Management of esophageal squamous cell carcinoma remains challenging, because detection often occurs at advanced stages and because of the high incidence of lymph node metastasis. This article focuses on whether lymphopenia is associated with response and tumor progression in patients with advanced esophageal squamous cell carcinoma who received concurrent chemotherapy.
Keywords: Esophageal squamous cell carcinoma, Lymphopenia, Radiation, Chemotherapy
Abstract
Background.
Lymphopenia occurs commonly in esophageal squamous cell carcinoma (ESCC) and may influence treatment outcomes. We aimed to examine its association with treatment response and tumor progression in patients with locally advanced ESCC treated with concurrent chemoradiotherapy (CCRT).
Materials and Methods.
A total of 286 patients with stage II–IVa ESCC treated with CCRT between 2015 and 2017 were analyzed. Total lymphocyte counts were assessed at baseline, weekly, and 4 weeks after CCRT. Pretreatment lymphopenia was defined as total lymphocyte count <1,000 cells per mm3 at diagnosis, and treatment‐related lymphopenia was defined as total lymphocyte count <200 cells per mm3 with 6 weeks after starting CCRT. Univariate and multivariate logistic regression methods were used to analyze factors associated treatment‐related lymphopenia and treatment response.
Results.
Lymphopenia was observed in 44 patients (15.4%) at initial diagnosis. Pretreatment lymphopenia was significantly associated with greater tumor length, worse T status, body mass index ≤18.5 kg/m2, and weight loss ≥3 kg in the previous 3 months. Six weeks after starting CCRT, 89 patients (31%) developed treatment‐related lymphopenia. Tumor progression and cancer‐related death were more frequently observed in treatment‐related lymphopenia group than those without (76.4% vs. 52.8% and 58.4% vs. 39.6%). A complete response (CR) was achieved in 62 patients (21.7%). In multivariate analysis, treatment‐related lymphopenia was significantly associated with lack of clinical CR, and older age, lower tumor location, greater tumor length, and larger planning target volume were independent predictors of treatment‐related lymphopenia.
Conclusion.
Treatment‐related lymphopenia during CCRT is an independent predictor for poor treatment response in ESCC.
Implications for Practice.
A total of 286 patients with locally advanced esophageal squamous cell carcinoma were treated with concurrent chemoradiotherapy (CCRT), and treatment‐related lymphopenia occurred in 31% of patients within 6 weeks from the start of CCRT. Treatment‐related lymphopenia was significantly associated with lack of treatment response, and older age, lower tumor location, greater tumor length, and larger planning target volume were independent predictors of treatment‐related lymphopenia. Lymphocyte count is an inexpensive biomarker that may be easily used by clinicians to identify patients who are most likely to benefit from CCRT.
摘要
背景。淋巴细胞减少症常见于食管鳞状细胞癌 (ESCC),可能影响其治疗结果。本文目的是研究淋巴细胞减少症与接受同步放化疗 (CCRT) 治疗的局部晚期 ESCC 患者的治疗反应和肿瘤进展之间的关系。
材料 和方法。分析 286 例于 2015 年至 2017 年期间应用 CCRT 治疗的 II‐IVa期 ESCC 患者的临床资料。测量基线、每周和 CCRT 4 周后的总淋巴细胞计数。治疗前淋巴细胞减少症定义为,诊断时淋巴细胞总数 < 1 000/mm3,治疗相关淋巴细胞减少症定义为,接受 CCRT 6 周后,淋巴细胞总数 <200/mm3。采用单因素和多因素逻辑回归分析治疗相关淋巴减少症的相关因素及治疗反应。
结果。初诊有淋巴细胞减少症患者 44 例 (15.4%)。治疗前淋巴细胞减少症与肿瘤长度较大、T 分期较差、体重指数 ≤18.5 kg/m2、前 3 个月体重减轻 ≥3 kg 显著相关。开始 CCRT 治疗 6 周后,89 名患者 (31%) 出现了与治疗相关的淋巴细胞减少症。治疗相关淋巴细胞减少症组的肿瘤进展和癌症相关死亡发生率高于无淋巴细胞减少症组(76.4% vs. 52.8%; 58.4% vs. 39.6%)。62 例患者 (21.7%) 完全缓解 (CR)。多因素分析结果显示,治疗相关淋巴细胞减少症与临床 CR 缺乏显著相关,年龄大、肿瘤位置低、肿瘤长度大、计划靶体积大是治疗相关淋巴细胞减少症的独立预测因子。
结论。CCRT 过程中出现治疗相关淋巴细胞减少症是 ESCC 治疗反应差的独立预测因子。
实践意义:286 例局部晚期食管鳞状细胞癌患者采用了同步放化疗 (CCRT) 治疗,31% 的患者在开始 CCRT 后 6 周内出现了治疗相关淋巴减少症。治疗相关淋巴细胞减少症与临床治疗反应缺乏显著相关,年龄大、肿瘤位置低、肿瘤长度大、计划靶体积大是治疗相关淋巴细胞减少症的独立预测因子。淋巴细胞计数是一种低成本的生物标志物,临床医生可以很容易地利用该指标识别 CCRT 治疗最为有效的患者。
Introduction
Esophageal squamous cell carcinoma (ESCC) is the fifth most common causes of cancer in China, with an annual mortality of nearly 100 per 100,000 [1]. The prognosis of this malignancy is extremely poor because of the high incidence of lymph node metastasis, with a 5‐year survival rate of only 25% [2], [3]. Because approximately 50% of patients with ESCC are detected at locally advanced stages, management of these patients remains challenging [4]. On the basis of Radiation Therapy Oncology Group 85‐01 trial results, concurrent chemoradiotherapy (CCRT) has been commonly recommended to patients with locally advanced cancer [5]. However, a greater number of clinical trials have definitely confirmed that preoperative CCRT benefits only 23%–49% of patients who get pathologic complete response (CR), and nearly half of these patients do not achieve a good response to treatment [6], [7]. Therefore, predictive biomarkers for tumor response to CCRT are needed to guide clinical practice and trial design.
The immune system plays a vital role in the prevention of cancer development and progression [8]. Of all the immune cells in the circulation, lymphocytes comprise approximate 30% of all human white blood cell population and are essential effector cells in the mediating cellular immunity against tumor cells [9]. Several previous studies have demonstrated that lower pretreatment lymphocyte counts are correlated with poor survival in multiple cancer types such as breast cancer, small cell lung cancer, pancreatic ductal adenocarcinoma, and cervical cancer [10], [11], [12], [13]. Lymphopenia is frequently observed following the administration of antineoplastic therapies for various kinds of cancer and has significant clinical consequences. For example, a recent study of patients with squamous cell head and neck cancer found that this patient population had normal total lymphocyte counts at baseline, but 2 months after beginning chemoradiation, 61% of patients developed severe treatment‐related lymphopenia, and patients with total lymphocyte counts <500 cells per mm3 had early tumor recurrence [14]. Treatment‐related lymphopenia was also observed in patients with cervical cancer who received chemoradiation. Up to 53% patients developed severe post‐treatment lymphopenia, and multivariate analysis demonstrated that post‐treatment lymphopenia had a 58% decrease of hazards of death [15].
However, the clinical and predictive value of lymphopenia in patients with ESCC remains largely unknown. This retrospective study was therefore performed to investigate whether lymphopenia is associated with response and tumor progression in patients with locally advanced ESCC who received CCRT.
Subjects, Materials, and Methods
Patient Section
This retrospective analysis was reviewed and approved by the institutional review board of Huai'an First Hospital. Patients with locally advanced ESCC treated with CCRT between January 2015 and December 2017 were identified. Inclusion criteria included the following: (a) biopsy‐confirmed ESCC, (b) clinically staged II–IVa according to the American Joint Committee on Cancer 6th edition of tumor‐node‐metastasis (TNM) classification for esophageal carcinoma, (c) measurable disease at baseline, (d) no prior therapy, (e) Eastern Cooperative Oncology Group performance status (ECOG PS) 0–2, and (f) at least four documented weekly absolute lymphocyte counts during CCRT. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) criteria were followed when possible for the design and analysis of the study.
Treatments and Assessments
All patients received three‐dimensional conformal radiotherapy (3D‐CRT) or intensity modulated radiotherapy (IMRT). A dose of 50–60 Gy (1.8–2.0 Gy per fraction, 5 days a week) started on the first day of chemotherapy. The gross tumor volume (GTV) was defined as any visible primary tumor on the computed tomography (CT) or esophageal barium image and clinical involved lymph node. The clinical target volume (CTV) included the GTV with 3‐cm craniocaudal margin, the metastatic lymph nodes, and regional lymph nodes. For upper thoracic tumor, the regional lymph nodes included bilateral supraclavicular and lymph node stations (LNS) 1R, 1L, 2R, 2L, 4R, 4L, 5, and 7; the regional lymph nodes for the middle thoracic tumor included LNS 1R, 1L, 2R, 2L, 4R, 4L, 5, 7, and 8; and the regional lymph nodes for the lower thoracic tumor included LNS 4R, 4L, 5, 7, 8 and left gastric lymph nodes (according to the 2014 International Association for the Study of Lung Cancer lymph node map [16]). The planning target volume (PTV) was defined as the CTV plus a 0.5–1‐cm margin. Two kinds of chemotherapy regimens were used in the study: (a) concurrent chemotherapy consisted of cisplatin (25 mg/m2 on day 1) and docetaxel (25 mg/m2 on day 1) weekly for 5 weeks. Three or four weeks after completion of CCRT, two additional cycles of consolidation chemotherapy (docetaxel 75mg/m2 on day 1 and cisplatin 25mg/m2 on days 1–3) were performed at 3‐ or 4‐weeks intervals. (b) Patients were administered oral S‐1 (70 mg/m2, twice per day) alone on days 1–14 and days 29–42.
Tumor response to treatment was evaluated by esophagography and chest CT scan 4 weeks after completion of CCRT as described previously [17]. The treatment response was assigned to one of two categories: CR or less than CR.
Treatment‐related toxicity was assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0.
Data Collection and Definitions
Variables including demographic, clinicopathologic, and treatment characteristics were obtained from electronic medical record system. Study variables collected at baseline include age, gender, body mass index (BMI), ECOG PS, history of tobacco exposure, tumor differentiation, clinical stage, grade, tumor location, complete blood count (including hemoglobin concentration and absolute lymphocyte counts), and serum albumin. Treatment‐related variables such as radiation dose, fractionation, PTV, mean lung dose (MLD), mean heart dose, and concurrent chemotherapy type were recorded. Absolute lymphocyte count was obtained at baseline, during (weekly), and 4 weeks after CCRT.
Pretreatment lymphopenia was defined as total lymphocyte count <1,000 cells per mm3 base on commonly accepted reference value [18]. The total lymphocyte count <200 cells per mm3 (grade IV, CTCAE 4.0) during CCRT weeks 1–6 was defined as treatment‐related lymphopenia in accordance with previous study [19]. Nutritional status was estimated by three parameters used in most screening tools; BMI, recent weight loss, and serum albumin. BMI was calculated as weight in kilograms divided by the square of height in meters. Underweight was defined as BMI less than 18.5 kg/m2, according to the World Health Organization recommendations for Asian populations. Recent weight loss was defined as weight loss ≥3 kg in the previous 3 months. Hypoalbuminemia was defined as serum albumin level less than 35 g/L.
Statistical Analysis
Data normality was assessed using Kolmogorov‐Smirnov tests. Patient and clinicopathologic characteristics were summarized by using descriptive statistics. Chi‐square tests (or Fisher's exact test) were used for proportional comparison. Univariate and multivariate logistic regression methods were used to analyze factors associated with treatment response and treatment‐related lymphopenia. Factors with p values <.1 on univariate analyses were entered as covariates in multivariate regression models. All statistical analyses were performed using SPSS Statistics version 20.0 (IBM, Armonk NY). A two‐side p value <.05 was considered statistically significant.
Results
Patient and Treatment Characteristics
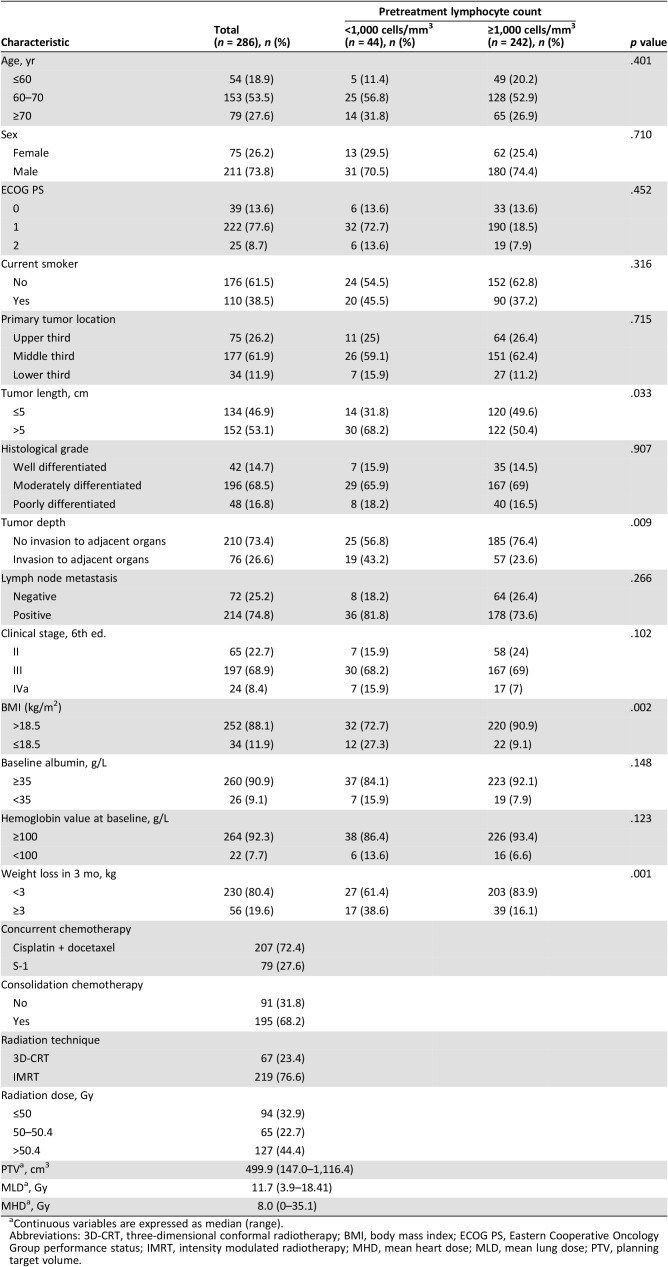
A total of 286 patients met the inclusion criteria and were finally included in this study. Patient and treatment characteristics are summarized in Table 1. All patients had histologically confirmed squamous cell carcinoma. Seventy‐four percent were male, and the median age at diagnosis was 67 years (range, 47–84). Most tumors (61.9%) originated from the middle thoracic esophagus. Fifty‐three percent of primary tumors were longer than 5 cm, with a median length of 5.6 cm (range, 2–11.5). Sixty‐five patients (22.7%) had stage II disease, 197 (68.9%) had stage III disease, and 24 (8.4%) had stage IVa disease. Regarding treatment details, 76.6% were treated with IMRT, and the rest were treated with 3D‐CRT. The median total radiation dose and fraction size were 50.4 Gy and 1.8 Gy per fraction, respectively. Radiation therapy (RT) was completed to at least of 50 Gy or more in 263 patients (92%), 10 patients (3.6%) were given 40–50 Gy, and 13 cases (4.4%) received less than 40 Gy because of treatment‐related toxicity. As for chemotherapy, the majority (72.3%) of patients received concurrent chemotherapy with cisplatin and docetaxel.
Table 1. Patient and treatment characteristics.
Continuous variables are expressed as median (range).
Abbreviations: 3D‐CRT, three‐dimensional conformal radiotherapy; BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance status; IMRT, intensity modulated radiotherapy; MHD, mean heart dose; MLD, mean lung dose; PTV, planning target volume.
Association of Pretreatment Lymphopenia with Baseline Variables
Prior to initiating treatment, 44 patients (15.4%) had lymphopenia. Patient and tumor characteristics separated by pretreatment lymphocyte count are shown in Table 1. Of the clinicopathological features analyzed, BMI ≤18.5 kg/m2 (27.3% vs. 9.1%, p = .002) and weight loss ≥3 kg in the previous 3 months (38.6% vs. 16.1%, p = .001) were more frequently observed in patients with pretreatment lymphopenia compared with those with lymphocyte count ≥1,000 cells per mm3. Additionally, tumor length was also significantly greater in the pretreatment lymphopenia group than in the normal lymphocyte count group (p = .033). Regarding clinical stage, the incidence of invasion to adjacent organs was significant higher in the pretreatment lymphopenia group than in the normal lymphocyte count group (p = .009). Otherwise, there were no significant differences between the two groups including age, sex, smoking history, lymph node metastasis, tumor location, and tumor differentiation (p > .05, Table 1).
Treatment‐Related Lymphopenia
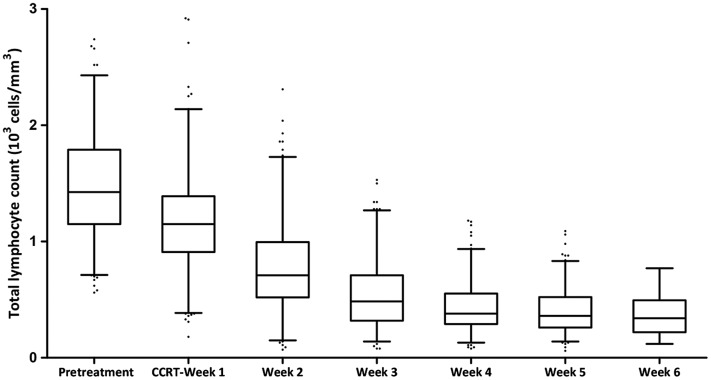
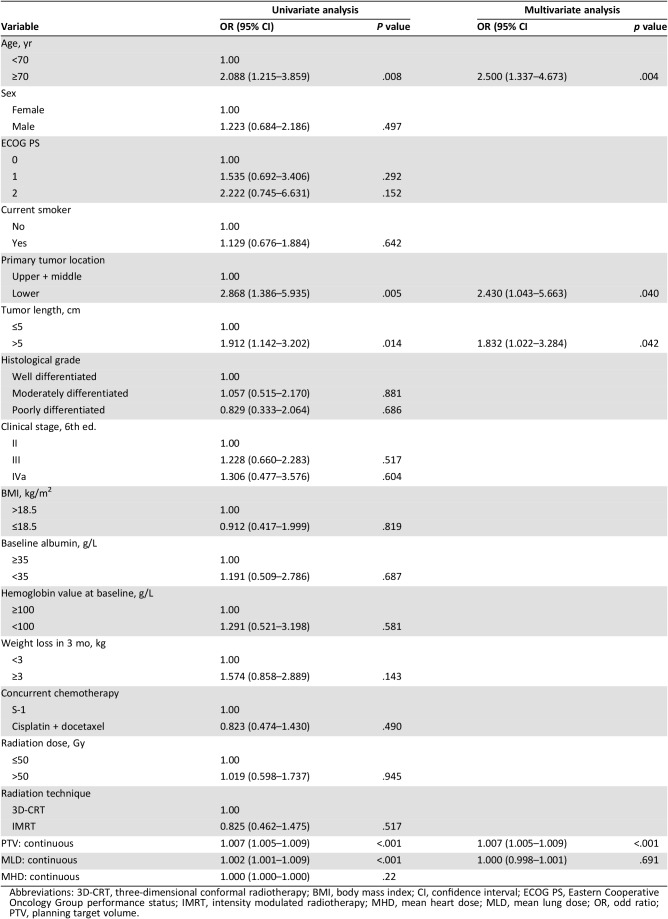
Lymphocyte count results recorded each week of CCRT are listed in Figure 1. The median pretreatment absolute lymphocyte count was 1,425 cells per mm3 (range, 560–3,830) and declined to 1,150, 710, 485, 380, 360, and 340 cells per mm3 from weeks 1–6, respectively. Then, the total lymphocyte count (median, 715 cells per mm3; range, 175–1,600) slowly increased 4 weeks after completion of treatment. During CCRT, a total of 89 patients (31%) had treatment‐related lymphopenia. Among them, 1 patient was first noted during the first week, 14 in the second week, 18 in the third week, 32 in the fourth week, 21 in the fifth week, and 3 in the sixth week. The incidence of treatment‐related lymphopenia in patients receiving RT with cisplatin and docetaxel was not significantly different from those receiving RT with S1 (30% vs. 34.2%, p = .568). Table 2 shows the univariate logistic regression analysis of potential factors associated with treatment‐related lymphopenia. Older age (p = .008), lower tumor location (p = .005), greater tumor length (p = .014), larger PTV volume (p < .001), and higher MLD (p < .001) were significantly associated with treatment‐related lymphopenia. The final multivariate analysis indicated that older age (odds ratio [OR], 2.500; 95% confidence interval [CI], 1.337–4.673; p = .005), lower tumor location (OR, 2.430; 95% CI, 1.043–5.663; p = .04), greater tumor length (OR, 1.832; 95% CI, 1.022–3.284; p = .042), and larger PTV (OR, 1.007; 95% CI, 1.005–1.009; p < .001) were independent predictors for treatment‐related lymphopenia (Table 2).
Figure 1.
Total lymphocyte count prior to treatment and during each week of concurrent chemoradiotherapy. Horizontal lines inside the box plots represent the median, boxes represent the interquartile range, and whiskers represent 2.5th and 97.5th percentiles.
Abbreviation: CCRT, concurrent chemoradiotherapy.
Table 2. Univariate and multivariate logistic regression analysis of factors associated with treatment‐related lymphopenia.
Abbreviations: 3D‐CRT, three‐dimensional conformal radiotherapy; BMI, body mass index; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; IMRT, intensity modulated radiotherapy; MHD, mean heart dose; MLD, mean lung dose; OR, odd ratio; PTV, planning target volume.
Association of Clinicopathologic Features, Lymphopenia, and CCRT Response
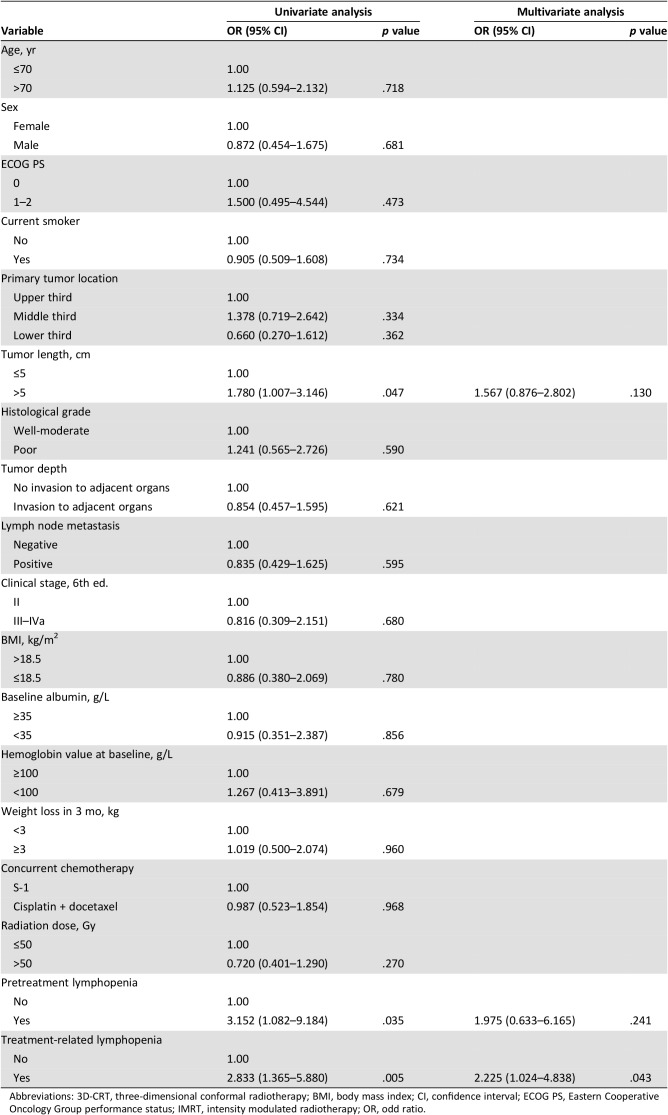
After CCRT, CR, partial response, no change, and progressive disease were achieved in 62 patients (21.7%), 136 patients (47.6%), 84 patients (29.3%), and 4 patients (1.4%), respectively. As shown in Table 3, the CR rate was significantly lower in patients with pretreatment lymphopenia than in those with pretreatment lymphocyte count ≥1,000 cells per mm3 (9.1% vs. 24%, p = .028). Furthermore, the CR rate in patients with treatment‐related lymphopenia was significantly different from that in patients with lymphocyte count ≥200 cells per mm3 (11.2% vs. 26.4%, p = .003). In univariate analysis, tumor length ≥5 cm (p = .047), pretreatment (p = .035), and treatment‐related lymphopenia (p = .005) were significant predictors of tumor response to treatment. In multivariate analysis, treatment‐related lymphopenia was the only independent variable significantly associated with lack of clinical CR (OR, 2.225; 95% CI, 1.024–4.838; p = .043, Table 4).
Table 3. Relationship between lymphopenia and response to concurrent chemoradiotherapy.
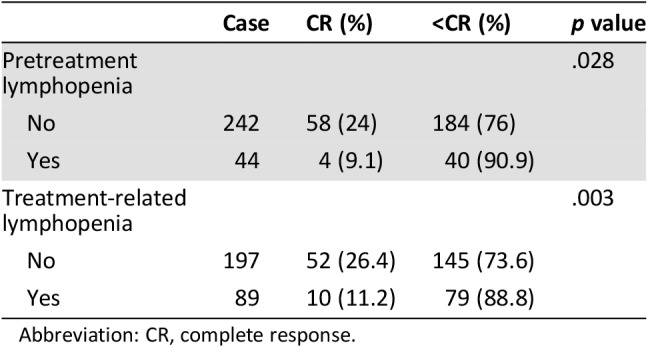
Abbreviation: CR, complete response.
Table 4. Univariate and multivariate logistic regression analysis of factors associated with complete response.
Abbreviations: 3D‐CRT, three‐dimensional conformal radiotherapy; BMI, body mass index; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; IMRT, intensity modulated radiotherapy; OR, odd ratio.
Lymphocyte Counts and Tumor Progression
As of July 2018, 172 of the 286 patients had tumor progression (109 with local recurrence, 47 with distant metastasis, and 16 with both local recurrence and distant metastasis), and deaths resulting from ESCC were identified in 130 patients (Table 5). Tumor progression and cancer‐related deaths were more frequently observed in the treatment‐related lymphopenia group than those in the post‐treatment lymphocyte count ≥200 cells per mm3 group (76.4% vs. 52.8%, p < .001 and 58.4% vs. 39.6%, p = .003, respectively). However, there were no significant differences in the incidence of tumor progression and cancer‐related death among patients with or without pretreatment lymphopenia (65.9% vs. 59.1%, p = .503; and 52.3% vs. 44.2%, p = .329, respectively).
Table 5. Patterns of failure according to pretreatment lymphopenia and treatment‐related lymphopenia.
Discussion
In the present study, we reviewed the clinical significance of lymphopenia in patients with locally advanced ESCC treated with CCRT. Our findings showed that the pretreatment lymphopenia was associated with malnutrition and aggressive clinicopathological feathers of patients with ESCC. Furthermore, our study demonstrated that CCRT for ESCC can dramatically reduce lymphocyte count during treatment, with approximately 31% patients developing severe lymphopenia during CCRT weeks 1–6. This study also confirmed that treatment‐related lymphopenia was significantly associated with CCRT response, and older age, greater tumor length, lower tumor location, and larger PTV were independent predictors for treatment‐related lymphopenia.
Recent studies have demonstrated that lymphocytes can specifically identify and kill tumor cells or release a serial of cytokines to activate host immune system [20]. A lower peripheral lymphocyte count may indicate a poor and insufficient lymphocyte‐medicated immune response to tumor progression. Patients with cancer frequently show decreased total lymphocyte counts at diagnosis. In the present study, we found that 15.4% of patients had lymphopenia at initial diagnosis, and patients with pretreatment lymphopenia had greater tumor length and advanced T status. This indicates that the disease itself is correlated with marked immunosuppression. The high incidence of pretreatment lymphopenia and its association with disease status have been reported in several previous studies. In patients with metastatic breast carcinoma (MBC), advanced soft‐tissue sarcoma, and non‐Hodgkin's lymphomas, the incidence of lymphopenia (<1,000 cells per mm3) before treatment was 25%, 24%, and 27%, respectively. And in patients with MBC, baseline lymphopenia was associated with bone metastasis and more than one metastatic site [21]. Fogar et al. reported that patients with locally advanced or metastatic pancreatic cancer have lower lymphocyte counts than patients with resectable tumors [22]. In addition, our results demonstrated that pretreatment lymphopenia was associated with worse nutritional status. These findings indicated that malnutrition and immune suppression have become common problems in patients with locally advanced ESCC.
The RT‐related lymphopenia was first investigated in 1916 and can occur after irradiation in a variety of different cancers including ESCC [23], [24]. Lymphocytes are the most radiosensitive cells with a dose required to kill 50% lymphocytes (D50) of as low as 1 Gy, and the D90 is nearly 2 Gy [18]. In the present study, nearly 31% patients developed grade IV lymphopenia, and the changes of total lymphocyte counts in patients receiving RT with cisplatin and docetaxel were similar in those receiving RT with S1. These findings are consistent with those seen in patients with locally advanced pancreatic adenocarcinoma receiving capecitabine or gemcitabine‐based chemoradiation [25]. Although both RT and chemotherapy have prolonged negative effects on immune system, recent studies indicated that local RT may play primary role in the etiology of treatment‐related lymphopenia. For example, Jian et al. recently analyzed treatment‐related lymphopenia in non‐small cell lung cancer and found that total lymphocyte counts were largely unchanged after two cycles of neoadjuvant chemotherapy. However, 2 months after the addition of RT, 50% patients had total lymphocyte counts <500 cells per mm3 [26]. Treatment‐related lymphopenia were also observed after RT in high‐grade gliomas, locally advanced cervical cancer, and rectal cancer, which contain little bone marrow or lymphatic tissue [27], [28], [29]. Currently, the exact mechanism underlying the observed lymphocyte reduction during RT remains unclear. It was hypothesized that irradiation of circulating blood pool might represent a possible mechanism. Yovino et al. used mathematical modeling to estimate the radiation dose to circulating lymphocytes during 60‐Gy radiation treatment for glioblastoma [30]. They found that a single fraction (2 Gy) delivered ≥0.5 Gy to 4.6% of the total blood pool. After 30 fractions (60 Gy), the mean dose to the circulating lymphocytes was 2.2 Gy, and 98.8% of circulating lymphocytes received at lease of 0.5Gy. During 6‐week treatment, circulating lymphocytes received a significant dose of radiation while passing through the lung. This could explain why higher mean lung doses were correlated with greater depletion of circulating lymphocytes in our study. In addition, this model also demonstrated that major decreases in the target volume size could significantly reduce the circulating lymphocytes dose [30]. Similarly, we found that PTV was inversely correlated with lymphocyte count in our study. Furthermore, unintentional RT to the lymphopoiesis sites like bone marrow and the thymus, which are the primary lymphoid organs, and spleen and lymph nodes, which are the secondary lymphoid organs, may be potential contributors [31].
CCRT is a standard treatment for locally advanced ESCC, and tumor response to CCRT is important for determining of success or failure of treatment [32]. Tumor response to local RT is not simply dependent on direct damage to irradiated tumor cells, also being largely affected by the systemic immune response [33]. In this study, we found that lymphopenia was significantly associated with lack of clinical response to CCRT in patients with ESCC. Recently, lymphopenia has been reported to be correlated with treatment response in certain solid tumors. Leibowitz‐Amit et al. found that baseline lymphocyte counts were significantly associated with response to platinum‐based neoadjuvant chemotherapy in muscle‐invasive bladder cancer [34]. Similarly, Kou et al. found that pretreatment lymphopenia was associated with poor tumor response to first‐line chemotherapy in metastatic ESCC [35]. In esophageal cancer, a recent study reported that maintaining a higher lymphocyte nadir was correlated with greater pathologic complete response [36]. In our study, the multivariate Cox analysis showed treatment‐related lymphopenia to be independent prognostic factor for treatment response. Therefore, treatment‐related lymphopenia might be used as an additional tool in identifying patients who are most likely to benefit from CCRT.
Given the strong association between treatment‐related lymphopenia and lack of clinical CR, strategies to reduce the risks of treatment‐related lymphopenia are rational. In our study, we found that older age, greater tumor length, lower tumor location, and larger PTV were independent predictors for treatment‐related lymphopenia. In contrast to other clinical parameters such as age, tumor location, and tumor size, PTV represents a parameter that can be modified with treatment plan. For example, shrinking radiation fields using limited‐field RT for glioblastoma has been correlated with less radiation‐related lymphopenia than standard‐field RT, and reduction of RT field does not seem to affect patient prognosis [37]. At present, the delineation of radiotherapeutic nodal clinical target volume for patients with ESCC has reached no global consensus until now. In our center, patients aged less than 80 years were treated with elective node irradiation (ENI), and the CTV included the GTV with 3‐cm craniocaudal margin, the metastatic lymph nodes, and high‐risk lymph nodal regions. However, the toxicities associated with ENI were severe, especially in patients who were treated with concurrent chemotherapy. Some recent reports showed that involved field irradiation (IFI; nodal target volume included only the malignant node) is a selective way of decreasing irradiation volume [38], [39]. In 2018, a meta‐analysis including 10 studies involving a total of 1,348 patients demonstrated no significant differences in the 1‐, 2‐, or 3‐local control rate or the 1‐, 2‐, or the 3‐year survival rate between the ENI and the IFI group, whereas the treatment‐related toxicities were significantly lower in the IFI group [40]. It is therefore possible that appropriate concurrent chemotherapy with IFI could lead to less treatment‐related lymphopenia, which could translate into more CR rates to CCRT.
This study is limited by its retrospective nature and relatively small sample sizes. In addition, the selection of treatment modalities and regimens were heterogeneous throughout this period, and the effects of RT and chemotherapy on lymphopenia could not be separately investigated. Therefore, the findings should be interpreted with some caution. Furthermore, survival analysis was not performed because of the short follow‐up time, and data related to additional treatment such as chemotherapy or radiotherapy to metastatic disease were not complete. As a result, our findings must be considered with these limitations in mind.
Conclusion
Treatment‐related lymphopenia is common and severe, and it seems to be an independent predictor of tumor response to treatment for patients with local ESCC treated with CCRT. Furthermore, the present study suggests that shrinking target volumes and reduction of mean lung dose may spare the circulating lymphocytes in patients at high risk of treatment‐related lymphopenia during CCRT.
Acknowledgments
This work was supported by grants from National Nature Science Foundation of China (Grant No. H1617/81602118).
Author Contributions
Conception/design: Xi‐Lei Zhou, Wei‐Guo Zhu, Yu‐Suo Tong
Provision of study material or patients: Wei‐Guo Zhu, Zhi‐Jian Zhu, Wan‐Wei Wang, Fu‐Zhi Ji, Yu‐Suo Tong
Collection and/or assembly of data: Xi‐Lei Zhou, Xue Deng, Wei‐Jing Tao, Fu‐Zhi Ji
Data analysis and interpretation: Xi‐Lei Zhou, Wei‐Guo Zhu, Yu‐Suo Tong
Manuscript writing: Xi‐Lei Zhou, Wei‐Guo Zhu, Zhi‐Jian Zhu, Yu‐Suo Tong
Final approval of manuscript: Xi‐Lei Zhou, Wei‐Guo Zhu, Zhi‐Jian Zhu, Wan‐Wei Wang, Xue Deng, Wei‐Jing Tao, Fu‐Zhi Ji, Yu‐Suo Tong
Disclosures
The authors indicated no financial relationships.
References
- 1.Zhao KL, Ma JB, Liu G et al. Three‐dimensional conformal radiation therapy for esophageal squamous cell carcinoma: Is elective nodal irradiation necessary? Int J Radiat Oncol Biol Phys 2010;76:446–451. [DOI] [PubMed] [Google Scholar]
- 2.Huang W, Huang Y, Sun J et al. Atlas of the thoracic lymph nodal delineation and recommendations for lymph nodal CTV of esophageal squamous cell cancer in radiation therapy from China. Radiother Oncol 2015;116:100–106. [DOI] [PubMed] [Google Scholar]
- 3.Herskovic A, Russell W, Liptay M et al. Esophageal carcinoma advances in treatment results for locally advanced disease: Review. Ann Oncol 2012;23:1095–1103. [DOI] [PubMed] [Google Scholar]
- 4.Sato S, Kunisaki C, Suematsu H et al. Impact of sarcopenia in patients with unresectable locally advanced esophageal cancer receiving chemoradiotherapy. In Vivo 2018;32:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper JS, Guo MD, Herskovic A et al. Chemoradiotherapy of locally advanced esophageal cancer: long‐term follow‐up of a prospective randomized trial (RTOG 85‐01). Radiation Therapy Oncology Group. JAMA 1999;281:1623–1627. [DOI] [PubMed] [Google Scholar]
- 6.van Hagen P, Hulshof MC, van Lanschot JJ et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 7.Courrech Staal EF, Aleman BM, Boot H et al. Systematic review of the benefits and risks of neoadjuvant chemoradiation for oesophageal cancer. Br J Surg 2010;97:1482–1496. [DOI] [PubMed] [Google Scholar]
- 8.Diakos CI, Charles KA, McMillan DC et al. Cancer‐related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493–e503. [DOI] [PubMed] [Google Scholar]
- 9.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afghahi A, Purington N, Han SS et al. Higher absolute lymphocyte counts predict lower mortality from early‐stage triple‐negative breast cancer. Clin Cancer Res 2018;24:2851–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki R, Lin SH, Wei X et al. Prognostic significance of pretreatment total lymphocyte count and neutrophil‐to‐lymphocyte ratio in extensive‐stage small‐cell lung cancer. Radio Oncol 2018;126:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark EJ, Connor S, Taylor MA et al. Preoperative lymphocyte count as a prognostic factor in resected pancreatic ductal adenocarcinoma. HPB (Oxford) 2007;9:456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YY, Choi CH, Sung CO et al. Clinical significance of changes in peripheral lymphocyte count after surgery in early cervical cancer. Gynecol Oncol 2012;127:107–113. [DOI] [PubMed] [Google Scholar]
- 14.Campian JL, Sarai G, Ye X et al. Association between severe treatment‐related lymphopenia and progression‐free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck 2014;36:1747–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu ES, Oduyebo T, Cobb LP et al. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol Oncol 2016;140:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El‐Sherief AH, Lau CT, Wu CC et al. International Association for the Study of Lung Cancer (IASLC) lymph node map: Radiologic review with CT illustration. Radiographics 2014;34:1680–1691. [DOI] [PubMed] [Google Scholar]
- 17.Zhou XL, Wang WW, Zhu WG et al. High expression of long non‐coding RNA AFAP1‐AS1 predicts chemoradioresistance and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Mol Carcinog 2016;55:2095–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grossman SA, Ellsworth S, Campian J et al. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw 2015;13:1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiraishi Y, Fang P, Xu C et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: A propensity matched analysis of the relative risk of proton versus photon‐based radiation therapy. Radio Oncol 2018;128:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Zhao Q, Deng W et al. Radiation‐related lymphopenia is associated with spleen irradiation dose during radiotherapy in patients with hepatocellular carcinoma. Radiat Oncol 2017;12:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray‐Coquard I, Cropet C, Van Glabbeke M et al. European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res 2009;69:5383–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fogar P, Sperti C, Basso D et al. Decreased total lymphocyte counts in pancreatic cancer an index of adverse outcome. Pancreas 2006;32:22–28. [DOI] [PubMed] [Google Scholar]
- 23.Wild AT, Herman JM, Dholakia AS et al. Lymphocyte‐sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2016;94:571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davuluri R, Jiang W, Fang P et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys 2017;99:128–135. [DOI] [PubMed] [Google Scholar]
- 25.Wild AT, Ye X, Ellsworth SG et al. The association between chemoradiation‐related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol 2015;38:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campian JL, Ye X, Brock M et al. Treatment‐related lymphopenia in patients with stage III non‐small‐cell lung cancer. Cancer invest 2013;31:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang J, DeWees TA, Badiyan SN et al. Clinical and dosimetric predictors of acute severe lymphopenia during radiation therapy and concurrent temozolomide for high‐grade glioma. Int J Radiat Oncol Biol Phys 2015;92:1000–1007. [DOI] [PubMed] [Google Scholar]
- 28.Santin AD, Hermonat PL, Ravaggi A et al. Effects of concurrent cisplatinum administration during radiotherapy vs. radiotherapy alone on the immune function of patients with cancer of the uterine cervix. Int J Radiat Oncol Biol Phys 2000;48:997–1006. [DOI] [PubMed] [Google Scholar]
- 29.Heo J, Chun M, Noh OK et al. Sustaining blood lymphocyte count during preoperative chemoradiotherapy as a predictive marker for pathologic complete response in locally advanced rectal cancer. Cancer Res Treat 2016;48:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yovino S, Kleinberg L, Grossman SA et al. The etiology of treatment‐related lymphopenia in patients with malignant gliomas: Modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest 2013;31:140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venkatesulu BP, Mallick S, Lin SH et al. A systematic review of the influence of radiation‐induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol 2018;123:42–51. [DOI] [PubMed] [Google Scholar]
- 32.Ishihara R, Yamamoto S, Iishi H et al. Factors predictive of tumor recurrence and survival after initial complete response of esophageal squamous cell carcinoma to definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys 2010;76:123–129. [DOI] [PubMed] [Google Scholar]
- 33.Chen HY, Xu L, Li LF et al. Inhibiting the CD8(+) t cell infiltration in the tumor microenvironment after radiotherapy is an important mechanism of radioresistance. Sci Rep 2018;8:11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leibowitz‐Amit R, Israel A, Gal M et al. Association between the absolute baseline lymphocyte count and response to neoadjuvant platinum‐based chemotherapy in muscle‐invasive bladder cancer. Clin Oncol (R Coll Radiol) 2016;28:790–796. [DOI] [PubMed] [Google Scholar]
- 35.Kou F, Lu Z, Li J et al. Pretreatment lymphopenia is an easily detectable predictive and prognostic marker in patients with metastatic esophagus squamous cell carcinoma receiving first‐line chemotherapy. Cancer Med 2016;5:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang P, Jiang W, Davuluri R et al. High lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancer. Radio Oncol 2018;128:584–590. [DOI] [PubMed] [Google Scholar]
- 37.Rudra S, Hui C, Rao YJ et al. Effect of radiation treatment volume reduction on lymphopenia in patients receiving chemoradiotherapy for glioblastoma. Int J Radiat Oncol Biol Phys 2018;101:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang L, Zhao X, Meng X et al. Involved field irradiation for the treatment of esophageal cancer is it better than elective nodal irradiation? Cancer Lett 2015;357:69–74. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Li M, Meng X et al. Involved‐field irradiation in definitive chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Radiat Oncol 2014;9:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng YJ, Jing SW, Zhu LL et al. Comparison of elective nodal irradiation and involved‐field irradiation in esophageal squamous cell carcinoma: A meta‐analysis. J Radiat Res 2018;59:604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]