This article reports on a prognostic model for use in patients with colorectal cancer initiating therapy with FOLFIRI/aflibercept to predict overall survival and aid in decision making, with the goal of optimizing treatment results in actual patient populations.
Keywords: Aflibercept, Colorectal cancer, Antiangiogenic, Real‐world data, Nomogram, Prognosis, Survival
Abstract
Introduction.
The VELOUR study evaluated the efficacy and safety of adding aflibercept to FOLFIRI (fluorouracil, leucovorin, irinotecan) in second‐line therapy for metastatic colorectal cancer (mCRC). However, a nomogram that can stratify patients according to prognosis is unavailable, and the frequency and effect of the pragmatic use of modified schedules in actual practice remains unknown.
Method.
The sample consists of 250 patients with mCRC treated with aflibercept and irinotecan‐based chemotherapy at nine Spanish academic centers between January 2013 and September 2015. The result of a Cox proportional hazards model regression for overall survival (OS), adjusted for covariates available in daily practice, was represented as a nomogram and web‐based calculator. Harrell's c‐index was used to assess discrimination.
Results.
The prognostic nomogram for OS includes six variables: Eastern Cooperative Oncology Group performance status, tumor location, number of metastatic sites, mutational status, better response to previous treatment(s), and carcinoembryonic antigen. The model is well calibrated and has acceptable discriminatory capacity (optimism‐corrected c‐index, 0.723; 95% confidence interval [CI], 0.666–0.778). Median OS was 6.1 months (95% CI, 5.1–8.8), 12.4 months (95% CI, 9.36–14.8), and 22.9 months (95% CI, 16.6–not reached) for high‐, intermediate‐, and low‐risk groups, respectively. Age, comorbidity, or use of modified FOLFIRI regimens did not affect prognosis in this series. Grade 3–4 adverse events were less common following modified schedules. The admission rate because of toxicity was higher in ≥65 years (9.7% vs. 19.6%; odds ratio, 2.26; p = .029).
Conclusion.
We have developed and internally validated a prognostic model for use in individuals with colorectal cancer initiating therapy with FOLFIRI‐aflibercept to predict both OS and the effect of pragmatic modifications of the classic regime on efficacy and safety. This can aid in decision making and in designing future trials.
Implications for Practice.
In this study, the authors developed and conducted the internal validation of a prognostic nomogram that makes it possible to stratify patients who are eligible for second‐line FOLFIRI‐aflibercept based on their probability of survival. This model was developed in a multicenter sample from nine Spanish hospitals. Furthermore, to increase the study's validity, the practical use of aflibercept in this setting was investigated, including doses or pragmatic modifications. The results suggest that the modified schedules often used in this daily clinical practice‐based patient population are associated with less severe toxicity without apparent detriment to survival endpoints. It is believed that these data complement the information provided by the VELOUR trial and are relevant for the oncologist in treating colon cancer in the second‐line setting.
摘要
介绍。VELOUR 研究对二线治疗中采用 FOLFIRI(氟尿嘧啶、甲酰四氢叶酸、伊立替康)联合阿帕西普治疗转移性结直肠癌 (mCRC) 的疗效和安全性进行了评估。然而,无法用列线图表示根据预后情况对患者进行的分层,且在临床实践中实际使用改良给药方案的频率和效果尚不可知。
方法。样本包括 2013 年 1 月至 2015 年 9 月期间,在西班牙 9 家学术中心接受阿帕西普和基于伊立替康化疗的 250 名mCRC患者。利用 Cox 比例风险回归模型得出的总生存期 (OS) 结果(根据临床实践中可用的协变量进行校正)可用列线图和基于网络的计算器表示。同时,利用 Harrell 的 C 指数来评估区分度。
结果。关于OS的预后列线图中包含六个变量:美国东部肿瘤协作组的体力状态、肿瘤位置、转移部位的数量、突变状态、对先前治疗的较好反应率及癌胚抗原。模型经过准确校准,具有合格的鉴别能力 [乐观校正 C 指数为 0.723;95% 置信区间(CI),0.666–0.778]。高风险、中度风险及低风险组的中位OS分别为 6.1 个月(95% CI,5.1–8.8)、12.4 个月(95% CI,9.36–14.8)及 22.9 个月(95% CI,16.6–未达到)。年龄、合并症或使用改良的 FOLFIRI 方案对本组预后无影响。在使用经改良的给药方案后,3‐4 级不良事件较为罕见。在 65 岁(含)以上的患者中,因毒性反应而入院的比率更高(9.7% vs. 19.6%;比值比,2.26;p = 0.029)。
结论。我们开发了一个预后模型并进行了内部验证,此模型可针对启用 FOLFIRI 联合阿帕西普治疗的结直肠癌患者预测其OS及实际改良传统治疗方案对药物疗效和安全性的影响。这有助于制定医疗决策和设计未来的试验。《肿瘤学家》
实践意义:在本研究中,作者编制了一份预后列线图并进行了内部验证,以便能够基于生存概率对符合 FOLFIRI 联合阿帕西普二线治疗的患者进行分层。此模型由 9 家西班牙医院利用多中心样本进行开发。此外,为提高本研究的有效性,我们研究了在此种情况下使用阿帕西普的实际情况,包括剂量或务实性改良。结果表明,经改良的给药方案常用于基于日常临床实践的患者群体中,与较少的严重毒性反应相关,但不会明显损害生存终点。我们认为,这些数据补充了 VELOUR 试验所提供的信息,有助于肿瘤学家研究结肠癌的二线治疗。
Introduction
Metastatic colorectal cancer (mCRC) is a common neoplasm, with a median survival of 30 months [1]. Standard initial therapy for fit patients consists of polychemotherapy, typically FOLFOX (fluorouracil, leucovorin, oxaliplatin) or FOLFIRI (fluorouracil, leucovorin, irinotecan) schedules associated with targeted molecular vascular endothelial growth factor (VEGF) or epidermal growth factor receptor (EGFR) therapies. The choice is conditioned by the tumor's molecular profile, location, and treatment aim [2]. For individuals who have received first‐line FOLFOX and bevacizumab, second‐line FOLFIRI with antiangiogenic agents [3], [4], [5] or with anti‐EGFR therapy for tumors with wild‐type KRAS, NRAS, or BRAF could be used [6], [7].
VEGF is a core regulator of angiogenesis in mCRC, and high VEGF expression is associated with poor prognosis. Blocking VEGF increases the efficacy of chemotherapy in first and successive lines [8]. Aflibercept (VEGF‐Trap; ziv‐aflibercept; Zaltrap) is a human recombinant fusion protein that acts as a “decoy receptor,” blocking angiogenesis by targeting VEGF‐A, VEGF‐B, and placental growth factor [9].
The pivotal, phase III VELOUR study evaluated the efficacy and safety of adding aflibercept to FOLFIRI as second‐line therapy in mCRC, following progression to an oxaliplatin‐based regimen [4]. Compared with placebo, aflibercept demonstrated significantly increased overall survival (OS) rates (hazard ratio [HR], 0.81; 95% confidence interval [CI], 0.71–0.93), as well as progression‐free survival (PFS; HR, 0.75; 95% CI, 0.66–0.86). Nevertheless, aflibercept was also associated with VEGF‐related toxicities, as well as increased grade 3–4 toxicity. Although the benefit was maintained across all strata [10], a post hoc analysis indicated that a profile of good and poor responders could be clinically defined (e.g., based on the Eastern Cooperative Oncology Group performance status [ECOG PS], time to relapse after adjuvant treatment, and number of metastases) [27]. Furthermore, another subanalysis confirmed that, despite efficacy data (PFS and OS) being consistent across age groups, grade 3–4 adverse events (AEs) were more common in the elderly [11]. Likewise, a registry of real‐world data suggested that clinical course during treatment with aflibercept (risk of progression or death) was heterogeneous and possibly affected by simple clinical variables [12].
All these data indicate that in the real world, where patients are often older, have comorbidities, and worse functional status, the risk‐benefit balance could differ from that initially expected [13]. Our group posed the working hypothesis that actual efficacy and safety outcomes might differ from the VELOUR study results, particularly bearing in mind that modified FOLFIRI regimens are commonly used with the aim of decreasing toxicity in vulnerable patients [14], [15], [16]. Therefore, the design of prognostic instruments in registries of real‐world data cannot be detached from the differential use of therapies in patients with unequal fitness. The correlation between the pragmatic variability of real‐world practice and prognostic factors must be fully analyzed before it can be generalized. Based on these premises, we have developed and internally validated a prognostic nomogram that can aid in optimizing treatment results in actual patient populations.
Materials and Methods
Participants and Study Design
A retrospective, observational, postmarketing study was conducted at nine Spanish university hospitals. Clinical and analytical data were obtained from patient histories and recorded by the same physicians caring for them; data were monitored in detail (A.F.M.) to filter inconsistencies, errors, or unjustified missing data.
Eligibility criteria included being adult (≥18 years) with histologically diagnosed, resistant or progressing mCRC, at least one oxaliplatin‐based first‐line chemotherapy or with tumor relapse in the first 6 months following adjuvant treatment (“rapid progressors”). Participants were to have received aflibercept with irinotecan‐based chemotherapy; recruitment was consecutive at each hospital. No intervention was performed, and treatment choice, clinical evaluations, and imaging studies were in line with each center's routine care practices.
The protocol was approved by a reference Clinical Research Ethics Committee (Galicia's CREC). All procedures carried out during the study period were performed in keeping with the Declaration of Helsinki of 1964. Those patients who were included and still alive when clinical information was collected provided written informed consent.
Definition of Variables and Endpoints
The primary aim of the study was the development and internal validation of a prognostic nomogram to predict OS after describing management patterns (modifications of standard treatment) and to evaluate survival and safety endpoints. PFS was defined as the time between initiating aflibercept and tumor progression or demise, censoring event‐free cases at last follow‐up. OS was established as the same time point until death from any cause.
Prior to devising the model, a descriptive analysis was made of possible treatment variability to verify that the sample had a comparable baseline hazard. Dose intensity (DI) was defined as the amount of drug administered per unit of time and expressed as mg/m2 per week. Cumulative dose was specified as total dose and reported as total mg/m2 administered. Relative dose intensity (RDI) was considered to be the DI administered with respect to the dose intensity scheduled for each regimen. Thus, three treatment groups were specified based on dose modifications: (a) FOLFIRI at the standard dose, the same dose and schedule as the VELOUR study [4]; (b) low‐dose FOLFIRI, for which the initial dosage included a reduction of at least 15% of one of the components; and (c) a subgroup labeled “other regimens” that included the suppression of the bolus and/or infusion of 5‐fluorouracil. Treatment groups were based on the regimen and dosage of the first cycle, regardless of subsequent dose reductions or discontinuations.
Potential predictors (see supplemental online Table 1) were chosen according to the criteria of being common and easily accessible at the beginning of aflibercept therapy and having demonstrated prognostic impact in at least one previous manuscript [17]. KRAS, NRAS, and BRAF mutational status was analyzed as per each center's clinical practice. The local researchers assessed tumor response using computerized tomographic images taken approximately every 3 months, in line with RECIST version 1.1 criteria [18]. This analysis refers to the maximum response achieved during treatment. Toxicities were categorized and recorded according to the grading scale in place during the treatment period (National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0) every 15 days [19]. The number of hospital admissions because of toxicities was likewise recorded.
Statistical Analysis
Categorical variables were analyzed by means of Pearson's chi‐squared (χ2) and linear‐by‐linear association tests, and continuous variables were assessed using Kruskal‐Wallis tests. Candidate predictors were initially chosen after a review of the literature and consulting with experts. These predictors were significant in univariate analyses. We applied a Cox proportional hazards (PH) regression model for OS, and internal validation was carried out by bootstrapping. Nonlinear effects were modeled by restricted cubic splines. Multiple imputation by fully conditional specification was used in multivariate analyses to deal with missing values [20]. The PH assumption for each predictor was verified using the Schoenfeld residuals test. For discriminatory capacity, 1,000 bootstrap replications served as internal validation subsets to estimate the bias‐corrected c‐index. The 1‐year calibration was gauged visually and by the Gronnesby‐Borgan goodness‐of‐fit test. Survival was estimated via the Kaplan‐Meier method. Stratified log‐rank tests were also applied. All statistical assessments were two‐sided, and p values <.05 were deemed statistically significant. Based on previous investigations, a 250‐patient sample with more than 100 events is considered sufficient to adjust a model of ten preset predictors [21]. This analysis was performed with SPSS version 23.0 software (SPSS Inc., Chicago, IL) and RStudio [22], including rms, mstate, and survival packages [23], [24].
Results
Patients
The sample includes 250 patients consecutively treated between January 2013 and September 2015. Table 1 illustrates participants’ characteristics. Subjects had a median age of 63 years (range 35–82); 44% (n = 111) were aged at least 65 years, and most exhibited good functional status (ECOG PS 0–1 in 96.4%). The most frequent first‐line regimen was CAPOX (capecitabine‐oxaliplatin) or FOLFOX (5‐fluorouracil‐oxaliplatin; n = 234, 93.6%; Table 1), combined with bevacizumab (n = 111, 44.4%) or anti‐EGFR (n = 50, 20%). The presence of KRAS, NRAS, and BRAF mutations was detected in 168 tumors (67.2%). The most common tumor localization was the left colon (39.6%), followed by the rectum (31.6%). The primary tumor was treated surgically in 66% (n = 164); metastases were synchronously diagnosed in 75%. In most patients, aflibercept was used with FOLFIRI in the second line (n = 226, 90.4%), although 7.2% received it in the third or fourth line, and 2.4% in rapid progressors after adjuvancy. Following aflibercept, patients received a median of one further line of treatment (range 0–3). Surgery was performed on metastases in 25 patients (10%), following aflibercept in 10 of them (4%). Median follow‐up in subjects still alive (after initiating aflibercept) was 8.8 months (95% CI, 7.0–10.6).
Table 1. Baseline characteristics of patients at initiation of treatment with aflibercept.
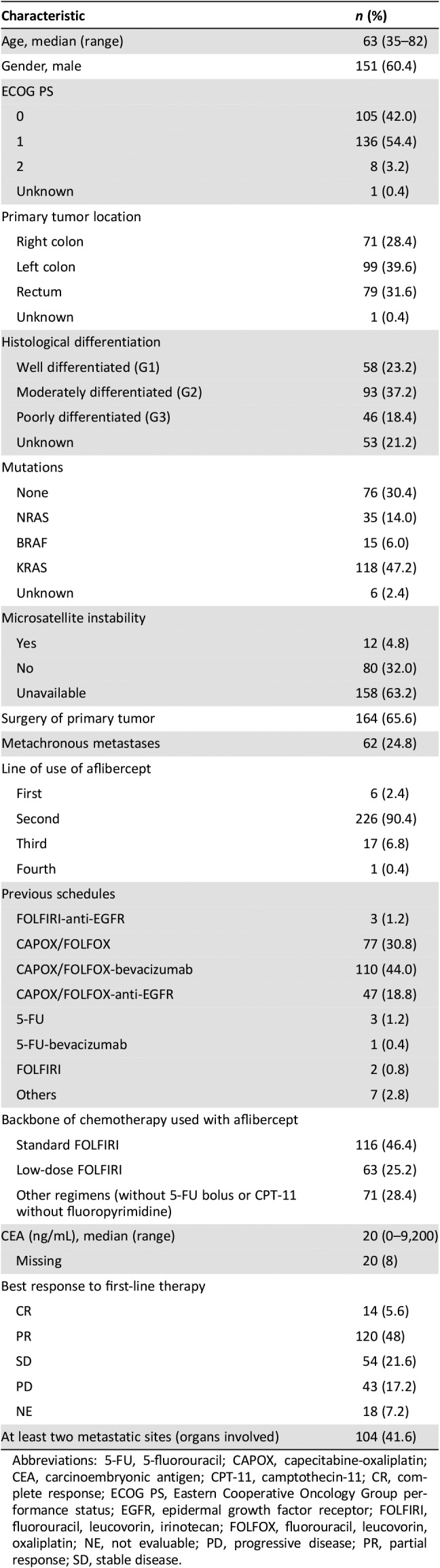
Abbreviations: 5‐FU, 5‐fluorouracil; CAPOX, capecitabine‐oxaliplatin; CEA, carcinoembryonic antigen; CPT‐11, camptothecin‐11; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; FOLFIRI, fluorouracil, leucovorin, irinotecan; FOLFOX, fluorouracil, leucovorin, oxaliplatin; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Aflibercept Dosage and Treatment Schedules
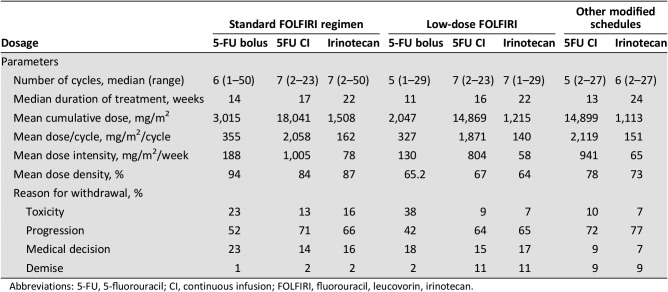
The participants received a median of six cycles of aflibercept (range 1–43), over a median of 14 weeks, with a mean dose per cycle of 3.9 mg/kg. The backbone of chemotherapy initially used with aflibercept consisted of FOLFIRI, the standard regimen, in 46%, low‐dose in 25%, and “other modified schedules” in 28.6% (FOLFIRI without 5‐fluorouracil bolus in 27%, irinotecan in monotherapy 1.2%, and others 0.4%; Table 1; for distribution by centers, supplemental online Table 2). Dosages of 5‐fluorouracil and irinotecan for each stratum are presented in Table 2. Of note is the relatively low mean RDI (<85%) in all subgroups, especially in those who received low‐dose FOLFIRI from the start (64%–67%). In contrast, treatment duration was comparable in the three strata. Aflibercept was discontinued because of toxicity (18%), progression (65%), medical decision (11%), and demise (4%).
Table 2. Fluorouracil and irinotecan dosages used together with aflibercept.
Abbreviations: 5‐FU, 5‐fluorouracil; CI, continuous infusion; FOLFIRI, fluorouracil, leucovorin, irinotecan.
Supplemental online Table 3 displays patients’ baseline characteristics by the most common backbone of chemotherapy. The low‐dose FOLFIRI stratum had the highest median age (66 vs. 63 years in the remaining strata; p = .040). The percentage of ECOG PS 0 was highest in the standard FOLFIRI schedule versus low‐dose or without bolus (54.3%, 31.7%, 31.4%; p = .001). The remaining characteristics examined revealed no significant differences across treatment groups.
Safety
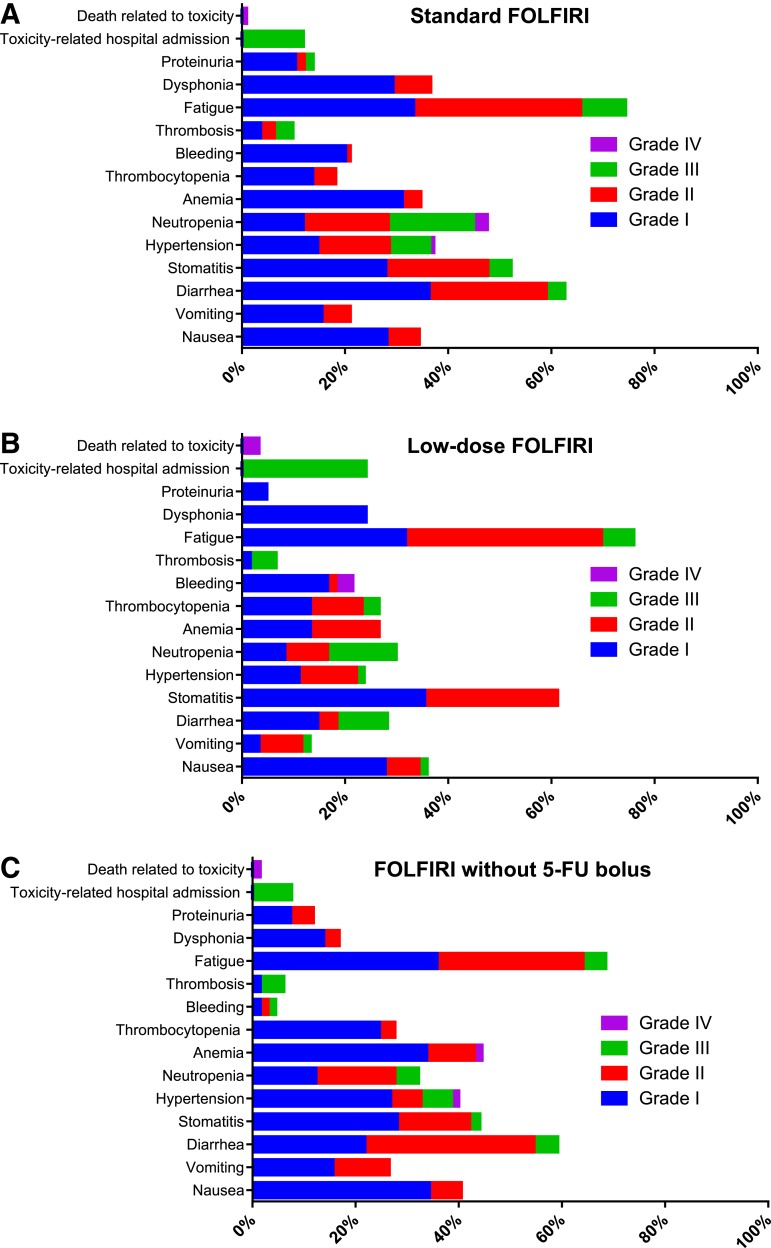
The most common AEs in the entire sample are shown in Table 3. The most prevalent toxicities were asthenia (73%), followed by mucositis (52%), neutropenia (38%), anemia (35%), and hypertension (34%). Most toxicities were mild, although 13.9% of the participants were admitted for grade 3–4 toxicity. Other frequent grade 3–4 toxicities were neutropenia (13%), hypertension (6.8%), asthenia (6.8%), diarrhea (5.4%), and thromboembolic disease (4.2%). Treatment‐related mortality was 1.6% (n = 4): two cases of severe hemorrhage, one gastrointestinal complication, and another febrile neutropenia. AEs stratified by type of FOLFIRI can be seen in Figure 1. Grade 3–4 AEs occurred more frequently with the standard schedule versus low‐dose FOLFIRI or without bolus (40%, 38%, 23%, respectively; linear‐by‐linear test, p = .033). Thus, grade 3–4 neutropenia developed more often in participants on standard FOLFIRI than those receiving low‐dose or without bolus (19.2%, 13.3%, and 4.6%, respectively; p = .018). Differences in grade 3–4 asthenia were likewise observed (36.6%, 24.1%, and 16.9%, respectively; p = .014); any grade bleeding (21.1%, 21.6%, and 4.6%; p = .009); dysphonia (36.6%, 24.1%, and 16.9%; p = .014), and hospitalizations for toxicity (11.9, 24.1%, and 8.6%; p = .039). No significant differences between treatment groups were identified in the remaining variables analyzed. Insofar as age is concerned, individuals aged at least 65 years exhibited more diarrhea, asthenia, and anemia. Moreover, the admission rate because of toxicity was also higher in these subjects (9.7% vs. 19.6%; odds ratio, 2.26; p = .029; supplemental online Fig. 1).
Table 3. Adverse events in the whole cohort (n = 250).
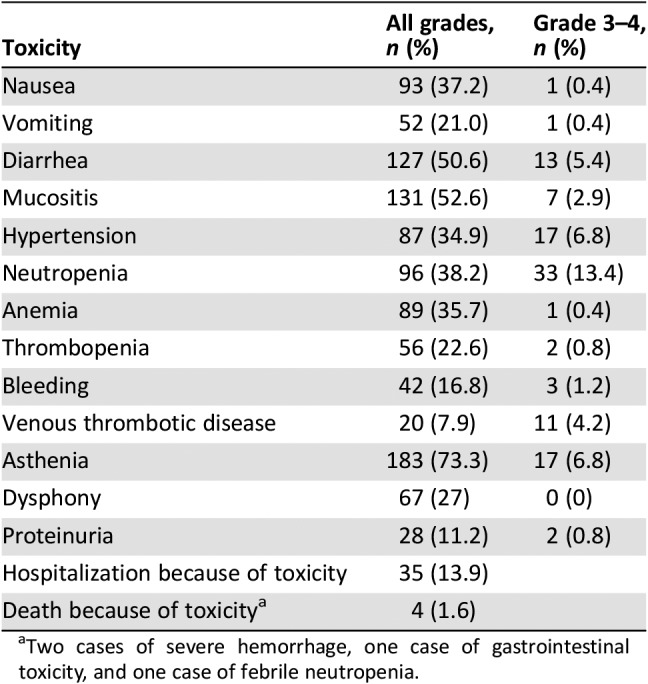
Two cases of severe hemorrhage, one case of gastrointestinal toxicity, and one case of febrile neutropenia.
Figure 1.
Adverse events stratified by FOLFIRI regimen. (A): Standard FOLFIRI. (B): Low‐dose FOLFIRI. (C): FOLFIRI without 5‐FU bolus.
Abbreviations: 5‐FU, 5‐fluorouracil; FOLFIRI, fluorouracil, leucovorin, irinotecan.
Efficacy
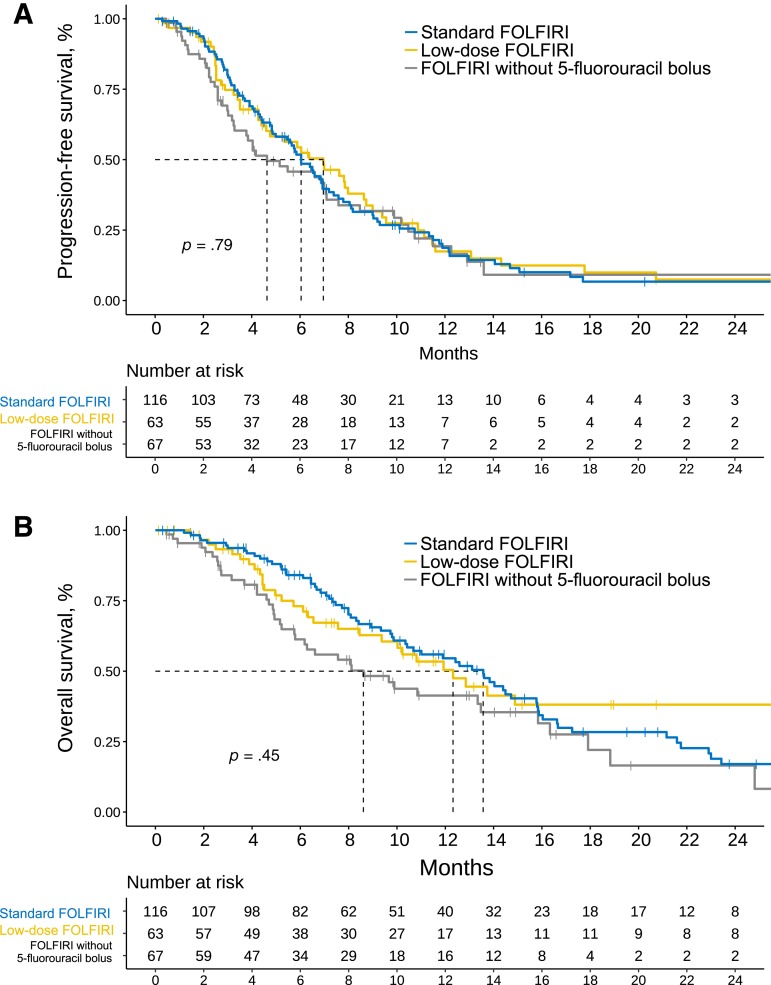
A total of 220 patients had measurable and response‐evaluable disease at approximately 3 months of treatment initiation. In these cases, the RECIST 1.1 evaluation at 3 months revealed 0 complete responses, 53 partial responses (24%), 84 stable disease (38%), and 83 tumor progression (38%). No statistical evidence was found, indicating that the dosages and treatment regimens modified the likelihood of response (χ2 = 2.055; degrees of freedom [d.f.] = 4; p = .720; supplemental online Fig. 2). At the time of analysis (March 2018), 189 progression events (78%) and 143 deaths (58%) were detected. Patients had a median PFS of 6.05 months (95% CI, 5.16–6.97) and OS of 12.4 months (95% CI, 10.2–14.4). Supplemental online Figure 3 is a survival graph. Figure 2A and B shows Kaplan‐Meier curves stratified by chemotherapy regimen. There was no statistical evidence of variations in PFS based on chemotherapy schedule: median PFS, 6.0 months (95% CI, 4.99–7.20) for the standard regimen; 6.97 months (95% CI, 4.6–8.7) for low‐dose; and 4.6 months (95% CI, 3.29–7.59) for “other regimens” (log‐rank test, χ2 = 0.6, d.f. = 2, p = .736). Similarly, no statistically significant differences were found in OS based on these same schedules or treatment groups: 13.5 (95% CI, 10.4–15.8), 12.32 (95% CI, 9.3–32.6), and 8.6 months (95% CI, 5.7–15.8), respectively (log‐rank test, χ2 = 4.9; d.f. = 2; p = .087). Subsequent systemic treatment was administered to 47% (n = 117), most often following standard FOLFIRI (53%) versus low‐dose FOLFIRI (33%), and FOLFIRI without bolus (49%; χ2 = 6.32; d.f. = 2; p = .042).
Figure 2.
Kaplan‐Meier survival estimates based on modifications to chemotherapy. (A): Progression‐free survival. (B): Overall survival.
Abbreviation: FOLFIRI, fluorouracil, leucovorin, irinotecan.
Development and Internal Validation of a Prognostic Nomogram
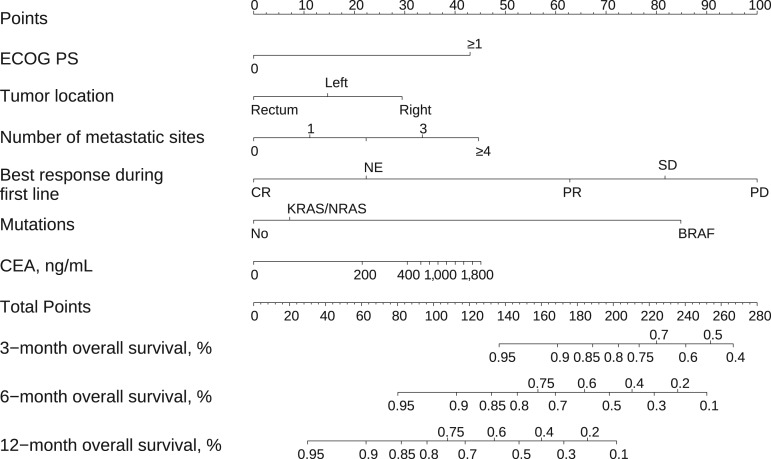
After verifying the uniformity of survival endpoints with respect to the pragmatic use of the treatment regimen, a prognostic nomogram was then created. Univariate Cox PH regression models for PFS and OS, including the effect of surgery, are shown in supplemental online Tables 4 and 5. Predictive factors for OS were ECOG PS 1 versus 0 (hazard ratio [HR] 2.58; p < .001), rectal localization (HR 0.52; p = .007), number of metastatic sites (continuous variable; HR 1.28; p = .037), BRAF mutations (HR 6.54; p < .001), complete response to first line (HR 0.10; p = .003), and carcinoembryonic antigen (CEA) levels (135 vs. 5 ng/mL; HR 1.41; p = .038; supplemental online Table 7). Age had no prognostic effect (e.g., in subjects >70 years, HR 1.07; 95% CI, 0.70–1.64). The nomogram is represented in Figure 3, and a web‐based calculator has been designed and is available at http://www.iricom.es/prognostictools/vtrap. The model was well calibrated (Groennesby‐Borgan score test: χ2 = 5.067; p = .535; supplemental online Fig. 4) and has an acceptable discriminatory capacity, with optimism‐corrected c‐index of 0.723 (95% CI, 0.666–0.778). Patients considered high (>164 points), intermediate (128–164 points), and low risk (<128) had a median OS time of 6.1 months (95% CI, 5.1–8.8), 12.4 months (95% CI, 9.36–14.8), and 22.9 months (95% CI, 16.6–not reached), respectively (log‐rank, p < .001; supplemental online Fig. 5). A sensitivity analysis illustrates that the model continues to be robust after excluding subjects who have undergone surgery for metastases. Additionally, the Akaike information criterion (AIC) does not support the inclusion of resection of the primary tumor or of metastases (ΔAIC 2.9 and 6.6, respectively). The multivariate Cox PH regression for PFS is shown in supplemental online Table 6.
Figure 3.
Predictive overall survival nomogram for patients treated with FOLFIRI‐aflibercept.
Abbreviations: CEA, carcinoembryonic antigen; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Discussion
Although several observational studies have corroborated the efficacy and safety data for FOLFIRI plus aflibercept [12], [25], [26], there is evidence that the clinical course of patients treated with second‐line antiangiogenic agents is variable. In this study, we have integrated putative factors that lead to efficacy and safety heterogeneity, developing and internally validating a prognostic nomogram for OS in patients who initiated FOLFIRI and aflibercept in routine conditions of care. Basically, the resulting model exhibited adequate calibration and acceptable discriminatory capacity; therefore, if externally validated by other international groups, it could prove to be valuable to stratify these patients. Regarding the previous literature, this nomogram can integrate the biological and clinical characteristics of the disease (mutations, tumor localization, CEA, ECOG PS, and number of metastatic sites) and identifies the variable of chemosensitivity to the previous line of chemotherapy as having the greatest weight. Two prior analyses revealed ECOG PS and number of metastatic sites to be important prognostic factors in individuals treated with aflibercept [12], [27]. High CEA levels [28], right‐sided colon tumor location [29], and BRAF mutations [30] correlated with poor prognosis in mCRC. Nevertheless, the VELOUR study evinced similar benefit, regardless of whether the primary was left‐ or right‐sided colon, and subjects with mutated BRAF in the placebo group had worse OS, although aflibercept showed superior efficacy in these cases [31]. The resulting nomogram is capable of correctly stratifying patients based on their risk of death and, in particular, correctly identifies individuals with a poor prognosis, with median OS of less than 6 months, who might be eligible to participate in clinical trials to improve outcomes.
Moreover, this analysis of prognostic factors has been expanded with an enriched description of the study population's baseline characteristics and details regarding their treatment at each center (e.g., supplemental online Table 2). We believe that this description is always essential before extrapolating the data from one setting to another. In addition, in actual conditions, potentially confounding variables must be assessed, as well as investigating all the strata of patients who may have a different baseline hazard for one reason or another. Thus, specific risks can be conditioned both by the greater vulnerability in the real world, as well as by pragmatic modifications to the schedules commonly being made by local oncologists, in pursuit of greater safety in groups that are frailer than those treated in trials [10], [12], [16]. In and of itself, this is certainly a valid research objective that should interest the clinician. This series confirms that, indeed, elderly patients are a commonly seen group who are prone to more serious AEs, as previously reported by other authors [11], which appears to justify clinicians’ use of modified regimens to reduce toxicities when confronted with this situation [16]. Therefore, in addition to helping us describe the usual management patterns in these individuals with this treatment alternative and in this specific clinical context, this analysis of routine clinical practice has enabled us to develop a nomogram with the potential to optimize treatment outcomes, as well as being applicable in developing subsequent clinical trials.
As for conditions under which it is possible to generalize, we have found that the characteristics here have been reasonably comparable to those of the VELOUR study, with the exception of the use of more than one prior treatment in 7.2%, greater prior exposure to bevacizumab (44% vs. 30% in VELOUR), as well as a slight increase in KRAS mutations (47% vs. 40% in VELOUR). Nonetheless, the most substantial difference lies in that more than half of the participants in our study received modified FOLFIRI schedules or FOLFIRI at nonstandard dosages schedules from the very beginning. This is consistent with the relatively low RDI (<85%) in all the strata, which comprises one of the model's key conditions for extrapolation. Furthermore, our series includes patients with a slightly older median age compared with the VELOUR trial (63 years, range 35–82, vs. 61 years, range 21–82 years), in addition to 44% of the cases being aged more than 65 years versus 36% in the pivotal trial. In our cohort, 42% and 54% presented with ECOG PS 0 and 1, whereas in VELOUR, these proportions were 57% and 40%, respectively. These two circumstances a priori make our population more similar to what is typically found in the clinical care setting. All in all, survival‐related endpoints in our cohort (median OS and PFS of 13.5 and 6.05 months, respectively) concur with those of the VELOUR study, 13.5 and 6.9 months [4]. We have not observed any differences in these endpoints according to age or type of chemotherapy used. However, as for best overall response, the rate of progressive disease in our registry (38%) was higher than in the experimental arm in VELOUR (10.4%), although in our study, we found no statistical evidence to indicate that the modifications to the schedules were the cause for them being less active. These changes can evidently be attributed to the fact that the evaluation was not centralized. Nonetheless, the most notable differences with the VELOUR study were seen regarding the toxicity profile, both in serious general AEs (e.g., rate of grade 3–4 diarrhea, 5.4% vs. 19.3%; grade 3–4 asthenia, 6.8% vs. 16.8%, in our series vs. in VELOUR, respectively), as well as class‐specific toxicity (e.g., hypertension, 6.8% vs. 19.3%, or grade 3–4 thrombosis, 4.2% vs. 7.8%). Our data reveal fewer AEs with modified schedules, not attributable to different treatment durations. Although the tolerance profile appears to be slightly better here than in the pivotal trial, it must be taken into account that serious toxicity‐related hospitalizations were still greater among elderly patients (≥65 years). This result essentially coincides with those of Ruff et al. who found an increase in the toxicity profile of individuals aged more than 65 years in an age‐based analysis of the VELOUR trial [11]. This group continues to be more complex in second‐line treatment for mCRC [13]. Therefore, much remains to be done in this field.
Insofar as limitations are concerned, the reader must be aware of the relatively small sample size, which precludes the detection of slight differences across strata. The study includes patients who would not have been eligible for VELOUR because of prior therapies, line of treatment, previous combination, etc. Other limitations are inherent to analyzing retrospective data, with the imprecision that typically entails, although grade 3–4 toxicity, tumor progression, and demise are generally robust events that are reliably recorded. Although no differences were observed in the appreciable magnitude in survival‐related endpoints according to stratum after adjusting for confounding factors, the presence of residual bias that could affect the apparent result of the modifications cannot be entirely ruled out.
With these limitations in mind, our data might be applicable in clinical practice for patients deemed candidates to receive FOLFIRI and aflibercept, who present a factor of vulnerability, such as old age, comorbidity, or impaired functional status, which suggests an increased risk of severe toxicity, raising the possibility of using an adapted or modified schedule. The model might be useful for patient counseling, patient stratification for clinical trials, or become an adjuvant tool for prognosis‐sensitive decision making. Nevertheless, the covariates of the model are prognostic factors, and their predictive ability has not been described before [31]; likewise, it cannot be ruled out that a subgroup of patients categorized as having a poor prognosis might benefit from chemotherapy. Furthermore, these data complement the efficacy and safety outcomes of the pivotal clinical trial by evaluating these parameters in a real‐world population and help to clarify the potential role of variables such as age and dose modifications in the final clinical benefit.
Conclusion
We have developed and internally validated a prognostic nomogram that is potentially useful in selecting patients to receive treatment with FOLFIRI and aflibercept within the context of routine clinical practice. Our data also confirm the efficacy and safety of this regimen even with modified schedules and introduce the concept of pragmatically individualized therapy as an attitude that can potentially mitigate serious risks, without substantially compromising efficacy.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
The authors thank Priscilla Chase Duran of PCD Translations. The translation of this manuscript has been covered by a grant from Sanofi España. It is an academic study in which the company has participated in no way in the study design, drafting, analysis, interpretation of results, or approval of the final text.
Contributed equally
Author Contributions
Conception/design: Ana Fernández Montes, Carlos López López, Paula Jiménez Fonseca, Alberto Carmona‐Bayonas
Provision of study material or patients: Ana Fernández Montes, Carlos López López, Guillem Argilés Martínez, David Páez López, Ana María López Muñoz, Beatriz García Paredes, David Gutiérrez Abad, Carmen Castañón López, Paula Jiménez Fonseca, Javier Gallego Plazas, María Carmen López Doldán, Eva Martínez de Castro, Manuel Sánchez Cánovas, María Tobeña Puyal, Beatriz Llorente Ayala, Ignacio Juez Martel, Mariana López Flores, Alberto Carmona‐Bayonas
Collection and/or assembly of data: Ana Fernández Montes, Carlos López López, Guillem Argilés Martínez, David Páez López, Ana María López Muñoz, Beatriz García Paredes, David Gutiérrez Abad, Carmen Castañón López, Paula Jiménez Fonseca, Javier Gallego Plazas, María Carmen López Doldán, Eva Martínez de Castro, Manuel Sánchez Cánovas, María Tobeña Puyal, Beatriz Llorente Ayala, Ignacio Juez Martel, Mariana López Flores, Alberto Carmona‐Bayonas
Data analysis and interpretation: Ana Fernández Montes, Carlos López López, Guillem Argilés Martínez, David Páez López, Ana María López Muñoz, Beatriz García Paredes, David Gutiérrez Abad, Carmen Castañón López, Paula Jiménez Fonseca, Javier Gallego Plazas, María Carmen López Doldán, Eva Martínez de Castro, Manuel Sánchez Cánovas, María Tobeña Puyal, Beatriz Llorente Ayala, Ignacio Juez Martel, Mariana López Flores, Alberto Carmona‐Bayonas
Manuscript writing: Ana Fernández Montes, Carlos López López, Paula Jiménez Fonseca, Alberto Carmona‐Bayonas
Final approval of manuscript: Ana Fernández Montes, Carlos López López, Paula Jiménez Fonseca, Alberto Carmona‐Bayonas
Disclosures
Ana Fernández Montes: Sanofi, Celgene, Roche, Servier, Amgen (C/A), Sanofi (RF); Carlos López López: Sanifo, Merck, Roche, Amgen (C/A, RF, SAB); Guillem Argilés Martínez: Bayer, Servier, Sanofi, Bristol‐Myers Squibb, Amgen, Merck, Roche (C/A), Bayer (RF), Bayer, Merck, Sanofi, Roche, Amgen, Servier (SAB); David Páez López: Amgen, Sanofi, Merck Serono, Servier (C/A); Beatriz García Paredes: Sanofi (C/A); Manuel Sánchez Cánovas: Leo Pharma (RF), Kyowakirin (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Van Cutsem E, Cervantes A, Adam R et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–1422. [DOI] [PubMed] [Google Scholar]
- 2.Moriarity A, O'Sullivan J, Kennedy J et al. Current targeted therapies in the treatment of advanced colorectal cancer: A review. Ther Adv Med Oncol 2016;8:276–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennouna J, Sastre J, Arnold D et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol 2013;14:29–37. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Tabernero J, Lakomy R et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin‐based regimen. J Clin Oncol 2012;30:3499–3506. [DOI] [PubMed] [Google Scholar]
- 5.Tabernero J, Yoshino T, Cohn AL et al. Ramucirumab versus placebo in combination with second‐line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first‐line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double‐blind, multicenter, phase 3 study. Lancet Oncol 2015;16:499–508. [DOI] [PubMed] [Google Scholar]
- 6.Peeters M, Price TJ, Cervantes A et al. Final results from a randomized phase 3 study of FOLFIRI±panitumumab for second‐line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:107–116. [DOI] [PubMed] [Google Scholar]
- 7.Sobrero AF, Maurel J, Fehrenbacher L et al. EPIC: Phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:2311–2319. [DOI] [PubMed] [Google Scholar]
- 8.Carrato A, Gallego‐Plazas J, Guillen‐Ponce C. Anti‐VEGF therapy: A new approach to colorectal cancer therapy. Expert Rev Anticancer Ther 2006;6:1385–1396. [DOI] [PubMed] [Google Scholar]
- 9.Syed YY, McKeage K. Aflibercept: A review in metastatic colorectal cancer. Drugs 2015;75:1435–1445. [DOI] [PubMed] [Google Scholar]
- 10.Tabernero J, Van Cutsem E, Lakomý R et al. Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: Prespecified subgroup analyses from the VELOUR trial. Eur J Cancer 2014;50:320–331. [DOI] [PubMed] [Google Scholar]
- 11.Ruff P, Van Cutsem E, Lakomy R et al. Observed benefit and safety of aflibercept in elderly patients with metastatic colorectal cancer: An age‐based analysis from the randomized placebo‐controlled phase III VELOUR trial. J Geriatr Oncol 2018;9:32–39. [DOI] [PubMed] [Google Scholar]
- 12.Feliu J, de Corcuera ID, Manzano JL et al. Effectiveness and safety of aflibercept for metastatic colorectal cancer: Retrospective review within an early access program in Spain. Clin Transl Oncol 2017;19:498–507. [DOI] [PubMed] [Google Scholar]
- 13.Tampellini M, Di Maio M, Baratelli C et al. Treatment of patients with metastatic colorectal cancer in a real‐world scenario: Probability of receiving second and further lines of therapy and description of clinical benefit. Clin Colorectal Cancer 2017;16:372–376. [DOI] [PubMed] [Google Scholar]
- 14.Jeon EK, Hong SH, Kim TH et al. Modified FOLFIRI as second‐line chemotherapy after failure of modified FOLFOX‐4 in advanced gastric cancer. Cancer Res Treat 2011;43:148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KP, Kim TW, Hong YS et al. Modified FOLFIRI (Irinotecan 150 mg/m2) compared to FOLFIRI (Irinotecan 180 mg/m2) in Korean patients with gastrointestinal cancer. J Clin Oncol 2015;33(suppl 15):3600A. [Google Scholar]
- 16.Visa L, Jiménez‐Fonseca P, Martínez EA et al. Efficacy and safety of chemotherapy in older versus non‐older patients with advanced gastric cancer: A real‐world data, non‐inferiority analysis. J Geriatr Oncol 2018;9:254–264. [DOI] [PubMed] [Google Scholar]
- 17.De Divitiis C, Nasti G, Montano M et al. Prognostic and predictive response factors in colorectal cancer patients: Between hope and reality. World J Gastroenterol 2014;20:15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 19.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. National Cancer Institute Web site. https://evs.nci.nih.gov/ftp1/CTCAE/About.html. Published 2009. Accessed April 27, 2018.
- 20.Liu Y, De A. Multiple imputation by fully conditional specification for dealing with missing data in a large epidemiologic study. Int J Stat Med Res 2015;4:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peduzzi P, Concato J, Kemper E et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373–1379. [DOI] [PubMed] [Google Scholar]
- 22.RStudio: Integrated development environment for R. Boston, MA: RStudio, Inc.; 2015.
- 23.Therneau TM, Lumley T. Package ‘survival.’ 2016.
- 24.De Wreede LC, Fiocco M, Putter H. The mstate package for estimation and prediction in non‐and semi‐parametric multi‐state and competing risks models. Comput Methods Programs Biomed 2010;99:261–274. [DOI] [PubMed] [Google Scholar]
- 25.Ivanova JI, Saverno KR, Sung J et al. Real‐world treatment patterns and effectiveness among patients with metastatic colorectal cancer treated with ziv‐aflibercept in community oncology practices in the USA. Med Oncol 2017;34:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pastorino A, Di Bartolomeo M, Maiello E et al. Aflibercept plus FOLFIRI in the real‐life setting: Safety and quality of life data from the Italian patient cohort of the Aflibercept Safety and Quality‐of‐Life Program Study. Clin Colorectal Cancer 2018;17:e457–e470. [DOI] [PubMed] [Google Scholar]
- 27.Chau I, Joulain F, Iqbal SU et al. A VELOUR post hoc subset analysis: Prognostic groups and treatment outcomes in patients with metastatic colorectal cancer treated with aflibercept and FOLFIRI. BMC Cancer 2014;14:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johanna L, Monika C, Henrik A et al. Serum HCGβ, CA 72‐4 and CEA are independent prognostic factors in colorectal cancer. Int J Cancer 2002;101:545–548. [DOI] [PubMed] [Google Scholar]
- 29.Byun JH, Ahn JB, Kim SY et al. The impact of primary tumor location in patients with metastatic colorectal cancer: A Korean Cancer Study Group CO12‐04 study. Korean J Intern Med 2019;34:165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bläker H, Alwers E, Arnold A et al. The association between mutations in BRAF and colorectal cancer‐specific survival depends on microsatellite status and tumor stage. Clin Gastroenterol Hepatol 2019;17:455–462.e6. [DOI] [PubMed] [Google Scholar]
- 31.Wirapati P, Pomella V, Vandenbosch B et al. Velour trial biomarkers update: Impact of RAS, BRAF, and sidedness on aflibercept activity. J Clin Oncol 2017;35(suppl 15):3538A.28862883 [Google Scholar]