This article evaluates prognostic biomarkers with a focus on primary tumor location in stage II colorectal cancer.
Keywords: Oncotype Recurrence Score assay, CDX2, Tumor location, Stage II colorectal cancer, Prognostic biomarkers
Abstract
Background.
Anatomic location of primary tumors across the colon correlate with survival in the metastatic setting, whereas left‐sided tumors may exhibit superior survival compared with right‐sided tumors. The Oncotype Recurrence Score (RS) assay is a clinically validated predictor of recurrence risk in patients with stage II colorectal cancer (CRC). Previous studies had indicated that without adjuvant chemotherapy, CDX2‐negative stage II CRC tumors are associated with a lower rate of disease‐free survival than CDX2‐positive stage II CRC tumors. We aimed to evaluate whether these two validated prognostic biomarkers may correlate with primary tumor location, and whether tumor location may reflect differential prognosis in stage II CRC.
Materials and Methods.
We retrospectively analyzed patients with T3 mismatch repair‐proficient (MMR‐P) stage II CRC for whom RS assay was performed. Pathological report was reviewed for exact primary tumor location and CDX2 immunostaining. RS and CDX2 expression were correlated with primary tumor location.
Results.
The analysis included 1,147 patients with MMR‐P stage II CRC (median age 69 years [range 29–93]). Tumor distribution across the colon was as follows: 46% (n = 551) were right‐sided and 54% (n = 596) were left‐sided. RS was higher in right‐sided tumors (p = .01). The RS results gradually decreased across the colon (cecum, highest score; sigmoid, lowest score; p = .04). Right‐sided tumors exhibited more CDX2‐negative tumors (p = .07).
Conclusion.
Our study indicates that right‐sided colorectal tumors may display worse prognosis compared with left‐sided tumors in MMR‐P stage II CRC. Primary tumor location may serve as a prognostic factor that should be taken into account for recurrence risk assessment and consideration of adjuvant treatment.
Implications for Practice.
Sidedness matters, even in stage II colorectal cancer (CRC). Using two previously established prognostic tools, the Oncotype DX assay and CDX2 expression, this study found that right‐sided tumors may display worse prognosis compared with left‐sided tumors in mismatch repair‐proficient stage II CRC. Therefore, primary tumor location should be taken into account for recurrence risk assessment and consideration of adjuvant treatment.
摘要
背景。在转移性结直肠癌中,原发性肿瘤的解剖位置与存活率相关,而与右侧肿瘤相比,左侧肿瘤可能表现出更高的存活率。肿瘤基因复发评分 (RS) 测定是经过临床验证的 II 期结直肠癌 (CRC) 患者复发风险的预测因子。既往研究已表明,如果没有辅助化疗,CDX2 阴性的 II 期 CRC 肿瘤与 CDX2 阳性的 II 期 CRC 肿瘤相比具有更低的无病生存率。我们的目的是评估这两种经过验证的预后生物标记物是否与肿瘤的原发部位相关,以及肿瘤部位是否可能反映出 II 期 CRC 的预后差异。
材料和方法。我们回顾性分析了具有 T3 错配修复‐无缺失 (MMR‐P) 的 II 期 CRC 患者,对他们进行了 RS 测定。回顾了病理报告中的确切原发肿瘤部位 和 CDX2 免疫染色。RS 和 CDX2 表达与肿瘤的原发部位相关。
结果。该分析包括 1 147 名患有 MMR‐P 的 II 期 CRC 患者 [中位年龄为 69 岁(年龄范围为 29‐93)]。结肠的肿瘤分布如下:46%(n = 551) 位于右侧, 54%(n = 596)位于左侧。RS 在右侧肿瘤中更高(p = 0.01)。RS 评分在结肠分布中逐渐减少(盲肠,最高分;乙状结肠,最低分; p = 0.04)。右侧肿瘤较多表现为 CDX2 阴性肿瘤(p = 0.07)。
结论。我们的研究表明,与 MMR‐P II 期 CRC 中的左侧肿瘤相比,右侧结直肠肿瘤可能表现出更差的预后。肿瘤的原发部位可作为一个预后因素,在进行复发风险评估和考虑辅助治疗时应该予以考虑。
实践意义:即使在 II 期结直肠癌 (CRC) 中,位于左侧还是右侧也很重要。使用两个先前建立的预后工具 ‐ Oncotype DX 测定和 CDX2 表达,本研究发现,与错配修复‐ 无缺失的 II 期 CRC 中的左侧肿瘤相比,右侧肿瘤可能表现出更差的预后。因此,在进行复发风险评估和考虑辅助治疗时,应考虑肿瘤的原发部位。
Introduction
Colorectal cancer (CRC) is one of the leading cancers in the Western world, with high recurrence incidence and poor prognosis. Although the development of early diagnosis and comprehensive treatment is dramatic, the mortality rate of colorectal cancer is still very advanced in both genders [1], [2]. At presentation, 25% of colon cancer cases are diagnosed as stage II, for which surgical resection remains the mainstay of treatment. Although surgery alone can be curative in most cases of localized colon cancer, 15%–20% of patients with stage II colon cancer will eventually experience disease recurrence [3]. However, the role of adjuvant chemotherapy remains controversial [4], [5], [6], [7], [8], [9], and identifying the patients who will benefit from the treatment remains a challenge.
Recent evidence indicates that the anatomic location of primary tumors across the colon correlates with survival in the metastatic setting; left‐sided tumors may exhibit superior survival compared with those that are right‐sided. It has been shown in a retrospective post hoc analysis of two phase III studies that were designed to compare the addition of either bevacizumab or cetuximab (CALGB/SWOG 80405 and FIRE‐3) in combination with chemotherapy as first‐line therapy for RAS wild‐type metastatic colorectal cancer that patients with left‐sided tumors had markedly better overall survival and objective response rate than those with right‐sided primary tumors. Furthermore, the benefit of anti‐epidermal growth factor receptor (EGFR) agents was markedly superior in left sided tumors compared with right‐sided tumors [10], [11]. Retrospective analysis of sidedness in other phase III and II studies evaluating the role of anti‐EGFR therapy in metastatic colorectal cancer revealed the same trend of better response to anti‐EGFR agents and better prognosis for left‐sided tumors. We aimed to evaluate whether sidedness may also prognosticate in the setting of stage II colorectal cancer. We used two factors that have been previously established as prognostic tools for stage II colorectal cancer: Oncotype DX colon cancer assay (Genomic Health, Redwood City, CA) and CDX2 expression.
The Oncotype DX colon cancer assay is a 12‐gene reverse transcriptase polymerase chain reaction‐based colon cancer assay designed to predict recurrence risk in patients with stage II and III colon cancer [12]. The assay is based on three stromal genes (BGN, FAP, INHBA), three cell cycle‐related genes (Ki‐67, C‐MYC, MYBL2), one early response gene (GADD45B), and five reference genes (ATP5E, GPX1, GPK1, UBB, VDAC2). It is a continuous variable ranging from 0 to 100, with low‐ (<30), intermediate‐ (31–40), and high‐recurrence (>41) risk groups representing 8%, 11%, and 25% risk of recurrence at 3 years, respectively [13]. The validation studies of the assay used archived samples from four major prospectively designed clinical trials (the Quick and Simple and Reliable study, the Cancer and Leukemia Group B 9581 study, the National Surgical Adjuvant Breast and Bowel Project C‐07 study, and the SUNRISE) [14], involving a total of 3,018 patients. These studies demonstrated that the Recurrence Score result is an independent predictor of recurrence and is able to predict the risk of recurrence beyond traditional clinical and pathological parameters. The greatest clinical benefit has been shown in average‐risk patients—a large group of approximately 70% stage II patients with T3 mismatch repair‐proficient (MMR‐P) tumors, for whom conventional prognostic factors are not informative [13], [15], [16], [17]. An additional validation study was performed on 279 patients from the Dutch Total Mesorectal Excision (TME) trial with stage II and III rectal cancer who were randomized to TME surgery alone. This study demonstrated that the 12‐gene Recurrence Score (RS) assay is a predictor of recurrence risk and cancer‐specific survival in patients with rectal cancer as well, suggesting a similar underlying biology in colon and rectal cancers [18]. It should be noted that the validation studies failed to demonstrate prediction of benefit from chemotherapy treatment.
CDX2 is a homeobox transcription factor that is a master regulator of intestinal development and oncogenesis and had recently been identified as a biomarker of mature colon epithelial tissue. Tumors enriched in cells with an undifferentiated, stem‐like phenotype might exhibit more aggressive clinical behavior. Previous studies found that tumors lacking CDX2 expression are often associated with several adverse prognostic variables such as high levels of ALCAM expression (characteristic of human colon cancer stem cell) and a high pathological grade. It has been shown that CDX2‐negative colorectal tumors are associated with a higher risk of recurrence and seem to benefit from adjuvant chemotherapy compared with CDX2‐positive colorectal tumors. This observation was noted not only in stage III but also in stage II disease [19], [20].
We aimed to evaluate whether these two prognostic biomarkers may correlate with primary tumor location, and whether tumor location may reflect differential prognosis in stage II colorectal cancer.
Materials and Methods
Patients and Study Design
This trial was a multicenter retrospective study that included Clalit Health Services (CHS) patients with stage II/III colorectal cancer who underwent the 12‐gene Recurrence Score assay between January 2011 and August 2016. The analysis was restricted to patients with MMR‐P tumors [21]. Pathological reports of the included patients were reviewed for exact primary tumor location and were correlated to the 12‐gene Recurrence Score assay and CDX2 expression. Rectal tumors were analyzed separately. The study was conducted in accordance with Good Clinical Practice guidelines and was approved by the institutional review board of the CHS as well as the institutional review boards of the participating institutions (Davidoff Cancer Center, Hadassah‐Hebrew University Medical Center, Kaplan Medical Center, Lin Medical Center, Rambam Healthcare Campus, Soroka University Medical Center, and Tel Aviv Sourasky Medical Center).
Recurrence Score Result Determination
The Recurrence Score results are derived from reference‐normalized gene expression measurements made by quantitative real‐time reverse transcriptase polymerase chain reaction using RNA extracted from a formalin‐fixed paraffin embedded tumor block obtained by surgical resection. The gene panel used for the assay comprises 12 genes: 7 cancer‐related genes, including 3 cell‐cycle genes, 3 stromal genes, and the early response gene, GADD45B, and 5 reference genes [18]. Stromal group score and cell‐cycle group score are calculated from reference‐normalized individual gene expression measurements, and an unscaled Recurrence Score result is determined using the following calculation: RSu = (0.15 × Stromal group score) − (0.3 × Cell‐cycle group score) + (0.15 × GADD45B). The Recurrence Score result is then rescaled from 0 to 100. Patients are categorized into three risk groups according to their Recurrence Score results: low (<30), intermediate (30–40), and high (≥41).
Immunohistochemical Analysis of CDX2 Expression
CDX2 staining was performed as a part of the routine pathological examination using a CDX2 antibody (clone EPR2764Y, CellMarque, AH‐Diagnostics, 1:100). The average nuclear CDX2 expression was estimated across the whole section, and tumors were classified by either “high/normal” or “low/absent” expression. Normal epithelial cells were used as an internal control [20]. All staining was evaluated by a gastrointestinal pathologist.
Statistical Analysis
Correlation of Recurrence Score results to tumor location was done using t test analysis when compared with the two colon groups of tumor location (left/right) and using one‐way analysis of variance test when compared with specific location. Fisher's exact test was used to analyze the correlation between CDX2 expression and tumor location. p < .05 was considered statistically significant. All analyses were conducted in SPSS software (IBM, Armonk, NY).
Results
Patients
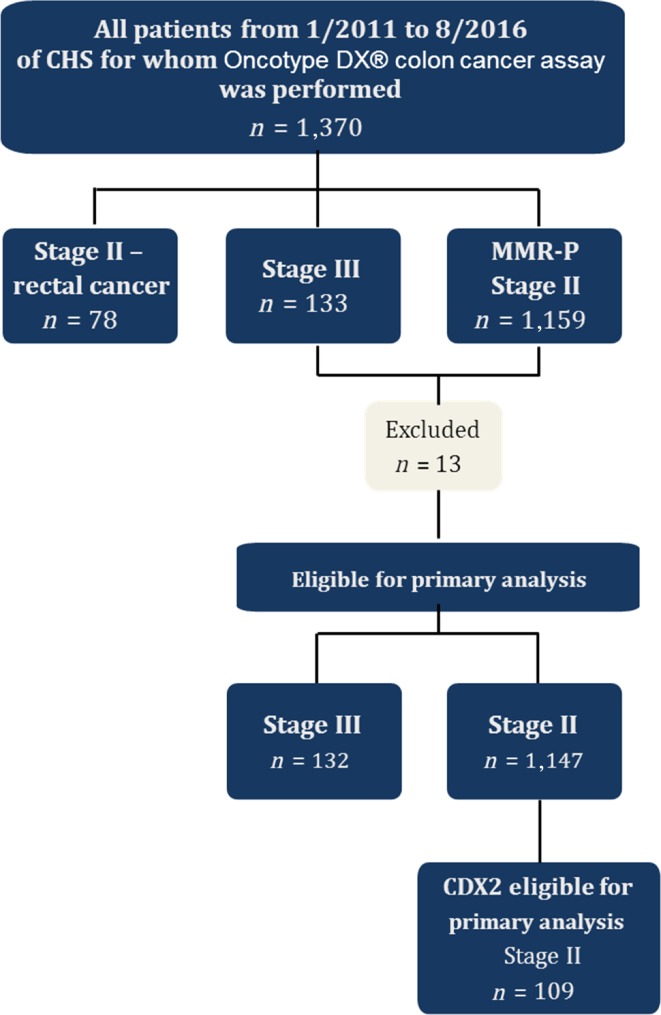
Of 1,370 patients of CHS for whom Oncotype DX colon cancer assay was performed, 1,357 were eligible for primary analysis; 13 patients were excluded because pathological review was not available. A total of 1,147 patients were diagnosed with stage II disease, and 132 were diagnosed with stage III disease; 78 patients with stage II rectal cancer were analyzed separately (Fig. 1). Median age was 69 years (range 30–90); left‐sided tumors were associated with younger patients (median age 68 vs. 72 years) and a higher incidence in males (56% vs. 44% in right‐sided tumors).
Figure 1.
Study flow chart.
Abbreviations: CHS, Clalit Health Services; MMR‐P, mismatch repair‐proficient.
Tumor distribution across the colon in the stage II cohort was as follows: 48% (n = 551) were right‐sided (cecum 17.2%, hepatic flexure 6.8%, transverse colon 15.3%, right‐sided unspecified 60.7%) and 52% (n = 596) were left‐sided (splenic flexure 8.8%, sigmoid colon 51.3%, rectosigmoid 17.5%, left‐sided unspecified 22.4%; Table 1).
Table 1. Patient characteristics.
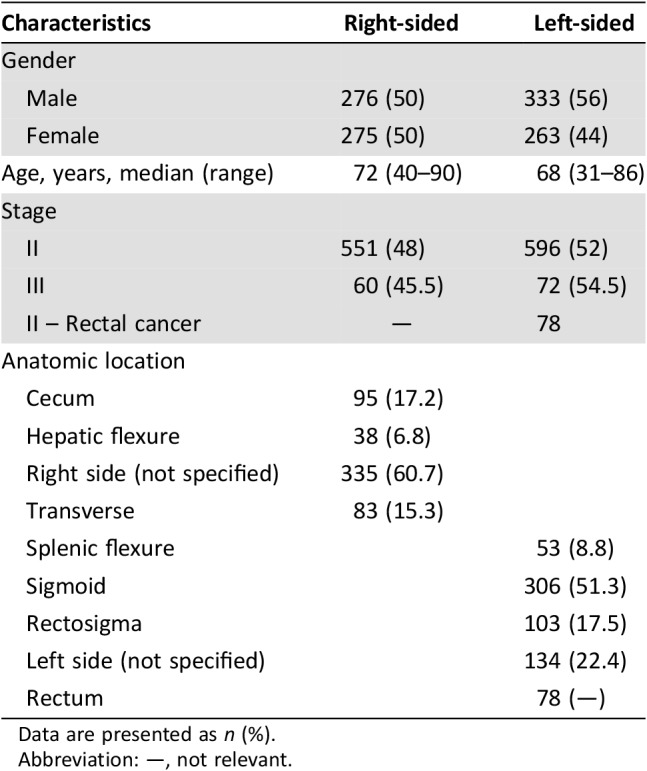
Data are presented as n (%).
Abbreviation: —, not relevant.
Recurrence Score Results According to Tumor Location
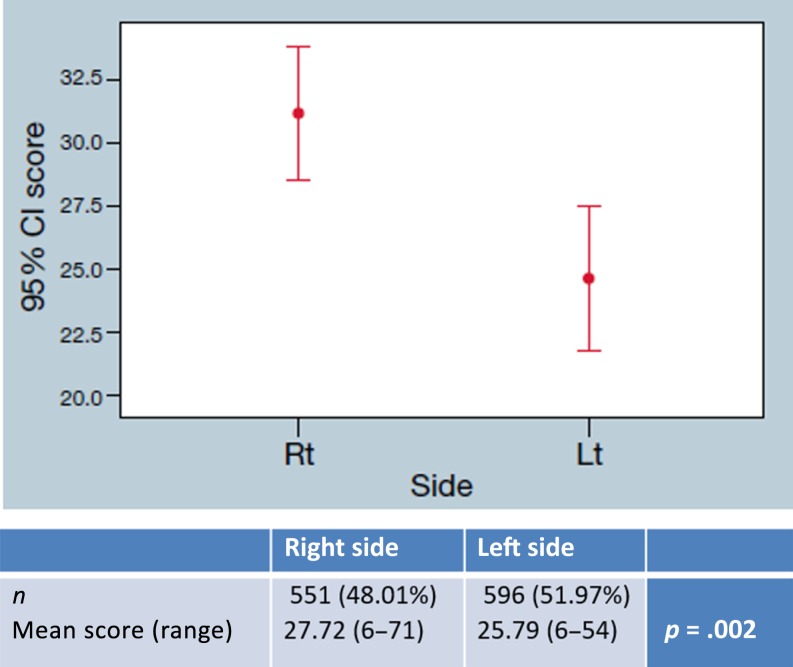
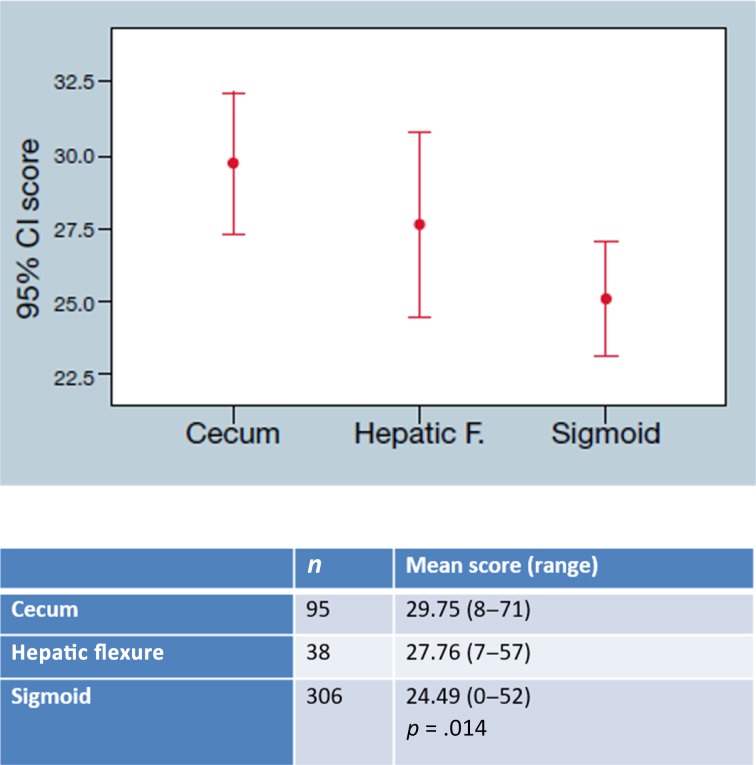
Stage II patients demonstrated a higher Recurrence Score in right‐sided tumors compared with left‐sided tumors, with a mean score of 27.72 (range 6–71) and 25.79 (range 6–54), respectively (p = .002; Fig. 2). Comparing the Recurrence Score in specific locations rather than left versus right revealed a gradual decrease across the colon, with the cecum‐located tumors receiving the highest Recurrence Score (29.75, range 8–71), hepatic flexure‐located tumors receiving a lower Recurrence Score (27.76, range 7–57), and the sigmoid‐located tumors receiving the lowest RS (24.49, range 0–52; p = .014; Fig. 3).
Figure 2.
Recurrence Score of stage II colon cancer: Mean Recurrence Score of left‐ and right‐side stage II colon cancer samples is presented with a confidence interval of 95%.
Abbreviations: CI, confidence interval; Lt, left; Rt, right.
Figure 3.
Recurrence Score of stage III colon cancer: Mean Recurrence Score of left‐ and right‐side stage III colon cancer samples is presented with a confidence interval of 95%.
Abbreviations: CI, confidence interval, Lt, left; Rt, right.
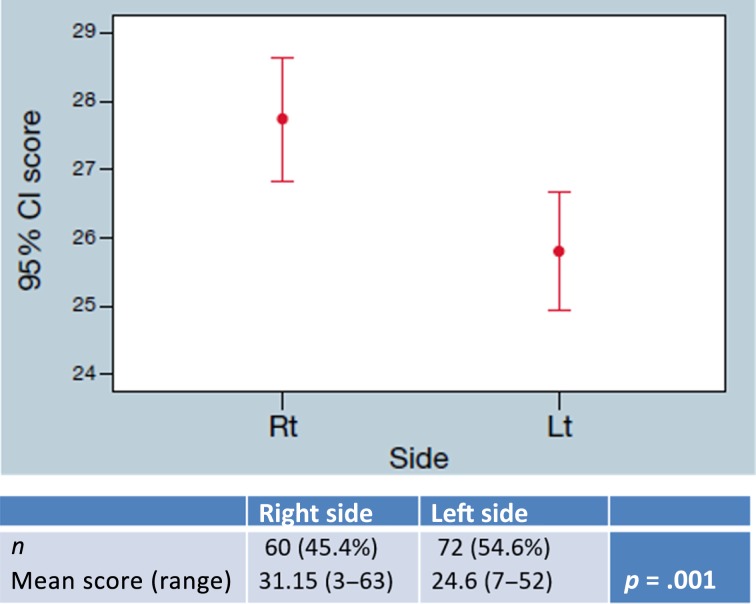
Similar results were demonstrated in stage III patients with mean Recurrence Score of 31.15 (range 3–63) in right‐sided tumors and 24.6 (range 7–52) in left‐sided tumors (p = .001; Fig. 4). Rectal tumors had a higher Oncotype DX colon cancer assay compared with left‐sided colon tumors in both stage II tumors (RS 27.06 vs. 25.79, p = .04) and stage III tumors (RS 27.15 vs. 24.6, p = .05).
Figure 4.
Gradient recurrence score of stage II colon cancer: Mean Recurrence Score across the colon (cecum, hepatic flexure, and sigmoid) in stage II colon cancer is significantly decreased (p = .014).
CDX2 Expression According to Tumor Location
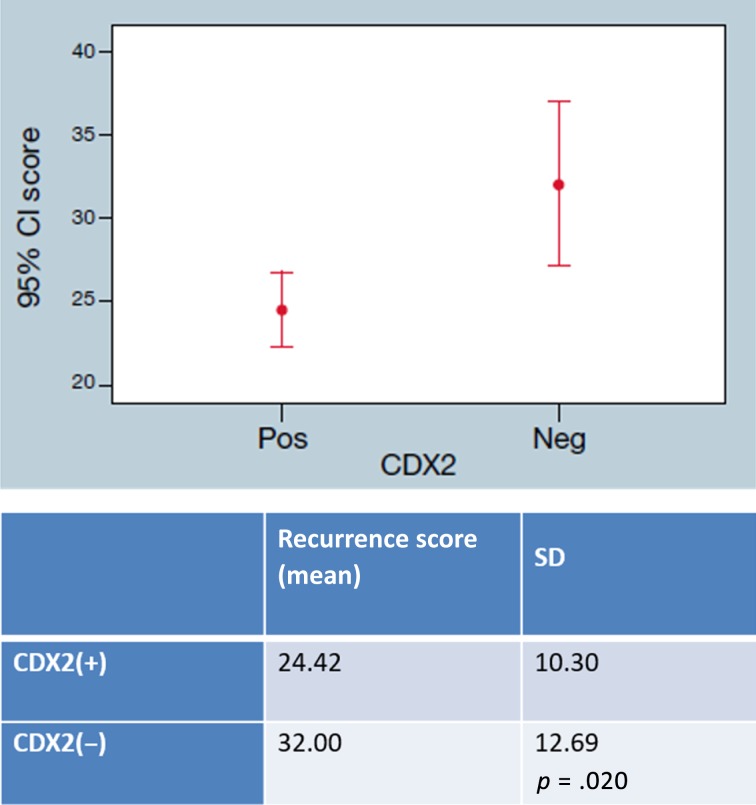
CDX2 status was available for 109 stage II patients. Right‐sided tumors exhibited more CDX2‐negative tumors compared with left‐sided tumors—35.8% (n = 19) and 16.1% (n = 9), respectively (p = .029; Table 2). CDX2‐negative tumors in general (both left‐ and right‐sided) had a higher RS: 32 versus 24.42 (p = .02; Fig. 5).
Table 2. CDX2 expression according to tumor location.
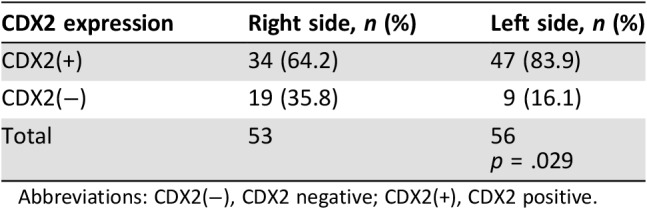
Abbreviations: CDX2(−), CDX2 negative; CDX2(+), CDX2 positive.
Figure 5.
Recurrence Score and CDX2 expression: Stage II colon cancer Recurrence Score in correlation with CDX2‐negative or ‐positive expression.
Abbreviations: CDX2(−), CDX2 negative; CDX2(+), CDX2 positive; CI, confidence interval; Neg, negative; Pos, positive.
Discussion
The clinical management of patients with stage II colon cancer remains controversial, and attempts are made to optimally define the patients who are at higher risk for recurrence and who may benefit from adjuvant chemotherapy. The aim of this large study was to evaluate whether tumor location may reflect differential prognosis in stage II colorectal cancer by examining two prognostic biomarkers: the Oncotype DX colon cancer assay and CDX2 expression. The results presented above, based upon more than 1,300 cases, indicate that right‐sided tumors displayed worse biological features manifested by significantly higher Recurrence Score compared with left‐sided tumors, as well as higher incidence of CDX2‐negative tumors. Despite the fact that the median RS for both right‐ and left‐sided tumors was below 30 and therefore considered low risk, the Recurrence Score is a continuous variable, and the difference between the groups was statistically significant and may indicate the differential biology of colon cancer across the colon. This observation is in correlation with recent data in the metastatic setting indicating worse prognosis for right‐sided tumors [11]. Nevertheless, in the metastatic setting, the data used only distinguished between the right and left colon in general, without referring to the specific segments across the colon. Most studies that were focusing on primary tumor location used a dichotomized distinction between right colon and left colon, with the differences attributed at least in part to the different embryonal origin. In our large cohort, we observed a gradient across the colon with tumors of the hepatic flexure representing lower recurrent score than the cecum and higher than the sigmoid colon. The main limitation is the relatively small number of patients in the subgroups analyzed, but nevertheless, these results together with the results of Salem et al. [22] raise the question of whether there is a broader clinically relevant spectrum of tumor location than right versus left.
With regard to CDX2 expression, it should be noted that the CDX2‐negative tumors represent a larger fraction in this cohort compared with the original Dalerba et al. cohort [19]. Our results are in concordance with recent studies that exhibited higher expression rates of CDX2 absence/loss in stage II tumors [23], [24]. Nevertheless, our results confirm the role of CDX2 expression as a prognostic factor, as has been previously demonstrated, and also display its correlation to tumor location. Although the biological correlation between the Oncotype DX colon cancer assay and CDX2 expression has not yet been established, we found that CDX2‐negative tumors are associated with a higher Recurrence Score.
The association between CDX2‐negative tumors and higher Recurrence Score might be attributed to the gene profile included in the assay. One example is the INHBA gene that encodes activin A, a ligand in the transforming growth factor β superfamily, which plays an important role in cell differentiation. Activin A expression has been implicated to be significantly increased in various types of cancer and correlates with cancer progression and metastasis [25], [26]. As described above, CDX2‐negative tumors are associated with high levels of ALCAM expression, which is characteristic of human colon cancer stem cell. Therefore, a possible explanation for the association is that both prognostic tools identify different molecular signatures of the undifferentiated tumors associated with worse outcome.
Two subgroups of patients included in this study require separate discussion. The first is a group of patients with stage III CRC (n = 132), for whom the Oncotype DX colon cancer assay was conducted within the framework of the clinical trial. As expected, stage III CRC tumors displayed a higher RS as compared with stage II tumors. Notably, in this group, there was also a significantly higher RS in right‐sided tumors compared with left‐sided tumors. This observation alongside the mounting evidence regarding stage IV disease supports the hypothesis that tumor location has an impact on prognosis of CRC regardless of disease stage.
The second group comprised patients with rectal cancer. In this relatively small group of patients, we found a higher Recurrence Score in both stage II and stage III patients compared with same stage left‐colon tumors. Because rectal tumors were not included in the large validation studies of the Oncotype DX colon cancer assay and were validated in a smaller study, these results represent a potential difference in prognosis, and further studies are required. Furthermore, recent studies demonstrate a different genomic profile of rectal tumors compared with those of the left colon, indicating that rectal cancer may represent a distinct entity that is not in continuum with colonic tumors [22], [27].
The main limitation of the study is the lack of long‐term survival data to indicate whether tumor sidedness is indeed a prognostic factor, as reflected by the adverse biological features observed in our study. Because the median follow‐up time is relatively short for a substantial fraction of the patients that were diagnosed in the last 2–3 years, survival analysis could not be performed for the entire cohort, and the data are being collected prospectively for future mature survival and recurrence analysis. Moreover, we could not evaluate the role of other potentially prognostic factors such as RAS and BRAF and their correlation to Oncotype RS or CDX2.
Conclusion
Right sidedness may reflect worse biological features in early colon cancer according to two validated prognostic tools. Future survival analysis may reveal whether primary tumor location may indeed serve as a prognostic factor that may be taken into account for recurrence risk assessment and consideration of adjuvant treatment. However, tumor location has not yet proved to be predictive.
Author Contributions
Conception/design: Irit Ben‐Aharon, Tal Goshen‐Lago, Baruch Brenner
Collection and/or assembly of data: Irit Ben‐Aharon, Tal Goshen‐Lago, Michal Sternschuss, Sara Morgenstern, Ravit Geva, Alexander Beny, Ygael Dror, Mariana Steiner, Ayala Hubert, Efraim Idelevich, Katerina Shulman, Moshe Mishaeli, Sophia Man, Nicky Liebermann, Lior Soussan‐Gutman, Baruch Brenner
Data analysis and interpretation: Irit Ben‐Aharon, Tal Goshen‐Lago, Michal Sternschuss, Sara Morgenstern, Ravit Geva, Alexander Beny, Ygael Dror, Mariana Steiner, Ayala Hubert, Efraim Idelevich, Katerina Shulman, Moshe Mishaeli, Sophia Man, Nicky Liebermann, Lior Soussan‐Gutman, Baruch Brenner
Manuscript writing: Irit Ben‐Aharon, Michal Sternschuss
Final approval of manuscript: Irit Ben‐Aharon, Tal Goshen‐Lago, Michal Sternschuss, Sara Morgenstern, Ravit Geva, Alexander Beny, Ygael Dror, Mariana Steiner, Ayala Hubert, Efraim Idelevich, Katerina Shulman, Moshe Mishaeli, Sophia Man, Nicky Liebermann, Lior Soussan‐Gutman, Baruch Brenner
Disclosures
Ravit Geva: Bristol‐Myers Squibb, Eli Lilly and Company, Medison, Roche, Novartis, Takeda, Merck Sharp & Dohme, Merck, Janssen (H), Bayer, Merck Sharp & Dohme, Novartis (SAB); Lior Soussan‐Gutman: Teva Pharmaceutical (E), Genomic Health (representative). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–386. [DOI] [PubMed] [Google Scholar]
- 3.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor ES, Greenblatt DY, LoConte NK et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol 2011;29:3381–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tournigand C, Andre T, Bonnetain F et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: Subgroup analyses of the multicenter international study of oxaliplatin, fluorouracil, and leucovorin in the adjuvant treatment of colon cancer trial. J Clin Oncol 2012;30:3353–3360. [DOI] [PubMed] [Google Scholar]
- 6.Yothers G, O'Connell MJ, Allegra CJ et al. Oxaliplatin as adjuvant therapy for colon cancer: Updated results of NSABP C‐07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quasar Collaborative Group , Gray R, Barnwell J et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet 2007;370:2020–2029. [DOI] [PubMed] [Google Scholar]
- 8.Gill S, Loprinzi CL, Sargent DJ et al. Pooled analysis of fluorouracil‐based adjuvant therapy for stage II and III colon cancer: Who benefits and by how much? J Clin Oncol 2004;22:1797–1806. [DOI] [PubMed] [Google Scholar]
- 9.Sargent D, Sobrero A, Grothey A et al. Evidence for cure by adjuvant therapy in colon cancer: Observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2009;27:872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venook AP, Niedzwiecki D, Innocenti F et al. Impact of primary (1°) tumor location on overall survival (OS) and progression‐free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 2016;34:3504–3504. [Google Scholar]
- 11.Tejpar S, Stintzing S, Ciardiello F et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild‐type metastatic colorectal cancer: Retrospective analyses of the CRYSTAL and FIRE‐3 trials. JAMA Oncol 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark‐Langone KM, Wu JY, Sangli C et al. Biomarker discovery for colon cancer using a 761 gene RT‐PCR assay. BMC Genomics 2007;8:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connell MJ, Lavery I, Yothers G et al. Relationship between tumor gene expression and recurrence in four independent studies of patients with stage II/III colon cancer treated with surgery alone or surgery plus adjuvant fluorouracil plus leucovorin. J Clin Oncol 2010;28:3937–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamanaka T, Oki E, Yamazaki K et al. 12‐gene recurrence score assay stratifies the recurrence risk in stage II/III colon cancer with surgery alone: The SUNRISE study. J Clin Oncol 2016;34:2906–2913. [DOI] [PubMed] [Google Scholar]
- 15.Gray RG, Quirke P, Handley K et al. Validation study of a quantitative multigene reverse transcriptase‐polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol 2011;29:4611–4619. [DOI] [PubMed] [Google Scholar]
- 16.Venook AP, Niedzwiecki D, Lopatin M et al. Biologic determinants of tumor recurrence in stage II colon cancer: Validation study of the 12‐gene recurrence score in cancer and leukemia group B (CALGB) 9581. J Clin Oncol 2013;31:1775–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yothers G, O'Connell MJ, Lee M et al. Validation of the 12‐gene colon cancer recurrence score in NSABP C‐07 as a predictor of recurrence in patients with stage II and III colon cancer treated with fluorouracil and leucovorin (FU/LV) and FU/LV plus oxaliplatin. J Clin Oncol 2013;31:4512–4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reimers MS, Kuppen PJ, Lee M et al. Validation of the 12‐gene colon cancer recurrence score as a predictor of recurrence risk in stage II and III rectal cancer patients. J Natl Cancer Inst 2014;106. [DOI] [PubMed] [Google Scholar]
- 19.Dalerba P, Sahoo D, Clarke MF. CDX2 as a prognostic biomarker in colon cancer. N Engl J Med 2016;374:2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen J, Eiholm S, Kirkeby LT et al. CDX2 downregulation is associated with poor differentiation and MMR deficiency in colon cancer. Exp Mol Pathol 2016;100:59–66. [DOI] [PubMed] [Google Scholar]
- 21.Sargent DJ, Marsoni S, Monges G et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil‐based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salem ME, Weinberg BA, Xiu J et al. Comparative molecular analyses of left‐sided colon, right‐sided colon, and rectal cancers. Oncotarget 2017;8:86356–86368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilati C, Taieb J, Balogoun R et al. CDX2 prognostic value in stage II/III resected colon cancer is related to CMS classification. Ann Oncol 2017;28:1032–1035. [DOI] [PubMed] [Google Scholar]
- 24.Graule J, Uth K, Fischer E et al. CDX2 in colorectal cancer is an independent prognostic factor and regulated by promoter methylation and histone deacetylation in tumors of the serrated pathway. Clin Epigenetics 2018;10:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seder CW, Hartojo W, Lin L et al. Upregulated inhba expression may promote cell proliferation and is associated with poor survival in lung adenocarcinoma. Neoplasia 2009;11:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Zhang N, Song R et al. Activin A expression in esophageal carcinoma and its association with tumor aggressiveness and differentiation. Oncol Lett 2015;10:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamas K, Walenkamp AM, de Vries EG et al. Rectal and colon cancer: Not just a different anatomic site. Cancer Treat Rev 2015;41:671–679. [DOI] [PubMed] [Google Scholar]