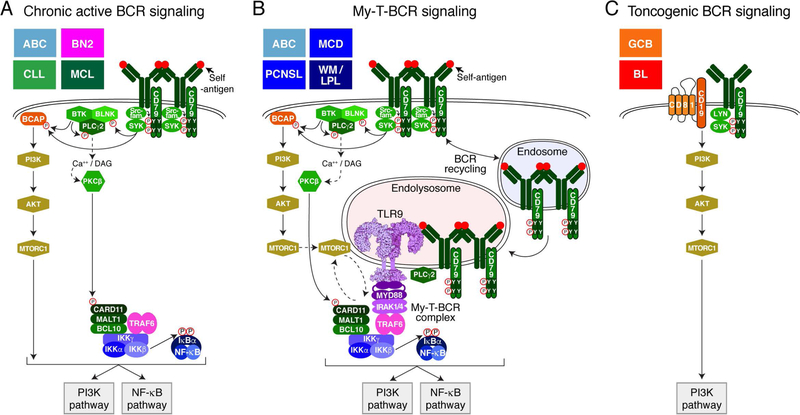
The first direct evidence connecting the BCR to survival of human lymphoma cells was provided with the discovery of chronic active BCR signaling in ABC DLBCL (Fig. 3A) (34). Previous work demonstrated that the ABC DLBCL cells are highly dependent on NF-κB for their viability (17, 39). This prompted a search for mechanisms that are responsible for the constitutive NF-κB activity in ABC cells. Large-scale loss-of-function RNA interference screens identified genes that were essential for the proliferation and/or survival of ABC cells (39). These screens revealed that the signaling adapter CARD11 is required for the survival of ABC, but not GCB, cell line models. This insight led to the discovery of oncogenic mutations in DLBCL tumors that target the coiled-coil (CC) region of CARD11, resulting in spontaneous formation of the CARD11, BCL10, MALT1 (CBM) complex, which activates IκB kinase β (IKKβ), the principle kinase in the classical NF-κB pathway (40). CARD11 mutations are present in ~15% of ABC tumors, but the trigger for NF-κB in the remainder of ABC cases was revealed by subsequent RNA interference screens that implicated the BCR (34). The viability of ABC lines with wild type CARD11 is sustained by signals generated by the BCR, which engage various effectors of BCR-dependent NF-κB activation (e.g. SYK, BLNK, PLCG2 and PRKCB) (34). This mode of BCR signaling is the sine qua non of ABC DLBCL and was dubbed “chronic active BCR signaling” to recognize its constitutive nature and its similarity to antigen-dependent BCR signaling. This pathway is rich in kinases that can be targeted by small-molecule inhibitors, including the selective and irreversible BTK inhibitor ibrutinib that is specifically toxic for ABC lines with chronic active BCR signaling (34, 41).
Figure 3.
Modes of BCR signaling in lymphoma. (A) Chronic active BCR signaling, (B) My-T-BCR signaling and (C) Toncogenic BCR signaling. The lymphoma subtypes that may utilize these different modes of BCR signaling are listed in the upper left-hand corner of each panel. See text for details.
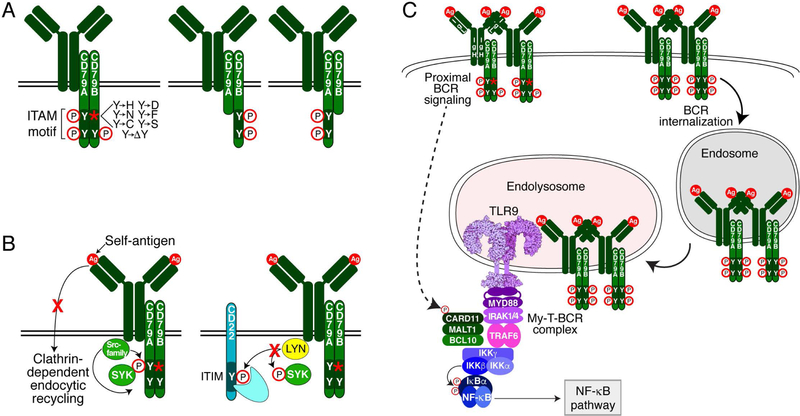
In conjunction with these functional genomic studies, targeted resequencing of genes in the BCR pathway revealed recurrent mutations affecting the ITAM motifs of both CD79B and CD79A, which in together are present in ~29% of ABC tumors, but are present in only 3% of GCB tumors (34, 37). The most frequent mutations change the first ITAM tyrosine in CD79B to another amino acid, eliminating this phosphorylation site (Fig. 4A). Importantly, CD79B mutant isoforms cannot initiate BCR signaling de novo, but rather potentiate ongoing BCR signaling, thus functioning more like a rheostat than an on/off switch (34). CD79A mutations are less common (~4% of ABC biopsies) and more heterogeneous, but typically remove the complete ITAM region. Initially, it seemed puzzling that mutations targeting tyrosine residues that are critical for downstream BCR signaling would be selected in lymphomas that depend on BCR signaling for survival. Further confounding was the observation that the CD79B/A mutant isoforms are not absolutely required for the survival of BCR-dependent ABC lines, since provision of either wild-type or mutant isoforms fully rescued survival following knockdown of the endogenous CD79B/A mutant isoforms. However, these mutant isoforms increase expression of the BCR on the plasma membrane to a greater extent that their wild-type counterparts, presumably enhancing proximal BCR signaling (Fig. 4B) (34, 41). Moreover, CD79B mutant isoforms do not activate LYN kinase as efficiently as the wild type CD79B isoform. LYN is unique among the SFKs in that it is a potent negative regulator of BCR signaling, and consequently, Lyn deficient mice develop autoimmune disease (42). Together, these observations suggest a model in which the CD79B/A mutant isoforms minimize negative feedback mechanisms, thereby enhancing BCR signaling (Fig. 4B).
Figure 4.
Mechanisms of CD79A/B ITAM mutations that sustain proximal BCR signaling. (A) Pictorial representation of common mutations affecting CD79B and CD79A. (B) CD79B Y196 mutations disrupt BCR internalization (left) and reduce LYN kinase activity, thereby abrogating LYN-dependent negative feedback mechanisms (right). (C) My-T-BCR signaling relies on two pools of BCR. One pool of BCR enriched for mutant isoforms of CD79B sustains proximal BCR signaling at the plasma membrane to activate the CBM complex. Another pool of BCR enriched for wild-type CD79B is preferentially internalized and ultimately interacts with TLR9 in endolysosomes to form the My-T-BCR. The My-T-BCR requires the CBM complex to activate IKK.
The lymphoma BCR mutations revealed a previously unappreciated dichotomy between the function of CD79A and CD79B, and between the first and second ITAM tyrosines (43). The CD79B ITAM in isolation cannot mediate BCR endocytosis due to cis-acting inhibitory residues within the ITAM, but the presence of the CD79A ITAM relieves this inhibition. Of the two tyrosines in CD79B, only the first is important for endocytosis (43). This leads to a simple hypothesis that the CD79A/B mutations increase the dwell time of the BCR on the plasma membrane, presumably augmenting proximal BCR signaling. An important genetic observation is that 94.2% of the CD79A/B mutations are heterozygous (37), meaning that a proportion of the CD79A-CD79B heterodimers in the BCR will both be fully wild type. Therefore, a complete model accounting for the selection of CD79A/B mutations in ABC DLBCL would posit that they prevent endocytosis and promote proximal signaling by the subset of BCRs with mutant isoforms while allowing the subset with wild type BCRs to transit internally (Fig. 4C). As will be discussed in detail below, one destination for this latter subset of BCRs is an endolysosomal compartment containing TLR9 (44)
BCR signaling in ABC DLBCL activates the NF-κB pathway (34), which is a feature of antigen-dependent but not tonic BCR signaling in normal mature B cells (44–46). Examination of BCR on the surface of DLBCL cells by total internal reflection fluorescence (TIRF) microscopy revealed distinct BCR puncta on the surface of ABC cell lines and patient samples (34). These clusters resembled that formed during acute BCR engagement by antigens on the surface of normal B cells (47). By contrast, the BCRs in GCB DLBCL cell lines and patient samples were diffusely distributed in the plasma membrane and did not form discernable clusters. Thus, the modes of BCR signaling in ABC and GCB DLBCL are fundamentally distinct in both their biochemical mechanisms and signaling consequences (see below).
5. Self-antigen-driven BCR signaling in lymphoid malignancies
Although chronic active BCR signaling in ABC DLBCL shares characteristics with antigen-dependent BCR signaling, this mode of BCR signaling in normal B cells is tightly regulated and self-limited by the availability of antigen and other negative controls. This observation led to the hypothesis that the constant engagement of the BCR by a self-antigen might account for the sustained nature of chronic active BCR signaling (48).
A role for antigenic stimulation of the BCR in the genesis of lymphoma has long been postulated (49). Indeed, certain forms of lymphoma are associated with viral or bacterial infections. For example, hepatitis C virus (HCV) infection is linked to the development of splenic marginal zone lymphoma (SMZL) (50). Treatment of SMZL patients with type I interferon to clear HCV infection eliminates the lymphoma in 75% of cases (51). These results could suggest antigenic stimulation of the BCR sustains lymphoma survival, or alternatively, it is possible that chronic infection results in an inflammatory environment that promotes lymphoma development. In the case of HCV-associated SMZL, a single BCR specific to a glycoprotein in the envelope has been described (52), implicating HCV-driven BCR signaling. Likewise, Helicobater pylori infection has been associated with gastric MALT lymphomas and antibiotic treatment often leads to remission of this lymphoma (53), either by removing the source of BCR-specific antigens or quenching the localized inflammation that provides more general stimulation for lymphoma growth. In addition to foreign antigens, BCR stimulation by self-antigens has been correlated with lymphomagenesis. Epidemiological studies have identified associations between autoimmune diseases, including systemic lupus erythematosus (SLE) and Sjogren’s syndrome, with an increased risk of lymphomas (54). However, the correlative nature of these observations makes it is difficult to establish causality and distinguish between potential oncogenic effects of inflammation and BCR signaling.
Mouse models of lymphoma provided more direct evidence that antigens can drive lymphomagenesis. Mice expressing a MYC transgene under the control of the IgH enhancer (EμMYC mice) develop lymphomas consisting of pro- and pre-B cells that do not express a BCR (55). Addition of a BCR transgene that is specific for hen egg lysozyme (BCRHEL) results in more aggressive tumors made up of mature naïve B cells (56). The further transgenic addition of soluble HEL (sHEL), the cognate antigen for BCRHEL, produced very aggressive tumors composed of GC-like B cells (56). In this case, enforced expression of the MYC oncogene prevents antigen-induced tolerance and allows the proliferation of B cells expressing BCRHEL and sHEL, whereas such B cells would be rendered anergic in the absence of MYC (57). Genetic knockdown of CD79A was lethal to tumor cell lines derived from either EμMYC/ BCRHEL or EμMYC/ BCRHEL/sHEL, indicating an essential role for both the BCR and its cognate antigen in driving lymphomas in this model system. Moreover, inhibition of SYK and BTK activity prolonged survival in mice with transplanted EμMYC/ BCRHEL/sHEL tumors (58, 59), confirming that these antigen-dependent tumors signaled through these proximal kinases, akin to chronic active BCR signaling in ABC DLBCL.
Circumstantial evidence for antigen-dependent BCR signaling in human lymphomas comes from the study of BCR V regions from malignant B cells. Sequence analysis of IgH V regions from cohorts of patients with chronic lymphocytic leukemia (CLL) has been used to distinguish two CLL subtypes. In one subtype with an indolent clinical course (M-CLL), SHM generates highly mutated IgH V regions (>2% mutant residues), whereas the other subtype (U-CLL) with an aggressive clinical course has few or no IgH V region mutations (<2% mutant residues) (60, 61). These CLL subtypes are also molecularly distinct, as judged by their differing gene expression signatures (62). Ensuing analysis of larger patient cohorts revealed that CLL recurrently utilizes certain V regions. The IgH locus codes for ~110 V genes, 23 D genes and 6 J genes that can be combined to create over 15,180 unique V regions. These V regions have been divided into over 300 subsets based on sequence homology to each other (63). The BCRs of both M-CLL and U-CLL cells preferentially utilize heavy chain variable (VH) segments VH4–34, VH1–69, VH3–31 and VH3–7 (64). Moreover, the CLL clones from over a third of patients express ‘stereotyped’ BCRs that are identical or nearly identical throughout their VH-DH-JH and VLJL sequences to BCRs present in unrelated CLL patients (65). The odds of two B cells having the same BCR V regions is vanishingly small, suggesting that stereotyped BCRs are selected in CLL to perform an essential function, most likely antigen binding. Similar BCR V region stereotypy has been described in mantle cell lymphoma (MCL) (66), again suggesting a role for antigen-mediated selection in the pathogenesis of this lymphoma type.
What is the nature of antigens that drive BCR selection and stereotypy in CLL and MCL? CLL BCRs have been proposed to be polyreactive (67) or bind to antigens derived from apoptotic cells (68, 69). Alternatively, a study found that all CLL-derived BCRs could induce spontaneous signaling when reconstituted in murine B cells lacking an endogenous BCR (70). The authors ascribed this to the ability of these BCRs to recognize a conserved framework 2 (FR2) residue in the BCR VH, a self-association that is akin to an idiotype/anti-idiotype interaction (71). As outlined below, this FR2 residue is also recognized by some BCRs in DLBCL (48). Nonetheless, this simple hypothesis did not account for the heterogeneity among CLL patients with respect to utilization of particular stereotypical BCRs by the malignant clone, a heterogeneity that has been linked to prognosis (72). A resolution of this paradox was provided by a structural analysis of CLL-derived stereotypic BCRs that showed that CLL BCRs do indeed react with themselves, but each stereotyped BCR binds to a different BCR residue (73). Remarkably, the self-association binding constant (KD) is lower for stereotypic BCRs that are associated with an indolent CLL course than for stereotypic BCRs that are associated with an aggressive clinical course.
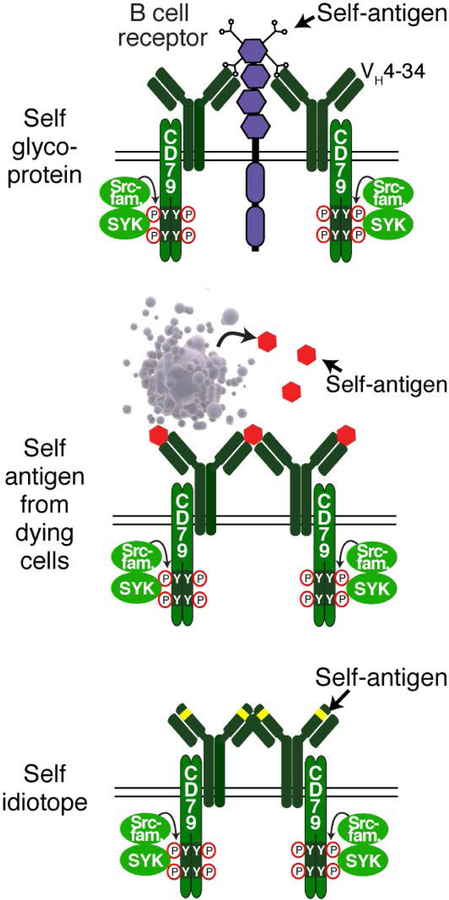
These studies do not distinguish between a role for BCR self-reactivity in initiating the oncogenic process versus a role in sustaining malignant cell survival and/or proliferation in an ongoing fashion. Evidence in favor of the latter hypothesis was provided by studying cell line models of ABC DLBCL with chronic active BCR signaling (Fig. 5) (48). In ABC DLBCL, a single VH gene segment, VH4–34, is utilized in roughly one third of cases (48) (Fig. 6A), in agreement with a study that identified enrichment of VH4–34 in non-GCB DLBCL cases (74). VH4–34+ BCRs are autoreactive, with specificity for glycoproteins that bear N-acetyl-lactosamine sugars (75). The binding of VH4–34+ BCRs to such cell surface glycoproteins requires particular residues within the VH framework 1 (FR1) region (76), and normally renders B cells anergic and incapable of participating in GC reactions (77). Interestingly, however, if anergic B cells are provided a co-stimulatory signal through Toll-like receptors, they are more prone to form germinal centers than non-anergic B cells (78), suggesting a mechanism by which anergic B cells might be precursors of GC-derived B cell malignancies.
Figure 5.
Diverse self-antigens initiate chronic active BCR signaling in lymphoma. Lymphomas expressing an IgVH4–34 BCR recognize N-acetyl-lactosamine sugars attached to cell surface proteins (top). Additionally, self-antigens found in debris from dying cells (middle) or found within the framework region of the BCR itself can drive chronic active BCR signaling. Adapted from ref. (48).
Figure 6.
Breaching immunological tolerance is critical to B cell transformation. (A) Frequency of IgVH4–34 expression within DLBCL gene expression subgroups (left) and genetic subtypes (right); (B) or within patients with indicated mutation; (C) or within patients with combined genetic alterations. The number of IgVH4–34+ patients within each classification is shown above each bar; p-values are listed in parentheses from Fisher’s exact test. (D) A model for how BCR and/or MYD88 mutations help to overcome mechanisms of B cell tolerance/anergy that normally silence low-affinity autoreactive BCRs. Data for panels A-C are taken from ref. (37) and ref. (48). Frequencies were normalized to reflect predicted population-based frequencies using gene expression subgroup distributions (201) as described (37).
The contribution of individual amino acids within the BCR VH to malignant survival was measured by testing the ability of mutated BCRs to rescue an ABC cell line from death following knockdown of its endogenous IgH gene (48). In one ABC line (HBL1) that has a VH4–34+ BCR, exogenous provision of a VH4–34 IgH isoform with a FR1 mutation that abrogates glycoprotein binding failed to rescue survival following knockdown of the endogenous VH4–34 IgH chain, as was also the case for patient-derived VH4–34+ IgH chains (48). This FR1 mutation also blocked the ability of these VH4–34+ BCRs to bind to the surface of malignant B cells. This suggests that chronic active BCR signaling in VH4–34+ ABC DLBCLs is maintained by continuous exposure of the BCR to its ligand on the surface of adjacent malignant cells in a tumor, thereby sustaining survival of the malignant cell.
Sugars appear to play a distinct but also essential role in BCR signaling in follicular lymphoma and GCB DLBCL (79–81). In normal B cells, N-linked glycosylation is restricted to the constant region (Fc) portion of the IgH chain but in FL and GCB DLBCL, SHM produces mutations in the IgVH and IgVL regions that introduce novel acceptor sites for N-linked glycosylation. In these cases, the BCR may be crosslinked by the binding of N-glycans to lectins present on the surface of tumor microenvironmental cells (82), thereby initiating constitutive BCR signaling.
In addition to glycoprotein binding by VH4–34+ BCRs, the BCRs in other ABC cases also react to autoantigens (48). Chronic active BCR signaling in the ABC cell line OCI-Ly10 is initiated by binding of the BCR to an antigen present in debris from apoptotic cells (48). Apoptotic debris is continually produced in DLBCL tumors since dying cells are often observed histologically. The survival of this cell line depended on the antigen specificity of the BCR since mutating charged amino acids within CDR2 and CDR3 of the IgVH3–7 region rendered the BCR insensitive to this antigen, and consequently this mutated BCR was unable to maintain cell viability. ABC patient-derived IgVH3–7 heavy chains could also sustain survival of this cell line, again in a fashion that depended on charged residues in CDR2. Since these charged CDR residues were introduced by SHM, this observation demonstrates that the process of lymphomagenesis selects for rare GC B cells that have BCR mutations that foster constitutive BCR signaling.
In another instance, the ABC cell line TMD8 relied on homotypic interactions with its own FR2 domain to sustain chronic active BCR signaling, similar to the phenomenon observed in CLL (70). Mutation of the residues responsible for FR2 binding prevent these BCRs from sustaining viability of this ABC cell line (48). Thus, multiple types of self-antigen can drive chronic active BCR signaling in ABC DLBCL, suggesting that selection for this phenotype may be an early event in lymphomagenesis.
6. Genetic abrogation of BCR anergy
While the majority of normal B cells are presumed to be autoreactive as they emerge from the bone marrow, these cells are rendered ineffectual by anergy and eventual deletion (83, 84). Given the apparent BCR autoreactivity in ABC lymphomas with chronic active BCR signaling, it is logical that this anergy checkpoint must be overcome for these B cells to become malignant. One of the characteristic features of anergic cells is low expression of the BCR on the plasma membrane, which presumably limits their ability to signal effectively (85). CD79B mutations that are acquired in ABC DLBCL block internalization of the BCR by inhibiting association of the BCR with clathrin coated pits, thereby supporting higher levels of proximal BCR signaling at the cell surface (34, 43). In addition, LYN, a kinase that promotes B cell anergy (77), is not activated efficiently by BCRs containing ITAM-mutant CD79B isoforms. CD79A mutations also potentiate BCR signaling (41), albeit in a mechanistically distinct fashion (see below).
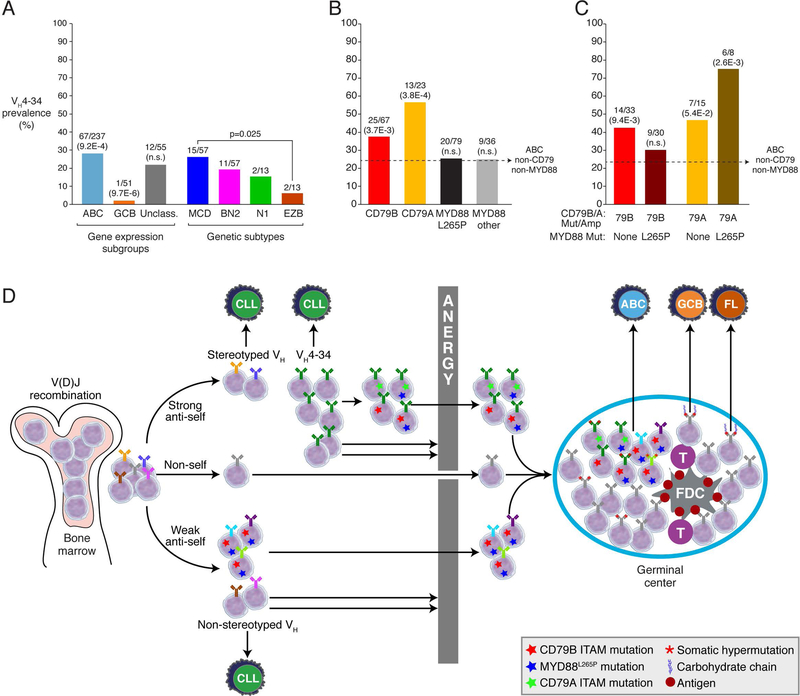
These observations suggest the hypothesis that the CD79B and CD79A mutations in DLBCL could be selected, in part, due to an ability to enhance signaling in anergic B cells, allowing them to engage in a GC response. Anergic B cells in the human are exemplified by the subset naïve blood B cells expressing a VH4–34+ heavy chain, which constitute ~7% of blood B cells (86). These anergic B cells react with self-glycoproteins, have an IgDhigh, IgMlow surface phenotype, fail to mobilize calcium upon BCR cross-linking and are excluded from GC reactions. As mentioned above, VH4–34 heavy chains are significantly overrepresented in the ABC subgroup and are underrepresented in the GCB subgroup (Fig. 6A) (48). We have extended this analysis by assessing the enrichment of VH4–34 heavy chains among various genetic subsets, using available data from 343 cases (Fig. 6A) (37, 48). The prevalence of VH4–34 among the DLBCL genetic subtypes mirrored the difference between the ABC and GCB subgroups, with the VH4–34 being significantly enriched in the ABC-enriched MCD subtype compared with the GCB-enriched EZB subtype.
Among all genetic aberrations in this cohort of DLBCL tumors, the most significantly associated with VH4–34 heavy chain utilization were mutations and/or amplification of CD79B or CD79A (p=1.0E-4), and this association was also true within the ABC subgroup (p=3.4E-3) (Fig. 6B). Both CD79B and CD79A aberrations were independently associated with VH4–34 utilization, with a prevalence of 37% and 56%, respectively. By contrast, VH4–34 was not significantly enriched among ABC tumors with the oncogenic MYD88L265P mutation (see below) or among tumors with other MYD88 mutations. Tumors with CD79B mutation or amplification (CD79BMut/Amp cases) in the absence of MYD88L265P were significantly enriched for VH4–34 (p=9.4E-3), but somewhat unexpectedly, those with a coincident MYD88L265P mutation were not (Fig. 6C). By contrast, the enrichment of VH4–34 among CD79AMut/Amp tumors (42% prevalence) was even more pronounced in those with coincident MYD88L265P (75% prevalence).
These genetic observations allow us to speculate that enhanced BCR signaling in tumors with either CD79BMut/Amp or CD79AMut/Amp can break B cell anergy, potentially allowing the B cell to enter into a GC reaction, where it may acquire additional genetic alterations on the road to DLBCL (Fig. 6D). The disparate effect of MYD88L265P on VH4–34 prevalence in the context of CD79BMut/Amp and CD79AMut/Amp might reflect quantitative or qualitative differences in oncogenic signaling between these genotypes. The CD79BMut/Amp/MYD88L265P genotype far outnumbers the CD79AMut/Amp/MYD88L265P genotype among ABC tumors (11.9% vs. 2.7%) and identifies tumors that are highly addicted to BCR signaling and responsive to ibrutinib (see below). One model to account for these observations would be that the CD79BMut/Amp/MYD88L265P genotype engenders such potent BCR signaling that B cells with relatively low self-antigen reactivity can become activated and enter into a GC center reaction, whereas the degree of BCR signaling in CD79AMut/Amp/MYD88L265P tumors may only be sufficient to promote GC differentiation of strongly self-reactive B cells, such as those with a VH4–34+ BCRs. In other words, the lower prevalence of VH4–34 among CD79BMut/Amp/MYD88L265P tumors reflects the dilution of VH4–34+ tumors by tumors with other V regions that have lower intrinsic self-reactivity. A test of this model and other scenarios awaits the reconstitution of these oncogenic DLBCL mutations in transgenic mice that are also engineered to develop B cell anergy.
7. IgM as the initiator oncogene in lymphoid malignancies
Under normal circumstances, naïve B cells co-express an IgM-BCR and an IgD-BCR on their surface, but often switch to an IgG-BCR in the GC. By contrast, B cell lymphomas that are reliant on chronic active BCR signaling or similar antigen-dependent BCR signaling often retain expression of an IgM-BCR, despite their apparent cell-of-origin being a GC or post-GC B cell (87). On some level, it is even surprising that malignant B cells express a BCR at all, in light of the AID-directed mutagenesis and frequent chromosomal translocations that target the immunoglobulin heavy and light chain loci in lymphomas. However, the signals that emanate from the BCR give malignant B cells a substantial growth and survival advantage, and failure to maintain BCR expression would reduce their competitive fitness relative to other tumor clones (88). This may be particularly true for the IgM-BCR, which potently promotes normal B cell proliferation, in contrast to the IgG-BCR, which preferentially triggers B cell differentiation programs (11, 89–91).
ABC DLBCL tumors express an IgM-BCR in ~80% of cases, compared to only ~34% in GCB DLBCL (23, 48). Somatic mutations targeting the switch μ region (Sμ), which is 5’ of the Cμ gene segment, are frequently acquired in ABC tumors and interfere with class switch recombination from IgM to another isotype (23). These mutations only target the in-frame, productive IgH allele, leaving the other non-productive allele to undergo CSR in most cases (92). In contrast to ABC DLBCL, GCB tumors do not have Sμ mutations and consequently, the majority have an IgG-BCR (48). In follicular lymphoma, the t(14;18) translocation that characterizes this disease prevents IgH transcription and instead places BCL2 under the control of the IgH enhancers. This IgH allele typically undergoes CSR, but remarkably, the non-translocated IgH allele remains IgM in the majority of cases. Although the mechanisms that maintain IgM expression in FL have not be investigated, this phenotypic selection strongly suggests that IgM is pro-oncogenic in this context (93).
All naïve mature B cells have an IgM-BCR but only a minority become lymphomas. As discussed above, BCR signaling in CLL often involves particular VH-DH-JH and VL-JL recombination events that create stereotyped BCRs, which are rare recombination events that are nonetheless selected independently in different CLL patients. Likewise, chronic active BCR signaling in ABC DLBCL can rely on particular somatic mutations that are acquired by the malignant clone and hence are rare in the B cell population as a whole. The somatically acquired mutations in FL that create novel N-linked glycosylation sites presumably allow the malignant B cell to engage in a type of BCR signaling in the tumor microenvironment that is unavailable to other B cells. It is plausible that these are early events in lymphomagenesis that may precede most other oncogenic aberrations that are acquired by the malignant cells.
Indeed, clonal proliferation of “normal” B cells occurs in SLE and could be viewed as quasi-oncogenic (94, 95). Similarly, cold agglutin disease, in which B cells make a pathogenic VH4–34+ antibody, has recently been shown to be due to a non-malignant, clonal proliferation of B cells that have acquired somatic mutations that improve binding to self-glycoproteins (96, 97). Remarkably, these non-malignant clones frequently acquire inactivating mutations in KMT2D (MLL2) and gain-of-function mutations in CARD11, two bona fide oncogenes in human lymphomagenesis, and some of the specific mutations reported in cold agglutin disease have also been observed in lymphoid malignances (37, 98). These considerations support the notion that IgM can be viewed as an oncogene that initiates proto-malignant expansion of normal B cells. Extended survival and rapid proliferation of such cells clearly requires cooperating oncogenic events, such as translocation of BCL2 and MYC.
8. MYD88 and BCR signaling
The BCR is not the only source of NF-κB activity in ABC DLBCL. RNA interference screens in DLBCL lines revealed that MYD88 is an essential gene in ABC DLBCL cell lines, but not in control GCB lines (35). MYD88 is an adaptor protein that coordinates a signaling complex connecting the interleukin-1 receptor (IL-1R) and Toll-like receptors (TLRs) to downstream kinases in IRAK family, including IRAK1 and IRAK4. Accordingly, IRAK1 and IRAK4 are also essential in ABC lines (35, 99). RNA-sequencing of DLBCL tumors uncovered a recurrent point mutation in MYD88 that changes the leucine at position 265 in the TIR domain to proline (MYD88L265P) (35). MYD88L265P is present in ~29% of ABC tumors, making it the more recurrent point mutation in this lymphoma subtype, but is rare or absent in GCB DLBCL, BL and primary mediastinal B cell lymphoma (PMBL). In addition to MYD88L265P, other mutations affecting the TIR domain of MYD88 are recurrent in ABC, GCB and Unclassified DLBCL tumors. Knockdown of MYD88L265P led to a substantial decrease in both NF-κB activity and STAT3 phosphorylation in ABC cell lines. Ectopic expression of MYD88L265P, but not wild type MYD88, rescued survival of ABC lines following knockdown of endogenous MYD88L265P expression, demonstrating that these ABC lines are addicted to this mutant MYD88 isoform (35). Moreover, ectopic expression of MYD88L265P or other mutant isoforms was sufficient to drive high levels of NF-κB activity relative to expression of wild type MYD88 (35). Computational modeling of the MYD88L265P surface suggested that increased homo-dimerization could contribute to its spontaneous activity (100, 101). Co-immunoprecipitation studies revealed that MYD88L265P selectively associates with a hyper-phosphorylated form of IRAK1 (35). This form of IRAK1 does not associate with wild type MYD88 or any other mutant MYD88 isoforms, suggesting that MYD88L265P has unique signaling properties, perhaps explaining its high incidence in ABC DLBCL.
Subsequent studies reported a high prevalence of MYD88L265P in other lymphoid malignancies, including a 90% prevalence in Waldenström’s macroglobulinemia (WM) (102). As a class, primary aggressive lymphomas occurring in extranodal sites are highly enriched for MYD88L265P, including primary central nervous center lymphoma (PCSNL; ~56%), primary testicular lymphoma (~73%), and primary cutaneous lymphoma (~83%) (reviewed in ref. (103)). These primary extranodal tumors constitute a subtype of DLBCL that typically has an ABC-like gene expression profile. Notably, MYD88L265P co-occurs with CD79B mutations in ABC DLBCL more often than expected by chance (35). This intersection is even more pronounced in primary extranodal tumors (103), raising the possibility that BCR signaling can collaborate with signaling by MYD88L265P. In BCR-dependent ABC lines expressing MYD88L265P, MYD88 knockdown or treatment with a MYD88 dimerization inhibitor decreased markers of proximal BCR signaling (41), suggesting a functional “cross-talk” between BCR and MYD88 signaling.
A clinical trial testing the efficacy of the BTK inhibitor, ibrutinib, in DLBCL provided additional evidence of a functional link between chronic active BCR signaling and MYD88L265P. This phase 1b/2 trial demonstrated correlated clinical responses with the mutational status of CD79B and MYD88 in ABC patients. A 37% objective response rate (ORR) (complete response (CR) + partial response (PR)) was achieved in ABC patients, compared with only a 5% ORR in GCB DLBCL, as predicted by pre-clinical studies (41). When subdivided by mutations, ABC cases with CD79B ITAM mutations had an improved 56% ORR, as compared with a 31% ORR in cases with wild type CD79B. Nonetheless, in absolute numbers, the majority of ibrutinib responders had wild type CD79B, demonstrating that chronic active BCR signaling can occur in the absence of BCR mutations, consistent with the possibility that self-antigens drive BCR signaling in these cases. Patients with tumors harboring both MYD88L265P and a CD79B mutation had an 80% ORR, whereas those with only a MYD88 mutation did not respond, again suggesting that the MYD88/CD79B double mutant genotype identifies tumors that are hyper-addicted to BCR-dependent NF-κB signaling and are therefore hyper-responsive to ibrutinib.
9. The My-T-BCR supercomplex
In normal cells, MYD88 can bind to the TIR domain of different TLRs to assemble and activate IRAK kinases. Whole-genome CRISPR-Cas9 screens identified TLR9 and its chaperones, UNC93B1 and CNPY3 as essential to the survival of ABC lines with mutant CD79B/A isoforms and MYD88L265P, but not in other ABC or GCB lines (44). Copy number gains and amplifications of MYD88, TLR9, UNC93B1 and CNPY3 are also recurrent in ABC biopsies, providing genetic evidence that the TLR9-MYD88 pathway has an important role in the pathogenesis of ABC DLBCL.
TLR9 function in ABC DLBCL was illuminated by an unbiased proteomic screen to identify TLR9-interacting proteins. TLR9 was fused to the promiscuous biotin ligase (BioID2, that can biotinylate proteins within a 10 nm distance (104),) and overexpressed in TLR9-dependent ABC lines. Mass spectrometry of biotinylated proteins purified from these cells identified CNPY3, MYD88, and the CD79A and CD79B subunits of the BCR, suggesting that TLR9 defines a supramolecular complex that includes the BCR and mutant MYD88.
Cooperative signaling between the BCR and TLRs has been reported previously in the context of normal and pathological immune responses in the mouse. For example, BCR ablation in mouse B cells impairs their proliferative response to TLR9 stimulation (105). Also, the antibody response to virus-like particles containing TLR9 ligands depends on MYD88 in GC B cells (106). In a murine model of SLE, pathological B cell proliferation depends on endosomal TLR7 and/or TLR9 binding ligands in complex self-antigens that are internalized by the BCR (107). Finally, MYD88L265P augments self-antigen-driven proliferation of mouse B cells in a TLR9-dependent fashion, especially in the presence of a Bcl2 transgene (108).
The proteomic data suggested a model in which TLR9 coordinates BCR and MYD88 signaling in a MyD88-TLR9-BCR (My-T-BCR) supercomplex (Fig. 3B). The interaction between the BCR, TLR9 and MYD88 was visualized in situ using the proximity ligation assay (PLA), an imaging technique that can identify protein-protein interactions within 40 nm of each other (109). An IgM and TLR9 PLA yielded robust puncta throughout the cytosol of ABC lines with MYD88L265P and chronic active BCR signaling, but this protein-protein interaction was not prominent in other ABC and GCB cell lines. The cytoplasmic location of the IgM:TLR9 interaction is consistent with the endolysosomal localization of TLR9, which is promoted by its chaperones UNC93B1 and CNPY3.
The PLA method also provided an opportunity to visualize the My-T-BCR in primary patient samples. Robust signals from an IgM:TLR9 PLA were detected in ABC DLBCL, PCNSL and WM biopsies, but were generally absent from GCB DLBCL, CLL and MCL samples. No IgM:TLR9 interactions were observed in normal B cells in and around the GC in normal tonsillar tissue, suggesting that the My-T-BCR supercomplex may be a feature of malignant but normal B cells. Of interest, TLR9 was never observed to interact with an IgG-BCR, suggesting a unique mode of cooperative signaling that is specific to the IgM-BCR. Indeed, MYD88L265P is preferentially enriched among IgM-BCR-expressing tumors (110). These findings suggest that the PLA method may prove useful in developing precision diagnostics to identify tumors with oncogenic My-T-BCR signaling (see below).
The My-T-BCR coordinates the majority of pro-survival NF-κB signaling in cells in which it is assembled, and this signaling occurs on the surface of endolysosomes (44). Various proteomic techniques revealed that the My-T-BCR supercomplex contains known mediators of NF-κB activation in ABC DLBCL, including the CBM complex, IκB kinase (IKK) in its phosphorylated and active form, and the NF-κB transcription factor subunit p50 (NFKB1). Thus, the My-T-BCR coordinates NF-κB signaling by MYD88 and the CBM, itself a multi-protein complex consisting and CARD11, BCL10, MALT1, and TRAF6 (111). Evidence that active NF-κB signaling takes place at the My-T-BCR was the presence in the supercomplex of the substrate of IKK, IκB alpha, in its phosphorylated form. Importantly, the My-T-BCR was disrupted by ibrutinib treatment, revealing why this drug potently inhibits NF-κB activation in these cells.
The My-T-BCR model accounts for two distinct pools of the BCR, each with an important and non-redundant function. BCR expressed on the surface is continually stimulated by self-antigens to promote chronic activation of signaling mediators proximal to the BCR, including SYK, BLNK and BTK. Proximal BCR signaling at the plasma membrane promotes My-T-BCR formation in two ways: (1) activation of PLCγ2 and PKCβ, which induce formation of the CBM complex, and (2) promotion of BCR internalization to Lamp1+ endolysosomes where the BCR can join TLR9 to form the My-T-BCR. As discussed above, the predominantly heterozygous nature of the CD79B and CD79A mutations in ABC DLBCL ensures that some pool of BCRs in CD79B/A mutant cases will be composed of wild type CD79B and CD79A subunits, which can be endocytosed and form the My-T-BCR, while other BCRs with mutant CD79B/A isoforms engage proximal signaling mediators on the plasma membrane. From this perspective, it is not surprising CD79B/A mutations often co-occur with MYD88L265P in ABC DLBCL and PCNSL, which often have My-T-BCR supercomplexes.
Unexpectedly, MYD88-BioID2 revealed that the My-T-BCR is in close proximity to the mTORC1 complex, which is also located in endolysosomal vesicles (112) and transduces PI3 kinase signaling to downstream targets of mTOR. Remarkably, ibrutinib cooperated with mTORC1 inhibitors in dispersing the My-T-BCR, leading to virtually complete inhibition of IKK phosphorylation as well as phosphorylation of the MTOR targets S6 kinase and 4E-BP1. The net result was strong cooperation between these two drugs in killing ABC cell lines in vitro and stalling the growth of ABC xenografts in vivo. These findings provide a mechanistic understanding of previous reports that documented synergism of ibrutinib and PI3 kinase pathway inhibitors in killing ABC cell lines (113–116), and provide a strong mechanistic rational for combination clinical trials that exploit the cooperation between mTOR and BCR-dependent NF-κB activation in sustaining the survival of ABC DLBCL cells with the My-T-BCR.
10. Toncogenic BCR signaling
Tonic BCR signaling was initially described in mature murine B cells based on the observation that conditional ablation of either IgH or CD79A in mature B cells causes a severe reduction of peripheral B cell numbers (117, 118). Further work demonstrated that constitutive PI3K, but not NF-κB, signaling could rescue B cells following ablation of the BCR, and that these cells could initiate GC reactions (45). However, an open question remained as to whether this type of BCR-dependent survival signaling plays a role in the pathogenesis of human lymphomas. Subsequently, PI3K signaling was identified as an essential survival pathway in both murine (119) and human (46) models of BL. This survival signaling depends on proximal BCR signaling since knockdown of CD79A or SYK in BL lines decreased phosphorylated AKT levels, as did treatment with PI3K inhibitors (46). The similarities between this mode of BCR signaling in BL and the essential BCR signaling in non-transformed mouse B cells led to suggestion that BLs co-opt tonic BCR signaling for their malignant survival.
In contrast to other forms of lymphoma, the essential nature of the BCR in GCB DLBCL has been unclear until recently. In initial RNA interference studies, quantitative knockdown of the BCR subunits CD79A and CD79B was not toxic for GCB lines, but did affect the viability of both ABC and BL lines. Also unlike ABC DLBCL, GCB cell lines do not have BCR clusters in the plasma as judged by TIRF microscopy (34, 46). As mentioned above, GCB DLBCL tumors do not often acquire mutations targeting the ITAM regions of CD79A or CD79B. Nor do they acquire mutations in TCF3 and ID3 that increase constitutive BCR signaling in BL by promoting TCF3-dependent repression of PTPN6, encoding the negative regulator of BCR signaling SHP-1 (46). However, GCB lines are sensitive to SYK inhibitors and SYK knockdown decreased PI3K activity (120, 121). The involvement of SYK downstream of several surface receptors, including integrins, as well as the off-target effects of some available SYK inhibitors left the direct role of the BCR in GCB DLBCL viability unresolved (122).
Genome-wide CRISPR screens revealed that a subset of GCB DLBCL lines are indeed dependent on the BCR subunits CD79A, CD79B for survival (44) (Fig. 3C). The BCR dependency of GCB lines had not been evident using RNA interference, which only partially knocks down gene expression, but is clear when BCR subunits are fully inactivated using the CRISPR methodology. The survival of BCR-dependent GCB lines also requires the proximal BCR signaling components LYN, CD19 and CD81, which are not required for the survival of ABC lines but are essential in BL lines (44). LYN is one of several SFKs that can initiate BCR signaling (123), but is redundant with BLK and FYN at several stages of B cell differentiation (4). While LYN kinase is essential in GCB lines, no single SFK is essential to ABC lines, possibly due to functional redundancy. Notably, the phosphatase CD45, a regulator of SFKs, is an essential gene in CRISPR screens of GCB lines, but by contrast, its deletion in ABC lines promoted their outgrowth. A related observation is that BCR-dependent differentiation in normal B cells is CD45-dependent whereas antigen-induced active BCR signaling is relatively independent of CD45 (124).
The proximal BCR tyrosine kinase SYK is essential in both ABC and GCB lines, which is notable given that small molecule SYK inhibitors are in clinical trials (see below). Nonetheless, the signal cascades stemming from SYK activity appear to differ between the DLBCL subtypes. SYK mediates PI3K signaling in normal B cells by several mechanisms, including direct interaction with the PI3K p85 subunit as well as phosphorylation of CD19 and BCAP (PIK3AP1) (reviewed in ref. (125)). As mentioned, CD19 was selectively essential in GCB lines, as was CD81, which facilitates CD19 localization to the plasma membrane (126). While ABC lines do not depend on CD19, they do require BCAP. Although CD19 and BCAP are functionally redundant in normal mouse B cells (127), these results indicate that this is not the case in DLBCL and suggest that each DLBCL subtype uses a distinct mechanism to engage PI3K (127).
Since tonic BCR signaling promotes the survival of all mature B cells, this process is presumed to be antigen-independent given the vast repertoire of BCRs expressed by normal B cells. In contrast to tonic BCR signaling, chronic active BCR signaling in ABC DLBCLs is antigen-dependent (see above). The antigen-independence of GCB lines was elegantly demonstrated by CRISPR-mediated replacement of the IgVH and IgVL CDR regions with sequences taken from normal B cell immunoglobulins, whereas similar substitutions in ABC cell lines made them less fit in competitive growth assays (128), in accord with other studies (48).
Taken together, these above observations suggest that the mode of constitutive BCR signaling in GCB DLBCL is distinct both from the autoantigen-dependent chronic active BCR signaling in ABC DLBCL. However, other considerations suggest that BCR signaling in GCB DLBCL is also distinct from tonic BCR signaling. CD19 and CD81 are unlikely to contribute to tonic BCR signaling since conditional deletion of CD19 or CD81 in the mouse does not promote massive loss of mature B cells, yet both proteins are required for the survival of BCR-dependent GCB DLBCL and BL lines. Likewise, B cell numbers are not reduced in LYN knockout mice, which instead accumulate autoimmune B cells, whereas BCR signaling in GCB lines is LYN-dependent. We therefore proposed the term “constitutive germinal center BCR signaling” to characterize the oncogenic BCR signaling in BL and GCB DLBCL (44). We now propose the less cumbersome term “toncogenic” BCR signaling to acknowledge that it promotes malignant B cell survival in a PI3K-dependent, NF-κB-independent fashion that is mechanistically distinct from tonic BCR signaling in normal mouse B cells and chronic active BCR signaling in ABC DLBCL (Fig. 3C).
11. Genetic alterations that augment BCR pathway activation
Available evidence from clinical trials of ibrutinib suggests that genetic aberrations targeting CD79B and CD79A may not fully account for the apparent addiction of some DLBCLs to BCR-dependent NF-κB activation. In a phase 1b/2 trial of ibrutinib in relapsed refractory DLBCL (41), the response rate was 31% in ABC DLBCL cases with wild-type CD79B and CD79A, and 64% of all responses were observed in this patient subset. In PCNSL, which has a high frequency of BCR subunit mutations (53%), ibrutinib was also highly active, but 28% of the responses occurred in cases with wild type CD79B (129, 130). While engagement of the BCR by self-antigens may be sufficient to account for ibrutinib-responsive BCR signaling in some cases, it is also possible that additional genetic abnormalities affecting other components in the BCR-dependent NF-κB signaling pathway may contribute to BCR signaling quantitatively or qualitatively (Fig. 1).
Among the first gain-of-function mutations described in DLBCL targeted the signaling adapter CARD11 in ~14% of DLBCL cases (37, 40), and these have since been observed in a variety of other mature B cell malignancies. In some cases, CARD11 mutations promoted ibrutinib resistance since they act downstream of BTK to foster the formation of large multi-protein signaling hub that activates NF-κB (34, 40). Normally, CARD11 activation is tightly controlled, requiring BTK-dependent PKCβ activity to phosphorylate an inhibitory “linker” domain in CARD11(131), leading to BCL10 recruitment and cooperative assembly of a cytoplasmic filament containing (at a minimum) MALT1 and TRAF6 (111, 132). Some CARD11 mutations do indeed promote ibrutinib resistance in ABC cell lines (34), and potentially also in some DLBCL tumors treated with ibrutinib (41, 130, 133–135).
Some caution in interpreting the impact of CARD11 mutations is warranted, however, since the signaling strength of different CARD11 mutant isoforms varies considerably, which correlates with their ability to form intracellular signaling foci (40). Indeed, weak CARD11 alleles render cells hyper-responsive to upstream signals (40), so that rather than conferring ibrutinib resistance, such mutations may identify tumors that are ibrutinib-sensitive. A comprehensive, quantitative analysis of the recurrent CARD11 mutant isoforms would therefore be needed before they could confidently be used as exclusion criteria in clinical trials of ibrutinib.
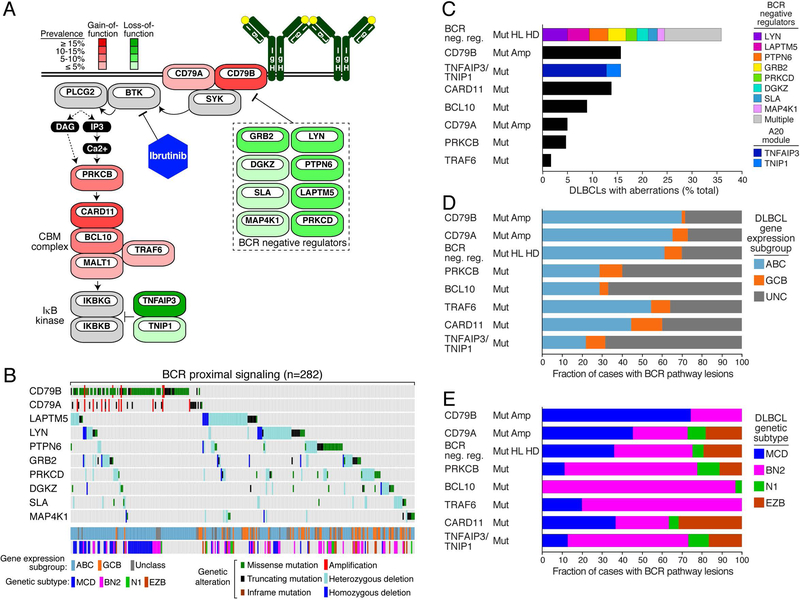
Recent comprehensive genomic profiling of DLBCL has uncovered new genetic aberrations that impinge on the BCR signaling pathway (37). DLBCLs frequently acquire inactivating mutations and deletions targeting an array of negative regulators of proximal BCR signaling (Fig. 7A). These can occur singly in tumors with wild type CD79B or CD79A, but often tumors acquire inactivating lesions in multiple negative regulators, suggesting that they quantitatively and additively augment BCR signaling (Fig. 7B). As a group, genetic aberrations affecting BCR negative regulators are more prevalent among DLBCL tumors than any other genetic aberrations that activate BCR-dependent NF-κB signaling (Fig. 7C).
Figure 7.
Genetic alterations of BCR pathway genes promote BCR signaling in DLBCL. (A) Frequency of gain-of-function (red) and loss-of-function (green) genetic alterations that affect the BCR pathway. (B) Oncoprint representation of the presence of genetic alterations in common BCR pathway genes in DLBCL. DLBCL subtypes and genetic classes are depicted. Cases lacking these alterations are not shown. The prevalence of BCR pathway mutations in (C) all DLBCL samples, (D) in ABC, GCB and Unclassified forms of DLBCL, or (E) within defined genetic subtypes of DLBCL. Data for panels B-E are taken from ref. (37). Frequencies in panels C-E were normalized to reflect predicted population-based frequencies using gene expression subgroup distributions (201) as described (37).
The DLBCL gene expression subgroups and genetic subtypes each have genetic abnormalities in the BCR pathway, but differ with respect to which particular BCR signaling components are targeted (Fig. 7D, 7E). MCD tumors are enriched for lesions targeting the BCR subunits CD79B and CD79A whereas BN2 tumors are instead enriched for lesions targeting distal components of the pathway, particularly PRKCB, BCL10, TRAF6 and the A20 negative regulatory module (i.e. TNFAIP3 and TNIP1). Cases with genetic aberrations targeting BCR negative regulators or CARD11 were drawn more equally from each genetic subtype. These observations make clear that aberrant activation of the BCR pathway is a unifying principle throughout DLBCL pathogenesis, but that the genetic trajectories of each DLBCL genetic subtype must impose distinct selective pressures that favor the acquisition of mutations targeting different BCR pathway components.
12. Therapy of lymphoma using BCR signaling inhibitors
Signal transduction through the BCR engages a multitude of protein and lipid kinases that can be inhibited with drugs that are currently in the clinic or in clinical development. As discussed above, lymphomas generally utilize either chronic active or toncogenic BCR signaling, each of which engages distinct signaling effectors (Fig. 3). The recent discovery of the My-T-BCR supercomplex revealed that chronic active BCR signaling may be further dissected into mechanistically distinct signaling modes that may influence the efficacy of various signaling inhibitors. Below, we review the more promising targets for BCR pathway inhibition in lymphoma, including the non-receptor tyrosine kinases BTK and SYK, as well as effectors in the PI3K pathway.
12.1. BTK
Following BCR activation in normal B cells, BTK is recruited to the BCR signalosome at the plasma membrane, where it is then activated, causing it to phosphorylate and activate the lipid hydrolase PLCγ2 (136, 137). BTK is an essential gene in ABC cell lines, but is dispensable in GCB lines (34, 44), indicating that BTK contributes to chronic active, but not toncogenic, BCR signaling. The BTK inhibitor ibrutinib, developed by Pharmacyclics, irreversibly binds to cysteine 481, which is adjacent to the active site of BTK (138). Only 9 other kinases have cysteine residues in homologous positions near their active sites, and consequently, off-target effects from ibrutinib are generally limited. The T cell-expressed kinase ITK is one of the other kinases targeted by ibrutinib, an activity that may be beneficial in certain scenarios. For example, ibrutinib therapy can improve the efficacy of anti-PD1/PDL1 therapies by skewing the ratio of Th1 and Th2 T cells (139).
Pre-clinical studies determined that ibrutinib is lethal to ABC DLBCL cell lines at low nM concentrations, but has virtually no effect on GCB lines at substantially higher doses (34, 41). Ibrutinib is also toxic for cultured primary CLL cells, even in the presence of stromal cells that mimic the tumor microenvironment (140, 141). More recently, Acerta Pharmaceuticals and AstraZeneca have developed a second generation BTK inhibitor, acalabrutinib, that also covalently binds to cysteine 481 of BTK. Acalabrutinib has improved specificity, with no activity against ITK and other kinases with cysteine residues proximal to their active site, and has clinical activity in CLL that is comparable to the efficacy of ibrutinib (142).
Ibrutinib has shown exceptional clinical activity as a single agent against indolent lymphomas, and was approved by the FDA for the treatment of MCL is 2013, CLL in 2014 and WM in 2015. Likewise, acalabrutinib was approved by the FDA for the treatment of MCL in 2017 and is pending approval for treating CLL. Ibrutinib is generally well tolerated at the standard dose of 560 mg per day, but severe adverse events include bleeding, atrial fibrillation, and diarrhea can occur (143). Acalabrutinib is reported to have similar types of adverse events, albeit at a potentially lower frequency (144).
As mentioned above, a phase 1b/2 clinical trial of ibrutinib monotherapy in relapsed/refractory DLBCL reported an objective response rate of 37% in the ABC subgroup compared with only 5% in GCB, consistent with the role of BTK in chronic active BCR signaling in ABC DLBCL but not in toncogenic BCR signaling GCB DLBCL (41). Correspondingly, ibrutinib treatment resulted in median overall survival of 10.3 months in patients with ABC DLBCL, but only 3.3 months in GCB patients. Exceptional responses to ibrutinib monotherapy were observed in 4/38 (10.5%) patients with relapsed/refractory ABC DLBCL, who survived at least 2 years, with one patient alive without evidence of disease after 8 years of ibrutinib monotherapy.
In an effort to identify genetic markers that predict ibrutinib response in ABC DLBCL, genes frequently mutated in this subgroup were sequenced and correlated with clinical responses. As noted above, ABC tumors harboring CD79B ITAM mutations were more likely to respond to ibrutinib monotherapy than tumors with wild-type CD79B (55% vs. 31%). However, a substantial number of ABC tumors with wild type CD79B responded, suggesting either that self-antigens are sufficient to drive chronic active BCR signaling in these cases or that other genetic lesions targeting negative regulators of BCR signaling play a role (34, 48). The presence of oncogenic MYD88 mutations alone had no effect on clinical response. However, ABC tumors with both MYD88L265P and a CD79B mutation had outstanding sensitivity to ibrutinib monotherapy, with an 80% (4/5) response rate (41). This double mutant genotype is the hallmark of the MCD subtype of ABC DLBCL and also of extranodal tumors such as PCNSL, which is not adequately treated by conventional chemotherapy approaches. Accordingly, ibrutinib monotherapy had exceptional activity in PCNSL, producing objective responses in 77–89% of cases (129, 130).
Cooperative signaling between the BCR and MYD88 is the hallmark of the My-T-BCR (44) and these results suggested that ABC cells that utilize the My-T-BCR to drive NF-κB activity may be particularly sensitive to ibrutinib. My-T-BCR detection in clinical biopsy samples by IgM:TLR9 PLA was exclusively found in ibrutinib-sensitive ABC tumors (44). None of the ABC tumors tested expressed mutant isoforms of MYD88, indicating that detection of the My-T-BCR by IgM:TLR9 PLA may have predictive value beyond mutational analysis (44). The My-T-BCR was also observed by PLA in 80% of PCNSL cases (44), consistent with the frequent co-occurrence of MYD88L265P and CD79B mutations in lymphomas and their exceptional sensitivity to ibrutinib (145–153). Hence, the My-T-BCR may serve as a predictive biomarker for ibrutinib responsiveness in aggressive lymphomas and PLA-based methods for its detection may complement genetic analysis, a possibility that should be evaluated prospectively in clinical trials of these agents.
12.2. SYK
SYK kinase transduces both chronic active and toncogenic BCR signals, making it an attractive therapeutic target to treat all BCR-dependent lymphomas. SYK inhibitors were the subject of the first preclinical studies of BCR pathway inhibitors in lymphoma. Fostamatinib (R788) is the pro-drug version of the SYK inhibitor R406. Fostamatinib treatment prolonged survival in mouse models of lymphoma that relied on either tonic or antigen-dependent BCR signaling (58), as well in Eμ-TCL1 mice, which model CLL (154). R406 treatment was toxic to cell line models of BL (58), GCB DLBCL (120) and ABC DLBCL (34), and to primary purified CLL cells (155). Unfortunately, fostamatinib had limited selectivity for SYK (156, 157), and although it demonstrated clinical activity against CLL and DLBCL (158), its toxicity profile precluded further clinical development. More selective SYK inhibitors are in development, including PRT062607 from Portola Pharmaceuticals and entospletinib (GS-9973) from Gilead Sciences, which have demonstrated selective toxicity in vitro (44, 159). A phase 2 study of entospetinib in relapsed/refractory indolent lymphomas and MCL demonstrated that this agent has manageable toxicity but modest efficacy as monotherapy (160), while a similar study in relapsed/refractory DLBCL yielded no objective responses (161). These findings suggest that SYK inhibitors may need to be combined with other targeted agents, as long as this can be done safely (see below).
12.3. PI3K and mTOR inhibitors
PI3K signaling is deregulated in many forms of human cancer (162), likely due to its ability to promote protein translation and nutrient utilization, thereby fostering malignant cell growth and survival. Like SYK, the PI3K pathway is engaged in both chronic active and toncogenic BCR signaling. PI3K is a lipid kinase that phosphorylates the 3-hydroxy position of PI(4,5)P2 to form phosphatidylinositol (3,4,5)-trisphosphate (PIP3). BCR-dependent PI3K activity results in localized increases in PIP3, which can recruit signaling effectors containing PH, FYVE and PX domains (163), including AKT and BTK. PI3K-dependent recruitment of the serine/threonine kinase AKT to the plasma membrane leads to its phosphorylation and activation by PDPK1. Active AKT has many potential substrates, but its phosphorylation and activation of mTOR is key since mTOR is a master regulator of cellular metabolism that is constitutively active in many lymphomas (164).
Three classes of PI3K exist, but only the class I PI3Ks signal proximal to the BCR (165). Class I PI3Ks contain a regulatory p85 subunit paired to one of four catalytic p110 subunits to form PI3Kα, PI3Kβ, PI3Kγ, PI3Kδ. A constitutively active form of PI3Kα can substitute for tonic BCR signaling (45), and PI3Kα and PI3Kδ are both essential for B cell development in mice (166). Pre-clinical studies demonstrated that inhibition of PI3K activity with the pan-class I PI3K inhibitor, BKM-120, is toxic for ABC lines harboring CD79B ITAM mutations (167), and for BL lines (46). However, PI3Kα and PI3Kβ are ubiquitously expressed, and pan-PI3K inhibitors such as BKM-120 can have significant adverse effects (168), which may limit their usage to treat GCB DLBCL and BL tumors that rely on toncogenic signaling. In contrast, PI3Kδ expression is restricted in expression to lymphocytes and is specifically activated following BCR ligation (169), making it an appropriate target in lymphomas with chronic active or toncogenic BCR signaling.
Idelalisib (CAL-101/GS-1101), a specific PI3Kδ inhibitor developed by Gilead Sciences, has been approved for the treatment of FL as well as relapsed/refractory CLL and small lymphocytic lymphoma (SLL). Copanlisib (BAY 80–6846) is a dual inhibitor of PI3Kδ and PI3Kα that is approved for treatment of relapsed/refractory FL. Pre-clinical studies of PI3K inhibitors in cell line models of ABC DLBCL demonstrated that selective PI3Kδ inhibition is quickly countered by an increase in PI3Kα activity, which can be targeted by copanlisib (114, 115).
There are numerous mTOR inhibitors available in the clinic, with rapamycin (sirolimus) being the first mTOR inhibitor discovered over 40 years ago (170). The related mTOR inhibitors temsirolimus and everolimus produced objective responses in 28% and 30% of patients with relapsed/refractory DLBCL, respectively, although the remissions were typically short lived (171, 172).
Going forward, studies of PI3K pathway inhibitors in DLBCL clinical trials should include molecular profiling since the cause and consequence of PI3K signaling differs between genomic subtypes. In GCB DLBCL, PI3K signaling is initiated by frequent genetic inactivation of PTEN and amplification of the miR17–92 locus, which encodes microRNAs that suppress PTEN expression (24, 173). ABC tumors lack these alterations, presumably because chronic active BCR signaling provides ample PI3K activation. Moreover, as discussed above, the mTORC1 complex is a component of the My-T-BCR supercomplex in ABC DLBCLs of the MCD subtype (44). Treatment of ABC lines with ibrutinib reduced the association of mTOR with other My-T-BCR components and decreased mTOR activation of downstream pathways. Treatment of these same lines with the mTOR inhibitor AZD2014 augmented the ability of ibrutinib to disrupt the My-T-BCR, resulting in a marked decline in NF-κB activity (44, 116), supporting clinical evaluation of this and other combinations targeting both BTK and the PI3K pathway in ABC DLBCL.
12.4. Other therapeutic targets in oncogenic BCR signaling pathways
As is obvious from the wiring diagram in Fig. 1, there are numerous enzymes in the BCR signaling pathway that could be used to attack BCR-dependent lymphomas. One interesting target is protein kinase C β (encoded by PRKCB), which lies downstream of BTK in the BCR signaling cascade (Fig. 1) and can inhibited by sotrastaurin (174, 175). Interestingly, sotrastaurin blocks chronic active BCR signaling in both ABC DLBCL and MCL models, which likely induce NF-κB by fundamentally different mechanisms. Whereas the chronic active BCR signaling in the ABC models depends on the My-T-BCR (Fig. 3B), the BCR-dependent NF-κB activation in MCL lines is presumably MYD88-independent since they lack MYD88L265P mutations. This latter, more “conventional” mode of chronic active BCR signaling (Fig. 3A) is also likely engaged in the BN2 subtype of DLBCL, which acquires activating mutations in PRKCB (37).
Toncogenic BCR signaling in GCB DLBCL and BL offers some unique therapeutic possibilities, including the Src-family kinase LYN. Normal B cell development is not compromised in Lyn-deficient mice due to functional redundancy with other Src family kinases (4). Moreover, LYN is not essential for the activation of mature B cells, and in fact inhibits BCR signaling in this context (176), as it does in ABC DLBCL (34). Thus, targeting of LYN would be predicted to have a good therapeutic window and would be specifically efficacious in lymphomas with toncogenic BCR signaling. The challenge this poses, however, is the identification of LYN-selective Src-family kinase inhibitors, since Src-family kinases perform diverse critical functions in multiple cells types.
A non-kinase target in chronic active BCR signaling is MALT1, which is a unique among proteases in cleaving substrates following arginine residues. Given the essential role of MALT1 in the CBM complex, several groups have identified MALT1 as a therapeutic target in ABC DLBCL and have identified prototype small molecule MALT1 inhibitors (167, 177–180). While these MALT1 inhibitors have selective toxicity for ABC DLBCL lines in vitro, clinical applications will require the development of much more potent inhibitors.
13. Resistance to BCR pathway inhibitors
BCR pathway inhibitors rarely elicit durable clinical responses in aggressive lymphomas. Generally, these responses last for a few months followed by progression as described above. In contrast, CLL is highly sensitive, with extended response durations. Resistance to ibrutinib monotherapy in CLL has been attributed to mutations targeting cysteine 481 on BTK, which destroy the ability of ibrutinib to covalently bind to BTK and are observed at high frequency in relapsed CLL (181, 182). These targeted mutations bear similarities to resistance mutations in other cancers, including the T790M mutation in EGFR that sterically blocks erlotinib from binding its active site (183) and the T315I mutation in BCR-ABL that blocks hydrogen bonding with imatinib (184). Additionally, gain-of-function mutations targeting PLCG2 have been observed at a lower frequency in ibrutinib-treated CLL patients, allowing CLL clones to bypass the need for BTK activity (181, 182). As with many malignancies, there is substantial sub-clonal heterogeneity in CLL. Resistance mutations likely pre-exist in subclones within the large pool of malignant CLL in each patient, potentially because they confer a modest growth advantage or ability to access supportive microenvironmental niches. Ibrutinib treatment provides the selective pressure to favor outgrowth of the mutant subclones. That said, the above ibrutinib resistance mutations and other rare genetic events only account for 28% of the patients who experience disease progression on ibrutinib, suggesting that other, perhaps non-genetic, mechanisms may contribute (185).
While a clinical trial demonstrated that ibrutinib can induce complete and partial responses in relapsed/refractory ABC DLBCL, the median PFS of these patients was only 6.41 months, indicating that ibrutinib resistance develops rapidly in this lymphoma subgroup (41). For example, a patient with extensive abdominal disease achieved a near CR after 3 weeks of ibrutinib monotherapy yet the lymphoma rapidly progressed by 6 weeks. Notably, the pre-treatment biopsy from this patient had a minor subclone harboring a CD79A mutation (~5% mutant allele frequency) that was much more prominent in a biopsy taken after only 3 days on treatment (~27% mutant allele frequency). In another study, the BTK C481S mutation that prevents ibrutinib binding was detected upon relapse in circulating tumor DNA of 2/3 patients (186). However, given the diversity of survival pathways in ABC DLBCL (reviewed in ref. (103)), it is conceivable that non-genetic mechanisms may contribute to ibrutinib resistance in this setting as well.
14. Combination Therapies
Aggressive lymphomas rely on an integrated network of cellular signaling cascades to promote malignant growth. Inhibition of a single pathway can be compensated by either mutation or biochemical re-wiring of these signal networks, which likely accounts for the limited frequency and durability of responses to individual targeted agents. Thus, inhibition of parallel survival pathways can not only result in synergistic killing of malignant cells, but also reduce the emergence of therapy resistance. The challenge is to identify drug combinations that can synergistically kill malignant B cells while minimizing side effects. In part, this can be done rationally by considering the wealth of data generated on the genetics, biology and oncogenic signaling in lymphoma. For example, ABC DLBCL relies on a minimum of 5 pathways to remain viable: (1) NF-κB; (2) PI3K/mTOR; (3) JAK1/STAT3; (4) BCL2 family; (5) lineage-defining transcription factors (reviewed in ref. (103)).
One strategy is to perform unbiased large-scale screens of clinically active compounds. One such screen profiled toxicity in an ABC DLBCL line treated with increasing doses of ibrutinib versus titrations of over 500 active compounds (113). These screens revealed synergy of ibrutinib in combination with inhibitors of SYK, the PI3K pathway, and the BCL2 family, as well as with standard chemotherapy agents. Additional studies observed synergy between ibrutinib and inhibitors of JAK1 (187), IRAK family kinases (99), BET proteins (188) and the lineage-defining transcription factor IRF4 (18).
These pre-clinical drug combination studies are already fueling clinical advances. The recent characterization of genetic subtypes within DLBCL defined the MCD subtype, in part, by the presence of CD79B and MYD88L265P mutations (37, 38). The MCD subtype is enriched for extranodal lymphomas of the ABC phenotype, including primary testicular lymphoma, primary cutaneous lymphoma and PCNSL. As mentioned above clinical trials evaluating response to ibrutinib monotherapy in PCNSL reported a high rate of responses (77–89%), but the responses were short-lived, with a median progression-free survival of 4.6 months (129, 130). Standard chemotherapy alone does not provide lasting benefit for the majority of these patients (189), potentially because the high NF-κB activity in these ABC-like tumors could block the apoptotic action of chemotherapy agents (190). The combination of ibrutinib with combination chemotherapy in the DA-TEDDi-R regimen produced complete responses in 86% of PCNSL patients that have been sustained for at least 2 years in 67% of patients with relapsed/refractory disease (129). These encouraging results await further confirmation in an ongoing phase 2 trial.
Recently, a double-blind phase 3 trial randomly assigned 838 patients with non-GCB DLBCL into two treatment arms comparing R-CHOP chemotherapy plus placebo, to R-CHOP plus ibrutinib (191). Younger patients under 60 years of age had significantly improved survival on the R-CHOP plus ibrutinib arm, with ibrutinib increasing the 5-year overall survival rate by roughly 12%. Patients over the age of 60 experienced increased toxicity when ibrutinib was included that led to discontinuation of both ibrutinib and chemotherapy, resulting in correspondingly worse clinical outcomes and accounting for the selective activity of ibrutinib in younger patients. This is the first randomized phase 3 trial in 17 years to report a regimen that produces a significantly higher rate of long-term survival than achieved with R-CHOP alone. The success of this platform may be extended in the future with the addition of targeted agents that synergize with ibrutinib.
This phase 3 trial used immunohistochemistry to select patients with non-GCB DLBCL. Most tumors were also analyzed by gene expression profiling and were found to be enriched for ABC and Unclassified cases, as expected. Interestingly, the effect of ibrutinib was not significantly greater in the pure ABC subset than in the entire cohort, possibly because the non-ABC tumors had genetic lesions in the BCR-dependent NF-κB pathway. In line with this hypothesis, genetic lesions in the BCR pathway are infrequent in GCB DLBCL, but are clearly overrepresented in both ABC and Unclassified DLBCL (Fig. 7D). An understanding of the molecular determinants of response to the combination of R-CHOP plus ibrutinib awaits the comprehensive genomic characterization of biopsies from this trial.
In another recent phase 2 trial, treatment naïve CLL patients were treated with a combination of ibrutinib and the BCL2-family inhibitor, venetoclax, both of which produce prolonged responses in CLL as monotherapy. An early report from this trial states that this regimen was safe and produced complete responses with no evidence of minimal residual disease in the blood in 9/11 (82%) of evaluable patients (192). If such combinations can overcome ibrutinib resistance mechanisms, one can envision a future in which CLL is either cured or maintained in long-term remission by the correct drug combination.
This wealth of knowledge could fuel decades of clinical trials, but care is needed to select drug combinations that are both efficacious and safe. Although many candidates for combination trials have known safety profiles as single agents, unexpected toxicities may occur in combination with other agents. For example, two patients died and others fell ill of pneumonitis on a combination trial of idelalisib and entospletinib, due to release of inflammatory cytokines (193). In PCNSL patients treated with the DA-TEDDi-R regimen mentioned above, opportunistic Aspergillosis infections were detected in the lung and/or brain in 7/18 patients and resulted in two deaths (129). Some of these infections occurred when the patients were only receiving ibrutinib monotherapy in a two-week window prior to the addition of chemotherapy, suggesting that these infections were potentially due to an effect of ibrutinib on the immune system. Interestingly, BTK mutant mice showed increased susceptibility to invasive and uncontrolled Aspergillus infections (129), providing evidence that the infections seen in PCNSL patients were an on-target consequence of ibrutinib, most likely due to the role of BTK in TLR signaling in innate immune cells (194). Steroids are also known to inhibit immune responses to Aspergillus (195), suggesting that the frequent treatment of PCNSL patients with steroids to minimize brain swelling likely contributed to the invasive Aspergillus infections. Invasive Aspergillus infections have also been reported in 33 CLL patients treated with ibrutinib, over half of whom were potentially immunosuppressed for other reasons, including by treatment with corticosteroids (196). While these infections can likely be prevented by anti-fungal prophylaxis, this example highlights the new spectrum of on-target toxicities that may arise as combinations of targeted agents are tested in the clinic.
15. An integrated approach to precision therapy of aggressive lymphomas
Patients with lymphoid malignancies and clinical investigators studying these diseases are fortunate to have an ever-increasing armamentarium of inhibitors targeting kinases in the BCR pathway at their disposal. When used as single agents, these BCR pathway inhibitors typically yield short-term responses followed by progression, although a small, but significant, fraction of patients can be effectively ‘cured’ by single agent therapies. The challenge ahead is two-fold: (1) identify drug combinations that can overcome drug resistance and relapse, while limiting unanticipated on-target and off-target toxicities; and (2) identify molecularly-defined subtypes of lymphoid malignancies for which particular BCR pathway inhibitors produce a high rate of meaningfully durable remissions. The identification and prioritization of drug combinations for testing in clinical trials should be based on a deep mechanistic understanding of oncogenic signaling and an appreciation of the parallel mechanisms that sustain survival of cancer cells. The identification of tumors likely to respond to certain drug combinations will require the application of genetic profiling (37, 38), RNA profiling (13, 197) and evaluation of biomarkers of BCR signaling, such as PLAs for protein-protein associations to detect active signaling even in the absence of a genetic marker (44). The path forward is clear: conduct large phase 2 trials with deep molecular profiling of tumors to identify those with the constellation of abnormalities that foster addiction to oncogenic survival pathways targeted by the drug combination under study.
Finally, an interesting opportunity for combination therapy has arisen with the success of CAR-T therapy for lymphoma and leukemia (198, 199) and the efficacy of an anti-CD47 monoclonal antibody (Hu5F9-G4) plus Rituximab in lymphoma (200). Though effective, these immunotherapies do not work in all patients and it is conceivable that in some cases, failure is essentially kinetic with tumor growth outstripping the rate at which immunotherapy can kill the malignant cells. Therapies targeting the BCR may improve outcomes in such patients by massively depleting the malignant tumor mass, allowing the immunotherapies a chance to clear the residual cells.
Acknowledgments
The authors thank Drs. Arthur L. Shaffer III, Jagan Muppidi and Raibatak Das for insightful discussions. This research was supported by the Intramural Research Program of the NIH, Center for Cancer Research, National Cancer Institute
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.SEER P. Surveillance Epidemiology and End Results wwwseercancergov.2002.
- 2.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reth M Antigen receptor tail clue. Nature 1989;338:383–384. [PubMed] [Google Scholar]
- 4.Saijo K, Schmedt C, Su IH, et al. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nat Immunol 2003;4:274–279. [DOI] [PubMed] [Google Scholar]
- 5.Turner M, Schweighoffer E, Colucci F, Di Santo JP, Tybulewicz VL. Tyrosine kinase SYK: essential functions for immunoreceptor signalling. Immunol Today 2000;21:148–154. [DOI] [PubMed] [Google Scholar]
- 6.Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J. B cell antigen receptor signaling 101. Mol Immunol 2004;41:599–613. [DOI] [PubMed] [Google Scholar]
- 7.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol 2012;30:429–457. [DOI] [PubMed] [Google Scholar]
- 8.Victora GD, Schwickert TA, Fooksman DR, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell 2010;143:592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominguez-Sola D, Victora GD, Ying CY, et al. The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nat Immunol 2012;13:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science 2002;297:2066–2070. [DOI] [PubMed] [Google Scholar]
- 11.Dogan I, Bertocci B, Vilmont V, et al. Multiple layers of B cell memory with different effector functions. Nat Immunol 2009;10:1292–1299. [DOI] [PubMed] [Google Scholar]
- 12.S. Swerdlow EC, Lee Harris N, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue 2008;
- 13.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503–511. [DOI] [PubMed] [Google Scholar]
- 14.Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A, Staudt LM. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci U S A 2003;100:9991–9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 2008;359:2313–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Read JA, Koff JL, Nastoupil LJ, Williams JN, Cohen JB, Flowers CR. Evaluating cell-of-origin subtype methods for predicting diffuse large B-cell lymphoma survival: a meta-analysis of gene expression profiling and immunohistochemistry algorithms. Clinical lymphoma, myeloma & leukemia 2014;14:460–467 e462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappa B activity is required for survival of activated B Cell-like diffuse large B cell lymphoma cells. J Exp Med 2001;194:1861–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Shaffer AL 3rd, Emre NC, et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell 2012;21:723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaffer AL, Emre NC, Lamy L, et al. IRF4 addiction in multiple myeloma. Nature 2008;454:226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein U, Casola S, Cattoretti G, et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol 2006;7:773–782. [DOI] [PubMed] [Google Scholar]
- 21.Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM, Singh H. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity 2006;25:225–236. [DOI] [PubMed] [Google Scholar]
- 22.Pasqualucci L, Compagno M, Houldsworth J, et al. Inactivation of the PRDM1/BLIMP1 gene in diffuse large B cell lymphoma. J Exp Med 2006;203:311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenz G, Nagel I, Siebert R, et al. Aberrant immunoglobulin class switch recombination and switch translocations in activated B cell-like diffuse large B cell lymphoma. J Exp Med 2007;204:633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenz G, Wright GW, Emre NC, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci U S A 2008;105:13520–13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang Y, Ortega-Molina A, Geng H, et al. CREBBP Inactivation Promotes the Development of HDAC3-Dependent Lymphomas. Cancer Discov 2017;7:38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortega-Molina A, Boss IW, Canela A, et al. The histone lysine methyltransferase KMT2D sustains a gene expression program that represses B cell lymphoma development. Nat Med 2015;21:1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Dominguez-Sola D, Hussein S, et al. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat Med 2015;21:1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Vlasevska S, Wells VA, et al. The CREBBP Acetyltransferase Is a Haploinsufficient Tumor Suppressor in B-cell Lymphoma. Cancer Discov 2017;7:322–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beguelin W, Popovic R, Teater M, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 2013;23:677–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caganova M, Carrisi C, Varano G, et al. Germinal center dysregulation by histone methyltransferase EZH2 promotes lymphomagenesis. The Journal of clinical investigation 2013;123:5009–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sneeringer CJ, Scott MP, Kuntz KW, et al. Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B-cell lymphomas. Proc Natl Acad Sci U S A 2010;107:20980–20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002;346:1937–1947. [DOI] [PubMed] [Google Scholar]
- 33.Morin RD, Mendez-Lago M, Mungall AJ, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 2011;476:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 2010;463:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011;470:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Compagno M, Lim WK, Grunn A, et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature 2009;459:717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz R, Wright GW, Huang DW, et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med 2018;378:1396–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 2018;24:679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ngo VN, Davis RE, Lamy L, et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature 2006;441:106–110. [DOI] [PubMed] [Google Scholar]
- 40.Lenz G, Davis RE, Ngo VN, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science 2008;319:1676–1679. [DOI] [PubMed] [Google Scholar]
- 41.Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 2015;21:922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hibbs ML, Tarlinton DM, Armes J, et al. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell 1995;83:301–311. [DOI] [PubMed] [Google Scholar]
- 43.Busman-Sahay K, Drake L, Sitaram A, Marks M, Drake JR. Cis and Trans Regulatory Mechanisms Control AP2-Mediated B Cell Receptor Endocytosis via Select Tyrosine-Based Motifs. PLoS One 2013;8:e54938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phelan JD, Young RM, Webster DE, et al. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature 2018;560:387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Srinivasan L, Sasaki Y, Calado DP, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell 2009;139:573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature 2012;490:116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tolar P, Hanna J, Krueger PD, Pierce SK. The constant region of the membrane immunoglobulin mediates B cell-receptor clustering and signaling in response to membrane antigens. Immunity 2009;30:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young RM, Wu T, Schmitz R, et al. Survival of human lymphoma cells requires B-cell receptor engagement by self-antigens. Proc Natl Acad Sci U S A 2015;112:13447–13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dameshek W, Schwartz RS. Leukemia and auto-immunization- some possible relationships. Blood 1959;14:1151–1158. [PubMed] [Google Scholar]
- 50.Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood 2011;117:1792–1798. [DOI] [PubMed] [Google Scholar]
- 51.Gisbert JP, Garcia-Buey L, Pajares JM, Moreno-Otero R. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Ther 2005;21:653–662. [DOI] [PubMed] [Google Scholar]
- 52.Quinn ER, Chan CH, Hadlock KG, Foung SK, Flint M, Levy S. The B-cell receptor of a hepatitis C virus (HCV)-associated non-Hodgkin lymphoma binds the viral E2 envelope protein, implicating HCV in lymphomagenesis. Blood 2001;98:3745–3749. [DOI] [PubMed] [Google Scholar]
- 53.Morgner A, Miehlke S, Fischbach W, et al. Complete remission of primary high-grade B-cell gastric lymphoma after cure of Helicobacter pylori infection. J Clin Oncol 2001;19:2041–2048. [DOI] [PubMed] [Google Scholar]
- 54.Bernatsky S, Ramsey-Goldman R, Joseph L, et al. Lymphoma risk in systemic lupus: effects of disease activity versus treatment. Annals of the rheumatic diseases 2014;73:138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adams JM, Harris AW, Pinkert CA, et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 1985;318:533–538. [DOI] [PubMed] [Google Scholar]
- 56.Refaeli Y, Young RM, Turner BC, Duda J, Field KA, Bishop JM. The B cell antigen receptor and overexpression of MYC can cooperate in the genesis of B cell lymphomas. PLoS Biol 2008;6:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goodnow CC, Brink R, Adams E. Breakdown of self-tolerance in anergic B lymphocytes. Nature 1991;352:532–536. [DOI] [PubMed] [Google Scholar]
- 58.Young RM, Hardy IR, Clarke RL, et al. Mouse models of non-Hodgkin lymphoma reveal Syk as an important therapeutic target. Blood 2009;113:2508–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young R, Smith A, Honigberg L, Refaeli Y. Abstract #1984: Btk is a novel therapeutic target to treat large B-cell lymphomas. AACR Meeting Abstracts 2009;2009:1984-. [Google Scholar]
- 60.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood 1999;94:1840–1847. [PubMed] [Google Scholar]
- 61.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 1999;94:1848–1854. [PubMed] [Google Scholar]
- 62.Rosenwald A, Alizadeh AA, Widhopf G, et al. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J Exp Med 2001;194:1639–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lefranc MP. IMGT unique numbering for the variable (V), constant (C), and groove (G) domains of IG, TR, MH, IgSF, and MhSF. Cold Spring Harb Protoc 2011;2011:633–642. [DOI] [PubMed] [Google Scholar]
- 64.Stamatopoulos K, Belessi C, Moreno C, et al. Over 20% of patients with chronic lymphocytic leukemia carry stereotyped receptors: Pathogenetic implications and clinical correlations. Blood 2007;109:259–270. [DOI] [PubMed] [Google Scholar]
- 65.Agathangelidis A, Darzentas N, Hadzidimitriou A, et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood 2012;119:4467–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hadzidimitriou A, Agathangelidis A, Darzentas N, et al. Is there a role for antigen selection in mantle cell lymphoma? Immunogenetic support from a series of 807 cases. Blood 2011. [DOI] [PubMed]
- 67.Herve M, Xu K, Ng YS, et al. Unmutated and mutated chronic lymphocytic leukemias derive from self-reactive B cell precursors despite expressing different antibody reactivity. The Journal of clinical investigation 2005;115:1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Catera R, Silverman GJ, Hatzi K, et al. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation. Mol Med 2008;14:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chu CC, Catera R, Zhang L, et al. Many chronic lymphocytic leukemia antibodies recognize apoptotic cells with exposed nonmuscle myosin heavy chain IIA: implications for patient outcome and cell of origin. Blood 2010;115:3907–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duhren-von Minden M, Ubelhart R, Schneider D, et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature 2012;489:309–312. [DOI] [PubMed] [Google Scholar]
- 71.Jerne NK. Towards a network theory of the immune system. Annales d’immunologie 1974;125C:373–389. [PubMed] [Google Scholar]
- 72.Baliakas P, Hadzidimitriou A, Sutton LA, et al. Clinical effect of stereotyped B-cell receptor immunoglobulins in chronic lymphocytic leukaemia: a retrospective multicentre study. Lancet Haematol 2014;1:e74–84. [DOI] [PubMed] [Google Scholar]
- 73.Minici C, Gounari M, Ubelhart R, et al. Distinct homotypic B-cell receptor interactions shape the outcome of chronic lymphocytic leukaemia. Nat Commun 2017;8:15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sebastian E, Alcoceba M, Balanzategui A, et al. Molecular characterization of immunoglobulin gene rearrangements in diffuse large B-cell lymphoma: antigen-driven origin and IGHV4–34 as a particular subgroup of the non-GCB subtype. The American journal of pathology 2012;181:1879–1888. [DOI] [PubMed] [Google Scholar]
- 75.Cappione AJ, Pugh-Bernard AE, Anolik JH, Sanz I. Lupus IgG VH4.34 antibodies bind to a 220-kDa glycoform of CD45/B220 on the surface of human B lymphocytes. J Immunol 2004;172:4298–4307. [DOI] [PubMed] [Google Scholar]
- 76.Richardson C, Chida AS, Adlowitz D, et al. Molecular basis of 9G4 B cell autoreactivity in human systemic lupus erythematosus. J Immunol 2013;191:4926–4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pugh-Bernard AE, Silverman GJ, Cappione AJ, et al. Regulation of inherently autoreactive VH4–34 B cells in the maintenance of human B cell tolerance. The Journal of clinical investigation 2001;108:1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sabouri Z, Schofield P, Horikawa K, et al. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proc Natl Acad Sci U S A 2014;111:E2567–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu D, McCarthy H, Ottensmeier CH, Johnson P, Hamblin TJ, Stevenson FK. Acquisition of potential N-glycosylation sites in the immunoglobulin variable region by somatic mutation is a distinctive feature of follicular lymphoma. Blood 2002;99:2562–2568. [DOI] [PubMed] [Google Scholar]
- 80.Radcliffe CM, Arnold JN, Suter DM, et al. Human follicular lymphoma cells contain oligomannose glycans in the antigen-binding site of the B-cell receptor. J Biol Chem 2007;282:7405–7415. [DOI] [PubMed] [Google Scholar]
- 81.Jardin F, Sahota SS, Ruminy P, et al. Novel Ig V gene features of t(14;18) and t(3;14) de novo diffuse large B-cell lymphoma displaying germinal center-B cell like and non-germinal center-B cell like markers. Leukemia 2006;20:2070–2074. [DOI] [PubMed] [Google Scholar]
- 82.Coelho V, Krysov S, Ghaemmaghami AM, et al. Glycosylation of surface Ig creates a functional bridge between human follicular lymphoma and microenvironmental lectins. Proc Natl Acad Sci U S A 2010;107:18587–18592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wardemann H, Nussenzweig MC. B-cell self-tolerance in humans. Adv Immunol 2007;95:83–110. [DOI] [PubMed] [Google Scholar]
- 84.Quach TD, Manjarrez-Orduno N, Adlowitz DG, et al. Anergic Responses Characterize a Large Fraction of Human Autoreactive Naive B Cells Expressing Low Levels of Surface IgM. J Immunol 2011;186:4640–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature 1991;353:765–769. [DOI] [PubMed] [Google Scholar]
- 86.Cappione A 3rd, Anolik JH, Pugh-Bernard A, et al. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. The Journal of clinical investigation 2005;115:3205–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shaffer AL, 3rd, Young RM, Staudt LM. Pathogenesis of human B cell lymphomas. Annu Rev Immunol 2012;30:565–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Varano G, Raffel S, Sormani M, et al. The B-cell receptor controls fitness of MYC-driven lymphoma cells via GSK3beta inhibition. Nature 2017;546:302–306. [DOI] [PubMed] [Google Scholar]
- 89.Martin SW, Goodnow CC. Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nat Immunol 2002;3:182–188. [DOI] [PubMed] [Google Scholar]
- 90.Horikawa K, Martin SW, Pogue SL, et al. Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. J Exp Med 2007;204:759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu W, Meckel T, Tolar P, Sohn HW, Pierce SK. Intrinsic properties of immunoglobulin IgG1 isotype-switched B cell receptors promote microclustering and the initiation of signaling. Immunity 2010;32:778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruminy P, Etancelin P, Couronne L, et al. The isotype of the BCR as a surrogate for the GCB and ABC molecular subtypes in diffuse large B-cell lymphoma. Leukemia 2011. [DOI] [PubMed]
- 93.Vaandrager JW, Schuuring E, Kluin-Nelemans HC, Dyer MJ, Raap AK, Kluin PM. DNA fiber fluorescence in situ hybridization analysis of immunoglobulin class switching in B-cell neoplasia: aberrant CH gene rearrangements in follicle center-cell lymphoma. Blood 1998;92:2871–2878. [PubMed] [Google Scholar]
- 94.Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature 1987;328:805–811. [DOI] [PubMed] [Google Scholar]
- 95.Tipton CM, Fucile CF, Darce J, et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol 2015;16:755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Malecka A, Troen G, Tierens A, et al. Immunoglobulin heavy and light chain gene features are correlated with primary cold agglutinin disease onset and activity. Haematologica 2016;101:e361–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Randen U, Troen G, Tierens A, et al. Primary cold agglutinin-associated lymphoproliferative disease: a B-cell lymphoma of the bone marrow distinct from lymphoplasmacytic lymphoma. Haematologica 2014;99:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Malecka A, Troen G, Tierens A, et al. Frequent somatic mutations of KMT2D (MLL2) and CARD11 genes in primary cold agglutinin disease. Br J Haematol 2018;183:838–842. [DOI] [PubMed] [Google Scholar]
- 99.Kelly PN, Romero DL, Yang Y, et al. Selective interleukin-1 receptor-associated kinase 4 inhibitors for the treatment of autoimmune disorders and lymphoid malignancy. J Exp Med 2015;212:2189–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Avbelj M, Wolz OO, Fekonja O, et al. Activation of lymphoma-associated MyD88 mutations via allostery-induced TIR-domain oligomerization. Blood 2014;124:3896–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhan C, Qi R, Wei G, Guven-Maiorov E, Nussinov R, Ma B. Conformational dynamics of cancer-associated MyD88-TIR domain mutant L252P (L265P) allosterically tilts the landscape toward homo-dimerization. Protein Eng Des Sel 2016;29:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N Engl J Med 2012;367:826–833. [DOI] [PubMed] [Google Scholar]
- 103.Young RM, Phelan JD, Shaffer AL, et al. Taming the Heterogeneity of Aggressive Lymphomas for Precision Therapy. Annu Rev Cancer Biology 2019;3:429–455. [Google Scholar]
- 104.Kim DI, Jensen SC, Noble KA, et al. An improved smaller biotin ligase for BioID proximity labeling. Molecular biology of the cell 2016;27:1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Otipoby KL, Waisman A, Derudder E, Srinivasan L, Franklin A, Rajewsky K. The B-cell antigen receptor integrates adaptive and innate immune signals. Proc Natl Acad Sci U S A 2015;112:12145–12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hou B, Saudan P, Ott G, et al. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity 2011;34:375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 2002;416:603–607. [DOI] [PubMed] [Google Scholar]
- 108.Wang JQ, Jeelall YS, Beutler B, Horikawa K, Goodnow CC. Consequences of the recurrent MYD88(L265P) somatic mutation for B cell tolerance. J Exp Med 2014;211:413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Soderberg O, Leuchowius KJ, Gullberg M, et al. Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods 2008;45:227–232. [DOI] [PubMed] [Google Scholar]
- 110.Martinez-Lopez A, Curiel-Olmo S, Mollejo M, et al. MYD88 (L265P) somatic mutation in marginal zone B-cell lymphoma. The American journal of surgical pathology 2015;39:644–651. [DOI] [PubMed] [Google Scholar]
- 111.David L, Li Y, Ma J, Garner E, Zhang X, Wu H. Assembly mechanism of the CARMA1-BCL10-MALT1-TRAF6 signalosome. Proc Natl Acad Sci U S A 2018;115:1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010;141:290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mathews Griner LA, Guha R, Shinn P, et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell-like diffuse large B-cell lymphoma cells. Proc Natl Acad Sci U S A 2014;111:2349–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paul J, Soujon M, Wengner AM, et al. Simultaneous Inhibition of PI3Kdelta and PI3Kalpha Induces ABC-DLBCL Regression by Blocking BCR-Dependent and -Independent Activation of NF-kappaB and AKT. Cancer Cell 2017;31:64–78. [DOI] [PubMed] [Google Scholar]
- 115.Pongas GN, Annunziata CM, Staudt LM. PI3Kdelta inhibition causes feedback activation of PI3Kalpha in the ABC subtype of diffuse large B-cell lymphoma. Oncotarget 2017;8:81794–81802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ezell SA, Mayo M, Bihani T, et al. Synergistic induction of apoptosis by combination of BTK and dual mTORC1/2 inhibitors in diffuse large B cell lymphoma. Oncotarget 2014;5:4990–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell 1997;90:1073–1083. [DOI] [PubMed] [Google Scholar]
- 118.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell 2004;117:787–800. [DOI] [PubMed] [Google Scholar]
- 119.Sander S, Calado DP, Srinivasan L, et al. Synergy between PI3K Signaling and MYC in Burkitt Lymphomagenesis. Cancer Cell 2012;22:167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen L, Monti S, Juszczynski P, et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood 2008;111:2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen L, Monti S, Juszczynski P, et al. SYK inhibition modulates distinct PI3K/AKT- dependent survival pathways and cholesterol biosynthesis in diffuse large B cell lymphomas. Cancer Cell 2013;23:826–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol 2010;10:387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gross AJ, Lyandres JR, Panigrahi AK, Prak ET, DeFranco AL. Developmental acquisition of the Lyn-CD22-SHP-1 inhibitory pathway promotes B cell tolerance. J Immunol 2009;182:5382–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cyster JG, Healy JI, Kishihara K, Mak TW, Thomas ML, Goodnow CC. Regulation of B-lymphocyte negative and positive selection by tyrosine phosphatase CD45. Nature 1996;381:325–328. [DOI] [PubMed] [Google Scholar]
- 125.Kulathu Y, Grothe G, Reth M. Autoinhibition and adapter function of Syk. Immunological reviews 2009;232:286–299. [DOI] [PubMed] [Google Scholar]
- 126.Maecker HT, Levy S. Normal lymphocyte development but delayed humoral immune response in CD81-null mice. J Exp Med 1997;185:1505–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aiba Y, Kameyama M, Yamazaki T, Tedder TF, Kurosaki T. Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood 2008;111:1497–1503. [DOI] [PubMed] [Google Scholar]
- 128.Havranek O, Xu J, Kohrer S, et al. Tonic B-cell receptor signaling in diffuse large B-cell lymphoma. Blood 2017;130:995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell 2017;31:833–843 e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grommes C, Pastore A, Palaskas N, et al. Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma. Cancer Discov 2017;7:1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sommer K, Guo B, Pomerantz JL, et al. Phosphorylation of the CARMA1 linker controls NF-kappaB activation. Immunity 2005;23:561–574. [DOI] [PubMed] [Google Scholar]
- 132.Qiao Q, Yang C, Zheng C, et al. Structural architecture of the CARMA1/Bcl10/MALT1 signalosome: nucleation-induced filamentous assembly. Mol Cell 2013;51:766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xu L, Tsakmaklis N, Yang G, et al. Acquired mutations associated with ibrutinib resistance in Waldenstrom macroglobulinemia. Blood 2017;129:2519–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bartlett NL, Costello BA, LaPlant BR, et al. Single-agent ibrutinib in relapsed or refractory follicular lymphoma: a phase 2 consortium trial. Blood 2018;131:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kanagal-Shamanna R, Jain P, Patel KP, et al. Targeted multigene deep sequencing of Bruton tyrosine kinase inhibitor-resistant chronic lymphocytic leukemia with disease progression and Richter transformation. Cancer 2019;125:559–574. [DOI] [PubMed] [Google Scholar]
- 136.Watanabe D, Hashimoto S, Ishiai M, et al. Four tyrosine residues in phospholipase C-gamma 2, identified as Btk-dependent phosphorylation sites, are required for B cell antigen receptor-coupled calcium signaling. J Biol Chem 2001;276:38595–38601. [DOI] [PubMed] [Google Scholar]
- 137.Ozdener F, Dangelmaier C, Ashby B, Kunapuli SP, Daniel JL. Activation of phospholipase Cgamma2 by tyrosine phosphorylation. Molecular pharmacology 2002;62:672–679. [DOI] [PubMed] [Google Scholar]
- 138.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A 2010;107:13075–13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci U S A 2015;112:E966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011;117:6287–6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood 2012;119:1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu J, Zhang M, Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. J Hematol Oncol 2016;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica 2018;103:874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med 2016;374:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Montesinos-Rongen M, Godlewska E, Brunn A, Wiestler OD, Siebert R, Deckert M. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol 2011;122:791–792. [DOI] [PubMed] [Google Scholar]
- 146.Bruno A, Boisselier B, Labreche K, et al. Mutational analysis of primary central nervous system lymphoma. Oncotarget 2014;5:5065–5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Braggio E, Van Wier S, Ojha J, et al. Genome-wide analysis uncovers novel recurrent alterations in primary central nervous system lymphomas. Clin Cancer Res 2015. [DOI] [PMC free article] [PubMed]
- 148.Nakamura T, Tateishi K, Niwa T, et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathology and applied neurobiology 2015. [DOI] [PubMed]
- 149.Vater I, Montesinos-Rongen M, Schlesner M, et al. The mutational pattern of primary lymphoma of the central nervous system determined by whole-exome sequencing. Leukemia 2015;29:677–685. [DOI] [PubMed] [Google Scholar]
- 150.Yamada S, Ishida Y, Matsuno A, Yamazaki K. Primary diffuse large B-cell lymphomas of central nervous system exhibit remarkably high prevalence of oncogenic MYD88 and CD79B mutations. Leuk Lymphoma 2015:1–5. [DOI] [PubMed] [Google Scholar]
- 151.Chapuy B, Roemer MG, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood 2016;127:869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fukumura K, Kawazu M, Kojima S, et al. Genomic characterization of primary central nervous system lymphoma. Acta Neuropathol 2016;131:865–875. [DOI] [PubMed] [Google Scholar]
- 153.Hattori K, Sakata-Yanagimoto M, Okoshi Y, et al. MYD88 (L265P) mutation is associated with an unfavourable outcome of primary central nervous system lymphoma. Br J Haematol 2016. [DOI] [PubMed]
- 154.Suljagic M, Longo PG, Bennardo S, et al. The Syk inhibitor fostamatinib disodium (R788) inhibits tumor growth in the Emu- TCL1 transgenic mouse model of CLL by blocking antigen-dependent B-cell receptor signaling. Blood 2010;116:4894–4905. [DOI] [PubMed] [Google Scholar]
- 155.Gobessi S, Laurenti L, Longo PG, et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia 2009;23:686–697. [DOI] [PubMed] [Google Scholar]
- 156.Braselmann S, Taylor V, Zhao H, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. The Journal of pharmacology and experimental therapeutics 2006;319:998–1008. [DOI] [PubMed] [Google Scholar]
- 157.Clemens GR, Schroeder RE, Magness SH, et al. Developmental toxicity associated with receptor tyrosine kinase Ret inhibition in reproductive toxicity testing. Birth Defects Res A Clin Mol Teratol 2009;85:130–136. [DOI] [PubMed] [Google Scholar]
- 158.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non Hodgkin’s lymphoma and chronic lymphocytic leukemia. Blood 2009. [DOI] [PMC free article] [PubMed]
- 159.Cheng S, Coffey G, Zhang XH, et al. SYK inhibition and response prediction in diffuse large B-cell lymphoma. Blood 2011;118:6342–6352. [DOI] [PubMed] [Google Scholar]
- 160.Andorsky DJ, Kolibaba KS, Assouline S, et al. An open-label phase 2 trial of entospletinib in indolent non-Hodgkin lymphoma and mantle cell lymphoma. Br J Haematol 2019;184:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Burke JM, Shustov A, Essell J, et al. An Open-label, Phase II Trial of Entospletinib (GS-9973), a Selective Spleen Tyrosine Kinase Inhibitor, in Diffuse Large B-cell Lymphoma. Clinical lymphoma, myeloma & leukemia 2018;18:e327–e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene 2008;27:5497–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol 2008;9:99–111. [DOI] [PubMed] [Google Scholar]
- 164.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012;149:274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.So L, Fruman DA. PI3K signalling in B- and T-lymphocytes: new developments and therapeutic advances. Biochem J 2012;442:465–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ramadani F, Bolland DJ, Garcon F, et al. The PI3K isoforms p110alpha and p110delta are essential for pre-B cell receptor signaling and B cell development. Science signaling 2010;3:ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kloo B, Nagel D, Pfeifer M, et al. Critical role of PI3K signaling for NF-kappaB-dependent survival in a subset of activated B-cell-like diffuse large B-cell lymphoma cells. Proc Natl Acad Sci U S A 2011;108:272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Heudel PE, Fabbro M, Roemer-Becuwe C, et al. Phase II study of the PI3K inhibitor BKM120 in patients with advanced or recurrent endometrial carcinoma: a stratified type I-type II study from the GINECO group. British journal of cancer 2017;116:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Okkenhaug K, Ali K, Vanhaesebroeck B. Antigen receptor signalling: a distinctive role for the p110delta isoform of PI3K. Trends in immunology 2007;28:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28:721–726. [DOI] [PubMed] [Google Scholar]
- 171.Smith SM, van Besien K, Karrison T, et al. Temsirolimus has activity in non-mantle cell non-Hodgkin’s lymphoma subtypes: The University of Chicago phase II consortium. J Clin Oncol 2010;28:4740–4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Witzig TE, Reeder CB, LaPlant BR, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia 2011;25:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pfeifer M, Grau M, Lenze D, et al. PTEN loss defines a PI3K/AKT pathway-dependent germinal center subtype of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A 2013;110:12420–12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Naylor TL, Tang H, Ratsch BA, et al. Protein kinase C inhibitor Sotrastaurin selectively inhibits the growth of CD79-mutant diffuse large B-cell lymphomas. Cancer Res 2011. [DOI] [PubMed]
- 175.Rahal R, Frick M, Romero R, et al. Pharmacological and genomic profiling identifies NF-kappaB-targeted treatment strategies for mantle cell lymphoma. Nat Med 2014;20:87–92. [DOI] [PubMed] [Google Scholar]
- 176.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity 1997;7:69–81. [DOI] [PubMed] [Google Scholar]
- 177.Ferch U, Kloo B, Gewies A, et al. Inhibition of MALT1 protease activity is selectively toxic for activated B cell-like diffuse large B cell lymphoma cells. J Exp Med 2009;206:2313–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hailfinger S, Lenz G, Ngo V, et al. Essential role of MALT1 protease activity in activated B cell-like diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A 2009;106:19946–19951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fontan L, Yang C, Kabaleeswaran V, et al. MALT1 small molecule inhibitors specifically suppress ABC-DLBCL in vitro and in vivo. Cancer Cell 2012;22:812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nagel D, Spranger S, Vincendeau M, et al. Pharmacologic inhibition of MALT1 protease by phenothiazines as a therapeutic approach for the treatment of aggressive ABC-DLBCL. Cancer Cell 2012;22:825–837. [DOI] [PubMed] [Google Scholar]
- 181.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med 2014;370:2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Furman RR, Cheng S, Lu P, et al. Ibrutinib resistance in chronic lymphocytic leukemia. N Engl J Med 2014;370:2352–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pao W, Miller VA. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directions. J Clin Oncol 2005;23:2556–2568. [DOI] [PubMed] [Google Scholar]
- 184.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001;293:876–880. Epub 2001 Jun 2021. [DOI] [PubMed] [Google Scholar]
- 185.Landau DA, Sun C, Rosebrock D, et al. The evolutionary landscape of chronic lymphocytic leukemia treated with ibrutinib targeted therapy. Nat Commun 2017;8:2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Scherer F, Kurtz DM, Newman AM, et al. Distinct biological subtypes and patterns of genome evolution in lymphoma revealed by circulating tumor DNA. Science translational medicine 2016;8:364ra155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rui L, Drennan AC, Ceribelli M, et al. Epigenetic gene regulation by Janus kinase 1 in diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A 2016;113:E7260–E7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ceribelli M, Kelly PN, Shaffer AL, et al. Blockade of oncogenic IkappaB kinase activity in diffuse large B-cell lymphoma by bromodomain and extraterminal domain protein inhibitors. Proc Natl Acad Sci U S A 2014;111:11365–11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rubenstein JL, Gupta NK, Mannis GN, Lamarre AK, Treseler P. How I treat CNS lymphomas. Blood 2013;122:2318–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. The Journal of clinical investigation 2001;107:241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Younes A, Sehn LH, Johnson P, et al. Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. J Clin Oncol 2019:JCO1802403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wierda W, Siddiqi T, Flinn I, et al. Phase 2 CAPTIVATE results of ibrutinib (ibr) plus venetoclax (ven) in first-line chronic lymphocytic leukemia (CLL). J Clin Oncol 2018;36, no. 15_suppl:7502–7502. [Google Scholar]
- 193.Barr PM, Saylors GB, Spurgeon SE, et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood 2016;127:2411–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Horwood NJ, Page TH, McDaid JP, et al. Bruton’s tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. J Immunol 2006;176:3635–3641. [DOI] [PubMed] [Google Scholar]
- 195.Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet 2003;362:1828–1838. [DOI] [PubMed] [Google Scholar]
- 196.Ghez D, Calleja A, Protin C, et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood 2018;131:1955–1959. [DOI] [PubMed] [Google Scholar]
- 197.Scott DW, Wright GW, Williams PM, et al. Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood 2014;123:1214–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010;116:4099–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Advani R, Flinn I, Popplewell L, et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N Engl J Med 2018;379:1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Scott DW, Mottok A, Ennishi D, et al. Prognostic Significance of Diffuse Large B-Cell Lymphoma Cell of Origin Determined by Digital Gene Expression in Formalin-Fixed Paraffin-Embedded Tissue Biopsies. J Clin Oncol 2015;33:2848–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]