SUMMARY
Background
HIV-1-specific broadly neutralizing antibodies such as VRC01 could promote HIV remission by halting viral replication and clearing infected cells. We conducted a randomised, double-blinded, placebo-controlled trial of VRC01 in adults who initiated antiretroviral therapy (ART) during acute HIV infection to evaluate whether VRC01 could promote sustained viral control off ART.
Methods
We recruited virally-suppressed, acutely-treated adults at the Thai Red Cross AIDS Research Centre in Bangkok, Thailand. We randomised participants in a 3:1 ratio using computer-generated lists with a blocking factor of 4, stratified by Fiebig stage, to receive VRC01 40mg per kg or placebo intravenously every three weeks for up to 24 weeks during analytic treatment interruption, followed by continued observation off all therapies. Participants were monitored closely and resumed ART if confirmed plasma HIV-1 RNA exceeded 1000 copies per mL. The primary outcomes were the frequency of serious adverse events and the proportion of participants with HIV-1 RNA <50 copies per mL at 24 weeks after treatment interruption. This trial was registered with ClinicalTrials.gov, number NCT02664415.
Findings
From 8 August 2016 to 9 January 2017, we enrolled 23 participants including four who were withdrawn prior to randomization and one who received a partial infusion without undergoing treatment interruption. Eighteen males aged 21-50 years received at least one full study infusion (13 VRC01, 5 placebo). Serum VRC01 concentrations in recipients were consistently above the target 50 μg per mL throughout dosing. There were no serious adverse events. One VRC01 recipient remained virally suppressed for 42 weeks. All other participants experienced HIV-1 RNA ≥1000 copies/mL and restarted ART before the 24-week endpoint assessment.
Interpretation
VRC01 monotherapy in individuals who initiated ART during acute HIV infection was well-tolerated but did not significantly impact the primary efficacy outcome for this study. Further development of VRC01 and other immunotherapies for HIV will likely occur as part of combination regimens that include multiple agents directed against unique therapeutic targets.
Funding
U.S. Department of the Army, U.S. National Institutes of Health, Thai Red Cross AIDS Research Centre.
BACKGROUND
Antiretroviral therapy (ART) reduces morbidity and mortality associated with human immunodeficiency virus (HIV) infection by suppressing viral replication.1 However, it does not eradicate infection from cellular reservoirs including latently-infected resting memory CD4+ T-cells in blood and lymphoid tissues2,3 that contribute to rebound viremia within weeks following ART discontinuation.4 Additionally, barriers to universal ART use include toxicities, costs, drug resistance, and the need for lifelong adherence. Therefore, treatment strategies that minimize ART exposure while conferring durable viral control and delayed disease progression are highly desirable.5
HIV-1-specific broadly-neutralizing antibodies may have potential therapeutic advantages over ART that could promote HIV remission (i.e. control of plasma viremia in the absence of ART). These antibodies bind virus and may facilitate clearance of infected cells.6 They may also enhance host humoral7 and cellular8 immune responses to HIV.
VRC01 is a broadly-neutralizing antibody that targets the CD4-binding site of the HIV-1 envelope protein and has demonstrated broad in vitro neutralization capacity against all major circulating subtypes.9 In non-human primate studies, a single dose of VRC01 protected against simian/human immunodeficiency viruses challenges,10 reduced acute viremia,11 and limited viral reservoir establishment.11 In clinical trials, a single dose of VRC01 decreased plasma HIV-1 RNA by 1 to 2 log10copies per mL in 75% of viremic volunteers.12 VRC01 infusions every three weeks during analytic treatment interruption in participants who initiated ART during chronic HIV-1 infection delayed viral rebound as compared to historic controls, although the vast majority in both groups experienced rebound within eight weeks.13
Individuals who have initiated ART during acute HIV infection are excellent candidates for evaluating novel strategies to achieve HIV remission. Early initiation of ART limits establishment of HIV reservoirs,14 enhances reservoir decay,15 limits viral genetic diversification,16 and, in some cases, is associated with post-treatment control.17
We conducted a randomised, double-blinded, placebo-controlled clinical trial of VRC01 administration during analytic treatment interruption in adults who began ART during acute HIV infection to evaluate whether VRC01 could promote sustained viral control off ART.
METHODS
Study Design
The RV397 study was a randomised, double-blind, placebo-controlled clinical trial conducted at the Thai Red Cross AIDS Research Centre in Bangkok, Thailand. The study was approved by institutional review boards at Chulalongkorn University, Bangkok, Thailand; Walter Reed Army Institute of Research, Silver Spring, MD, USA; and all collaborating institutions.
Participants
We recruited participants from ongoing cohorts18,19 who received ART during acute HIV infection via a separately-funded protocol (ClinicalTrials.gov NCT00796263). Participants were offered enrollment in RV397 if they satisfied the following criteria: 20-50 years old, initiated ART during Fiebig stages I-III, prescribed ART for >24 months, HIV-1 RNA <50 copies per mL on three consecutive measurements, CD4+ T-cell count >400 cells per μL, integrated HIV-1 DNA <10 copies per 106 peripheral blood mononuclear cells, and in generally good health. Participants provided written informed consent prior to enrollment.
Randomization and Masking
We randomised participants in a 3:1 ratio to receive either VRC01 40 mg per kg or placebo intravenously every three weeks for up to 24 weeks, with stratification of randomization by Fiebig stage at HIV diagnosis. The study statistician used SAS version 9⋅4 PROC PLAN (SAS Institute, Cary, NC USA) to generate a randomization list using a blocking factor of 4. The study statistician prepared separate lists for each Fiebig stage (I, II or III) using different seeds and provided the electronic lists to the study pharmacist by encrypted file transfer. On confirmation of participant eligibility, the next treatment assignment on the appropriate randomization list was assigned by the unblinded pharmacist and recorded in the list. The appropriate study product was prepared by the pharmacist for infusion and labeled with the participant’s study identification number and date. Once prepared for infusion, VRC01 and the normal saline placebo were indistinguishable visually. To maintain masking, the unblinded pharmacist and blinded clinical staff did not communicate about treatment assignments.
Procedures
To minimize the risk of emerging drug resistance, participants receiving a non-nucleoside reverse transcriptase inhibitor at enrollment had this agent replaced with a protease inhibitor for four weeks prior to randomization. VRC01 was administered at a dose of 40 mg per kg intravenously every three weeks for up to 24 weeks. Normal saline was administered as a placebo using the same dosing interval. Analytic treatment interruption began the day of the first administration of VRC01 or placebo.
Participants were monitored for HIV-1 viremia with at least weekly quantitative or qualitative nucleic acid testing for the first 32 weeks after ART cessation and obligatory twice weekly testing during weeks 2-6 and 26-30, when risk for viral rebound was considered greatest (appendix p 11). ART was resumed in cases of confirmed HIV-1 RNA >1,000 copies per mL or other pre-specified virologic, immunologic, or clinical evidence of disease progression. Study infusions were discontinued after 24 weeks or if ART was resumed. Participants who were virally suppressed without an indication for ART resumption at 24 weeks continued intensive monitoring for an additional 24 weeks with no VRC01 or placebo administration.
Upon ART resumption, participation in this study ended and participants were offered enrollment in RV412 (clinicatrials.gov NCT02761200) with up to 48 weeks of additional observation, including assessments for re-suppression of HIV.
HIV-1 genetic sequences were generated and subtype was determined by single genome amplification from plasma samples. Human leukocyte antigen (HLA) genotyping was performed using next-generation sequencing.
After enrollment into RV397, HIV-1 RNA was measured in real-time either quantitatively using the COBAS TaqMan HIV-1 Test v2⋅0 (Roche Diagnostics, Branchburg, NJ, USA) with lower measurement limit of 20 copies per mL or qualitatively using the Aptima HIV-1 RNA Qualitative Assay (Hologic, Inc, San Diego, CA) with lower limit of detection around 60 copies per mL. Any positive qualitative test was confirmed by the quantitative assay. An in-house ultrasensitive hybrid real time/digital PCR single copy assay optimized for HIV subtype CRF01_AE was used retrospectively to determine HIV-1 RNA levels with lower measurement limit of 0·45 copies per mL.
Outcomes
The primary objectives of this study were to evaluate the safety of VRC01 and its ability to maintain viral suppression during analytic treatment interruption. The primary outcomes assessed were the frequency of serious adverse events up to 10 weeks after the last study infusion and the proportion of participants demonstrating viral suppression (HIV-1 RNA <50 copies per mL) at 24 weeks after ART cessation. Secondary outcomes specified in the research protocol included time to viral rebound and level of rebound viremia after cessation of ART; time to ART resumption for any reason after cessation of ART; HIV-1 RNA via single copy assay; CD4+ T-cell counts; cell-associated HIV-1 RNA and DNA; neuropsychological battery performance; control and attention testing performance; and hospitalizations and non-AIDS related conditions. Outcomes not described in this manuscript may be combined with results of other studies that employed analytic treatment interruption to explore thematic areas, such as neuropsychiatric implications of treatment interruption and interventions to achieve HIV remission.
Serum VRC01 concentration was measured via enzyme-linked immunosorbent assay using a murine anti-VRC01 monoclonal antibody. Viral neutralization was evaluated to determine the median infective dose for selected time points against a panel of pseudoviruses inclusive of sensitive and resistant strains. The presence of anti-VRC01 antibodies in serum was evaluated using an electrochemiluminescence bridging assay.
Total and integrated HIV-1 DNA were quantified by nested PCR. Tat/rev induced limiting dilution assay was used to measure the frequency of cells with inducible multiply-spliced HIV-1 RNA using modified primers and probes specific for the CRF01_AE subtype. Memory CD4+ T-cell-associated HIV-1 RNA was measured using RT-qPCR on FACS sorted cells.
HIV-1 serology was assessed using second-, third-, and fourth-generation diagnostic assays. Antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cell-mediated phagocytosis (ADCP) were measured by flow cytometry. Additional details of laboratory methods are in the Supplementary Appendix (pp 2-10).
Statistical Analyses
With planned enrollment of 24 participants, this study had 84% power to detect an absolute increase of 65% in viral suppression at 24 weeks among VRC01 recipients compared to an expected 15% among placebo recipients.17
As per protocol, the primary safety analyses included all participants exposed to any study product and the primary efficacy analyses included all participants who received at least one full dose of study product. Baseline participant characteristics were summarized with descriptive statistics. The number and percentage of participants experiencing adverse events were tallied by treatment group. Time-to-event analyses were performed by generating Kaplan-Meier curves and applying the log-rank test. Spearman’s correlation coefficient was calculated to evaluate associations between continuous variables and time to viral rebound. For all analyses, a two-sided type I error of 5% was considered statistically significant. Randomization was stratified by Fiebig stage; analyses did not adjust for Fiebig stage or other potentially confounding factors. Analyses were performed using GraphPad Prism 7⋅0 (GraphPad Software, San Diego, CA, USA) and Stata 13⋅1 (StataCorp LP, College Station, TX, USA). The protocol safety review team reviewed safety data at least weekly and an external safety monitoring committee reviewed these data every six months. This trial was registered with ClinicalTrials.gov, number NCT02664415.
Role of the Funding Source
The funders were involved in the study design, study operations, data collection, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
RESULTS
This study targeted enrollment of 24 participants. However, enrollment was halted early because changes in VRC01 packaging and stability testing procedures introduced barriers to the importation of study product after study initiation. From 8 August 2016 to 9 January 2017, 23 participants were enrolled. Of these, four were withdrawn prior to randomization and 19 were randomised with five assigned to placebo and 14 to VRC01 (Figure 1). One VRC01 recipient experienced severe generalised urticaria during the first study infusion and did not complete the infusion or undergo treatment interruption. Eighteen male participants received at least one full study infusion, underwent treatment interruption, and were included in all non-safety analyses (Table 1 and appendix p 22). Clinical activities for this study were completed on August 4, 2017, and the final participant to exit the study was followed after ART resumption in RV412 through July 11, 2018.
Figure 1. CONSORT Flow Diagram.

This diagram displays the progression of participants through screening, enrollment, allocation, and follow-up in the RV397 parallel, randomised clinical trial of VRC01 40 mg/kg or placebo administered intravenously to participants who initiated antiretroviral therapy during acute HIV infection, using the standard recommended by the CONSORT Group. The study had a target enrollment of 24 participants, but enrollment was halted early because changes in VRC01 packaging and stability testing procedures introduced barriers to the importation of study product into Thailand. Twenty-three participants were enrolled, four were withdrawn prior to randomization because of the unavailability of study product in-country, and 19 were randomised with five assigned to the placebo arm and 14 to VRC01. One participant in the VRC01 arm experienced severe generalised urticaria during the first study infusion and did not complete the infusion or undergo treatment interruption. This participant was included in analyses of safety data but no other analyses. Eighteen participants completed at least one study product infusion, underwent treatment interruption, and were included in all analyses.
Table 1.
Characteristics of Participants who Completed at Least One Study Product Infusion
| Characteristics | Overall (N=18) | Placebo (N=5) | VRC01 (N=13) |
|---|---|---|---|
|
At Randomization | |||
| Age, years | |||
| Median (range) | 29 (21 – 50) | 25 (23-48) | 32 (21-50) |
| Risk Group, n (%) | |||
| Men who have Sex with Men | 18 (100) | 5 (100) | 13 (100) |
| Weight, kilograms | |||
| Median (range) | 68 (53 – 85) | 62 (54 – 67) | 70 (53 – 85) |
| HIV-1 Subtype, n (%) | |||
| CRF01_AE | 12 (66) | 4 (80) | 8 (61) |
| B | 4 (22) | 1 (20) | 3 (23) |
| CRF01_AE and B co-infection | 1 (6) | 0 (0) | 1 (8) |
| CRF01_AE/B/C recombinant | 1 (6) | 0 (0) | 1 (8) |
| CD4+ T-cell Count, cells per μL | |||
| Median (range) | 717 (402 – 1032) | 562 (431-735) | 769 (402-1032) |
| CD4/CD8 Ratio | |||
| Median (range) | 1⋅1 (0⋅6 – 1⋅8) | 0⋅9 (0⋅7-1⋅8) | 1⋅1 (0⋅6-1⋅6) |
| Duration from ART Initiation, years | |||
| Median (range) | 3⋅0 (2⋅2 – 6⋅6) | 2⋅7 (2⋅3-3⋅8) | 3⋅1 (2⋅3-6⋅6) |
| Duration of Viral Suppression, years | |||
| Median (range) | 2⋅5 (1⋅8 – 6⋅4) | 2⋅6 (1⋅8 – 3⋅6) | 2⋅4 (1⋅8 – 6⋅4) |
| ART Regimen, n (%) | |||
| Tenofovir-emtricitabine-efavirenz | 16 (88) | 5 (100) | 11 (84) |
| Tenofovir-emtricitabine-raltegravir | 1 (6) | 0 (0) | 1 (8) |
| Tenofovir-emtricitabine-rilpivirine | 1 (6) | 0 (0) | 1 (8) |
| At ART Initiation | |||
| Fiebig stage*, n (%) | |||
| I (RNA+, p24−) | 1 (6) | 0 (0) | 1 (8) |
| II (p24+, IgM−) | 10 (55) | 3 (60) | 7 (54) |
| III (IgM+, WB−) | 7 (39) | 2 (40) | 5 (38) |
| HIV-1 RNA, log10copies per mL | |||
| Median (range) | 5⋅9 (4⋅4 – 7⋅1) | 6⋅0 (5⋅5 – 7⋅1) | 5⋅8 (4⋅4 – 7⋅1) |
| CD4+ T-cell Count, cells per μL | |||
| Median (range) | 303 (124 – 702) | 286 (207 – 303) | 388 (124 – 702) |
| CD4/CD8 Ratio | |||
| Median (range) | 0⋅7 (0⋅2 – 1⋅8) | 0⋅8 (0⋅;6 – 1⋅5) | 0⋅6 (0⋅2 – 1⋅8) |
Fiebig staging was performed on the day of enrollment into RV254; participants initiated ART at median 0 (range 0-4) days after enrollment.
A median of ten HIV-1 near-full-length genomes were amplified from plasma samples collected during acute HIV infection from each participant, revealing a predominance of infection with CRF01_AE viruses. Comparison of env sequences obtained upon viral rebound after analytic treatment interruption to those sampled before ART initiation during acute HIV infection suggested that viral rebound was not due to evolution of genetic patterns known to be linked to VRC01 resistance. Sequencing of host HLA alleles revealed no significant differences in distribution between the VRC01 and placebo groups (appendix pp 23-25).
There were no serious adverse events. Infusion-related adverse events were observed in three of eight (37·5%) placebo infusions and ten of 33 (30·3%) VRC01 infusions (appendix p 26). All infusion-related adverse events were mild except for two among VRC01 recipients: one moderate infusion site bruising and one severe generalised urticaria. Infusion-unrelated adverse events were similar between the two arms (appendix pp 27-30). There were no hospitalizations, no cases of acute retroviral syndrome, and no participants with new drug resistance mutations at ART resumption (appendix p 31). The median CD4 change from ART interruption to resumption was −29 (range −258 to +258) cells per μL. No anti-VRC01 antibodies were observed at the time of ART resumption (appendix p 32).
All 18 participants restarted ART due to confirmed HIV-1 RNA ≥1000 copies per mL (Figure 2A). Only one participant (3499), a VRC01 recipient, achieved the primary efficacy endpoint of viral suppression at 24 weeks after ART cessation. Placebo recipients had their first HIV-1 RNA ≥20 copies per mL at median 14 (range 14-29) days after analytic treatment interruption compared to median 29 (range 9-296) days among VRC01 recipients (Figure 2B, p=0⋅051). Placebo recipients had their first HIV-1 RNA ≥1000 copies per mL at median 14 (range 14-32) days compared to 33 (range 13-305) days among VRC01 recipients (Figure 2C, p=0⋅010). Excluding participant 3499 with prolonged viral suppression, VRC01 recipients had their first HIV-1 RNA ≥20 copies per mL at median 26 (range 9-65) days (p=0⋅08 compared to placebo) and their first HIV-1 RNA ≥1000 copies per mL at median 33 (range 13-67) days (p=0⋅02 compared to placebo). The ultrasensitive single copy assay revealed that 11 of 18 participants had low-level viremia prior to detectability on the routine assay and two participants had detectable HIV-1 RNA on the day of analytic treatment interruption, including one VRC01 recipient with HIV-1 RNA 2⋅1 copies per mL and one placebo recipient with detectable but not quantifiable HIV-1 RNA <0⋅45 copies per mL (appendix p 12). Placebo and VRC01 recipients had similar median HIV-1 RNA levels at ART resumption (3845 [range 1401-26865] vs. 3440 [1587-31807] copies per mL, p=0⋅588) and achieved viral re-suppression <20 copies per mL at median 28 (range 6-34) and 20 (range 7-28) days, respectively (Figure 2D and 2E, p=0⋅083).
Figure 2. Plasma HIV-1 RNA after Treatment Interruption and ART Resumption.
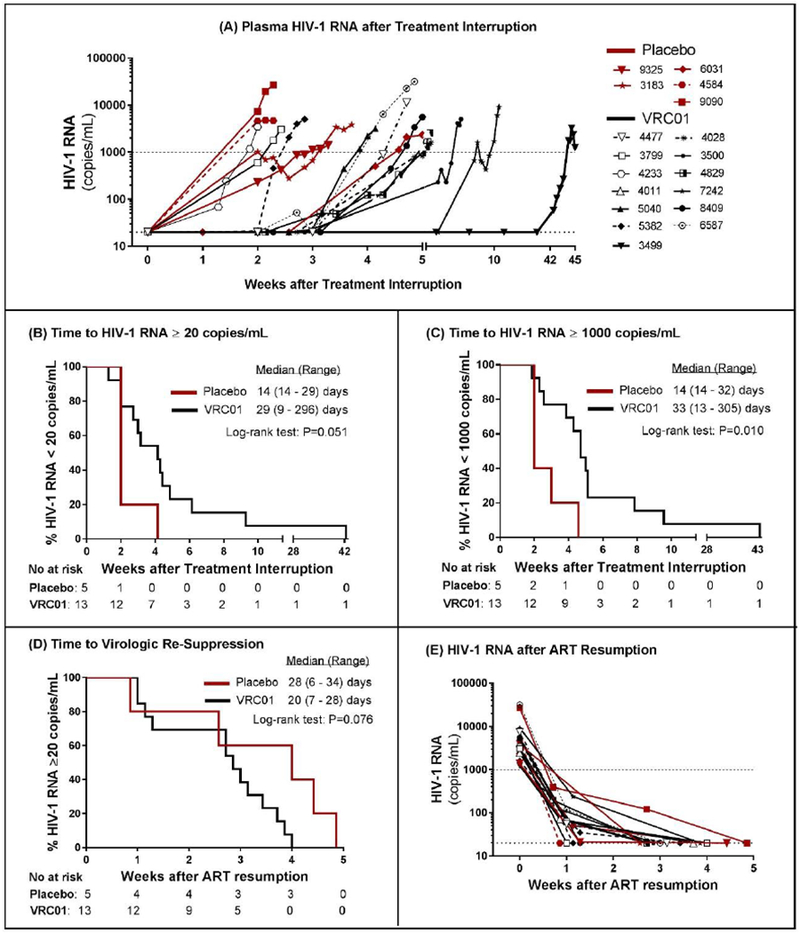
Plasma HIV-1 RNA for placebo (red) and VRC01 (black) recipients shows viral rebound by week 24 in all except one VRC01 recipient (panel A). Time to HIV-1 RNA ≥20 copies per mL did not reach the threshold for statistical significance comparing VRC01 to placebo recipients (panel B). Time to HIV-1 RNA ≥1000 copies per mL, the threshold for ART resumption, was significantly delayed in VRC01 compared to placebo recipients (panel C). After ART resumption, there was no difference in the time to achieving viral suppression <20 copies/mL between VRC01 and placebo recipients (panel D). All participants achieved undetectable HIV-1 RNA within 5 weeks (panel E).
Individual serum VRC01 concentrations among recipients were consistently above 50 μg per mL throughout the dosing period (Figure 3A and appendix p 13). After the first infusion, population-level geometric mean serum VRC01 concentration was 370 (range 249-444) μg per mL at one week and 290 (range 213-366) μg per mL at two weeks. Among the 12 VRC01 recipients who experienced viral rebound during the infusion period, the median VRC01 concentration at ART resumption was 277 (range 174-479) μg per mL. Participant 3499, with prolonged viral suppression, maintained a serum VRC01 concentration >50 μg per mL for at least 12 weeks after his final infusion and had serum VRC01 concentration 7⋅5 μg per mL at ART resumption 18 weeks after his final infusion (Figure 3B). To confirm that the infused antibody maintained its biological activity, HIV-1 neutralization activity of serum after VRC01 infusion was assessed. Potent in vitro neutralization of VRC01-sensitive strains MW965, PVO.04, and Q23.17 was observed in serum from all participants at all post-infusion time points (appendix p 14), including 18 weeks after the final infusion in participant 3499 (appendix p 15).
Figure 3. Serum VRC01 Concentrations.

Serum VRC01 concentrations are plotted longitudinally for all participants (panel A) and participant 3499 (panel B). In all VRC01 recipients, the target trough of 50 μg per mL was exceeded throughout the dosing interval. No VRC01 was detected in the serum of placebo recipients. The timing of each study infusion is indicated by arrows and the timing of ART resumption is indicated by a vertical dashed line.
The frequency of HIV-infected CD4+ T-cells, measured by total and integrated HIV-1 DNA, remained stable in the VRC01 group throughout the study but increased slightly in the placebo group at the time of ART resumption with a return after six months to levels observed before analytic treatment interruption (Figure 4A and 4B). Neither total nor integrated HIV DNA at analytic treatment interruption correlated with time to viral rebound ≥20 copies per mL (Figure 4C and 4D). Tat/rev induced limiting dilution assay measures were below the limit of detection in the majority of samples tested at all time points (appendix p 16). There were numerically fewer RNA-containing memory CD4+ T-cells at the time of ART resumption among VRC01 recipients as compared to placebo, but the difference was not statistically significant (appendix p 17). Median RNA copies per cell were not different between arms (appendix p 17).
Figure 4. Total and Integrated HIV DNA.
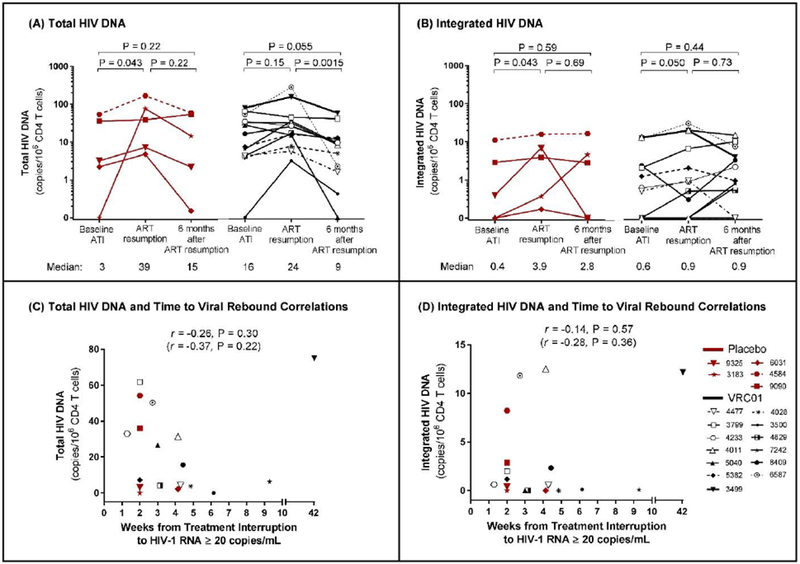
The frequencies of CD4+ T-cells that harbor total (panel A) and integrated (panel B) HIV-1 DNA remained low in both the placebo (red) and VRC01 (black) arms throughout the period before, during, and after treatment interruption. Median values for the total and integrated HIV-1 DNA measurements for each arm and at each time point are reported below the x-axis. Viral rebound was associated with a small, transient increase in total and integrated HIV-1 DNA in the placebo group at the time of ART resmnption with a return at six months after ART resmnption to levels observed before analytic treatment interruption. There were no significant differences in total or integrated HIV-1 DNA between placebo and VRC01 recipients at any of the time points tested (all p>0⋅05). There was no statistically significant correlation (p<0⋅05) between total HIV-1 DNA (panel C) or integrated HIV-1 DNA (panel D) and time to HIV-1 RNA ≥20 copies per mL. Correlation analyses were repeated with only VRC01 recipients (r and p in parentheses).
All participants had reactive second- and third-generation HIV diagnostic immunoassays at the time of analytic treatment interruption while three were non-reactive by fourth-generation immunoassay. Of these, two seroconverted by the time of ART resumption and one remained non-reactive. ADCC responses were not detectable prior to ART initiation during acute HIV infection, but were detected at the time of analytic treatment interruption, ART resumption, and 24 weeks later, with no differences between VRC01 and placebo recipients (appendix p 18). In contrast, ADCP responses were already detectable during acute HIV infection, with higher MN-specific ADCP activity among placebo recipients as compared to VRC01 recipients during acute HIV infection and at the time of ART resumption (appendix p 18). VRC01 concentration did not correlate with concurrently measured ADCC or ADCP responses (appendix p 19). Measurements of ADCC, ADCP, and CD4/CD8 ratio did not correlate with time to HIV-1 RNA ≥20 copies per mL (appendix p 20). At the time of ART resumption, the frequencies of activated effector CD8+ T-cells (Ki67+Bcl-21oCD8+ and CD38+HLA-DR+CD8+) were lower in VRC01 recipients versus placebo recipients and did not correlate with time to HIV-1 RNA ≥20 copies per mL (appendix p 22).
DISCUSSION
In this study of acutely-treated HIV-infected adults, only one participant—a VRC01 recipient—achieved the primary efficacy endpoint of HIV-1 RNA <20 copies/mL at 24 weeks after analytic treatment interruption. On average, VRC01 recipients experienced an approximately two-week delay in viral rebound as compared to contemporaneous placebo controls. This difference was statistically significant but not of sufficient clinical significance to alter standard HIV treatment practices. The median time to HIV-1 RNA ≥20 copies per mL among our VRC01 recipients was similar to that observed in chronically-treated HIV-infected participants who received VRC01.13 A study of a related broadly-neutralizing antibody, 3BNC117, pre-screened chronically-treated HIV-infected participants for susceptible virus and observed a longer time to viral rebound.20 However, these studies used historic controls rather than contemporaneous placebo controls.
A small HIV reservoir size is proposed to enhance the chance of HIV remission.21 However, in our study, viral rebound was not substantially delayed as compared to prior studies of chronically-treated individuals despite total HIV-1 DNA levels before treatment interruption that were 10-100 times lower.13,20 The pace of viral rebound may have been influenced by limited HIV-specific immune responses following rapid viral suppression with early ART as well as unique host (Thai) and virus (CRF01_AE) characteristics.22
Participants in this study experienced viral rebound despite serum VRC01 levels 3-10 times the target trough of 50 μg per mL sufficient to neutralize 91% of primary multi-subtype HIV-1 isolates evaluated by a single round of infection by Env-pseudoviruses in TZM-bl cells.9 Other assays, such as those assessing neutralization of replicating virus derived from outgrowth cultures, may provide different results. There was an expected dose-response relationship between serum VRC01 concentration and neutralization activity against a standardized panel of pseudoviruses. Serum VRC01 levels achieved in this study were similar to those reported by others.12,13,23 However, the optimal dose of VRC01 is unknown and it is possible that a higher dose may be safe and more effective. Studies in chronically HIV-infected participants showed that VRC01 alone was insufficient to suppress viremia and resistance developed during viremia on VRC01.13 Our ongoing analysis of pseudovirus neutralization data from 12 VRC01 recipients during acute HIV infection and eight at the time of viral rebound indicated two cases of pre-existing phenotypic resistance to VRC01 without emergence of resistance (data not shown).
One participant demonstrated viral suppression for 42 weeks after analytic treatment interruption despite a number of characteristics previously shown to be associated with rapid disease progression and viral rebound. This participant had the highest total HIV DNA in CD4+ T-cells in the study,24 CD4/CD8 ratio <1,25 and unfavorable HLA-B alleles.26 This highlights the knowledge gap in understanding biomarkers for post-treatment control.
The generation of CD8+ T-cells after broadly-neutralizing antibody administration during acute viremia in rhesus macaques was possibly driven by immune complexes and likely contributed to long lasting viral control.8 In our study that initiated VRC01 infusions at the time of analytic treatment interruption in aviremic participants, the induced CD8+ T-cell responses were lower in VRC01 than placebo recipients at ART resumption, despite delayed viral rebound in the former group. It is possible that VRC01 reduced HIV-1 antigen exposure during analytic treatment interruption that would have been required for a robust response. We cannot exclude that CD8+ T-cells reduced HIV-infected cells in tissues. Their potential role in suppressing or delaying HIV rebound after broadly-neutralizing antibody administration requires further evaluation.
This study demonstrated that closely-monitored treatment interruption with a low viral load threshold for ART resumption can be performed safely to evaluate the impact of therapeutic interventions. Optimal ART resumption criteria after analytic treatment interruption would maintain participant safety while enabling assessment of scientific endpoints of interest. In this study, ART was restarted when HIV-1 RNA was 1000 copies per mL or greater for at least three days and no lasting adverse impact of the treatment interruption was observed. There were no cases of acute retroviral syndrome and no new drug resistance mutations. A small increase in total HIV-1 DNA with analytic treatment interruption in the placebo group was reversed following re-treatment, as seen in ART interruption studies enrolling chronically-treated HIV-infected participants.27 This strategy enabled evaluation of initial viral rebound kinetics that could not have been evaluated with a lower threshold for ART re-initiation, such as HIV-1 RNA of 20 or 50 copies per mL, but did not allow for evaluation of other endpoints such as viral set point. Furthermore, this strategy may miss cases of spontaneous viral re-suppression following an initial rebound, as has been observed in some studies with longer analytic treatment interruption that permitted a greater magnitude of viremia.28 An even higher threshold, such as 10,000 copies per mL, and allowance for sustained viremia over a longer period of time could be scientifically valuable if proven safe.
It is important to note that findings from a relatively small, carefully-selected participant population composed primarily of Thai men with HIV-1 subtype CRF01_AE may not be generalisable to other populations. The number of randomised participants was less than targeted, which reduced the study’s power to detect significant effects.
It is likely that durable control of viremia using immunotherapies such as broadly-neutralizing antibodies will require multiple agents with different therapeutic targets, as demonstrated in non-human primate models 11,29 and one recent human clinical trial.30 Combination immunotherapy administered during acute infection may also facilitate HIV remission by limiting reservoir formation and promoting potent, HIV-specific immune responses.8 Further research is needed to characterize correlates of post-treatment control with and without receipt of investigational products such as VRC01.
In summary, VRC01 was safe when administered during analytic treatment interruption but did not significantly impact the proportion of participants with viral suppression at 24 weeks after ART cessation. VRC01 monotherapy modestly delayed viral rebound. Optimization of strategies that use broadly-neutralizing antibodies and other immunotherapies to induce HIV remission will likely require the development and testing of combination approaches.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Antiretroviral therapy (ART) suppresses human immunodeficiency virus (HIV-1) replication but does not eradicate infection. Latent cellular reservoirs of HIV-1 contribute to rebound viremia that generally occurs within weeks of ART discontinuation. HIV-1-specific broadly-neutralizing antibodies could promote durable control of plasma viremia in the absence of ART by facilitating clearance of infected cells and virions, and enhancing host immune responses to HIV-1. VRC01 is a broadly-neutralizing antibody that targets the CD4-binding site of the HIV-1 envelope protein and has demonstrated broad in vitro neutralization capacity against all major circulating subtypes. We searched PubMed using the terms “HIV,” “VRC01,” and “broadly neutralizing antibody” for articles published through September 30, 2018, without restriction on article type. We also tracked clinical trials of VRC01 and other broadly-neutralizing antibodies registered with ClinicalTrials.gov. Several studies of VRC01 and similar antibodies in non-human primate models demonstrated antiviral activity with reduction of viremia and blunted reservoir formation. In clinical trials, VRC01 was safe and well-tolerated in both HIV-uninfected and HIV-infected volunteers. Antiviral activity was confirmed in vivo by a reduction in plasma viremia with VRC01 administration. VRC01 infusions every three weeks during analytic treatment interruption in participants who initiated ART during chronic HIV-1 infection delayed viral rebound as compared to historic controls, although the vast majority in both groups experienced rebound within eight weeks. We found no randomised controlled trials of VRC01 and no reports of administration to participants who initiated ART during acute HIV infection.
Added value of this study
In this randomised, double-blinded, placebo-controlled clinical trial of VRC01 administration during analytic treatment interruption in adults who began ART during acute HIV infection, VRC01 was safe and well-tolerated but did not significantly impact the proportion of participants with viral suppression at 24 weeks after ART cessation. On average, viral rebound was delayed by about two weeks among VRC01 recipients as compared to placebo controls. One VRC01 recipient remained virally suppressed for 42 weeks and all other participants restarted ART due to confirmed viremia of 1000 copies per mL or greater within 8 weeks, demonstrating that monotherapy with VRC01 is insufficient to control viral replication even within a favorable population of individuals who initiated ART during acute HIV infection. There were no serious adverse events, no cases of acute retroviral syndrome, no new drug resistance mutations, and no lasting increases in total or integrated HIV-1 DNA, demonstrating that closely-monitored treatment interruption can be performed safely to evaluate the impact of therapeutic interventions.
Implications of all the available evidence
For most individuals with HIV, durable control of viremia will likely require combination therapy with multiple broadly-neutralizing antibodies and agents with different therapeutic targets. Closely-monitored treatment interruption with a low viral load threshold for ART initiation is a safe strategy for evaluating the efficacy of these therapies, but studies that include longer analytic treatment interruption and permit a greater magnitude of viremia should be considered and evaluated for safety. Further research is needed to characterize correlates of post-treatment control with and without receipt of investigational products such as VRC01.
ACKNOWLEDGMENTS
This work was supported by cooperative agreements [W81XWH-07-2-0067, W81XWH-11-2-0174] between The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of the Army; by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (NIH) and the Delaney AIDS Research Enterprise to find a cure [1U19AI096109]; via federal funds from the National Cancer Institute at the NIH [Contract No. HHSN261200800001E]; and by an intramural grant from the Thai Red Cross AIDS Research Centre. JA and LT are partially funded by the National Institute of Allergy and Infectious Diseases [R01AI108433]. Antiretroviral therapy in RV254 was supported by the Thai Government Pharmaceutical Organization, Gilead, Merck and ViiV Healthcare.
We thank our study participants and staff from the Thai Red Cross AIDS Research Centre, Chulalongkorn University and AFRIMS for their valuable contributions to this study. We are grateful to the Thai Government Pharmaceutical Organization, Gilead, Merck, and ViiV Healthcare for providing antiretroviral medications.
The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army, the Department of Defense, the National Institutes of Health, or the Department of Health and Human Services. The investigators have adhered to the policies for protection of human subjects as prescribed in AR-70.
Footnotes
Prior Presentation: This work was presented, in part, at the International AIDS Society Conference on HIV Science in Paris, France, 23-26 July 2017.
DECLARATION OF INTERESTS
Trevor Crowell has received a speaker fee from Gilead Sciences. Nicolas Chomont has served on the scientific advisory board of Theravectys. Jintanat Ananworanich has participated in advisory meetings for ViiV Healthcare, Merck, AbbVie, Gilead, and Roche. The remaining authors report no relevant conflicts of interest.
DATA SHARING STATEMENT
The Henry M. Jackson Foundation for the Advancement of Military Medicine (HJF) and the U.S. Department of the Army are committed to safeguarding the privacy of research participants. De-identified participant-level data and accompanying research resources are available upon request. Distribution of data will require compliance with all applicable regulatory and ethical processes, including establishment and approval of an appropriate data-sharing agreement. The research protocol, informed consent documents, and instructions for submitting data requests can be found at https://www.hivresearch.org/RV397_Protocol.
Contributor Information
Trevor A. Crowell, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA.
Donn J. Colby, SEARCH, The Thai Red Cross AIDS Research Center, Bangkok, Thailand.
Suteeraporn Pinyakorn, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA.
Carlo Sacdalan, SEARCH, The Thai Red Cross AIDS Research Center, Bangkok, Thailand.
Amélie Pagliuzza, Centre de Recherche du CHUM and Department of Microbiology, Infectiology and Immunology, Université de Montréal, Quebec, Canada.
Jintana Intasan, SEARCH, The Thai Red Cross AIDS Research Center, Bangkok, Thailand.
Khunthalee Benjapornpong, SEARCH, The Thai Red Cross AIDS Research Center, Bangkok, Thailand.
Kamonkan Tangnaree, SEARCH, The Thai Red Cross AIDS Research Center, Bangkok, Thailand.
Nitiya Chomchey, SEARCH, The Thai Red Cross AIDS Research Center, Bangkok, Thailand.
Eugène Kroon, SEARCH, The Thai Red Cross AIDS Research Center, Bangkok, Thailand.
Mark S. de Souza, Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA; SEARCH, The Thai Red Cross AIDS Research Center, Bangkok, Thailand.
Sodsai Tovanabutra, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA.
Morgane Rolland, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA.
Michael A. Eller, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA.
Dominic Paquin-Proulx, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA.
Diane L. Bolton, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA.
Andrey Tokarev, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA.
Rasmi Thomas, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA.
Hiroshi Takata, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA.
Lydie Trautmann, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA.
Shelly J. Krebs, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA.
Kayvon Modjarrad, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA.
Adrian B. McDermott, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Robert T. Bailer, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Nicole Doria-Rose, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Bijal Patel, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Robert J. Gorelick, AIDS and Cancer Virus Program, Frederick National Laboratory for Cancer Research, Frederick, MD, USA.
Brandie A Fullmer, AIDS and Cancer Virus Program, Frederick National Laboratory for Cancer Research, Frederick, MD, USA.
Alexandra Schuetz, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA; Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand.
Pornsuk V. Grandin, Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand.
Robert J. O’Connell, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand.
Julie E. Ledgerwood, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Barney S. Graham, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Randall Tressler, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
John R. Mascola, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Nicolas Chomont, Centre de Recherche du CHUM and Department of Microbiology, Infectiology and Immunology, Université de Montréal, Quebec, Canada.
Prof Nelson L. Michael, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA.
Merlin L. Robb, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA.
Nittaya Phanuphak, SEARCH, The Thai Red Cross AIDS Research Center, Bangkok, Thailand.
Prof Jintanat Ananworanich, U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA; SEARCH, The Thai Red Cross AIDS Research Center, Bangkok, Thailand; Department of Global Health, University of Amsterdam, Amsterdam, the Netherlands.
REFERENCES
- 1.Palella FJ Jr., Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. The New England journal of medicine 1998; 338(13): 853–60. [DOI] [PubMed] [Google Scholar]
- 2.Chun TW, Stuyver L, Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proceedings of the National Academy of Sciences of the United States of America 1997; 94(24): 13193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997; 278(5341): 1295–300. [DOI] [PubMed] [Google Scholar]
- 4.Rothenberger MK, Keele BF, Wietgrefe SW, et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proceedings of the National Academy of Sciences of the United States of America 2015; 112(10): E1126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deeks SG, Lewin SR, Ross AL, et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med 2016; 22(8): 839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun TW, Murray D, Justement JS, et al. Broadly neutralizing antibodies suppress HIV in the persistent viral reservoir. Proceedings of the National Academy of Sciences of the United States of America 2014; 111(36): 13151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoofs T, Klein F, Braunschweig M, et al. HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science 2016; 352(6288): 997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura Y, Gautam R, Chun TW, et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 2017; 543(7646): 559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu X, Yang ZY, Li Y, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 2010; 329(5993): 856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gautam R, Nishimura Y, Pegu A, et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 2016; 533(7601): 105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolton DL, Pegu A, Wang K, et al. Human Immunodeficiency Virus Type 1 Monoclonal Antibodies Suppress Acute Simian-Human Immunodeficiency Virus Viremia and Limit Seeding of Cell-Associated Viral Reservoirs. Journal of virology 2016; 90(3): 1321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch RM, Boritz E, Coates EE, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med 2015; 7(319): 319ra206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bar KJ, Sneller MC, Harrison LJ, et al. Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. The New England journal of medicine 2016; 375(21): 2037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ananworanich J, Chomont N, Eller LA, et al. HIV DNA Set Point is Rapidly Established in Acute HIV Infection and Dramatically Reduced by Early ART. EBioMedicine 2016; 11: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buzon MJ, Martin-Gayo E, Pereyra F, et al. Long-term antiretroviral treatment initiated at primary HIV-1 infection affects the size, composition, and decay kinetics of the reservoir of HIV-1-infected CD4 T cells. Journal of virology 2014; 88(17): 10056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts HE, Hurst J, Robinson N, et al. Structured observations reveal slow HIV-1 CTL escape. PLoS Genet 2015; 11(2): e1004914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS pathogens 2013; 9(3): e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robb ML, Eller LA, Kibuuka H, et al. Prospective Study of Acute HIV-1 Infection in Adults in East Africa and Thailand. The New England journal of medicine 2016; 374(22): 2120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Souza MS, Phanuphak N, Pinyakorn S, et al. Impact of nucleic acid testing relative to antigen/antibody combination immunoassay on the detection of acute HIV infection. Aids 2015; 29(7): 793–800. [DOI] [PubMed] [Google Scholar]
- 20.Scheid JF, Horwitz JA, Bar-On Y, et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 2016; 535(7613): 556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinkevych M, Cromer D, Tolstrup M, et al. HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5–8 Days--Implications for HIV Remission. PLoS pathogens 2015; 11(7): e1005000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colby DJ, Trautmann L, Pinyakorn S, et al. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med 2018; 24(7): 923–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledgerwood JE, Coates EE, Yamshchikov G, et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol 2015; 182(3): 289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams JP, Hurst J, Stohr W, et al. HIV-1 DNA predicts disease progression and post-treatment virological control. Elife 2014; 3: e03821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurst J, Hoffmann M, Pace M, et al. Immunological biomarkers predict HIV-1 viral rebound after treatment interruption. Nat Commun 2015; 6: 8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park YJ, Etemad B, Ahmed H, et al. Impact of HLA Class I Alleles on Timing of HIV Rebound After Antiretroviral Treatment Interruption. Pathog Immun 2017; 2(3): 431–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarridge KE, Blazkova J, Einkauf K, et al. Effect of analytical treatment interruption and reinitiation of antiretroviral therapy on HIV reservoirs and immunologic parameters in infected individuals. PLoS pathogens 2018; 14(1): e1006792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sneller MC, Justement JS, Gittens KR, et al. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med 2017; 9(419): eaan8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barouch DH, Whitney JB, Moldt B, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 2013; 503(7475): 224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendoza P, Gruell H, Nogueira L, et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 2018; 561(7724): 479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


