Abstract
It has been known for over four decades that prenatal alcohol exposure (PAE) can adversely affect neurodevelopment and behavior (NDB). Yet, early detection of altered NDB due to PAE continues to present a major clinical challenge. Identification of altered NDB in the first two years of life, before higher order cognitive processes develop, invites early interventions for affected children to improve long-term outcomes. Studies published in English from January of 1980 to July of 2018 were identified in PubMed/Medline. The review focused on prospective birth cohort studies which used standardized NDB assessments in children up to 2 years of age, wherein PAE was the main exposure and NDB was the main outcome. NDB was categorized into the domains of neurocognitive, adaptive, and self-regulation based on the 2016 Updated Clinical Guidelines for diagnosing FASD. An initial search resulted in 1,867 articles for which we reviewed abstracts; 114 were selected for full-text review and 3 additional abstracts were identified through review of references in eligible publications. Thirty-one publications met criteria and were included; of these, 24 reported neurocognitive outcomes, 24 reported adaptive behavior outcomes, and 12 reported outcomes in the domain of self-regulation. Although self-regulation was assessed in the fewest number of studies, 8/12 (75%) reported PAE-associated deficits. In contrast, results were mixed for the other two domains: 13/24 (54%) of the selected studies that included neurocognitive outcomes showed poorer performance following PAE, and 8/24 (33%) studies that assessed adaptive functioning found significant differences between PAE and comparison infants. There is considerable evidence to support the value of early-life assessments of infant NDB when PAE is known or suspected. More studies focusing on infant self-regulation, in particular, are needed to determine the utility of early evaluation of this critical developmental domain in infants with PAE.
INTRODUCTION
Since the broader recognition of alcohol as a teratogen in the 1970s (Jones and Smith, 1973), clinical research has demonstrated a wide range of adverse effects related to prenatal alcohol exposure (PAE), which are encompassed under the umbrella term of fetal alcohol spectrum disorders (FASD). FASD is estimated to affect 1.1–4.8% of school-aged children in the U.S., with approximately 2.3% global prevalence (May et al., 2018, Roozen et al., 2016). While the hallmark physical abnormalities of fetal alcohol syndrome (FAS) are most well-known and represent the most visible of alcohol’s teratogenic effects, it is increasingly recognized that the neurodevelopmental and behavioral (NDB) outcomes related to PAE pose the greatest challenges across an individual’s lifespan (Reid et al., 2015). NDB outcomes of PAE commonly lead to significant disruptions in education, social relationships, employment status, and involvement in illegal activities (Freunscht and Feldmann, 2011, Gagnier et al., 2011). Early FASD diagnosis and treatment are known protective factors against these challenges (Streissguth et al., 2004, Alex and Feldmann, 2012). Yet, in the majority of cases, diagnostic referral and subsequent treatment is not initiated until a child is well into their school-age years (average 9–10 years of age) and has already experienced significant difficulties with learning, classroom behaviors, and formation of positive social relationships (Astley, 2010, Streissguth et al., 2004). Studies show this delay could be further exacerbated in children without full FAS and those who are not in the custody of a biological parent (Bakhireva et al., 2018a). On average, referral for FASD diagnosis occurs at 9 years of age; however, more than one-half of children who eventually receive a diagnosis of FASD exhibited some form of measurable NDB deficit in the first three years of life, and fewer than one-third of affected children are referred for diagnosis in time to receive early intervention (Olson et al., 2007).
There are numerous factors which make the early identification of PAE-related NDB deficits challenging. First, screening pregnant women for alcohol use, e.g. by screening questionnaires and/or ethanol biomarkers, has not been universally implemented (Waterman et al., 2013). Maternal self-report might be subject to bias (Eichler et al., 2016), and use of ethanol biomarkers in pregnancy remains largely experimental or reserved for surveillance purposes (Bakhireva and Savage, 2011, Bakhireva et al., 2017, Montag, 2016). Second, in cases where PAE is known, improper or inadequate training, education, and resources among healthcare professionals may contribute to suboptimal follow-up of the child (Gahagan et al., 2006). Third, children with heavy PAE might be disproportionately affected by severe adverse socio-environmental contexts (Lange et al., 2013) that impose greater urgency when assessing and prioritizing intervention needs. Multiple studies, including our own, indicate that caregiver instability poses a major risk factor hindering the accurate and timely diagnosis of FASD (Bakhireva et al., 2018a, Astley, 2010) in as many as 87% of cases (Chasnoff et al., 2015), often due to lack of reliable history of PAE (Bakhireva et al., 2018a). Fourth, and of central interest in this review paper, prevailing FASD diagnostic approaches lean heavily upon the ability to measure social, cognitive, or behavioral delays or deficits which may not easily be assessed until after a child has surpassed the toddler years and entered the classroom environment (Hoyme et al., 2016, Olson et al., 2007, Streissguth et al., 1994). Even among the minority (~10%) of patients with FASD that have facial features specific to FAS, diagnosis rarely is established until age 4 years or older (Moberg et al., 2014).
The identification of a specific NDB phenotype of FASD has occupied ongoing interest among researchers (Kodituwakku, 2009, Mattson et al., 2011, Quattlebaum and O’Connor, 2013, Nash et al., 2013, Stevens et al., 2013, Coriale et al., 2013, Olson et al., 1998) owing to the obvious clinical utility of establishing such a profile in a spectrum of disorders which, more often than not, lacks physical evidence. Yet, evidence of significant social, cognitive, and behavioral impairment related to PAE is observed throughout the lifespan of affected individuals (Streissguth et al., 1994). Recently, a general consensus has emerged regarding the recognition of three key NDB diagnostic domains: neurocognitive, adaptive, and self-regulation. These domains of impairment are now included in the Updated Clinical Guidelines for Diagnosing FASD (Hoyme et al., 2016) and the proposed DSM-5 criteria for diagnosing neurodevelopmental disorders associated with PAE (ND-PAE) (Kable et al., 2016). The delineation of these domains within the rubrics of the diagnostic guidelines represents an important step towards clarifying the NDB phenotype of FASD. However, the identification of these domains has occurred largely through case ascertainment research conducted among school-age children (May et al., 2018), while characterization of NDB impairment in the age range of 0–2 years remains the least specific (Hoyme et al., 2016).
Several important reviews have been published in the last decade which summarize the scientific progress that has been made regarding the identification of a neuropsychological profile or phenotype of FASD (Kodituwakku, 2009, Mattson et al., 2011). However, to our knowledge, these have been restricted to or primarily focused on the outcomes of children greater than two years of age. This has led to a persistent gap in knowledge concerning the early identification of adverse effects in infants and toddlers with PAE which continues to pose a major obstacle to providing appropriate early interventions (Hoyme et al., 2016, Coles et al., 2000). The present systematic review critically examines what has been learned in the past four decades about NDB outcomes related to PAE in the first two years of life. In particular, we attempt to map the existing research in this age group onto the rubric of NDB domains (adaptive, neurocognitive, and self-regulation) according to the prevailing diagnostic criteria for FASD/ND-PAE outlined in the updated 2016 Guidelines (Hoyme et al., 2016) and DSM-5 (Kable et al., 2016).
METHODS
Selection criteria and literature search
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines in planning and executing this review of the literature (Moher et al., 2009). Studies included in this review were identified through two main sources. A comprehensive PubMed search was performed covering English language articles published between January 1980 and July 2018. We incorporated both keywords and controlled vocabulary (Medical Subject Headings, MeSH terms) in our search strategy. Various terms associated with alcohol, infant development or behavior, cognition, and pregnancy were determined by the authors, with the search performed by an experienced health sciences librarian (S.M.). The search strategy used to identify articles for inclusion is available upon request. Additional articles were identified through reviewing the reference lists of eligible manuscripts.
Eligibility
Inclusion criteria for this review were as follows: 1) human, prospective cohort design; 2) prenatal enrollment with prospective (during pregnancy) assessment of alcohol use; 3) published in English; 4) published between January of 1980 and July of 2018; 5) PAE was a primary exposure of interest; 6) NDB outcomes, which fell into categories of neurocognition, self-regulation, and adaptive behavior, were primary outcomes of interest; 7) NDB was measured using a structured or standardized assessment; and 8) cohort infants were assessed between ages 3 months and 2 years. The following exclusion criteria were also applied: 1) studies measuring only physiological responses (e.g., eyeblink conditioning, cortisol levels, or heart rate variability) as indicators of stress or information processing; 2) studies which did not independently measure PAE separately from co-exposures (i.e., cocaine, marijuana, etc.); 3) nested case-control studies, such as primary grouping based on FASD with matched controls; and 4) publications containing data analyses which overlapped with other reports (same cohorts) meeting eligibility criteria.
Selection of studies
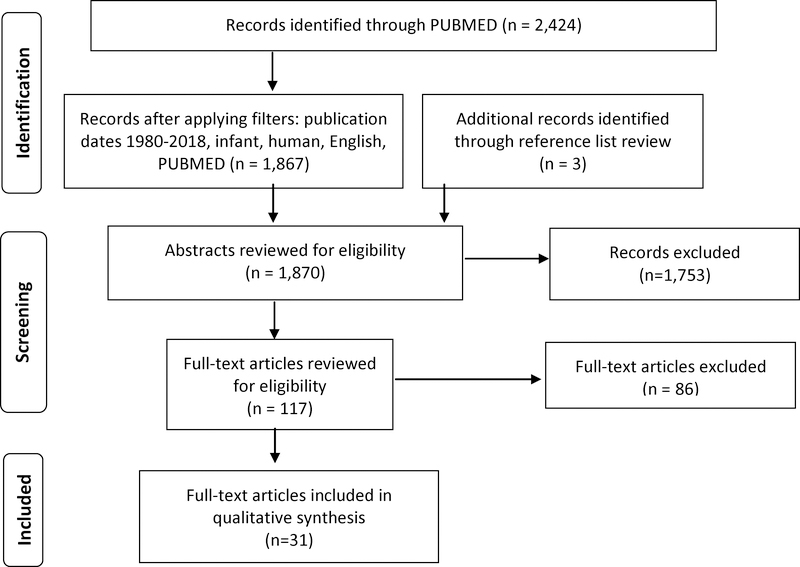
A total of 2,987 citations were retrieved from the PubMed Medline database. The search strategy incorporated text words and MeSH terms as follows: ((alcohol AND (pregnancy or pregnant) AND ((infant and (development or behavior)))) OR ((((((((((“Cognition”[Mesh]) OR “Language”[Mesh]) OR “Affect”[Mesh]) OR “Infant Behavior”[Mesh]) OR “Neurodevelopmental Disorders”[Mesh]) OR “Neuropsychological Tests”[Mesh])) AND (“Fetal Alcohol Spectrum Disorders”[Mesh] OR “Ethanol”[Mesh] OR “Alcohol-Induced Disorders”[Mesh] OR “Alcohol Drinking”[Mesh]))) AND ((“Mothers”[Mesh]) OR “Pregnancy”[Mesh])). After applying specific filters (publication date, English language, infant birth-23 months, human) the results list contained 1,867 citations. Also included were three additional citations identified through a reference list review. Abstracts of the citations containing the applicable terms were reviewed (N=1,870), and relevant articles (N=117) were chosen for full-text review. When the eligibility of a full-text article was unclear or in question, a final decision was reached by consensus among co-authors. Of the full-texts we reviewed, 86 articles were excluded (Figure 1), and our final sample size of included studies was 31 (N=31).
Figure 1.
Selection of Articles for Review
Behavioral domain classification
Key NDB assessments used in the included publications were ‘mapped’ onto the corresponding NDB domains of neurocognition, self-regulation, and adaptive behaviors (Table 1). Each of the included publications was additionally categorized based on the assessed domains (Table 2). Both Table 1 and Table 2 classifications were determined following the domain definitions outlined in the Updated Clinical Guidelines for Diagnosing FASD (Hoyme et al., 2016) and the proposed DSM-5 criteria for diagnosing ND-PAE (Kable et al., 2016). All co-authors agreed on the final domain classifications of included studies herein presented, while acknowledging that such categorization is somewhat subjective.
Table 1.
Common Assessment Tools and Corresponding NeuroDevelopmental or Behavioral Domain.
| Assessment Name | Primary Domain |
|---|---|
| Mental Development Index/Cognitive - Bayley Scales of Infant Development | Neurocognitive* |
| Fagan Test of Infant Intelligence | Neurocognitive |
| Cross-Modal Transfer | Neurocognitive |
| Visual Recognition Memory | Neurocognitive |
| Griffiths Mental Development | Neurocognitive, Adaptive |
| Reynell Comprehensive and Expressive Language | Adaptive |
| Expressive and Receptive Scales of the Sequenced Inventory of Communication Development | Adaptive |
| Psychomotor Development Index/Motor Scale - Bayley Scales of Infant Development | Adaptive |
| Infant Behavior Questionnaire | Self-Regulation |
| Play/Complexity of Play (Spontaneous, Elicited, Sustained) | Self-Regulation |
| Behavior Rating Scale | Self-Regulation |
| Infant Behavior Record | Self-Regulation |
| Still-Face Paradigm | Self-Regulation |
| Infant/Toddler Sensory Profile | Self-Regulation |
| Brief Infant/Toddler Social Emotional | Self-Regulation |
| Parenting Stress Index | Self-Regulation |
| Difficult Temperament Scale of the Infant | Self-Regulation |
On newer versions of the BSID, specific items within this portion of the assessment may overlap with other domains; however, it has traditionally been used as a measure of cognition and mental performance and has been categorized here as such to remain consistent with many of the large, older studies in this review. On a more general note, the authors acknowledge there may be significant overlap of the domains captured.
Table 2.
Systematic Review: Individual Study Characteristics and Key Findings
| Publication | Study Design | Domain Evaluated | Outcome Measures/Scales | Key findings | ||
|---|---|---|---|---|---|---|
| Neurocognitive | Self-Regulation | Adaptive | ||||
| (Streissguth et al., 1980) | Cohort, Seattle, USA N= 462 at 8m PAE level: Continuous measure AA/day1 before pregnancy recognition (pre-recognition) and in the 5th month of pregnancy |
X | X | Bayley Scales of Infant Development-I (BSID): Mental Development Index (MDI), Psychomotor Development Index (PDI) | Pre-recognition PAE was associated with MDI, and later-pregnancy PAE was associated with PDI. The threshold where PAE infants differ from controls on MDI and PDI was ~2–4 drinks/day. | |
| (Gusella and Fried, 1984) | Cohort, Ottawa, Canada, N=84 at 13m PAE level: Continuous measure AA/day averaged per trimester and across pregnancy |
X | X | X | BSID-I: MDI, PDI; Infant Behavioral Record (IBR) | PAE (any trimester) was associated with lower MDI; PAE was not associated with PDI or IBR. |
| (Larsson et al., 1985) | Cohort, Sweden, N=73 at 18m-27m PAE level: <30g/day1, 30–125g/day, or >125g/day |
X | X | Griffith’s Mental Development Scale (GMDS) | PAE >125g/day was associated with lower personal/social, eye/hand coordination, and cognitive performance scores. PAE levels ≤125g were not associated with the GMDS. | |
| (Coles et al., 1987) | Cohort, Atlanta, USA, N=60 at 6m PAE level: 1st trimester PAE only or Continuous PAE (mean 12.2AA/wk throughout pregnancy) |
X | X | BSID-I: MDI, PDI | Lower MDI and PDI scores were observed in the PAE groups, but differences did not reach significance. | |
| (Fried and Watkinson, 1988) | Cohort, Canada, N=217 at 12m and N=153 at 24m PAE level: Heavy (>0.85 AA/day) vs. None/minimal (<0.14 AA/day) |
X | X | X | BSID-I: MDI and PDI; IBR; Reynell Comprehensive and Expressive language | PAE was associated with lower MDI and Reynell Comprehension scores at 24m. These associations were not present at 12m. PAE was not associated with PDI or IBR at either assessment age. |
| (Greene et al., 1990) | Cohort, Cleveland, USA, N=279 at 12m and N=275 at 24m PAE Level: Michigan Alcohol Screening Test (MAST) score >5 and averaged AA/day |
X | Expressive and Receptive Scales of the Sequenced Inventory of Communication Development | No association was found between PAE and language scores at either time point. | ||
| (Autti-Ramo and Granstrom, 1991a) | Cohort, Finland, N=109 at 18m PAE level: None-slight (<28g/wk), moderate (28–140g/wk or 110–630g/month), heavy during trimesters 1 and 2 (>140g/wk or >630g/month), continuous heavy |
X | X | FMTOT: Fine motor development; LTOT: Language development MTOT: Mental development (sum of FMTOT and LTOT) | Heavy (trimesters 1–2) and continuous heavy PAE were associated with poorer performance on motor, speech, and mental development scores. | |
| (Forrest et al., 1991) | Cohort, Scotland, N=592 at 18m PAE level: Mild (1–49g/wk), moderate (50–99g/wk), heavy (≥100g/wk) |
X | X | BSID-I: MDI, PDI | No significant differences were observed at any PAE level. | |
| (Autti-Ramo and Granstrom, 1991b) | Cohort, Finland, N=80 at 3–5m and 5–8m PAE level: None-slight (<28g/wk), moderate (28–140g/wk or 110–630g/month), heavy during trimesters 1 and 2 (>140g/wk or >630g/month), continuous heavy |
X | Münchener Funktionelle Entwicklungsdiagnostik (MFED) |
At 3–5m: PAE longer duration associated with poorer performance in sitting, supine, and prehension 5–8m: PAE longer duration associated with poorer active speech development, comprehension, and social. |
||
| (Jacobson et al., 1993b) | Cohort, Detroit, USA, N=403 at 6.5m, 12m, and 13m PAE level: AA/day at conception ≥0.5 and AA/day during pregnancy (2nd and 3rd trimesters) |
X | X | Visual Recognition Memory (VRM); Cross-modal Transfer; Play: Spontaneous, Elicited, and Sustained directed activity (SDA) | PAE at conception and later in pregnancy were independently associated with poorer Spontaneous and Elicited Play. Only PAE at conception was associated with SDA. PAE later in pregnancy was associated with poorer speed of processing on both VRM and Cross-Modal Transfer. | |
| (Jacobson et al., 1993a) | Cohort, Detroit, USA, N=382 at 13m PAE level: AA/day at conception ≥0.5 and AA/day during pregnancy (2nd and 3rd trimesters) |
X | X | BSID-I: MDI, PDI | AA/day in pregnancy was associated with poorer MDI scores. AA/day ≥0.5 in pregnancy was associated with a 2-fold increased likelihood of scoring in lowest 10th percentile of MDI. AA/day ≥2 in pregnancy was associated with a 5-fold increased likelihood of scoring in the bottom 10th percentile of PDI (p<0.001). | |
| (Streissguth et al., 1994) | Cohort, Seattle, USA N= 496 at 18m PAE level: Continuous measure AA/day (on average, >1 drink/day pre-recognition) |
X | X | BSID-I: MDI, PDI | PAE was not associated with MDI or PDI. | |
| (Olsen, 1994) | Cohort, Denmark, N=276 at 18m PAE Level: ≥5 drinks/wk in 1st trimester |
X | X | BSID-I: MDI and PDI | PAE was not associated with MDI or PDI. | |
| (Richardson et al., 1995) | Cohort, Pittsburgh, USA, N=592 at 9m and N=645 at 19m PAE level: Light-to moderate (≥3 SDU/wk) vs. none-to-minimal (<3 SDU/wk) |
X | X | BSID-I: MDI and PDI | PAE was not associated with MDI or PDI at either assessment age. | |
| (Jacobson et al., 1996) | Cohort, Detroit, USA, N=72–480 (range) at 6.5m, 12m, and 13m PAE level: AA/day at conception ≥0.5 and AA/day during pregnancy (2nd and 3rd trimesters) |
X | X | X | BSID-I: MDI and PDI; Fagan Test of Infant Intelligence (FTII); Play: Spontaneous and Elicited | PAE was associated with poorer Elicited Play at 12m and MDI at 13m; when stratified by maternal age (≤30 years vs >30 years), differences were significant only for maternal age >30. PAE was not associated with the PDI, FTII, or Spontaneous Play. |
| (Seagull et al., 1996) | Cohort, Detroit, USA, N=120 at 11m PAE Level: Averaged AA/day: Light (<1 drink/day), Heavy (1+ drink/day), Binge (>5 drinks/day) |
X | X | BSID-I: MDI and PDI | PAE (any level) was associated with poorer MDI scores.2 PAE was not associated with PDI. | |
| (Kaplan-Estrin et al., 1999) | Cohort, Detroit, USA, N=92 for MDI and N=89 for PDI at 13m PAE level: AA/day at conception ≥0.5 and AA/day during pregnancy (2nd and 3rd trimesters) |
X | X | BSID-I: MDI, PDI | Periconceptional AA/day was associated with poorer MDI and PDI; AA/day during pregnancy was associated with MDI but not with PDI. | |
| (Backstrand et al., 2001) | Cohort, Mexico, N=70 at 6m PAE level: No use, Any use (median consumption among those with ‘any use’ was 11.8g/day, approx.. 1 drink/day)3 |
X | X | BSID-II: MDI, PDI | PAE at >130g/wk was associated with poorer MDI; however, after removing 4 outliers with >450kcal/day from alcohol consumption, PAE was associated with better MDI scores. No associations with PDI. | |
| (Jacobson et al., 2002) | Cohort, Detroit, USA, N=354 at 6.5m-13m PAE Level: AA/day at conception ≥0.5 and AA/day during pregnancy (2nd and 3rd trimesters) |
X | X | X | FTII; Cross-modal transfer; Play: Spontaneous; BSID-I: MDI and PDI | PAE in trimesters 2–3 was associated with poorer FTII, Cross-modal transfer, Spontaneous Play, and MDI. No associations with PDI. |
| (Lowe et al., 2006) | Cohort, Albuquerque, USA, N=76 at 6m PAE level: Positive TWEAK (≥2) or AUDIT (≥5) and reported Continuous (≥1 drink/day) or Binge (≥3 drinks/occasion) drinking in pregnancy |
X | Still Face Paradigm (SFP) | Although PAE was associated with increased positive affect during the first play episode (pre-stressor), in the post-stressor (recovery play) episode PAE was associated with increased infant negative affect.2 | ||
| (Molteno et al., 2010) | Cohort, South Africa, N=107 at 13m PAE level: >1.0 AA/day or >2 binge episodes at conception and across pregnancy |
X | Play: Spontaneous and Elicited | PAE at conception and across pregnancy was associated with poorer Elicited Play. PAE was not associated with Spontaneous Play after controlling for social environment. | ||
| (Alvik et al., 2011) | Cohort, Norway, N=1,330 at 6m PAE level: No binge, <1 binge/wk, ≥1binge/wk (only weeks 0–6 of pregnancy were examined) |
X | Modified Difficult Temperament Scale of the Infant Characteristics Questionnaire (DTS) | Binge ≥1time(s)/wk was associated with poorer overall DTS and poorer performance on the Demanding, Irritable and Uneasy subscales. | ||
| (Fraser et al., 2012) | Cohort, Quebec, N=195 at 6m PAE level: None, any, ≥0.5 AA/day, weekly use, any binge (≥5 drinks), <5 binges, >2 binges/month, and able to consume 13 drinks without passing out |
X | FTII | PAE was not associated with the FTII. | ||
| (Molteno et al., 2014) | Cohort, South Africa, N=144 at 6.5m PAE level: >1.0 AA/day or >2 binge episodes; stratified by AA/day, drinks per occasion, and frequency, at conception and across pregnancy |
X | Alarm Distress Baby Scale (social withdrawal); Emotionality Activity Shyness (EAS) Temperament Scale | PAE frequency at conception and across pregnancy were associated with social withdrawal. PAE drinks per occasion (but not average AA/day or frequency) at conception and across pregnancy were associated with decreased activity. | ||
| (Polanska et al., 2015) | Cohort, Poland, N= 458 at 12m, N=315 at 24m PAE level: ≥1 drink/month |
X | X | BSID-III | PAE was not associated with any BSID-III measures. | |
| (Lehikoinen et al., 2016) | Cohort, Finland, N=42 at 12m PAE level: ≥8 on AUDIT |
X | X | GMDS (1996 Revision) | PAE was not associated with GMDS scores. | |
| (Donald et al., 2016) | Cohort, South Africa, N=73 at 6m PAE level: ≥11 on ASSIST questionnaire and drank ≥2times/wk OR ≥2 drinks/occasion in any trimester |
X | X | BSID-III | PAE was associated with poorer socio-emotional outcomes. PAE was not associated with any other BSID-III sub-scales. | |
| (Bandoli et al., 2016) | Cohort, Ukraine, N=304 at 6m and 12m PAE Level: Weekly binge (5+ drinks) or ≥5 occasions of 3–4 drinks or ≥10 drinking occasions in the month around conception or month before enrollment (average 19 weeks gestation) |
X | X | BSID-II: MDI, PDI | Periconceptional drinking was associated with MDI and PDI at both time points. | |
| (Halliday et al., 2017) | Cohort, Australia, N=554 for BSID-III and N=948 for Sensory Profile at 24m PAE level: Low (≤20g/occasion and ≤70g /wk), Moderate (21–49g/occasion and ≤70g /wk), high (>70g/wk), binge (≥50g/occasion) |
X | X | X | BSID-III, Infant/Toddler Sensory Profile (ITSP), Brief Infant/Toddler Social Emotional | Binge exposure in trimester 1 only, or exposure to any level of alcohol across all three trimesters was associated with poorer outcomes on sensation avoidance of the ITSP. PAE (any level/timing) was not associated with any BSID-III or BITSEA measures after adjusting for covariates. |
| (Lowe et al., 2017) | Cohort, Albuquerque, USA, N=91 at 6m PAE level: ≥3 drinks/wk or ≥ 2 binge episodes around conception and confirmed drinking in pregnancy (any binge or ≥3 drinks/wk or positive ethanol biomarker in pregnancy) |
X | SFP | In univariate analysis, PAE was associated with a reduction in infant positive affect across repeated measures of the SFP; differences became non-significant after adjustment for covariates. | ||
| (Bakhireva et al., 2018b) | Cohort, Albuquerque, USA, N=93 at 6m PAE Level: ≥3 drinks/wk or ≥ 2 binge episodes around conception and confirmed drinking in pregnancy (any binge or ≥3 drinks/wk or positive ethanol biomarker in pregnancy) |
X | X | X | BSID-III, Parenting Stress Index (PSI), Infant Behavior Questionnaire-Revised (IBQ-R), Infant/Toddler Sensory Profile (ITSP) | PAE was associated with poorer outcomes on the ‘difficult child’ and ‘total stress’ PSI scales, as well as ‘negative affect’ on the IBQ-R. PAE was not associated with any BSID-III outcomes. |
AA/day indicates absolute ounces of alcohol per day (0.5 AA/day being roughly equivalent to 1 standard drink unit [SDU]) unless otherwise indicated by grams (1 SDU=14 grams)
Gender effect was observed.
The alcohol consumed in the study was pulque, a traditional drink in Mexico containing high nutrient levels and generally lower alcohol levels.
Briefly, neurocognitive development in infants and toddlers includes ‘global’ indices of intelligence and learning ability (Hoyme et al., 2016, Kable et al., 2016). It is important to note that what are believed to be among the most important and easily-measured (with standardized assessments) neurocognitive indices in PAE-affected individuals (e.g., executive functioning [EF] and intelligence quotient [IQ]) are underdeveloped in infants and toddlers, and thus were not considered in this review. Self-regulation in infants and toddlers includes soothability, social-emotional withdrawal/engagement, affect, stress reactivity, sensory processing, and complexity of play (Hoyme et al., 2016); in older children, other key features of self-regulation include behavioral regulation, impulse control, and attention (Kable et al., 2016). Finally, adaptive behaviors specific to infants and toddlers include gross motor and feeding skills; more generally and in older children, they also include speech, language and communication, social skills, and daily living skills (Hoyme et al., 2016, Kable et al., 2016).
RESULTS
The effects of PAE on NDB in children aged 3 months to 2 years were assessed in 31 prospective birth cohort studies that met our review criteria. Of our final sample of eligible articles, publications examining the neurocognitive (77%, n=24) and adaptive (77%, n=24) behavioral domains were most prevalent, while only 39% (n=12) of eligible publications examined behavioral outcomes that we classified within the self-regulation domain. The majority (61%) utilized a version of the Bayley Scales of Infant Development (BSID) which, in most cases, included both neurocognitive and adaptive behavior portions of the test. A total of 18 additional structured assessments were utilized across included publications; however, none of these were included in more than a few publications, and several were only used in only one publication, preventing generalizable findings related to specific structured assessments.
PAE effects on the neurocognitive domain
A total of 24 publications included in this review contained a specific assessment of neurocognitive behavior, predominantly the Mental or Cognitive scales of the BSID. BSID Mental/Cognitive scale findings related to PAE in the large cohort studies reviewed here were mixed. Notably, PAE was associated with poorer BSID Mental/Cognitive scores in the Detroit (r=−0.22 at 13 months, p<0.05 for a one-sided test; (Kaplan-Estrin et al., 1999)), Ottawa (r=−0.19 at 13 months, p<0.05; (Gusella and Fried, 1984)), and Ukraine (β=−2.91 at 6 months, β=−2.67 at 12 months, p’s<0.05; (Bandoli et al., 2016)) cohorts. No significant differences on the BSID Mental/Cognitive scores in relation to PAE were found in the Australia (β=3.06, 95% CI: −1.19 to 7.30 at 24 months; (Halliday et al., 2017)), New Mexico (mean cognitive scores in the moderate/mild PAE group: 102.4 ± 7.5 vs. No PAE: 101.3 ± 9.3 at 6 months, p>0.05; (Bakhireva et al., 2018b)), and Scotland (PAE 50–99g AA/week: β=1.57; PAE ≥ 100 g AA/week: β=−2.00; all analyses at 18 months, p’s>0.05; (Forrest et al., 1991)) cohorts. Interestingly, in the Seattle cohort, BSID Mental/Cognitive scores were poorer in children with PAE at age 8 months (Streissguth et al., 1980), but normalized when re-assessed at 18 months (Streissguth et al., 1994). Moreover, none of the studies assessing children aged 14 – 24 months found associations between PAE and poorer mental/cognitive performance on the BSID. In another cohort based in rural Central Mexico, an association was found between heavy PAE and poorer mental performance on the BSID, while moderate PAE was associated with improved mental performance in infants aged 6 months. However, this cohort focused on a traditional beverage called Pulque, which could have unique nutritional benefits, coupled with a low-ethanol content, thus possibly preventing direct comparison with PAE from wine, beer, or liquor (Backstrand et al., 2001). In total, approximately half of studies assessing the neurocognitive domain found significant negative effects of PAE in association with a neurocognitive outcome during the first 2 years of life. Overall, 13 of 24 publications (54%) that assessed the neurocognitive domain demonstrated significant deficits with PAE.
Self-Regulation
Self-regulation assessments were only included in 39% of the publications in our review. However, the majority (75%) of publications reporting on self-regulation outcomes found significant delays or deficits in this domain among infants with PAE. Only three publications reporting a self-regulation outcome in relation to PAE found no effect in this domain, and two of these were from the Ottawa cohort, which utilized the Infant Behavior Record (IBR) at multiple assessment ages. The third of these, from our own cohort, utilized the Still-Face Paradigm (SFP) in 6-month-old infants. We found significantly lower positive infant affect across repeated measures in the univariate analyses, but these differences did not reach significance after adjusting for covariates (Lowe et al., 2017).
A variety of self-regulation measures were reviewed, including interactive and observational play paradigms (i.e., SFP, Complexity of Play), and caregiver-completed questionnaires, such as the Behavior Rating Scale, Infant/Toddler Sensory Profile (ITSP), IBR and Infant Behavior Questionnaire (IBQ). As most of these were included in only one or two studies, there is insufficient evidence to suggest that any one is more or less sensitive than others; however, it is noteworthy that most did detect significant problem areas in a PAE group.
Some key findings within the self-regulation domain include the following: PAE was significantly associated with temperament in 6-month infants in the Norway cohort (OR 3.3, 95% CI: 1.4–7.9 using the Difficult Temperament Scale; (Alvik et al., 2011)), increased emotional withdrawal in the South Africa cohort (β=0.26, p<0.05 for an association between more frequent drinking and the Alarm Distress Baby Scale; (Molteno et al., 2014)), and the “Difficult Child” scale of the Parenting Stress Index in the New Mexico cohort (β=13.9, p=0.05; (Bakhireva et al., 2018b)). The New Mexico cohort also found PAE to be significantly related to increased infant negative affect (β=8.60, p=0.008 using the IBQ; (Bakhireva et al., 2018b)). In the Australia cohort (Halliday et al., 2017), binge exposure in early pregnancy with no more than low-to-moderate continued exposure was associated with abnormal sensory avoidance on the ITSP (OR 1.88, 95% CI: 1.03–3.41). PAE throughout pregnancy was also strongly negatively correlated with elicited play (where infant play is initiated through modeling behaviors of an examiner) in 13-month infants in South Africa (r=−.34, p<0.001) and somewhat attenuated by adjustment for socio-environmental confounders (β=0.022, p<0.05; (Molteno et al., 2010)). With only two exceptions where children up to 24 months of age were assessed (Halliday et al., 2017, Fried and Watkinson, 1988), self-regulation assessments reviewed here were predominantly used in infants between 6 months and 13 months of age.
Adaptive behavior
Adaptive behavior was assessed in 24 of the included publications. Compared to the other domains, publications in this domain reported the fewest associations with PAE, with only one-third finding a significant association. Among those which found significant associations between PAE and an adaptive behavior measure, two well-characterized cohorts (Seattle and Detroit) independently produced findings of PAE dose-response effects in 8 month-old (Streissguth et al., 1980) and 13 month-old infants (Jacobson et al., 1993a) associated with deficits on the BSID Motor scale; however, the effect in the Detroit cohort decreased after adjustment for socio-environmental factors and prenatal co-exposures. Additionally, while its results did not reach significance, the Atlanta cohort findings are worth mentioning. In this cohort, continuous PAE was associated with an average score more than 10 points lower than those with only early PAE or no PAE (BSID Motor scores among abstainers=113.9±15.9 vs. early pregnancy PAE only=113.7±15.3 vs. continuous PAE=101.6±22.9; (Coles et al., 1987)).
While the BSID Motor scale was the most prominent structured assessment used in this domain, several studies used other assessments to specifically examine speech development and comprehension as it relates to PAE. In the Finland cohort, increased PAE duration and intensity were associated with poorer speech and comprehension outcomes at 5–8 months and 18 months of age (Autti-Ramo and Granstrom, 1991a, Autti-Ramo and Granstrom, 1991b), while PAE was associated with poorer Reynell Comprehension and Expressive Language outcomes in the Canada cohort in infants assessed at 24 months of age (β=−0.18, p<0.05), but not at 12 months (Fried and Watkinson, 1988). In the Cleveland cohort, no associations were observed in language outcomes at either 12 or 24 months of age (Greene et al., 1990).
DISCUSSION
It is widely acknowledged that indicators of brain vulnerability related to PAE exist in infants (Hoyme et al., 2016), with some indicators potentially being measurable at the time of birth in severe cases (Streissguth et al., 1994, Coles et al., 2000). However, global measures of infant and toddler development have yielded inconsistent results, possibly due to low sensitivity of the assessment tools to identify deficits in very young children (Streissguth et al., 1994, O’Connor et al., 1986, Fried and Watkinson, 1988, Larsson et al., 1985, Forrest et al., 1991, Autti-Ramo and Granstrom, 1991a, Autti-Ramo and Granstrom, 1991b, Jacobson et al., 1996, Jacobson et al., 1993a, Greene et al., 1990, Richardson et al., 1995). Meanwhile, standardized assessments that assess higher-order cognitive processes, such as IQ, executive functioning, verbal memory, and mathematical problem-solving abilities, have been largely successful in identifying PAE-related deficits in older children (Kodituwakku, 2009, Streissguth et al., 1994, Mattson et al., 2011). As these higher-order levels of functioning are underdeveloped in infants and toddlers, an NDB profile specific to infants with PAE continues to remain somewhat elusive.
While the specific aims and results of the included studies are varied, they broadly support the continued pursuit of an NDB phenotype that would facilitate meaningful clinical assessments in this youngest age group to identify PAE-related delays or deficits before affected children reach school age. Findings from this review also lend important insights into the use of various assessment measures. One important consideration that follows from our findings is in regards to the use of global tests of intelligence in infants with PAE. Our review of the literature indicates that such tests might produce inconsistent results among infants with PAE, which may be related to the lack of clear guidelines about the best ages to assess this domain in infants. In the meta-analysis findings of Testa, Quigley, and Das Eiden (2003), who quantitatively examined 9 studies using the BSID Mental/Cognitive scale in children with PAE aged 0–2 years, it was similarly concluded that after adjusting for covariates, this particular assessment has not consistently demonstrated an ability to detect significant differences in PAE vs non-PAE children at any level of exposure (Testa et al., 2003).
The BSID Motor scale was the most commonly-used assessment of adaptive behavior, and it also produced inconsistent results with respect to the effect of PAE. In fact, two-thirds of studies found no association of this domain with PAE. This finding contrasts with recent meta-analysis findings in children over the age of 5 years by Lucas et al., which indicated that general motor skills are significantly altered in children with PAE using common standardized assessments (Lucas et al., 2014). Given that motor skills specifically, and adaptive behaviors more generally, develop at varying rates among typically-developing infants under age 2 (Hadders-Algra, 2018), it is possible that normal developmental variation in this age group is a unique challenge to assessing PAE’s association with outcomes in this domain, and may be more appropriate for children older than 2 years. The studies in our review also suggest that outcomes in this domain may be more susceptible to effects related to PAE dose and frequency/duration (Coles et al., 1987, Jacobson et al., 1993a, Streissguth et al., 1980).
Overall, growing research points to the underestimation of developmental delay in infants and toddlers assessed using scales from the BSID, both in the general population and among high-risk infants (Martin et al., 2013), with some calling for revised BSID norms in the latest version (Anderson and Burnett, 2017). It is possible that the BSID, both Mental/Cognitive and Motor scale components, may only have a modest level of sensitivity for predicting long-term developmental delay (Luttikhuizen dos Santos et al., 2013), although sensitivity may increase concordant with increased age of assessment (Koseck and Harris, 2004).
Some comment on the quality of included studies and interpretation of findings is needed. Studies in which infant NDB effects were found in association with periconceptional alcohol use but not gestational alcohol use (Bandoli et al., 2016) might represent possible underreporting of alcohol use during pregnancy. However, it may also be related to the assessment of gestational drinking later in pregnancy when many women have substantially reduced or stopped alcohol use, while the periconceptional period might be indicative of alcohol use in early pregnancy. It is important to note that additional factors, such as variations in dose and timing of PAE, risk or protective modifying factors, specific assessment methods, study participant age, and statistical power are important considerations when attempting to interpret the findings. Finally, reliance on structured questionnaires involves inherent limitations of potential bias from both participants and researchers. Objective physiological measures, such as those obtained through rigorous standardized examiner-administered evaluation or neuroimaging (Stephen et al., 2018), and other experimental approaches including eye movement (Zhang et al., 2019) and still-face paradigm (Lowe et al., 2006), might be more sensitive to the identification of PAE effects in young children and requires continued study through prospective cohorts.
Although this review did not examine the effect of early interventions, a goal of early-life NDB assessment is to facilitate this for patients at risk of NDB adverse outcomes. Pilot studies indicate that the earlier in life FASD-targeted early interventions are provided, the better the long-term outcomes are (Yazdani et al., 2009, Hopkins et al., 2008, Zarnegar et al., 2016). It is important to note that FASD diagnosis need not preclude early interventions when an NDB delay is identified. Future research should strive to broaden the horizons for early intervention in PAE-affected infants, as the first two years of any child’s life are critical. Early life experiences have been shown to affect brain development dramatically and produce measurable, long-lasting alterations in specific regions of the brain that are associated with behavioral and cognitive functioning (Smith et al., 2011, Graham et al., 2015). Our own research has suggested that supportive parenting interaction style, which is modifiable through teaching and specialized support, might be a key early intervention area that could benefit NDB outcomes in alcohol- and drug-exposed infants during the first year of life (Lowe et al., 2017). Future studies can use the information in this report to more closely investigate and identify which assessments or assessment domains should become the focus of research and, ultimately, shape clinical guidelines for infant evaluations and the development of early interventions. Future similar studies are also needed to examine the literature on NDB outcomes in newborns (0–2 months of age) and older toddlers (25–35 months of age) with PAE, as these age groups might have unique assessment and early intervention needs that could be met before entering pre-school.
To our knowledge, this is the first comprehensive review of the literature concerning NDB outcomes related to PAE in children aged 3 months to 2 years. Despite over four decades of research with well-characterized prospective birth cohorts, diagnosis of children with FASD prior to their reaching school-age remains uncommon. The general findings of the current review suggest that the domain of self-regulation may be an under-recognized area of manifestation of PAE effects in the youngest age group of patients. Notably, the self-regulation studies reviewed here frequently utilized brief, parent-completed questionnaires. Parent-reported measures may introduce bias, and be less reliable or valid compared to standardized testing in identifying deficits. However, they have advantages for use as a screening tool. These questionnaires can be used in a variety of clinical contexts, are low-cost, and require relatively little time or specialized training to administer and score.
While the large majority of examined publications in this review focused on assessments of neurocognitive and adaptive behaviors in infants, these studies were less consistently able to identify significant deficits or delays. More in-depth meta-analyses concerning the use of specific assessments in infants and toddlers with PAE is greatly needed. Additional future directions for research include the identification of ideal assessment ages for specific standardized assessments; further exploration of sex differences within validated tests; the potential influences of genetic and epigenetic factors on NDB outcomes associated with PAE; and, the relations between dose, timing, and frequency of PAE with regards to specific NDB outcomes.
ACKNOWLEDGEMENTS
We would like to thank Carolyn Parshall, M.P.H. and Dominique Rodriguez, M.A. for their assistance in retrieving and reviewing literature for this project. Authors have no conflicts of interest to disclose.
FUNDING
Funding support includes NIH/NIAAA grants 1 R01 AA021771 and 2 R01 AA021771-06
REFERENCES
- ALEX K & FELDMANN R 2012. Children and adolescents with fetal alcohol syndrome (FAS): better social and emotional integration after early diagnosis. Klin Padiatr, 224, 66–71. [DOI] [PubMed] [Google Scholar]
- ALVIK A, TORGERSEN AM, AALEN OO & LINDEMANN R 2011. Binge alcohol exposure once a week in early pregnancy predicts temperament and sleeping problems in the infant. Early Hum Dev, 87, 827–33. [DOI] [PubMed] [Google Scholar]
- ANDERSON PJ & BURNETT A 2017. Assessing developmental delay in early childhood - concerns with the Bayley-III scales. Clin Neuropsychol, 31, 371–381. [DOI] [PubMed] [Google Scholar]
- ASTLEY SJ 2010. Profile of the first 1,400 patients receiving diagnostic evaluations for fetal alcohol spectrum disorder at the Washington State Fetal Alcohol Syndrome Diagnostic & Prevention Network. Can J Clin Pharmacol, 17, e132–64. [PubMed] [Google Scholar]
- AUTTI-RAMO I & GRANSTROM ML 1991a. The effect of intrauterine alcohol exposition in various durations on early cognitive development. Neuropediatrics, 22, 203–10. [DOI] [PubMed] [Google Scholar]
- AUTTI-RAMO I & GRANSTROM ML 1991b. The psychomotor development during the first year of life of infants exposed to intrauterine alcohol of various duration. Fetal alcohol exposure and development. Neuropediatrics, 22, 59–64. [DOI] [PubMed] [Google Scholar]
- BACKSTRAND JR, ALLEN LH, MARTINEZ E & PELTO GH 2001. Maternal consumption of pulque, a traditional central Mexican alcoholic beverage: relationships to infant growth and development. Public Health Nutr, 4, 883–91. [DOI] [PubMed] [Google Scholar]
- BAKHIREVA LN, GARRISON L, SHRESTHA S, SHARKIS J, MIRANDA R & ROGERS K 2018a. Challenges of diagnosing fetal alcohol spectrum disorders in foster and adopted children. Alcohol, 67, 37–43. [DOI] [PubMed] [Google Scholar]
- BAKHIREVA LN, LOWE J, GARRISON LM, CANO S, LEYVA Y, QEADAN F & STEPHEN JM 2018b. Role of caregiver-reported outcomes in identification of children with prenatal alcohol exposure during the first year of life. Pediatr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKHIREVA LN & SAVAGE DD 2011. Focus on: biomarkers of fetal alcohol exposure and fetal alcohol effects. Alcohol Res Health, 34, 56–63. [PMC free article] [PubMed] [Google Scholar]
- BAKHIREVA LN, SHARKIS J, SHRESTHA S, MIRANDA-SOHRABJI TJ, WILLIAMS S & MIRANDA RC 2017. Prevalence of Prenatal Alcohol Exposure in the State of Texas as Assessed by Phosphatidylethanol in Newborn Dried Blood Spot Specimens. Alcohol Clin Exp Res, 41, 1004–1011. [DOI] [PubMed] [Google Scholar]
- BANDOLI G, COLES CD, KABLE JA, WERTELECKI W, GRANOVSKA IV, PASHTEPA AO & CHAMBERS CD 2016. Assessing the Independent and Joint Effects of Unmedicated Prenatal Depressive Symptoms and Alcohol Consumption in Pregnancy and Infant Neurodevelopmental Outcomes. Alcohol Clin Exp Res, 40, 1304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHASNOFF IJ, WELLS AM & KING L 2015. Misdiagnosis and missed diagnoses in foster and adopted children with prenatal alcohol exposure. Pediatrics, 135, 264–70. [DOI] [PubMed] [Google Scholar]
- COLES CD, KABLE JA, DREWS-BOTSCH C & FALEK A 2000. Early identification of risk for effects of prenatal alcohol exposure. J Stud Alcohol, 61, 607–16. [DOI] [PubMed] [Google Scholar]
- COLES CD, SMITH IE & FALEK A 1987. Prenatal alcohol exposure and infant behavior: immediate effects and implications for later development. Adv Alcohol Subst Abuse, 6, 87–104. [DOI] [PubMed] [Google Scholar]
- CORIALE G, FIORENTINO D, DI LAURO F, MARCHITELLI R, SCALESE B, FIORE M, MAVIGLIA M & CECCANTI M 2013. Fetal Alcohol Spectrum Disorder (FASD): neurobehavioral profile, indications for diagnosis and treatment. Riv Psichiatr, 48, 359–69. [DOI] [PubMed] [Google Scholar]
- DONALD KA, FOUCHE JP, ROOS A, KOEN N, HOWELLS FM, RILEY EP, WOODS RP, ZAR HJ, NARR KL & STEIN DJ 2016. Alcohol exposure in utero is associated with decreased gray matter volume in neonates. Metab Brain Dis, 31, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EICHLER A, GRUNITZ J, GRIMM J, WALZ L, RAABE E, GOECKE TW, BECKMANN MW, KRATZ O, HEINRICH H, MOLL GH, FASCHING PA & KORNHUBER J 2016. Did you drink alcohol during pregnancy? Inaccuracy and discontinuity of women’s self-reports: On the way to establish meconium ethyl glucuronide (EtG) as a biomarker for alcohol consumption during pregnancy. Alcohol, 54, 39–44. [DOI] [PubMed] [Google Scholar]
- FORREST F, FLOREY CD, TAYLOR D, MCPHERSON F & YOUNG JA 1991. Reported social alcohol consumption during pregnancy and infants’ development at 18 months. Bmj, 303, 22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER SL, MUCKLE G, ABDOUS BB, JACOBSON JL & JACOBSON SW 2012. Effects of binge drinking on infant growth and development in an Inuit sample. Alcohol, 46, 277–83. [DOI] [PubMed] [Google Scholar]
- FREUNSCHT I & FELDMANN R 2011. Young adults with Fetal Alcohol Syndrome (FAS): social, emotional and occupational development. Klin Padiatr, 223, 33–7. [DOI] [PubMed] [Google Scholar]
- FRIED PA & WATKINSON B 1988. 12- and 24-month neurobehavioural follow-up of children prenatally exposed to marihuana, cigarettes and alcohol. Neurotoxicol Teratol, 10, 305–13. [DOI] [PubMed] [Google Scholar]
- GAGNIER KR, MOORE TE & GREEN M 2011. A need for closer examination of FASD by the criminal justice system: has the call been answered? J Popul Ther Clin Pharmacol, 18, e426–39. [PubMed] [Google Scholar]
- GAHAGAN S, SHARPE TT, BRIMACOMBE M, FRY-JOHNSON Y, LEVINE R, MENGEL M, O’CONNOR M, PALEY B, ADUBATO S & BRENNEMAN G 2006. Pediatricians’ knowledge, training, and experience in the care of children with fetal alcohol syndrome. Pediatrics, 118, e657–68. [DOI] [PubMed] [Google Scholar]
- GRAHAM AM, PFEIFER JH, FISHER PA, LIN W, GAO W & FAIR DA 2015. The potential of infant fMRI research and the study of early life stress as a promising exemplar. Dev Cogn Neurosci, 12, 12–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENE T, ERNHART CB, MARTIER S, SOKOL R & AGER J 1990. Prenatal alcohol exposure and language development. Alcohol Clin Exp Res, 14, 937–45. [DOI] [PubMed] [Google Scholar]
- GUSELLA JL & FRIED PA 1984. Effects of maternal social drinking and smoking on offspring at 13 months. Neurobehav Toxicol Teratol, 6, 13–7. [PubMed] [Google Scholar]
- HADDERS-ALGRA M 2018. Early human motor development: From variation to the ability to vary and adapt. Neurosci Biobehav Rev, 90, 411–427. [DOI] [PubMed] [Google Scholar]
- HALLIDAY JL, MUGGLI E, LEWIS S, ELLIOTT EJ, AMOR DJ, O’LEARY C, DONATH S, FORSTER D, NAGLE C, CRAIG JM & ANDERSON PJ 2017. Alcohol consumption in a general antenatal population and child neurodevelopment at 2 years. J Epidemiol Community Health, 71, 990–998. [DOI] [PubMed] [Google Scholar]
- HOPKINS RB, PARADIS J, ROSHANKAR T, BOWEN J, TARRIDE JE, BLACKHOUSE G, LIM M, O’REILLY D, GOEREE R & LONGO CJ 2008. Universal or targeted screening for fetal alcohol exposure: a cost-effectiveness analysis. J Stud Alcohol Drugs, 69, 510–9. [DOI] [PubMed] [Google Scholar]
- HOYME HE, KALBERG WO, ELLIOTT AJ, BLANKENSHIP J, BUCKLEY D, MARAIS AS, MANNING MA, ROBINSON LK, ADAM MP, ABDUL-RAHMAN O, JEWETT T, COLES CD, CHAMBERS C, JONES KL, ADNAMS CM, SHAH PE, RILEY EP, CHARNESS ME, WARREN KR & MAY PA 2016. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON JL, JACOBSON SW & SOKOL RJ 1996. Increased vulnerability to alcohol-related birth defects in the offspring of mothers over 30. Alcohol Clin Exp Res, 20, 359–63. [DOI] [PubMed] [Google Scholar]
- JACOBSON JL, JACOBSON SW, SOKOL RJ, MARTIER SS, AGER JW & KAPLAN-ESTRIN MG 1993a. Teratogenic effects of alcohol on infant development. Alcohol Clin Exp Res, 17, 174–83. [DOI] [PubMed] [Google Scholar]
- JACOBSON SW, CHIODO LM, SOKOL RJ & JACOBSON JL 2002. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics, 109, 815–25. [DOI] [PubMed] [Google Scholar]
- JACOBSON SW, JACOBSON JL, SOKOL RJ, MARTIER SS & AGER JW 1993b. Prenatal alcohol exposure and infant information processing ability. Child Dev, 64, 1706–21. [PubMed] [Google Scholar]
- JONES KL & SMITH DW 1973. Recognition of the fetal alcohol syndrome in early infancy. Lancet, 302, 999–1001. [DOI] [PubMed] [Google Scholar]
- KABLE JA, O’CONNOR MJ, OLSON HC, PALEY B, MATTSON SN, ANDERSON SM & RILEY EP 2016. Neurobehavioral Disorder Associated with Prenatal Alcohol Exposure (ND-PAE): Proposed DSM-5 Diagnosis. Child Psychiatry Hum Dev, 47, 335–46. [DOI] [PubMed] [Google Scholar]
- KAPLAN-ESTRIN M, JACOBSON SW & JACOBSON JL 1999. Neurobehavioral effects of prenatal alcohol exposure at 26 months. Neurotoxicol Teratol, 21, 503–11. [DOI] [PubMed] [Google Scholar]
- KODITUWAKKU PW 2009. Neurocognitive profile in children with fetal alcohol spectrum disorders. Dev Disabil Res Rev, 15, 218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSECK K & HARRIS SR 2004. Changes in performance over time on the Bayley Scales of Infant Development II when administered to infants at high risk of developmental disabilities. Pediatr Phys Ther, 16, 199–205. [DOI] [PubMed] [Google Scholar]
- LANGE S, SHIELD K, REHM J & POPOVA S 2013. Prevalence of fetal alcohol spectrum disorders in child care settings: a meta-analysis. Pediatrics, 132, e980–95. [DOI] [PubMed] [Google Scholar]
- LARSSON G, BOHLIN AB & TUNELL R 1985. Prospective study of children exposed to variable amounts of alcohol in utero. Arch Dis Child, 60, 316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHIKOINEN A, ORDEN MR, HEINONEN S & VOUTILAINEN R 2016. Maternal drug or alcohol abuse is associated with decreased head size from mid-pregnancy to childhood. Acta Paediatr, 105, 817–22. [DOI] [PubMed] [Google Scholar]
- LOWE J, HANDMAKER N & ARAGON C 2006. Impact of mother interactive style on infant affect among babies exposed to alcohol in utero. Infant Ment Health J, 27, 371–382. [DOI] [PubMed] [Google Scholar]
- LOWE J, QEADAN F, LEEMAN L, SHRESTHA S, STEPHEN JM & BAKHIREVA LN 2017. The effect of prenatal substance use and maternal contingent responsiveness on infant affect. Early Hum Dev, 115, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCAS BR, LATIMER J, PINTO RZ, FERREIRA ML, DONEY R, LAU M, JONES T, DRIES D & ELLIOTT EJ 2014. Gross motor deficits in children prenatally exposed to alcohol: a meta-analysis. Pediatrics, 134, e192–209. [DOI] [PubMed] [Google Scholar]
- LUTTIKHUIZEN DOS SANTOS ES, DE KIEVIET JF, KONIGS M, VAN ELBURG RM & OOSTERLAAN J 2013. Predictive value of the Bayley scales of infant development on development of very preterm/very low birth weight children: a meta-analysis. Early Hum Dev, 89, 487–96. [DOI] [PubMed] [Google Scholar]
- MARTIN AJ, DARLOW BA, SALT A, HAGUE W, SEBASTIAN L, MCNEILL N & TARNOW-MORDI W 2013. Performance of the Parent Report of Children’s Abilities-Revised (PARCA-R) versus the Bayley Scales of Infant Development III. Arch Dis Child, 98, 955–8. [DOI] [PubMed] [Google Scholar]
- MATTSON SN, CROCKER N & NGUYEN TT 2011. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev, 21, 81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAY PA, CHAMBERS CD, KALBERG WO, ZELLNER J, FELDMAN H, BUCKLEY D, KOPALD D, HASKEN JM, XU R, HONERKAMP-SMITH G, TARAS H, MANNING MA, ROBINSON LK, ADAM MP, ABDUL-RAHMAN O, VAUX K, JEWETT T, ELLIOTT AJ, KABLE JA, AKSHOOMOFF N, FALK D, ARROYO JA, HERELD D, RILEY EP, CHARNESS ME, COLES CD, WARREN KR, JONES KL & HOYME HE 2018. Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. Jama, 319, 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOBERG DP, BOWSER J, BURD L, ELLIOTT AJ, PUNYKO J & WILTON G 2014. Fetal alcohol syndrome surveillance: age of syndrome manifestation in case ascertainment. Birth Defects Res A Clin Mol Teratol, 100, 663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOHER D, LIBERATI A, TETZLAFF J & ALTMAN DG 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol, 62, 1006–12. [DOI] [PubMed] [Google Scholar]
- MOLTENO CD, JACOBSON JL, CARTER RC, DODGE NC & JACOBSON SW 2014. Infant emotional withdrawal: a precursor of affective and cognitive disturbance in fetal alcohol spectrum disorders. Alcohol Clin Exp Res, 38, 479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLTENO CD, JACOBSON JL, CARTER RC & JACOBSON SW 2010. Infant Symbolic Play as an Early Indicator of Fetal Alcohol-Related Deficit. Infancy, 15, 586–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTAG AC 2016. Fetal alcohol-spectrum disorders: identifying at-risk mothers. Int J Womens Health, 8, 311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASH K, STEVENS S, ROVET J, FANTUS E, NULMAN I, SORBARA D & KOREN G 2013. Towards identifying a characteristic neuropsychological profile for fetal alcohol spectrum disorders. 1. Analysis of the Motherisk FASD clinic. J Popul Ther Clin Pharmacol, 20, e44–52. [PubMed] [Google Scholar]
- O’CONNOR MJ, BRILL NJ & SIGMAN M 1986. Alcohol use in primiparous women older than 30 years of age: relation to infant development. Pediatrics, 78, 444–50. [PubMed] [Google Scholar]
- OLSEN J 1994. Effects of moderate alcohol consumption during pregnancy on child development at 18 and 42 months. Alcohol Clin Exp Res, 18, 1109–13. [DOI] [PubMed] [Google Scholar]
- OLSON HC, FELDMAN JJ, STREISSGUTH AP, SAMPSON PD & BOOKSTEIN FL 1998. Neuropsychological deficits in adolescents with fetal alcohol syndrome: clinical findings. Alcohol Clin Exp Res, 22, 1998–2012. [PubMed] [Google Scholar]
- OLSON HC, JIRIKOWIC T, KARTIN D & ASTLEY S 2007. Responding to the challenge of early intervention for fetal alcohol spectrum disorders. Infants & Young Children, 20, 172–189. [Google Scholar]
- POLANSKA K, MUSZYNSKI P, SOBALA W, DZIEWIRSKA E, MERECZ-KOT D & HANKE W 2015. Maternal lifestyle during pregnancy and child psychomotor development - Polish Mother and Child Cohort study. Early Hum Dev, 91, 317–25. [DOI] [PubMed] [Google Scholar]
- QUATTLEBAUM JL & O’CONNOR MJ 2013. Higher functioning children with prenatal alcohol exposure: is there a specific neurocognitive profile? Child Neuropsychol, 19, 561–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REID N, DAWE S, SHELTON D, HARNETT P, WARNER J, ARMSTRONG E, LEGROS K & O’CALLAGHAN F 2015. Systematic Review of Fetal Alcohol Spectrum Disorder Interventions Across the Life Span. Alcohol Clin Exp Res, 39, 2283–95. [DOI] [PubMed] [Google Scholar]
- RICHARDSON GA, DAY NL & GOLDSCHMIDT L 1995. Prenatal alcohol, marijuana, and tobacco use: infant mental and motor development. Neurotoxicol Teratol, 17, 479–87. [DOI] [PubMed] [Google Scholar]
- ROOZEN S, PETERS GJ, KOK G, TOWNEND D, NIJHUIS J & CURFS L 2016. Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcohol Clin Exp Res, 40, 18–32. [DOI] [PubMed] [Google Scholar]
- SEAGULL FN, MOWERY JL, SIMPSON PM, ROBINSON TR, MARTIER SS, SOKOL RJ & MCCARVER-MAY DG 1996. Maternal assessment of infant development: associations with alcohol and drug use in pregnancy. Clin Pediatr (Phila), 35, 621–8. [DOI] [PubMed] [Google Scholar]
- SMITH GC, GUTOVICH J, SMYSER C, PINEDA R, NEWNHAM C, TJOENG TH, VAVASSEUR C, WALLENDORF M, NEIL J & INDER T 2011. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol, 70, 541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEPHEN JM, FLYNN L, KABELLA D, SCHENDEL M, CANO S, SAVAGE DD, RAYBURN W, LEEMAN LM, LOWE J & BAKHIREVA LN 2018. Hypersynchrony in MEG spectral amplitude in prospectively-identified 6-month-old infants prenatally exposed to alcohol. Neuroimage Clin, 17, 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEVENS SA, NASH K, FANTUS E, NULMAN I, ROVET J & KOREN G 2013. Towards identifying a characteristic neuropsychological profile for fetal alcohol spectrum disorders. 2. Specific caregiver-and teacher-rating. J Popul Ther Clin Pharmacol, 20, e53–62. [PubMed] [Google Scholar]
- STREISSGUTH AP, BARR HM, MARTIN DC & HERMAN CS 1980. Effects of maternal alcohol, nicotine, and caffeine use during pregnancy on infant mental and motor development at eight months. Alcohol Clin Exp Res, 4, 152–64. [DOI] [PubMed] [Google Scholar]
- STREISSGUTH AP, BARR HM, SAMPSON PD & BOOKSTEIN FL 1994. Prenatal alcohol and offspring development: the first fourteen years. Drug Alcohol Depend, 36, 89–99. [DOI] [PubMed] [Google Scholar]
- STREISSGUTH AP, BOOKSTEIN FL, BARR HM, SAMPSON PD, O’MALLEY K & YOUNG JK 2004. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr, 25, 228–38. [DOI] [PubMed] [Google Scholar]
- TESTA M, QUIGLEY BM & EIDEN RD 2003. The effects of prenatal alcohol exposure on infant mental development: a meta-analytical review. Alcohol Alcohol, 38, 295–304. [DOI] [PubMed] [Google Scholar]
- WATERMAN EH, PRUETT D & CAUGHEY AB 2013. Reducing fetal alcohol exposure in the United States. Obstet Gynecol Surv, 68, 367–78. [DOI] [PubMed] [Google Scholar]
- YAZDANI P, MOTZ M & KOREN G 2009. Estimating the neurocognitive effects of an early intervention program for children with prenatal alcohol exposure. Can J Clin Pharmacol, 16, e453–9. [PubMed] [Google Scholar]
- ZARNEGAR Z, HAMBRICK EP, PERRY BD, AZEN SP & PETERSON C 2016. Clinical improvements in adopted children with fetal alcohol spectrum disorders through neurodevelopmentally informed clinical intervention: A pilot study. Clin Child Psychol Psychiatry, 21, 551–567. [DOI] [PubMed] [Google Scholar]
- ZHANG C, PAOLOZZA A, TSENG PH, REYNOLDS JN, MUNOZ DP & ITTI L 2019. Detection of Children/Youth With Fetal Alcohol Spectrum Disorder Through Eye Movement, Psychometric, and Neuroimaging Data. Front Neurol, 10, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]