Latin American countries have had one of the largest increases in clinical trial participation. This article analyzes the advantages and challenges to conducting precision oncology clinical trials in Latin America.
Keywords: Precision oncology, Clinical trials, Global oncology, Cancer
Abstract
The participation of patients in precision oncology trials needs to fulfill molecular‐based selection criteria. This strongly limits accrual, and as a consequence, screening successes have decreased, costs have increased, and fewer subjects are enrolled. To achieve narrowed targets, studies have been forced to be multicenter and multinational to reach a larger pool of candidates. However, this globalization faces many challenges, as, for example, in the case of precision oncology trials. These trials have a complex structure that is dependent upon a high‐tech infrastructure and knowledge in a dynamic environment. Given the movement of precision clinical cancer research to regions other than Europe and the U.S., it is important to evaluate the feasibility of performing such trials in lower‐middle‐ and low‐income countries. Here we critically discuss the advantages of conducting precision oncology clinical trials in Latin America and make suggestions on how to overcome the main challenges involved.
Implications for Practice.
Precision clinical trials in oncology are studies that require candidates to have tumors with specific molecular alterations, which are considered the target for the trial experimental therapy. Because many molecular alterations are rare, fewer patients are enrolled. This has led to trials being forced to be multicenter and multinational, including trials in Latin America. This article discusses the challenges and opportunities to conduct precision oncology trials in Latin America, aiming to help sponsors and investigators to solve complex issues that ultimately lead to more of such trials being run in the region, potentially benefiting more Latin American patients with cancer.
Introduction
The global movement of interactions of companies, citizens, and governments from different nations toward clinical research activities has gained consistency in the last decades. The largest average annual growth in clinical trial participation occurred in lower‐middle‐income countries (LMIC; 33%) and low‐income countries (LIC; 21%) [1], with Asian and Latin American countries showing the largest increase (30% and 12%, respectively), and three Latin American countries were ranked among the top 25 countries with higher participation in pharmaceutical clinical trials: Argentina, Brazil, and Mexico [2]. Yet conducting clinical research in LMIC and LIC needs several considerations. Two decades ago, concerns were raised because of previous research experiments in Africa. Trials with azidothymidine (AZT) to prevent human immunodeficiency virus vertical transmission were considered unethical because AZT was not accessible in Africa at that time and the comparator was considered placebo [3]. This historical example brings up the principle that the ethics for trial design and conduct are universal. The application of ethical principles to research in developing or emerging countries requires the support and involvement of the host country as well as an understanding of how conditions in the host country may differ from conditions in the partner countries. Consequently, different types of clinical trial designs and the proper selection of the comparator intervention should be carefully evaluated by research actors in LMIC and LIC [1], [4].
Precision oncology trials (POTs) bear the concept that using molecular information about patients and their diseases enriches participants’ selection for a given trial [5]. The use of a “new taxonomy” that defines diseases by their underlying molecular drivers to guide targeted therapies holds the promise to deliver superior outcomes to patients [6]. Participation in POTs needs to fulfill molecular‐based selection criteria, which strongly limits accrual, and as a consequence, screening successes have decreased, costs have increased, and fewer subjects are enrolled [7], [8]. To recruit a selected patient population, studies have become multicenter and multinational to reach a larger pool of potential research participants. Therefore, globalization of POTs is necessary to allow faster trial enrollment and to shorten the timelines for clinical testing completion [9], [10]. But precision oncology clinical trials carry a complex structure that requires multiple and specific stakeholders: genomically stratified population, next‐generation sequencing (NGS) scientists, bioinformaticians, biotechnology companies, governmental staff prepared to regulate biotechnology and to handle innovative products, investors, attorneys with expertise in clinical trials, genomics and human genetics aplications, health care payers, and others. Given the globalization of precision clinical cancer research and the need to include more regions other than Europe and the U.S., is it feasible for those trials to be conducted in Latin America?
General Ethical Aspects that Regulate Clinical Research—Particularities in Latin America
The current ethical and regulatory system for research activities involving human subjects were developed in response to deliberated abuses in the past, in which the most infamous were the Nazi Medical World War II crimes and the experiments conducted on black people in Tuskegee, Alabama. Ethical guidelines in human protection are constantly overseen by the Declaration of Helsinki (DOH; which was last updated in Brazil in 2013). And ethical codes have become the basis for clinical research regulations, such as the Nuremberg Code (1947), the Belmont Report (1979), the International Conference of Harmonization and Good Clinical Practice (ICH‐GCP; 1996), and the Council for International Organization of Medical Sciences (2002), which have been adopted by several nations and by Latin America in the “Documiento de las Américas” of the Pan American Health Organization [11].
An essential requirement of any clinical research is to guarantee that patients accept participation voluntarily and that ethical bodies critically review the scientific protocol and consent forms. Patient subjects must be fully aware of protocol procedures and must understand properly the risks and benefits involved. They also have to be informed about treatment alternatives, if applicable. The information provided in the informed consent form (ICF) must be objective, adapted to local culture, and presented in lay terms. The process of signing the ICF should avoid any possibility of coercion, even if nonintentional. ICFs from multinational trials often contain an enormous list of potential adverse events, which are described in medical terms such as “neutropenia,” “hand‐foot skin reaction,” and “increase in QT intervals.” In POTs, genetic tests are sometimes described in detail, making ICFs even more difficult to understand [12]. This is of particular concern in low socioeconomic settings [13], [14] and when there are language barriers, as, for example, in some populations in Latin America such as the Quechua‐speaking people in Peru, where the presence of an institutional or patient‐related translator is necessary. Additionally, high illiteracy rates and low educational status in some areas in Latin America are extra pitfalls [15].
Technology can be used to improve the informed consent process. Besides written information, visual techniques such as graphics and/or videos can be provided in electronic informed consent devices (e‐ICFs) in order to leverage the comprehension of fundamentals of POTs [12]. In fact, the last version of the ICH‐GCP guidelines was revised to highlight the current global level and the even more complex protocols as well as the potential usefulness of digital applications such as mobile and real‐time data capturing systems.
Precision Oncology Trials and Their Challenges
After the publication of the Human Genome Project in 2003, we witnessed an explosion of knowledge, technology, and studies pushed by the new paradigm of the genome era. The concept of cancer as a single disease remains in the past, and instead, we now recognize a constellation of diseases that can be divided into multiple subtypes based on genetic, proteomic, metabolomic, and epigenomic profiles. The movement toward the personalized treatment and better prediction of therapeutic responses has led scientists to adopt the term “precision medicine” to refer accurately to the use of genomic tools in human research, risk assessment, diagnostic categories, and therapeutic strategies in medicine. The NGS technologies (using DNA, RNA, or methylation sequencing), genetic computational solutions for omics data, and large‐scale biologic databases such as The Cancer Genome Atlas and the Catalogue of Somatic Mutations in Cancer [16] have unfolded new perspectives on how to precisely attack malignancies, uncovering novel genomic signatures and targetable key genomic changes in the tumors. Thus, the acceleration of precision oncology holds a great hope not only for the most common cancers but also for rare subtypes.
(Re)use of Human Biological Sample and Its Genomic Data
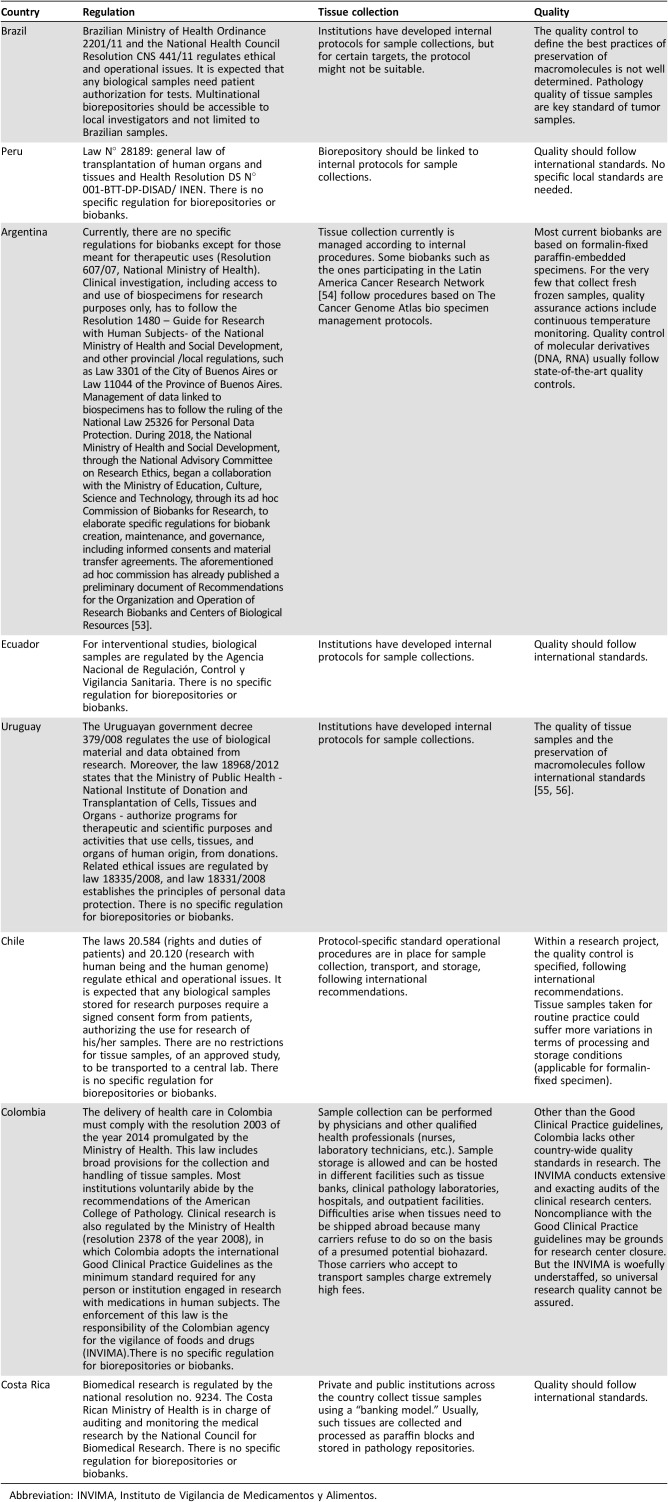
The concepts and utility of POTs are apparently in place; however, this is just one part of a larger picture. For many decades, tumor biological samples were collected once at diagnosis, and small biopsy fragments were enough for hematoxilin and eosin staining and a few immunohistochemistry markers. Currently, more biological materials at multiple timepoints are necessary for diagnosis, molecular characterization, and evaluation of response to targeted therapy. In addition, promising markers emerge every day from translational research, bringing up new challenges to ongoing trials that will have to incorporate each of these new elements to the research enterprise. Some Ethics Committees request a specific informed consent including all the biomarkers to be analyzed. This ICF often becomes obsolete and inappropriate for use in unforeseen secondary research aims, imposing an enormous obstacle to the reuse of collected material [17]. In addition, the majority of sample collections are project‐driven collections (“biorepositories”) that restrict their use to a single researcher or a single research group. The use of tumor banks could overcome these challenges. Donors providing samples for biobanks have the option to provide a general consent, in lieu of a consent for each new research project. Although biobanks are increasingly recognized as vital to developing cancer research, few biobanks exist in Latin America. With the exception of Brazil, research tumor banks in other Latin American countries operate without specific regulation (Table 1).
Although biobanks are increasingly recognized as vital to developing cancer research, few biobanks exist in Latin America. With the exception of Brazil, research tumor banks in other Latin American countries operate without specific regulation.
Table 1. Latin America tumor banking requirements per country.
Abbreviation: INVIMA, Instituto de Vigilancia de Medicamentos y Alimentos.
Genomic Data Handling and Privacy/Discrimination Concerns
Generation and analysis of genomic data require expensive and sophisticated equipment and highly trained personel that are restricted to some research centers and are not widely available in Latin America [18]. To generate comprehensive data on Latin American patients with cancer, POTs should be collaborative and preferentially multinational. The amount of genomic data generated currently by research is enormous, and it is desirable that results are shared in public databases. The aim is to translate this huge amount of data into clinical practice, and the big challenge is how to do this while assuring the privacy of the participants and the confidentiality of their genetic information. In other words, genomic and clinical characteristics should be available and anonymous at the same time, never allowing the link between a specific research participant and his/her genetic information. Issues with the maintenance of privacy, data security, and confidentiality of research‐related genetic information have raised ethical concerns, and the absence of clear regulations or policies may impair the recruitment of patients into future POTs.
From patients’ perpectives, concerns with confidentiality are related to potential use of genetic information for discrimination, as, for example, when applying for a job or purchasing health insurance. Such risk is related to sensitive information on a patient's genetic background (e.g., germline mutations or polimorphisms) but that has a faint association with tumor genetic alterations (e.g., somatic mutations, methylation, mutational burden, etc.).
Recently, a Brazilian law to protect individual data will come into force in the next 2 years (PLC 53/2018). The objective is to safeguard privacy rights to individuals. Clearer requirements would be necessary for collection, storage, manipulation, and sharing of personal data with companies while promoting tecnological development. An important aspect to be regulated involves making authorizations to study personal data more flexible [19]. Although this Brazilian law constitutes progress, there are no specific legal protection initiatives against genetic discrimination by employers, health insurers, and others in Latin America such as the Genetic Information Nondiscrimination Act passed into law in 2008 in the U.S. [20].
Access to Health Care and Novel Targeted Therapies
The selection of participants in POTs is not efficient as many have to be screened to identify the eligible participants, but the tumor response rates tend to be higher. For example, it is estimated that only 1.5% of patients with relapsed and refractory solid tumors will benefit from targeted drugs for identified mutations [8]. This should be clearly explained to candidates in the consenting process. Moreover, once clinically relevent genetic information is available, for example, when a druggable mutation is revealed (i.e., crizotinib for ALK translocation), investigators have the ethical obligation to indicate the best treatment based on this new information while halting any clinical research activity [21]. However, most targeted therapies are expensive and may not be accessible to many patients. For example, the advances in the molecular profiling and treatment of non‐small cell lung cancer over the last 10 years have not equally benefited patients worldwide. In Latin America, a very bureaucratic drug regulatory approval process, affordability of extremely expensive targeted therapies, and lack of widespread access to genetic diagnostic tools pose huge challenges to the access of adequate lung cancer treatment [22], [23]. This situation, particulary in countries like Brazil, Argentina, Ecuador, and Uruguay, where the state by law has to provide comprehensive, universal, and equal access to the health care system, may trigger lawsuits against the government to pay for unaffordable (and sometimes, experimental) drugs [21], [24]. Paradoxically, the judicialization of health care increases inequity in access to drugs because it is not pursued by citizens who can't afford a lawyer [25].
Patients’ Preferences and Values
From a patient‐centered perspective, personalized medicine should consider broader aspects of patient preferences and values [26]. In Brazil, where great emphasis is put on physicians as decision makers, the survival time is more valuable for patients with cancer than for health care professionals, whereas quality of life is more important for heath care professionals than for patients with cancer [27]. Behavioral sciences are examining patients’ attitudes and preferences to understand the engagement in treatment decision making [28]. And patients’ individuality strongly influences, if not determines, treatment decisions. Evidence per se is not enough, and one has to consider how patients envision the outcomes related to current standard of care (SOC) practices (efficacy, quality of life, safety) to make clinical judgments and decisions, including the decision to enroll in a clinical trial [28], [29]. In this aspect, the individualized approach of POTs, with recruitment usually based on biomarkers, is associated with high expectancies of success and lower tolerance of uncertainty for a given study outcome. However, even if a new therapeutic strategy is supported by a strong background and rationale on molecular mechanisms and preclinical evidence, adequately powered trials are often needed to confirm efficacy and safety of a new targeted drug [30].
Thus, clinical trials in the era of precision medicine still take participants on a journey with essentially unknown outcomes. Potential adverse effects, chances to be assigned to control arms with placebo or with SOC drugs, and high likelihood of being deemed not eligible because of biomarkers restriction may influence patients’ motivation to enroll into POTs. It is important to present the aforementioned scenario a priori to patients and to referring physicians to help them to guide their decision on whether or not to refer or to enroll in a POT. Valid and easy‐to‐understand ICFs/e‐ICFs definitely aid in this time‐consuming process that requires caution, attention, and respect for patients’ needs and cultural beliefs.
Latin America Clinical Research Environment
Conducting precision trials in Latin America is undoubtely a great opportunity for scientific advances. Middle America comprises a population of approximately 50 million people, of whom approximately 50% are ethnically characterized as admixed (Mestizos). Reports regarding molecular autosome estimates of parental ancestry indicate that Amerindian ancestries are prevalent in Mexico and Guatemala, whereas in the Caribbean area, African ancestors predominate. South America has approximately 422 million people, and the influence of European and Asian ancestry is more marked in this region. Because of this rich mixture of ancestry, Latin America presents a unique population for clinical, social, environmental, and genetic characteristics in cancer research.
Cancer epidemiology in Latin America differs from that found in other regions, having, for example, higher incidences of gastric, cervical, and gallbladder cancers [31], [32]. The region also combines modern medical facilities, well‐trained investigators, and premier research groups. Although most Latin American investigators and study coordinators have no formal training in clinical research [33], those who work in large academic centers generally learn the concepts of clinical cancer research through practice and international training and/or networking. National cooperative groups have facilitated the participation of Latin American sites in global academic research initiatives, such as the Chilean Cooperative Group for Oncological Research and the Peruvian Cooperative Oncology Group, which is an active member of the Breast International Group and the International Breast Cancer Study Group. Multinational initiatives of specialized research groups such as the Latin American Cooperative Oncology Group, the U.S. Latin American Cancer Research Network, and the Latin American Cancer Research Network [34] are contributing to the capacity building for high‐quality research participation in the region [33], [35]. Clinical oncology groups also promote discussions and improvements fostering local development of research activities such as the Latin American Society of Clinical Oncology and the Latin American Federation of Societies of Cancerology [36]. Despite these efforts, the activity of clinical trials in Latin America represents only 5.3% of all clinical trials worldwide. For cancer trials (neoplasm, tumor, malignancy, oncology, neoplastic syndrome, and neoplastic disease), Latin America represents 4.0% of the global activity (source: clinicaltrials.gov on October 28, 2018). Reasons for such low numbers are numerous. For example, Brazilian authorities classify clinical research activities as risky drug trials, and the regulation for human research is the same for all types of research—from pharma‐sponsored phase I–III trials to academic studies. Such requirements in oncology trials result in very high costs for simple studies that are almost prohibitive for academic studies; thus, they end up becoming pharma‐sponsored trials [37].
Education Level and the Understanding of Informed Consent Forms
Latin America comprises low‐ and mid‐per‐capita‐income countries in a quite heterogeneous scenario [38]. Limited access to education results in a high percentage of illiteracy. Although it is common knowledge that most patients consider the ICF difficult to understand, in LIC and LMIC, this scenario is likely to be even worse [10], [13], [39]. Such unequal economic distribution and heterogeneous educational background is a critical combination that may result in recruitment of potentially vulnerable subjects. Such individuals’ freedom and capability to protect themselves from intended or inherent risks is variably abbreviated, from decreased freewill to inability to make informed choices [40]. For example, we performed a transversal study to evaluate the readability and complexity of ICFs of phase III cancer trials using widely available software (Flesch Index and Flesch Kincaid Index) among 137 patients with cancer who had been enrolled into clinical trials in an academic center in Sao Paulo, Brazil. We found that the complexity of the ICFs required at least 18 years of education, whereas half of the patients had attended less than 8 years of school [15]. Such results provide a clue of the unrealistic content of the ICFs (required by regulatory agencies) and to understand the misconception of human beings used as “guinea pigs” [36].
National Cancer Registries and Geographic Distribution of Cancer Care
Another regional problem is the paucity of accurate cancer registries [41]. Cancer registries provide the data‐driven foundation for cancer control efforts. Innumerous initiatives for the systematic collection, storage, analysis, interpretation, and reporting of data on subjects with cancer are being done in Latin America. Uruguay, for example, besides having a cancer registry with nationwide coverage (run under the Comisión Honoraria de Lucha contra el Cáncer), has developed and implemeted the national Oncology Electronic Health Record that includes information about genetic and nongenetic risk factors, tumor molecular profile data, and clinical‐pathological characteristics. The Argentinian National Cancer Institute has made progress in establishing national computer registers on types of tumors (the RITA registry), cases of pediatric oncology (ROHA), epidemiological surveillance (SIVER‐Ca), and the recently created SITHER, which collects reported cases of tumors with hereditary and familial characteristics. This model served for diagnosis of weaknesses and difficulties in carrying out molecular epidemiology projects. The new Brazilian law (PLC 14/2018), dated June 25, 2018, establishes that any and all cancer‐related health and disease events will now have mandatory notification in public and private health services throughout the country [42]. The law takes effect in 180 days after the due date and requires regulation from the Ministry of Health. However it will take some time for this to become reality because the law requires investments and training on the part of academic institutions.
Despite these initiatives, accurate and robust cancer registries are still under development in several Latin American countries. Of note, most patients with cancer in Latin America are treated in large academic public institutions, where most clinical trials are also conducted. Centralizing cancer treatment and trials in big centers (often in major cities) provides a more homogeneous oncologic care and consistent setting for trial enrollment in terms of therapeutic protocols, technology, working staff, and quality data for cancer registries. Nevertheless, such a strategy also reduces access to oncologic care and may limit patient recruitment and cancer registries in rural areas. In countries such as Peru, Ecuador, and Bolivia, for example, more than half of the population live in the rural countryside.
Genetic Research and Regulatory Agencies
Research regulation in Latin America has been developed for decades, but still, for some countries such as Brazil, it has one of the longest timelines for a trial protocol review and approval. Many efforts have been made to enable good and faster regulatory review, but in the case of genetic data handling, the requirements are sometimes unpredictable [43]. The review directives upon ethical principles may also vary considerably across Latin America, depending on the institutional experience. Genetic research projects submitted to ethics committees may be delayed if the committee is not familiar with the theme.
In terms of technology, research sites also vary considerably. Laboratories proficient in next‐generation sequencing and other genetic tests performed under fast‐delivering logistics are limited to few academic institutions or private laboratories. In 2017, only 221 next‐generation sequencing plataforms developing cancer genomics projects existed in Latin America [44]. Thus, samples collected in global studies end up in central laboratories usually located in developed countries with generation of a multinational biorepository. In this scenario, the regulation for the use of samples (rights and limitations) and the administrators’ responsibilities over the samples pose obstacles to reaching consensus among research colaborators and regulatory agencies. Moreover, the regulation of sample‐derived genetic data sharing is in its infancy in Latin America. A big effort is being made in Argentina regarding training of institutional review boards about genomic data information and analyses through the recent creation of the National Ethics Advisor Committee, which is in charge of establishing the basic criteria for ethical evaluation of clinical research, including genomic data manipulation. This Committee has a specific Advisory Commission for Biorepositories and Genomic Data, in which genomic researchers, clinicians, and ethicists are currently discussing the minimal requirements needed for good quality and ethics aspects of running precision medicine research in the country. In Ecuador, the government has also created the Ethics Advisor Committee to facilitate the undertaking of research.
Recommendations involving data privacy and confidentiality of individual identity are key points of data handling. In that sense, Argentina is currently updating its law on personal data handling with a chapter devoted to genomic information. Therefore, researchers are encouraged to obtain consent for future use and broad sharing of genomic and phenotypic data, balancing the responsibility of protecting participants' interests with the potential loss of opportunities for future research. In Uruguay, the government constituted a National Commission of Ethics in Research, whose main responsibility is to examine the ethical aspects of research with human beings and to update and adapt the applicable regulations.
Precision Randomized Placebo‐Controlled Cancer Trials in Latin America
The use of placebo is a well‐recognized method to control for observation bias [45]. Placebo responses consider an individual's genetic predisposition, personality, type of disease (e.g., tumor with spontaneous shrinkage), and values that could also interfere with therapeutic outcomes [5]. Thus, such factors justify the use of placebos in POTs. Brazil is the Latin American country with the largest number of clinical trials [2], [46]. It is also known for its strong opposition against the flexible use of placebo. Both the Federal Council of Medicine and the National Health Council have published resolutions regulating the use of placebo exclusively in clinical trials, preventing their use if there is any effective therapeutic method already in place [47]—although sometimes the interpretation of “effective” by this council is not standard. Likewise, in Uruguay, the current national regulations adhered to the 2000 version of the DOH without taking into account subsequent revisions, which flexibilizes the use of placebo and the best existing treatment in controlled clinical trials (article 29 of the DOH).
The use of placebos also represents a known barrier to trial enrollment. If compared with U.S. patients with cancer, Brazilian patients with cancer demonstrate lower interest in participating in POTs. In a cross‐sectional survey with Brazilian patients with cancer and investigators, authors observed that 41% of patients were not willing to participate in trials with a placebo‐exclusive arm, and half of investigators objected to recommend a POT to patients because they “felt uncomfortable to offer no treatment to their patients” [48], [49].
POTs in Latin America and the Use of High‐End Next‐Generation Sequencing Technology
POTs are able to gather elements in clinical data to classify and measure associations between environmental exposures, clinical consequences, and genetic profile. The analyses of the large amount of data generated need a technological approach to integrate any data from a myriad of distinct sources; this is a key solution to refine patient particularities, but will create a tremendous volume of information that would cause conventional processing methods to simply collapse. Big Data analytics covers data retrieval and handling and analyses of massive, diverse data sets of genomic and clinical data. An analytic result may reveal hidden patterns, uncryptic not obvious correlations, and many other types of patterns (or lack of any) [50]. Precision trials would frequently need to find a specific patient in a huge population or the study should be placed in a particular population with higher probability for eligible candidates.
Latin America's leading markets have a proven appetite for technology adoption. Brazil (at number 2 in the world), Mexico (6), and Argentina (8) are among the highest‐ranked markets worldwide in time per week spent on the internet, much of it on mobile devices [51]. Technology companies are also fast growing in Argentina, which is on the verge of a boom [52]. This is a particularly good scenario to run POTs in Latin America in the field of patient recruitment, as these patients are likely to better accept digital language and innovative approaches using high‐end sequencing technology. However, Latin America currently needs improvements in local accessibility of biotechnology suppliers, particularly in genomic analysis, to centralize and coordinate multinational trials [36].
The lack of local quality reinforcement regulations also plays against the necessary investment on quality assurance of public and private genomic analysis providers. Political decisions regarding protective economic rules for locally developed tests and more strict quality controls should be necessary to assure the necessary genomic analytic capabilities.
With respect to molecular analyses of tumors, Latin America faces difficult challenges in implementing integrated analysis of germline and somatic variants in cancer care. In Argentina and Brazil, for example, both private and public institutions suffer from the high cost of imported standard lab equipment and molecular biology reagents for precision medicine especially when large‐scale purchases for analysis cannot be guaranteed. Even when infrastructure and equipment as well as expert human resources can be attained, local high‐quality genomic analysis is hard to sustain becacuse of the aforementioned costs. The lack of local quality reinforcement regulations also plays against the necessary investment on quality assurance of public and private genomic analysis providers. Political decisions regarding protective economic rules for locally developed tests and more strict quality controls should be necessary to assure the necessary genomic analytic capabilities.
As for routine molecular diagnosis, several pharmaceutical companies have until recently used a business model in several Latin American countries, including Argentina, Colombia, and Brazil, based on provision of the companion diagnostic assay used to guide prescription of targeted drugs. However, this model is currently being changed to a market‐based, competitive approach, in which competitive providers of private medicine purchase such genetic tests before allowing coverage for the targeted drug. This strategy would be beneficial in terms of assuring quality but more difficult in terms of provision for local laboratories. In Colombia, these industry‐sponsored mutation analyses of some genes (i.e., RAS, EGFR, BRAF), although widely available, are concentrated to a few laboratories in major cities, with long waiting times between test request and results. Additionally, there is general poor access to rare clinically relevant mutations without appeal to big pharma. From the practical point of view, only centers equipped with access to technology are in a position to run POTs in Colombia, mostly located in Bogota, the capital.
Lastly, another barrier for successful enrollment in POTs stems from the way cancer care is reimbursed to treatment providers. Fee‐for‐service is the predominant reimbursement model in some Latin American nations, such as Colombia and Brazil, which may disincentivize some treating physicians to refer patients to a different center.
Recommendations to Overcome the Challenges and Improve the Participation of Latin America in POTs
In spite of all these caveats and challenges, some Latin American institutions are already capable of conducting POTs. We describe biobanking requirements per country as to facilitate collaborations with those Latin American countries (Table 1). To expand the conduct of POTs to more Latin American centers, some crucial obstacles have to be overcome. Below, we propose an expert panel list of recommendations (Table 2) and potential solutions to boost the dissemination of POTs in our region. Three authors (R.J.A., R.S.C.G., and R.R.) initially contacted key research leaders in their Latin American countries to discuss the manuscript and recommendations. Because this project did not receive or have financial support, we conducted all discussions by teleconference and e‐mails. All recommendations and requirements were careflully revised and approved by the authors. Each author described their local regulatory characteristics (Table 1).
Table 2. Recommendations to improve the participation of Latin America in precision oncology clinical trials.
Abbreviation: e‐ICFs, electronic informed consent forms; GCP, Good Clinical Practice; ICF, informed consent form; ICH, International Conference of Harmonization; IRBs, institutional review boards; POTs, precision oncology trials.
Conclusion
POTs involve a complex scenario that is not limited to genetic and clinical data handling. They need accessible technology requiring staff trained at a high level to interpret useful information. The ethical oversight has to be more dynamic and personalized to local environments while maintaining moral fundamentals. A comprehensive strategy should be applied that involves improvements in staff training, adaptations of infrastructure, updated investigators’ ability to navigate in local regulatory environments, and better access to technology and health care. We conclude that POTs are feasible in Latin America but are still limited to few investigators and institutions from academic centers. Here we recommended some actions to personalize and boost POTs across Latin America. With the inevitable globalization of clinical cancer research, it is crucial to have Latin America on board in order to improve the external validity of trials’ results and to provide more access to new drugs for Latin American patients with cancer.
Author Contributions
Conception/design: Roberto Jun Arai, Rodrigo Santa Cruz Guindalini, Andrea Sabina Llera, Juan Manoel O'Connor, Bettina Muller, Mauricio Lema, Helano C. Freitas, Tannia Soria, Lucía Delgado, Denis Landaverde, Paola Montenegro, Rachel P. Riechelmann
Collection and/or assembly of data: Roberto Jun Arai, Rodrigo Santa Cruz Guindalini, Andrea Sabina Llera, Juan Manoel O'Connor, Bettina Muller, Mauricio Lema, Helano C. Freitas, Tannia Soria, Lucía Delgado, Denis Landaverde, Paola Montenegro, Rachel P. Riechelmann
Manuscript writing: Roberto Jun Arai, Rodrigo Santa Cruz Guindalini, Andrea Sabina Llera, Juan Manoel O'Connor, Bettina Muller, Mauricio Lema, Helano C. Freitas, Tannia Soria, Lucía Delgado, Denis Landaverde, Paola Montenegro, Rachel P. Riechelmann
Final approval of manuscript: Roberto Jun Arai, Rodrigo Santa Cruz Guindalini, Andrea Sabina Llera, Juan Manoel O'Connor, Bettina Muller, Mauricio Lema, Helano C. Freitas, Tannia Soria, Lucía Delgado, Denis Landaverde, Paola Montenegro, Rachel P. Riechelmann
Disclosures
Helano C. Freitas: Pfizer, Merck Sharp & Dohme (C/A), AstraZeneca (SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Eccard R, Amato AA, Guilhem DB et al. Globalization of clinical trials: Ethical and regulatory implications. Int J Clin Trials 2016;3:1–8. [Google Scholar]
- 2.Thiers FA, Sinskey AJ, Berndt ER. Trends in the globalization of clinical trials. Nat Rev Drug Discov 2008;7:13–14. [Google Scholar]
- 3.Zion D. Ethical considerations of clinical trials to prevent vertical transmission of HIV in developing countries. Nat Med 1998;4:11–12. [DOI] [PubMed] [Google Scholar]
- 4.Varmus H, Satcher D. Ethical complexities of conducting research in developing countries. N Engl J Med 1997;337:1003–1005. [DOI] [PubMed] [Google Scholar]
- 5.Katsnelson A. Momentum grows to make ‘personalized’ medicine more ‘precise.’ Nat Med 2013;19:249. [DOI] [PubMed] [Google Scholar]
- 6.Blau CA, Liakopoulou E. Can we deconstruct cancer, one patient at a time? Trends Genet 2013;29:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denicoff AM, McCaskill‐Stevens W, Grubbs SS et al. The National Cancer Institute‐American Society of Clinical Oncology Cancer Trial Accrual Symposium: Summary and recommendations. J Oncol Pract 2013;9:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad V. Perspective: The precision‐oncology illusion. Nature 2016;537:S63–S63. [DOI] [PubMed] [Google Scholar]
- 9.DiMasi J, Hansen R, Grabowski H. The price of innovation: New estimates of drug development costs. J Health Econ 2003;22:151–185. [DOI] [PubMed] [Google Scholar]
- 10.Arai RJ, Mano MS, de Castro G et al. Building research capacity and clinical trials in developing countries. Lancet Oncol 2010;11:712–713. [DOI] [PubMed] [Google Scholar]
- 11.Etienne CF. Annual Report of the Director 2018. Pan American Health Organization. 2018. Available at https://www.paho.org/annual‐report‐of‐the‐director‐2018/en. Accessed March 14, 2019.
- 12.Grady C, Cummings SR, Rowbotham MC et al. Informed consent. N Engl J Med 2017;376:856–867. [DOI] [PubMed] [Google Scholar]
- 13.Verástegui EL. Consenting of the vulnerable: The informed consent procedure in advanced cancer patients in Mexico. BMC Med Ethics 2006;7:E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arai RJ, Hoff PM, De Castro G Jr, et al. Ethical responsibility of phase 0 trials. Clin Cancer Res 2009;15:1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miranda Vda C, Fede AB, Lera AT, et al. How to consent without understanding? [in Portuguese]. Rev Assoc Med Bras 2009;55:328–334. [DOI] [PubMed] [Google Scholar]
- 16.Forbes SA, Beare D, Bindal N et al. COSMIC: High‐Resolution Cancer Genetics Using the Catalogue of Somatic Mutations in Cancer. In: Current Protocols in Human Genetics. 2016:10.11.1‐10.11.37. [DOI] [PubMed] [Google Scholar]
- 17.Rance B, Canuel V, Countouris H et al. Integrating heterogeneous biomedical data for cancer research: the CARPEM infrastructure. Appl Clin Inform 2016;7:260–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutzwiller F, Blank PR. Current challenges in handling genetic data. Swiss Med Wkly 2014;144:1–2. [DOI] [PubMed] [Google Scholar]
- 19.Guaracho RF. A privacidade, as novas regras de proteção de dados e o futuro digital. Estadão 2018. Available at https://politica.estadao.com.br/blogs/fausto‐macedo/tres‐vereadores‐deixarao‐camara‐de‐sao‐paulo‐em‐2019/. Accessed March 14, 2019.
- 20.Green RC, Lautenbach D, McGuire AL. GINA, genetic discrimination, and genomic medicine. N Engl J Med 2015;372:397–399. [DOI] [PubMed] [Google Scholar]
- 21.Paim J, Travassos C, Almeida C et al. The Brazilian health system: History, advances, and challenges. Lancet 2011;377:1778–1797. [DOI] [PubMed] [Google Scholar]
- 22.Raez LE, Cardona AF, Santos ES et al. The burden of lung cancer in Latin‐America and challenges in the access to genomic profiling, immunotherapy and targeted treatments. Lung Cancer. 2018;119:7–13. [DOI] [PubMed] [Google Scholar]
- 23.Raez LE, Santos ES, Rolfo C et al. Challenges in facing the lung cancer epidemic and treating advanced disease in Latin America. Clin Lung Cancer 2017;18:e71–e79. [DOI] [PubMed] [Google Scholar]
- 24.Nisihara RM, Possebom AC, de Martino Cruvinel Borges L et al. Demanda judicial de medicamentos na Justiça Federal do Estado do Paraná. Judic demand Medicat through Fed Justice State Paraná 2017;15:85–91. [Google Scholar]
- 25.Biehl J, Socal MP, Amon JJ. The judicialization of health and the quest for state accountability: Evidence from 1,262 lawsuits for access to medicines in southern Brazil. Health Hum Rights 2016;18:209–220. [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein DB, Tate SK, Sisodiya SM. Pharmacogenetics goes genomic. Nat Rev Genet 2003;4:937–947. [DOI] [PubMed] [Google Scholar]
- 27.de Araujo Toloi D, Critchi G, Mangabeira A et al. Living better or living longer? Perceptions of patients and health care professionals in oncology. Ecancermedicalscience 2015;9:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arai RJ, Santana Longo E, Sponton MH et al. Bringing a humanistic approach to cancer clinical trials. Ecancermedicalscience 2017;11:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bae J. Value‐based medicine: Concepts and application. Epidemiol Health 2015;37:e2015014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunter DJ. Uncertainty in the era of precision medicine. N Engl J Med 2016;375:711–713. [DOI] [PubMed] [Google Scholar]
- 31.Torre LA, Bray F, Siegel RL et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 32.Mano MS, Arai RJ, Hoff PM. Rare tumors research in emerging countries. Rare Tumors 2010;2:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coronel E, Fregni F. Clinical research in Latin America: Scientific production in high impact clinical research journals from 2000 to 2010. Int J Clin Trials 2015;2:28–33. [Google Scholar]
- 34.Investigators of the US–Latin America Cancer Research Network . Translational cancer research comes of age in Latin America. Sci Transl Med 2015;7:319fs50. [DOI] [PubMed] [Google Scholar]
- 35.LACOG . Latin American Cooperative Oncology Group. 2018. Available at www.lacog.org.br. Accessed March 14, 2019.
- 36.Rolfo C, Caglevic C, Bretel D et al. Cancer clinical research in Latin America: current situation and opportunities. Expert opinion from the first ESMO workshop on clinical trials, Lima, 2015. ESMO Open 2016;1:e000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizzo LV, Fernando L, Camargo A. Clinical research in Brazil. Einstein (Sao Paulo) 2013;11:vii–viii. [DOI] [PMC free article] [PubMed]
- 38.Gaviria, DG. How can Latin America escape it's middle‐income trap? World Economic Forum. 2016. Available at https://www.weforum.org/agenda/2016/09/how‐can‐latin‐america‐escape‐its‐middle‐income‐trap/. Accessed March 14, 2019.
- 39.Jefford M, Moore R. Improvement of informed consent and the quality of consent documents. Lancet Oncol. 2008;9:485–493. [DOI] [PubMed] [Google Scholar]
- 40.Shivayogi P. Vulnerable population and methods for their safeguard. Perspect Clin Res 2013;4:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goss PE, Lee BL, Badovinac‐crnjevic T et al. The Lancet Oncology Comissão Planejamento do controle do câncer na América Latina e no Caribe. Lancet Oncol 2013;14. [Google Scholar]
- 42.Federal S. Projeto de Lei da Câmara n° 14, de 2018. 2018.
- 43.Bujar M, Patel P, Liberti L. The changing regulatory environment in Latin America Focus on good review practices. Cent Innov Regul Sci 2015. Available at http://www.cirsci.org/wp-content/uploads/2016/01/CIRS-RD-Briefing-58-FINAL-for-distribution.pdf. Accessed March 14, 2019.
- 44.Torres Á, Oliver J, Frecha C et al. Cancer genomic resources and present needs in the Latin American region. Public Health Genomics 2017;20:194–201. [DOI] [PubMed] [Google Scholar]
- 45.Araujo RLC, Riechelmann RP, eds. Methods and biostatistics in oncology. Cham, Switzerland: Springer International, 2018. [Google Scholar]
- 46.Atal I, Trinquart L, Porcher R et al. Differential globalization of industry‐ and non‐industry‐sponsored clinical trials. PLoS One 2015;10:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fregnani JHTG, Carvalho AL, Paranhos FRL et al. Eticidade do uso de placebo em pesquisa clínica: proposta de algoritmos decisórios. Rev Bioética 2015;23:456–467. [Google Scholar]
- 48.Fede AB, Miranda MC, Lera AT et al. Placebo‐controlled trials (PCT) in cancer research: Patient and oncologist perspectives. J Clin Oncol 2010;28:e19626–e19626. [Google Scholar]
- 49.Comis RL. Public attitudes toward participation in cancer clinical trials. J Clin Oncol 2003;21:830–835. [DOI] [PubMed] [Google Scholar]
- 50.Raghupathi W, Raghupathi V. Big data analytics in healthcare: Promise and potential. Health Inf Sci Syst 2014;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.We are social. Digital in 2017: Global Overview. 2017. Available at https://wearesocial.com/special‐reports/digital‐in‐2017‐global‐overview. Accessed March 14, 2019.
- 52.Reuters. This South American country's tech sector is on the cusp of a boom. Fortune. October 21, 2016. Available at http://fortune.com/2016/10/21/argentina-tech-sector-boom/. Accessed March 14, 2019.
- 53.Grupo Ad Hoc de Biobancos, Ministerio de Ciencia, Tecnología e Innovación Productiva. Recomendaciones para la organización y funcionamiento de Biobancos/Centros. Available at http://www.celulasmadre.mincyt.gob.ar/Documentos/Recomend‐Biobancos.pdf. Accessed March 14, 2019.
- 54.National Cancer Institute. United States‐Latin America Cancer Research Network (US‐LA CRN). 2014. Available at https://www.cancer.gov/about-nci/organization/cgh/research/us-la-crn. Accessed March 14, 2019.
- 55.Marodin G, Salgueiro JB, Motta Mda L et al. Brazilian guidelines for biorepositories and biobanks of human biological material. Rev Assoc Med Bras (1992) 2013;59:72–77. [PubMed] [Google Scholar]
- 56.Hugo A, Froes J, Campos M et al. Tumor banking for health research in Brazil and Latin America: Time to leave the cradle. Appl Cancer Res 2017;11:4–8. [Google Scholar]
- 57.Vanaken H. eConsent study provides insights to shape industry adoption. Appl Clin Trials 2016;25. [Google Scholar]
- 58.Lunshof JE, Chadwick R, Vorhaus DB et al. From genetic privacy to open consent. Nat Rev Genet 2008;9:406–411. [DOI] [PubMed] [Google Scholar]
- 59.Arai R, Noronha I, Nicolau J et al. Academic health centers: Integration of clinical research with healthcare and education. Comments on a workshop. Clinics (Sao Paulo) 2018;73(suppl 1):e515s. [DOI] [PMC free article] [PubMed]