Comprehensive geriatric assessment (CGA) is composed of a coordinated multidisciplinary assessment that identifies medical, social, or psychological problems and leads to the development of relevant interventions and guides therapeutic decisions. CGA is the best assessment of the physiological age, life expectancy, and functional reserve of a patient. This article describes the use of CGA in published clinical trials dedicated to older cancer patients and assesses the evolution in CGA implementation from the founding of the International Society of Geriatric Oncology through the subsequent 10 years.
Keywords: Comprehensive geriatric assessment, Older patients, Clinical trials
Abstract
Background.
The objective of this study was to describe the implementation of comprehensive geriatric assessment (CGA) in clinical trials dedicated to older patients before and after the creation of the International Society of Geriatric Oncology in the early 2000s.
Subjects, Materials, and Methods.
All phase I, II, and III trials dedicated to the treatment of cancer among older patients published between 2001 and 2004 and between 2011 and 2014 were reviewed. We considered that a CGA was performed when the authors indicated an intention to do so in the Methods section of the article. We collected each geriatric domain assessed using a validated tool even in the absence of a clear CGA, including nutritional, functional, cognitive, and psychological status, comorbidity, comedication, overmedication, social status and support, and geriatric syndromes.
Results.
A total of 260 clinical trials dedicated to older patients were identified over the two time periods: 27 phase I, 193 phase II, and 40 phase III trials. CGA was used in 9% and 8% of phase II and III trials, respectively; it was never used in phase I trials. Performance status was reported in 67%, 79%, and 75% of phase I, II, and III trials, respectively. Functional assessment was reported in 4%, 11%, and 13% of phase I, II, and III trials, respectively. Between the two time periods, use of CGA increased from 1% to 11% (p = .0051) and assessment of functional status increased from 3% to 14% (p = .0094).
Conclusion.
The use of CGA in trials dedicated to older patients increased significantly but remained insufficient.
Implications for Practice.
This article identifies the areas in which research efforts should be focused in order to offer physicians well‐addressed clinical trials with results that can be extrapolated to daily practice.
Introduction
Comprehensive geriatric assessment (CGA) is composed of a coordinated multidisciplinary assessment that identifies various medical, social, or psychological problems and leads to the development of relevant interventions and guides therapeutic decisions [1], [2]. CGA is the best assessment of physiological age, life expectancy, and functional reserve of a patient [3]. CGA includes validated measures of geriatric assessment across the domains of functional status, comorbid medical conditions, psychological state, social support, nutritional status, cognitive function, and medications. In older patients with cancer, CGA has a strong and consistent prognostic value for chemotherapy completion [4], survival [4], [5], [6], severe toxicity [7], [8], [9], early death [10], and quality of life [5] and might also be predictive of treatment benefit [11]. It has also been shown to help identify problems that would not have been considered otherwise or to modify treatment decisions in terms of dosage, intensity, therapeutic regimens, or supportive care [12]. Given its prognostic and predictive value, CGA should be considered a means of patient selection or a stratification factor for data analysis and randomization in clinical trials [13]. Moreover, without CGA information, it is difficult to evaluate the fitness of older individuals included in a clinical trial, limiting extrapolation of the results to the general older population. Indeed, classical oncology tools of functional status assessment such as the Eastern Cooperative Oncology Group (ECOG) or Karnofsky performance status (PS) have been shown to poorly reflect functional impairment in older patients with cancer [14]. In 2000, the International Society of Geriatric Oncology (SIOG) was founded with the goal to foster the development of health professionals in the field of geriatric oncology, in order to optimize treatment of older adults with cancer. SIOG decided to promote efforts in three strategic directions: education, clinical practice, and research [15], [16]. Concerning research, SIOG expressed the objective to increase the relevance of clinical trials for older patients, which implies implementing CGA in clinical trials.
The objective of the present study was therefore to describe the use of CGA in published clinical trials dedicated to older patients with cancer and to assess the evolution in CGA implementation at the time of the SIOG creation and 10 years after.
Subjects, Materials, and Methods
Trial Selection
In January 2015, two of the authors (O.L.S. and J.P.) identified all reports of clinical trials (phase I, phase II, and phase III trials) assessing therapies for hematological or solid tumors and dedicated to older patients (≥60 years) or older patients along with unfit patients (impaired functional status or comorbidities). Included reports were published in English between January 1, 2001, and December 31, 2004 (first time period, i.e., “pre‐geriatric oncology era”), or between January 1, 2011, and December 31, 2014 (second time period, i.e., “geriatric oncology era”), to assess the evolution in CGA implementation between the two time periods. The early 2000s is considered to be the starting point of research focusing on older patients with cancer as relevant guidelines were published at this time [16], and a minimum of 10 years is necessary to observe the impact of such guidelines on clinical trials, as several years will pass between the drafting of a protocol and publishing results. The methodology of this systematic review has been published previously [17]. The full Search strategy is reported in supplemental online Appendix S1.
Data Extraction
Data extraction was performed by the same authors who carried out the initial article selection (O.L.S. and J.P.). The data were cross‐verified by the two data extractors. The collected variables were study design, year of publication, tumor site, source of trial funding, journal impact factor, regions in which trials were conducted, type of investigational therapy, cancer stage, and the notion of a geriatric assessment and each component evaluated. We considered that a CGA was performed when the authors intended in their patients and methods section to perform such an assessment. We also collected each geriatric domain assessed using a validated tool by authors even in the absence of a clear CGA. Domains collected were nutritional status, functional status, cognitive status, psychological status, comorbidity, comedication, overmedication, social status and support, and presence of geriatric syndromes [18]. ECOG or Karnofsky PS data were also collected.
Statistical Analysis
Most of the analyses performed were descriptive. Qualitative data were described by percentage, and statistical comparisons were performed when appropriate using chi‐square or Fisher's exact test as appropriate. Quantitative data were described by median values and interquartile ranges, and statistical comparisons were performed using the Wilcoxon test or the Kruskall‐Wallis test as appropriate. All analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL) or R software version 3.3.1 (http://www.R-project.org/).
Results
Characteristics of the Selected Clinical Trials
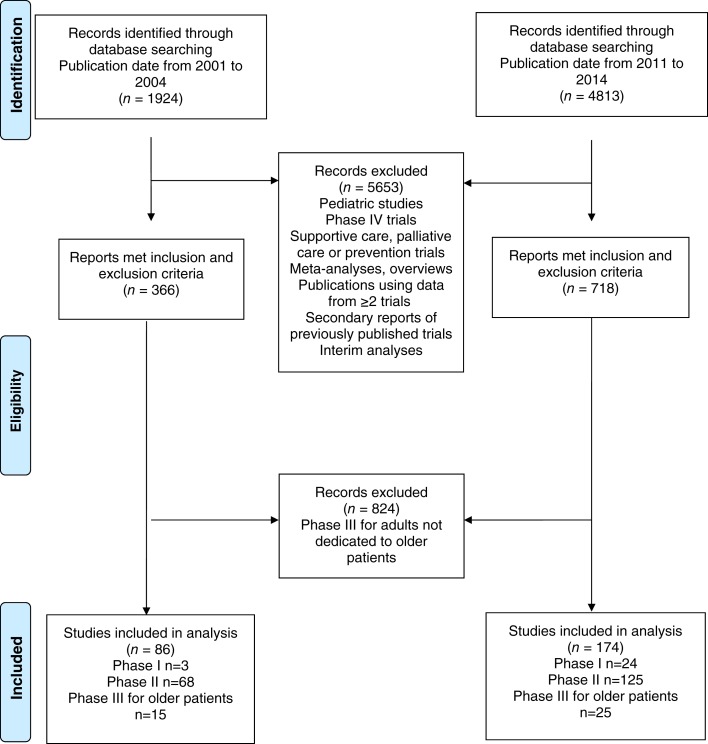
A total of 260 clinical trials including only older patients (or older patients along with unfit patients—impaired functional status or comorbidities) over the two time periods were identified: 27 phase I clinical trials, 193 phase II trials, and 40 phase III clinical trials (Fig. 1). In the “pre‐geriatric oncology era,” 3 phase I trials, 68 phase II clinical trials, and 15 phase III clinical trials were identified; in the “geriatric oncology era,” we identified 24 phase I trials, 125 phase II clinical trials, and 25 phase III clinical trials. Study characteristics of included trials are presented in supplemental online Tables S1–S3. Median (interquartile range) age of patients included in phase I trials was 72 years (68–76), that of patients included in phase II trials was 72 years (72–76), and that of patients included in phase III trials was 72 years (68–74).
Figure 1.
Flow diagram.
Use of CGA and Components of CGA Evaluated in Dedicated Clinical Trials
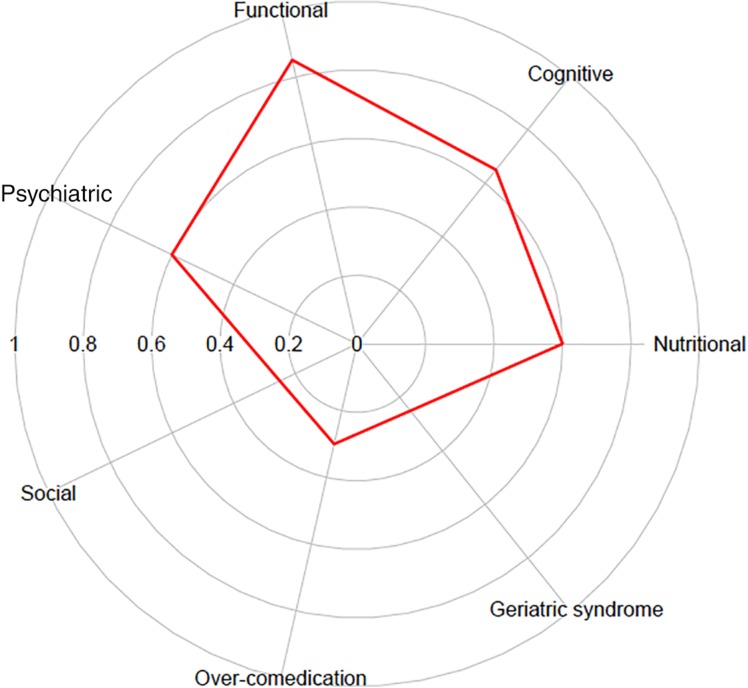
CGA was used in 9% of dedicated phase II (17/193) and 8% of dedicated phase III trials (3/40); it was never used in dedicated phase I trials. ECOG or Karnofsky PS was reported in 67% of phase I trials, 79% of phase II trials, and 75% of phase III trials. Functional assessment was reported for 4% of dedicated phase I, 11% of dedicated phase II, and 13% of dedicated phase III trials. Comorbidities were reported for 31% of dedicated phase II trials and 33% of dedicated phase III trials, and cognitive status was reported for 7% of dedicated phase II trials and 13% of phase III trials (Table 1). When a CGA was used in a trial (n = 20), a median of 5 domains were reported (range: 2–7; Fig. 2).
Table 1. Use of CGA and its components in dedicated clinical trials.
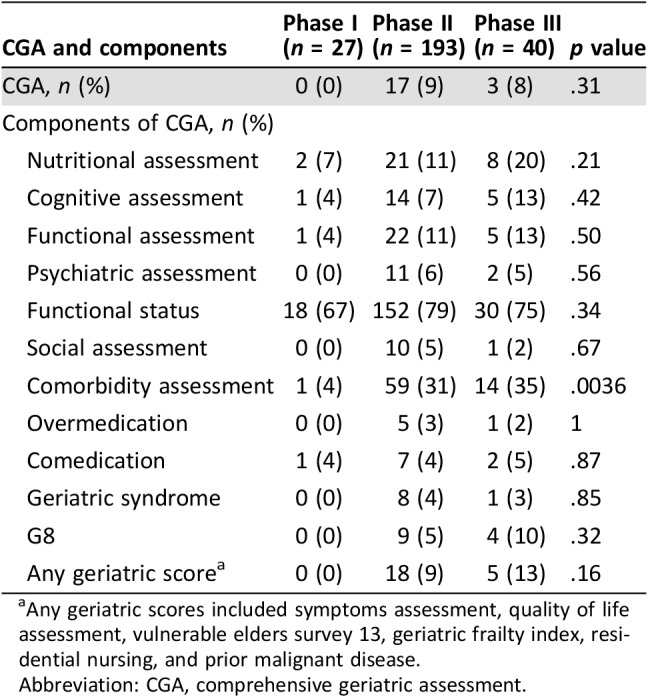
Any geriatric scores included symptoms assessment, quality of life assessment, vulnerable elders survey 13, geriatric frailty index, residential nursing, and prior malignant disease.
Abbreviation: CGA, comprehensive geriatric assessment.
Figure 2.
Frequency of geriatric dimensions reporting in comprehensive geriatric assessment (n = 20).
Evolution of CGA and its Components Between the Two Time Periods
The use of CGA within clinical trials increased from 1% to 11% between the two periods considered (p = .0051; Table 2). The assessment of functional status increased from 3% to 14% (p = .0094), the assessment of comorbidities increased from 19% to 33% (p = .013), and psychiatric assessment increased from 1% to 7% (p = .066). There was a trend toward increase in the assessment of cognitive status (p = .086). Nutritional assessment remained stable (12% in both periods).
Table 2. Geriatric assessment criteria used according to the considered period.
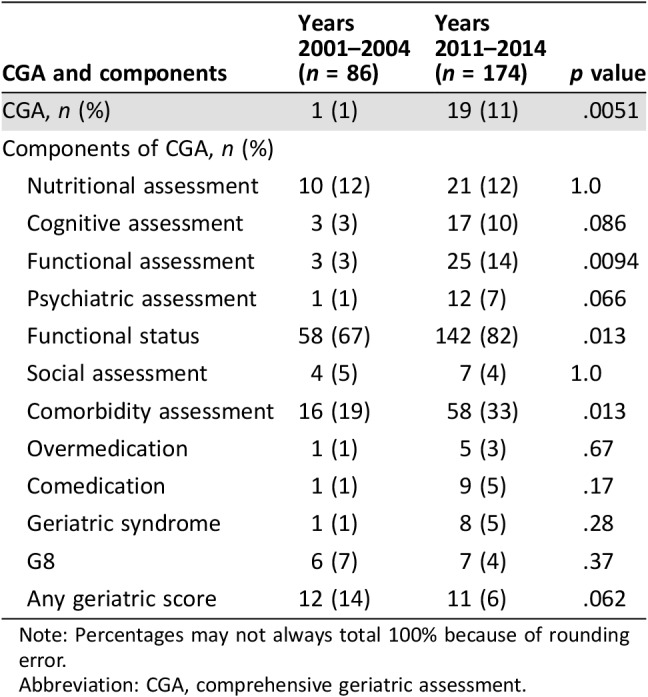
Note: Percentages may not always total 100% because of rounding error.
Abbreviation: CGA, comprehensive geriatric assessment.
Characteristics of Trials According to the Use of CGA in Clinical Trials
The use of CGA was more frequent among trials investigating solid tumors compared with hematology trials (11% vs. 3%, p = .017) and among regions where trials were conducted (p = .0053; Table 3). Industrial funding and type of investigational therapy were not significantly different according to the use of CGA.
Table 3. Factors associated with the use of CGA in clinical trials.
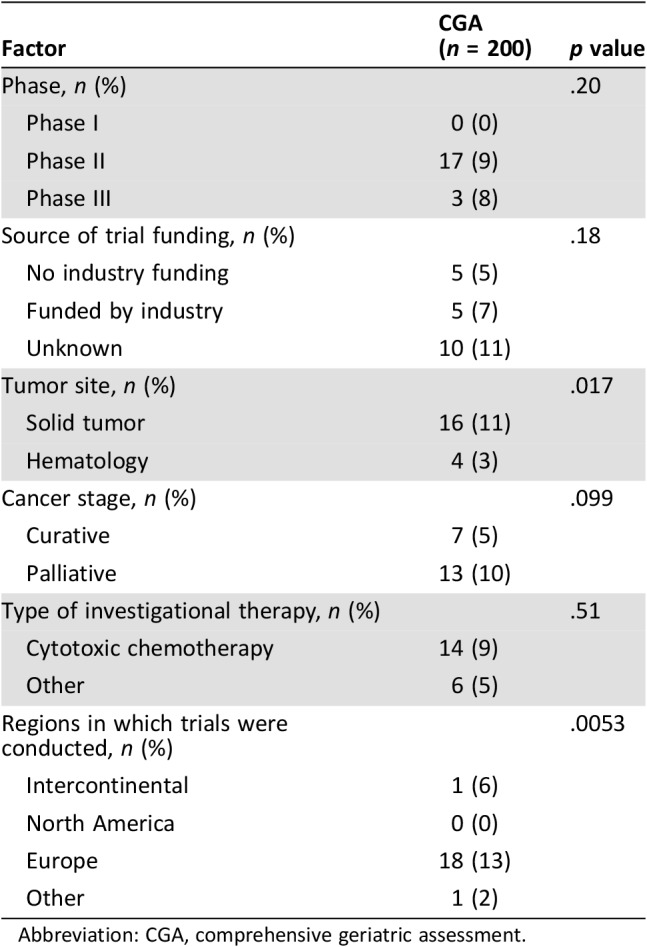
Abbreviation: CGA, comprehensive geriatric assessment.
Discussion
We found that the use of CGA in clinical trials dedicated to older patients increased significantly between the two periods considered, yet the level of CGA use remained surprisingly low in the geriatric oncology era. There are several reasons that might explain the lack of CGA in clinical trials. For instance, its implementation is limited by the amount of time needed to conduct a CGA and the human resources required (i.e., its economic cost). However, a large, prospective, multicenter cohort study conducted in Belgium that included 1,967 patients demonstrated its feasibility in daily practice [19]; therefore, its implementation in clinical trials should be possible. In countries or institutions where CGA is not routinely performed, its implementation in clinical trials would require additional efforts to organize and fund CGA, but one could expect that the ongoing expansion of CGA in daily practice will make it easier to implement it in future trials. Screening tools to identify patients who could benefit from a CGA may also be an interesting option to reduce the burden associated with assessing all patients comprehensively; examples include the G8 [20], the Cancer and Leukemia Group B (CALGB) tool [21], the Vulnerable Elders Survey‐13, and the Groningen Frailty Index [22]. The infrequent use of CGA in clinical trials could also be seen as a reflection of the poor involvement of geriatricians in the design of oncology trials. To reinforce collaboration between geriatricians and oncologists, geriatric skills should be integrated into the training of oncologists, and vice versa [23]. Another limitation of CGA use in a clinical trial setting is its lack of standardization. This is reflected herein by the number of domains assessed for a CGA that varied from 2 to 7. However, some efforts toward this have been made; for instance, the SIOG guidelines recommend that CGA include at least an assessment of cognitive and emotional status, activities of daily living (ADL), instrumental ADL, home environment, social support, nutrition, comorbidities, and polymedication [24]. The Geriatric Core Dataset was developed recently [25]. Following a consensus approach, a panel of 14 geriatricians from oncology clinics identified a set of geriatric data to be collected in cancer trials of older patients including (a) social assessment: living alone or support requested to stay at home; (b) functional autonomy: ADL questionnaire and short instrumental ADL questionnaire; (c) mobility: Timed Up and Go test; (d) nutrition: weight loss during the past 6 months and body mass index; (e) cognition: Mini‐Cog test; (f) mood: mini‐Geriatric Depression Scale; and (g) comorbidity: updated Charlson Comorbidity Index.
Recently, the European Organisation for Research and Treatment (EORTC), the SIOG, the Alliance for Clinical Trials in Oncology, the American Society of Clinical Oncology, and the CALGB published guidelines on the design of clinical trials in older patients with cancer [21], [26], [27], [28]. These all recommend the integration of a CGA in future studies because it will give information on overall status of older patients included [21], [26], [27]. Physicians could therefore be able to identify whether patients included in the trials are similar to those treated in daily clinical practice. CGA is consequently important for extrapolation of data. The CALGB also identified that integration of CGA in clinical trials would permit detection of clinical information that would otherwise be unrecognized such as cognitive impairment [21]. This information is of the utmost importance when older patients with cancer included in clinical trials consent to participate. Moreover, integration of CGA could help identify factors that are predictive of toxicity or mortality other than cancer itself [21]. The EORTC also mentioned that the inclusion of CGA in clinical trials could help clarify the prognostic value of CGA itself [26]. The recent publication of these guidelines in 2011 and 2013 may help further increase the use of CGA in clinical trials in the future, but it may be necessary for the European Medicines Agency and U.S. Food and Drug Administration to require CGA in trials dedicated to older populations. Other important aspects that we need to take into account in order to improve the relevance of clinical trials dedicated to older patients include the use of broader eligibility criteria in order to make clinical trials more representative of real‐life patients [29], [30], the use of endpoints relevant to older patients such as quality of life [27], the stratification of investigational treatment according to patients fitness, and the development of novel trial designs such as extended trials or prospective cohorts [31].
The study, however, has some limitations. It is a retrospective systematic review of published trials, and publication bias was not taken into account [32], which can be significant, especially in older patients with comorbidities or impaired functional status. Indeed, because of increased toxicity and decreased tolerance [33], authors and sponsors (such as industry [34]) may be reluctant to publish trial reports with negative results and editors might be less likely to accept them [35]. A second limitation is that it takes many years from the drafting of a protocol to publication of results. As a consequence, the maximal effect of the recent guidelines published by the SIOG, EORTC, Alliance for Clinical Trials in Oncology, and CALGB was not reached during the second period considered herein.
Conclusion
The use of CGA in clinical trials dedicated to older patients remains low. Dissemination into daily practice, and efforts made by learned societies to integrate CGA into study designs, could help increase its use.
See http://www.TheOncologist.com for supplemental material available online.
Author Contributions
Conception/design: Olivia Le Saux, Julien Péron
Provision of study material or patients: Olivia Le Saux, Julien Péron
Collection and/or assembly of data: Olivia Le Saux
Data analysis and interpretation: Olivia Le Saux, Claire Falandry, Hui K. Gan, Benoit You, Gilles Freyer, Julien Péron
Manuscript writing: Olivia Le Saux, Claire Falandry, Hui K. Gan, Benoit You, Gilles Freyer, Julien Péron
Final approval of manuscript: Olivia Le Saux, Claire Falandry, Hui K. Gan, Benoit You, Gilles Freyer, Julien Péron
Disclosures
The authors indicated no financial relationships.
References
- 1.Ellis G, Whitehead MA, Robinson D et al. Comprehensive geriatric assessment for older adults admitted to hospital: Meta‐analysis of randomised controlled trials. BMJ 2011;343:d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohile SG, Dale W, Somerfield MR et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol 2018;36:2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korc‐Grodzicki B, Holmes HM, Shahrokni A. Geriatric assessment for oncologists. Cancer Biol Med 2015;12:261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aaldriks AA, Maartense E, le Cessie S et al. Predictive value of geriatric assessment for patients older than 70 years, treated with chemotherapy. Crit Rev Oncol Hematol 2011;79:205–212. [DOI] [PubMed] [Google Scholar]
- 5.Biesma B, Wymenga AN, Vincent A et al. Quality of life, geriatric assessment and survival in elderly patients with non‐small‐cell lung cancer treated with carboplatin‐gemcitabine or carboplatin‐paclitaxel: NVALT‐3 a phase III study. Ann Oncol 2011;22:1520–1527. [DOI] [PubMed] [Google Scholar]
- 6.Girones R, Torregrosa D, Gomez‐Codina J et al. Lung cancer chemotherapy decisions in older patients: The role of patient preference and interactions with physicians. Clin Transl Oncol 2012;14:183–189. [DOI] [PubMed] [Google Scholar]
- 7.Freyer G, Geay JF, Touzet S et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: A GINECO study. Ann Oncol 2005;16:1795–1800. [DOI] [PubMed] [Google Scholar]
- 8.Hurria A, Togawa K, Mohile SG et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol 2011;29:3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Extermann M, Chen H, Cantor AB et al. Predictors of tolerance to chemotherapy in older cancer patients: A prospective pilot study. Eur J Cancer 2002;38:1466–1473. [DOI] [PubMed] [Google Scholar]
- 10.Soubeyran P, Fonck M, Blanc‐Bisson C et al. Predictors of early death risk in older patients treated with first‐line chemotherapy for cancer. J Clin Oncol 2012;30:1829–1834. [DOI] [PubMed] [Google Scholar]
- 11.Tucci A, Ferrari S, Bottelli C et al. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer 2009;115:4547–4553. [DOI] [PubMed] [Google Scholar]
- 12.Caillet P, Laurent M, Bastuji‐Garin S et al. Optimal management of elderly cancer patients: Usefulness of the Comprehensive Geriatric Assessment. Clin Interv Aging 2014;9:1645–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon R. Clinical trials for predictive medicine: New challenges and paradigms. Clin Trials 2010;7:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Repetto L, Fratino L, Audisio RA et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology Study. J Clin Oncol 2002;20:494–502. [DOI] [PubMed] [Google Scholar]
- 15.Lichtman SM, Balducci L, Aapro M. Geriatric oncology: A field coming of age. J Clin Oncol 2007;25:1821–1823. [DOI] [PubMed] [Google Scholar]
- 16.Balducci L. Geriatric oncology: Challenges for the new century. Eur J Cancer 2000;36:1741–1754. [DOI] [PubMed] [Google Scholar]
- 17.Le Saux O, Falandry C, Gan HK et al. Inclusion of elderly patients in oncology clinical trials. Ann Oncol 2016;27:1799–1804. [DOI] [PubMed] [Google Scholar]
- 18.Wellens NI, Deschodt M, Flamaing J et al. First‐generation versus third‐generation comprehensive geriatric assessment instruments in the acute hospital setting: A comparison of the Minimum Geriatric Screening Tools (MGST) and the interRAI Acute Care (interRAI AC). J Nutr Health Aging 2011;15:638–644. [DOI] [PubMed] [Google Scholar]
- 19.Kenis C, Bron D, Libert Y et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: Results of a prospective multicentric study. Ann Oncol 2013;24:1306–1312. [DOI] [PubMed] [Google Scholar]
- 20.Martinez‐Tapia C, Canoui‐Poitrine F, Bastuji‐Garin S et al. Optimizing the G8 screening tool for older patients with cancer: Diagnostic performance and validation of a six‐item version. The Oncologist 2016;21:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurria A, Cirrincione CT, Muss HB et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol 2011;29:1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Decoster L, Van Puyvelde K, Mohile S et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: An update on SIOG recommendations. Ann Oncol 2015;26:288–300. [DOI] [PubMed] [Google Scholar]
- 23.Maggiore RJ, Callahan KE, Tooze JA et al. Geriatrics fellowship training and the role of geriatricians in older adult cancer care: A survey of geriatrics fellowship directors. Gerontol Geriatr Educ 2018;39:170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wildiers H, Heeren P, Puts M et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paillaud E, Soubeyran P, Caillet P et al. Multidisciplinary development of the Geriatric Core Dataset for clinical research in older patients with cancer: A French initiative with international survey. Eur J Cancer 2018;103:61–68. [DOI] [PubMed] [Google Scholar]
- 26.Pallis AG, Ring A, Fortpied C et al. EORTC workshop on clinical trial methodology in older individuals with a diagnosis of solid tumors. Ann Oncol 2011;22:1922–1926. [DOI] [PubMed] [Google Scholar]
- 27.Wildiers H, Mauer M, Pallis A et al. End points and trial design in geriatric oncology research: A joint European Organisation for Research and Treatment of Cancer—Alliance for Clinical Trials in Oncology—International Society Of Geriatric Oncology position article. J Clin Oncol 2013;31:3711–3718. [DOI] [PubMed] [Google Scholar]
- 28.Hurria A, Levit LA, Dale W et al. Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology Statement. J Clin Oncol 2015;33:3826–3833. [DOI] [PubMed] [Google Scholar]
- 29.Lichtman SM, Harvey RD, Damiette Smit MA et al. Modernizing clinical trial eligibility criteria: recommendations of the American Society of Clinical Oncology‐Friends of Cancer Research Organ Dysfunction, Prior or Concurrent Malignancy, and Comorbidities Working Group. J Clin Oncol 2017;35:3753–3759. [DOI] [PubMed] [Google Scholar]
- 30.Kim ES, Bruinooge SS, Roberts S et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J Clin Oncol 2017;35:3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soto‐Perez‐De‐Celis E, Lichtman SM. Considerations for clinical trial design in older adults with cancer. Expert Opin Investig Drugs 2017;26:1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Easterbrook PJ, Berlin JA, Gopalan R et al. Publication bias in clinical research. Lancet 1991;337:867–872. [DOI] [PubMed] [Google Scholar]
- 33.Wedding U, Honecker F, Bokemeyer C et al. Tolerance to chemotherapy in elderly patients with cancer. Cancer Control 2007;14:44–56. [DOI] [PubMed] [Google Scholar]
- 34.Als‐Nielsen B, Chen W, Gluud C et al. Association of funding and conclusions in randomized drug trials: A reflection of treatment effect or adverse events? JAMA 2003;290:921–928. [DOI] [PubMed] [Google Scholar]
- 35.van Lent M, Overbeke J, Out HJ. Role of editorial and peer review processes in publication bias: Analysis of drug trials submitted to eight medical journals. PLoS One 2014;9:e104846. [DOI] [PMC free article] [PubMed] [Google Scholar]