This article assesses long‐term, real‐world treatment patterns and clinical outcomes of patients with lung neuroendocrine tumors (NET) at four tertiary cancer centers in the U.S., considering the FDA‐approved treatment and NCCN recommendations. A retrospective patient chart review was performed to determine treatment patterns and clinical outcomes for patients with advanced lung NETs.
Keywords: Lung neuroendocrine tumor, Treatment patterns, Somatostatin analogs, Real‐world analysis
Abstract
Background.
Using data from four tertiary referral centers in the U.S., we assessed real‐world treatment patterns and clinical outcomes of patients with advanced lung neuroendocrine tumors (NETs).
Subjects, Materials, and Methods.
We performed a retrospective chart review of adult patients with locally advanced/metastatic (typical/atypical) lung NETs treated between July 2011 and December 2014. Index date was histologically confirmed typical/atypical carcinoid tumor diagnosis date. Data included baseline characteristics, treatment patterns, progression, death, and lung NET‐related health care resource use from index date through last contact/death. Time to treatment discontinuation and first progression, time from first to second progression, and overall survival (OS) were estimated using Kaplan‐Meier analysis.
Results.
We identified 83 patients; 19 (23%) had functional NET. First‐line treatments included somatostatin analogs (SSAs) alone (56%) or in combination with other therapies (6%), cytotoxic chemotherapy (20%), external beam radiation therapy (EBRT) (9%), liver‐directed therapy (LDT) (4%), and everolimus/other (5%). Sixty patients had second‐line therapy including SSA alone (18%) or in combination (40%), cytotoxic chemotherapy (17%), everolimus (12%), LDT (7%), EBRT (3%), and other treatments (3%). Median time (months) to first‐line discontinuation were as follows: SSAs, 43.3; cytotoxic chemotherapy, 3.6. Overall median time (months) to investigator‐assessed progression following treatment initiation was 12.4. Median OS (months) following treatment initiation was 66.4 for all patients and 81.5 for patients receiving SSAs.
Conclusion.
SSAs, alone and in combination, are common treatments for advanced lung NETs. Patients have additional treatment options and relatively long survival compared with patients with other advanced cancers. Treatment pattern assessment following approval of newer treatments is needed.
Implications for Practice.
Somatostatin analogs (SSAs), cytotoxic chemotherapy, EBRT, liver‐directed therapy, and targeted therapies are common treatments for locally advanced/metastatic (typical/atypical) lung neuroendocrine tumors (NETs). SSAs alone or in combination with other treatment modalities were the most common first‐ and second‐line therapy, followed by cytotoxic chemotherapy. Patients continued treatment with SSAs long‐term with median treatment duration of 43 months. Median overall survival was 66 months following initiation of first‐line therapy for all patients. Treatment pattern assessment beyond the time period of this study is needed given recent U.S. Food and Drug Administration approvals for additional treatments for lung NETs that will likely be incorporated in the treatment landscape.
Introduction
Neuroendocrine tumors (NETs) are slow‐growing malignancies arising from neuroendocrine cells throughout the body [1]. Low‐ and intermediate‐grade lung NETs are known as typical and atypical pulmonary carcinoids, respectively. Together they make up the second most common category of NETs (behind gastrointestinal NETs) but account for only 1%–2% of all lung tumors [2]. Functional tumors cause distinct syndromes such as carcinoid syndrome (CS) when they secrete peptides and neuroamines [3].
Although NETs are considered rare, increasing incidence has been demonstrated using cancer registry data in the U.S. From 1973 to 2012, incidence of lung NET in the U.S. rose from 0.3 new cases to 1.6 new cases per 100,000 persons, likely due to an increase in imaging procedures in medicine [4]. Low‐dose computed tomography scans for lung cancer screening in smokers may also contribute to enhanced detection of lung NETs [2].
Currently, the only treatment approved by the U.S. Food and Drug Administration (FDA) for NET of the lung is everolimus, which was approved in 2016 [5]. Somatostatin analogs (SSAs), including octreotide and lanreotide, benefit patients with symptoms of hormone secretion, and recently telotristat ethyl has also become available as a treatment for CS diarrhea [6]. Although lanreotide was approved in 2014 by the FDA for improvement of progression‐free survival among patients with well‐ or moderately differentiated, locally advanced or metastatic gastroenteropancreatic (GEP)‐NET [7] and for the treatment of CS in 2017 [8], the ongoing phase III SPINET trial is assessing lanreotide for the treatment of well‐differentiated typical or atypical lung NETs [9]. The FDA approved the peptide receptor radionuclide therapy (PRRT) 177Lu‐dotatate for GEP‐NET but not lung NET in January 2018 [10]. Depending on the clinical characteristics of lung NET, the National Comprehensive Cancer Network (NCCN) guidelines in 2018 recommend, with different category of evidence, treatment with SSAs octreotide and lanreotide, everolimus, PRRT with 177Lu‐dotatate, and cytotoxic chemotherapies [11].
The value of SSAs and other treatments in first, second, and subsequent lines of therapy for lung NET has not been described. The objective of this study was to assess long‐term, real‐world treatment patterns and clinical outcomes of patients with lung NET at four tertiary cancer centers given there is only one recently FDA‐approved treatment and the NCCN recommendations include several treatments. We performed a retrospective chart review of patients to describe treatment patterns and clinical outcomes for patients with advanced lung NETs treated at four major tertiary care centers in the U.S.
Subjects, Materials, and Methods
Study Design and Study Population
This study was a multicenter, noninterventional, retrospective chart review among patients with lung NET, conducted at MD Anderson Cancer Center (MDACC) in Houston, TX; Dana‐Farber Cancer Institute (DFCI) in Boston, MA; UCSF Helen Diller Family Comprehensive Cancer Center in San Francisco, CA; and Robert H. Lurie Comprehensive Cancer Center in Chicago, IL. The cancer centers included in this study have sizeable populations of NET patients with long duration of follow‐up, allowing for the assessment of long‐term outcomes such as progression and survival.
Eligible patients included adults diagnosed with locally advanced or metastatic NET of lung origin with histologic diagnosis of typical or atypical carcinoid tumor. Patients were required to be treated with SSAs, targeted therapy (e.g., everolimus, sunitinib, bevacizumab), cytotoxic chemotherapy (e.g., capecitabine, carboplatin, cisplatin, etoposide, temozolomide), PRRT, liver‐directed therapy (LDT; e.g., transarterial chemoembolization, radioembolization, hepatic arterial embolization), or interferon‐alfa between July 2011 and December 2014 (i.e., identification period); patients were permitted to have initiated therapy prior to July 2011. Eligible patients may have received some of their care outside of the institution, provided that they received comprehensive care at the institution, had at least two visits to the institution in the 14 months prior to the patient's last visit, and their advanced lung NET treatment and clinical outcomes information was available. Patients with poorly differentiated histology such as large cell neuroendocrine carcinoma or small cell lung carcinoma, gastrointestinal NET, pancreatic NET, mixed tumor types (e.g., NET plus other histology, goblet cell carcinoid, composite carcinoid, adenocarcinoid), or NET of unknown primary site were excluded.
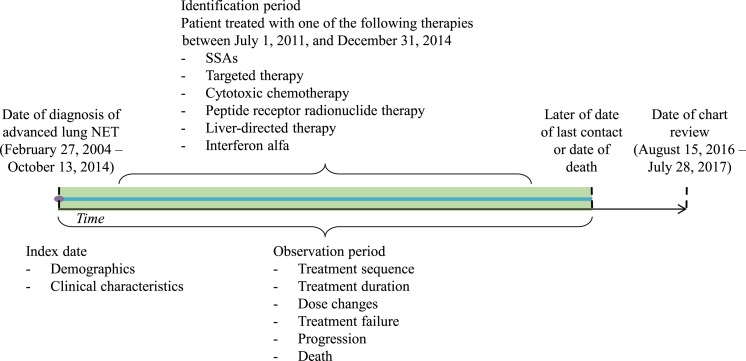
The observation period for a given patient was the time from date of diagnosis of advanced lung NET (index date) until the later of the date of last contact or death (Fig. 1). Baseline patient characteristics during the preindex period included demographics, comorbidities, treatment history, and disease characteristics such as whether the patient had a hereditary cancer syndrome and whether the patient's NET was functioning, based on medical notes. Data on treatments, including types of treatment (i.e., pharmacological, surgical, LDT, and radiotherapy), treatment doses, dose modifications, dates of treatment initiation, termination, or discontinuation as recorded in medical charts, and reasons for discontinuation, were collected for the observation period.
Figure 1.
Study design.Abbreviations: NET, neuroendocrine tumor; SSA, somatostatin analog.
For the treatment pattern analysis, only pharmacological therapies, LDT, and radiotherapy were considered. Information on surgeries, such as debulking procedures, were collected but not included in the treatment pattern analysis; surgery as treatment for metastatic NET is only possible when there are limited sites of disease and when radical resection is possible for all the sites [2]. In determining treatment sequence, treatment discontinuation was defined as the first 1‐month gap between treatments for the same therapy, with the exception of LDT, for which the gap was 6 months between LDT treatments.
Time to treatment discontinuation was defined as the time from initiation of a therapy to its discontinuation for any reason. Overlap of individual pharmacological or medical procedures longer than 14 days were classified as combination treatment regimen. Multiple LDT procedures occurring within a 6‐month period were considered one LDT regimen. Addition of a new agent demarcated the line of treatment (e.g., first line and second line of therapy). Data on treatments used at the time of disease progression and following progression were collected.
Data for assessment of clinical outcomes included dates and characteristics of tumor progressions, as well as date of death. For tumor progressions, physicians assessed radiologists' notes in the medical charts and used them to determine whether a patient's status improved (responded), stayed the same (stabilized), or worsened (progressed). Lung NET‐related health care resource utilization (HRU) data included number and length of inpatient stays, emergency room visits, and number and type of oncologist visits.
Clinical research coordinators (CRCs) at the hospitals screened patient records and identified the records of eligible patients based on the inclusion criteria. CRCs then entered data from the patient charts into an electronic case report form via a secure website. Data abstraction was conducted between August 15, 2016, and July 28, 2017. Data were deidentified and complied with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act. All study materials were approved by local Institutional Review Boards at each of the four institutions.
Statistical Analysis
Data collected from each center were pooled for the analysis. Descriptive statistics were calculated using frequencies and proportions for categorical variables and means, and standard deviations and medians for continuous variables. A Sankey treatment sequence flow chart was developed to show flow of treatments by line of therapy over time. A GRAPHx flow chart was developed, in which each colored segment indicates a treatment, and the multicolored line segments reflect treatment sequences over time for individual patients.
Time to treatment discontinuation, time to first physician‐assessed progression, time from first physician‐assessed progression to second physician‐assessed progression, and overall survival from time of first‐line treatment initiation were estimated using Kaplan‐Meier analysis, in which patients who did not experience the event were censored from the analysis. The time origin was set at the initiation of pharmacological therapies, LDT, or radiotherapy for the time‐to‐event analyses of treatment discontinuation, first physician‐assessed progression, and overall survival. Incidence rates were calculated to summarize lung NET‐related hospitalizations, emergency room visits, and outpatient visits from diagnosis of advanced lung NET to the later of the date of last contact or death. A Poisson probability density function was used to calculate confidence intervals of incidence rates.
All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Eighty‐three eligible patients were included in the study (41 MDACC, 27 DFCI, 9 UCSF, 6 Northwestern).
Table 1 summarizes baseline demographic and clinical characteristics of the study population. Among the 83 patients included in this study, about half were female (52%) and the majority were white (78%); mean age at advanced lung NET diagnosis was 60 years. The earliest recorded advanced NET diagnosis was in February 2004, and the latest recorded date of contact was in July 2017. Patients were followed for a median of 49.1 months (range: 4.0–155.4) since diagnosis of advanced lung NET. A minority of patients were diagnosed with functional NETs (23%), all of whom had carcinoid syndrome. Most common carcinoid syndrome symptoms were flushing (63%) and diarrhea (42%). Mean number of metastatic sites at advanced NET diagnosis was 1.3 sites. Ki‐67 proliferation index was available for 48% of patients, with a median proliferation index of 10%. Mitotic rate was available for 45% of patients, with a median rate of 2 mitoses per 10 high power fields (HPF). Among the 37 patients for whom the mitotic rate was known, 41% had <2 mitotic figures per 10 HPF, and 59% had ≥2 mitotic figures per 10 HPF. Twenty‐one patients (25%) received at least one NET treatment in a clinical trial setting.
Table 1. Demographic and clinical characteristics at baseline.
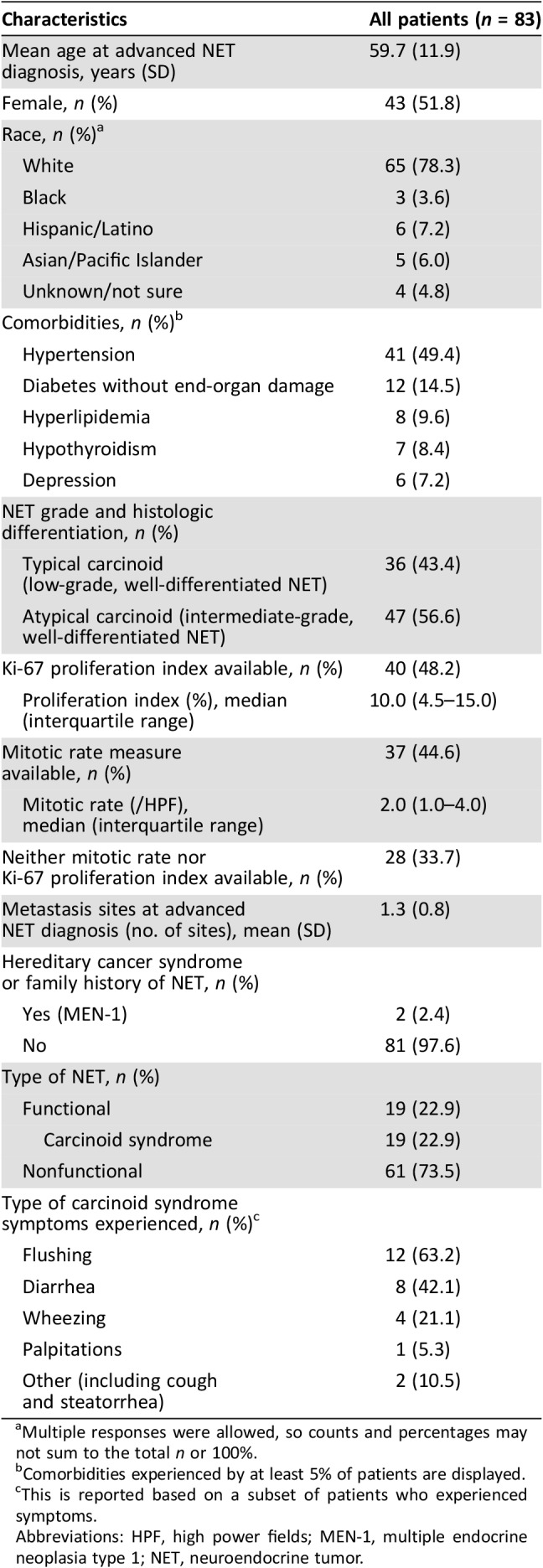
Multiple responses were allowed, so counts and percentages may not sum to the total n or 100%.
Comorbidities experienced by at least 5% of patients are displayed.
This is reported based on a subset of patients who experienced symptoms.
Abbreviations: HPF, high power fields; MEN‐1, multiple endocrine neoplasia type 1; NET, neuroendocrine tumor.
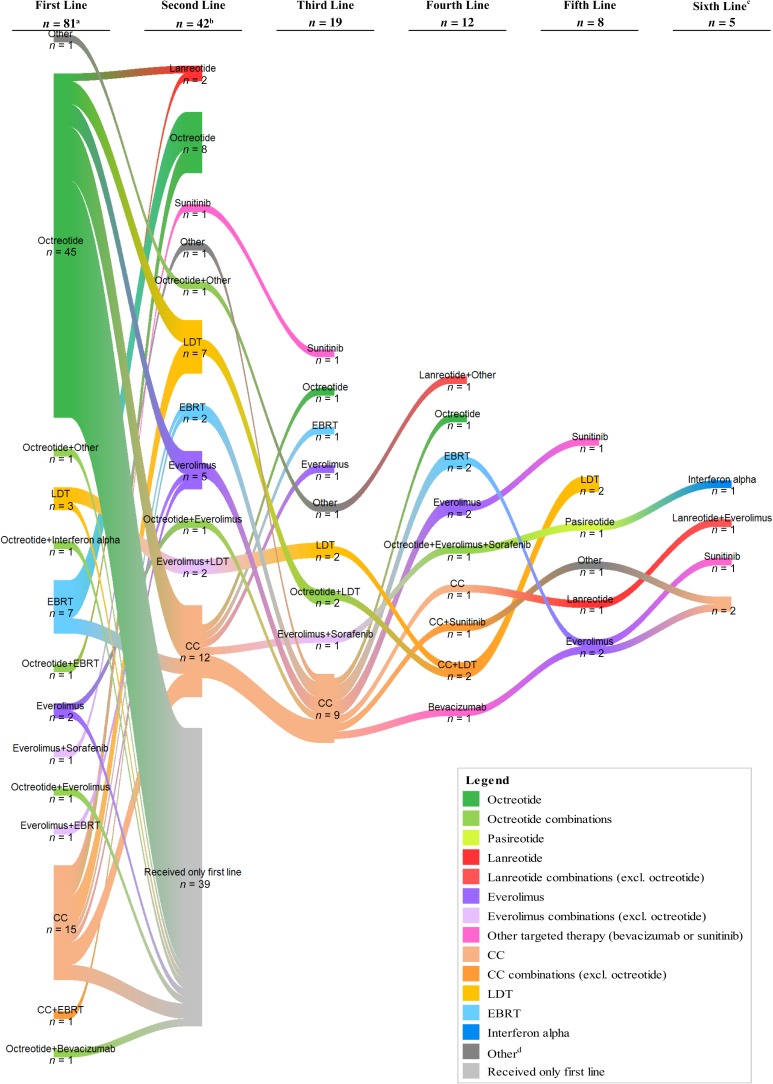
Table 2 displays first‐ and second‐line treatment regimens for the study population. For first‐line therapy, the majority of patients were treated with SSAs alone (45 [56%]) or in combination (5 [6%]) as first‐line therapy; 16 (20%) patients were treated with cytotoxic chemotherapy, and the remaining 15 (18%) were treated with EBRT, LDT, everolimus (alone or in combination), or other therapies. Octreotide was the only SSA used for first‐line therapy. In total, 60 patients (72%) received second‐line therapy, of whom 24 (40%) were treated with SSAs in combination with other therapies, and 11 (18%) switched to SSAs only. Octreotide was the second‐line SSA treatment for nearly all those treated with SSA; two patients were treated with lanreotide and one with a combination of octreotide and pasireotide. In second‐line, 10 (17%) patients were treated with cytotoxic chemotherapy, and the remaining 15 (25%) had treatment with everolimus, sunitinib, LDT, EBRT, or other therapies.
Table 2. Treatment regimens for the first two lines of treatment.
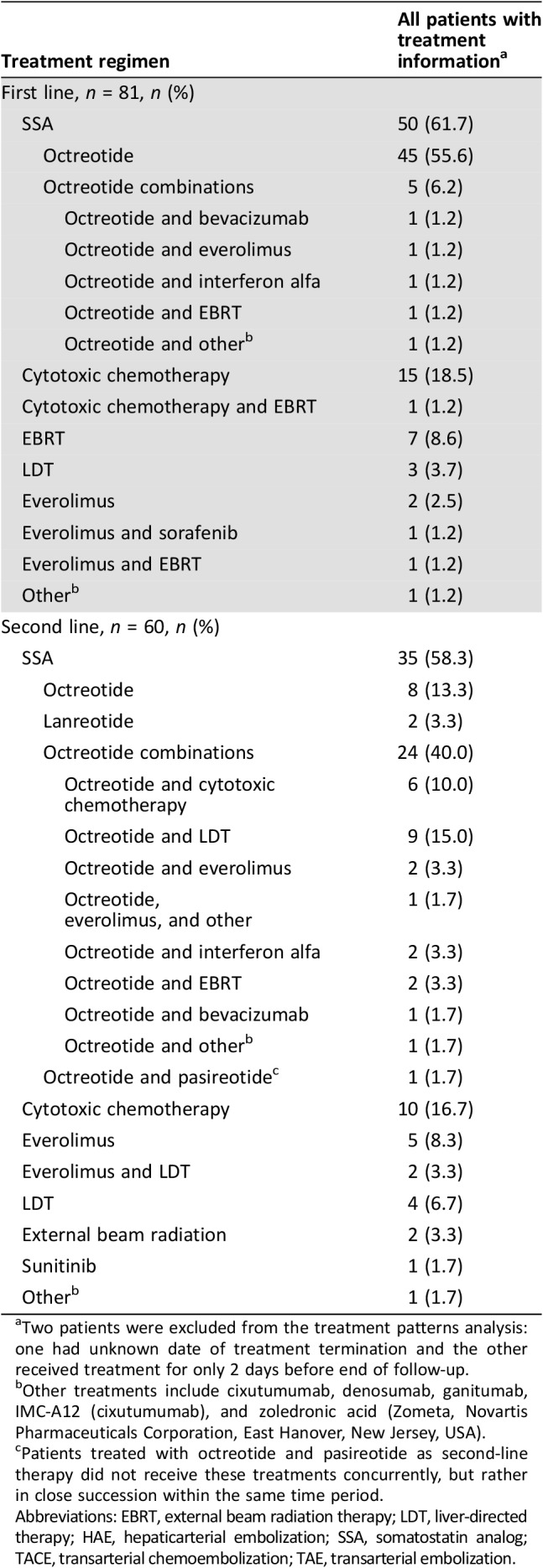
Two patients were excluded from the treatment patterns analysis: one had unknown date of treatment termination and the other received treatment for only 2 days before end of follow‐up.
Other treatments include cixutumumab, denosumab, ganitumab, IMC‐A12 (cixutumumab), and zoledronic acid (Zometa, Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA).
Patients treated with octreotide and pasireotide as second‐line therapy did not receive these treatments concurrently, but rather in close succession within the same time period.
Abbreviations: EBRT, external beam radiation therapy; LDT, liver‐directed therapy; HAE, hepaticarterial embolization; SSA, somatostatin analog; TACE, transarterial chemoembolization; TAE, transarterial embolization.
Figure 2 illustrates the switches in therapy from first‐line to second‐line and up to six lines of therapy. SSAs were the dominant first‐line therapy. Patients were treated with SSAs in later lines as well, but the proportion of patients using other therapies such as cytotoxic chemotherapy increased in subsequent lines.
Figure 2.
Sankey diagram of treatment sequences. aTwo patients were excluded from the treatment patterns analysis: one had unknown date of treatment termination and the other received treatment for only 2 days before end of follow‐up. bThe count of patients for the second‐line treatment does not include the 39 patients shown in the diagram who continued their first‐line treatment. cOnly one patient received more than six lines of treatment. dOther therapy includes aflibercept, bevacizumab, cabozantinib, cixutumumab, cyberknife radiotherapy, denosumab, ganitumab, IMC‐A12 (cixutumumab), lapatinib, samarium‐153, stereotactic body radiation therapy, sirolimus, sorafenib, stereotactic radiation therapy, sunitinib, and Zometa (zoledronic acid;). Abbreviations: CC, cytotoxic chemotherapy; EBRT, external beam radiation therapy; LDT, liver‐directed therapy.
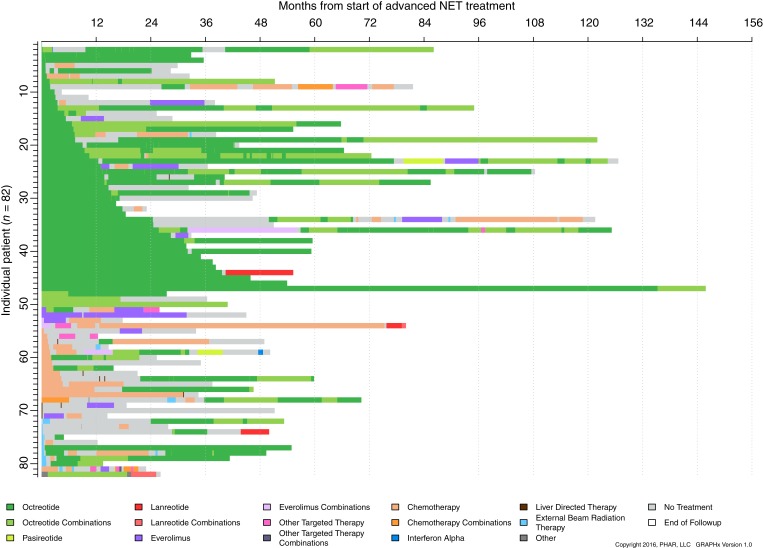
Figure 3 illustrates treatment duration and sequence. SSAs and octreotide specifically appear the most frequent therapy with a median of 43.3 (95% confidence interval [CI]: 25.4 to not reached) months until discontinuation of first‐line therapy for SSAs. Those with first‐line octreotide treatment and nonfunctional NET had a median of 29.3 months until discontinuation, whereas the median was not reached for patients with functional NET treated with first‐line octreotide. Median time to first‐line discontinuation was 3.6 (95% CI: 2.4–5.3) months for cytotoxic chemotherapy and 2.7 (95% CI: 1.9–31.8) months for targeted therapy.
Figure 3.
GRAPHx diagram of treatment sequences and durations of treatment by patient.Abbreviation: NET, neuroendocrine tumor.
In assessing SSA dosing, only four patients (5%) were treated with lanreotide with doses ranging from 60 mg/4 weeks (n = 1) to 120 mg/4 weeks (n = 2); one patient had unknown dosage; only one patient changed dose (from 90 to 60 mg/4 weeks because of toxicity). In evaluating octreotide doses administered during the observation period, most patients (85%) were known to initiate octreotide with a dose of 30 mg/4 weeks or less (supplemental online Fig. 1). Dose modification occurred in 23 (35%) patients receiving octreotide with a maximum of five instances of modification (supplemental online Table 1). The most frequently reported reasons for dose changes were disease progression or patient preference. The median (interquartile range) dose ever administered was 30 mg/4 weeks (20, 30). Supplemental online Figure 2 displays the patterns of octreotide dose changes observed in at least 1% of patients among those with known octreotide doses.
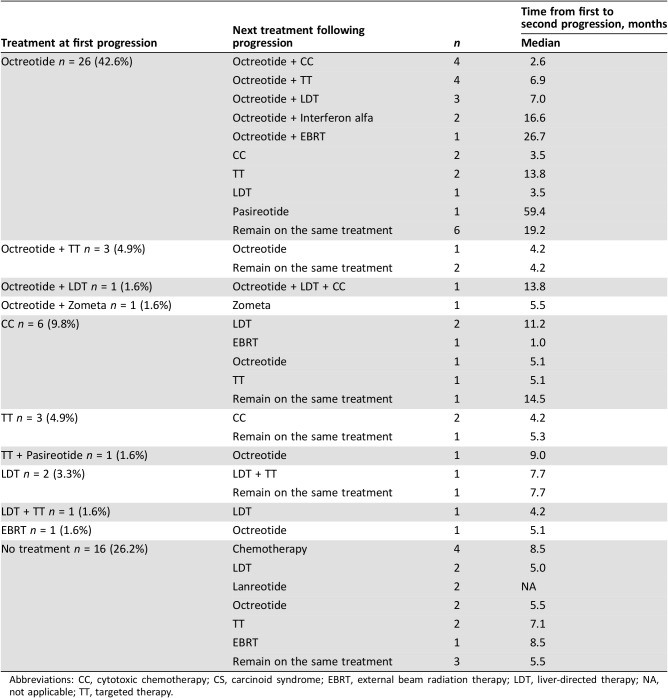
Median time to first progression as assessed by a physician following treatment initiation was 12.4 (95% CI: 9.9–15.7) months for all patients. For the 61 patients who initiated treatment for advanced lung NET and progressed, Table 3 shows the treatments at time of first physician‐assessed progression, next treatment received, and median time from first to second physician‐assessed progression.
Table 3. Treatment sequence from first physician‐assessed tumor progression and analysis of time to next progression (n = 61).
Abbreviations: CC, cytotoxic chemotherapy; CS, carcinoid syndrome; EBRT, external beam radiation therapy; LDT, liver‐directed therapy; NA, not applicable; TT, targeted therapy.
Among patients with advanced lung NETs, median overall survival after initiating first‐line therapy was 66.4 (95% CI: 40.8–108.3) months; 47% of patients died. Patients with first‐line treatment with SSAs (octreotide) had median overall survival of 81.5 (95% CI: 43.4–126.6) months (functional NET: 66.4; nonfunctional NET: 81.5).
Patients with advanced lung NETs had the following rates of lung NET‐related HRU (per person‐year): 0.09 (95% CI: 0.07–0.13) emergency room visits, 0.23 (95% CI: 0.19–0.029) hospitalizations, and 10.16 (95% CI: 9.84–10.50) outpatient visits.
Discussion
This study conducted at four tertiary academic medical centers characterizes treatments and outcomes among patients with advanced well‐differentiated lung NET during a time when everolimus became the only FDA‐approved treatment. This study demonstrated that SSAs were a primary treatment choice for the majority of patients with advanced well‐differentiated lung NET. Sixty‐two percent of patients were treated with SSAs for their first‐line therapy, and a significant number of patients continued with treatment with SSA alone or SSA with another therapy during subsequent lines of therapy. Cytotoxic chemotherapy, EBRT, LDT, and targeted therapy were common first‐line treatment, with nearly 20% of patients being treated with cytotoxic chemotherapy in first line.
Octreotide was the most frequent SSA treatment. There was limited use of lanreotide likely because the study evaluated patients receiving treatment between July 2011 and December 2014, prior to the FDA approval of lanreotide for GEP‐NET in 2014. The ongoing phase III SPINET trial is assessing lanreotide for the treatment of well‐differentiated typical or atypical lung NETs [9]. There was minimal use of targeted therapies, including everolimus, as a first‐line therapy in the current study, likely because the FDA approved everolimus for lung NET treatment in February 2016, and the current study required patients to have initiated treatment prior to that time [5].
Similarly, no use of PRRT was observed in the current study, recognizing that 177Lu‐dotatate was FDA approved in January 2018 for GEP‐NET indication and is not yet well studied in lung NETs [10].
The NCCN treatment guidelines have changed over the past several years to include more recently available treatments. The high rate of use of SSA was consistent with NCCN treatment guidelines during the study time period. The guidelines from NCCN in 2012 for metastatic NET listed octreotide with category of evidence and consensus 2A; for clinically significant progressive disease, the guidelines listed LDT (category 2B), everolimus (category 3), and cytotoxic chemotherapy (category 3) [12]. The current guidelines from NCCN have expanded such that the 2018 guidelines include the following treatments with category of evidence and consensus 2A for advanced or metastatic lung NET: SSAs octreotide and lanreotide if somatostatin receptor positive, everolimus, and PRRT with 177Lu‐dotatate if somatostatin receptor positive and progression on octreotide or lanreotide treatment. Depending on the Ki‐67 proliferation index and mitotic index, current recommended treatment for intermediate‐grade (atypical) lung NET also includes the cytotoxic chemotherapies (category 2A) cisplatin/etoposide, carboplatin/etoposide, and temozolomide [11]. An analysis of more recent data might reflect use of therapies not available during the current study's time period and also reflect changes in NCCN guidelines. Notably, the category of evidence for cytotoxic chemotherapy for patients with atypical carcinoid tumor has become stronger in recent guidelines.
In addition to characterizing the various treatments, dose data for octreotide, the most common SSA treatment in this study, were analyzed, and the analysis showed most octreotide administrations were given at the standard dose of 30 mg/4 weeks for CS treatment. Furthermore, whereas prior studies on resource use for patients with NET have focused on all‐cause use [3], [13], this study adds to the literature by showing lung NET‐related resource use following advanced lung NET diagnosis.
Other observational studies have shown SSAs are common for first‐line treatment for NETs, but to varying degrees. Variations in first‐line use of SSAs may be due to differences across study populations. Study time periods in relation to the time of availability of particular SSAs may also impact the reported use of particular SSAs by patients. Strosberg et al. [13] reported 77% of patients with lung/gastrointestinal (GI) NET in an analysis of physician‐reported data from academic and community settings were ever treated with SSAs; however, this study population included patients who may have had surgery as their only treatment, whereas the current study required patients to have some nonsurgical treatment. In a claims data analysis of a commercially insured population in 2007–2010, Chuang et al. [3] reported that among those treated with SSAs or chemotherapy following carcinoid or pancreatic islet‐cell tumor diagnosis, 92% were treated with long‐acting octreotide, 27% were treated with short‐acting octreotide, and 1.4% were treated with lanreotide depot during the 12 months immediately following diagnosis.
Treatment for lung NET specifically has not been as widely assessed, but a recent publication showed high use of cytotoxic chemotherapy for this population of patients with NET. In a U.S. claims analysis among patients treated for lung NET during a time period similar to that of the current study, Broder et al. [14] reported that among pharmacologically treated patients with lung NET, first‐line therapy was 78% cytotoxic chemotherapy, 18% SSA, and 1% targeted therapy. It is not possible to ascertain whether the higher reported use of cytotoxic chemotherapy among the population in Broder et al. [14] could be due to patient clinical characteristics, such as a high proportion of atypical carcinoids, poorly differentiated or high‐grade lung cancers, or small cell cancers. In the study by Broder et al. [14], fewer patients (only 8%) received second‐line therapy compared with patients in the current study. SSAs were the most common second‐line treatment in that study.
The current study's examination of treatment flow from first‐line to subsequent lines of therapy demonstrated that patients continued treatment with SSAs throughout their treatment course. Similar to the current study, Broder et al. [14] reported first‐line treatment was longer for SSAs relative to other pharmacologic treatment. There may be underlying differences in clinical and demographic characteristics of patients receiving different therapies, which may influence treatment duration.
Median time to first physician‐assessed progression was 12.4 (95% CI: 9.9–15.7) months from initiation of first‐line therapy and 12.9 months (95% CI: 9.9–31.8) from initiation of first‐line treatment with SSA, in the current study. In an institutional database study by Ter‐Minassian et al. [15], progression‐free survival from initiation of an SSA was 1.2 (95% CI: 1.0–1.6) years for patients with NET of non‐small bowel. A small French study reported median progression‐free survival of 17.4 (95% CI: 8.7–26.0) months for patients with pulmonary carcinoids and treated with SSAs [16]. A small retrospective Swedish study [17] reported a median progression‐free survival of 5.3 months for patients with metastatic bronchial NETs treated with single‐agent temozolomide. In the study by Ter‐Minassian et al. a longer time to disease progression and longer progression‐free survival were associated with longer overall survival, showing the importance of studying progression endpoints [15].
Median overall survival was observed to be 66 months following initiation of first‐line treatment for lung NET in the current study; 47% of patients with lung NET died. The Surveillance, Epidemiology, and End Results (SEER) database study by Dasari et al. [4] reported that patients with NETs in the pancreas and lung were found to have the worst median overall survival, with a median of 5.5 years for all stages of lung NET and only 6 months for lung NET patients with distant metastasis; in contrast, those with GI NET had a median overall survival of 16.2 years [4]. A single‐site chart review study in France reported median overall survival of 58.4 months (95% CI: 44.2–102.7) for patients with pulmonary carcinoid treated with SSAs [16]. Shen et al. [18] analyzed SEER‐Medicare data and reported that the death rate was nearly fourfold higher among those with NETs with primary sites of the larynx, lung, or other respiratory organs versus those with a primary site of the small intestine.
Although this study used data collected from cancer centers with detailed clinical data, the current study has some limitations that should be noted. Treatment sequences and discontinuation information are based on patient medical records. Short‐ and long‐acting octreotide could not be distinguished in the data and were therefore reported together. However, assuming that dosage amounts of <10 mg/4 weeks referred to short‐acting octreotide, we find that <5% of patients were treated with short‐acting octreotide; thus, conclusions here are most likely applicable to long‐acting octreotide. Treatment with SSAs consisted almost entirely of octreotide, as lanreotide was only approved, and not for lung NET specifically, in December 2014 [7]. In addition, everolimus was only recently approved for treating lung NET in 2016 [5], and PRRT was approved in 2018 and only for GEP‐NET [10]; considering these recent approvals, the treatment landscape may continue to evolve. Tumor progression was based on radiologist assessment or physician notes, which is subject to physician's assessment. In the current study, only 40% of all scans were assessed using RECIST criteria, indicating that real‐world treatment settings for NET are unlikely to incorporate RECIST criteria when assessing patients. Furthermore, detection bias may have occurred as patients with more aggressive diseases may receive more frequent radiographic scans. Results reported in this study are based on data collected at four cancer referral centers and may not reflect practice patterns observed in other institutions. Given that the data set was somewhat small, we did not conduct single‐center analyses. Lack of information about patients' care at outside institutions may have resulted in underreporting of treatments and health care resource use. Lastly, this study is descriptive—no statistical comparisons were performed.
Conclusion
Despite these limitations, this study used detailed clinical data and a long follow‐up duration up to 10 years, and the results are reflective of treatment patterns for patients with lung NET at U.S. tertiary care facilities. This study showed that SSAs, at the recommended dose, are common treatment for long‐term treatment of lung NET both alone and in combination with other therapies and as both initial and subsequent treatment. We further demonstrate the common use of multiple lines of therapy for these patients and a relatively long overall survival of patients with advanced lung NET treated at tertiary referral centers. Assessments of treatment patterns in the near future are needed to better understand how newer treatments for lung NET may impact treatment patterns and clinical outcomes.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We thank Caroline Korves, ScD, of Analysis Group, Inc. for her assistance with developing this manuscript. This research was funded by Novartis Pharmaceuticals Corporation, East Hanover, NJ.
Editor's Note: See the companion paper, “Real‐World Treatment Patterns and Clinical Outcomes in Advanced Gastrointestinal Neuroendocrine Tumors (GI NET): A Multicenter Retrospective Chart Review Study” by Matthew H. Kulke, Al B. Benson, Arvind Dasari et al., on page 1056 of this issue.
Author Contributions
Conception/design: Arvind Dasari, Emily K. Bergsland, Al B. Benson, Beilei Cai, Lynn Huynh, Todor Totev, Jerome Shea, Mei Sheng Duh, Maureen P. Neary, Cecile G. Dagohoy, Brandon E. Shih, Victoria E. Maurer, Jennifer Chan, Matthew H. Kulke
Provision of study material or patients: Arvind Dasari, Emily K. Bergsland, Al B. Benson, Cecile G. Dagohoy, Brandon E. Shih, Victoria E. Maurer, Jennifer Chan, Matthew H. Kulke
Collection and/or assembly of data: Lynn Huynh, Todor Totev, Jerome Shea, Mei Sheng Duh
Data analysis and interpretation: Arvind Dasari, Emily K. Bergsland, Al B. Benson, Beilei Cai, Lynn Huynh, Todor Totev, Jerome Shea, Mei Sheng Duh, Maureen P. Neary, Cecile G. Dagohoy, Brandon E. Shih, Victoria E. Maurer, Jennifer Chan, Matthew H. Kulke
Manuscript writing: Arvind Dasari, Emily K. Bergsland, Al B. Benson, Beilei Cai, Lynn Huynh, Todor Totev, Jerome Shea, Mei Sheng Duh, Maureen P. Neary, Cecile G. Dagohoy, Brandon E. Shih, Victoria E. Maurer, Jennifer Chan, Matthew H. Kulke
Final approval of manuscript: Arvind Dasari, Emily K. Bergsland, Al B. Benson, Beilei Cai, Lynn Huynh, Todor Totev, Jerome Shea, Mei Sheng Duh, Maureen P. Neary, Cecile G. Dagohoy, Brandon E. Shih, Victoria E. Maurer, Jennifer Chan, Matthew H. Kulke
Disclosures
Emily K. Bergsland: UpToDate (H), Novartis Pharmaceuticals Corporation (C/A); Al B. Benson: Bristol‐Myers Squibb, Guardant Health, Eli Lilly & Co, Exelixis, Purdue Pharma, Harborside, Xcenda, NCCN, Emron, inVentiv Health Inc, Axio, Genentech, Bayer, Merck, Rafael Pharmaceuticals, Astellas Pharma, Terumo (CA), Novartis Pharmaceuticals Corporation, Acerta, Celegene, Advanced Accelerator Applications, Infinity Pharmaceuticals, Merck Sharp and Dohme, Taiho Pharmaceutical, Bristol‐Myers Squibb, Medimmune/AstraZeneca, Xencor, Bristol‐Myers Squibb (RF); Beilei Cai: Novartis Pharmaceuticals Corporation (E, OI); Lynn Huynh: Novartis Pharmaceuticals Corporation (RF), Analysis Group, Inc. (E); Todor Totev: Novartis Pharmaceuticals Corporation (RF), Analysis Group, Inc. (E); Mei Sheng Duh: Novartis Pharmaceuticals Corporation (RF), Analysis Group, Inc. (E); Maureen P. Neary: Novartis Pharmaceuticals Corporation (E); Jennifer Chan: Novartis Pharmaceuticals Corporation, Ipsen (SAB), Ipsen (H), Sanofi, Eli Lilly & Co (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Yao JC, Hassan M, Phan A et al. One hundred years after ‘carcinoid’: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 2.Hilal T. Current understanding and approach to well differentiated lung neuroendocrine tumors: An update on classification and management. Ther Adv Med Oncol 2017;9:189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuang CC, Bhurke S, Chen SY et al. Clinical characteristics, treatment patterns, and economic burden in patients treated for neuroendocrine tumors in the United States: A retrospective cohort study. J Med Econ 2015;18:126–136. [DOI] [PubMed] [Google Scholar]
- 4.Dasari A, Shen C, Halperin D et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novartis Pharmaceuticals Corporation . FDA approves new indication for Novartis drug afinitor® for progressive, nonfunctional GI and lung neuroendocrine tumors (NET). 2016. Available at https://www.novartis.com/news/media‐releases/fda‐approves‐new‐indication‐novartis‐drug‐afinitor‐progressive‐nonfunctional‐gi‐and‐lung‐neuroendocrine‐tumors‐net. Accessed September 14, 2017.
- 6.U.S. Food and Drug Administration . Highlights of prescribing information. Xermelo (telotristat ethyl). February 2017. Available at https://www.xermelo.com/Media/Default/pdfs/Product_Info_telotristat_ethyl.pdf. Accessed August 15, 2018.
- 7.U.S. Food and Drug Administration . Highlights of prescribing information. Somatuline depot (lanreotide). December 2014. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022074s011lbl.pdf. Accessed January 25, 2017.
- 8.Ipsen Biopharmaceuticals Inc . U.S. FDA approves new indication for Ipsen's somatuline®depot (lanreotide) injection for the treatment of carcinoid syndrome. 2017. Available at https://www.ipsen.com/websites/IPSENCOM‐PROD/wp‐content/uploads/2017/09/16000129/18‐09‐2017‐Approval‐Somatuline‐US‐carcinoid‐syndrom‐FINAL.pdf. Accessed February 19, 2018.
- 9.Reidy‐Lagunes D, Kulke M, Wolin E et al. PUB119 lanreotide in patients with lung neuroendocrine tumors: The randomized double‐blind placebo‐controlled international phase 3 SPINET study. J Thorac Oncol 2017;12:S1516–S1517. [Google Scholar]
- 10.U.S. Food and Drug Administration . FDA approves new treatment for certain digestive tract cancers. 2018. Available at https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm594043.htm. Accessed March 20, 2018.
- 11.National Comprehensive Cancer Network clinical practice guidelines in oncology: Neuroendocrine and adrenal tumors. Version 2.2018. [DOI] [PubMed]
- 12.Kulke MH, Benson AB 3rd, Bergsland E et al. Neuroendocrine tumors. J Natl Compr Canc Netw 2012;10:724–764. [DOI] [PubMed] [Google Scholar]
- 13.Strosberg J, Casciano R, Stern L et al. United States‐based practice patterns and resource utilization in advanced neuroendocrine tumor treatment. World J Gastroenterol 2013;19:2348–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broder MS, Cai B, Chang E et al. Real‐world treatment patterns for lung neuroendocrine tumors: A claims database analysis. Oncology 2018;94:281–288. [DOI] [PubMed] [Google Scholar]
- 15.Ter‐Minassian M, Zhang S, Brooks NV et al. Association between tumor progression endpoints and overall survival in patients with advanced neuroendocrine tumors. The Oncologist 2017;22:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan I, Le Teuff G, Guigay J et al. Antitumour activity of somatostatin analogues in sporadic, progressive, metastatic pulmonary carcinoids. Eur J Cancer 2017;75:259–267. [DOI] [PubMed] [Google Scholar]
- 17.Crona J, Fanola I, Lindholm DP et al. Effect of temozolomide in patients with metastatic bronchial carcinoids. Neuroendocrinology 2013;98:151–155. [DOI] [PubMed] [Google Scholar]
- 18.Shen C, Xu Y, Dasari A et al. Octreotide LAR dosage and survival among elderly patients with distant‐stage neuroendocrine tumors. The Oncologist 2016;21:308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]