Abstract
Lessons Learned.
The combination of ofatumumab and bendamustine in elderly patients with diffuse large B‐cell lymphoma demonstrated modest efficacy compared with standard of care.
The poor response may have been due to patient age and the high rate of treatment discontinuation.
Background.
This phase II trial evaluated the efficacy of bendamustine and ofatumumab in elderly patients with newly diagnosed diffuse large B‐cell lymphoma (DLBCL) who were not candidates for rituximab cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP).
Methods.
Patients received IV 90 mg/m2 bendamustine on days 1 and 2 of cycles 1 through 6 and IV 1,000 mg ofatumumab on days 1 and 8 of cycle 1 and on day 1 of cycles 2 through 6. Both drugs were administered at the U.S. Food and Drug Administration‐approved dose for combination therapy. All patients received premedications before each infusion of ofatumumab and hematopoietic growth factors. Treatment was administered in 21‐day cycles, with restaging after cycle 3 and cycle 6. The primary endpoint was complete response rate (CRR).
Results.
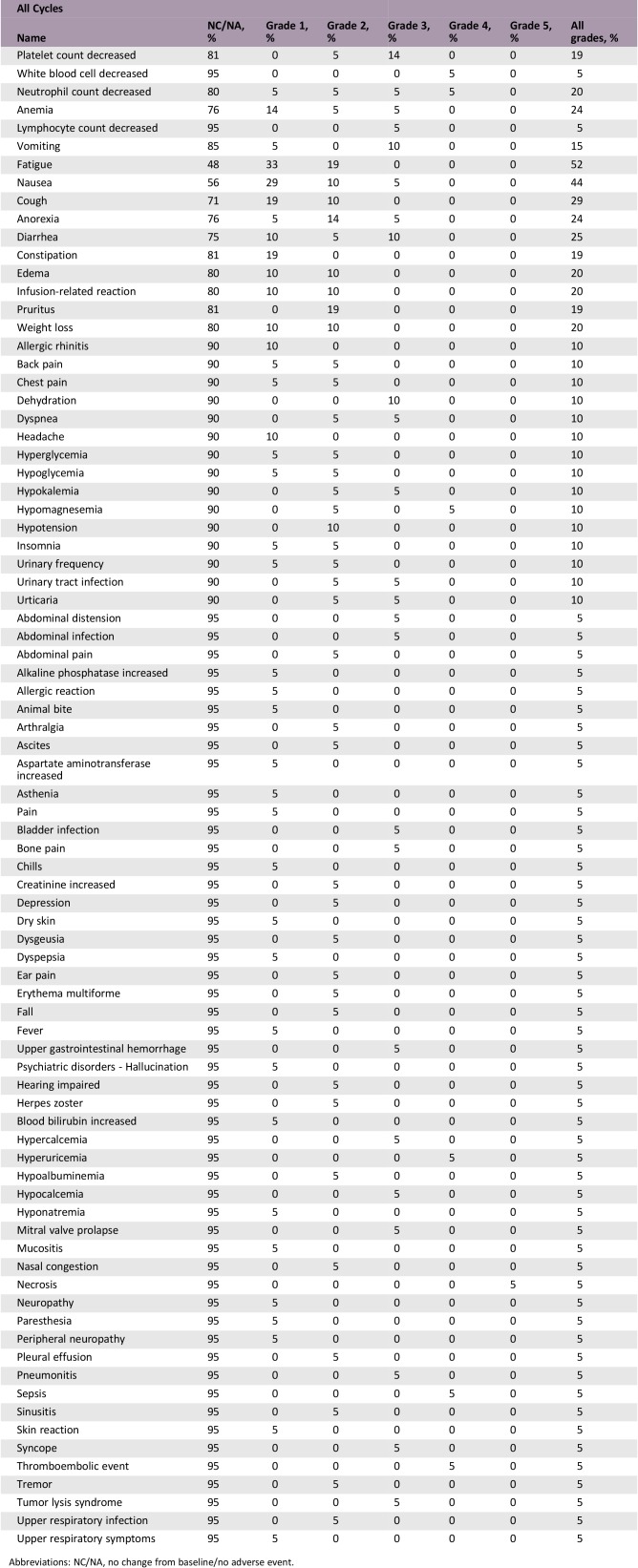
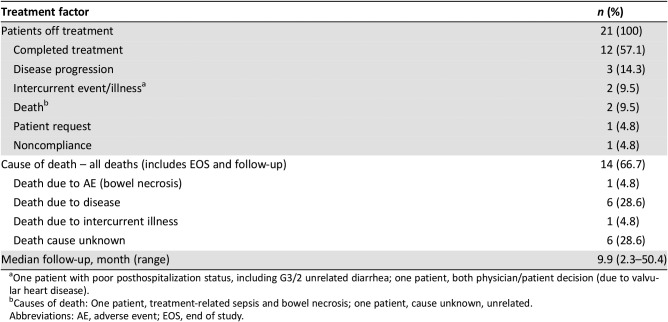
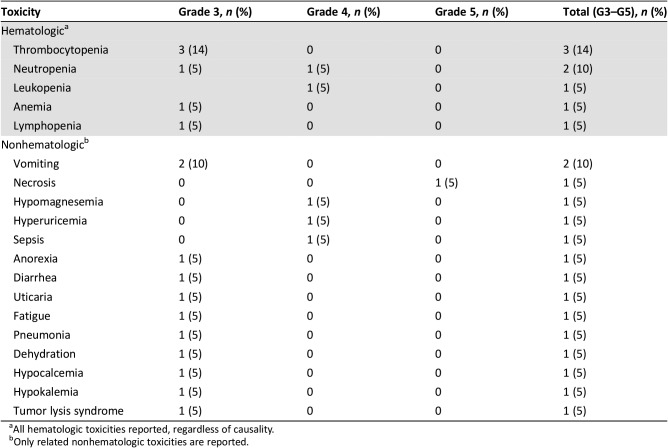
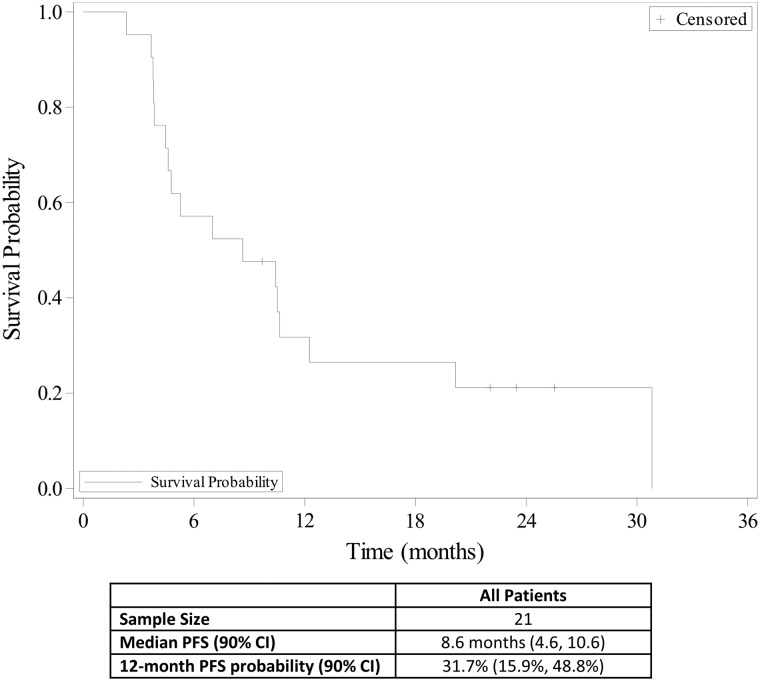
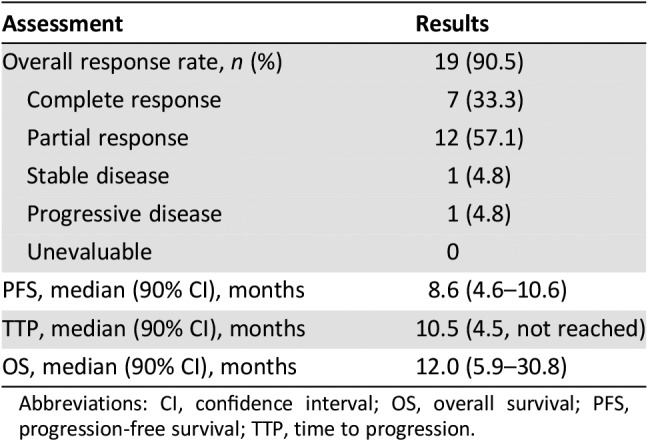
Twelve of 21 enrolled patients completed treatment; median age was 83 years. The most common reasons for treatment discontinuation were disease progression (three patients), intercurrent illness (two patients), and death (one patient due to drug‐related sepsis and bowel necrosis and one patient due to unknown cause). Thrombocytopenia (14%), neutropenia (10%), diarrhea (10%), vomiting (10%), and dehydration (10%) were the most common grade ≥3 treatment‐related adverse events. The overall response rate was 90.5% and the CRR was 33.3%. Median progression‐free survival (PFS) and overall survival (OS) were 8.6 and 12.0 months, respectively.
Conclusion.
The combination of ofatumumab and bendamustine is feasible in elderly patients with DLBCL.
Abstract
经验总结
• 在老年弥漫大 B 细胞淋巴瘤患者中,苯达莫司汀联合奥法单抗与标准治疗相比疗效有限。
• 有效率低的原因可能是患者年龄与治疗终止率高的影响。
摘要
背景。本项 II 期试验评估了苯达莫司汀联合奥法单抗在初诊老年弥漫大 B 细胞淋巴瘤 (DLBCL) 而不适合利妥昔单抗、环磷酰胺、阿霉素、长春新碱和泼尼松 (R‐CHOP) 化疗患者中的疗效。
方法。患者在第一疗程至第六疗程的第 1 天和第 2 天接受静脉注射 90 mg/m2 苯达莫司汀,第一疗程的第 1 天和第 8 天以及第二疗程至第六疗程的第 1 天静脉注射 1 000 mg 奥法单抗。这两种药物的联合使用剂量符合美国食品和药物管理局批准。所有患者在每次输注奥法单抗和造血生长因子前均接受了药物前治疗。治疗疗程为 21 天,第 3 和第 6 个疗程后再分期。主要终点为完全缓解率 (CRR)。
结果。21 名参与患者中有 12 名完成治疗;中位年龄为 83 岁。停止治疗最常见的原因为疾病进展(3 例)、并发疾病(2 例)和死亡(1 例死于药物相关败血症和肠坏死,1 例原因不明)。3 级及以上治疗相关不良事件主要为血小板减少 (14%)、中性粒细胞减少 (10%)、腹泻 (10%)、呕吐 (10%) 和脱水 (10%)。总缓解率为 90.5%,CRR为 33.3%。中位无进展生存期 (PFS) 和总生存期 (OS) 分别为 8.6 个月和 12.0 个月。
结论。奥法单抗联合苯达莫司汀在老年 DLBCL 的治疗中具有可行性。
Discussion
The R‐CHOP combination is considered standard of care for patients with DLBCL [1], although there is concern about increased toxicity in the elderly population [2]. Older patients with DLBCL have been shown to have a worse outcome than corresponding younger patients on the same treatment regimen [3]. A lower tolerance to treatment, comorbidities, and an inferior immunosurveillance have been analyzed and reviewed as important causes for the differences in outcome between young and older patients with DLBCL [2]. For this reason, alternative effective treatment modalities with less toxicity are required in the elderly population.
Bendamustine is an alkylating agent that causes intra‐ and interstrand cross‐links between DNA bases [4]. Studies of the combination of bendamustine and rituximab in elderly patients have demonstrated a complete response rate of approximately 50%, and the combination was associated with lower rates of grade ≥3 hematologic toxicities than R‐CHOP [5], [6], [7].
Ofatumumab is fully human anti‐CD20 antibody, well tolerated by elderly patients, that induces B‐cell lysis primarily through complement‐dependent cytotoxicity and antibody‐dependent cell‐mediated cytotoxicity [8]. The antibody recognizes a different epitope of the CD20 molecule than rituximab [9], [10].
In this study, we evaluated the safety and efficacy of ofatumumab plus bendamustine for the treatment of DLBCL in the elderly population. The drug combination is safe, but efficacy was modest. At 33.3% (Table 1), the complete response rate was lower than the historic CRRs of approximately 50% in elderly patients treated with bendamustine plus rituximab [5], [6], [7]. However, it should be noted that the median PFS and median OS in this study, at 8.6 months and 12 months respectively, were generally consistent with those observed in similar populations treated with bendamustine plus rituximab [5], [6], [7]. The poor response rate seen here may have been due, in part, to patient age and general health. The inclusion criteria for this study required patients to be ≥70 years old and also to be considered poor candidates for R‐CHOP therapy. Elderly patients unable to tolerate R‐CHOP treatment may still derive some benefit from this treatment regimen. Further studies are needed to better identify less toxic, but more efficacious, therapies for DLBCL for patients too frail to receive R‐CHOP.
Table 1. Treatment response (n = 21).
Abbreviations: CI, confidence interval; OS, overall survival; PFS, progression‐free survival; TTP, time to progression.
Trial Information
- Disease
Lymphoma – non‐Hodgkins
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
None
- Type of Study – 1
Phase II
- Type of Study – 2
Single arm
- Primary Endpoint
Complete response rate
- Secondary Endpoint
Progression‐free survival
- Secondary Endpoint
Overall response rate
- Secondary Endpoint
Overall survival
- Investigator's Analysis
Level of activity did not meet planned endpoint
Drug Information
- Drug 1
- Generic/Working Name
Bendamustine
- Trade Name
Treanda
- Company Name
Cephalon, Inc.
- Drug Type
Antineoplastic/cytotoxic
- Drug Class
Alkylating agent
- Dose
90 milligrams (mg) per squared meter (m2)
- Route
IV
- Schedule of Administration
Days 1 and 2 of cycles 1 through 6
- Drug 2
- Generic/Working Name
Ofatumumab
- Trade Name
Arzerra
- Company Name
GlaxoSmithKline
- Drug Type
Antibody
- Drug Class
CD20
- Dose
1000 milligrams (mg) per flat dose
- Route
IV
- Schedule of Administration
Days 1 and 8 during cycle 1 only and on day 1 of cycles 2 through 6
Patient Characteristics
- Characteristic
(n = 21), n (%)
- Median age, years (range)
83 (73–88)
- Sex
- Male
9 (42.9)
- Female
12 (57.1)
- Race
- White
20 (95.2)
- American Indian/Alaskan Native
1 (4.8)
- Modified Ann Arbor stage at diagnosis
- Stage III
14 (66.7)
- Stage IV
7 (33.3)
- Median B2‐microglobin (range)
3 (0–7)
- B2‐microglobin normality
- Abnormal
18 (85.7)
- Normal
3 (14.3)
- Cancer Types or Histologic Subtypes
DLBCL, 21
Primary Assessment Method
- Title
Complete Response (CR)
- Number of patients screened
21
- Number of patients enrolled
21
- Number of patients evaluable for toxicity
21
- Number of patients evaluated for efficacy
21
- Evaluation method
International Working Group for Response Categories
- Response Assessment CR
n = 7 (33.3%)
- Response Assessment PR
n = 12 (57.1%)
- Response Assessment SD
n = 1 (4.8%)
- Response Assessment PD
n = 1 (4.8%)
- Response Assessment Other
n = 0 (0%)
- (Median) Duration Assessments PFS
8.6 months, CI: 90%
- (Median) Duration Assessments TTP
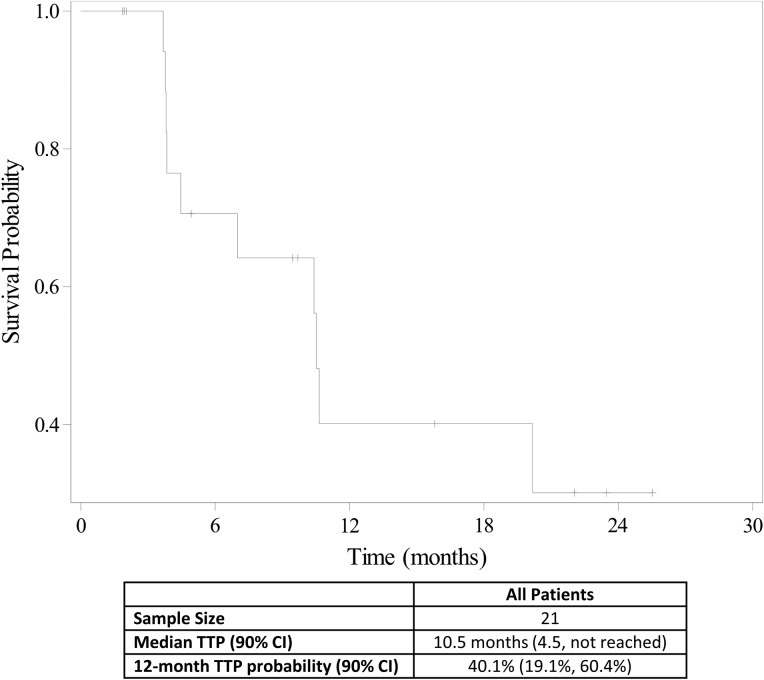
10.5 months, CI: 90%
- (Median) Duration Assessments OS
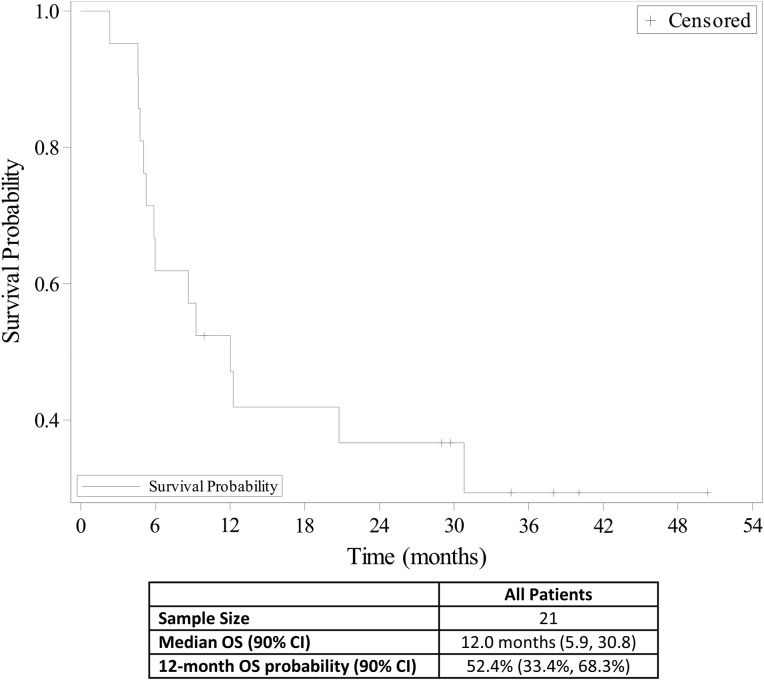
12.0 months, CI: 90%
Adverse Events
Abbreviations: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Level of activity did not meet planned endpoint
Over 50% of patients with diffuse large B‐cell lymphoma (DLBCL) are 65 years of age or older [5], and older patients with DLBCL have been shown to have a worse outcome than younger patients [6]. In this study, we evaluated the safety and efficacy of bendamustine plus the anti‐CD20 monoclonal antibody ofatumumab for the treatment of DLBCL in older patients who were not good candidates for rituximab cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP) therapy. Treatment summary is shown in Table 2. The most common grade ≥3 AEs were thrombocytopenia (14%), neutropenia (10%), diarrhea (10%), vomiting (10%), and dehydration (10%; Table 3). The overall response rate was 90.5%, and the complete response (CR) rate was 33.3%. Median progression‐free survival (PFS) was 8.6 months (Fig. 1), median time to progression was 10.5 months (Fig. 2), and median overall survival was 12.0 months (Fig. 3). The study was closed early because of low accrual. This study demonstrated the safety of the bendamustine plus ofatumumab combination for the treatment of DLBCL in this patient population. However, with a CR rate of 33.3%, this drug combination showed modest efficacy compared with standard of care, but median survival was comparable to bendamustine plus rituximab.
Table 2. Treatment summary (n = 21).
One patient with poor posthospitalization status, including G3/2 unrelated diarrhea; one patient, both physician/patient decision (due to valvular heart disease).
Causes of death: One patient, treatment‐related sepsis and bowel necrosis; one patient, cause unknown, unrelated.
Abbreviations: AE, adverse event; EOS, end of study.
Table 3. Toxicities grade ≥3 (n = 21).
All hematologic toxicities reported, regardless of causality.
Only related nonhematologic toxicities are reported.
Figure 1.
Progression‐free survival (n = 21).
Abbreviations: CI, confidence interval; PFS, progression‐free survival.
Figure 2.
Time to progression (n = 21).
Abbreviations: CI, confidence interval; TTP, time to progression.
Figure 3.
Overall survival (n = 21).
Abbreviations: CI, confidence interval; OS, overall survival.
The study was discontinued early because of low enrollment rates. The low CR rate for patients on this study regimen may have dampened enthusiasm for later patient enrollment. In addition, the common use of other treatment regimens such as rituximab plus bendamustine may have resulted in fewer patients entering the study. The combination of rituximab plus bendamustine treatment regimens has demonstrated some efficacy in older patients with DLBCL [5], [6], [7], but there remains a critical need for safer and more effective therapies.
Although the efficacy of ofatumumab plus bendamustine as first‐line treatment for DLBCL in older patients was modest, elderly patients unable to tolerate R‐CHOP treatment may still derive some benefit from this treatment regimen. The drug combination was safe in the study population, and both PFS and overall survival were similar to those seen in patients treated with rituximab and bendamustine [5], [6], [7]. There may also be some use for ofatumumab in treating rituximab‐refractory patients. Ofatumumab targets a different epitope on the CD20 molecule [9], [10] than rituximab, and the drug has been shown to be active in patients with rituximab‐refractory follicular lymphoma [11]. Similarly, it may have efficacy in the treatment of rituximab‐refractory DLBCL.
Figures and Tables
Acknowledgments
The authors thank all participating patients, their families, and site personnel members for their very important contributions to this clinical trial. The authors would also like to thank Laura M. DeBusk, Ph.D., for medical writing assistance and editorial support.
Footnotes
ClinicalTrials.gov Identifier: NCT01626352
Sponsor: Sarah Cannon Research Institute
Principal Investigator: Ian W. Flinn
IRB Approved: Yes
Disclosures
Ian W. Flinn: Agios, ArQule, Beigene, Calithera, Celgene, Constellation, Curis, Forma, Forty Seven, Genentech, Gilead, Incyte, Infinity, Janssen, Kite Pharma, Merck Novartis, Pfizer, Pharmacyclics, Portola, Roche, Seattle Genetics, Takeda, Teva, TG Therapeutics, Trillium, Verastem (RF—Institutional); Jack Erter: Genzyme, Sanofi (C/A), Sirtex, Gilead, Alexion (H); Jesus G. Berdeja: Takeda, Bristol‐Meyers Squibb, Karyopharm, CRISPR, Celgene, Kite, Servier (C/A), Abbvie, Amgen, Bluebird, Bristol‐Meyers Squibb, Celgene, Genentech, Glenmark, Janssen, Novartis, Poseida, Sanofi, Takeda, Teva (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.National Comprehensive Cancer Network . Clinical Practice Guidelines in Oncology B‐Cell Lymphomas. Version 3.2017, March 27, 2017. https://www.nccn.org/professionals/physician_gls/pdf/b‐cell.pdf. Accessed August 14, 2017.
- 2.Gutierrez A, Mestre F, Perez‐Manga G et al. Diffuse large B‐cell lymphoma in the older. Crit Rev Oncol Hematol 2011;78:59–57. [DOI] [PubMed] [Google Scholar]
- 3.Aapro MS, Cameron DA, Pettengell R et al. EORTC guidelines for the use of granulocyte‐colony stimulating factor to reduce the incidence of chemotherapy‐induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer 2006;42:2433–2435. [DOI] [PubMed] [Google Scholar]
- 4.Coiffier B, Radford J, Bosly A et al. A multicentre, phase II trial of ofatumumab monotherapy in relapsed/progressive diffuse large B‐cell lymphoma. Br J Haematol 2013;163:334–342. [DOI] [PubMed] [Google Scholar]
- 5.Weidmann E, Neumann A, Fauth F et al. Phase II study of bendamustine in combination with rituximab as first‐line treatment in patients 80 years or older with aggressive B‐cell lymphomas. Ann Oncol 2011;22:1839–1844. [DOI] [PubMed] [Google Scholar]
- 6.Storti S, Spina M, Pesce EA et al. Rituximab plus bendamustine as front‐line treatment in frail elderly (>70 years) patients with diffuse large B‐cell non‐Hodgkin's lymphoma: A phase II multicenter study of the Fondazione Italiana Linfomi. Haematologica 2018;103:1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SI, Grover NS, Olajide O et al. A phase II trial of bendamustine in combination with rituximab in older patients with previously untreated diffuse large B‐cell lymphoma. Br J Haematol 2016;175:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li B, Zhao L, Guo H et al. Characterization of a rituximab variant with potent antitiumor activity against rituximab‐resistant B‐cell lymphoma. Blood 2009;114:5007–5015. [DOI] [PubMed] [Google Scholar]
- 9.Teeling JL, Mackus WJ, Wiegman LJ et al. The biological acitivity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol 2006;177:362–371. [DOI] [PubMed] [Google Scholar]
- 10.Du J, Yang H, Guo Y et al. Structure of the Fab fragment of therapeutic antibody ofatumumab provides insights into the recognition mechanism with CD20. Mol Immunol 2009;46:2419–2423. [DOI] [PubMed] [Google Scholar]
- 11.Czuczman MS, Fayad L, Delwail V et al. Ofatumumab monotherapy in rituximab‐refractory follicular lymphoma: Results from a multicenter study. Blood 2012;119:3698–3704. [DOI] [PubMed] [Google Scholar]