Abstract
Background:
Indications for partial nephrectomy (PN) in the treatment of renal cell carcinoma are evolving, particularly for larger, more complex tumors.
Objective:
Compare single-institution outcomes for minimally invasive partial nephrectomy (MIPN) and open partial nephrectomy (OPN) for tumors >4–7 cm.
Design, setting, and participants:
A total of 2290 patients underwent PN from 2002 to 2010 at Memorial Sloan-Kettering Cancer Center; 280 had >4–7 cm renal cortical tumors. Of these 280 patients, 230 had pT1b, 48 had pT3a, and 2 had angiomyolipomas; 226 underwent OPN and 54 underwent MIPN (16 robot-assisted and 37 laparoscopic procedures). Perioperative management was uniform on the clinical pathway. Perioperative data, clinicopathologic variables, complications within 30 d, and oncologic outcomes were reviewed.
Measurements:
Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation. Complications were reported from prospectively collected data based on a modified Clavien system. The Fisher exact and Mann-Whitney U tests were used for descriptive statistical analysis. Kaplan-Meier methods were used to estimate survival.
Results and limitations:
Median follow-up for OPN and MIPN was 29 and 13 mo, respectively. There were no statistically significant differences in age, gender, preoperative American Society of Anesthesiologists score, laterality, histologic subtype, tumor size, tumor stage, or margin status between procedures. Univariate analysis revealed significantly greater values in the OPN group for preoperative eGFR, renal artery clamp time, estimated blood loss, use of renal hypothermia, and length of stay. Differences in overall survival and recurrence-free survival were not statistically significant; however, short median follow-up times limit comparison. There was no significant difference in the number of complications grade ≥3 (p = 0.1) or urine leaks requiring intervention (p = 0.7). Limitations include the retrospective nature of the study and the possibility of selection bias.
Conclusions:
OPN and MIPN procedures performed in patients with tumors >4–7 cm offer acceptable and comparable results in terms of operative, functional, and convalescence measures, regardless of approach.
Keywords: Renal cell carcinoma, Partial nephrectomy, Radical nephrectomy, Small renal masses, Minimally invasive surgery
1. Introduction
Partial nephrectomy (PN) is the standard of care for small renal masses and selected larger tumors that are technically amenable to nephron-sparing techniques. With experience, the boundaries of what is considered manageable by PN have expanded to include more complex and challenging cases involving multiple, central, and larger masses. From an oncologic perspective, PN has been found to be comparable with radical nephrectomy (RN) for appropriately selected patients with small (<4 cm) renal cancers [1–3]. The preservation of renal function associated with PN [4–6], combined with equivalent oncologic outcomes and recognition of the association between chronic kidney disease (CKD) and medical morbidity [7,8], has led the American Urological Association and European Association of Urology to declare PN as the procedure of choice when feasible [9,10]. In selected series, PN for larger tumors (≥4 cm) has been suggested to provide similar oncologic outcomes and complications rates to RN either by the open [11–14] or the laparoscopic approach [15–17].
Alterations and developments in surgical technique are often intended to minimize perioperative morbidity while optimizing oncologic outcomes. Minimally invasive partial nephrectomy (MIPN), for example, is associated with reduced postoperative pain and hospital costs while maintaining comparable 5-yr oncologic outcomes compared with open partial nephrectomy (OPN) [18,19]. These techniques appear transferrable to larger and more complex cases; at Memorial Sloan-Kettering Cancer Center (MSKCC), the indications for PN were initially limited to lesions <4 cm but have expanded progressively to include any lesion that is technically feasible to remove, including nearly 80% of pT1b cases in 2009. The current study evaluates a contemporary cohort of patients who underwent PN either by minimally invasive (robot-assisted or laparoscopic nonrobotic) or OPN for renal tumors that were >4–7 cm and confined to the kidney without vascular invasion (including pT1b and pT3a by the 2002 American Joint Committee on Cancer definition) [20,21]. Surgical complications as well as oncologic and functional outcomes were assessed and compared between these approaches.
2. Materials and methods
After institutional review board approval was obtained, we identified 2290 PN procedures performed by 15 urologic oncology surgeons at MSKCC between January 2002 and February 2010. Renal tumors >4–7 cm were identified in 280 (12%) of these patients, and their medical records were retrospectively reviewed. Of the 280 patients, 230 had pT1b, 48 had pT3a, and 2 had angiomyolipomas. A total of 226 underwent OPN and 54 underwent MIPN (16 robot-assisted and 37 laparoscopic procedures). These cases comprised 57% and 46% of patients treated surgically for tumors >4–7 cm by the open or minimally invasive approach, respectively, during this period.
Patient demographics (gender, age), clinical characteristics (body mass index, American Society of Anesthesiologists [ASA] score, history of diabetes or hypertension), surgical characteristics (estimated blood loss, renal artery clamp time, transfusion rates, use of renal hypothermia, conversion of laparoscopic procedure to open surgery and from PN to RN), postsurgical complications (up to 30 d after surgery), pathologic characteristics, oncologic (recurrence rates and cancer-specific mortality), and renal function outcomes (estimated glomerular filtration rate [eGFR]) were compared for each technique. All perioperative management and follow-up was uniform using a common clinical pathway. Complication data were prospectively collected and retrospectively audited using the modified Clavien scale [22,23]. Urine leak was defined as a persistent drainage of urine for >7 d postoperatively or percutaneous drainage of an urinoma [22]. The eGFR was calculated with the Chronic Kidney Disease Epidemiology Collaboration equation [24]. Chronic kidney disease was diagnosed according to the National Kidney Foundation Dialysis Outcomes Quality Initiative Clinical Practice Guidelines [25].
2.1. Surgical technique
Standard laparoscopic, robot-assisted, and open PN procedures were performed. The laparoscopic approach included modified flank position and a three- to four-port method of transperitoneal nephrectomy. The robotic approach was completed via transperitoneal or retroperitoneal access using four to five ports (camera port, working arm ports [with a third arm if required for exposure], and assistant port). Open procedures included a variety of incisions between the 10th or 11th interspace, with most of them mini-flank incisions at the 11th rib [26,27]. Vascular control was obtained with a bulldog clamp except in selected patients who did not undergo renal artery occlusion. Cold ischemia, when utilized, was achieved with the described techniques of ice slush in the open procedures and by cold intravascular perfusion for minimally invasive procedures [28]. Reconstruction of collecting system defects was completed with interrupted sutures, and parenchymal hemostasis was achieved with absorbable hemostatic agents in bolster configuration and matrix injection [29].
2.2. Statistical analysis
The baseline characteristics of the OPN and MIPN groups were compared using the Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables. The Kaplan-Meier method was used to estimate survival probabilities, and a Cox proportional hazards regression model was used to adjust for preoperative eGFR. Disease recurrence was defined as any documented radiographic growing mass or pathologically proven failure in the operative site, regional lymph nodes, or distant metastasis. All statistics were performed using IBM SPSS Statistics v.19 (IBM Corp., Armonk, NY, USA).
3. Results
Median follow-up for OPN and MIPN was 29 and 13 mo, respectively (p < 0.001). Preoperative characteristics were comparable between types of procedure (Table 1), although patients in the MIPN group had statistically lower preoperative eGFR (OPN: 68 ml/min per 1.73 m2; MIPN: 61 ml/min per 1.73 m2; p = 0.01). Patients undergoing OPN under ischemia conditions had significantly longer renal artery clamp time (42 min vs 37 min; p = 0.006) and use of renal hypothermia (78% vs 7%; p = <0.001) compared with MIPN (Table 2). Those undergoing MIPN required longer median operative times (OPN: 159 min; MIPN: 242 min; p < 0.01), although median postoperative length of stay was shorter (OPN: 4 d; MIPN: 3 d; p = 0.007).
Table 1 –
Demographic and clinical characteristics of patients treated with partial nephrectomy for 4–7 cm renal cortical tumors
| OPN n = 226 |
% | MIPN n = 54 |
% | P | |
|---|---|---|---|---|---|
| Age, yr | |||||
| Median (IQR) | 61 (51–68) | - | 59(51–67) | - | 0.7 |
| Gender | |||||
| Male | 170 | 75 | 35 | 65 | 0.1 |
| ASA score | |||||
| 1 and 2 | 127 | 56 | 24 | 44 | 0.1 |
| 3 and 4 | 99 | 44 | 30 | 56 | |
| BMI, kg/m2, median (IQR) | 29 (26–33) | 31 (27–36) | 0.06 | ||
| Hypertension | 118 | 52 | 27 | 50 | 0.8 |
| Diabetes | 30 | 13 | 9 | 17 | 0.5 |
| Preoperative eGFR, ml/min per 1.73 m2, median (IQR) | 68 (55–78) | - | 61 (53–68) | - | 0.01 |
| Laterality (left) | 123 | 54 | 25 | 46 | 0.3 |
| Length of follow-up, mo, median (IQR) | 29 (14–53) | - | 13 (6–25) | - | - |
OPN = open partial nephrectomy; MIPN = minimally invasive partial nephrectomy; IQR = interquartile range; ASA = American Society of Anesthesiologists; BMI = body mass index; eGFR = estimated glomerular filtration rate.
Table 2 –
Perioperative characteristics of partial nephrectomies for 4–7 cm renal cortical tumors
| OPN | MIPN | P | |||
|---|---|---|---|---|---|
| n = 226 | % | n = 54 | % | ||
| Median estimated blood loss, ml, median (IQR) | 400 (200–600) | - | 300 (144–438) | - | 0.03 |
| Transfusion | 33 | 15 | 3 | 6 | 0.1 |
| Renal artery clamped | 184 | 81 | 50 | 93 | 0.06 |
| Renal artery clamp time, min, median (IQR) | 42 (35–50) | - | 37 (31–41) | - | 0.006 |
| Hypothermic ischemia | 177 | 78 | 4 | 7 | <0.001 |
| Conversion | |||||
| To open | - | - | 3 | 6 | |
| To radical | 27 | 12 | 4 | 7 | 0.5 |
| Length of stay, d, median (IQR) | 4 (3–5) | - | 3 (2–5) | - | 0.007 |
| 6-mo eGFR, ml/min per 1.73 m2, median (IQR) | 55 (43–65) | 56 (51–74) | 0.1 | ||
| 6-mo eGFR change in baseline, ml/min per 1.73 m2, median (IQR) | −10 (−18 to −0.8) | - | −5 (−13 to +4) | - | 0.5 |
OPN = open partial nephrectomy, MIPN = minimally invasive partial nephrectomy, IQR = interquartile range, eGFR = estimated glomerular filtration rate.
There was a statistically significant difference in median estimated blood loss in the OPN group (400 vs 300; p = 0.03). Notably, nine of the patients requiring transfusions in the OPN group did not undergo renal artery clamping. Of the remaining 22 OPN cases receiving blood, 3 were transfused intraoperatively and 19 postoperatively for low hemoglobin levels or symptoms. Three MIPN patients required blood transfusions. Two were intraoperative for bleeding and ultimately were converted to an RN after open exploration was unsuccessful in controlling hemostasis.
Renal function at 6 mo after surgery (Table 2) was not significantly different between the techniques. Median eGFR change from baseline at 6 mo postoperatively was −10 ml/min per 1.73 m2 and −5 ml/min per 1.73 m2 for the OPN and MIPN group, respectively (p = 0.5). Most of the patients had pT1b renal cell carcinomas (Table 3). Postoperative pain medication data (Table 4) revealed no significant difference in the use of narcotics (p = 0.4) or acetaminophen (p = 0.3) when comparing OPN and MIPN patients. However, OPN patients used more ibuprofen equivalents (p = 0.002). Three MIPNs were converted to open (6%) with two of these resulting in RN. A total of four conversions to RN (7%) occurred in the MIPN group (two open as just cited and two laparoscopic); 27 planned OPN cases (12%) were converted to RN. No renal units were subsequently lost or removed.
Table 3 –
Pathologic characteristics of patients who received a partial nephrectomy for 4–7 cm renal cortical tumors
| OPN | MIPN | P | |||
|---|---|---|---|---|---|
| n = 226 | % | n = 54 | % | ||
| Histology | |||||
| Clear cell | 125 | 55 | 34 | 63 | 0.5 |
| Papillary | 39 | 17 | 7 | 13 | - |
| Chromophobe | 31 | 14 | 8 | 14 | - |
| Unclassified | 8 | 4 | 1 | 2 | - |
| AML | 2 | 1 | 0 | - | - |
| Oncocytoma | 18 | 8 | 1 | 2 | - |
| Other | 3 | 1 | 3 | 6 | - |
| Tumor size, cm, median (IQR) | 5.0 (4.5–5.5) | - | 5.2 (4.5–5.9) | - | 0.4 |
| Positive margin | 11 | 5 | 2 | 4 | 0.5 |
| Stage | |||||
| pT1b | 183 | - | 47 | - | 0.4 |
| pT3a | 41 | - | 7 | - | - |
OPN = open partial nephrectomy; MIPN = minimally invasive partial nephrectomy; AML = angiomyolipoma; IQR = interquartile range.
Table 4 –
Postoperative pain medication use of patients who received a partial nephrectomy for a T1b renal cortical tumor
| OPN | MIPN | P | |
|---|---|---|---|
| Morphine equivalent, mg, mean ± SD | 379±637 | 343 ± 327 | 0.4 |
| Acetaminophen equivalent, mg, mean ± SD | 1297± 1056 | 2200±2111 | 0.3 |
| Ibuprofen equivalent, mg, mean ± SD | 1972± 1103 | 927± 711 | 0.002 |
OPN = open partial nephrectomy; MIPN = minimally invasive partial nephrectomy; SD = standard deviation.
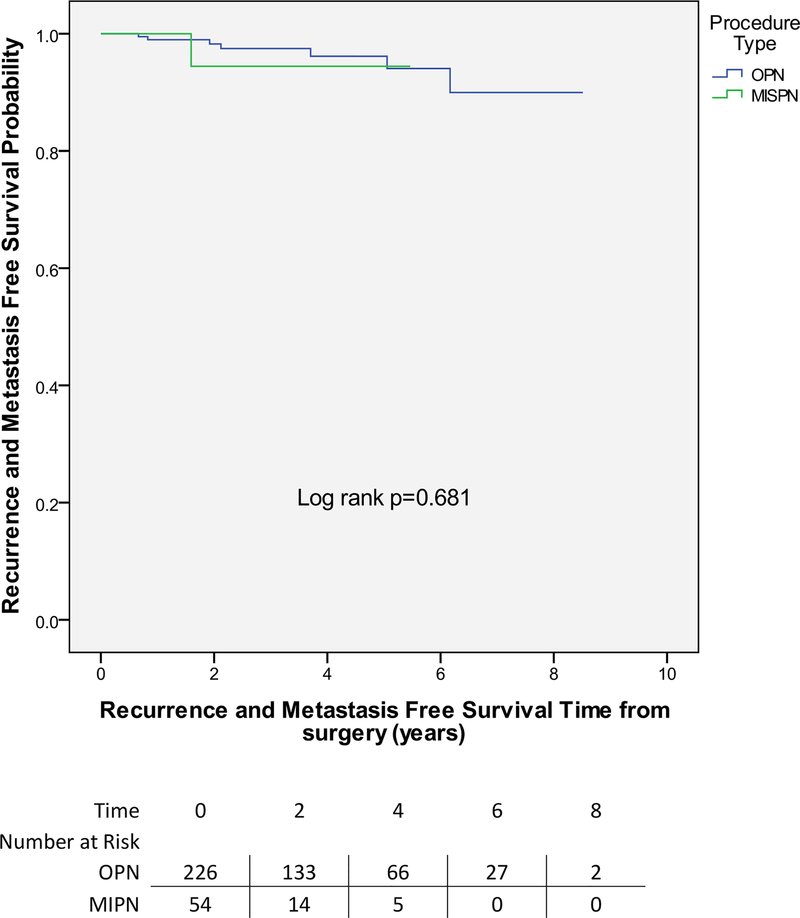
There were no cancer-specific deaths. Two local recurrences and five metastasis events occurred in the OPN group (median: 2 mo to event) (Fig. 1). Only one metastatic event was noted in the MIPN group. On univariate analysis, we did not find any evidence that procedure type was associated with recurrence-free survival (RFS) (log rank 0.7).
Fig. 1 – Recurrence-free survival comparing open partial nephrectomy versus minimally invasive partial nephrectomy.
MIPN = minimally invasive partial nephrectomy; OPN = open partial nephrectomy.
There was no statistically significant difference in the number of complications grade ≥3 (OPN 7%; MIPN 15%; p = 0.1) or occurrences of urine leak requiring intervention (OPN 4%; MIPN 4%; p = 0.7) (Table 5). In the MIPN group, there were more overall complications across all grades (33% vs 20%; p = 0.05). The positive margin rate was not significantly different between techniques (OPN: 5%; MIPN: 4%; p = 0.5).
Table 5 –
Postoperative complication differences
| OPN (%) | MIPN (%) |
OR | 95% CI | P | |
|---|---|---|---|---|---|
| Any complication | 46 (20) | 18 (33) | 2.0 | 1.0–3.8 | 0.05 |
| Grade ≥3 complications | 16 (7) | 8 (15) | 2.3 | 0.9–5.7 | 0.1 |
| Any urine leak | 25 (11) | 5 (9) | 0.8 | 0.3–2.3 | 0.8 |
| Urine leak requiring intervention | 10 (4) | 2 (4) | 0.4 | 0.1–3.3 | 0.7 |
OPN = open partial nephrectomy; MIPN = minimally invasive partial nephrectomy; OR = odds ratio; CI = confidence interval.
4. Discussion
Progress in surgical technique and an enhanced understanding the biology of renal masses has led to an increasing use of nephron-sparing surgical approaches [30] and minimally invasive techniques for larger [15,17] and more complicated renal masses [31]. The results of the present study suggests that MIPN and OPN surgical approaches for renal masses >4–7 cm are comparable, without a significant impact on critical outcomes including overall and cancer-specific survival (CSS), positive margin rates, rates of grade ≥3 complications, or rates of urine leak requiring intervention.
Our institutional management strategies for renal masses focus on approaches intended to account for tumor biology while optimizing functional renal preservation and local tumor control. Under these strategies, PN is currently considered and potentially executed for any renal mass whenever technically feasible, regardless of size. This is particularly true if the mass is exophytic or polar in location. The development of this experience, demonstrated graphically in Figure 2, has grown gradually, from <26% of cases in 2002 to 81% of both open and minimally invasive procedures by the end of 2009. Although technically more challenging in this setting, the data regarding outcomes presented here are similar to those previously reported for smaller renal masses treated surgically [21,22]. These outcomes likely represent the evolution of multiple learning curves related to patient management, including a more thorough preoperative assessment and hence more educated case selection, technical advances related to surgical technique and instrumentation, and cognitive growth related to an improved understanding of cancer biology and patient physiology. In combination, this gained experience appears to span both open and minimally invasive approaches, demonstrating that PN for larger tumors is feasible with results that may be independent of surgical approach.
Fig. 2 – Change in utilization of partial nephrectomy as a percentage of all surgery (radical and partial nephrectomy) for renal masses pathologically >4–7 cm in size (pT1b and pT3a), 2002–2009.
MIS = minimally invasive surgery.
The primary limitation of our study is its retrospective nature, with the inherent limitations of a single-center nonprospective nonrandomized series. It is particularly difficult to account for biases including case selection, case complexity, and surgical learning curve. The concept of controlling for case complexity with nephrometry scoring systems [32] is of interest, yet these systems await validation and are of limited use in homogeneous cohorts like ours: The patients evaluated in this study all had tumors >4–7 cm that were deemed amenable to PN.
There are inherent difficulties with any comparative study of open and minimally invasive techniques. The open surgical experience is certainly more mature, which makes a controlled comparison with the earliest experience with both laparoscopic and robotic procedures inherently difficult. However, the similar growth curves in adapting to PN procedures for these larger tumors from 4 to 7 cm is indicative of a broader institutional evolution in approach to renal masses. Despite these limitations, we see little in the way of differences in outcomes between the two approaches at our center. There has not been a shift in case volume away from open procedures over time, but instead there has been a parallel growth in minimally invasive procedures. A prospective multi-institutional randomized trial would be the best way to evaluate if surgical approach affects short- and long-term outcomes.
Oncologic outcomes are of primary concern in the surgical approach to renal tumors, both large and small. There were no cancer-related deaths in these patients, but our RFS rates were 94% in the MIPN group and 96% in the OPN group at 5-yr follow-up, which are consistent with rates seen in other series evaluating individual surgical techniques. Leibovich et al [14] reported a 5-yr CSS rate of 95% and 98% for patients with tumors measuring 4–7 cm who had undergone open PN and open RN, respectively. In a series comparing laparoscopic RNs and PNs for tumors ≥4 cm, Simmons et al [17] described a 97% CSS rate in both groups at a median follow-up of 57 mo and 44 mo, respectively. Becker et al [33] found overall survival to be 95% and CSS 100% at 5 yr for patients receiving OPN.
Positive margin rates for both the OPN and MIPN groups were 5% and 4%, respectively. In other published series, positive margin rates range from 1.3% [2] to 3.8% [34] for patients who underwent OPN for pT1b tumors, whereas positive margins for pT1b lesions in laparoscopic series range from 0% to 5.3% [15–17].The published robotic series for lesions >4 cm had no positive margins [35,36], which may to a certain extent reflect case selection (our robotic series also had no positive margins). In the final years of this series, we treated approximately 80% of tumors >4–7 cm with PN, indicating an ambitious nephron-sparing surgical program that includes deep tumors found within the renal hilum and central sinus, and may in part explain the slightly higher positive margin rates in this series.
A significantly longer renal artery clamp time was noted in the OPN compared with the MIPN group (42 min vs 37 min; p = 0.006) which differs from some of the published literature [37], although it is consistent with other series [18]. We believe a large contributing factor was the significant use of renal hypothermia (78% vs 7%; p = <0.001) in the OPN group compared with MIPN (Table 2). In the Cleveland Clinic experience [37], almost all PNs are done under warm ischemia, in which a shorter ischemia time has clearly been linked to improved renal functional outcomes [38]. In addition, the preoperative difference in eGFR between our two study groups was statistically significant, although the median 6-mo postoperative eGFR was within the range for stage 3 CKD in both groups. The degree of decrease related to surgery was similar to that in the published literature [39].
PN can be performed safely although with some increased risk of unique complications [22] compared with RN. Published data from patients with tumors ≥4 cm identify a 12% rate of surgical complications and a 5% rate of urinary fistula [2] for OPN; complication rates for laparoscopic series appear to range from 20% to 37% [16,17] with a urinary fistula rate of 7% [16]. This appears comparable with the one study of robot-assisted PN for tumors ≥4 cm that cites a urinary fistula rate of 5% [35]. Our standardized prospective institutional complication reporting system with use of detailed retrospective review criteria for adverse events previously demonstrated a higher rate of actual adverse event detection and reporting [40]. This may account for the higher observed rate of overall complications relative to other published reports, although less so our rate of grade ≥3 complications (OPN 7%, MIPN 15%) that compares with events in other large series [2].The association between complications and medical comorbidities is well documented, and the proportion of higher ASA scores in this series are notable, with scores >3 in 44% and 56% of OPN and MIPN cases, respectively, compared with 35% of those reported in other combined series [2].
5. Conclusions
With appropriate experience, PN for renal tumors >4–7 cm can be safely utilized in most cases by either open or minimally invasive surgery with comparable oncologic and functional outcomes regardless of choice of approach.
Take-home message.
Open and minimally invasive partial nephrectomy (PN) for tumors 4–7 cm in size appear to be comparable in terms of operative, functional, and convalescence outcomes. PN for larger renal lesions is an appropriate alternative to radical nephrectomy for many cases when performed by experienced surgeons, regardless of surgical approach.
Acknowledgments
Funding/Support and role of the sponsor: None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- [1].Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol 2000;163:442–5. [PubMed] [Google Scholar]
- [2].Patard JJ, Pantuck AJ, Crepel M, et al. Morbidity and clinical outcome of nephron-sparing surgery in relation to tumour size and indication. Eur Urol 2007;52:148–54. [DOI] [PubMed] [Google Scholar]
- [3].Mabjeesh NJ, Avidor Y, Matzkin H. Emerging nephron sparing treatments for kidney tumors: a continuum of modalities from energy ablation to laparoscopic partial nephrectomy. J Urol 2004;171:553–60. [DOI] [PubMed] [Google Scholar]
- [4].Lau WK, Blute ML, Weaver AL, Torres VE, Zincke H. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc 2000;75:1236–42. [DOI] [PubMed] [Google Scholar]
- [5].McKiernan J, Simmons R, Katz J, Russo P. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology 2002;59:816–20. [DOI] [PubMed] [Google Scholar]
- [6].Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 2006;7:735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305. [DOI] [PubMed] [Google Scholar]
- [8].Astor BC, Hallan SI, Miller ER III, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol 2008;167:1226–34. [DOI] [PubMed] [Google Scholar]
- [9].Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol 2009;182:1271–9. [DOI] [PubMed] [Google Scholar]
- [10].Ljungberg B, Cowan NC, Hanbury DC, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58:398–406. [DOI] [PubMed] [Google Scholar]
- [11].Weight CJ, Larson BT, Gao T, et al. Elective partial nephrectomy in patients with clinical T1b renal tumors is associated with improved overall survival. Urology 2010;76:631–7. [DOI] [PubMed] [Google Scholar]
- [12].Mitchell RE, Gilbert SM, Murphy AM, Olsson CA, Benson MC, McKiernan JM. Partial nephrectomy and radical nephrectomy offer similar cancer outcomes in renal cortical tumors 4 cm or larger. Urology 2006;67:260–4. [DOI] [PubMed] [Google Scholar]
- [13].Antonelli A, Cozzoli A, Nicolai M, et al. Nephron-sparing surgery versus radical nephrectomy in the treatment of intracapsular renal cell carcinoma up to 7 cm. Eur Urol 2008;53:803–9. [DOI] [PubMed] [Google Scholar]
- [14].Leibovich BC, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol 2004;171:1066–70. [DOI] [PubMed] [Google Scholar]
- [15].Deklaj T, Lifshitz DA, Shikanov SA, Katz MH, Zorn KC, Shalhav AL. Laparoscopic radical versus laparoscopic partial nephrectomy for clinical T1bN0M0 renal tumors: comparison of perioperative, pathological, and functional outcomes. J Endourol 2010;24:1603–7. [DOI] [PubMed] [Google Scholar]
- [16].Rais-Bahrami S, Romero FR, Lima GC, et al. Elective laparoscopic partial nephrectomy in patients with tumors >4 cm. Urology 2008;72:580–3. [DOI] [PubMed] [Google Scholar]
- [17].Simmons MN, Weight CJ, Gill IS. Laparoscopic radical versus partial nephrectomy for tumors >4 cm: intermediate-term oncologic and functional outcomes. Urology 2009;73:1077–82. [DOI] [PubMed] [Google Scholar]
- [18].Lane BR, Gill IS. 5-Year outcomes of laparoscopic partial nephrectomy. J Urol 2007;177:70–4; discussion 74. [DOI] [PubMed] [Google Scholar]
- [19].Permpongkosol S, Bagga HS, Romero FR, Sroka M, Jarrett TW, Kavoussi LR. Laparoscopic versus open partial nephrectomy for the treatment of pathological T1N0M0 renal cell carcinoma: a 5-year survival rate. J Urol 2006;176:1984–8; discussion 1988–9. [DOI] [PubMed] [Google Scholar]
- [20].Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg 2002;87:13–5. [PubMed] [Google Scholar]
- [21].Greene FLP, Page DL, Fleming ID, et al. AJCC cancer staging handbook, ed 6 New York, NY: Springer-Verlag; 2002. [Google Scholar]
- [22].Stephenson AJ, Hakimi AA, Snyder ME, Russo P. Complications of radical and partial nephrectomy in a large contemporary cohort. J Urol 2004;171:130–4. [DOI] [PubMed] [Google Scholar]
- [23].Nogueira L, Katz D, Pinochet R, et al. Critical evaluation of perioperative complications in laparoscopic partial nephrectomy. Urology 2010;75:288–94. [DOI] [PubMed] [Google Scholar]
- [24].Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–47. [DOI] [PubMed] [Google Scholar]
- [26].Diblasio CJ, Snyder ME, Russo P. Mini-flank supra-11th rib incision for open partial or radical nephrectomy. BJU Int 2006;97:149–56. [DOI] [PubMed] [Google Scholar]
- [27].Russo P Partial nephrectomy for renal cancer (part II): the impact of renal ischaemia, patient preparation, surgical approaches, management of complications and utilization. BJU Int 2010;105:1494–507. [DOI] [PubMed] [Google Scholar]
- [28].Marley CS, Siegrist T, Kurta J, et al. Cold intravascular organ perfusion for renal hypothermia during laparoscopic partial nephrectomy. J Urol 2011;185:2191–5. [DOI] [PubMed] [Google Scholar]
- [29].Nogueira L, Katz D, Pinochet R, Kurta JM, Coleman JA. Comparison of gelatine matrix-thrombin sealants used during laparoscopic partial nephrectomy. BJU Int 2008;102:1670–4. [DOI] [PubMed] [Google Scholar]
- [30].Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Wei JT. National utilization trends of partial nephrectomy for renal cell carcinoma: a case of underutilization? Urology 2006;67:254–9. [DOI] [PubMed] [Google Scholar]
- [31].Dulabon LM, Kaouk JH, Haber GP, et al. Multi-institutional analysis of robotic partial nephrectomy for hilar versus nonhilar lesions in 446 consecutive cases. Eur Urol 2011;59:325–30. [DOI] [PubMed] [Google Scholar]
- [32].Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009;182:844–53. [DOI] [PubMed] [Google Scholar]
- [33].Becker F, Siemer S, Hack M, Humke U, Ziegler M, Stockle M. Excellent long-term cancer control with elective nephron-sparing surgery for selected renal cell carcinomas measuring more than 4 cm. Eur Urol 2006;49:1058–63; discussion 1063–4. [DOI] [PubMed] [Google Scholar]
- [34].Yossepowitch O, Thompson RH, Leibovich BC, et al. Positive surgical margins at partial nephrectomy: predictors and oncological outcomes. J Urol 2008;179:2158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gupta GN, Boris R, Chung P, Marston Linehan W, Pinto PA, Bratslavsky G. Robot-assisted laparoscopic partial nephrectomy for tumors greater than 4 cm and high nephrometry score: feasibility, renal functional, and oncological outcomes with minimum 1 year follow-up. Urol Oncol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Patel MN, Krane LS, Bhandari A, et al. Robotic partial nephrectomy for renal tumors larger than 4 cm. Eur Urol 2010;57:310–6. [DOI] [PubMed] [Google Scholar]
- [37].Gill IS, Matin SF, Desai MM, et al. Comparative analysis of laparoscopic versus open partial nephrectomy for renal tumors in 200 patients. J Urol 2003;170:64–8. [DOI] [PubMed] [Google Scholar]
- [38].Thompson RH, Lane BR, Lohse CM, et al. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol 2010;58:340–5. [DOI] [PubMed] [Google Scholar]
- [39].Adamy A, Favaretto RL, Nogueira L, et al. Recovery of renal function after open and laparoscopic partial nephrectomy. Eur Urol 2010;58:596–601. [DOI] [PubMed] [Google Scholar]
- [40].Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol 2009;55:164–74. [DOI] [PubMed] [Google Scholar]