Abstract
Lessons Learned.
This single‐arm, phase II study shows that concurrent EGFR‐tyrosine kinase inhibitor plus thoracic radiotherapy as the first‐line treatment for stage IV non‐small cell lung cancer harboring EGFR active mutations provides long‐term control for the primary lung lesion, and 1‐year progression‐free survival (PFS) rate and median PFS are numerically higher than those of the erlotinib monotherapy.
Serious adverse events are acceptable, although grade >3 radiation pneumonitis occurred in 20% of patients.
Background.
Studies show effective local control by EGFR‐tyrosine kinase inhibitor (TKI) combined with radiotherapy at metastatic sites in advanced lung cancer harboring EGFR active mutations. Salvage local radiotherapy is associated with prolonged progression‐free survival (PFS) in local disease during EGFR‐TKI treatment. However, no prospective study has been reported on concurrent EGFR‐TKI and radiotherapy for primary lung lesions. This study investigated the efficacy and safety of first‐line EGFR‐TKI combined with thoracic radiotherapy in treating stage IV non‐small cell lung cancer (NSCLC) harboring EGFR active mutations.
Methods.
We conducted a single‐arm, phase II clinical trial. Each patient received EGFR‐TKI (erlotinib 150 mg or gefitinib 250 mg per day) plus thoracic radiotherapy (54–60 Gy/27–30 F/5.5–6 w) within 2 weeks of beginning EGFR‐TKI therapy until either disease progression or intolerable adverse events (AEs) appeared.
Results.
From January 2015 to March 2018, 401 patients were screened, and 10 patients (5 male and 5 female) were eligible. These patients had a median age of 55 years (40–75) and median follow‐up of 19.8 months (5.8–34). The 1‐year PFS rate was 57.1%, median PFS was 13 months, and median time to progression of irradiated lesion (iTTP) was 20.5 months. Objective response rate (ORR), was 50% and disease control rate (DCR) was 100%. The most common grade ≥3 AEs were radiation pneumonitis (20%) and rash (10%). One patient died after rejecting treatment for pneumonitis. The others received a full, systematic course of glucocorticoid therapy. Pneumonitis was all well controlled and did not relapse.
Conclusion.
Concurrent EGFR‐TKI plus thoracic radiotherapy as the first‐line treatment for stage IV NSCLC harboring EGFR active mutations shows a long‐term control of primary lung lesion. The 1‐year PFS rate and median PFS of this combined therapy are numerically higher than those of the erlotinib monotherapy. The risk of serious adverse events is acceptable.
Abstract
经验总结
本项研究为单臂 II 期研究,结果表明胸部放疗联合 EGFR‐酪氨酸激酶抑制剂在 EGFR 活性突变的 IV 期非小细胞肺癌患者一线治疗中,可以长期控制原发肺病灶,而且 1 年无进展生存 (PFS) 率和中位 PFS 高于厄洛替尼单药治疗。
严重不良事件的发生率可接受,尽管3 级以上放射性肺炎发生率为 20%。
摘要
背景。研究表明,EGFR 酪氨酸激酶抑制剂 (TKI) 联合放疗可有效局部控制 EGFR 活性突变的晚期肺癌转移部位。在 EGFR‐TKI 治疗过程中,挽救性局部放疗可延长局部疾病的无进展生存期 (PFS)。然而,目前还没有 EGFR‐TKI 联合放疗治疗原发肺病灶的前瞻性研究报道。本研究探讨了 EGFR‐TKI 联合胸部放疗在 EGFR 活性突变的 IV 期非小细胞肺癌 (NSCLC) 一线治疗中的疗效和安全性。
方法。研究为单臂 II 期临床试验。所有患者在开始 EGFR‐TKI 治疗后 2 周内接受 EGFR‐TKI(每天厄洛替尼 150 mg或 吉非替尼250 mg)联合胸部放疗(54‐60 Gy/ 27‐30 F/ 5.5‐6 w),直到出现疾病进展或无法忍受的不良事件 (AE)。
结果。筛查 2015 年 1 月至 2018 年 3 月期间的 401 例患者,其中 10 例患者符合条件(男性 5 例,女性 5 例)。这些患者的中位年龄为 55 岁(40‐75 岁),中位随访时间为 19.8 个月(5.8‐34 个月)。1 年 PFS 率为 57.1%,中位 PFS 为 13 个月,中位放射病灶至进展时间 (iTTP) 为 20.5 个月。客观有效率 (ORR) 为 50%,疾病控制率 (DCR) 为 100%。最常见的 3 级以上AE为放射性肺炎 (20%) 和皮疹 (10%)。1 例患者在拒绝接受肺炎治疗后死亡。其他患者接受了完整、系统的糖皮质激素治疗。肺炎得到很好的控制,没有复发。
结论。EGFR‐TKI 联合胸部放疗在 EGFR 活性突变的 IV 期 NSCLC 患者一线治疗中,可以长期控制原发肺病灶。这种联合疗法的 1 年 PFS 率和中位 PFS 数值上高于厄洛替尼单药治疗。严重不良事件的发生率可接受。
Discussion
Our previous study revealed that local radiation was associated with prolonged PFS in patients with EGFR‐mutant advanced lung cancer who experienced local progression. It was also reported that EGFR‐TKI could increase radiosensitivity and that radiotherapy could reduce EGFR‐TKI resistance. Therefore, EGFR‐TKI plus thoracic radiotherapy could be a promising strategy for treating patients with advanced NSCLC.
We conducted this prospective study to explore the efficacy and safety of concurrent EGFR‐TKI plus thoracic radiotherapy as the first‐line treatment for stage IV limited metastatic NSCLC harboring EGFR mutations (no more than ten distant lesions). The primary endpoint was 1‐year PFS rate. In the ENSURE study, the 1‐year PFS rate of patients treated with erlotinib monotherapy was 43%, and the median PFS was 11.0 months. In the current study, the estimated 1‐year PFS rate was 60%. Considering a 20% dropout rate and one‐sided α = .1, β = .2, a sample size of 47 patients was needed according to the Freedman rule from the Contract Research Organization of Brightech (Chengdu, China).
A total of 401 patients with NSCLC were screened. Ultimately, ten patients (five males and five females) with a median age of 55 (40–75) years were eligible. Tumors in four patients displayed an exon 21 L858R mutation, and six had exon 19 deletion. All patients had good baseline performance status (PS, 0–1). One patient received gefitinib, and the others received erlotinib. The radiotherapy design was 54–60 Gy/27–30 F/5.5–6 w. The median radiation dose to both lungs in all the recruited cases was as follows: normal bilateral lung volume receiving at least 20 Gy, 12% ± 4%; mean lung dose, 5.25 ± 3.75 Gy. All the patients completed radiotherapy. By October 8, 2018, the median follow‐up time for all ten patients was 19.8 months (5.8–34.0 months). By the last follow‐up, five patients were alive. One‐year PFS was 57.1%. Kaplan‐Meier analysis showed that the median PFS was 13.0 months (95% confidence interval [CI] 4.9–21.1 months), and the median iTTP was 20.5 months (95% CI, 10.6–25.5 months). Five patients were assessed as PR (50%), five as SD (50%), and no patient as PD. ORR was 50%, and DCR was 100%. Rash (5/10), radiation pneumonitis (4/10), and diarrhea (2/10) were the most common adverse events. Treatment‐related grade ≥3 radiation pneumonitis occurred at a rate of 20% (2/10) and rash at 10% (1/10). Radiation pneumonitis occurred at an average 40 days after radiation. One patient died after rejecting any treatment for pneumonitis, whereas the others recovered after a full, systematic course of glucocorticoid therapy.
To the best of our knowledge, this is the first study to focus on the efficacy and safety of concurrent EGFR‐TKI and thoracic radiotherapy as the first‐line treatment for stage IV NSCLC harboring EGFR active mutations. Our study found that combined therapy can achieve long‐term control of the primary lung lesion. The 1‐year PFS rate and median PFS of this combined therapy are numerically higher than those of erlotinib monotherapy. The risk of serious adverse events is acceptable.
Trial Information
- Disease
Lung cancer – NSCLC
- Stage of Disease/Treatment
Primary
- Prior Therapy
None
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Single arm
- Primary Endpoint
1‐year progression‐free survival rate
- Secondary Endpoint
Progression‐free survival
- Secondary Endpoint
Overall survival
- Secondary Endpoint
Safety
- Secondary Endpoint
Overall response rate
- Secondary Endpoint
Time to progression of irradiated lesion
- Additional Details of Endpoints or Study Design
- The study recruited patients with pathologically confirmed stage IV NSCLC harboring active EGFR mutations. The patients should not have received previous systemic therapy or treatment with any other investigational agents or have participated in any other clinical trials. Patients between the ages of 18 and 75, with Eastern Cooperative Oncology Group (ECOG) performance status 0~2, with no more than ten metastatic lesions, no brain metastases or serious functional damage of important organs were eligible. All patients signed an informed consent document after careful discussion of risk and potential benefit. Radiation therapy had to begin within 2 weeks of EGFR‐TKI therapy.
- Investigator's Analysis
Active and should be pursued further
Drug Information
- Drug 1
- Generic/Working Name
Erlotinib
- Drug Class
EGFR
- Dose
150 milligrams (mg) per flat dose
- Route
Oral (p.o.)
- Schedule of Administration
150 mg per day until either disease progression or intolerable AEs appeared.
- Drug 2
- Generic/Working Name
Gefitinib
- Drug Class
EGFR
- Dose
250 milligrams (mg) per flat dose
- Route
Oral (p.o.)
- Schedule of Administration
250 mg per day until either disease progression or intolerable AEs appeared.
Patient Characteristics
- Number of Patients, Male
5
- Number of Patients, Female
5
- Stage
Stage IV
- Age
Median (range): 55 (40–75) years
- Number of Prior Systemic Therapies
Median (range): 0
- Performance Status: ECOG
-
0 — 6
1 — 4
2 — 0
3 — 0
Unknown — 0
- Cancer Types or Histologic Subtypes
Lung adenocarcinoma, 10
Primary Assessment Method
- Title
New assessment
- Number of Patients Screened
401
- Number of Patients Enrolled
10
- Number of Patients Evaluable for Toxicity
10
- Number of Patients Evaluated for Efficacy
10
- Evaluation Method
RECIST version 1.1
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 5 (50%)
- Response Assessment SD
n = 5 (50%)
- Response Assessment PD
n = 0 (0%)
- (Median) Duration Assessments PFS
13 months; CI, 8.4–15.4
- Outcome Notes
- From January 2015 to March 2018, 401 patients were screened, and 10 patients (5 male and 5 female) were eligible with a median age of 55 years (40–75) and median follow‐up of 19.8 months (5.8–34). The 1‐year PFS rate was 57.1%; median PFS was 13 months, and median iTTP was 20.5 months. ORR was 50%, and DCR was 100%. The most common grade ≥3 AEs were radiation pneumonia (20%) and rash (10%).
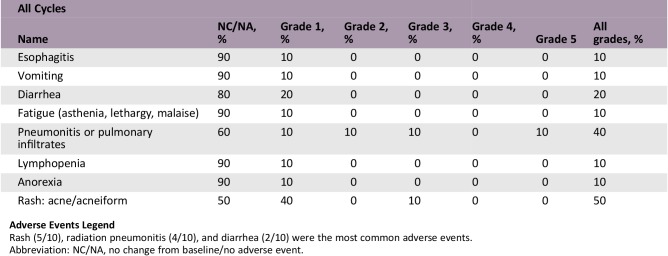
Adverse Events
Adverse Events Legend
Rash (5/10), radiation pneumonitis (4/10), and diarrhea (2/10) were the most common adverse events.
Abbreviation: NC/NA, no change from baseline/no adverse event.
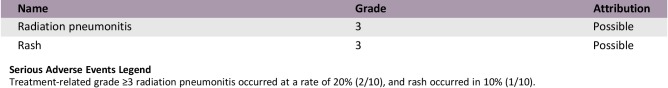
Serious Adverse Events
Serious Adverse Events Legend
Treatment‐related grade ≥3 radiation pneumonitis occurred at a rate of 20% (2/10), and rash occurred in 10% (1/10).
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Investigator's Assessment
Active and should be pursued further
Most patients receiving first‐generation EGFR‐tyrosine kinase inhibitor (TKI) undergo disease progression after 8.4 to 13.1 months of treatment, and the majority have local progression [1], [2], [3]. It has been reported that EGFR‐TKI could increase radiosensitivity and that radiotherapy could reduce EGFR‐TKI resistance [4], [5]. Moreover, several studies showed effective local control by EGFR‐TKI combined with radiotherapy in metastatic sites of advanced non‐small cell lung cancer (NSCLC) harboring EGFR active mutations [6], [7], [8]. Our previous study also revealed that local radiation prolonged progression‐free survival (PFS) in patients with EGFR‐mutant advanced lung cancer who acquired local progression after EGFR‐TKI therapy [9]. Therefore, we hypothesized that there would be an improved efficacy in concurrent EGFR‐TKI and local radiotherapy. No prospective study of concurrent EGFR‐TKI and radiotherapy for primary lung cancer has yet been reported. Thus, we conducted this single‐arm phase II study to investigate the efficacy and safety of EGFR‐TKI with concurrent thoracic radiotherapy in newly diagnosed stage IV NSCLC harboring EGFR active mutations
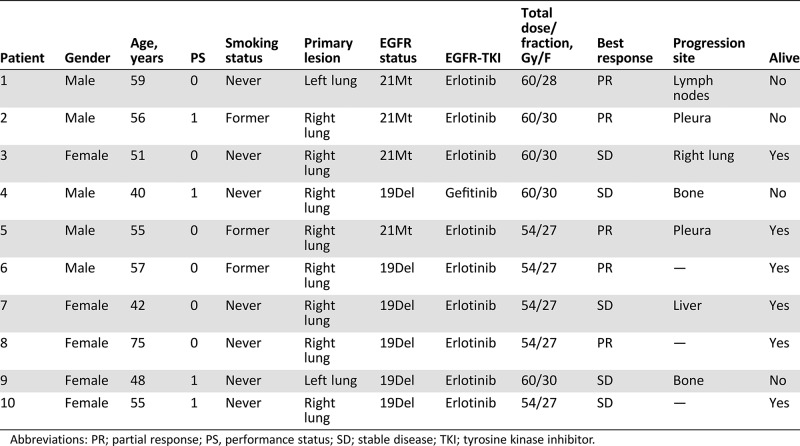
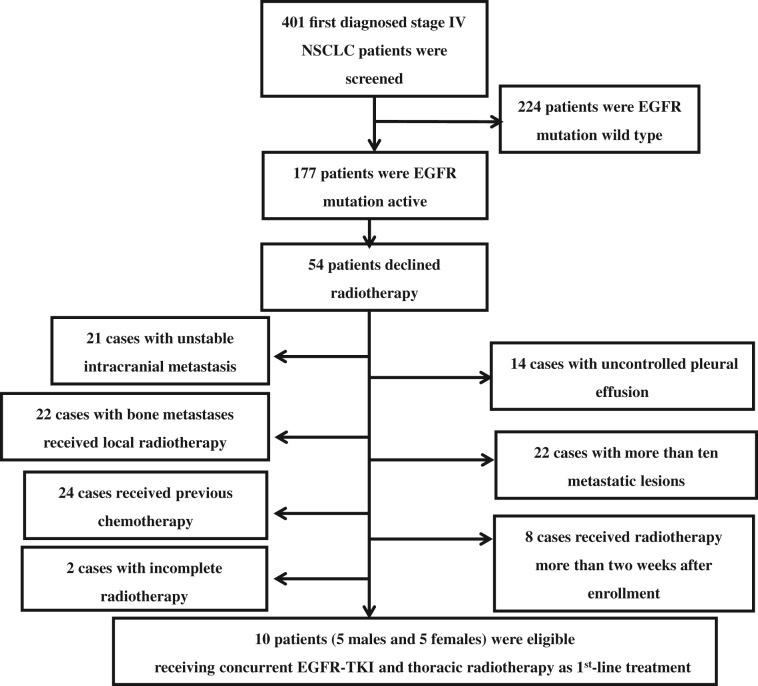
From January 2015 to March 2018, a total of 401 patients with NSCLC were screened, including sequencing tumors to determine mutation status. EGFR mutation analysis was performed by staff members from Pathology Department of our hospital during screening using the SuperARMS assay (AmoyDx, Xiamen, China). Finally, ten eligible patients (five male and five female) with a median age of 55 (40–75) years were enrolled (Fig. 1). The basic clinical characteristics of these ten cases are listed in Table 1. Nine patients had bone metastases, eight had lymph nodes metastases, and three had lung metastases. The trial was closed prematurely because of the low acceptance of thoracic radiotherapy plus EGFR‐TKI treatment in patients.
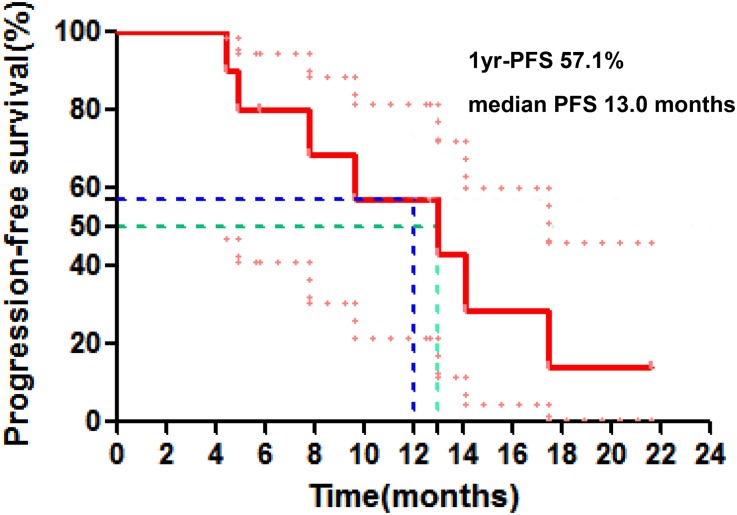
Figure 1.
Progression‐free survival.
Abbreviation: PFS, progression‐free survival.
Table 1. Basic clinical characteristics.
Abbreviations: PR; partial response; PS, performance status; SD; stable disease; TKI; tyrosine kinase inhibitor.
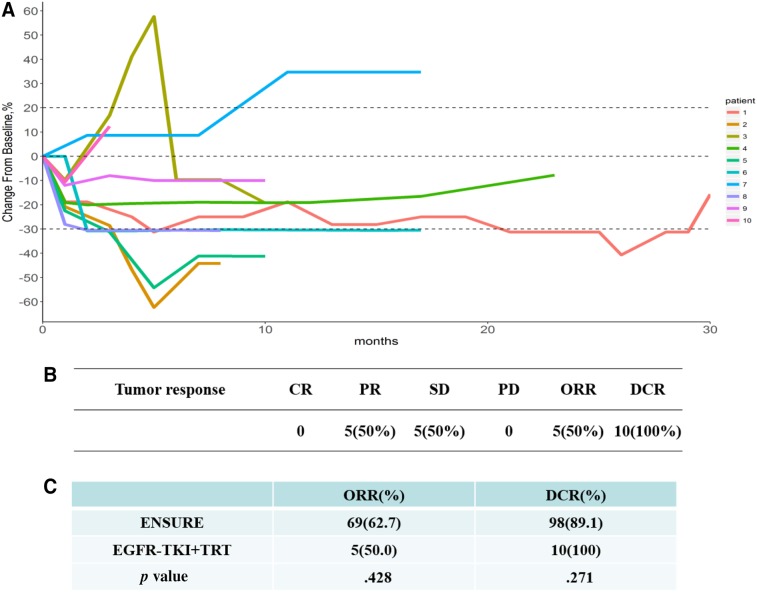
One patient received gefitinib, and the others received erlotinib. EGFR‐TKI was used until disease progression or intolerability of adverse events appeared. The plan of radiotherapy was 54–60 Gy/27–30 F/5.5–6 w. There was no limit to the maximum size of primary tumor, but normal bilateral lung volume receiving at least 20 Gy (V20) was strictly controlled to be less than 20%. Usually, primary tumors smaller than 7 cm in diameter were suitable to deliver concurrent EGFR‐TKI plus thoracic radiotherapy. At the last follow‐up on October 8, 2018, five patients were alive. We compared our results with those in the ENSURE study, which randomized first‐line erlotinib against gemcitabine/cisplatin in stage IIIB/IV EGFR mutation‐positive NSCLC. Concurrent EGFR‐TKI plus thoracic radiotherapy as the 1st‐line treatment for patients with stage IV NSCLC harboring EGFR active mutations showed numerically higher 1‐ year PFS rate (57.1% vs. 43%) and median PFS (13.0 vs. 11.0 months; Fig. 2). In addition to stage IV disease, patients with IIIB disease were enrolled into the ENSURE study, which may contribute to a longer PFS and therefore minimize the gain by the addition of radiation therapy. A spider plot was used to show the dynamic changes in the maximum diameter of primary lung lesion. Five patients were assessed as having partial response (50%), five as having stable disease (50%), and no patient as having progressive disease or complete response. Compared with the ENSURE study, there was no significant difference in objective response rate (50% vs. 62.7%, p = .43) or disease control rate (100% vs. 89.1%, p = .27; Fig. 3). Median time to progression of irradiated lesion in the current study was 20.5 months (95% confidence interval, 10.6–25.5 months). Similarly, Wang et al. [10] found that EGFR‐TKI concurrent with thoracic radiotherapy in treating stage IIIB/IV NSCLC had a local control rate of 96% for thoracic tumor, with 1‐year PFS rate of 42%. However, only one patient's EGFR mutation status was confirmed in that study.
Figure 2.
Tumor response. (A): Spider plot of dynamic changes in the maximum diameter of the tumor. (B): Best tumor response. (C): Compared with the ENSURE study, there was no significant difference in ORR (50%, p = .43) or DCR (100%, p = .27). ORR = CR + PR; local control rate = CR + PR + SD.
Abbreviations: CR, complete response; DCR, disease control rate; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease; TKI, tyrosine kinase inhibitor; TRT, thoracic radiotherapy.
Figure 3.
Flow chart of patient enrollment process.
Abbreviations: NSCLC, non‐small cell lung cancer; TKI, tyrosine kinase inhibitor.
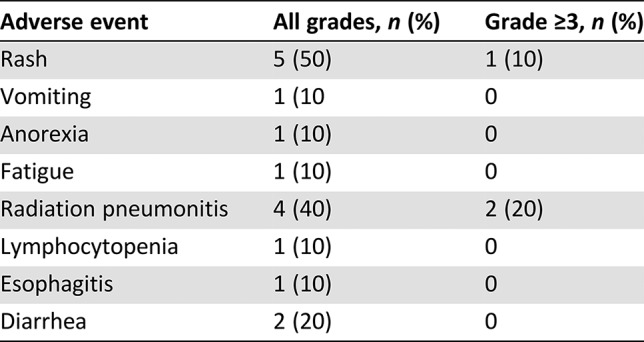
In the current study, rash (5/10), radiation pneumonitis (4/10) and diarrhea (2/10) were the most common adverse events at any grade. The incidence of treatment‐related grade ≥3 radiation pneumonitis and rash was 20% (2/10) and 10% (1/10), respectively (Table 2). Radiation pneumonitis occurred at an average 40 days after radiation. Two patients were assessed as grade 2 adverse events and one as grade 3 adverse event. One patient (grade 5) died, rejecting any treatment, whereas the others recovered after receiving a full, systematic course of glucocorticoid therapy. Previous studies also reported that the risk of radiation pneumonitis reached 22–32% during concurrent chemotherapy and radiotherapy, consistent with the current trial [11], [12]. The mean lung dose (MLD) and V20 were the two most important parameters of radiation pneumonitis [13], [14], which were strictly controlled in our study (MLD = 5.25 ± 3.75 Gy, V20 = 12 ± 4%). The incidence of rash in the current study was not higher than that in the study using erlotinib monotherapy [15]. Thus, the risk of serious adverse events is acceptable in concurrent EGFR‐TKI and radiotherapy. Yoshida et al. [16] reported that lung was the most frequent site of treatment failures during EGFR‐TKI treatment. Chen et al. [17] investigated the recurrence patterns of advanced non‐small cell lung cancer of 318 patients treated with gefitinib and found that lung (62.34%) was the most common initial failure site. And patients with failure in the lung had a shorter median PFS time. Wang et al. [10] found that EGFR‐TKI concurrent with thoracic radiotherapy in treating stage IIIB/IV NSCLC had a local control rate of 96% for thoracic tumor. We thus combined thoracic radiotherapy to control the primary lung lesions, finding that the median time to progression of irradiated lesion reached 20.5 months.
Table 2. Adverse events.
In conclusion, concurrent EGFR‐TKI plus thoracic radiotherapy as the first‐line treatment for stage IV NSCLC harboring EGFR active mutations shows a long‐term control of primary lung lesion. The 1‐year PFS rate and median PFS of this combined therapy are numerically higher than those of erlotinib monotherapy. The risk of serious adverse events is acceptable. A multicenter randomized controlled clinical trial with a larger sample size is needed to confirm the efficacy and safety of first‐line concurrent EGFR‐TKI and thoracic radiotherapy for stage IV NSCLC.
Figure and Tables
Acknowledgments
We thank The National Key Research and Development Project, 2016YFC0106400; Clinical Innovation Foundation of Army Medical University, yclkt‐201408, and Wu Jieping Medical Foundation, 320.6799.15037. We thank the patients and investigators who were involved in this study. We also thank Brightech company (Chengdu, China) for being the Contract Research Organization, Zong‐Ren Xu (The First Affiliated Hospital of Chongqing Medical University, Chongqing, China) for generating charts, and Blake Wassom (University of North Texas Health Science Center, Fort Worth, TX) for English language editing.
Footnotes
ClinicalTrials.gov Identifier: NCT02353741
Sponsor: Xinqiao Hospital of Chongqing
Principal Investigators: Jianguo Sun, Yuzhong Duan, Bo Zhu
IRB Approved: Yes
Contributor Information
Bo Zhu, Email: bo.zhu@tmmu.edu.cn.
Yuzhong Duan, Email: duanyuzhong@sina.com.
Jianguo Sun, Email: sunjg09@aliyun.com.
Disclosures
The authors indicated no financial relationships.
References
- 1.Wu YL, Zhou C, Liam CK et al. First‐line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer: Analyses from the phase III, randomized, open‐label, ENSURE study. Ann Oncol 2015;26:1883–1889. [DOI] [PubMed] [Google Scholar]
- 2.Zhou C, Wu YL, Chen G et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): A multicentre, open‐label, randomised, phase 3 study. Lancet Oncol 2011;12:735–742. [DOI] [PubMed] [Google Scholar]
- 3.Goto K, Nishio M, Yamamoto N et al. A prospective, phase II, open‐label study (JO22903) of first‐line erlotinib in Japanese patients with epidermal growth factor receptor (EGFR) mutation‐positive advanced non‐small‐cell lung cancer (NSCLC). Lung Cancer 2013;82:109–114. [DOI] [PubMed] [Google Scholar]
- 4.Shintani S, Li C, Mihara M et al. Enhancement of tumor radioresponse by combined treatment with gefitinib (Iressa, ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, is accompanied by inhibition of DNA damage repair and cell growth in oral cancer. Int J Cancer 2003;107:1030–1037. [DOI] [PubMed] [Google Scholar]
- 5.Tsai YC, Ho PY, Tzen KY et al. Synergistic blockade of EGFR and HER2 by new‐generation EGFR tyrosine kinase inhibitor enhances radiation effect in bladder cancer cells. Mol Cancer Ther 2015;14:810–820. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Deng L, Zhou X et al. Concurrent brain radiotherapy and EGFR‐TKI may improve intracranial metastases control in non‐small cell lung cancer and have survival benefit in patients with low DS‐GPA score. Oncotarget 2017;8:111309–111317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang T, Min W, Li Y et al. Radiotherapy plus EGFR TKIs in non‐small cell lung cancer patients with brain metastases: An update meta‐analysis. Cancer Med 2016;5:1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Q, Zhou F, Liu H et al. Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first‐line EGFR‐TKIs. J Thorac Oncol 2018;13:1383–1392. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Li Y, Xia L et al. Continued EGFR‐TKI with concurrent radiotherapy to improve time to progression (TTP) in patients with locally progressive non‐small cell lung cancer (NSCLC) after front‐line EGFR‐TKI treatment. Clin Transl Oncol 2018;20:366–373. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Xia TY, Wang YJ et al. Prospective study of epidermal growth factor receptor tyrosine kinase inhibitors concurrent with individualized radiotherapy for patients with locally advanced or metastatic non‐small‐cell lung cancer. Int J Radiat Oncol Biol Phys 2011;81:e59–e65. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Liao Z, Wei X et al. Analysis of clinical and dosimetric factors associated with treatment‐related pneumonitis (TRP) in patients with non‐small‐cell lung cancer (NSCLC) treated with concurrent chemotherapy and three‐dimensional conformal radiotherapy (3D‐CRT). Int J Radiat Oncol Biol Phys 2006;66:1399–1407. [DOI] [PubMed] [Google Scholar]
- 12.Verma V, Simone CN, Werner‐Wasik M. Acute and late toxicities of concurrent chemoradiotherapy for locally‐advanced non‐small cell lung cancer. Cancers (Basel) 2017;9:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernando ML, Marks LB, Bentel GC et al. Radiation‐induced pulmonary toxicity: A dose‐volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys 2001;51:650–659. [DOI] [PubMed] [Google Scholar]
- 14.Graham MV, Purdy JA, Emami B et al. Clinical dose‐volume histogram analysis for pneumonitis after 3D treatment for non‐small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1999;45:323–329. [DOI] [PubMed] [Google Scholar]
- 15.Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR‐TKI treatment for EGFR mutation‐positive non‐small cell lung cancer. Lung Cancer 2015;88:74–79. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida T, Yoh K, Niho S et al. RECIST progression patterns during EGFR tyrosine kinase inhibitor treatment of advanced non‐small cell lung cancer patients harboring an EGFR mutation. Lung Cancer 2015;90:477–483. [DOI] [PubMed] [Google Scholar]
- 17.Chen MZ, Zhong W, Zhang L et al. Recurrence patterns of advanced non‐small cell lung cancer treated with gefitinib. Chin Med J (Engl) 2013;126:2235–2241. [PubMed] [Google Scholar]