Abstract
Twenty-nine protein kinase inhibitors have been used to treat human diseases. Out of these, two are Rho-associated protein kinase (ROCK) 1 and 2 inhibitors. The ROCKs heavily influence neuronal architecture and structural plasticity, and ROCKs are putative drug targets for various brain disorders. While the pan-ROCK inhibitor Fasudil has been clinically approved to treat hypertension, heart failure, glaucoma, spinal cord injury, and stroke, a barrier to progress on this therapeutic avenue is the lack of experimental comparisons between pharmacologic and genetic manipulation of ROCKs. Our study begins to address this question using parallel approaches to study behavior in mice that were treated with Fasudil or were heterozygous for ROCK1 or ROCK2. Adult mice treated with Fasudil for thirty days displayed reduced time spent in the open arms of the elevated plus maze, whereas activity in the open field was more analogous to mock-treated animals. Both male and female adult ROCK1+/− and ROCK2+/− mice exhibited reduced time spent in open arms of the elevated plus maze compared to littermate controls. However, ROCK1 or ROCK2 heterozygosity did not alter performance in the open field or Y-maze. These results indicate that chronic treatment with Fasudil induces anxiety-like behaviors that are likely the consequence of ROCK1 and/or ROCK2 inhibition. Our findings may have implications for several ongoing clinical trials using Fasudil or other ROCK-based therapeutics.
Keywords: Fasudil, Rho kinase, ROCK1, ROCK2, Anxiety
1. Introduction
Rho-associated coiled-coil containing kinases (ROCK) are members of the AGC family of serine/threonine kinases and are well-characterized regulators of actin–myosin-mediated cytoskeleton contractility. In mammals ROCKs exist as two isoforms, ROCK1 and ROCK2, and share 65% similarity in their amino acid sequences and 92% identity in their kinase domains [1]. ROCKs are ubiquitously expressed and phosphorylate a number of substrates that heavily influence cellular morphology, adhesion, and motility [2]. Through these actions, ROCK1 and ROCK2 are considered therapeutic targets for cancer, asthma, insulin resistance, kidney failure, osteoporosis, and erectile dysfunction [3–8]. Additionally, ROCKs are implicated in a number of neurodegenerative disorders, including glaucoma, spinal cord injury, stroke, Alzheimer’s disease, Frontotemporal Dementia, Parkinson’s disease, and Amyotrophic Lateral Sclerosis [9–17].
Small-molecule inhibitor studies have provided the majority of knowledge on ROCKs in the brain, with Fasudil and Y-27632 being the most widely characterized drugs. These pan-ROCK inhibitors are not selective to ROCK1 or ROCK2, and at doses typically used for preclinical in vivo experiments, likely inhibit other kinases, including PKA and PKC [18]. Hence, it is challenging to assign outcomes from pan-ROCK inhibitor studies to disruption of ROCK1 and/or ROCK2 activity. Therefore, pharmacological experiments should be confirmed by RNAi or knock-out animals. Despite these limitations, enthusiasm for ROCK-based therapeutics is growing, and Fasudil continues to yield promising results in preclinical studies tackling various brain disorders [19, 20].
Genetic confirmation of ROCK-based drug inhibition studies has been limited due to the complications of homozygous knockout mice on mixed genetic backgrounds [21–23]. To overcome this barrier, we independently generated new ROCK1+/− and ROCK2+/− mice on the C57BL/6N background to compare the effects of pan-ROCK inhibitors with ROCK1 or ROCK2 deficiency [24]. Our report and others indicate that ROCK1+/− and ROCK2+/− mice develop normally, but despite this, in vivo studies of ROCK1 or ROCK2 heterozygous models are rare [17, 24, 25]. Previous studies by Saitoh et al. demonstrated that intracerebroventricular delivery of Y-27632 to adult mice reduced time spent in the open arms of the elevated plus maze compared to vehicle controls [26]. Based on this, we explored how chronic oral delivery of Fasudil impacts anxiety-like behaviors, and in parallel we tested whether mice genetically deficient for ROCK1 or ROCK2 displayed a similar behavioral profile as Fasudil-treated animals.
2. Methods
2.1. Animals
All experimental procedures were performed under a protocol approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham (UAB). Generation of ROCK1+/− mice and ROCK2+/− mice were previously described [24]. Briefly, C57BL/6N-Rock1<tm1b(NCOM)Mfgc>/Tcp were made as part of the NorCOMM2 project at the Toronto Centre for Phenogenomics and were obtained from the Canadian Mouse Mutant Repository [27]. C57BL/6N-Rock2tm1a(KOMP)Wtsi mice were made from ES cells purchased from the International Mouse Phenotyping Consortium at the University of California, Davis. For more information or to obtain KOMP products go to www.komp.org or email service@komp.org. All mice were kept in a facility with a 12 hour light/dark cycle. All behavioral testing was performed during the light cycle. Mice were placed in the testing room at a minimum of one hour before testing for acclimation. Each apparatus was disinfected with 2% chlorhexidine prior to testing. Each apparatus was cleaned with 70% ethanol after each experiment. All testing was conducted at the same time each day on consecutive days.
2.2. Behavior
The elevated plus maze apparatus (EPM; Med Associates) was 1 m high with 2 in wide arms. Two opposite arms had 8 in high black walls, while the other opposing arms were open. Each mouse was placed in the center of the maze and freely explored for 5 minutes. Exploration into arms was recorded and traced by the manufacturer’s software (CleverSys). Percent time in open arms was calculated by dividing the time in open arms by total time. For open field assessment, mice were placed into a 16 in x 16 in plexiglass box (Med Associates) with opaque walls. Mice explored for 10 minutes, and ambulatory distance and ambulatory counts were determined by the manufacturer’s software (CleverSys). Y-maze testing was conducted as previously described [28]. The Y-maze consisted of three arms (38.1 cm long, 8.9 cm wide, 12.7 cm high) made of white plexiglass with randomly placed visual cues in each arm. Mice were placed in the center of the maze and allowed to explore for 5 minutes. Activity was recorded and tracked with video tracking software (Cleversys). An alternation was defined as sequential entries into each arm without re-entry into the previously explored arm. The percent of correct alternations was calculated by dividing the total number of spontaneous alternations by the total number of arm entries.
2.3. Oral gavage
Six month old C57BL/6J mice (The Jackson Laboratory Stock No: 000664) were treated once daily with mock (H20) or Fasudil (Selleckchem #S1573) for 30 days via oral gavage using plastic gavage tips (Instech Catalog #FTP-20–38). Treatment was given at 2:00 pm daily throughout the entirety of the treatment regimen. Fasudil was dissolved in H20 fresh each day at a concentration of 10 mg/kg (200 μL total volume per animal per day). For all experiments, age-matched and sex-matched animals were used. Additional details on mouse sex are provided in figure legends.
2.4. Statistical analysis
The effect of ROCK1 or ROCK2 heterozygosity was determined by comparing with ROCK1 or ROCK2 homozygotes, respectively. Two-way ANOVA was employed to assess a main effect of sex among the experimental variables, including Fasudil-treatment and ROCK1 or ROCK2 heterozygosity. There was no main effect of sex; therefore the two sexes were collapsed in the subsequent analyses. All analyses were conducted with Prism 6.0 (GraphPad Software, La Jolla, CA). Significance level was set at P < 0.05, and data were reported as mean ± standard error of the mean. Comparison of genotype or mock versus Fasudil was conducted by unpaired parametric two-tailed student’s t-test.
3. Results
3.1. Fasudil treatment induces anxiety-like behaviors
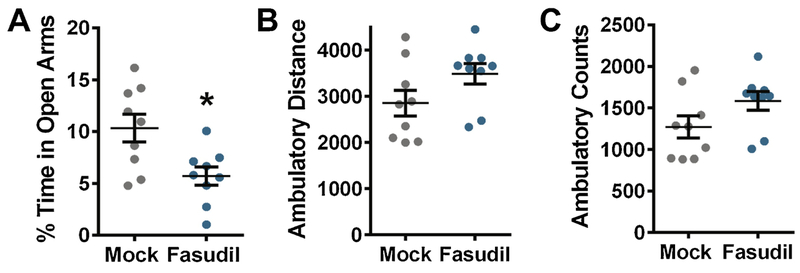
Six month old C57BL/6J mice were dosed with 10 mg/kg Fasudil or equivalent volume H20 (mock) by oral gavage once a day for 30 days. At the first dosing and the end of 30 days, all mice were weighed, revealing that Fasudil treatment did not alter weight. Behaviors were assessed over the final 3 days of drug treatment and included elevated plus maze (EPM) and open field. EPM was used to assess potential anxiety-like behaviors [29]. The amount of time spent in the open arms of the EPM was reduced significantly in mice treated with Fasudil compared to mock (P=0.0109) (Fig. 1A). Results from the open field test indicated that locomotor activity was slightly increased in mice exposed to Fasudil as measured by ambulatory distance and counts. However, the comparisons between mock- and Fasudil-treated mice in the open field were not significant (Fig. 1 B, C). These results indicate that exposure to Fasudil for 30 days induces anxiety-like behavior in adult mice.
Figure 1.
Fasudil induces anxiety-like behaviors. All mice were 6 months old. N=9 (5 males, 4 females) for both Mock and Fasudil-treated mice. (A) Fasudil-treated mice spent less time in the open arms of the elevated plus maze (EPM) compared to mock (t-test: *P= 0.0109, t=2.880, df=16). (B-C) Open field test. (B) Ambulatory distance and (C) ambulatory counts were measured. Lines represent the mean ± standard error of the mean.
3.2. Genetic reduction of ROCK1 or ROCK2 induces anxiety-like behaviors
Differences in weight, general health, in-cage behavior or food consumption was not observed in male or female adult ROCK1+/− and ROCK2+/− mice compared to ROCK1+/+ and ROCK2+/+ littermates, respectively. It is important to note that ROCK1+/+ and ROCK1+/− mice are littermates and represent a genetic lineage, while ROCK2+/+ and ROCK2+/− mice are littermates and represent a separate genetic lineage. Similar to Fasudil-treated mice, the amount of time spent in the open arms was reduced significantly in male and female adult ROCK1+/− or ROCK2+/− mice compared to ROCK1+/+ or ROCK2+/+, respectively (P=0.0024 for ROCK1+/− and P=0.0486 for ROCK2+/−) (Fig. 2 A, E). Locomotor activity was similar among all genotypes in the open field test (Fig. 2 B, C, F, G) and no difference in working memory was observed in Y-maze (Fig. 2 D, H). These results indicate that ROCK1 or ROCK2 heterozygosity induces anxiety without grossly affecting general activity or basic working memory components.
Figure 2.
ROCK1 or ROCK2 heterozygosity induces anxiety-like behaviors. All mice were age-matched (6 months old) and sex-matched. (A) ROCK1+/− mice (N=17: 9 males, 8 females) spent less time in the open arms of the elevated plus maze (EPM) compared to ROCK1+/+ (N=15: 8 males, 7 females) (t-test: **P=0.0024, t=3.318, df=30). (B-C) Open field test for ROCK1+/+ (N=20: 10 males, 10 females) and ROCK1+/− (N=19: 10 males, 9 females) mice. (B) Ambulatory distance and (C) ambulatory counts were measured. (D) ROCK1+/+ (N=20: 10 males, 10 females) and ROCK1+/− (N=19: 10 males, 9 females) mice performed similarly in Y-maze. (E) ROCK2+/− mice (N=15: 8 males, 7 females) spent less time in the open arms of the EPM compared to ROCK2+/+ (N=13: 7 males, 6 females) (t-test: *P=0.0486, t=2.069, df=26). (F-G) Open field test for ROCK2+/+ and ROCK2+/− mice (N=16 for both genotypes: 8 males, 8 females) show similar (F) ambulatory distance and (G) ambulatory counts. (H) ROCK2+/+ and ROCK2+/− mice (N=17 for both genotypes: 9 males, 8 females) performed similarly in Y-maze. Lines represent the mean ± standard error of the mean. R1+/+, ROCK1+/+; R1+/−, ROCK1+/−; R2+/+, ROCK2+/+; R2+/+, ROCK2+/−.
4. Discussion
ROCKs are attractive drug targets, and out of the 29 protein kinase inhibitors that have been used to treat human diseases, two are pan-ROCK inhibitors: Fasudil and Ripasudil [20]. The findings herein indicate that chronic treatment of mice with Fasudil induces anxiety-like behaviors, which are comparable to mice that are heterozygous for ROCK1 or ROCK2. Therefore, we conclude that the effects of Fasudil exposure in this study are the consequence of ROCK1 and/or ROCK2 inhibition. While locomotor activity and working memory were normal in mice heterozygous for ROCK1 or ROCK2, both genotypes spent reduced time in the open arms of the EPM compared to ROCK1+/+ and ROCK2+/+ littermates. Similarly, Fasudil-treated animals spent less time in the open arms of the EPM.
Analogous to our findings herein, Saitoh et al. showed that mice treated with the pan-ROCK inhibitor Y-27632 spent reduced time in the open arms of the EPM compared to vehicle controls [26]. Moreover, Y-27632 did not produce significant effects on exploratory behavior or locomotor activity in the EPM. These results are similar to our pharmacologic and genetics studies using the open field test. Finally, Saitoh et al. found no significant effect of Y-27632 on spontaneous alterations in the Y-maze, which matched our results on ROCK1+/− and ROCK2+/− mice. In a contrasting study, mice exposed to the pan-ROCK inhibitor Y-27632 did not display anxiety in the EPM or changes in novel object recognition tests but did exhibit enhanced spatial discrimination in Y-maze [30]. These contrasting outcomes may be attributed to possible off-target peripheral effects of Y-27632, different target-engagement profile for Y-27632 in comparison with Fasudil, or the difference in drug-delivery methods. Alternatively, haploinsufficiency of ROCK1 or ROCK2 from birth rather than inhibition of ROCKs in adulthood could contribute to the observed discrepancies. To our knowledge, the results herein are the first reported behavior examinations that assess the impact of ROCK1 or ROCK2 heterozygosity in rodents. Based on the similarity of EPM phenotypes, our findings suggest potential overlap in ROCK1 and ROCK2 function with respect to behavior at the organismal level.
The medial prefrontal cortex (mPFC) is required for normal anxiety-related behaviors in the EPM [31]. Moreover, rodent avoidance of the open arms in the EPM is linked to alterations in the firing rate of single neurons in the mPFC [32]. Previous work from our group demonstrated a role for ROCK1 and ROCK2 in mPFC structural plasticity. Specifically, haploinsufficiency of ROCK1 increased dendrite length and dendritic arbor complexity of layer 2/3 pyramidal neurons in the mPFC [24]. Other studies showed that intracerebroventricular delivery of Fasudil increased dendrite length in CA1 pyramidal neurons [33], and homozygous deletion of ROCK2 on the CD-1 genetic background increased dendrite length and arbor complexity in hippocampal neurons [17]. Together, these studies point toward a role for ROCKs in regulating neuronal architecture at the dendrite level which could influence behavior; however ROCKs also affect neuronal microstructure, including dendritic spines. Although heterozygosity of ROCK1 or ROCK2 did not alter dendritic spine density among layer 2/3 pyramidal neurons in the mPFC, ROCK1+/− mice displayed reduced spine length on apical dendrites and reduced spine head diameter on basal dendrites. Similarly, ROCK2+/− mice exhibited reduced spine head diameter on basal dendrites but showed increased spine length on apical dendrites [24]. Collectively, these findings suggest a possible link between changes in dendritic spine head diameter and neuronal firing rate among neurons in the mPFC which could influence performance on the EPM.
Another possibility is that the effects of Fasudil or ROCK1 or ROCK2 heterozygosity outside the central nervous system contributed to the behavioral outcomes on the EPM. For instance, blood pressure reduction is a well-characterized effect of pan-ROCK inhibitors, including Fasudil, and is likely due to ROCK1 inhibition [34]. However, hypertension can be associated with anxiety in rodents, and presumably, inhibiting or reducing ROCKs would partially alleviate hypertensive effects, which does not correlate to the observed behavior on the EPM [35]. The findings herein are informative given the continued development of pan-ROCK and ROCK isoform-selective inhibitors to treat human diseases [20]. Although we show that there are over-lapping behavior phenotypes under experimental conditions of chronic pan-ROCK inhibition or ROCK1 or ROCK2 heterozygosity, more work is required to understand the specific functional differences between ROCK1 and ROCK2 in the brain. Future studies using conditional ROCK1 and ROCK2 knockout mice will help define isoform-specific functions in a cell-type or time-dependent manner to better assess the potential for ROCK-based therapeutics in adolescence and aging.
Highlights for Greathouse etal.
The clinically approved pan-ROCK inhibitor Fasudil induces anxiety-like behavior.
ROCK1 or ROCK2 heterozygosity induces anxiety-like behavior.
Locomotor activity and working memory is not altered by ROCK1 or ROCK2 heterozygosity.
Acknowledgements
We thank Courtney K. Walker for critical reading of this manuscript. This work was supported by the National Institutes of Health through NIA AG054719–01 to J.H.H. and NIA AG061800–01 to J.H.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors have nothing to disclose and have no conflicts of interest.
References
- [1].Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S, ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice, FEBS Lett 392(2) (1996) 189–93. [DOI] [PubMed] [Google Scholar]
- [2].Julian L, Olson MF, Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions, Small GTPases 5 (2014) e29846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schaafsma D, Gosens R, Zaagsma J, Halayko AJ, Meurs H, Rho kinase inhibitors: a novel therapeutical intervention in asthma?, Eur J Pharmacol 585(2–3) (2008) 398–406. [DOI] [PubMed] [Google Scholar]
- [4].Rath N, Olson MF, Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy, EMBO Rep 13(10) (2012) 900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Albersen M, Shindel AW, Mwamukonda KB, Lue TF, The future is today: emerging drugs for the treatment of erectile dysfunction, Expert Opin Emerg Drugs 15(3) (2010) 467–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Olson MF, Applications for ROCK kinase inhibition, Curr Opin Cell Biol 20(2) (2008) 242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee DH, Shi J, Jeoung NH, Kim MS, Zabolotny JM, Lee SW, White MF, Wei L, Kim YB, Targeted disruption of ROCK1 causes insulin resistance in vivo, J Biol Chem 284(18) (2009) 11776–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Komers R, Oyama TT, Beard DR, Tikellis C, Xu B, Lotspeich DF, Anderson S, Rho kinase inhibition protects kidneys from diabetic nephropathy without reducing blood pressure, Kidney Int 79(4) (2011) 432–42. [DOI] [PubMed] [Google Scholar]
- [9].Challa P, Arnold JJ, Rho-kinase inhibitors offer a new approach in the treatment of glaucoma, Expert Opin Investig Drugs 23(1) (2014) 81–95. [DOI] [PubMed] [Google Scholar]
- [10].Henderson BW, Gentry EG, Rush T, Troncoso JC, Thambisetty M, Montine TJ, Herskowitz JH, Rho-associated protein kinase 1 (ROCK1) is increased in Alzheimer’s disease and ROCK1 depletion reduces amyloid-beta levels in brain, J Neurochem 138(4) (2016) 525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Herskowitz JH, Feng Y, Mattheyses AL, Hales CM, Higginbotham LA, Duong DM, Montine TJ, Troncoso JC, Thambisetty M, Seyfried NT, Levey AI, Lah JJ, Pharmacologic inhibition of ROCK2 suppresses amyloid-beta production in an Alzheimer’s disease mouse model, J Neurosci 33(49) (2013) 19086–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gentry EG, Henderson BW, Arrant AE, Gearing M, Feng Y, Riddle NC, Herskowitz JH, Rho Kinase Inhibition as a Therapeutic for Progressive Supranuclear Palsy and Corticobasal Degeneration, J Neurosci 36(4) (2016) 1316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gunther R, Balck A, Koch JC, Nientiedt T, Sereda M, Bahr M, Lingor P, Tonges L, Rho Kinase Inhibition with Fasudil in the SOD1(G93A) Mouse Model of Amyotrophic Lateral Sclerosis-Symptomatic Treatment Potential after Disease Onset, Front Pharmacol 8 (2017) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Koch JC, Tonges L, Barski E, Michel U, Bahr M, Lingor P, ROCK2 is a major regulator of axonal degeneration, neuronal death and axonal regeneration in the CNS, Cell Death Dis 5 (2014) e1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tatenhorst L, Eckermann K, Dambeck V, Fonseca-Ornelas L, Walle H, Lopes da Fonseca T, Koch JC, Becker S, Tonges L, Bahr M, Outeiro TF, Zweckstetter M, Lingor P, Fasudil attenuates aggregation of alpha-synuclein in models of Parkinson’s disease, Acta Neuropathol Commun 4 (2016) 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shibuya M, Hirai S, Seto M, Satoh S, Ohtomo E, Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial, J Neurol Sci 238(1–2) (2005) 31–9. [DOI] [PubMed] [Google Scholar]
- [17].Duffy P, Schmandke A, Sigworth J, Narumiya S, Cafferty WB, Strittmatter SM, Rho-associated kinase II (ROCKII) limits axonal growth after trauma within the adult mouse spinal cord, J Neurosci 29(48) (2009) 15266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Davies SP, Reddy H, Caivano M, Cohen P, Specificity and mechanism of action of some commonly used protein kinase inhibitors, Biochem J 351(Pt 1) (2000) 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Defert O, Boland S, Rho kinase inhibitors: a patent review (2014 – 2016), Expert opinion on therapeutic patents 27(4) (2017) 507–515. [DOI] [PubMed] [Google Scholar]
- [20].Feng Y, LoGrasso PV, Defert O, Li R, Rho Kinase (ROCK) Inhibitors and Their Therapeutic Potential, J Med Chem (2015). [DOI] [PubMed] [Google Scholar]
- [21].Zhou Z, Meng Y, Asrar S, Todorovski Z, Jia Z, A critical role of Rho-kinase ROCK2 in the regulation of spine and synaptic function, Neuropharmacology 56(1) (2009) 81–9. [DOI] [PubMed] [Google Scholar]
- [22].Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, Noda Y, Matsumura F, Taketo MM, Narumiya S, ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles, J Cell Biol 168(6) (2005) 941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, Oshima M, Taketo MM, Narumiya S, Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death, Mol Cell Biol 23(14) (2003) 5043–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Greathouse KM, Boros BD, Deslauriers JF, Henderson BW, Curtis KA, Gentry EG, Herskowitz JH, Distinct and complementary functions of rho kinase isoforms ROCK1 and ROCK2 in prefrontal cortex structural plasticity, Brain structure & function 223(9) (2018) 4227–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang YM, Bo J, Taffet GE, Chang J, Shi J, Reddy AK, Michael LH, Schneider MD, Entman ML, Schwartz RJ, Wei L, Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis, FASEB J 20(7) (2006) 916–25. [DOI] [PubMed] [Google Scholar]
- [26].Saitoh A, Yamada M, Yamada M, Kobayashi S, Hirose N, Honda K, Kamei J, ROCK inhibition produces anxiety-related behaviors in mice, Psychopharmacology 188(1) (2006) 1–11. [DOI] [PubMed] [Google Scholar]
- [27].Bradley A, Anastassiadis K, Ayadi A, Battey JF, Bell C, Birling MC, Bottomley J, Brown SD, Burger A, Bult CJ, Bushell W, Collins FS, Desaintes C, Doe B, Economides A, Eppig JT, Finnell RH, Fletcher C, Fray M, Frendewey D, Friedel RH, Grosveld FG, Hansen J, Herault Y, Hicks G, Horlein A, Houghton R, Hrabe de Angelis M, Huylebroeck D, Iyer V, de Jong PJ, Kadin JA, Kaloff C, Kennedy K, Koutsourakis M, Lloyd KC, Marschall S, Mason J, McKerlie C, McLeod MP, von Melchner H, Moore M, Mujica AO, Nagy A, Nefedov M, Nutter LM, Pavlovic G, Peterson JL, Pollock J, Ramirez-Solis R, Rancourt DE, Raspa M, Remacle JE, Ringwald M, Rosen B, Rosenthal N, Rossant J, Ruiz Noppinger P, Ryder E, Schick JZ, Schnutgen F, Schofield P, Seisenberger C, Selloum M, Simpson EM, Skarnes WC, Smedley D, Stanford WL, Stewart AF, Stone K, Swan K, Tadepally H, Teboul L, Tocchini-Valentini GP, Valenzuela D, West AP, Yamamura K, Yoshinaga Y, Wurst W, The mammalian gene function resource: the International Knockout Mouse Consortium, Mamm Genome 23(9–10) (2012) 580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li Z, Hall AM, Kelinske M, Roberson ED, Seizure resistance without parkinsonism in aged mice after tau reduction, Neurobiology of aging 35(11) (2014) 2617–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hogg S, A review of the validity and variability of the elevated plus-maze as an animal model of anxiety, Pharmacol Biochem Behav 54(1) (1996) 21–30. [DOI] [PubMed] [Google Scholar]
- [30].Christie KJ, Turbic A, Turnley AM, Adult hippocampal neurogenesis, Rho kinase inhibition and enhancement of neuronal survival, Neuroscience 247 (2013) 75–83. [DOI] [PubMed] [Google Scholar]
- [31].Shah AA, Sjovold T, Treit D, Inactivation of the medial prefrontal cortex with the GABAA receptor agonist muscimol increases open-arm activity in the elevated plus-maze and attenuates shock-probe burying in rats, Brain Res 1028(1) (2004) 112–5. [DOI] [PubMed] [Google Scholar]
- [32].Adhikari A, Topiwala MA, Gordon JA, Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity, Neuron 71(5) (2011) 898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Couch BA, DeMarco GJ, Gourley SL, Koleske AJ, Increased dendrite branching in AbetaPP/PS1 mice and elongation of dendrite arbors by fasudil administration, J Alzheimers Dis 20(4) (2010) 1003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Feng Y, LoGrasso PV , Rho kinase inhibitors: a patent review (2012 – 2013), Expert opinion on therapeutic patents 24(3) (2014) 295–307. [DOI] [PubMed] [Google Scholar]
- [35].Duchemin S, Belanger E, Wu R, Ferland G, Girouard H, Chronic perfusion of angiotensin II causes cognitive dysfunctions and anxiety in mice, Physiology & behavior 109 (2013) 63–8. [DOI] [PubMed] [Google Scholar]