Table 3. Adverse events in the whole cohort (n = 250).
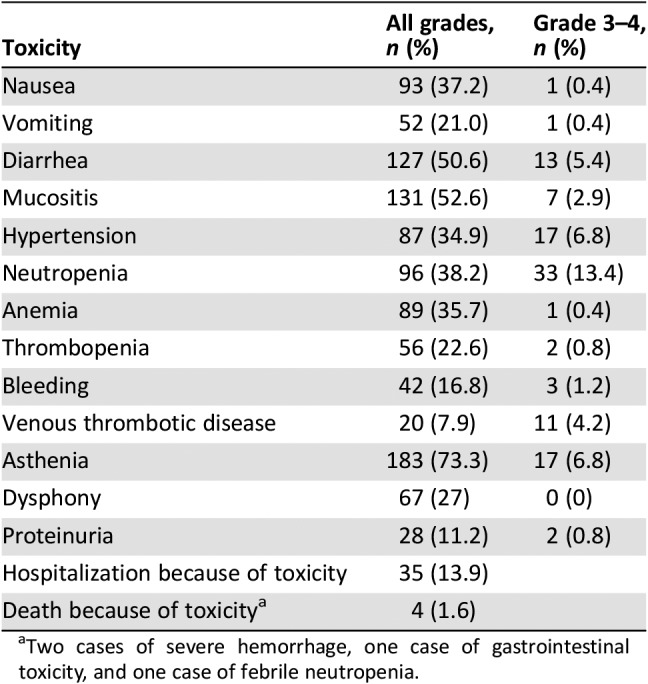
Two cases of severe hemorrhage, one case of gastrointestinal toxicity, and one case of febrile neutropenia.

Two cases of severe hemorrhage, one case of gastrointestinal toxicity, and one case of febrile neutropenia.