Summary
Background:
Accurately estimating rates of HIV disease progression and retention on antiretroviral therapy (ART) can help inform interventions to help control HIV microepidemics and mathematical models used to inform health resource allocation decisions. Our objective was to estimate on-ART CD4+ cell count progression, mortality, ART dropout, and ART re-initiation rates using a continuous time multi-state Markov model. Secondly, we aimed to validate health state transition probability estimates to ensure they accurately reproduced the regional HIV microepidemics across the United States.
Methods:
We considered a cohort of patients from the HIV Research Network (HIVRN) cohort, a consortium of 17 adult and pediatric HIV care providers located in the northeastern (n=8), southern (n=5), and western United States (n=4). Individuals aged ≥15 years who were in care (defined as one CD4 test and one HIV care visit) with at least one ART prescription (2010–2015) were included in the analysis. Continuous-time multi-state Markov models were used to estimate transitions between CD4 strata, and between on- and off-ART states. We examined and adjusted for differences in transition rates by region, race/ethnicity, gender, HIV risk group, and baseline clinical status.
Findings:
The median age of the 32,242 individuals included in the analysis was 44 years [IQR: 35–51]. Over a median follow-up of 4.9 years [2.6–6.0], 26.7% interrupted ART and 4.1% died. Women, men who have sex with men and individuals with no prior ART utilization had increased rates of CD4 improvement whereas Blacks and people who inject drugs had increased rates of ART dropout, and faster disease progression. Regardless of CD4 strata, individuals had increased hazard rates of ART dropout if they were from the South (aHR 1.91–2.45) or the West (aHR 1.29–1.66), compared to individuals from the Northeast.
Interpretation:
This study illustrates heterogeneities in disease progression during ART and ART retention rates across race/ethnicity, HIV risk groups and regions. These differences should be viewed as targets for intervention and should be incorporated in mathematical models of regional HIV microepidemics in the United States.
Introduction
More than 1.1 million people were living with HIV in the United States (US) by the end of 2015. Despite the dramatic decreases in mortality and HIV transmission achieved in the US, inadequate retention on antiretroviral therapy (ART) and suboptimal continuity of care are among the biggest challenges to realize the full public health benefits from sustained viral suppression,1,2 and public health departments are continuously under pressure to use available resources efficiently.
The HIV epidemic in the US features substantial geographic variation and is best characterized as a set of diverse microepidemics dispersed mostly across large urban centers.3 Given the heterogeneity in care engagement4 and structural conditions affecting the HIV response across cities,3 locally-oriented combination implementation strategies are imperative to prioritize resources according to the greatest public health benefit.
Dynamic HIV transmission models are increasingly being used to facilitate decision-making in HIV/AIDS.5,6 These models can capture all relevant costs and benefits attributable to HIV care interventions over an extended time horizon;7 however, accurately estimating rates of disease progression and ART retention that capture the heterogeneity across individuals and settings is of central importance to accurately predict the current and future burden of disease for the HIV-infected population.8
Given the strong relationship between CD4+ T-lymphocyte counts and the risk of mortality,9 healthcare costs10 and health-related quality of life,11 modelling disease progression via transition between CD4-based health states is commonplace in health economic evaluations. Markov chains have previously been applied to model disease progression via CD4 cell count deterioration.12,13 Multi-state Markov regression models can account for competing risks and censored observations from observational cohort data and are thus suited to estimate health state transition probabilities between multiple disease stages.14,15 Furthermore, generating estimates using multivariable regression models can account for characteristics of both individuals and settings to represent the heterogeneity observed across microepidemics.
Previous studies have estimated HIV disease progression using multi-state Markov models;12 however, these estimates have usually not been validated for use in the modeling of specific locations and contexts. For instance, observed differences across cities in the availability and distribution of HIV care may impact ART engagement.3 Generally, the credibility of model inferences are largely determined by the quality and representativeness of input data used for key parameters. Thus, explicit assessment of the representativeness of disease progression estimates used for locally-oriented modelling can help decision makers evaluate their validity and therefore the credibility of the model’s inferences.
Our objective was to estimate on-ART CD4 progression, mortality, ART dropout, and ART re-initiation rates using a continuous time multi-state Markov model. Secondly, we aimed to validate health state transition probability estimates to ensure they accurately reproduced the current state of diverse regional HIV microepidemics across the United States.
Methods
Study population
The HIV Research Network (HIVRN) is a consortium of 17 adult and pediatric HIV care providers located in the northeastern (n=8), southern (n=5), and western United States (n=4). HIVRN sites abstract specified data elements from individuals’ medical records, and abstracted data are assembled into a single database after quality assurance review. All sites participating in the HIVRN for all years between 2002 and 2015 were included in the analysis.
We considered all individuals reporting male or female gender age ≥ 15 years who were in HIV care (defined as having at least one CD4 count and one HIV primary care outpatient visits in a one calendar year period) between January 1st, 2010 and December 31st, 2015. Individuals in HIV care without prior ART utilization at the start of the study period were included upon ART initiation from the date of initiation up to September 30th, 2015 in order to have at least a 3-month period of follow-up. Consequently, individuals without any ART utilization prior to the study period or throughout the study period, i.e. they did not initiate ART, were not considered for inclusion. We did not consider transgender people for inclusion given the insufficient number of observations. We excluded individuals with a single observed disease status measurement (either a CD4 measurement while on ART, off-ART status, or death) or missing covariate information. Loss to follow-up was defined as having no records of medical care at HIVRN sites for at least 18 months prior to the study end date. The end of the observation period for individuals lost to follow-up was set to either the date of their last HIVRN visit date or the final day of their medication supply.
Measures
HIV disease progression for individuals on ART, mortality, ART dropout and ART re-initiation rates were of primary interest in this study. Disease progression was represented by changes in longitudinally-collected CD4 measurements, and all CD4 measurements while on ART during the period of January 1st, 2010 to December 31st, 2015 were included in the analysis. We categorized CD4 cell count measurements as ≥500, 200–499, and <200 per μL. The CD4 metric is known to exhibit considerable variability resulting from intra-individual temporal fluctuation and measurement error16 that may lead to biased estimates of disease progression. To address these potential issues, we applied an “ad hoc smoothing” technique shown to perform best in terms of bias reduction, whereby transitions between CD4 categories were only allowed when 2 consecutive CD4 measurements were observed.17 We classified individuals as off ART if there was a minimum 90-day gap without an active ART prescription.13
In order to generate estimates of disease progression and ART retention capturing heterogeneity across settings and individuals, we included covariates to adjust for differences in transition rates by region (Northeast, South, West), sex (male, female), race/ethnicity (Black, Hispanic/Latino, and non-Hispanic white/others), and HIV risk group (men who have sex with men (MSM), people who inject drugs (PWID), MSM who inject drugs (MWID), and heterosexual (HET)). To address multicollinearity between gender and risk group covariates, individuals were categorized in mutually exclusive categories of HET female, HET male or MSM and we defined PWID status as a binary variable (yes/no).
We also considered other potentially confounding factors hypothesized to influence disease progression and ART retention, including age at study entry, time since diagnosis and whether an individual had prior ART utilization at study entry (yes/no). Since sustained high plasma viral load (pVL) periods are associated with CD4 declines, we also controlled for an individuals’ historical average pVL prior to study entry as measured by the area under the log pVL curve. Lastly, we did not control for insurance status as it is often considered as a potentially mediating factor of individual characteristics (e.g., race/ethnicity, risk group) that are of primary interest to our analysis.18
Statistical analysis
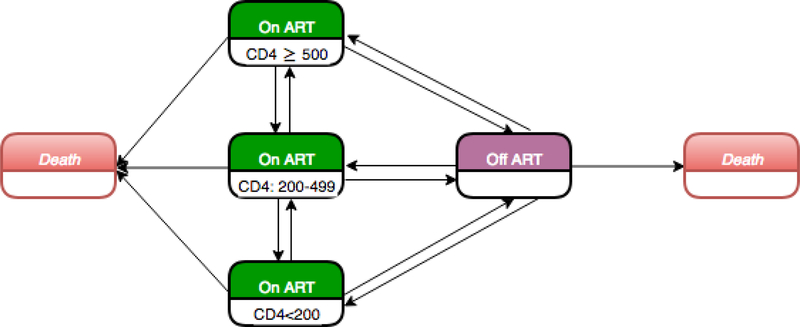
We used a parametric, continuous-time, Multi-state Markov model to estimate monthly transition probabilities between CD4 categories, and between on- and off-ART states, controlling for the covariates listed above. The estimation was operationalized by a matrix with 14 instantaneous transitions among five states (three on ART states defined as CD4 ≥500, 200–499, and <200, off ART and death), allowing for transitions to and from given states where possible (Figure 1). Multi-state Markov models efficiently handle heavily censored data, such as when the exact time of disease onset is unknown or when an individual is observed over a portion of their disease history.19 Further, the variation in elapsed time between CD4 measurements is inherently controlled for in this methodology.19
Figure 1.
Diagram for the Multi-state Markov model.
ART: Antiretroviral treatment; CD4: CD4 T-lymphocyte cell count per µL.
The effects of the covariates in this model are assumed to be multiplicative and constant over time, both assumptions being consistent with the conventional proportional hazards model. Therefore, the interpretation of exponentiated coefficient estimates are equivalent to that of the adjusted hazard ratio in a Cox model. The likelihood ratio test was used to select covariates which improved model fit, and the most parsimonious model was chosen. The analysis was conducted on the full sample and on region-specific study samples to examine potential differences in covariate effects across regions.
In order to generate a matrix of transitions probabilities for each region and for each subgroup defined by gender, race/ethnicity and HIV risk group, we estimated all monthly transition probabilities, except mortality, by using region-specific stratified models for each subgroup, holding the other covariates at the region-specific study sample average level. Given the low number of deaths captured in the data within each region, mortality estimates during ART for each subgroup were obtained from the full sample model consisting of all three regions.
Model validation
We utilized transition probability estimates from the region-specific model to project over a 5-year period the number of individuals expected to be in each health state at 3-month intervals, equivalent to the median time between observations in the study sample. The estimated probability of being in health state i at time t given an individual’s initial health state r is thus P(t)r,i. Assuming all individuals are at time t = 0 at study entry, and given n(t) individuals observed at time t, the expected number of individuals in health state i at time t is n(t)P(t)r,i.
The selection of validation targets depends on the quality of available data,20 and an external validation is considered dependent if the source for the targets was used to estimate model parameters.21 We derived validation targets from the observed number of individuals in each health state at each interval, and we assumed an individuals’ health state was the same as their previous when it was not observed at time t. We compared projections from the multi-state Markov model to the observed CD4 disease progression during ART, proportion off ART and mortality over a 5-year period. The percentage deviation from each target was calculated at 3-month intervals, and we calculated the mean percentage deviation to evaluate how close the model projections were to the targets.22
Role of the funding source
The funders had no direct role in the conduct of the analysis or the decision to submit the manuscript for publication. The corresponding author had final responsibility for the decision to submit for publication and full access to all the data.
Results
A total of 38,472 individuals met our inclusion criteria and were in HIV care during the study period. We excluded 3,376 (8.8%) due to having a single observed disease status measurement, and another 2,854 (7.4%) due to missing covariate information. Among the, 32,242 (83.8%) included in the study sample, the majority were male (75.4%) and the median age was 44 years [interquartile range (IQR), 35–51 years]. At study entry, 27,550 (85.4%) were on ART including 8,342 (25.9%) who had no prior ART utilization. Over a median follow-up of 4.9 years [IQR, 2.6–6.0 years], 8,614 individuals (26.7%) interrupted ART. Table 1 provides baseline characteristics on the study sample by region.
Table 1.
Characteristics of the HIV Research Network study participants, by region (N=32,242)
| Region |
|||
|---|---|---|---|
| Characteristics | Northeast n=13,563 (42.1%) |
South n=13,345 (41.4%) |
West n=5,334 (16.5%) |
| Age at study entry (Median (IQR)) | 45.4 [36.5, 51.7] | 43.3 [33.1, 50.4] | 44.3 [36.4, 50.8] |
| Follow-up in years(Median(IQR)) | 4.8 [2.8, 6.0] | 5.1 [2.6, 6.0] | 5.3 [2.5, 6.0] |
| Male | 10,224 (75.4) | 9,486 (71.1) | 4,595 (86.1) |
| Race/ethnicity | |||
| Black | 5,866 (43.3) | 7,627 (57.2) | 1,072 (20.1) |
| Hispanic / Latino | 3,749 (27.6) | 2,439 (18.3) | 1,308 (24.5) |
| non-Hispanic white / others | 3,948 (29.1) | 3,279 (24.6) | 2,954 (55.4) |
| HIV risk group | |||
| MSM | 6,768 (49.9) | 5,483 (41.1) | 3,344 (62.7) |
| PWID | 1,580 (11.6) | 1,336 (10.0) | 362 (6.8) |
| MWID | 272 (2.0) | 316 (2.4) | 363 (6.8) |
| HET | 4,943 (36.4) | 6,210 (46.5) | 1,265 (23.7) |
| Year of diagnosis | |||
| ≤ 1996 | 4,701 (34.7) | 2,875 (21.5) | 1,634 (30.6) |
| 1997–2006 | 5,261 (38.8) | 5,318 (39.9) | 2,057 (38.6) |
| 2007–2015 | 3,601 (26.6) | 5,152 (38.6) | 1,643 (30.8) |
| Year of ART initiation | |||
| ≤ 1996 | 690 (5.1) | 673 (5.0) | 517 (9.7) |
| 1997–2006 | 3,289 (24.2) | 4,398 (33.0) | 1,729 (32.4) |
| 2007–2015 | 4,555 (33.6) | 6,488 (48.6) | 2,282 (42.8) |
| Unknown | 5,029 (37.1) | 1,786 (13.4) | 806 (15.1) |
| No prior ART utilization at study entry | 2,610 (19.2) | 4,377 (32.8) | 1,355 (25.4) |
MSM: men who have sex with men; PWID: people who inject drugs; MWID: MSM who inject drugs;
HET: heterosexual; ART: Antiretroviral treatment.
The five-state, multivariable multi-state Markov was fitted to produce parameter estimates for each of the 14 possible transitions modeled. Selected independent determinants of transition rates from the full sample model are shown in Table 2 (full results on appendix page 4). We found that both heterosexual females and MSM had an increased hazard of CD4 improvement when compared to heterosexual males, and that the improvement was more likely to be sustained. For example, the adjusted hazard ratios (aHR) during ART for transitions from CD4 200–499 to ≥500 and from CD4 ≥500 to 200–499 among heterosexual females were 1.35 (95% confidence interval [CI], 1.26–1.45) and 0.71 (95% CI, 0.65–0.77), respectively. In contrast, when compared to heterosexual male individuals with CD4 200–499, MSM had reduced hazard of ART drop out (aHR: 0.89 [95% CI, 0.82–0.96]) whereas heterosexual females had increased hazard of ART drop out (aHR: 1.14 [95% CI, 1.04–1.23]). Individuals with no prior ART utilization had increased hazard of sustained CD4 improvement (aHRs from 1.31–1.39) and reduced hazard of CD4 deterioration (aHRs 0.84–0.86). Regardless of CD4 strata, individuals had increased hazard rates of ART dropout if they were from the South (aHRs from 1.91–2.45) or the West (aHRs from 1.29–1.66), compared to individuals from the Northeast. However, those from the South were more likely to re-initiate ART (aHRs from 1.30–1.79) whereas individuals from the West were less likely (aHRs from 0.49–0.94). Moreover, in analysis of the full sample model, reporting injection drug use was independently associated with increased rates of ART dropout and faster disease progression (CD4 decline or death) (Table 2). Finally, our results were robust to sensitivity analysis examining potential bias from exclusion of individuals with missing covariate information (appendix pages 8–9).
Table 2.
Multivariate multi-state Markov model results of disease progression during ART, mortality and ART engagement from full sample (N=32,242)
| HET female | MSM | Black | Hispanic/Latino | PWID | No prior ART* | South | West | |
|---|---|---|---|---|---|---|---|---|
| Reference | HET male | non-Hispanic white/others | non-PWID | Prior ART | Northeast | |||
| Transitions | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) |
| CD4 improvement during ART | ||||||||
| <200 to 200–499 | 1.37 (1.24,1.51) | 1.25 (1.14,1.37) | 1.08 (0.98,1.19) | 1.06 (0.95,1.18) | 0.98 (0.88,1.10) | 1.39 (1.26,1.53) | 1.16 (1.07,1.26) | 1.05 (0.94,1.17) |
| 200–499 to ≥500 | 1.35 (1.26,1.45) | 1.10 (1.03,1.18) | 0.98 (0.92,1.05) | 0.93 (0.87,1.00) | 0.97 (0.90,1.05) | 1.31 (1.22,1.40) | 0.85 (0.80,0.89) | 0.84 (0.78,0.90) |
| CD4 deterioration during ART | ||||||||
| ≥500 to 200–499 | 0.71 (0.65,0.77) | 0.85 (0.79,0.91) | 1.13 (1.06,1.22) | 1.18 (1.09,1.27) | 1.23 (1.13,1.33) | 0.86 (0.78,0.94) | 1.18 (1.11,1.26) | 1.18 (1.09,1.28) |
| 200–499 to <200 | 1.12 (1.00,1.26) | 0.82 (0.74,0.92) | 1.11 (0.99,1.25) | 1.02 (0.90,1.15) | 1.23 (1.10,1.38) | 0.84 (0.73,0.95) | 1.01 (0.91,1.11) | 1.04 (0.92,1.19) |
| ART dropout | ||||||||
| ≥500 to off-ART | 1.04 (0.95,1.12) | 0.88 (0.81,0.95) | 0.97 (0.90,1.04) | 0.73 (0.67,0.80) | 1.36 (1.25,1.49) | 1.10 (1.00,1.20) | 2.45 (2.29,2.62) | 1.66 (1.51,1.82) |
| 200–499 to off-ART | 1.14 (1.04,1.23) | 0.89 (0.82,0.96) | 1.12 (1.03,1.21) | 0.91 (0.83,1.00) | 1.45 (1.34,1.58) | 0.92 (0.84,1.01) | 1.95 (1.81,2.10) | 1.41 (1.27,1.56) |
| <200 to off-ART | 1.00 (0.89,1.13) | 0.91 (0.82,1.02) | 1.23 (1.09,1.39) | 0.89 (0.77,1.03) | 1.42 (1.26,1.61) | 0.73 (0.64,0.84) | 1.91 (1.71,2.13) | 1.29 (1.10,1.51) |
| ART re-initiation | ||||||||
| Off-ART to ≥500 on-ART | 1.39 (1.27,1.52) | 1.25 (1.14,1.38) | 1.02 (0.94,1.12) | 1.20 (1.08,1.32) | 0.88 (0.79,0.96) | 1.14 (1.03,1.26) | 1.30 (1.21,1.41) | 0.49 (0.43,0.55) |
| Off-ART to 200–499 on-ART | 1.02 (0.93,1.11) | 1.02 (0.93,1.12) | 1.16 (1.05,1.27) | 1.37 (1.23,1.52) | 1.02 (0.93,1.12) | 0.86 (0.78,0.96) | 1.43 (1.32,1.55) | 0.59 (0.52,0.67) |
| Off-ART to <200 on-ART | 0.81 (0.73,0.90) | 0.82 (0.74,0.91) | 1.39 (1.24,1.57) | 1.55 (1.36,1.77) | 0.94 (0.84,1.05) | 0.60 (0.52,0.68) | 1.79 (1.62,1.99) | 0.94 (0.80,1.09) |
| Mortality | ||||||||
| ≥500 to death | 0.77 (0.51,1.15) | 0.80 (0.53,1.20) | 0.88 (0.61,1.26) | 0.50 (0.31,0.83) | 2.31 (1.62,3.29) | 0.76 (0.42,1.36) | 1.24 (0.89,1.73) | 1.05 (0.68,1.63) |
| 200–499 to death | 1.01 (0.75,1.38) | 0.70 (0.51,0.95) | 0.81 (0.60,1.08) | 0.54 (0.38,0.78) | 1.56 (1.18,2.06) | 0.91 (0.60,1.36) | 1.12 (0.86,1.46) | 1.28 (0.93,1.76) |
| <200 to death | 1.08 (0.85,1.37) | 1.12 (0.90,1.40) | 0.90 (0.72,1.13) | 0.77 (0.59,1.01) | 1.61 (1.30,2.00) | 0.93 (0.72,1.21) | 1.93 (1.56,2.40) | 1.37 (1.02,1.84) |
| Off-ART to death | 1.34 (0.80,2.22) | 1.21 (0.70,2.08) | 0.63 (0.39,1.01) | 0.74 (0.41,1.33) | 1.36 (0.86,2.16) | 0.75 (0.36,1.54) | 1.15 (0.76,1.76) | 0.38 (0.18,0.80) |
Bold indicates statistically significant adjusted hazard ratios at p<0.05
ART: Antiretroviral treatment; CD4: CD4 T-lymphocyte cell count per µL; dx: Diagnosis; aHR: adjusted hazard ratio; CI: confidence interval.
HET: heterosexual; MSM: men who have sex with men; PWID: people who inject drugs.
No prior ART utilization at study entry compared to prior ART utilization at study entry.
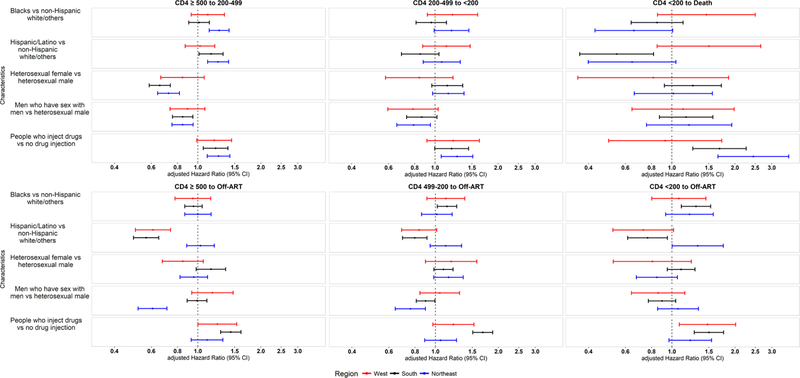
In the region-specific stratified models, we found the hazard ratios of death from CD4<200 and ART dropout across CD4 categories to be highly heterogeneous for PWID (Figure 2). For example, injection drug use was associated with increased hazard of death from CD4<200 in the Northeast (aHR: 2.45 [95% CI: 1.66–3.62]), and South (aHR: 1.69 [95% CI: 1.26–2.27]), but not in the West. In contrast, CD4 deterioration for PWID was generally consistent across regions (appendix pages 5–7 present full results for the region-specific stratified models). In addition, results from the region-specific models highlighted heterogeneity in CD4 deterioration for heterosexual females (compared to heterosexual males), with aHRs associated with CD4 deterioration from ≥500 to 200–499 ranging from 0.66 [95% CI: 0.59–0.74] in the South to 0.85 [95% CI: 0.67–1.07] in the West. We also found heterogeneity across regions in the reduced hazard of ART dropout from CD4>200 among MSM (compared to heterosexual males), with the largest reductions in the Northeast (aHR: 0.61 [95% CI: 0.52–0.71] from CD≥500; aHR: 0.76 [95% CI: 0.64–0.90] from CD4 200–499) compared to the other two regions (aHRs ranged from 0.90 [95% CI: 0.81–1.00] to 1.17 [95% CI: 0.94–1.47]) (Figure 2).
Figure 2.
Adjusted hazard ratios associated with CD4 deterioration during ART, including mortality, and ART dropout, by region.
ART: Antiretroviral treatment; CD4: CD4 T-lymphocyte cell count per µL.
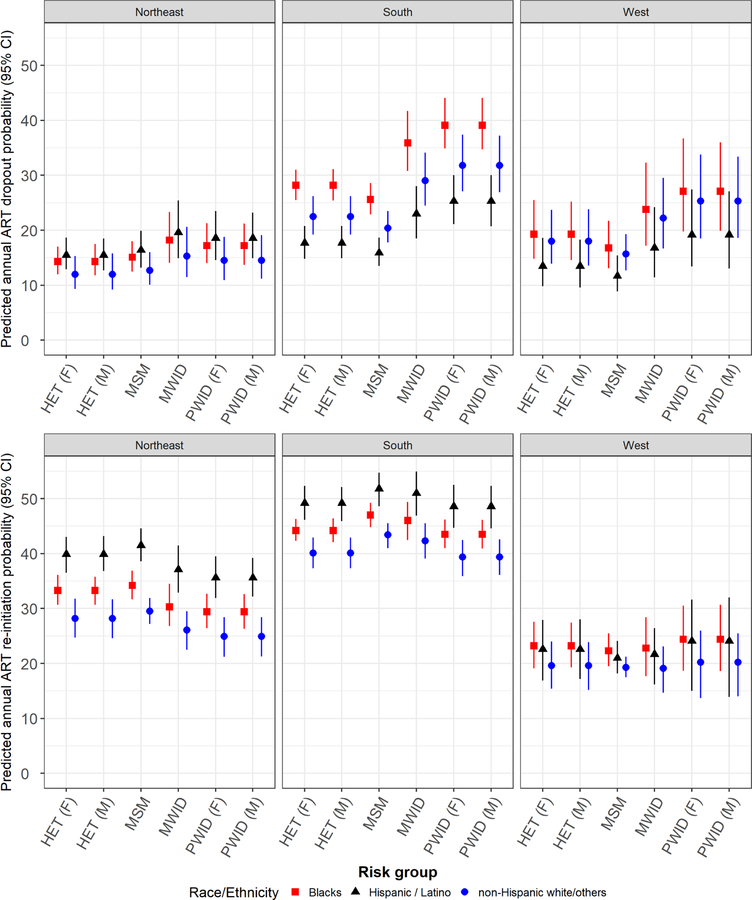
We present in Figure 3 the predicted probability of ART dropout among individuals with CD4<200 and of any ART re-initiation regardless of CD4 count, by risk group and region. The fitted values were estimated on an annual basis, generated from the region-specific stratified multivariate multi-state Markov models, and using mean values of covariates observed in the region-specific study samples. The annual probability of ART dropout was markedly higher in the southern region across all risk groups, and ranged from 16% for Hispanic / Latino MSM to 39% for Black PWID. Compared to the other regions, differences were the greatest for southern Blacks. For example, male PWIDs were 23% more likely to dropout compared to those in the Northeast and heterosexual females 8% more likely than those in the western region. Furthermore, fitted values within the Northeast region were more homogeneous across risk groups and race/ethnicity compared to the other regions (complete fitted values for monthly transition probabilities, by region & subgroups, on appendix pages 10–22).
Figure 3.
Predicted annual probability of ART dropout among individuals with CD4<200 (top) and of any ART re-initiation regardless of CD4 count (bottom) by region, risk group and race/ethnicity.
CD4: CD4 T-lymphocyte cell count per μL; MSM: Men who have sex with men; PWID: People who inject drugs; MWID: MSM who inject drugs; HET: Heterosexuals; F: Female; M: Male. Predicted values from region-specific stratified models for each gender, risk group and race/ethnicity adjusting for age at study entry, prior ART utilization at study entry (yes/no) and historical average plasma viral load (pVL) (control values equal to the region-specific study sample average level).
Validation results from comparing the multi-state Markov model projections to the validation targets are presented in Table 3. The dependent validation showed the model to slightly under-predict individuals in each CD4 strata during ART for the western region, ranging from −2.2% for individuals with CD4≥500 to −7.4% for those with CD4 200–499. In contrast, the model slightly over-predicted individuals in only two CD4 strata during ART for the southern region, by 1.6% for individuals with CD4 200–499 and 6.3% for those with CD4<200. Mean deviations for each CD4 strata in the Northeast were relatively small and ranged from −2.1% for CD4≥500 to 1.0% for CD4<200. The probability of being off ART was over-predicted across regions from 3.5% (South) to 22.7% (West), while mortality was under predicted across regions from −4.4% (Northeast) to −18.4% (West).
Table 3.
Comparison of multi-state Markov model projections and validation targets.
| Mean deviation from regional stratified model projections |
|||
|---|---|---|---|
| Regional validation targets | Northeast | South | West |
| CD4 during ART | |||
| ≥500 | −2.1% | 0.0% | −2.2% |
| 200–499 | 0.6% | 1.6% | −7.4% |
| <200 | 1.0% | 6.3% | −7.1% |
| Off-ART | 15.9% | 3.5% | 22.7% |
| Mortality | −4.4% | −11.8% | −18.4% |
ART: Antiretroviral treatment; CD4: CD4 T-lymphocyte cell count per µL.
Discussion
This study illustrates the heterogeneity found across settings and individuals in ART engagement and its effect on HIV disease progression. We found the probability of disease progression during ART and ART retention rates varied across HIV risk groups, race/ethnicity and regions of the United States. Prior studies have estimated CD4 transition probabilities using comparable methodologies.12,13 However, this multivariable regression analysis conducted using region-specific models to jointly estimate the ART dropout and re-initiation in addition to CD4 progression is the first, to our knowledge, to demonstrate heterogeneity across individuals and settings. These findings have important implications. Clearly, simulation models which employ CD4 transition probabilities estimated from national estimates that do not account for race/ethnicity or HIV risk groups may mischaracterize the optimal set of interventions for treating and preventing HIV across distinct microepidemics. This is an important result to communicate as public health departments aim to implement locally-oriented combination implementation strategies necessary to achieve the ambitious goal of eliminating the HIV epidemic in the United States.
Our findings also have important implications for clinical practice. Delivering continuous care to people living with HIV (PWH) is critical to the control HIV microepidemics23,24 and the differences we found should be viewed as targets for interventions. As more than a quarter of all individuals included in our study interrupted ART at least once, treatment initiation must be recognized as an opportunity to improve ART persistence. Our results that individuals without prior ART experience had accelerated CD4 improvement and had a lower hazard of discontinuing ART are consistent with prior findings of successive treatment episodes that tended to decrease in duration whereas episodes off ART remained relatively constant.25 Given the paucity of evidence on effective and cost-effective interventions to improve ART persistence26,27 careful attention must be given to emerging evidence of promising ART engagement programs that target recently diagnosed individuals and individuals in need of comprehensive care assistance, particularly in states that did not expand Medicaid under the Affordable Care Act.
In addition, the increased hazard of both ART dropout and re-initiation in the full sample model for individuals in the southern region are indicative of substantial levels of ‘churn’ (a cyclical process of engagement, disengagement, and reengagement in HIV care) among PWH.1 This pattern was also noticeable when comparing other race/ethnic groups to non-Hispanic whites. Compared to individuals in the Northeast, those in the South with higher CD4 counts had the highest hazard of ART dropout. Furthermore, those off ART and with low CD4 counts had the highest hazard of ART re-initiation. With HIVRN sites in the South all located in states that did not expand Medicaid under the Affordable Care Act, this observed gradient effect of CD4 count levels may be indicative of the disparity in access and quality of care between the South and Northeast. This disparity between the South and other regions of the United States has also been noted in studies on HIV-related mortality, which is now predominantly concentrated in poorer southern counties.28 Addressing the complex needs of PWH struggling to remain engaged in ART is a key strategy in the United States’ new initiative for “Ending the HIV Epidemic”. It is essential to address the ART persistence challenges to realize the reductions in morbidity, mortality and transmission associated with sustained viral suppression.
Models used to make recommendations of targeted, locally-oriented combination implementation strategies should accurately reflect the current state of a given microepidemic. Several factors might explain the relatively larger deviations of our model projections from mortality and off-ART targets. Prior studies have suggested a higher risk of mortality among individuals lost to follow-up compared to those retained in care,29 and individuals lost to follow-up were more likely to be in poorer health as measured by CD4 counts. Therefore, our analysis using multi-state Markov models that assume non-informative censoring might have resulted in the underestimation of mortality. Moreover, the estimation of ART re-initiation rates by CD4 category, not feasible in our study, might have improved off-ART model projection. Our validation results thus offer further support for the need to locally validate models, a best practice that can be supported by the calibration of key input parameters.21,30 Additionally, conducting one-way sensitivity analyses and probabilistic sensitivity analysis in economic evaluations to quantify the uncertainty in model recommendations resulting from parameters with greater uncertainty is critical. Efforts to collect population-based health administrative and surveillance data that could be used for estimating disease progression with greater precision would greatly enhance the validity of modeling recommendations. Given the paucity of data describing disease processes that are specific to the distinct microepidemics across the United States, increasing transparency and standardizing methods for model calibration and validation can ultimately promote better integration of locally-oriented modelling in decision-making.
This study has some limitations that are worth noting. First, although HIVRN sample demographics are comparable to those of PWH in the United States in general, our results may not generalize to all HIV care sites. However, most of the study population received care from urban academic and community-based clinics, thus closely mirroring the HIV epidemic in the United States, which is dispersed mostly across large urban centers.3 In addition, our analysis included multiple sites across broad geographic areas affording greater generalizability than single-site studies. Second, our analysis was restricted to males and females with at least two disease status measurements and with complete covariate information. Comparatively, a greater proportion of excluded individuals were MSM, had been diagnosed after 2007, and had no prior ART utilization (appendix page 3). In addition, our results may not be generalizable to individuals identifying as transgender or non-binary as our analysis was restricted to individuals reporting sex categorized as male or female. Third, the determination of ART interruption and loss to follow-up relied on medication prescription information and receipt of care in the HIVRN network, respectively. HIVRN data does not always reliably capture HIV treatment received elsewhere but this potential measurement bias did not impact disease progression estimates during ART. Data linkages to other databases such as Medicaid Claim data and AIDS Drug Assistance Program (ADAP) data could help improve measures of both ART and HIV care retention. Fourth, we could not determine how immune recovery may have been affected by previous ART regimens among treatment experienced individuals; however, we accounted for differences in disease progression between ART naïve and treatment experienced individuals. Lastly, multi-state Markov models assume non-informative censoring and definitions of loss to follow-up in longitudinal studies may influence event rates.19 Our multivariable regression analysis accounted for several factors that could be related to propensity of censorship thus mitigating potential attrition biases.
This study reveals heterogeneities in disease progression on ART and ART retention rates across racial and ethnic groups, HIV risk groups and regions. These differences should be viewed as targets for intervention and otherwise should be incorporated in mathematical models of regional HIV microepidemics in the United States.
Supplementary Material
Research-in-Context.
Previous Evidence
We searched PubMed for titles and abstracts of literature published in English between Jan 1, 1996, and July 13, 2018, using the search terms “HIV” AND “CD4” AND “Antiretroviral Therapy” AND (“Survival” OR “Mortality” OR “Prognosis” OR “Disease progression” OR “CD4 change” OR “Treatment interruption” OR “ART dropout” OR “ART interruption” OR “ART retention” OR “ART adherence”) AND ((“Determinants” OR “Predictors” OR “Factors” OR “Disparity”) OR (“Rate”, OR “Estimate”)). Search results were reviewed to identify publications reporting estimates and determinants of rates of disease progression (measured by CD4 changes) and/or ART retention. A number of factors have been noted in the literature to correlate with accelerated disease progression, including male, injection drug use and race/ethnicity minorities, whereas earlier diagnosis and treatment initiation and improved treatment adherence were associated with improved prognosis. In addition, younger age, injection drug use, and race/ethnicity minorities have been frequently found to be associated with reduced ART retention or adherence. Among them, three studies adopted multivariable multi-state Markov models to estimate rates of CD4 progression adjusting for a list of key covariates: one focused on the natural history of CD4 progression among seroconverter cohorts from 25 countries, while the other two were conducted in a Canadian setting, estimating probability of CD4 progression and mortality among population-based samples who have initiated ART, and interrupted ART, respectively. However, there was no study reporting estimates on rates of CD4 decline and ART retention accounting for heterogeneity across subgroups and geographic regions in the United States.
Added value of this study
We have used comprehensive clinical and ART prescription data from a large multi-site observational cohort to estimate rates of CD4 progression and mortality during ART and ART retention for several population subgroups across geographic regions of the United States. Using a continuous multi-state Markov model, we found that women, men who have sex with men and individuals with no prior ART utilization had increased rates of CD4 improvement whereas Blacks and people who inject drugs had increased rates of ART dropout, and faster disease progression. Finally, regardless of CD4 strata, individuals from the South or the West had increased hazard rates of ART dropout compared to individuals from the Northeast.
Implications of all the available evidence
Our study highlights the heterogeneity in HIV disease progression and ART retention across geographic locations and subgroups. These differences should be viewed as targets for intervention and otherwise should be incorporated in mathematical models of regional HIV microepidemics in the United States. Simulation models which employ CD4 transition probabilities estimated from national estimates that do not account for race/ethnicity or HIV risk groups may mischaracterize the absolute and relative cost-effectiveness of interventions to treat and prevent HIV in distinct microepidemics. This is an important result to communicate as public health departments aim to implement locally-oriented combination implementation strategies to reduce the public health burden of HIV/AIDS.
Acknowledgments
Funding for this study was provided by the US National Institutes of Health, National Institute on Drug Abuse (R01DA041747-02S1) awarded to Simon Fraser University and the HIV Research Network (HIVRN) is supported by the Agency for Healthcare Research and Quality (HHSA290201100007C) and the Health Resources and Services Administration (HHSH250201600009C). Further HIVRN acknowledgements for participating sites and principal investigators are on appendix page 2.
Funding: US National Institutes of Health, Agency for Healthcare Research and Quality, Health Resources and Services Administration.
Declaration of interests
AN reports grants from the Gilead FOCUS program, outside the submitted work. JAA reports grants from Johns Hopkins University Federal Subcontract, during the conduct of the study; personal fees from Janssen, personal fees from Merck, grants from Frontier technology, grants and personal fees from Viiv, grants from Shionogi, grants from Gilead, outside the submitted work. RDM reports grants from the Agency for Healthcare Research and Quality and the Health Resources and Services Administration during the conduct of the study. KAG is a paid consultant to Simon Fraser University, and this arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. BN reports grants from the US National Institutes of Health, National Institute on Drug Abuse during the conduct of the study. All other authors declare no competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nachega JB, Uthman OA, del Rio, Mugavero MJ, Rees H, Mills EJ. Addressing the Achilles’ heel in the HIV care continuum for the success of a test-and-treat strategy to achieve an AIDS-free generation. Clin Infect Dis 2014; 59(1): S21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA internal medicine 2015; 175(4): 588–96. [DOI] [PubMed] [Google Scholar]
- 3.Panagiotoglou D, Olding M, Enns B, et al. Building the case for localized approaches to HIV: structural conditions and health system capacity to address the HIV/AIDS epidemic in six US cities. AIDS and Behavior 2018;22(9):3071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). Monitoring selected national HIV prevention and care objectives by using HIV surveillance data: United States and 6 dependent areas, 2015. HIV Surveillance Supplemental Report 2018. 22(2). [Google Scholar]
- 5.Long E, Brandeau M, Owens D. The cost-effectiveness and population outcomes of expanded HIV screening and antiretroviral treatment in the United States. Ann Intern Med 2010. 153(12): 778–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiseman V, Mitton C, Doyle-Waters M, et al. Using economic evidence to set healthcare priorities in low-income and lower-middle-income countries: A systematic review of methodological frameworkds. Health Econ 2016; 25(S1): 140–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garnett G, Cousens S, Hallett T, Steketee R, Walker N. Mathematical models in the evaluation of health programmes. Lancet 2011; 378(9790): 515–25. [DOI] [PubMed] [Google Scholar]
- 8.Rebeiro PF, Gange SJ, Horberg MA, et al. Geographic variations in retention in care among HIV-infected adults in the United States. PLoS One 2016; 11(1): e0146119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nosyk B, Min JE, Evans E, et al. The effects of Opioid Substitution Treatment and Highly Active Antiretroviral Therapy on the cause-specific risk of mortality among HIV-positive people who inject drugs. Clin Infect Dis 2015; 61(7): 1157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy A, Johnston K, Annemans L, Tramarin A, Montaner J. The impact of disease stage on direct medical costs of HIV management: a review of the international literature. PharmacoEconomics 2010; 28 Suppl 1: 35–47. [DOI] [PubMed] [Google Scholar]
- 11.Kauf TL, Roskell N, Shearer A, et al. A predictive model of health state utilities for HIV patients in the modern era of highly active antiretroviral therapy. Value Health 2008; 11(7): 1144–53. [DOI] [PubMed] [Google Scholar]
- 12.Nosyk B, Min J, Lima VD, Yip B, Hogg RS, Montaner JS. HIV-1 disease progression during highly active antiretroviral therapy: an application using population-level data in British Columbia: 1996–2011. Journal of acquired immune deficiency syndromes 2013; 63(5): 653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Min JE, Zang X, et al. Characterizing Human Immunodeficiency Virus Antiretroviral Therapy Interruption and Resulting Disease Progression Using Population-Level Data in British Columbia, 1996–2015. Clinical Infectious Diseases 2017; 65(9): 1496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borg S, Persson U, Jess T, et al. A maximum likelihood estimator of a Markov model for disease activity in Crohn’s disease and ulcerative colitis for annually aggregated partial observations. Medical Decision Making 2010; 30(1): 132–42. [DOI] [PubMed] [Google Scholar]
- 15.Nichol MB, Knight TK, Wu J, et al. Transition Probabilities and Predictors of Adherence in a California Medicaid Population Using Antihypertensive and Lipid-Lowering Medications. Value Health 2009; 12(4): 544–50. [DOI] [PubMed] [Google Scholar]
- 16.Hoover DR, Graham NM, Chen B, et al. Effect of CD4+ cell count measurement variability on staging HIV-1 infection. J Acquir Immune Defic Syndr 1992; 5(8): 794–802. [PubMed] [Google Scholar]
- 17.Sypsa V, Touloumi G, Kenward M, Karafoulidou A, Hatzakis A. Comparison of smoothing techniques for CD4 data in a Markov model with states defined by CD4: an example on the estimation of the HIV incubation time distribution. Stat Med 2001. 20(24): 3667–76. [DOI] [PubMed] [Google Scholar]
- 18.Fleishman JA, Yehia BR, Moore RD, Gebo KA, Agwu AL. Disparities in receipt of antiretroviral therapy among HIV-infected adults (2002–2008). Medical care 2012; 50(5): 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson CH. Multi-state models for panel data: the msm package for R. Journal of Statistical Software 2011; 38(8): 1–29. [Google Scholar]
- 20.Vanni T, Karnon J, Madan J, et al. Calibrating models in economic evaluation. Pharmacoeconomics 2011; 29(1): 35–49. [DOI] [PubMed] [Google Scholar]
- 21.Eddy D, Hollingworth W, Caro J, et al. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making 2012; 32(5): 733–43. [DOI] [PubMed] [Google Scholar]
- 22.Taylor DC, Pawar V, Kruzikas D, et al. Methods of model calibration: observations from a mathematical model of cervical cancer. Pharmacoeconomics 2010; 28(11): 995–1000. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg AE, Purcell DW, Gordon CM, Barasky RJ, Del Rio C. Addressing the challenges of the HIV continuum of care in high-prevalence cities in the United States. JAIDS Journal of Acquired Immune Deficiency Syndromes 2015; 69: S1–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clinical Infectious Diseases 2013; 57(8): 1164–71. [DOI] [PubMed] [Google Scholar]
- 25.Nosyk B, Lourenço L, Min JE, et al. Characterizing retention in HAART as a recurrent event process: insights into ‘cascade churn’. AIDS 2015; 29(13): 1681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanters S, Park JJ, Chan K, et al. Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta-analysis. The Lancet HIV 2017; 4(1): e31–e40. [DOI] [PubMed] [Google Scholar]
- 27.Nosyk B, Krebs E, Eyawo O, Min JE, Barrios R, Montaner JS. Cost-effectiveness analysis along the continuum of HIV care: how can we optimize the effect of HIV treatment as prevention programs? Curr HIV/AIDS Rep 2014; 11(4): 468–78. [DOI] [PubMed] [Google Scholar]
- 28.El Bcheraoui C, Mokdad AH, Dwyer-Lindgren L, et al. Trends and patterns of differences in infectious disease mortality among US counties, 1980–2014. JAMA 2018; 319(12): 1248–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chammartin F, Zürcher K, Keiser O, et al. Outcomes of Patients Lost to Follow-up in African Antiretroviral Therapy Programs: Individual Patient Data Meta-analysis. Clinical infectious diseases 2018. [DOI] [PMC free article] [PubMed]
- 30.Enns EA, Cipriano LE, Simons CT, Kong CY. Identifying best-fitting inputs in health-economic model calibration: a Pareto frontier approach. Medical decision making 2015; 35(2): 170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.