Abstract
Chronic stress is often associated with a dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, which can greatly increase risk for a number of stress-related diseases, including neuropsychiatric disorders. Despite a striking sex-bias in the prevalence of many of these disorders, few preclinical studies have examined female subjects. Hence, the present study aimed to explore the effects of chronic stress on the basal and acute stress-induced activity of the HPA axis in the female C57BL/6 mouse. We used a chronic variable stress (CVS) paradigm in these studies, which successfully induces physiological and behavioral changes that are similar to those reported for some patients with mood disorders. Using this model, we found pronounced, time-dependent effects of chronic stress on the HPA axis. CVS-treated females exhibited adrenal hypertrophy, yet their pattern of glucocorticoid secretion in the morning resembled that of controls. CVS-treated and control females had similar morning basal corticosterone (CORT) levels, which were both significantly elevated following a restraint stressor. Although morning basal gene expression of the key HPA-controlling neuropeptides corticotropin releasing hormone (CRH), arginine vasopressin (AVP) and oxytocin (OT) was unaltered within the paraventricular nucleus (PVN) by CVS, CVS altered the PVN OT and AVP mRNA responses to acute restraint. In control females, acute stress decreased AVP, but not OT mRNA; whereas, in CVS females, it decreased OT, but not, AVP mRNA. Unlike the morning pattern of HPA activity, in the evening, CVS-treated females showed increased basal CORT with hypoactive responses of CORT and PVN c-Fos immunoreactivity to restraint stress. Furthermore, CVS elevated evening PVN CRH and OT mRNAs in the PVN, but it did not influence anxiety- or depressive-like behavior after a light/dark box or tail suspension test. Taken together, these findings indicate that CVS is an effective model for HPA axis dysregulation in the female mouse and may be relevant for stress-related diseases.
Keywords: chronic variable stress, corticotropin releasing hormone (CRH), corticosterone, oxytocin, vasopressin (AVP), female
1. Introduction
The allostatic load hypothesis posits that an organism’s repeated efforts to maintain homeostasis in response to chronic exposure to stressors induce a state of dysregulation referred to as an “allostatic state,” which can have important health consequences. One common consequence is increased susceptibility to mood disorders such as anxiety and depression [1]. Indeed, chronic interpersonal stress increases the risk of developing depression [2], and stressful and negative life events are associated with adverse symptom trajectories for individuals with anxiety or depression [3]. Many of these disorders are more prevalent in women relative to men, even after controlling for social and cultural effects [4, 5]. Despite this sex-bias, the vast majority of preclinical research modeling mood disorders has focused exclusively on male subjects [6], leaving a critical need for further investigation of sex-specific effects within mood disorder models.
The allostatic load hypothesis has been modeled using a chronic variable stress (CVS) paradigm, which exposes rodents to a variety of mild stressors applied in an unpredictable manner over a prolonged period of time [7]. This model reliably induces physiological and behavioral changes that are similar to those in patients with mood disorders, and shows high predictive validity in responsiveness to antidepressant medication [8]. CVS has also been found to reproduce the hyperactivation of the hypothalamic-pituitary-adrenal (HPA) axis observed in some patients with mood disorders [9], as indicated by upregulated corticotropin releasing hormone (CRH) and arginine vasopressin (AVP) mRNAs within the paraventricular nucleus (PVN) of the hypothalamus coupled with elevated plasma corticosterone (CORT) levels [10–12]. Although the source of altered HPA axis function following CVS is not well-characterized, the hormone oxytocin (OT) is an attractive target for alteration following chronic stress exposure. In addition to this neuropeptide’s mediation of HPA axis function [13], OT mRNA has been shown to be elevated in patients with mood disorders [14]. It has also been reported to be increased [15] or decreased [16] by CVS in the male rat.
Some evidence suggests that sex may influence the effects of CVS on both HPA axis function and affective behavior in the rat [17, 18], but the consequences of CVS exposure for the female mouse are less understood. Thus, the present set of experiments sought to investigate the impact of CVS on female HPA axis function in the C57BL/6 mouse. These studies aimed to first identify consequences of CVS on basal and stress-induced activity of the HPA axis. Because of known circadian rhythms in the activity of the HPA axis, we examined HPA function in both the morning and evening [19–24]. Secondly, these studies sought to characterize the effects of CVS on neuropeptide expression and anxiety- and depressive-like behavior in the female mouse.
2. Methods
2.1. Subjects
Two-month-old female C57BL/6 mice were purchased from Charles Rivers Laboratories (Wilmington, MA) and maintained on a 12:12 light cycle (lights on at 0600 h) in the Colorado State University Laboratory Animal Research facility. Subjects were pair-housed in one of two colony rooms and access to food and water was available ad libitum. All animal protocols were approved by the Colorado State University Institutional Animal Care and Use Committee and were performed in accordance with the guidelines of Colorado State University, the National Institutes of Health, and the Association for Assessment and Accreditation of Laboratory Animal Care International.
To control for potential effects of changing gonadal hormone levels across the estrous cycle, all female subjects were examined outside of the proestrous phase of the cycle when estradiol levels are highest [25]. Estrous cyclicity was monitored for approximately two weeks prior to and on the day of sacrifice by daily vaginal lavage (collected between 0900–1100 h) using a 0.9% saline solution. Samples were stained with methylene blue (0.05%) and visualized using light microscopy as previously reported [26].
2.2. Experiment 1. Effect of CVS on the morning HPA axis response to a novel, acute stressor
Adult female mice were exposed to CVS daily, over a six-week period, as previously described [27]. Briefly, subjects experienced an average of two stressors per day, administered at variable time points. Stressors included three hours of occupying a cage with damp bedding, no bedding, or bedding soiled by same-sex unfamiliar mice, three hours of cage tilt (approximately 45°), one hour of exposure to cat odor, eight hours of white noise (85 dB) and overnight exposure to overhead light. Control subjects were housed in a separate colony room to prevent unintended stress exposure. For more details on the protocol used for CVS, see Tables S1 and S2.
After six weeks of CVS, CVS-treated (n=9) and control (n=9) subjects were exposed to 20 minutes of restraint stress in closed, ventilated conical tubes inside their home cages between 0900 and 1100 h or were left unperturbed. Subjects were anesthetized with isoflurane, weighed, and decapitated immediately following the restraint stress. Nonstressed animals were killed within one minute of first disturbance of their home cage. Upon decapitation, trunk blood was collected from all animals into chilled tubes containing 0.5 M ethylenediaminetetraacetic acid and aprotinin (4 mg/ml; Sigma-Aldrich, St. Louis, MO) and centrifuged at 3000 rpm for 12 minutes in a Beckman J6 centrifuge. Plasma was isolated and stored at −20°C until assayed for CORT and OT by radioimmunoassay (RIA). Brains and adrenal glands were also collected shortly following decapitation, flash-frozen and stored at −80°C.
2.3. Experiment 2. Effect of CVS on the evening HPA axis response to an acute stressor
Adult female mice were exposed to CVS or control conditions daily, over a six-week period as in Experiment 1. However, in Experiment 2, the stressors presented twice-daily included 30 minutes of restraint stress in addition to those listed above (Tables S1 and S2). CVS-treated (n=8) and control (n=6–7) subjects were exposed to 20 minutes of restraint stress in closed, ventilated conical tubes in their home cages or were left undisturbed in their home cages approximately one hour prior to dark cycle onset (1700 h). Trunk blood was collected immediately after the 20-minute restraint stressor, and plasma was isolated and stored until it was assayed for CORT by RIA as in Experiment 1.
A separate cohort of CVS-treated (n=8) and control (n=8) subjects were examined for stressor-induced neuronal activation in the PVN. Subjects were exposed to 20 minutes of restraint stress within closed, ventilated conical tubes in their home cages at 1700 h (lights off at 1800 h). Animals were released back into their home cages until approximately 90 minutes after onset of the restraint stress, at which point they were anesthetized with isofluorane and transcardially perfused with 0.1M phosphate buffered saline followed by 4% paraformaldehyde for measurement of c-Fos.
2.4. Experiment 3. Effect of CVS on anxiety- and depressive-like behavior and on PVN neuropeptide gene expression
As in Experiment 2, female mice were exposed to a six-week CVS protocol. At the end of six weeks of CVS, CVS-treated (n=8) and control (n=7) subjects were habituated to a behavioral testing room in the afternoon (1400–1600h) for 30 minutes prior to a light/dark box test. The apparatus (40 cm x 40 cm) consisted of two chambers separated by a barrier with a door permitting entry between chambers. The light chamber was illuminated to 2000 lm, while the dark chamber was measured at approximately 10 lm. Each subject was placed in the light chamber facing the doorway, and behavior was video recorded (Bunker Hill Security) for five minutes. Videos were scored by an experimenter blind to treatment condition. All testing occurred between 1400–1600 h. The apparatus was sanitized before and after each subject using a 70% ethanol solution.
A separate group of CVS-treated (n=11) and control (n=7) subjects were habituated to a behavioral testing room for 30 minutes prior to a tail suspension test. Each subject was suspended from a horizontal bar for ten minutes by its tail from a height of 12 inches with a section of tape while behavior was video recorded. All testing was conducted between 1400–1600 h. Given the tendency of C57BL/6 mice to climb their tails during this test [20], climbing was prevented by slipping a short segment from a plastic straw over each subject’s tail prior to suspension. The tail suspension apparatus was sanitized before and after each subject using a 70% ethanol solution. Duration of immobility was subsequently scored from video footage by an experimenter that was blind to treatment group.
Two hours after testing in the light/dark box or tail suspension test, CVS-treated (n=6) and control (n=6) subjects were anesthetized with isoflurane and decapitated. Brains were flash-frozen in 2-methylbutane (−40°C) and stored at −80°C. Brains were used for droplet digital PCR (ddPCR) to examine neuropeptide gene expression in the PVN.
2.5. Radioimmunoassays (RIAs)
Plasma CORT was measured as previously described [21]. Briefly, plasma was diluted (1:25) in PBS and plasma binding proteins were denatured by heating to 65°C for one hour. Diluted plasma samples were incubated overnight at 4°C with rabbit anti-CORT antiserum (1:1200, MP Biomedicals, Sonon OH) and 3H-CORT (PerkinElmer, Boston, MA) in 0.01 M PBS containing 0.1% gelatin. Dextran coated charcoal was used to separate antibody bound CORT from free CORT. Standard curves were constructed from dilutions of CORT (4-pregnen-11β, 21-diol-3, 20-dione; Steraloids, Wilton, NH; 5–500 ng/ml). For all assays, the intra-assay coefficient of variation was less than 10%.
To measure circulating OT levels, plasma was subjected to an extraction protocol that was adapted from a previous report [28] using methanol. Briefly, 2.0 ml methanol was added to all samples and, following vortex and centrifugation, the supernatant was further subjected to cold precipitation (−20°C) for 24 hours. After three rounds of cold precipitation, the supernatant was dried under nitrogen at 45°C and resuspended in RIA buffer to the same volume as the starting amount. This product was then used in the protocol for an OT RIA kit (RK-051–01; Phoenix Pharmaceuticals, Burlingame, CA) following manufacturer’s instructions. The extraction procedure resulted in the recovery of 69.1% of the starting material as determined by spiking samples with known amounts of 125I-oxytocin. The intra-assay coefficient of variation was 4.5%.
2.6. In situ hybridization
16-μm brain sections were cut in the coronal plane using a CM3050 S cryostat (Leica, Wetzlar). Sections containing the PVN were cut at −20°C, thaw-mounted onto positively charged slides (Superfrost Plus, VWR Scientific, West Chester, PA), and stored at −80°C. In situ hybridization was performed as previously described [27]. Briefly, tissue was thawed to room temperature, fixed, acetylated, delipidated, dehydrated in graded ethanols and air - dried. 48-bp oligonucleotide mRNA probes for Crh (5’CAGTTTCCTGTTGCTGTGAGCTTGCTGAGCTAACTGCTCTGCCCGGGC-3’), Ot (5’-AAGCAGGCAGCAAGCGAGACTGGGGCAGGCCATGGCG ATGGTGCTCAG-3’) and Avp (5’GTAGACCCGGGGCTTGGCAGAATCCACGGAC TCCCGTGTCCCAGCCAG-3’) were end-labeled with [35S] using terminal deoxynucleotidyl transferase (Thermo Scientific, Waltham, MA) and added to hybridization solution at a concentration of 20 × 106 cpm/mL. Brain sections were incubated in this hybridization solution at 37°C overnight and then washed and dehydrated in a series of solutions with increasing levels of ethanol. To examine hybridization intensity, slides were exposed to X-ray film (Carestream Kodak Biomax MR, Carestream, Rochester, NY) for 4 days (Crh), 1 day (Avp), or 14 hours (Ot) to generate autoradiograms. Film autoradiograms were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD) to quantify optical density in 4–6 PVN containing tissue sections. The density of exposed pixels in each half of the PVN for all sections was measured using a template of fixed size and expressed as arbitrary density units (ADUs). Background density in an adjacent area without labeling was subtracted from each measurement and resulting ADUs were averaged to obtain a single value per animal for statistical analysis. All density calculations were performed by an experimenter blind to treatment condition.
2.7. Droplet digital PCR (ddPCR)
Flash-frozen brains were sectioned by cryostat (Leica CM3050 S, Leica Biosystems) at 300 μm, and the PVN was isolated via micropunch with a 0.98 mm diameter cannula. RNA extraction of PVN tissue was performed using an RNeasy mini kit (Qiagen, Germantown, MD) according to the manufacturer’s instructions. Total RNA was quantitated using an Epoch Microplate Spectrophotometer and Gen5 v. 1.11 data analysis software (BioTek, Winooski, VT). cDNA was generated using an iScript cDNA synthesis kit (Bio-Rad, Munich, Germany). For each ddPCR reaction, 5 uL of template DNA was added to a master mix containing primers for CRH (F: 5’-ATGCTGCTGGTG GCTCTGTC; R: 5’ GGATCAGAACCGGCTGAGGT-3’) or OT (F: 5’-AAGGGAGCTGCAGTGGAGTA-3’; R: 5’-AGACTGGCAGGGCGAAG-3’), Evagreen (Bio-Rad, Munich, Germany) and RNAse free water. 20 uL of sample and 70 uL of droplet generation oil (Bio-Rad, Munich, Germany) were pipetted into DG8 Cartridge (Bio-Rad, Munich, Germany) wells and secured with a gasket. Droplets were then generated in a QX200 droplet generator (Bio-Rad, Munich, Germany), transferred to a 96-well plate, and heat-sealed. PCR was performed in a thermal cycler (C1000 Touch, Bio-Rad, Munich, Germany) using the following protocol: 95°C for 10 minutes (1 cycle), 95°C for 30 seconds then 60°C for 1 minute (40 cycles), 4°C for 5 minutes (1 cycle), 90°C for 5 minutes (1 cycle), and hold at 4°C. The ramp rate was set at 2°C/second, the sample volume at 40 µl, and the heated lid at 105°C. After PCR amplification, droplets were analyzed in a QX200 droplet reader (Bio-Rad, Munich, Germany), and the absolute template expression in copies/ul input was quantified using QuantaSoft software (Bio-Rad, Munich, Germany). All values were normalized to the amount of cDNA loaded in each reaction, which was calculated based on cDNA concentrations quantified using the Quant-iT OliGreen ssDNA Assay Kit (Thermo Scientific, Waltham, MA) according to manufacturer’s instructions.
2.8. Immunohistochemistry
Brains from perfused animals in Experiment 2 were collected and post-fixed for 24 hours in 4% paraformaldehyde at 4°C, then infiltrated with 30% sucrose at 4°C until equilibrated. Immunohistochemistry was performed on the fixed brain tissue to examine changes in PVN neuronal activation. Fixed brains were sectioned into four series of 35 μm thickness on a cryostat at −16°C, then immunohistochemistry was performed on a series of free-floating sections. Tissue was washed in 0.1 M PBS, blocked in 5% normal donkey serum (Jackson Laboratories, Bar Harbor, ME) for one hour at room temperature, then incubated overnight in a previously validated goat anti-c-Fos primary antibody (1:2000; Santa Cruz Biotechnology, Dallas TX) [29]. The next day, tissue was washed in a PBS-Triton X solution, then incubated in donkey anti-goat Alexa Fluor (AF) 568 (Jackson Laboratories, Bar Harbor, ME) for two hours at room temperature. After washing in PBS-Triton X, tissue was mounted onto glass slides and coverslipped with ProLong Gold Antifade Mountant (Thermo Fisher). Fluorescence was visualized using an LSM 880 confocal scanning microscope (Carl Zeiss, Jena, Germany) and 10X objective (Zeiss Plan-Apochromat 10X/0.45∞/0.17), and 25 um-thick Z-stacks (1 um thick optical sections) were taken through the PVN.
Immunohistochemical analysis of Z-stack images was conducted using Imaris v8.0 (Bitplane, Concord, MA). For each subject, 2–3 images per hemisphere were captured for the PVN. For each image, the number of c-Fos-immunoreactivity (ir) expressing neurons was determined using the automated counting application in Imaris v8.0 and manually checked and scored by an investigator that was blind to treatment condition. Values from all images were averaged together for each subject.
2.9. Statistical Analysis
All statistical analyses were performed using the Prism statistical program (GraphPad Software, La Jolla, CA). The effects of CVS treatment on adrenal weights, evening OT mRNA levels, c-Fos-ir expressing cell numbers and affective behaviors were examined using Student’s t-tests. For evening CRH mRNA levels, effects of CVS were assessed with Welch’s t-test. Plasma CORT levels and morning mRNA expression levels were analyzed by two-way (CVS treatment X restraint stress) ANOVAs. All post hoc analyses were performed with the Fisher’s Least Significant Difference test. Significance was set at p<0.05.
3. Results
3.1. Experiment 1. Effect of CVS on the morning HPA axis response to a novel, acute stressor
To initially assess the influence of CVS on the activity of the HPA axis, we compared weights of adrenal glands collected from CVS-treated versus control subjects. Adrenal weight was significantly increased following CVS when expressed as percentage of body weight [p<0.001] (Fig. 1).
Figure 1.
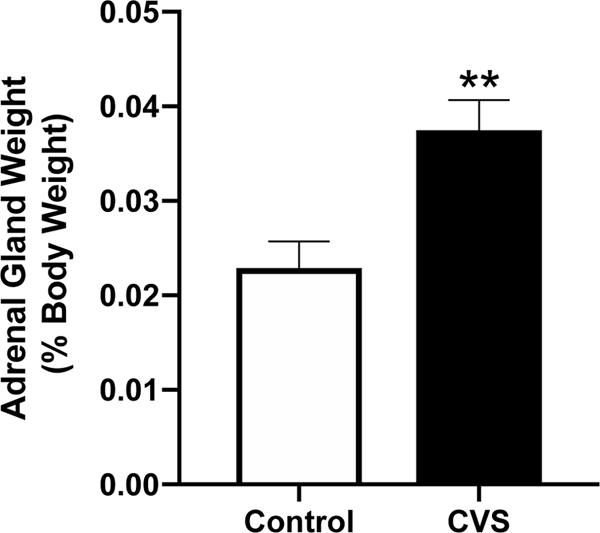
Adrenal weights (% of body weight) of intact female mice exposed to six weeks of chronic variable stress (CVS) and control subjects. Each bar represents the mean ± SEM of n=14 /group. **=p<0.001.
We further examined morning plasma CORT levels immediately following a novel, 20-minute restraint stressor versus basal, non-stress levels in CVS- and control-treated subjects. Regardless of previous exposure to CVS, restraint stress significantly elevated plasma CORT levels. Accordingly, a two-way (CVS x restraint stress) ANOVA showed a significant effect of restraint stress [F(1,32)=641.5; p<0.0001], but not a CVS or interaction effect (Fig. 2).
Figure 2.
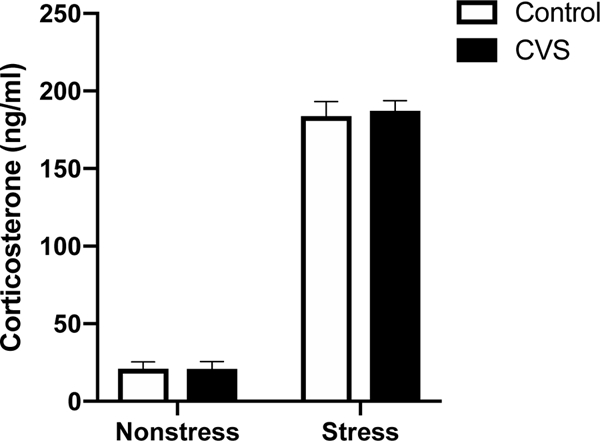
Morning (0900–1100 h) plasma corticosterone levels in control or chronic variable stress (CVS)-treated female mice collected at baseline (nonstress) or 20 minutes after initiation of restraint stress. Each bar represents the mean ± SEM of n=9/group.
Morning neuropeptide expression in the PVN was significantly altered by restraint stress, but in a neuropeptide-specific manner. Two-way ANOVAs revealed that there was no acute stressor effect on CRH (Fig. 3a) mRNA levels, whereas there was an effect of restraint stress to decrease AVP [F(1,32)=4.754; p<0.05; Fig. 3b] and OT [F(1,30)=11.14; p<0.01; Fig. 3c] mRNA levels in both CVS-treated and control females. Because we originally hypothesized that CVS would alter the HPA axis response to an acute, novel stressor, we performed post hoc analyses to examine the effect of restraint stress within CVS and control groups. Acute restraint stress significantly decreased OT mRNA in CVS (p<0.01) but not control females (p=0.2139). In contrast, restraint stress decreased AVP mRNA in control (p<0.05) but not CVS females (p=0.3602).
Figure 3.
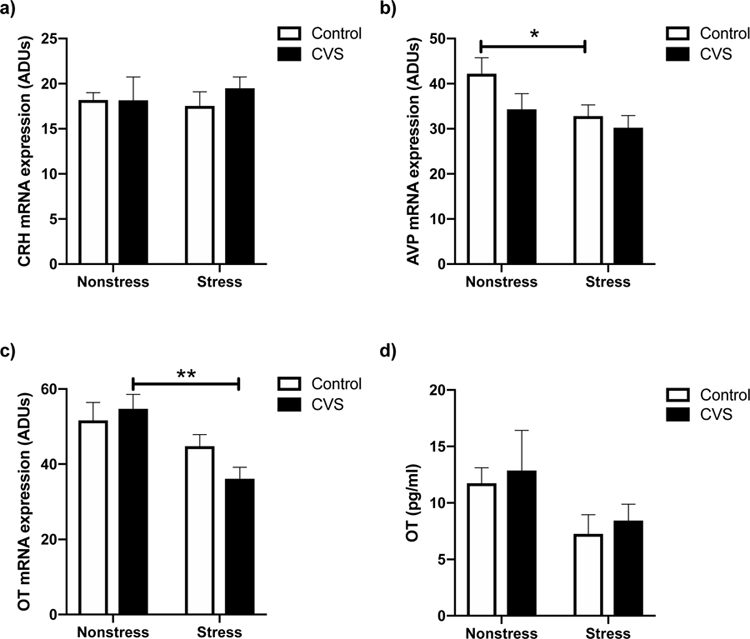
Morning (0900–1100 h) baseline (nonstress) and 20 minute restraint stress-induced levels of corticotropin releasing hormone (CRH) mRNA (a), arginine vassopressin (AVP) mRNA (b), and oxytocin (OT) mRNA (c) within the paraventricular nucleus (PVN) of female mice exposed to six weeks of chronic variable stress (CVS) versus control subjects. Panel d shows morning basal and restaint induced plasma OT levels in control and CVS females. Each bar represents the mean ± SEM of n=6–9 /group. *=p<0.05, and **=p<0.01 versus the nonstressed group of the same treatment. ADUs= Arbitrary density units.
To determine if the effects of acute restraint stress on PVN Ot gene expression in CVS-and control-treated subjects aligned with changes in circulating OT protein, plasma OT levels were also examined. Two-way ANOVA revealed a trend for restraint to decrease plasma OT in both CVS- and control-treated animals [F(1,31)=3.987; p=0.0547] (Fig. 3d).
3.2. Experiment 2. Effect of CVS on the evening HPA axis response to an acute stressor
To examine evening HPA axis activity in CVS-treated versus control subjects, we first assessed plasma CORT levels at 1700 h after 20 minutes of restraint stress compared to basal values. A two-way ANOVA showed a significant CVS by restraint stress interaction [F(1,25)=11.72; p<0.01] (Fig. 4). Post hoc contrasts revealed that CVS-treated females had elevated nonstress CORT levels (p<0.05) and decreased stress-induced CORT levels (p<0.05) compared to those of control females. Moreover, whereas there was a trend for restraint stress to increase CORT in control females (p=0.0833), CVS females exhibited a significant drop in plasma CORT in response to acute restraint (p<0.01).
Figure 4.
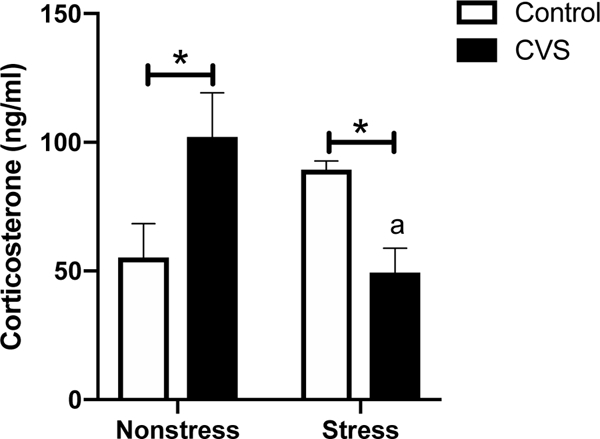
Evening (1700 h) plasma corticosterone levels after 20 minutes of restraint stress compared to nonstress values in chronic variable stress (CVS) and control females. Each bar represents the mean ± SEM of n=6–8 p/group. *=p<0.05 for control versus CVS females within each treatment group. a=p<0.01 for CVS nonstress verus CVS stress females.
We next sought to investigate changes in the PVN that may contribute to the CVS-dependent effects of acute stress on evening plasma CORT levels. In the evening, CVS significantly reduced PVN neuronal activation, as measured by c-Fos-ir, 90 minutes after onset of the 20 minute restraint stressor [p<0.0001] (Fig. 5).
Figure 5.
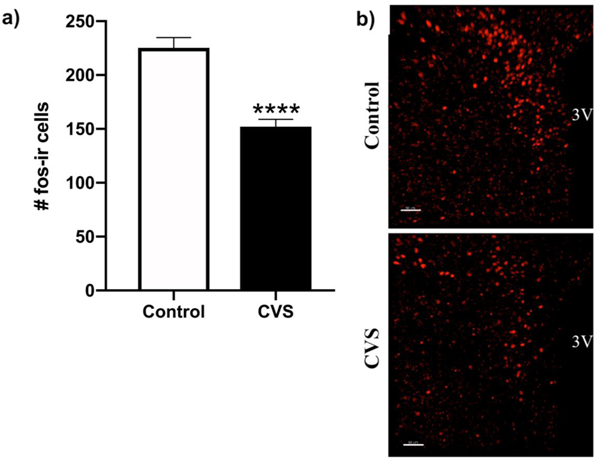
Evening c-Fos immunoreactivity (fos-ir) after acute restraint stress starting at 1700 h is reduced in the paraventricular nucleus (PVN) of female chronic variable stress (CVS)-treated subjects. a) Cells counts represent a single hemisphere of the PVN, averaged across 3–6 images encompassing the anterior and mid subregions. Each bar represents the mean ± SEM of 8 subjects per group. b) Photomicrographs show representative examples of fos-ir neurons in the PVN. 3V= 3rd ventricle. Calibration bar = 50 nm. ****= p<0.0001.
3.3. Experiment 3. Effects of CVS on anxiety- and depressive-like behavior and on PVN neuropeptide gene expression
Behaviors in the light/dark box and the tail suspension test were analyzed to assess the effect of CVS exposure on anxiety- and depressive-like behavior, respectively. For the light/dark box, no differences in time spent in the light compartment of the box (Control: M=116.37±18.67; CVS: M=96.17±10.17), entries into the light compartment (Control: M=6.67±1.05; CVS: M=7.67±0.60), or initial latency to enter the light compartment (Control: M=47.37±4.96; CVS: M=50.94±10.99) were observed. In addition, no differences in the percent duration of immobility (Control: M=47.37±4.96; CVS: M=56.59±3.91) were seen in the tail suspension test.
Although no effects of CVS were observed on behaviors in the light/dark box or the tail suspension test, CVS altered neuropeptide gene expression within the PVN. Two hours after behavioral testing, there was a trend for CVS to increase CRH mRNA [Control: M=5.48±1.40; CVS: M=35.63±13.48; p=0.0969], and OT mRNA was significantly elevated by CVS [p<0.05; Fig. 6].
Figure 6.
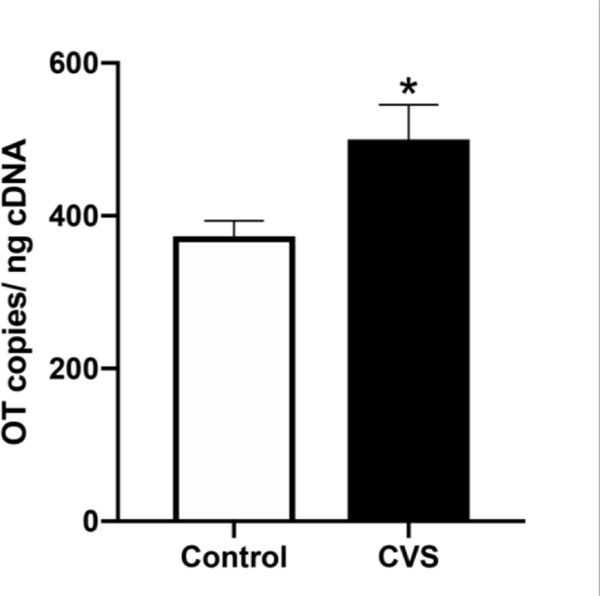
Evening (1600–1800 h) levels of oxytocin (OT) mRNA within the paraventricular nucleus (PVN) of female mice exposed to six weeks of chronic variable stress (CVS) and control subjects who all underwent testing for anxiety- and depressive-like behavior. Each bar represents the mean ± SEM of n=6 /group. *=p<0.05.
4. Discussion
CVS is an established rodent model for studying affective disorders, inducing behavioral and physiological changes in the male rodent that parallel depressive and anxiety disorders in humans [8, 30, 31]. As observed in some patients with mood disorders, CVS induces alterations in HPA axis activity indicative of hyperactivation or dysregulation [9–11, 16, 18]. Despite the considerable literature on CVS effects in the male rodent, very few studies to date have investigated the consequences of CVS in females, and fewer still have examined effects in the female mouse. In the present studies we sought to characterize the effects of CVS exposure on HPA axis function in female C57BL/6 mice. The results of these studies indicate that CVS effectively induces physiological but not behavioral alterations in the female C57BL/6 mouse. CVS-treated female mice ultimately appear to show time-dependent dysregulation of the HPA axis. In the morning, females exhibited similar levels of basal CORT, which were significantly elevated by a novel, acute stressor, irrespective of CVS exposure and its effect to increase adrenal weight. Whereas acute restraint stress revealed effects of CVS on Ot and Avp gene expression, morning basal OT, AVP, and CRH mRNAs were unaltered by CVS. Alternatively, in the evening, CVS-treated females showed increased basal CORT and a hypoactive CORT response to stress, as well as decreased stress-induced neuronal activation in the PVN. Elevations in evening PVN CRH and OT mRNAs were also observed after CVS and behavioral testing. Thus, CVS may be a potent model for dysregulation of the HPA axis in the female mouse.
4.1. CVS and the morning activity of the HPA axis
Adrenal hypertrophy is a consequence of CVS commonly observed in male rodents [32, 33]. While a recent study by Dadomo et al. (2018) found no effect of a more severe CVS schedule on adrenal weight in female CD1 mice [34], our subjects demonstrated significant adrenal hypertrophy at the end of the study, as indicated by increased adrenal weight. Elevated adrenal weight following CVS has been linked to subregion-specific increases in cell number and size in the male rat [32]. These structural changes could impart enhanced responsivity of the adrenal gland to adrenocorticotrophic hormone (ACTH), thereby increasing glucocorticoid release.
Despite the increase in adrenal weight observed following CVS in female mice, we did not detect an effect of CVS on morning basal or acute stress-induced glucocorticoid levels. Regardless of previous CVS exposure, female mice exhibited similar baseline levels of plasma CORT, which were significantly increased following 20 minutes of novel restraint stress. These results are well aligned with those of another study demonstrating that CVS and control females had similar elevations in plasma CORT after a novel forced swim stress [35]. Notably, our findings oppose those of previous studies conducted in the CVS-treated female rat [10, 36], and, more recently, in the female mouse [34], showing elevations in basal plasma CORT levels. This discrepancy could be due to a number of factors, including varying durations of CVS exposure or the varying stressors incorporated. Additionally, subjects in the present study were exposed to isoflurane anesthesia immediately prior to collection of plasma. Isoflurane has been reported to affect CORT levels in male and female rats [37], although C57Bl/6 mice may not be as sensitive [38]. Nonetheless, our findings suggest that CVS may not have a strong influence on the morning activity of the HPA axis in female mice, as has been shown previously in male mice [33, 39–41].
Contrary to the findings of numerous CVS studies in male rodents [16, 33], PVN Crh expression at both basal and acute stress-generated levels was unaffected after six weeks of CVS exposure. While several groups have observed an elevation in PVN CRH levels in male rodents [16, 33], research in female rats has found either elevated hypothalamic CRH mRNA [10], or no change [18, 36, 42] following CVS. Thus, our findings are supported by previous studies showing no effect of CVS on Crh expression in female rats. As CRH plays an essential role in the activation of the HPA axis and in stimulating the production of CORT, it is perhaps not surprising that we also did not observe differences in basal plasma CORT levels between CVS-treated and control female subjects.
Similar to Crh gene expression, morning basal PVN Avp expression was unaffected by CVS, as has been previously reported in female rats [42]. However, a notable effect of CVS on the Avp response to a novel, acute stressor was observed. Restraint stress significantly decreased PVN AVP mRNA in control but not CVS-treated animals, suggesting that CVS inhibits the Avp response to acute stress. The effects of acute restraint stress on Avp expression are currently not well understood. Some studies in male rats have shown no alterations in AVP mRNA levels four [43, 44] or six [45] hours after acute restraint, while others report that a single period of restraint increases parvocellular PVN AVP heteronuclear RNA as early as 30 minutes and mRNA after 90 minutes or two hours [46, 47]. In the present study, AVP mRNA was decreased within 20 minutes following the onset of restraint, a surprisingly rapid change for mRNA. Accordingly, this decrease argues for an increase in mRNA degradation rather than decreased transcription. In support of this hypothesis, both cyclic adenosine monophosphate and glucocorticoids have been shown to alter AVP mRNA stability in parvocellular PVN neurons [48]. CVS, therefore, could ultimately influence mRNA stability to inhibit the Avp response to acute restraint stress.
Whether or not the downstream consequences of CVS on AVP mRNA following a novel, acute stressor relate to the activity of the HPA axis remains to be determined. In humans and rats, AVP is co-expressed in some parvocelluar CRH neurons, where it acts synergistically with CRH to stimulate ACTH production by the anterior pituitary [49, 50]. In contrast, in the mouse PVN, very little overlap of AVP- and CRH-ir has been found [51], yet a role for AVP as an ACTH secretagogue has still been identified [52, 53]. The rapid changes in AVP gene expression we observed may ultimately alter downstream glucocorticoid production as a result. However, the reduction in AVP mRNA found following restraint stress likely occurred too quickly to impact the amount of AVP peptide available for release during the restraint [54]. Thus, it is not unexpected that glucocorticoid levels substantially increased in both control and CVS subjects despite the effect of CVS to inhibit the Avp response to acute restraint stress. Rather, such CVS- and restraint stress-dependent changes in AVP mRNA may alter the accumulation of peptide that drives future glucocorticoid responses. A more thorough time course study would be necessary to demonstrate such an effect. Because we examined mRNA expression in parvocellular and magnocellular neurons collectively in these studies, we also cannot exclude the possibility that CVS alters the Avp response to restraint stress, with consequences for behavioral and physiological processes outside of the HPA axis [44, 55–57].
Of interest, although the restraint-induced effect on Avp was found in control animals only, a restraint-induced decrease in OT mRNA was found in the CVS groups only. This suggests the possibility of a switch in CVS-treated animals to a more OT-dependent mechanism of HPA axis regulation. Like Avp, morning basal Ot expression in the PVN was unaltered by CVS, paralleling our previous findings in female mice that showed no changes in PVN OT mRNA after CVS and behavioral testing [27]. Although restraint stress has been shown to induce activation of PVN OT neurons [58], OT mRNA [44] and OT secretion [59] in male rats, we also observed no effect of restraint stress on OT mRNA in control animals in our studies. In contrast, CVS-treated female mice showed a rapid reduction in OT mRNA following restraint. This rapid change in mRNA, as discussed for AVP, is likely explained by increased mRNA degradation rather than reduced Ot transcription, but further investigation is necessary.
The effects of CVS on the Ot response to a novel, acute stressor likely do not immediately influence glucocorticoid production by the HPA axis, as we observed no CVS-dependent changes in restraint-stimulated CORT levels. Instead, changes in OT mRNA following 20 minutes of restraint stress may alter OT peptide stores that in turn influence future glucocorticoid production. PVN OT is not only believed to act locally to inhibit CRH neurons under basal conditions, but it also has been found to potentiate CRH-induced secretion of ACTH at the level of the pituitary [13, 60]. Thus, if the stress-generated decrease in OT mRNA observed in CVS-treated animals ultimately results in a decrease of locally released OT peptide, then this may lead to increased glucocorticoid production relative to controls in CVS-treated females. While such a finding would align well with previous reports of elevated plasma CORT following CVS [10, 34, 36], a further time course of plasma CORT levels following the 20-minute restraint stress is necessary to assess this possibility. An alternate possibility is that the decrease in OT mRNA eventually produces a decrease in OT in the general circulation, which may act on the pituitary gland to decrease ACTH and CORT synthesis and secretion. Supporting this possibility, we observed a trend for restraint stress to decrease plasma levels of OT irrespective of CVS treatment. Lastly, we cannot discount the possibility that CVS alters stress-induced Ot expression in magnocellular neurons to control behaviors or physiological functions beyond the HPA axis [55, 57, 61].
4.2. CVS and the evening activity of the HPA axis
Although we did not observe an effect of CVS on morning basal CORT levels, we did find that CVS elevated evening levels. Thus, at least in the evening, CVS-treated females exhibited hyperactivity of the HPA axis, as has been previously suggested in female rodents [10, 34, 36]. When challenged with an acute restraint stressor, however, CVS-treated females showed a blunted CORT response, potentially related to their evening rise in CORT levels. These findings notably contrast with the results of a previous study in female rats showing similar elevations in plasma CORT levels following a novel stressor in chronically stressed and control subjects when tested in the morning [35]. Such discrepancies likely are the result of differences in the time of sampling (i.e. morning versus evening). It is also possible that the blunted evening stress response we observed in our subjects is reflective of the familiarity of the stressor, as acute restraint stress is one of the manipulations used in our model. Simpkiss et al. (2003) observed a blunted ACTH response to restraint stress in CVS-treated male rats familiar with the manipulation, but a normal response in restraint-naïve CVS-treated subjects [41]. Alternatively, research conducted in the male rat by a different group has demonstrated a non-specific attenuation of HPA axis reactivity following CVS as indicated by a blunted corticosterone response to novel stressors [27, 31]. Future research will examine the effects of a novel stressor on the evening CORT response in CVS-treated subjects and will determine whether our observed blunted response is sex-specific in the mouse. Nonetheless, the blunted evening stress response did not occur in control animals in our studies, suggesting that it was not simply related to the familiarity of the restraint stressor in CVS animals.
Paralleling their pattern of evening, stress-induced glucocorticoid secretion, CVS-treated female mice had decreased neuronal activation in the PVN after acute restraint, as measured by c-Fos-ir. Although previous studies in female rodents are lacking, the nature of CVS’s effects on stress-provoked activation of the PVN has shown inconsistencies in the male rat CVS literature. Increased [62] and decreased [39] expression or no change [63] in c-Fos-ir within this brain area have all been reported. Thus, the decrease in PVN neuronal activation we observed following CVS and restraint is not unprecedented and moves us closer to understanding the neurobiological mechanisms that drive the evening CORT response to stress in CVS-treated females.
4.3. CVS and the morning versus evening activity of the HPA axis
We cannot exclude the possibility that the differential effects of CVS on the morning versus evening activity of the HPA axis we observed are due to the presence versus absence of the restraint stressor in the CVS protocols employed. Thus, our findings must be taken with the caveat that subtle variations in the CVS protocols could drive time-dependent differences in the activity of the HPA axis. A previous study comparing HPA function following acute restraint stress in CVS-treated male rats that were familiar and unfamiliar with the restraint stressor revealed that stressor familiarity greatly influences the ACTH response [41]. However, this study also reported no differences in the CORT response to restraint in restraint-naïve versus familiar CVS-treated subjects, suggesting that restraint stressor incorporation in the evening CVS protocol may not have altered the findings of our studies [41]. Further studies are ultimately necessary to determine if the differences in the morning and evening HPA activity we observed depend on whether or not the CVS paradigm incorporates restraint stress.
In our studies, CVS seemed to dysregulate HPA axis activity in a time-dependent manner, apparently via alterations in circadian control. This conclusion is supported by evidence of a circadian rhythm for CORT secretion, as well as for Crh, Avp, and Ot gene expression [19–24]. Furthermore, in BALB/c male mice, CVS has been shown to elevate and phase-shift serum CORT levels in the evening, indicating a time-dependent overactivation of the HPA axis [64]. Specifically, peak CORT levels were observed later in the evening in CVS-treated versus control BALB/c mice. Thus, the elevated basal evening, but not morning, CORT levels following CVS we observed in the present studies may reflect a similar phase shift in serum CORT levels. Notably, Takahashi et al. (2013) did not observe elevated or altered rhythmicity of serum CORT in C57BL/6 male mice following CVS. This discrepancy could result from a sex difference in CVS effects on the circadian regulation of the HPA axis in C57BL/6 mice or from differences in the durations of the CVS protocol employed. The potentially altered CORT rhythmicity following CVS in our studies could also be a result of sleep disruption/ deprivation, as we exposed mice to various stressors during the light cycle for an especially prolonged period. Sleep deprivation has been shown to produce increases in plasma ACTH and CORT levels [65], as well as a marked effect on the circadian secretion pattern of CORT [66]. Given the apparent influence of CVS on CORT rhythmicity in our studies, it seems likely that the circadian control of PVN neuropeptide gene expression is similarly disrupted, but this remains to be determined.
4.4. CVS and anxiety- and depressive-like behavior
We observed no effects of CVS on anxiety- or depressive-like behavior. While our findings may reflect a sex difference in vulnerability to CVS in terms of behavior, they may also be indicative of strain-specific effects on affective behavior. A study by Mineur et al. (2006) found that while male but not female C57BL/6 mice showed increased immobility in the tail suspension test following CVS, time in the light portion of the light/dark box was decreased following CVS in both male and female BALB/cJ mice, but not in either sex for C57BL/6 animals [30]. Future studies utilizing both sexes are needed to thoroughly characterize the effects of CVS on behavioral outcomes, as well as on measures of HPA axis activity. While our subjects were tested during the dark phase, at least two hours after the last CVS stressor, it is also possible that behavioral testing during the light phase would have shown different results. A study by Huynh et al. (2011) found that female rats exposed to chronic restraint stress showed a decrease in a combined Z-score of two tests of depressive-like behavior (forced swim test and sucrose preference test) compared with unstressed females when tested during the light phase but not the dark phase. However, the effect of chronic stress on depressive-like behavior in female rats was small, as no significant effect on depressive-like behavior was found during either light phase when examining behaviors on each test individually [67]. Since HPA axis dysfunction is also associated with cardiac and metabolic disease, conditions linked to chronic stress exposure [68], it may be more apt to investigate CVS exposure in female mice as a potential model for disorders outside of anxiety and depression.
Despite the relative “stress resiliency” of C57BL/6 mice when compared with other murine strains [69], our findings indicate that our CVS paradigm has neurobiological effects for female C57BL/6 mice, which are well-positioned to influence the activity of the HPA axis. The combination of CVS and behavioral testing elevated levels of OT mRNA in the female PVN. Given the observed increase in CRH mRNA (albeit at the trend level), this simultaneous elevation in OT mRNA may reflect an attempt to compensate for the increased activation of CRH neurons. OT is often viewed as an inhibitor of HPA axis activity [13]. Accordingly, a previous study conducted in male rats found that intracerebroventricular administration of OT attenuated elevated parvocellular PVN CRH mRNA levels induced by CVS, and that PVN OT mRNA levels were negatively correlated with CRH mRNA levels within this region [15]. Because using ddPCR precluded the analysis of magnocellular and parvocellular regions of the PVN individually in our studies, we cannot restrict our findings to the parvocellular PVN. Thus, our results support the possibility that CVS increases OT mRNA in magnocellular PVN neurons, as has previously been reported in male rats [15]. Although changes in mRNA levels do not necessarily reflect changes in neuropeptide secretion or neurotransmission, the observed increase in PVN OT mRNA may correlate with an increase in magnocellular OT neuronal activation and a subsequent increase in the rate of OT secretion in and around the PVN. Consistent with this interpretation, local dendritic release of OT has been shown to influence nearby cells in a paracrine fashion, and central OT administration inhibits the CRH mRNA response to stress [70, 71]. However, an upregulation of local OT release can act independently of axonal release. Consequently, our findings may also indicate an increase in OT available to potentiate CRH-induced secretion of ACTH at the level of the pituitary and thereby increase HPA axis activity [60, 72]. Further investigation of how CVS influences the highly complex relationship between PVN Crh and Ot gene expression to alter HPA axis activity following behavioral testing is ultimately necessary.
To avoid the potential effects of changing gonadal hormone levels across the estrous cycle, all subjects in the present study were tested outside of the proestrous phase. Previous studies assessing female C57BL/6 or C57BL/6J mice showed that, when tested in proestrus, subjects showed decreased duration of immobility in the tail suspension test [73] and increased pituitary CRH binding protein expression [74]. In the rat, higher peak ACTH and CORT responses to stress have been reported during proestrus compared to the estrous and diestrous phases [75]. Given the influence of estrous cyclicity on HPA axis activity and affective behavior in the rodent, it is possible that effects of CVS on the female mouse may be altered by the stage of the estrous cycle during which they are tested. Such estrous cycle variations will be an important topic of future investigation, as they may ultimately explain some sex differences found following CVS.
While a more thorough battery of behavioral tests is needed to unveil novel effects of CVS exposure on affective behavior in the female mouse, our findings collectively suggest that the female C57BL/6 mouse may be better suited as a model of altered HPA axis function or of stress-associated autonomic disorders, such as metabolic and cardiovascular disease. CVS in our studies ultimately appeared to dysregulate HPA axis activity in a time-dependent manner, likely via alterations in the circadian control of the HPA axis. Future studies of CVS and the circadian regulation of the HPA axis will ultimately improve our understanding of the CVS-induced HPA dysregulation thought to increase risk for stress-related autonomic disorders.
Supplementary Material
Highlights.
Female mice exhibit time-dependent HPA axis dysregulation after chronic stress
Chronic stress alters the morning responses of hypothalamic mRNAs to acute stress
Chronic stress changes evening basal and acute stress-induced corticosterone levels
Acknowledgements
This research was supported by the National Institutes of Health (Grant RO1-DK105826 to R.J.H.).
Financial Support: This research was supported by the National Institutes of Health (RO1-DK105826).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Summary: The authors have nothing to disclose.
References
- [1].McEwen BS Mood disorders and allostatic load. Biol Psychiatry 2003, 54:200–7. [DOI] [PubMed] [Google Scholar]
- [2].Vrshek-Schallhorn S, Stroud CB, Mineka S, Hammen C, Zinbarg RE, Wolitzky-Taylor K, Craske MG Chronic and episodic interpersonal stress as statistically unique predictors of depression in two samples of emerging adults. J Abnorm Psychol 2015, 124:918–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nandi A, Beard JR, Galea S Epidemiologic heterogeneity of common mood and anxiety disorders over the lifecourse in the general population: A systematic review. BMC Psychiatry 2009, 9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Piccinelli M, Wilkinson G Gender differences in depression. critical review. Br J Psychiatry 2000, 177:486–92. [DOI] [PubMed] [Google Scholar]
- [5].Bekker MH, van Mens-Verhulst J Anxiety disorders: Sex differences in prevalence, degree, and background, but gender-neutral treatment. Gender medicine 2007, 4:S193. [DOI] [PubMed] [Google Scholar]
- [6].Palanza P Animal models of anxiety and depression: How are females different?. Neuroscience & Biobehavioral Reviews 2001, 25:219–33. [DOI] [PubMed] [Google Scholar]
- [7].Willner P, Towell A, Sampson D, Sophokleous S, Muscat R Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987, 93:358–64. [DOI] [PubMed] [Google Scholar]
- [8].Willner P The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol Stress 2016, 6:78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Swaab DF, Bao AM, Lucassen PJ The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev 2005, 4:141–94. [DOI] [PubMed] [Google Scholar]
- [10].Liu L, Yang J, Qian F, Lu C Hypothalamic-pituitary-adrenal axis hypersensitivity in female rats on a post-weaning high-fat diet after chronic mild stress. Exp Ther Med 2017, 14:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Franco AJ, Chen C, Scullen T, Zsombok A, Salahudeen AA, Di S, Herman JP, Tasker JG Sensitization of the hypothalamic-pituitary-adrenal axis in a male rat chronic stress model. Endocrinology 2016, 157:2346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Meynen G, Unmehopa UA, van Heerikhuize JJ, Hofman MA, Swaab DF, Hoogendijk WJ Increased arginine vasopressin mRNA expression in the human hypothalamus in depression: A preliminary report. Biol Psychiatry 2006, 60:892–5. [DOI] [PubMed] [Google Scholar]
- [13].Neumann ID Involvement of the brain oxytocin system in stress coping: Interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res 2002, 139:147–62. [DOI] [PubMed] [Google Scholar]
- [14].Dai D, Li Q, Zhu Q, Hu S, Balesar R, Swaab D, Bao A Direct involvement of androgen receptor in oxytocin gene expression: Possible relevance for mood disorders. Neuropsychopharmacology 2017, 42:2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zheng J, Babygirija R, Bulbul M, Cerjak D, Ludwig K, Takahashi T Hypothalamic oxytocin mediates adaptation mechanism against chronic stress in rats. Am J Physiol Gastrointest Liver Physiol 2010, 299:946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Flak JN, Jankord R, Solomon MB, Krause EG, Herman JP Opposing effects of chronic stress and weight restriction on cardiovascular, neuroendocrine and metabolic function. Physiol Behav 2011, 104:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vieira JO, Duarte JO, Costa-Ferreira W, Morais-Silva G, Marin MT, Crestani CC Sex differences in cardiovascular, neuroendocrine and behavioral changes evoked by chronic stressors in rats. Prog Neuropsychopharmacol Biol Psychiatry 2018, 81:426–37. [DOI] [PubMed] [Google Scholar]
- [18].Duncko R, Kiss A, Skultetyova I, Rusnak M, Jezova D Corticotropin-releasing hormone mRNA levels in response to chronic mild stress rise in male but not in female rats while tyrosine hydroxylase mRNA levels decrease in both sexes. Psychoneuroendocrinology 2001, 26:77–89. [DOI] [PubMed] [Google Scholar]
- [19].Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N Regulation of ACTH secretion: Variations on a theme of B. Recent Prog Horm Res 1987, 43:113–73. [DOI] [PubMed] [Google Scholar]
- [20].Watts AG, Tanimura S, Sanchez-Watts G Corticotropin-releasing hormone and arginine vasopressin gene transcription in the hypothalamic paraventricular nucleus of unstressed rats: Daily rhythms and their interactions with corticosterone. Endocrinology 2004, 145:529–40. [DOI] [PubMed] [Google Scholar]
- [21].Maejima Y, Takahashi S, Takasu K, Takenoshita S, Ueta Y, Shimomura K Orexin action on oxytocin neurons in the paraventricular nucleus of the hypothalamus. Neuroreport 2017, 28:360–6. [DOI] [PubMed] [Google Scholar]
- [22].Kwak SP, Morano MI, Young EA, Watson SJ, Akil H Diurnal CRH mRNA rhythm in the hypothalamus: Decreased expression in the evening is not dependent on endogenous glucocorticoids. Neuroendocrinology 1993, 57:96–105. [DOI] [PubMed] [Google Scholar]
- [23].Muglia LJ, Jacobson L, Weninger SC, Luedke CE, Bae DS, Jeong KH, Majzoub JA Impaired diurnal adrenal rhythmicity restored by constant infusion of corticotropin-releasing hormone in corticotropin-releasing hormone-deficient mice. J Clin Invest 1997, 99:2923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nicolaides NC, Charmandari E, Chrousos GP, Kino T Circadian endocrine rhythms: The hypothalamic-pituitary-adrenal axis and its actions. Ann N Y Acad Sci 2014, 1318:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Butcher RL, Collins WE, Fugo NW Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology 1974, 94:1704–8. [DOI] [PubMed] [Google Scholar]
- [26].Marcondes FK, Bianchi FJ, Tanno AP Determination of the estrous cycle phases of rats: Some helpful considerations. Braz J Biol 2002, 62:609–14. [DOI] [PubMed] [Google Scholar]
- [27].Borrow AP, Bales NJ, Stover SA, Handa RJ Chronic variable stress induces sex-specific alterations in social behavior and neuropeptide expression in the mouse. Endocrinology 2018, 159:2803–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moenter SM, Caraty A, Locatelli A, Karsch FJ Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: Existence of a preovulatory GnRH surge. Endocrinology 1991, 129:1175–82. [DOI] [PubMed] [Google Scholar]
- [29].Isingrini E, Perret L, Rainer Q, Amilhon B, Guma E, Tanti A, Martin G, Robinson J, Moquin L, Marti F Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat Neurosci 2016, 19:560. [DOI] [PubMed] [Google Scholar]
- [30].Mineur YS, Belzung C, Crusio WE Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res 2006, 175:43–50. [DOI] [PubMed] [Google Scholar]
- [31].Hu C, Luo Y, Wang H, Kuang S, Liang G, Yang Y, Mai S, Yang J Re-evaluation of the interrelationships among the behavioral tests in rats exposed to chronic unpredictable mild stress. PLoS One 2017, 12:e0185129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. American journal of physiology-endocrinology and metabolism 2006, 291:E973. [DOI] [PubMed] [Google Scholar]
- [33].Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology 2006, 147:2008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dadomo H, Gioiosa L, Cigalotti J, Ceresini G, Parmigiani S, Palanza P What is stressful for females? differential effects of unpredictable environmental or social stress in CD1 female mice. Horm Behav 2018, 98:22–32. [DOI] [PubMed] [Google Scholar]
- [35].Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z Chronic mild stress impact: Are females more vulnerable?. Neuroscience 2005, 135:703–14. [DOI] [PubMed] [Google Scholar]
- [36].Uban KA, Comeau WL, Ellis LA, Galea LA, Weinberg J Basal regulation of HPA and dopamine systems is altered differentially in males and females by prenatal alcohol exposure and chronic variable stress. Psychoneuroendocrinology 2013, 38:1953–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bekhbat M, Merrill L, Kelly SD, Lee VK, Neigh GN Brief anesthesia by isoflurane alters plasma corticosterone levels distinctly in male and female rats: Implications for tissue collection methods. Behav Brain Res 2016, 305:122–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pomplun D, Mohlig M, Spranger J, Pfeiffer AF, Ristow M Elevation of blood glucose following anaesthetic treatment in C57BL/6 mice. Horm Metab Res 2004, 36:67–9. [DOI] [PubMed] [Google Scholar]
- [39].Ostrander MM, Ulrich-Lai YM, Choi DC, Flak JN, Richtand NM, Herman JP Chronic stress produces enduring decreases in novel stress-evoked c-fos mRNA expression in discrete brain regions of the rat. Stress 2009, 12:469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bielajew C, Konkle A, Merali Z The effects of chronic mild stress on male Sprague–Dawley and long evans rats: I. biochemical and physiological analyses. Behav Brain Res 2002, 136:583–92. [DOI] [PubMed] [Google Scholar]
- [41].Simpkiss JL, Devine DP Responses of the HPA axis after chronic variable stress: Effects of novel and familiar stressors. Neuroendocrinol Lett 2003, 24:97–103. [PubMed] [Google Scholar]
- [42].Lan N, Hellemans KG, Ellis L, Weinberg J Exposure to chronic mild stress differentially alters corticotropin-releasing hormone and arginine vasopressin mRNA expression in the stress-responsive neurocircuitry of male and female rats prenatally exposed to alcohol. Alcohol Clin Exp Res 2015, 39:2414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Harbuz MS, Jessop DS, Lightman SL, Chowdrey HS The effects of restraint or hypertonic saline stress on corticotrophin-releasing factor, arginine vasopressin, and proenkephalin A mRNAs in the CFY, sprague-dawley and wistar strains of rat. Brain Res 1994, 667:6–12. [DOI] [PubMed] [Google Scholar]
- [44].Hesketh S, Jessop DS, Hogg S, Harbuz MS Differential actions of acute and chronic citalopram on the rodent hypothalamic-pituitary-adrenal axis response to acute restraint stress. J Endocrinol 2005, 185:373–82. [DOI] [PubMed] [Google Scholar]
- [45].Pinnock SB, Herbert J Corticosterone differentially modulates expression of corticotropin releasing factor and arginine vasopressin mRNA in the hypothalamic paraventricular nucleus following either acute or repeated restraint stress. Eur J Neurosci 2001, 13:576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Herman JP In situ hybridization analysis of vasopressin gene transcription in the paraventricular and supraoptic nuclei of the rat: Regulation by stress and glucocorticoids. J Comp Neurol 1995, 363:15–27. [DOI] [PubMed] [Google Scholar]
- [47].Ma XM, Levy A, Lightman SL Rapid changes in heteronuclear RNA for corticotrophin-releasing hormone and arginine vasopressin in response to acute stress. J Endocrinol 1997, 152:81–9. [DOI] [PubMed] [Google Scholar]
- [48].Kuwahara S, Arima H, Banno R, Sato I, Kondo N, Oiso Y Regulation of vasopressin gene expression by cAMP and glucocorticoids in parvocellular neurons of the paraventricular nucleus in rat hypothalamic organotypic cultures. J Neurosci 2003, 23:10231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Antoni FA Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol 1993, 14:76–122. [DOI] [PubMed] [Google Scholar]
- [50].Aguilera G, Liu Y The molecular physiology of CRH neurons. Front Neuroendocrinol 2012, 33:67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Biag J, Huang Y, Gou L, Hintiryan H, Askarinam A, Hahn JD, Toga AW, Dong HW Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: A study of immunostaining and multiple fluorescent tract tracing. J Comp Neurol 2012, 520:6–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lolait SJ, Stewart LQ, Jessop DS, Young WS, O’Carroll AM The hypothalamic-pituitary-adrenal axis response to stress in mice lacking functional vasopressin V1b receptors. Endocrinology 2007, 148:849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Muller MB, Landgraf R, Preil J, Sillaber I, Kresse AE, Keck ME, Zimmermann S, Holsboer F, Wurst W Selective activation of the hypothalamic vasopressinergic system in mice deficient for the corticotropin-releasing hormone receptor 1 is dependent on glucocorticoids. Endocrinology 2000, 141:4262–9. [DOI] [PubMed] [Google Scholar]
- [54].Watts AG Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: A complexity beyond negative feedback. Front Neuroendocrinol 2005, 26:109–30. [DOI] [PubMed] [Google Scholar]
- [55].Ludwig M Functional role of intrahypothalamic release of oxytocin and vasopressin: Consequences and controversies. Am J Physiol 1995, 268:537. [DOI] [PubMed] [Google Scholar]
- [56].Albers HE The regulation of social recognition, social communication and aggression: Vasopressin in the social behavior neural network. Horm Behav 2012, 61:283–92. [DOI] [PubMed] [Google Scholar]
- [57].McCann SM, Gutkowska J, Antunes-Rodrigues J Neuroendocrine control of body fluid homeostasis. Braz J Med Biol Res 2003, 36:165–81. [DOI] [PubMed] [Google Scholar]
- [58].Miyata S, Itoh T, Lin SH, Ishiyama M, Nakashima T, Kiyohara T Temporal changes of c-fos expression in oxytocinergic magnocellular neuroendocrine cells of the rat hypothalamus with restraint stress. Brain Res Bull 1995, 37:391–5. [DOI] [PubMed] [Google Scholar]
- [59].Lang RE, Heil JW, Ganten D, Hermann K, Unger T, Rascher W Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology 1983, 37:314–6. [DOI] [PubMed] [Google Scholar]
- [60].Gibbs DM, Vale W, Rivier J, Yen SS Oxytocin potentiates the ACTH-releasing activity of CRF(41) but not vasopressin. Life Sci 1984, 34:2245–9. [DOI] [PubMed] [Google Scholar]
- [61].Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology 2011, 36:2159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kormos V, Gaspar L, Kovacs LA, Farkas J, Gaszner T, Csernus V, Balogh A, Hashimoto H, Reglodi D, Helyes Z, Gaszner B Reduced response to chronic mild stress in PACAP mutant mice is associated with blunted FosB expression in limbic forebrain and brainstem centers. Neuroscience 2016, 330:335–58. [DOI] [PubMed] [Google Scholar]
- [63].Choi DC, Evanson NK, Furay AR, Ulrich-Lai YM, Ostrander MM, Herman JP The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinology 2008, 149:818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Takahashi K, Yamada T, Tsukita S, Kaneko K, Shirai Y, Munakata Y, Ishigaki Y, Imai J, Uno K, Hasegawa Y, Sawada S, Oka Y, Katagiri H Chronic mild stress alters circadian expressions of molecular clock genes in the liver. Am J Physiol Endocrinol Metab 2013, 304:301. [DOI] [PubMed] [Google Scholar]
- [65].Hipolide DC, Suchecki D, Pimentel de Carvalho Pinto A, Chiconelli Faria E, Tufik S, Luz J Paradoxical sleep deprivation and sleep recovery: Effects on the hypothalamic-pituitary-adrenal axis activity, energy balance and body composition of rats. J Neuroendocrinol 2006, 18:231–8. [DOI] [PubMed] [Google Scholar]
- [66].Retana-Marquez S, Bonilla-Jaime H, Vazquez-Palacios G, Dominguez-Salazar E, Martinez-Garcia R, Velazquez-Moctezuma J Body weight gain and diurnal differences of corticosterone changes in response to acute and chronic stress in rats. Psychoneuroendocrinology 2003, 28:207–27. [DOI] [PubMed] [Google Scholar]
- [67].Huynh TN, Krigbaum AM, Hanna JJ, Conrad CD Sex differences and phase of light cycle modify chronic stress effects on anxiety and depressive-like behavior. Behav Brain Res 2011, 222:212–22. [DOI] [PubMed] [Google Scholar]
- [68].Murphy MO, Loria AS Sex-specific effects of stress on metabolic and cardiovascular disease: Are women at higher risk?. Am J Physiol Regul Integr Comp Physiol 2017, 313:R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chan JC, Houghton AB, Bale TL Strained in planning your mouse background? using the HPA stress axis as a biological readout for backcrossing strategies. Neuropsychopharmacology 2017, 42:1749–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ludwig M, Leng G Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci 2006, 7:126–36. [DOI] [PubMed] [Google Scholar]
- [71].Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: Partial action within the paraventricular nucleus. J Neuroendocrinol 2000, 12:235–43. [DOI] [PubMed] [Google Scholar]
- [72].Neumann ID, Krömer SA, Toschi N, Ebner K Brain oxytocin inhibits the (re) activity of the hypothalamo–pituitary–adrenal axis in male rats: Involvement of hypothalamic and limbic brain regions. Regul Pept 2000, 96:31–8. [DOI] [PubMed] [Google Scholar]
- [73].Meziane H, Ouagazzal A, Aubert L, Wietrzych M, Krezel W Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: Implications for phenotyping strategies. Genes, Brain and Behavior 2007, 6:192–200. [DOI] [PubMed] [Google Scholar]
- [74].Speert DB, McClennen SJ, Seasholtz AF Sexually dimorphic expression of corticotropin-releasing hormone-binding protein in the mouse pituitary. Endocrinology 2002, 143:4730–41. [DOI] [PubMed] [Google Scholar]
- [75].Viau V, Meaney MJ Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology 1991, 129:2503–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


