Predictive markers for immune‐related adverse events (irAEs) are not available. This article evaluates the association of peripheral blood markers with the onset of irAEs in patients with non‐oncogene addicted advanced non‐small cell lung cancer treated with immune‐checkpoint inhibitors.
Keywords: Lung cancer, Immunotherapy, Immune‐related adverse events, Platelet‐to‐lymphocyte ratio, Neutrophil‐to‐lymphocyte ratio, Predictive markers
Abstract
Background.
Immune‐checkpoint inhibitors (ICIs) are now standard of care for advanced non‐small cell lung cancer (NSCLC). Unfortunately, many patients experience immune‐related adverse events (irAEs), which are usually mild and reversible, but they require timely management and may be life threatening. No predictive markers of irAEs are available.
Materials and Methods.
The neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) were evaluated in patients with NSCLC consecutively treated with ICIs. Prespecified cutoff values of NLR and PLR were used and related to outcome and onset of irAEs. A control group of patients with advanced NSCLC not receiving ICIs was included.
Results.
The study included 184 patients: 26 (14.1%) received pembrolizumab upfront, and 142 (77%) received ICIs (pembrolizumab, nivolumab or atezolizumab) after one or more lines of chemotherapy. The median number of ICIs cycles was six (range, 1–61). The median progression‐free survival and overall survival were 4.8 (95% CI, 3.4–6.3) and 20.6 (95% CI, 14.7–26.5) months, respectively. Sixty patients (32.6%) developed irAEs, mainly grade 1–2 (65.0%), causing ICI interruption in 46 cases (25.0%). Low NLR and low PLR at baseline were significantly associated with the development of irAEs (odds ratio [OR], 2.2; p = .018 and OR, 2.8; p = .003, respectively). Multivariate analyses confirmed PLR as independent predictive marker of irAEs (OR, 2.3; p = .020).
Conclusion.
NLR and PLR may predict the appearance of irAEs in non‐oncogene‐addicted aNSCLC, although this conclusion warrants prospective validation.
Implications for Practice.
This study was designed to investigate the role of blood biomarkers in predicting the occurrence of immune‐related adverse events (irAEs) in patients with advanced non‐small cell lung cancer receiving immunotherapy. The results of the study suggest a potential predictive role of neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio as markers for irAE development in this category of patients. These data provide rationale for an easy and feasible application to be validated in clinical practice.
摘要
背景。免疫检查点抑制剂 (ICI) 药物目前是治疗晚期非小细胞肺癌 (NSCLC) 的标准药物。不幸的是,许多患者会出现免疫相关不良事件 (irAE),虽然此类事件通常并不严重而且可逆,但需要及时治疗而且可能会危及生命。目前还没有 irAE 的预测标志物。
材料和方法。评估连续接受 ICI 治疗的 NSCLC 患者的中性粒细胞淋巴细胞比 (NLR) 和血小板淋巴细胞比 (PLR)。使用预先指定的 NLR 和 PLR 临界值,该值与 irAE 结局和发病相关。对照组选择未接受 ICI 治疗的晚期 NSCLC 患者。
结果。研究包括 184 名患者:26 例 (14.1%) 预先接受帕博利珠单抗 (Pembrolizumab) 治疗,142 例 (77%) 在经过一线或多线化疗之后接受 ICI(帕博利珠单抗、纳武单抗或阿特朱单抗)治疗。中位ICI 周期为 6(范围:1‐61)。中位无进展生存期和总生存期分别为 4.8 个月(95% CI, 3.4‐6.3) 和 20.6 个月 (95% CI, 14.7‐26.5)。60 例 (32.6%) 患者发生 irAE,以 1‐2 级为主 (65.0%),其中 46 例 (25.0%) 中断 ICI 治疗。基线 NLR 低和 PLR 低与 irAE 的发生显著相关 [分别为:比值比 (OR), 2.2;p = 0.018, OR, 2.8; p = 0.003]。多变量分析证实 PLR 是 irAE 的独立预测标志物 (OR, 2.3; p = 0.020)。
结论。NLR 和 PLR 可能预测非嗜癌性 aNSCLC 中是否会出现 irAE,但这一结论仍需接受前瞻性验证。
实践意义:本研究旨在探讨血液生物标志物在预测晚期非小细胞肺癌患者免疫相关不良事件 (irAE) 发生中的作用。研究结果表明,中性粒细胞淋巴细胞比和血小板淋巴细胞比是预测此类患者是否发生 irAE 的潜在预测标志物。这些数据为简单可行的应用提供了理论依据,有待临床实践验证。
Introduction
In the latest years, immunotherapy has rapidly become one of the mainstays of modern oncology. In particular, immune‐checkpoints inhibitors (ICIs) have radically changed the treatment of non‐oncogene‐addicted advanced non‐small cell lung cancer (aNSCLC). Nivolumab was the first drug demonstrating a significant benefit over standard chemotherapy for previously treated patients with aNSCLC, whereas pembrolizumab is now considered the preferred first‐line treatment for patients with strong expression of PD‐L1 in tumor cells [1], [2], [3]. Soon after, the combination of chemotherapy with ICIs has demonstrated clear superiority compared with chemotherapy alone irrespectively of PD‐L1 status, further reshaping the therapeutic landscape and increasing the percentage of patients who may benefit from immunotherapy [4], [5], [6], [7].
As real‐world experience with ICIs continues to confirm that a subset of patients may achieve remarkable and durable responses, no reliable predictive markers are available yet. This would be crucial for a number of reasons. Actually, the identification of predictive biomarkers would ease optimal treatment choice especially for patients who might not have the chance of further treatment lines or may not tolerate combination strategies; it would avoid unnecessary toxicities in patients with minimal chance to respond, and it would save health care costs. PD‐L1 tumor proportion score (TPS) has shown limitations as a predictive biomarker, especially for pretreated patients [8]. On the other side, circulating markers, such as neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR), have been suggested as relevant in predicting clinical benefit in non‐small cell lung cancer, as well as in other malignancies [9], [10], [11], [12]. Both these ratios seem to reflect the balance between nonspecific inflammation and immunoreaction, potentially impacting on the response to ICIs.
Although ICIs are better tolerated compared with conventional chemotherapy, there is a spectrum of unique adverse events, known as immune‐related adverse events (irAEs), caused by activation of an immune response against healthy tissues, that need close monitoring and specific management [13], [14]. The majority of irAEs are mild and manageable when rapidly recognized and properly treated. Nevertheless, these specific toxicities may require long‐term steroids or endocrine replacement therapy, and in selected cases they cause hospitalization. No markers are available to predict the onset and severity of irAEs, although baseline serum proteomic profiling and in particular soluble CD163 and CXCL5 may have a role in melanoma [12], [15], [16], [17].
The aim of the present study is to evaluate the association of peripheral blood markers (NLR and PLR) with the onset of irAEs in patients with aNSCLC treated with ICIs.
Materials and Methods
Patients
Consecutive patients with aNSCLC treated at the Veneto Institute of Oncology (Padova, Italy) and at the Department of Oncology, San Bortolo General Hospital (Vicenza, Italy), between August 2013 and April 2018 were retrospectively reviewed. Inclusion criteria were availability of clinical database, adequate follow‐up, and treatment with ICIs according to clinical practice. A minimum follow‐up time of 3 months was required for the inclusion in the study because of the higher probability of developing irAEs within the first 12 weeks [14].
Since June 2017, patients with PD‐L1 TPS ≥50% received pembrolizumab flat dose (200 mg) every 3 weeks as first‐line therapy. The treatment was administered for a maximum of 2 years until radiological or clinical progression, unacceptable toxicity, or death from any cause. Previously treated patients received nivolumab 3 mg/kg administered every 2 weeks, pembrolizumab 2 mg/kg every 3 weeks, or atezolizumab 1,200 mg every 3 weeks until radiological progression, unacceptable toxicity, or death from any cause. Patients with immune‐related comorbidities, baseline pulmonary interstitial diseases, or acute or chronic hepatitis B or C viral infections were excluded.
As internal validation, we evaluated a control group of patients with aNSCLC consecutively referring to the Veneto Institute of Oncology between January 2014 and December 2016, treated with systemic treatment (first‐line treatment with platinum‐based chemotherapy) and not receiving ICIs.
Patients’ data collected at baseline included patient demographics, Eastern Cooperative Oncology Group performance status (ECOG PS) at time of ICI start, smoking history, and comorbidities. Tumor data collected included histology and molecular results for EGFR, ALK, MET, HER‐2, K‐RAS, ROS‐1, BRAF, and PD‐L1 status, when available. Radiological imaging performed before the start of ICIs and during treatment was reviewed. Toxicity data reported by treating physician during and after treatment with ICIs were recorded. Baseline blood counts data (defined as the most recent blood count within 1 week before ICI initiation and including absolute neutrophil count, absolute lymphocyte count, and platelet count) were used to calculate NLR (absolute neutrophil count/absolute lymphocyte count) and PLR (platelet count/lymphocyte count).
The ethics committees of the two institutions approved the study. Signed informed consent was obtained, whenever feasible, for collection, analysis, and publication of data, according to the Italian data protection authority dispositions. The study was performed in accordance with the Declaration of Helsinki.
Statistical Analysis
The primary aim was to evaluate the association between peripheral blood markers and the onset of irAEs.
Secondary aims were to evaluate the impact of NLR and PLR on outcome in terms of radiological response (RR), overall survival (OS), and progression‐free survival (PFS).
IrAEs were defined as AEs possibly related to an immune dysregulation and requiring frequent monitoring or specific treatment with immune suppression and/or endocrine replacement therapy.
RR was assessed according to RECIST version 1.1; disease control rate (DCR) was defined as complete response plus partial response plus stable disease. Radiological response rate was defined as partial response plus complete response.
PFS was calculated from the first day of treatment with ICIs to the first sign of disease progression or death. OS was calculated from the first day of treatment with ICIs to death from any cause. For the control group, we considered the beginning of first‐line therapy as the beginning time for survival analyses.
Patients were dichotomized according to prespecified cutoff values of NLR ≥3 (high NLR [H‐NLR]) versus <3 (low NLR [L‐NLR]) [9], [18] and PLR ≥180 (high PLR [H‐PLR]) versus <180 (low PLR [L‐PLR]) [19]. The cutoffs were chosen according to literature references [9], [18], [19].
Variables were presented by using median value for continuous variables and percentages (numbers) for categorical variables, and their relationship with occurrence of irAE was assessed using the Mann‐Whitney test and the chi‐squared test as appropriate.
The association between NLR and PLR and the onset of irAEs was analyzed by univariate and multivariable logistic regression models, and results were reported using odds ratio (OR) with 95% confidence interval (CI). The median PFS and OS were estimated by using Kaplan‐Meier methods, and the log‐rank test was used to compare survival between groups. Hazard ratios (HRs) and 95% CI were calculated with the Cox regression method. Statistical significance level was set at p < .05 for all tests. All statistical analyses were performed with SPSS 20.0 software (SPSS Inc., Chicago, IL).
Results
Study Population and Outcome
A total of 184 patients with aNSCLC treated with ICIs were included. Patients were predominantly male (68%), smokers (87%) and had a good PS (ECOG PS of 0 or 1 in 83% of cases). At the time of analysis, the median follow‐up time was 56.3 months (range, 3.4–59.2 months). Seventy‐nine patients not receiving ICIs were evaluated as control group.
Table 1 summarizes patients’ clinical features.
Table 1. Clinical features and treatment of the study population.
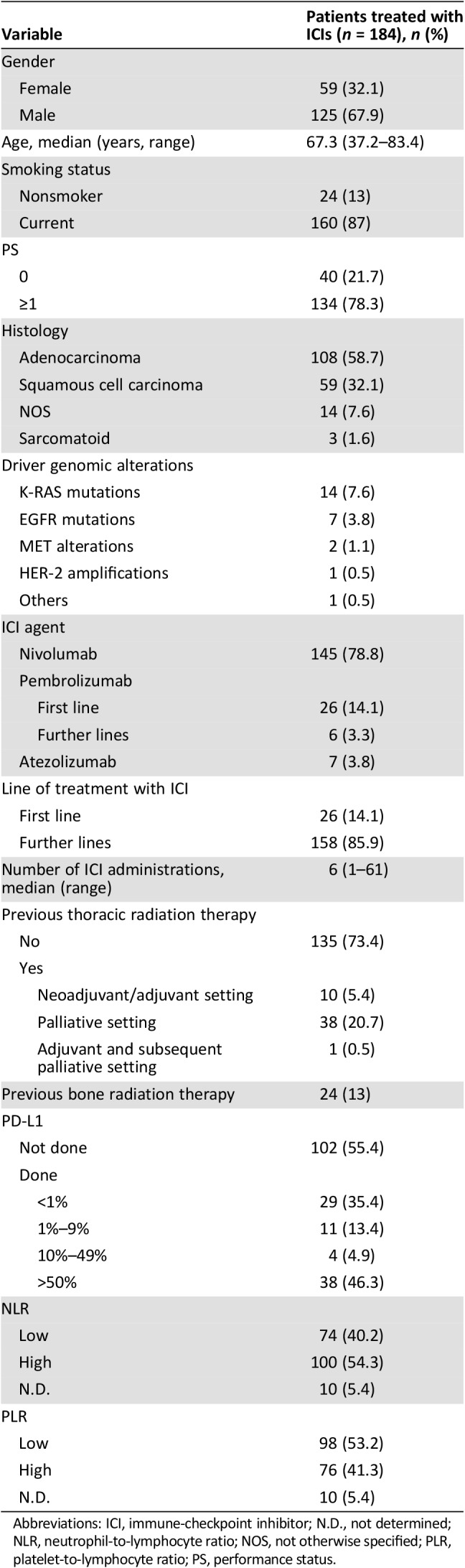
Abbreviations: ICI, immune‐checkpoint inhibitor; N.D., not determined; NLR, neutrophil‐to‐lymphocyte ratio; NOS, not otherwise specified; PLR, platelet‐to‐lymphocyte ratio; PS, performance status.
Twenty‐six patients diagnosed with non‐small cell lung cancer with PD‐L1 TPS ≥50% received pembrolizumab as first‐line treatment. Other patients were treated with nivolumab (142 patients, 78.8%), atezolizumab (7 patients, 3.8%), and pembrolizumab (6 patients, 3.2%) mainly in second‐ or third‐line setting (142 patients, 77.2%). Except for the ones treated with pembrolizumab, patients were not selected for PD‐L1 expression. The median number of ICI administrations was 6 (range, 1–61); 65.2% of patients discontinued ICI because of disease progression (120 out of 184 patients). The only other reason for ICI permanent discontinuation was the development of irAE (see the subsection on Immune‐Related Adverse Events).
One patient achieved complete response (0.5%), 44 patients (23.9%) achieved partial response, 53 (28.8%) patients experienced stable disease, and 86 (46.7%) had progressive disease as best radiological response. DCR was 53.3%. The median PFS was 4.8 months (95% CI, 3.4–6.3 months), and median OS 20.6 months (95% CI, 14.7–26.5 months).
In univariate analysis, patients’ PS was the only clinical feature that had significant impact both on OS (HR, 2.305; 95% CI, 1.642–3.236; p < .001) and on PFS (HR, 2.254; 95% CI, 1.600–3.177; p < .001; supplemental online Table 1). A higher number of treatments for advanced disease before ICIs administration had a significant association only with OS (HR, 0.611; 95% CI, 0.481–0.776; p < .001), possibly because of selection bias. In multivariate analysis patients’ PS confirmed its significant impact both on PFS (HR, 1.721; 95% CI, 1.202–2.466; p = .003) and on OS (HR, 1.616; 95% CI, 1.125–2.320; p = .009; data not shown).
The median PFS among patients treated with first‐line ICI was 4.1 months (95% CI, 1.4–6.9 months), and median OS was 36.4 months (95% CI, not evaluable). Patients who received ICIs after progression on platinum‐based chemotherapy had a median PFS of 4.8 months (95% CI, 3.0–6.7 months) and a median OS of 20.9 months (95% CI, 15.1–26.9 months).
Interestingly, in this subset of patients, PD‐L1 expression on tumor cells, both as continuous and as dichotomized variable (positivity defined as PD‐L1 TPS ≥1%), had no impact on outcome, in terms of DCR, PFS, and OS (data not shown).
Immune‐Related Adverse Events
Sixty patients (32.6%) experienced a total of seven different irAE categories (Table 2). Baseline clinical features between patients with or without irAE were not significantly different (chi‐square test; supplemental online Table 2). The median number of ICI administrations was four (range, 1–49), and the median number of weeks before the onset of any irAEs was 12.3 (range, 1.0–107.3 weeks). Twenty‐five patients (41.7%) developed any irAEs within 12 weeks from the first ICI administration.
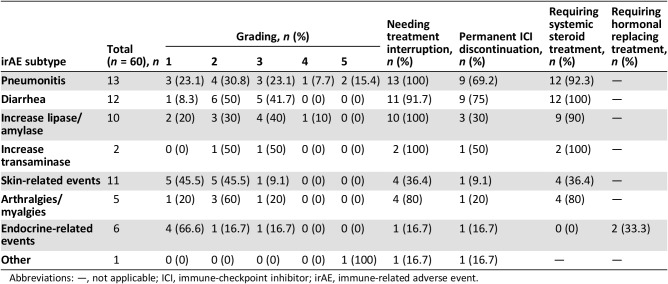
Table 2. Immune‐related adverse events of the study population.
Abbreviations: —, not applicable; ICI, immune‐checkpoint inhibitor; irAE, immune‐related adverse event.
Five patients (19.2%) receiving first‐line pembrolizumab and 55 patients (34.8%) receiving ICIs in further lines experienced irAEs. Distribution of irAEs, severity of irAEs, and number of ICI administrations before the onset of any irAEs did not significantly differ between these two groups of patients (p = .116: chi square test, p = .178 Mann‐Whitney test and p = .196, Mann‐Whitney test, respectively).
IrAEs were mainly mild (grade 1–2 in 65% of cases). Nevertheless, we retrieved two serious toxicities (one case of grade 4 asymptomatic serum increase of pancreatic enzyme and one case of grade 4 immune‐related pneumonitis) and three fatal irAEs (two immune‐related pneumonitis and one treatment‐related cardiac event).
IrAEs caused treatment interruption in 46 cases (76.7%), the discontinuation was permanent in 26 of them. These patients had a median time to progressive disease of 17.8 months (95% CI, 2.4–33.3 months). irAEs requiring specific medication were 45 (75%): 43 patients required systemic steroidal treatment because of pneumonitis (n = 12), diarrhea (n = 12), asymptomatic serum increase of pancreatic (n = 9) or liver enzymes (n = 2), arthralgia (n = 4), or cutaneous rash (n = 4); one patient with endocrine irAE needed hormonal replacement therapy, and one started thyrostatic treatment for hyperthyroidism.
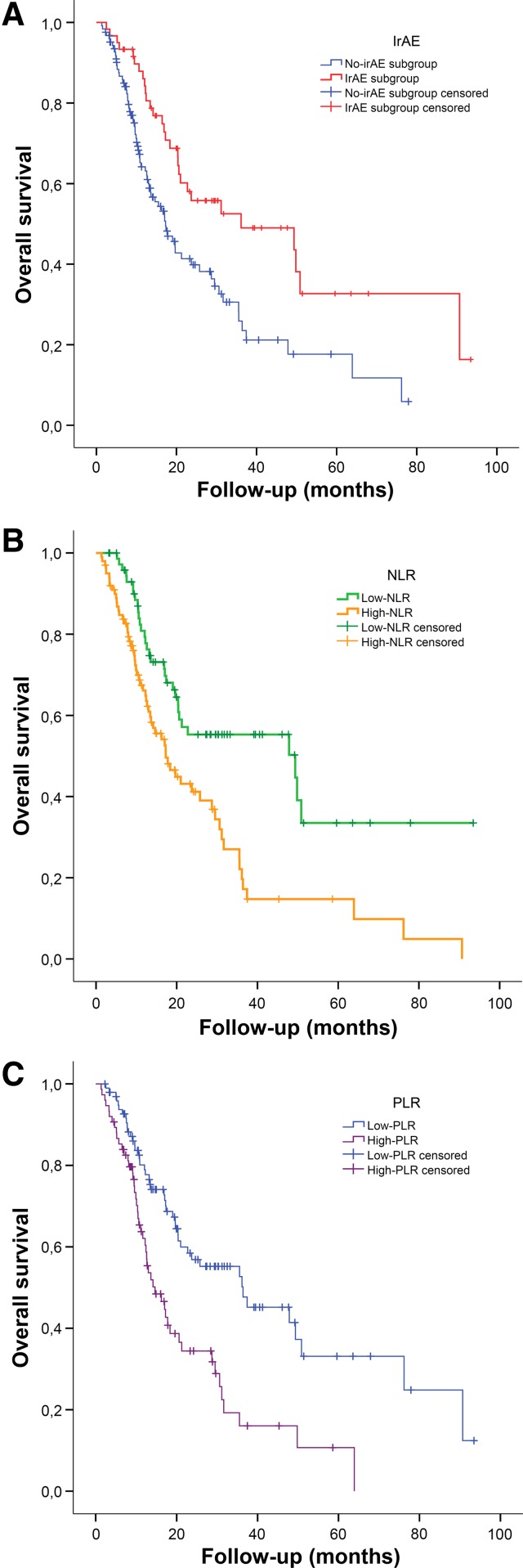
Patients who developed any irAE had a significantly better DCR compared with non‐irAE patients (68.3% vs. 46.8%; p = .008; supplemental online Table 3). The median PFS among irAE patients (8.8 months; 95% CI, 2.5–15 months) was longer than in non‐irAE patients (3.1 months; 95% CI, 1–4.1 months; p < .001; HR, 0.488; 95% CI, 0.325–0.735; p = .001; supplemental online Table 1). Similarly, patients with irAE had a better median OS (36.1 months; 95% CI, 4.7–67.5 months) than the non‐irAE counterpart (17.3 months; 95% CI, 12.9–21.7; p = .002; HR, 0.497; 95% CI, 0.320–0.773; p = .002; Fig. 1A; supplemental online Table 1). Among irAE patients, neither the subtype of adverse event, its grading (except for G5), nor the need of permanent interruption of ICI administration had any significant impact either on PFS or on OS.
Figure 1.
Overall survival of study population. Overall survival according to onset of immune‐related adverse events (A), neutrophil‐to‐lymphocyte ratio (B), and platelet‐to‐lymphocyte ratio (C).
Abbreviations: irAE, immune‐related adverse event; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio.
NLR and PLR Analyses
Baseline complete blood cell counts were available for 174 (94.6%) out of 184 patients treated with ICIs. Among 79 patients treated exclusively with chemotherapy, 57 (72.2%) had baseline complete blood counts available for NLR and PLR calculation.
Among patients treated with ICIs, 100 (57.5%) out 174 had an NLR ≥3 (H‐NLR), and 76 (43.7%) had a PLR ≥180 (H‐PLR).
L‐NLR and L‐PLR were significantly related with a better PS (p < .001 and p = .007, chi‐square test).
Distribution of NLR and PLR values significantly differed between patients treated with ICIs in first‐line setting rather than in further lines: median NLR of 4.50 (range, 1.98–29.48) versus 3.25 (range, 0.78–20.86; p = .007, Mann‐Whitney test), respectively, and median PLR of 239.47 (range, 92.24–537.14) versus 160.78 (range, 36.99–730.23; p = .005, Mann‐Whitney test) were observed.
Among patients with available data on PD‐L1 TPS (82 patients, 44.6%), H‐NLR showed a correlation with PD‐L1 ≥ 1% (p = .049, chi‐square test), whereas PLR showed no correlation with PD‐L1 expression on tumor cells.
Predictive Markers for irAEs
We first investigated the association of irAEs onset with baseline clinical features. Worse PS was not related with higher risk for irAEs. Increased number of cycles of treatments was not significantly associated with higher probability of developing irAEs (OR, 1.336; 95% CI, 1.009–1.785; p = .05). No association was found between PD‐L1 expression and irAEs onset (p = .481, chi‐square test). In our study population 49 patients (26.6%) received radiation therapy on thoracic field and 24 patients (13%) were treated with radiotherapy on lumbosacral or hips bone lesions. In our experience, none of these treatments affected the overall risk of irAEs (p = .716 and p = .935, respectively, chi‐square test). This was confirmed also when we considered the impact of thoracic radiotherapy on immune‐related pneumonitis and the impact of bone radiotherapy on diarrhea (p = .107 and p = .704, respectively, chi‐square test).
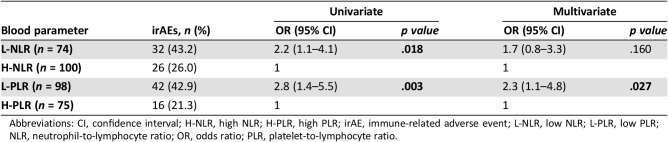
The occurrence of any irAE was associated with baseline L‐NLR and L‐PLR. OR for L‐NLR was 2.2 (95% CI, 1.1–4.1; p = .018), and OR for L‐PLR was 2.8 (95% CI, 1.4–5.5; p = .003). Multivariate model confirmed only L‐PLR as independent predictive factor (OR, 2.3; 95% CI, 1.1–4.8; p = .027; Table 3).
Table 3. Correlation between NLR and PLR and irAE development.
Abbreviations: CI, confidence interval; H‐NLR, high NLR; H‐PLR, high PLR; irAE, immune‐related adverse event; L‐NLR, low NLR; L‐PLR, low PLR; NLR, neutrophil‐to‐lymphocyte ratio; OR, odds ratio; PLR, platelet‐to‐lymphocyte ratio.
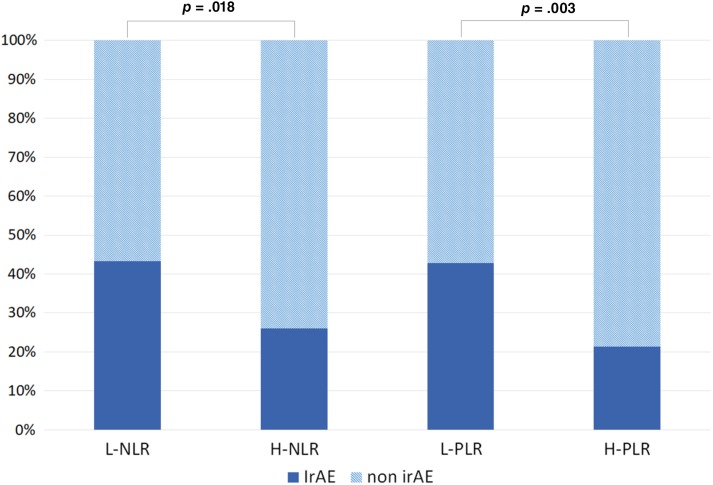
Among patient with L‐PLR the percentage of patients who developed irAE was 42.9% versus 21.3% among patients with H‐PLR. 48% of patients with baseline L‐PLR and L‐NLR experienced irAEs (Fig. 2). In order to exclude the potential confounding effect of PLR impact on survival, the cumulative incidence of irAE was estimated using a competing risk analysis, accounting for death as a competing risk, and the impact of PLR on irAE development was still significant (p = .005, K‐sample test; supplemental online Fig. 2).
Figure 2.
Distribution of irAE development according to baseline dichotomized neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio.
Abbreviations: H‐NLR, high neutrophil‐to‐lymphocyte ratio; H‐PLR, high platelet‐to‐lymphocyte ratio; irAE, immune‐related adverse event; L‐NLR, low neutrophil‐to‐lymphocyte ratio; L‐PLR, low platelet‐to‐lymphocyte ratio.
Among patients experiencing any irAE, 72.4% had PLR lower than 180 (p = .003, chi‐square test; data not shown).
The number of patients included in each subgroup experiencing a different kind of toxicity was too low to draw any conclusion about the potential specific effect of predictive biomarkers on each irAE.
NLR and Outcome
Patients with baseline L‐NLR had a better DCR than the ones with H‐NLR (67.6% vs. 43.4%; p = .002; supplemental online Table 3).
The median PFS was longer for patients with L‐NLR (7.4 months; 95% CI, 5.0–9.8 months) compared with H‐NLR ones (3.1 months; 95% CI, 2.2–3.9 months; p = .003; HR, 0.557; 95% CI, 0.378–0.820; p = .003; supplemental online Table 1). Likewise, median OS was significantly longer for patients with NLR <3 (49.3 months; 95% CI, 7.4–91.3 months) than for patients with NLR ≥3 (17.3 months; 95% CI, 12.1–22.5 months; p < .001; HR, 0.468; 95% CI, 0.304–0.720; p = .001; Fig. 1B; supplemental online Table 1).
This impact on patients’ survival was confirmed also in multivariate analysis (HR, 1.149; 95% CI, 1.080–1.186; p < .001), including PS, number of previous treatments, and the occurrence of irAE as covariates (supplemental online Table 1).
For patients who received pembrolizumab upfront NLR dichotomization using 3 as cutoff value did not have significant impact on outcome, probably because of the low numbers of events observed.
Patients eligible for ICIs after previous systemic treatment showed a significant relationship between baseline NLR and survival outcome: 8.3 months (95% CI, 4.4–12.3 months) of median PFS for patients with L‐NLR versus 2.9 months (95% CI, 1.8–4.1 months) of H‐NLR ones (p = .001, log‐rank; HR, 0.486; 95% CI, 0.310–0.762; p = .002) and 22.7 months (95% CI, 0.3–45.2 months) of median OS for patients with L‐NLR versus 17.8 months (95% CI, 11.9–23.6 months) of H‐NLR ones (p = .016, log‐rank; HR, 0.568; 95% CI, 0.357–0.904; p = .017).
PLR and Outcome
PLR levels did not show statistically significant association either with DCR (supplementary online Table 3) or with radiological response rate. H‐PLR patients had a response rate of 23% versus 28% for L‐PLR ones (p = .350). Patients with H‐PLR had significantly shorter PFS than those with L‐PLR. The median PFS was 2.9 months (95% CI, 1.9–4.0 months) in patients with H‐PLR versus 7.3 months (95% CI, 4.4–10.2 months) in patients with L‐PLR (p = .004); HR was 1.709 (95% CI, 1.178–2.478; p = .005; supplemental online Table 1). A similar impact was observed on median overall survival: patients with H‐PLR had 14.7 months (95% CI, 9.6–19.7 months), whereas L‐PLR ones had 36.4 months (95% CI, 16.4–56.4 months; log‐rank p < .001; HR, 2.239; 95% CI, 1.478–3.392; p < .001) of median OS (Fig. 1C; supplemental online Table 1).
No effect of PLR on OS was observed at multivariate analysis including PS, number of previous treatments, irAE and NLR as covariates, whereas L‐NLR confirmed its predictive value with an HR of 1.098 (95% CI, 1.032–1.169; p = .003; supplemental online Table 1).
PLR had no significant impact on the outcome of patients eligible for upfront treatment with ICIs. On the contrary, the median PFS for patients receiving ICIs after previous systemic treatment was 7.9 months (95% CI, 5.2–10.7 months) for patients with L‐PLR versus 2.8 months (95% CI, 2.1–3.5 months) for those with H‐PLR (p < .001, log‐rank; HR, 0.497, 95% CI, 0.333–0.742; p = .001). The median OS was 36.1 months (95% CI, 13.9–58.4 months) for patients with L‐PLR versus 16.2 months (95% CI, 11.7–20.8 months) for H‐PLR ones (p = .001, log‐rank; HR, 0.473; 95% CI, 0.307–0.729; p = .001).
Control Group: Effect of Peripheral Blood Markers on Outcome
Baseline characteristics of control group patients are summarized in supplemental online Table 4.
Patients received a median of one line of chemotherapy (range, 1–3); median PFS and median OS were 4.9 months (95% CI, 4.0–5.7 months) and 9.6 months (95% CI, 7.4–11.9 months), respectively. NLR affected the PFS of patients treated with chemotherapy but had no significant impact on OS.
Patients with H‐NLR had a median PFS of 4.3 months (95% CI, 3.4–5.3 months) versus 5.9 months for L‐NLR (95% CI, 2.9–9.9 months; log‐rank p = .033). The median OS was 7.5 months (95% CI, 5.8–9.1 months) for the H‐NLR group and 12 months for L‐NLR one (95% CI, 6.9–17.2 months; log‐rank p = .92; supplemental online Fig. 1A).
PLR had no effect on outcome. Patients with H‐PLR had a similar PFS (4.6 months; 95% CI, 3.6–5.7 months) to those with L‐PLR (5.3 months; 95% CI, 3.6–6.9 months; p = .100). The median OS was 7.1 months (95% CI, 3.1–11.1 months) for the H‐PLR group and 10.1 months for L‐PLR one (95% CI, 5.6–16.2 months; log‐rank p = .318; supplemental online Fig. 1B). No correlation was observed between NLR or PLR and DCR.
Discussion
The role of immunotherapy in the treatment of aNSCLC is rapidly increasing and its introduction in clinical practice has changed the clinicians’ perspectives on treatment and outcome of patients with non‐oncogene‐addicted aNSCLC. Recent data suggest that the majority of patients with aNSCLC will receive ICIs as first‐line treatment in the near future, mainly in combination with chemotherapy [4], [5], [6], [7].
Better toxicity profile and improved quality of life have been reported for ICIs in monotherapy compared with standard chemotherapy [1], [2], [3], whereas their addition to platinum‐based doublet increases toxicity with respect to chemotherapy alone [4], [6]. Rapid detection and correct management are crucial for a proper management of irAE. In addition, the time of irAE presentation may be delayed, potentially requiring specific monitoring even after the conclusion of active treatment [20], [21]. Sometimes, and especially when not treated timely, irAEs require hospitalization, thus increasing the treatment costs significantly.
With the exception of the presence of preexisting immune disorders, no other predictive markers are currently used to predict the risk of irAEs [12]. On the other hand, several reports are consistent in describing a correlation between immune‐related toxicity and clinical benefit from ICIs administration [22], [23], [24], [25]. The biological explanation for this phenomenon is unclear.
Our study represents a real‐life observation concerning the onset and management of irAEs in aNSCLC in clinical practice and has the primary aim to identify circulating markers able to predict the onset of irAEs.
In our study population, the rate of irAE was similar to that reported in large real‐life studies [26], [27], and our data confirmed the association of irAEs with improved outcomes in aNSCLC [22]. Using the published cutoff of NLR and PLR, a correlation with the risk of irAEs was demonstrated, although only PLR maintained a significant correlation at multivariate analysis [9], [18], [19]. Two studies conducted in patients with aNSCLC treated with nivolumab used 5 as cutoff for NLR because of the higher median value of patients’ baseline NLR [28], [29]. A higher proportion of patients with poor PS might explain this discrepancy, taking into account our data suggesting that a lower NLR is significantly related to a better PS (in the first study patients with ECOG PS >1 were 25% vs. 16% in our population; in the second one only 45 patients had information about PS status).
Mechanisms of immune‐related toxicity are not fully clarified. ICIs may unmask low‐level self‐reacting T cells, but macrophage‐mediated toxicity and production of antibodies by activated B‐cells are also plausible [30]. In a recent collection of pathological samples from patients who developed gastrointestinal immune‐mediated toxicity, the main feature was CD8‐positive T‐cell infiltration [31]. In addition, in pancreatic cancer, elevated levels of NLR were associated with elevated levels of peripheral blood regulatory T cells, whose role in immune tolerance is well known [32]. These observations lay the basis for studying biological rationale for our observation about predictive role of NLR and PLR.
Moreover, the observation that patients treated with ICIs in first‐line setting have higher NLR and PLR suggests that prior chemotherapy might have an impact on the balance between nonspecific inflammation and immunoreaction.
We acknowledge the limitations of our study, mainly because of its retrospective nature. Nevertheless, our data derive from a relatively large series of consecutively treated patients, reflecting a real‐world scenario and including a control group of patients treated without ICIs, to evaluate the potential prognostic role of NLR in aNSCLC. The results found in the control group are overall consistent with recently published data [11], although they differ from what has been previously observed in other malignancies, in which prognostic role of NLR has been observed among patients treated with chemotherapy [33], [34], [35], [36]. Using a different cutoff of 3.7, Berardi and colleagues demonstrated NLR to be an independent prognostic factor for both PFS and OS in patients with non‐small cell lung cancer treated with first‐line therapies, including chemotherapy and targeted therapies [37].
The possibility of predicting the onset of irAEs has great relevance in clinical practice, because it may impact the clinical monitoring during treatment and after the conclusion of ICIs administration. The identification of patients at higher risk of irAEs will be more and more important when considering the possible future introduction of immunotherapy in adjuvant setting (several trials are currently ongoing), the future availability of flat‐dose monthly schedule administration for nivolumab, and the introduction of combination strategies in clinical practice [38]. If our results are further validated, baseline PLR may be used as a tool to identify patients that require more frequent clinical monitoring. The timely identification of irAEs is thus essential for a proper management and to reduce the risk of hospitalization and, consequently, reduce the costs of treatment. Finally, the probability of irAEs should be considered when selecting frail patients for combination strategies including chemotherapy and immunotherapy in first‐line settings.
Conclusion
We have demonstrated an association between baseline PLR and the probability of irAEs in a real‐life scenario of aNSCLC. These results may have relevant impact in the management of patients with aNSCLC in clinical practice, especially for clinically selected subpopulations, and warrant further prospective validation and confirmation in patients treated with chemotherapy and immunotherapy.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Footnotes
For Further Reading: Narjust Duma, Azzouqa Abdel‐Ghani, Siddhartha Yadav et al. Sex Differences in Tolerability to Anti‐Programmed Cell Death Protein 1 Therapy in Patients with Metastatic Melanoma and Non‐Small Cell Lung Cancer: Are We All Equal? The Oncologist first published on April 29, 2019; doi:10.1634/theoncologist.2019‐0094.
Implications for Practice: The results of this study suggest that women may be at a higher risk for immune‐related adverse events (irAEs) compared with men when treated with anti‐programmed cell death protein 1 therapy. In addition, women were more likely to develop certain irAEs, including endocrinopathies and pneumonitis. Close follow‐up of women undergoing treatment with immune checkpoint inhibitors will allow clinicians to diagnose these treatment‐related complications early, potentially reducing their associated morbidity and mortality. In addition, a possible association between irAEs and response to therapy was observed.
Contributor Information
PierFranco Conte, Email: pierfranco.conte@unipd.it.
Laura Bonanno, Email: laura.bonanno@iov.veneto.it.
Author Contributions
Conception/design: Alberto Pavan, Lorenzo Calvetti, Laura Bonanno
Provision of study material or patients: Valentina Guarneri, Giuseppe Aprile, PierFranco Conte
Collection and/or assembly of data: Alberto Pavan, Lorenzo Calvetti, Alessandro Dal Maso, Ilaria Attili, Giulia Pasello, Laura Bonanno
Data analysis and interpretation: Alberto Pavan, Paola Del Bianco, Valentina Guarneri, Giuseppe Aprile, PierFranco Conte, Laura Bonanno
Manuscript writing: Alberto Pavan, Laura Bonanno
Final approval of manuscript: Alberto Pavan, Lorenzo Calvetti, Alessandro Dal Maso, Ilaria Attili, Paola Del Bianco, Giulia Pasello, Valentina Guarneri, Giuseppe Aprile, PierFranco Conte, Laura Bonanno
Disclosures
The authors indicated no financial relationships.
References
- 1.Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non–small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non–small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reck M, Rodríguez‐Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD‐L1–positive non–small‐cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi L, Rodríguez‐Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non–small‐cell lung cancer. N Engl J Med 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 5.Paz‐Ares LG, Luft A, Ali T et al. Phase 3 study of carboplatin‐paclitaxel/nab‐paclitaxel (Chemo) with or without pembrolizumab (Pembro) for patients (Pts) with metastatic squamous (Sq) non‐small cell lung cancer (NSCLC). J Clin Oncol 2018;36(suppl 15):105A. [Google Scholar]
- 6.Socinski MA, Jotte RM, Cappuzzo F et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288–2301. [DOI] [PubMed] [Google Scholar]
- 7.Jotte RM, Cappuzzo F, Vynnychenko I et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab‐paclitaxel vs carboplatin + nab‐paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018;36(suppl 18):LBA9000A. [Google Scholar]
- 8.Melosky B, Chu Q, Juergens RA et al. Breaking the biomarker code: PD‐L1 expression and checkpoint inhibition in advanced NSCLC. Cancer Treat Rev 2018;65:65–77. [DOI] [PubMed] [Google Scholar]
- 9.Nakaya A, Kurata T, Yoshioka H et al. Neutrophil‐to‐lymphocyte ratio as an early marker of outcomes in patients with advanced non‐small‐cell lung cancer treated with nivolumab. Int J Clin Oncol 2018;23:634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo A, Franchina T, Ricciardi GRR et al. Baseline neutrophilia, derived neutrophil‐to‐lymphocyte ratio (dNLR), platelet‐to‐lymphocyte ratio (PLR), and outcome in non small cell lung cancer (NSCLC) treated with Nivolumab or Docetaxel. J Cell Physiol 2018;233:6337–6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mezquita L, Auclin E, Ferrara R et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non‐small cell lung cancer. JAMA Oncol 2018;4:351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopkins AM, Rowland A, Kichenadasse G et al. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br J Cancer 2017;117:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haanen JB a. G, Carbonnel F, Robert C et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2017;28:iv119–iv142. [DOI] [PubMed] [Google Scholar]
- 15.Patil PD, Burotto M, Velcheti V. Biomarkers for immune‐related toxicities of checkpoint inhibitors: Current progress and the road ahead. Expert Rev Mol Diagn 2018;18:297–305. [DOI] [PubMed] [Google Scholar]
- 16.Gowen MF, Giles KM, Simpson D et al. Baseline antibody profiles predict toxicity in melanoma patients treated with immune checkpoint inhibitors. J Transl Med 2018;16:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujimura T, Sato Y, Tanita K et al. Serum levels of soluble CD163 and CXCL5 may be predictive markers for immune‐related adverse events in patients with advanced melanoma treated with nivolumab: A pilot study. Oncotarget 2018;9:15542–15551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akinci Ozyurek B, Sahin Ozdemirel T, Buyukyaylaci Ozden S et al. Prognostic value of the neutrophil to lymphocyte ratio (NLR) in lung cancer cases. Asian Pac J Cancer Prev 2017;18:1417–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu X, Sun S, Gao XS et al. Prognostic value of platelet to lymphocyte ratio in non‐small cell lung cancer: Evidence from 3,430 patients. Sci Rep 2016;6:23893–23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Champiat S, Lambotte O, Barreau E et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann Oncol 2016;27:559–574. [DOI] [PubMed] [Google Scholar]
- 21.Michot JM, Bigenwald C, Champiat S et al. Immune‐related adverse events with immune checkpoint blockade: A comprehensive review. Eur J Cancer 2016;54:139–148. [DOI] [PubMed] [Google Scholar]
- 22.Haratani K, Hayashi H, Chiba Y et al. Association of immune‐related adverse events with nivolumab efficacy in non‐small‐cell lung cancer. JAMA Oncol 2018;4:374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanlorenzo M, Vujic I, Daud A et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol 2015;151:1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeman‐Keller M, Kim Y, Cronin H et al. Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune‐related adverse events and association with outcomes. Clin Cancer Res 2016;22:886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura Y, Tanaka R, Asami Y et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: A multi‐institutional retrospective study. J Dermatol 2017;44:117–122. [DOI] [PubMed] [Google Scholar]
- 26.Girard N, Audigier Valette C, Cadranel J et al. 1302PD IFCT‐1502 CLINIVO: Real‐life experience with nivolumab in 600 patients (pts) with advanced non‐small cell lung cancer (NSCLC): Efficacy and safety of nivolumab and post‐nivolumab treatment in the French Expanded Access Program (EAP). Ann Oncol 2017;28(suppl 5):1302PDA.28368455 [Google Scholar]
- 27.Crinò L, Bidoli P, Delmonte A et al. Italian nivolumab expanded access programme: Efficacy and safety data in squamous non‐small cell lung cancer patients. J Thorac Oncol 2017;12:S1336–S1337. [Google Scholar]
- 28.Bagley SJ, Kothari S, Aggarwal C et al. Pretreatment neutrophil‐to‐lymphocyte ratio as a marker of outcomes in nivolumab‐treated patients with advanced non‐small‐cell lung cancer. Lung Cancer 2017;106:1–7. [DOI] [PubMed] [Google Scholar]
- 29.Diem S, Schmid S, Krapf M et al. Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) as prognostic markers in patients with non‐small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176–181. [DOI] [PubMed] [Google Scholar]
- 30.Chen DS, Mellman I. Oncology meets immunology: The cancer‐immunity cycle. Immunity 2013;39:1–10. [DOI] [PubMed] [Google Scholar]
- 31.Collins M, Michot JM, Danlos FX et al. Inflammatory gastrointestinal diseases associated with PD‐1 blockade antibodies. Ann Oncol 2017;28:2860–2865. [DOI] [PubMed] [Google Scholar]
- 32.Chen L. Co‐inhibitory molecules of the B7‐CD28 family in the control of T‐cell immunity. Nat Rev Immunol 2004;4:336–347. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Oh SY, Kim SH et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer 2013;13:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pine JK, Morris E, Hutchins GG et al. Systemic neutrophil‐to‐lymphocyte ratio in colorectal cancer: the relationship to patient survival, tumour biology and local lymphocytic response to tumour. Br J Cancer 2015;113:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi S, Basso M, Strippoli A et al. Are markers of systemic inflammation good prognostic indicators in colorectal cancer? Clin Colorectal Cancer 2017;16:264–274. [DOI] [PubMed] [Google Scholar]
- 36.Vernieri C, Mennitto A, Prisciandaro M et al. The neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratios predict efficacy of platinum‐based chemotherapy in patients with metastatic triple negative breast cancer. Sci Rep 2018;8:8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berardi R, Rinaldi S, Santoni M et al. Prognostic models to predict survival in patients with advanced non‐small cell lung cancer treated with first‐line chemo‐ or targeted therapy. Oncotarget 2016;7:26916–26924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Attili I, Passaro A, Pavan A et al. Combination immunotherapy strategies in advanced non‐small cell lung cancer (NSCLC): Does biological rationale meet clinical needs? Crit Rev Oncol Hematol 2017;119:30–39. [DOI] [PubMed] [Google Scholar]