This article reports on the efficacy of eribulin for the treatment of advanced invasive lobular carcinoma compared with invasive ductal carcinoma.
Keywords: Chemotherapy, Eribulin, Invasive lobular carcinoma, Invasive ductal carcinoma, Pooled analysis
Abstract
Background.
Data on the efficacy of chemotherapy regimens in patients with advanced invasive lobular carcinoma (ILC) of the breast are limited. We investigated the efficacy of single‐agent eribulin for the treatment of advanced ILC when compared with invasive ductal carcinoma (IDC).
Patients and Methods.
Results from the eribulin arms of two phase III studies (305 [EMBRACE] and 301) and a single‐arm, phase II study were pooled. The studies involved patients with metastatic breast cancer who had previously received treatment with an anthracycline and a taxane. In all three studies, the dose of eribulin mesylate was 1.4 mg/m2 given on days 1 and 8 of a 21‐day cycle. Overall survival (OS), progression‐free survival (PFS), and response rates in patients with ILC were assessed and compared with data from patients with IDC.
Results.
In total, 1,152 patients were included in this analysis (118 patients with ILC and 1,034 patients with IDC). Median OS was similar in patients with ILC and IDC (13.4 vs. 13.5 months; hazard ratio [HR], 1.10; 95% confidence interval [CI], 0.87–1.38); as was median PFS (4.1 vs. 3.6 months; HR, 0.91; 95% CI, 0.72–1.14). There were no major differences in response rates between the two groups.
Conclusion.
This retrospective analysis suggests that eribulin demonstrates similar efficacy in patients with ILC and IDC with metastatic disease who have previously received an anthracycline and a taxane.
Implications for Practice.
Data on the efficacy of chemotherapy regimens in patients with advanced invasive lobular carcinoma (ILC) of the breast are limited. This pooled retrospective analysis of three clinical studies demonstrates that the magnitude of benefit of eribulin in the metastatic setting did not differ between patients with ILC versus invasive ductal carcinoma (IDC), even when restricting for patients with estrogen receptor‐positive/HER2‐negative IDC.
摘要
背景。关于化疗方案对晚期乳腺浸润性小叶癌 (ILC) 患者的有效性的数据十分有限。我们研究了单药艾日布林治疗晚期 ILC 对比浸润性导管癌 (IDC) 的有效性。
患者和方法。我们对来自两项 III 期研究 [305 (EMBRACE)和 301] 和一项单组 II 期研究的艾日布林组结果进行了汇总。这些研究涉及既往曾接受蒽环霉素和紫杉烷治疗的转移性乳腺癌患者。在所有三项研究中,在一个为期 21 天的周期中,甲磺酸艾日布林在第 1 天和第 8 天的剂量为 1.4 mg/m2。我们对 ILC 患者的总生存期 (OS)、无进展生存期 (PFS) 以及反应率进行评估,并将它们与 IDC 患者的数据进行对比。
结果。本分析中共包含 1 152 名患者(118 名 ILC 患者和 1 034 名 IDC 患者)。ILC 患者与 IDC 患者中的中位 OS 相似 [13.4 个月vs. 13.5 个月;风险比 (HR),1.10;95% 置信区间 (CI),0.87–1.38];中位 PFS 也是如此(4.1 个月vs. 3.6 个月;HR,0.91;95% CI,0.72–1.14)。两组之间的反应率没有重大差异。
结论。本回顾性分析表明,艾日布林在既往曾接受蒽环霉素和紫杉烷治疗且患有转移性疾病的 ILC 患者和 IDC 患者中的有效性相似。
实践意义:关于化疗方案对晚期乳腺浸润性小叶癌 (ILC) 患者的有效性的数据十分有限。本次关于三项临床研究的汇总式回顾性分析证明,即使限制在雌激素受体‐阳性/HER2‐阴性 IDC 患者时,艾日布林在转移性疾病中的有益程度在 ILC 患者与浸润性导管癌 (IDC) 患者之间也没有任何差别。
Introduction
Invasive lobular carcinoma (ILC) is the second most common histologic subtype of breast cancer, accounting for approximately 10%–15% of invasive breast cancers [1]. ILC has unique clinicopathologic features compared with invasive ductal carcinoma (IDC), the most common histologic subtype [1], [2]. ILC has been associated with older age [3], larger tumor size at presentation, and a unique pattern of disease dissemination [2]. The biological hallmark of ILC is the loss of the cell adhesion molecule E‐cadherin, which likely explains the elevated incidence of metastasis to unusual sites, such as the gastrointestinal tract, pelvic organs, and retroperitoneum [4]. ILC is typically characterized as having low to intermediate histologic grade and is often estrogen receptor (ER)‐positive, progesterone receptor (PgR)‐positive, and human epidermal growth factor receptor 2 (HER2)‐negative [5].
Data from retrospective studies point to differences in response to systemic therapy for patients with ILC versus IDC [2]. Retrospective analyses conducted using data from large phase III studies suggest a partial resistance to tamoxifen for patients with ILC. However, outcomes are similar when comparing patients with ILC and IDC treated with aromatase inhibitors [6], [7]. Several studies have shown that ILC is associated with a poorer response to neoadjuvant chemotherapy compared with IDC [8], [9]. The results of a meta‐analysis that included 17 studies showed that the pathologic complete response (pCR) rate to neoadjuvant treatment in patients with ILC was 5.9% compared with 16.7% in those with IDC [10]. Although definitive data are not available to explain the reduced pCR rates in ILC versus IDC, an enrichment of low‐proliferative genomic subsets in ILC tumors may account for this difference. In contrast, similar findings, in terms of efficacy, have been reported after trastuzumab therapy in patients with HER2‐positive early breast cancer with ILC or IDC [11]. However, HER2‐positive ILC accounts for a very small fraction of ILCs (approximately 3%–5%) [12].
Compared with the early‐stage setting, there are limited data on the efficacy of chemotherapy and survival outcomes for patients with metastatic ILC. It is unclear if inherent biological features of early‐stage ILC informs chemotherapy benefit in an advanced setting.
In this retrospective analysis, we leveraged data from three eribulin mesylate studies, comprising two phase III trials and one phase II trial, to describe the relative efficacy of chemotherapy for patients with ILC when compared with IDC. The analysis was performed using data from studies evaluating the role of eribulin for patients previously treated with an anthracycline and a taxane. Safety data were not evaluated in this analysis. The availability of patient‐level data from clinical trials allowed us to precisely measure overall response data from a single‐agent chemotherapy treatment and report on survival outcomes for patients with metastatic ILC.
Materials and Methods
Study Design
Data from patients who received eribulin in two phase III studies and one single‐arm phase II study were pooled. Detailed methods for these studies have been published previously [13], [14], [15] and are briefly summarized here. Study 305 (EMBRACE; NCT00388726) was a phase III, multicenter, open‐label, randomized study of intravenous eribulin mesylate 1.4 mg/m2 (equivalent to 1.23 mg/m2 eribulin expressed as free base) on days 1 and 8 of a 21‐day cycle versus treatment of physician's choice (2:1 ratio) in women with metastatic breast cancer (MBC). The key inclusion criteria included two to five lines of previous chemotherapy (treatment with an anthracycline and a taxane was required), in which at least two of these regimens were for locally recurrent breast cancer or MBC. Study 301 (NCT00337103) was a phase III, multicenter, open‐label, randomized study of intravenous eribulin mesylate 1.4 mg/m2 on days 1 and 8 versus capecitabine 1,250 mg/m2 orally twice daily on days 1 and 14, both in 21‐day cycles (1:1 ratio) in women with MBC. The key inclusion criteria included three or fewer prior chemotherapy regimens (treatment with an anthracycline and a taxane was required), with two or fewer regimens for advanced breast cancer or MBC. Study 211 (NCT00246090) was a phase II, multicenter, open‐label, single‐arm study of intravenous eribulin mesylate 1.4 mg/m2 on days 1 and 8 of a 21‐day cycle in women with MBC. The key inclusion criteria included two to five prior chemotherapy regimens, including an anthracycline, a taxane, and capecitabine, with at least one regimen for advanced breast cancer or MBC. All three clinical studies in this analysis required informed patient consent and were approved by ethical review boards.
Study Objectives and Assessments
This retrospective analysis assessed whether the efficacy of eribulin differed in patients with ILC or IDC tumors. Patients with either ILC or IDC were identified from the baseline disease characteristics from each study. Diagnosis of histological subtype (i.e., lobular vs. ductal carcinoma) was prospectively collected in the study electronic case report forms and defined locally at each participating center. The end points of this analysis were overall survival (OS), progression‐free survival (PFS), and investigator‐evaluated tumor response rates.
OS was measured from date of randomization until date of death from any cause, or last date known alive or data cutoff (censored date). PFS was measured from date of randomization to date of recorded disease progression, death from any cause, or censored date. Tumor response was determined according to RECIST version 1.0 [16]. Response was classified as complete response (CR), partial response (PR), progressive disease, or stable disease (SD). Tumor response was confirmed by a second examination performed at least 4 weeks after the criteria for response were met. Overall response rate was defined as CR + PR; clinical benefit rate (CBR) was defined as CR + PR + SD ≥6 months; and disease control rate (DCR) was defined as CR + PR + SD.
Statistical Analysis
OS and PFS Kaplan‐Meier curves were presented by ILC and IDC and adjusted by study. Hazard ratios (HRs) of ILC over IDC were calculated using a Cox regression model stratified by ER and HER2 status, number of prior lines of therapy for advanced disease, and study. A stratified log‐rank test with the same stratification factors was used to calculate p values. For the subgroup analysis of patients with ER‐positive/HER2‐negative breast cancer, the Cox regression model was stratified by study, and p values were calculated using a log‐rank test stratified by study.
Results
Patients
This analysis included 1,152 of the 1,353 patients treated with eribulin in the three clinical trials; histology was not recorded in 201 of 1,353 patients (Fig. 1, Table 1). As expected, there were substantially fewer patients with ILC (n = 118) compared with IDC (n = 1,034) in this analysis. More patients with ILC had ER‐positive disease (68.6%) and/or PgR‐positive disease (55.1%) than those with IDC (59.9% and 47.7%, respectively). In addition, fewer patients with ILC had received at least three lines of chemotherapy prior to receiving eribulin than those with IDC (52.5% vs. 61.4%, respectively).
Figure 1.
Patient flow.
Abbreviations: IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; ITT, intent to treat.
Table 1. Patient demographics and baseline characteristics.

Unless otherwise noted.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; PgR, progesterone receptor.
Efficacy
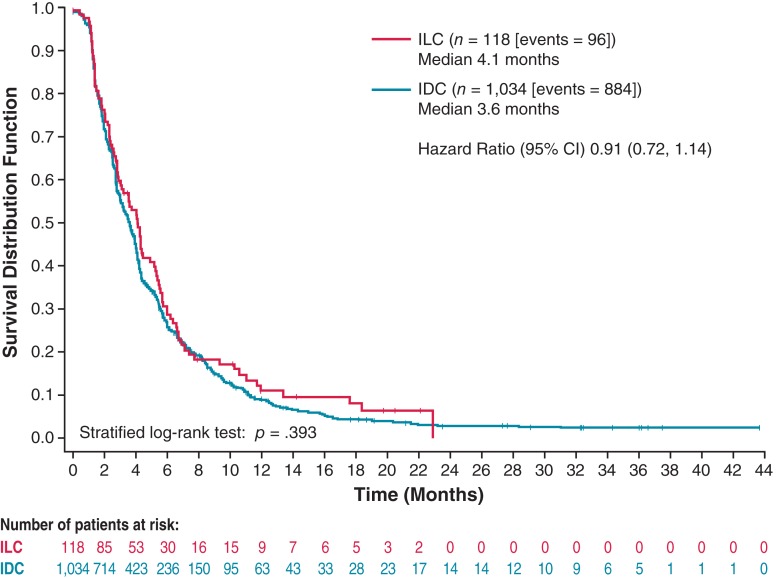
The median OS for eribulin‐treated patients with ILC and IDC was 13.4 months and 13.5 months, respectively (HR, 1.10; 95% confidence interval [CI], 0.87–1.38; nominal p = .431; Fig. 2). The median PFS for patients with ILC was 4.1 months compared with 3.6 months for patients with IDC (HR, 0.91; 95% CI, 0.72–1.14; nominal p = .393; Fig. 3). Median OS with eribulin was similar between patients with ILC and patients with IDC with ER‐positive, HER2‐negative disease (13.2 months vs. 15.0 months, respectively; HR, 1.24; 95% CI, 0.98–1.56; nominal p = .072; Fig. 4). Similarly, median PFS with eribulin was similar between patients with ILC and patients with IDC with ER‐positive, HER2‐negative disease (4.1 months vs. 4.0 months, respectively; HR, 0.94; 95% CI, 0.75–1.18; nominal p = .611; Fig. 5).
Figure 2.
Kaplan‐Meier estimate of overall survival for eribulin‐treated patients with invasive lobular carcinoma and invasive ductal carcinoma.
Abbreviations: CI, confidence interval; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
Figure 3.
Kaplan‐Meier estimate of progression‐free survival for eribulin‐treated patients with invasive lobular carcinoma and invasive ductal carcinoma.
Abbreviations: CI, confidence interval; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
Figure 4.
Kaplan‐Meier estimate of overall survival for eribulin‐treated patients with invasive lobular carcinoma and invasive ductal carcinoma (ILC vs. IDC estrogen receptor‐positive/human epidermal growth factor receptor 2‐negative).
Abbreviations: CI, confidence interval; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
Figure 5.
Kaplan‐Meier estimate of progression‐free survival for eribulin‐treated patients with invasive lobular carcinoma and invasive ductal carcinoma (ILC vs. IDC estrogen receptor‐positive/human epidermal growth factor receptor 2‐negative).
Abbreviations: CI, confidence interval; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
No patients in the ILC group achieved CR, and 17 patients achieved PR; in the IDC group, 4 patients achieved CR and 142 patients achieved PR (Table 2). The objective response rate was similar in patients with ILC (15.5%) compared with those with IDC (14.8%). The DCR (69.1% vs. 67.5% in the ILC and IDC patient groups, respectively) and CBR (29.1% vs. 28.6% in the ILC and IDC patient groups, respectively) were also similar (Table 2).
Table 2. Best overall tumor responses by investigator review in eribulin‐treated patients with invasive lobular carcinoma and invasive ductal carcinoma.

ORR = PR + CR.
DCR = PR + CR + SD.
CBR = PR + CR + SD ≥6 months.
Abbreviations: CBR, clinical benefit rate; CR, complete response; CI, confidence interval; DCR, disease control rate; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Discussion
In this retrospective analysis from three prospective clinical trials, we demonstrated that the magnitude of benefit of eribulin in the metastatic setting did not differ between patients with ILC and IDC. Our analysis evaluated patients who had received previous chemotherapy regimens, including an anthracycline and a taxane. In this analysis, OS, PFS, and response rates were found to be similar between the two breast cancer subtypes. Of relevance, similar results were observed when restricting for hormone receptor‐positive IDC.
Previous studies have consistently shown that patients with ILC do not respond as well to neoadjuvant chemotherapy as patients with IDC [8], [9], [10]. This may have led to a general assumption that patients with ILC will respond relatively poorly to systemic chemotherapy as their breast cancer advances. However, data to support or counter this assumption are lacking. In contrast, our results suggest that patients with MBC respond equally well to eribulin treatment regardless of ILC or IDC breast cancer subtype.
We acknowledge several limitations to the current post hoc, retrospective analysis. The number of ILC cases included is small and limits our ability to derive definitive conclusions. The selection of a patient population exposed to previous lines of therapies in the metastatic setting may have affected our ability to identify differences in the patterns of response between ILC and IDC tumors. It is unclear if the magnitude of benefit of systemic chemotherapy would be similar in earlier lines of treatment for metastatic disease. It is also uncertain if the pattern of response of treatment would be similar if patients were exposed to a different chemotherapy agent. It would have been informative to report on potential differences between eribulin and capecitabine using data from Study 301; however, this analysis could not be performed because of the small number of patients with ILC. One may also hypothesize that a longer duration of hormonal treatment for patients with advanced ILC when compared with those with IDC could affect OS measured from the time of initiation of chemotherapy. In the current study, we were not able to perform an analysis controlling for the duration of prior hormonal therapy.
Differences in sites of metastatic disease and number of metastatic sites in ILC and IDC may have influenced the results of this analysis. Given that ILC is the “prototype” of hormone receptor‐positive breast cancer, we expected a higher incidence of bone metastases in the ILC subset [17]. Although it is important to report on sites of metastasis for the studied population, this information was not available for the current analysis. The lack of a central review of histological subtype, HER2 status, and hormone receptor status represents another limitation. Lastly, as safety data were not included in this analysis, it is not possible to assess benefit and risk in this group of patients. Despite these limitations, to the best of our knowledge, this is the first attempt to report on the relative efficacy of eribulin for patients with ILC using patient‐level data from clinical trials.
A previous retrospective analysis comparing outcomes for patients with metastatic hormone receptor‐positive breast cancer included 437 patients with IDC and 131 patients with ILC [18]. Interestingly, in that study, patients with ILC had more bone metastases at diagnosis (46.5% vs. 34.8% for patients with ILC vs. IDC, respectively) and a smaller percentage, although not statistically significant, of patients with multiple metastatic sites (23.7% vs. 30.9% for patients with ILC vs. IDC, respectively). Time to initiation of chemotherapy since diagnosis of metastatic disease was longer for ILC than IDC. In agreement with the data presented in the current analysis, patients with ILC and IDC had similar OS (ILC vs. IDC; 29 vs. 25 months; p = .53). OS estimates were similar for patients with ILC and IDC despite a longer prior exposure to endocrine therapy in the ILC setting.
In our experience, clinicians usually rely on data from early‐stage disease to guide treatment decisions in the metastatic setting, and it remains unclear if clinicopathologic features defined at baseline (e.g., histological subtype, proliferative activity) are also informative in the advanced setting. This is particularly true for the subset of hormone receptor‐positive breast cancer previously exposed to multiple lines of therapy. Retrospective studies have indicated the emergence of somatic genetic mutations in ER‐positive tumors previously exposed to endocrine therapies [19] with potential implications for treatment decisions. This observation illustrates the need to routinely include biopsies from metastatic tumor samples in prospective clinical trials.
Conclusion
This retrospective analysis suggests that single‐agent eribulin demonstrates similar efficacy in patients with ILC or IDC, with locally advanced or metastatic disease who have previously received an anthracycline and a taxane. Despite limitations, the current results provide three important messages: (a) biological tumor features derived from early‐stage tumor specimens are not always informative in the advanced setting, and prospective collection of metastatic tumor specimens remains an important research priority; (b) data collection in prospective clinical trials should include additional information on prior treatments given to participants prior to study enrollment and additional information on treatment beyond progression; and (c) although the current evidence does not point to survival differences for patients with ILC when compared with IDC, the unique biologic features of ILC and differences in patterns of disease progression highlight the need for additional studies with a focus on ILC.
Acknowledgments
The authors thank Yi He (Eisai Inc., Woodcliff Lake, NJ) for his contributions to the statistical analyses. Editorial support was provided by Oxford PharmaGenesis, Newtown, PA. This work was supported by Eisai Inc. Funding for all editorial support was provided by Eisai Inc. The studies contributing to this analysis were funded by Eisai Inc. No grant number is applicable.
Author Contributions
Conception/design: José Pérez‐Garcia, Javier Cortés, Otto Metzger Filho
Provision of study material or patients: José Pérez‐Garcia, Javier Cortés
Collection and/or assembly of data: José Pérez‐Garcia, Javier Cortés, Otto Metzger Filho
Data analysis and interpretation: José Pérez‐Garcia, Javier Cortés, Otto Metzger Filho
Manuscript writing: José Pérez‐Garcia, Javier Cortés, Otto Metzger Filho
Final approval of manuscript: José Pérez‐Garcia, Javier Cortés, Otto Metzger Filho
Disclosures
José Pérez‐Garcia: Roche, Eli Lilly (SAB); Javier Cortés: Roche, Novartis, Eisai, Celgene, Pfizer (H), Roche, Celgene, AstraZeneca, Cellestia Biotech, Biothera, Merus (C/A); Otto Metzger Filho: AbbVie, Eisai, Genentech, Pfizer, Roche (RF). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Li CI, Anderson BO, Daling JR et al. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA 2003;289:1421–1424. [DOI] [PubMed] [Google Scholar]
- 2.Barroso‐Sousa R, Metzger‐Filho O. Differences between invasive lobular and invasive ductal carcinoma of the breast: Results and therapeutic implications. Ther Adv Med Oncol 2016;8:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iorfida M, Maiorano E, Orvieto E et al. Invasive lobular breast cancer: Subtypes and outcome. Breast Cancer Res Treat 2012;133:713–723. [DOI] [PubMed] [Google Scholar]
- 4.Ciriello G, Gatza ML, Beck AH et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 2015;163:506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rakha EA, Ellis IO. Lobular breast carcinoma and its variants. Semin Diagn Pathol 2010;27:49–61. [DOI] [PubMed] [Google Scholar]
- 6.Metzger Filho O, Giobbie‐Hurder A, Mallon E et al. Relative effectiveness of letrozole compared with tamoxifen for patients with lobular carcinoma in the BIG 1‐98 trial. J Clin Oncol 2015;33:2772–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knauer M, Gruber C, Dietze O et al. Survival advantage of anastrozol compared to tamoxifen for lobular breast cancer in the ABCSG‐8 study. Cancer Res 2015;75(suppl 9):S2‐06A. [Google Scholar]
- 8.Wenzel C, Bartsch R, Hussian D et al. Invasive ductal carcinoma and invasive lobular carcinoma of breast differ in response following neoadjuvant therapy with epidoxorubicin and docetaxel + G‐CSF. Breast Cancer Res Treat 2007;104:109–114. [DOI] [PubMed] [Google Scholar]
- 9.Loibl S, Volz C, Mau C et al. Response and prognosis after neoadjuvant chemotherapy in 1,051 patients with infiltrating lobular breast carcinoma. Breast Cancer Res Treat 2014;144:153–162. [DOI] [PubMed] [Google Scholar]
- 10.Petrelli F, Barni S. Response to neoadjuvant chemotherapy in ductal compared to lobular carcinoma of the breast: A meta‐analysis of published trials including 1,764 lobular breast cancer. Breast Cancer Res Treat 2013;142:227–235. [DOI] [PubMed] [Google Scholar]
- 11.Metzger‐Filho O, Procter M, de Azambuja E et al. Magnitude of trastuzumab benefit in patients with HER2‐positive, invasive lobular breast carcinoma: Results from the HERA trial. J Clin Oncol 2013;31:1954–1960. [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Dabbs DJ, Shuai Y et al. Classical‐type invasive lobular carcinoma with HER2 overexpression: Clinical, histologic, and hormone receptor characteristics. Am J Clin Pathol 2011;136:88–97. [DOI] [PubMed] [Google Scholar]
- 13.Cortes J, O’Shaughnessy J, Loesch D et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open‐label randomised study. Lancet 2011;377:914–923. [DOI] [PubMed] [Google Scholar]
- 14.Cortes J, Vahdat L, Blum JL et al. Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J Clin Oncol 2010;28:3922–3928. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman PA, Awada A, Twelves C et al. Phase III open‐label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 2015;33:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–216. [DOI] [PubMed] [Google Scholar]
- 17.Metzger‐Filho O, Sun Z, Viale G et al. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node‐negative disease: Results from International Breast Cancer Study Group Trials VIII and IX. J Clin Oncol 2013;31:3083–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobbezoo D, Truin W, Voogd A et al. The role of histological subtype in hormone receptor positive metastatic breast cancer: Similar survival but different therapeutic approaches. Oncotarget 2016;7:29412–29419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeselsohn R, Buchwalter G, De Angelis C et al. ESR1 mutations ‐ A mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol 2015;12:573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]