Abstract
Lessons Learned.
GC1118 is a novel fully human anti‐epidermal growth factor receptor (EGFR) antibody with unique binding epitopes and different ligand‐binding inhibitory activity compared with cetuximab or panitumumab.
GC1118 showed promising antitumor activity, especially in patients with colorectal cancer resistant to prior EGFR antibody. Skin toxicities were more common and diarrhea was less frequent compared with other anti‐EGFR antibodies.
Background.
GC1118 is a novel monoclonal antibody targeting epidermal growth factor receptor (EGFR) with more potent ligand inhibition than cetuximab or panitumumab. We conducted a first‐in‐human, phase I study of GC118 in patients with refractory solid tumors.
Methods.
In the dose escalation part, GC1118 was administered on days 1, 8, 15, and 22, followed by a 2‐week rest, during which dose‐limiting toxicities (DLTs) were evaluated. In the expansion part, patients were enrolled into three cohorts (Cohort 1 [C1], patients with colorectal cancer [CRC] without prior anti‐EGFR treatment; Cohort 2 [C2], patients with CRC with tumors resistant to anti‐EGFR therapy; Cohort 3 [C3], EGFR‐overexpressing gastric cancer).
Results.
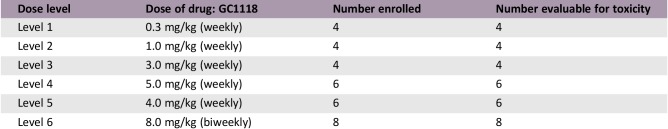
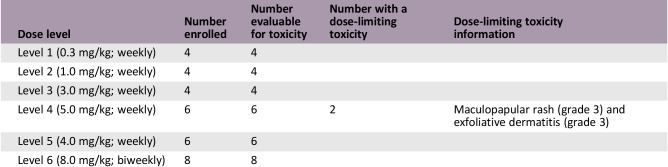
In the dose escalation part, 24 patients were treated at five dose levels: 0.3, 1.0, 3.0, 4.0, and 5.0 mg/kg. In the 5.0 mg/kg cohort, two patients experienced DLTs (skin toxicities). The maximum‐tolerated dose (MTD) was 4.0 mg/kg. Common adverse events were skin toxicities. In the expansion part, 39 patients were enrolled. In Cohort 1, stable disease (SD) was observed in 58%; in Cohort 2, partial response (PR) 17% and SD 8%; in Cohort 3, PR 8% and SD 17%.
Conclusion.
GC1118 showed promising antitumor activity and was well tolerated. Infrequent diarrhea compared with other anti‐EGFR antibodies might be advantageous for further development.
Abstract
经验总结
• GC1118 是一种完全人源新型抗表皮生长因子受体 (EGFR) 抗体,与西妥昔单抗或帕尼单抗相比具有独特的结合表位和不同的配体结合抑制活性。
• GC1118 显示出良好的抗肿瘤活性,尤其适用于对既往EGFR抗体产生耐药性的结直肠癌患者。与其他抗EGFR抗体相比,其皮肤毒性更为常见,但腹泻的发生率较低。
摘要
背景。GC1118 是一种新型单克隆抗体,靶向抑制表皮生长因子受体 (EGFR),且其配体抑制作用要强于西妥昔单抗或帕尼单抗。我们针对难治性实体瘤患者开展了首次应用于人体的 I 期 GC118 研究。
方法。在剂量递增阶段,在第 1 天、第 8 天、第 15 天及第 22 天给予 GC1118,然后停药 2 周,在此期间评估剂量限制性毒性 (DLT)。在剂量扩展阶段,将患者纳入到三个队列中[队列 1(C1),既往未经抗EGFR治疗的结直肠癌(CRC)患者;队列 2(C2),肿瘤对抗EGFR治疗耐药的CRC患者;队列 3(C3),EGFR过表达的胃癌患者]。
结果。在剂量递增阶段,24 名患者接受了 5 个剂量水平的治疗:0.3、1.0、3.0、4.0 及 5.0 mg/kg。在 5.0 mg/kg 剂量的队列中,两名患者出现DLT(皮肤毒性)。最大耐受剂量 (MTD) 为 4.0 mg/kg。常见的不良事件是皮肤毒性。在剂量扩展阶段,纳入了 39 名患者参与研究。在队列 1 中,病情稳定 (SD) 为 58%;在队列 2 中,部分缓解 (PR) 为 17%,SD为 8%;在队列 3 中,PR为 8%,SD为 17%。
结论。GC1118 显示出良好的抗肿瘤活性,且具有良好的耐受性。与其他抗EGFR抗体相比,较少出现腹泻,这可能有利于进一步研发。
Discussion
The EGFR pathway plays a critical role in carcinogenesis. Anti‐EGFR antibodies such as cetuximab and panitumumab are effective in some tumor types including head and neck cancer and CRC. GC1118, a novel human anti‐EGFR antibody, binds to nonoverlapping epitopes of EGFR, distinct from those bound by cetuximab and panitumumab. GC1118 exhibited potent inhibitory effects on both high‐ and low‐affinity ligand‐induced EGFR signaling pathways, whereas the suppressive effects of cetuximab and panitumumab were limited to low‐affinity EGFR ligands. Based on these promising preclinical data, this first‐in‐human, phase I study was conducted to investigate the MTD, safety, pharmacokinetics, and preliminary efficacy of GC1118 in patients with advanced solid tumors.
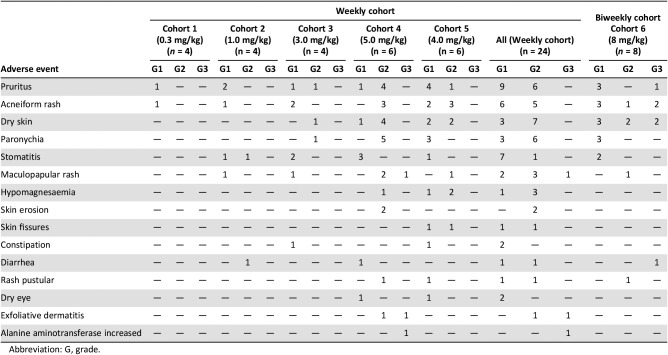
During the DLT evaluation period in the dose escalation part, GC1118 was administered on days 1, 8, 15, and 22, followed by a 2‐week rest, at five dose levels: 0.3 mg/kg (n = 4), 1.0 mg/kg (n = 4), 3.0 mg/kg (n = 4), 4.0 mg/kg (n = 6), and 5.0 mg/kg (n = 6). Thereafter, GC1118 was administered every week without a rest period. In the 5.0 mg/kg cohort, two patients experienced DLTs (grade 3 skin toxicities). The MTD was determined as 4.0 mg/kg. Adverse events (AEs) included skin toxicities (pruritus [63%], acneiform rash [46%], dry skin [42%], paronychia [38%], and maculopapular rash [25%]) and stomatitis (33%). Diarrhea developed only in two patients (grade ≤2). In pharmacokinetic analysis, systemic exposure to GC1118 increased in a greater‐than‐dose‐proportional manner as the dose was increased. Considering the toxicity and pharmacokinetic data, the recommended phase II dose of GC1118 was determined as 4.0 mg/kg/week.
In the expansion part, 39 patients were enrolled (Cohort 1 [patients with CRC without prior anti‐EGFR treatment], n = 14; Cohort 2 [patients with CRC resistant to prior anti‐EGFR therapy], n = 12; Cohort 3 [patients with gastric cancers with EGFR overexpression (2+ or 3+ by immunohistochemistry)], n = 13) and 12 patients were response‐evaluable in each cohort. GC1118 (4.0 mg/kg) was administered every week. In Cohort 1, SD was observed in 58% (7/12). In Cohort 2, two patients (17%; 2/12) achieved PR and one SD (8%). In Cohort 3, PR was 8% and SD 17% (Table 1). Skin toxicity (all grade) was observed in 90% of patients (35/39), stomatitis in 21% (all grade 1/2), and diarrhea in 8% (all grade 1/2). Compared with cetuximab or panitumumab, GC1118 showed markedly less diarrhea and far more frequent skin AEs.
Table 1. Efficacy in the cohort expansion part.
Abbreviations: CI, confidence interval; CR, complete response; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease.
In conclusion, GC1118 administered on a weekly schedule was well tolerated and showed promising antitumor activity, especially in patients with CRC resistant to prior EGFR antibody treatment (PR, 17%), even in this heavily treated population. Less frequent diarrhea compared with other anti‐EGFR antibodies might be unique and advantageous for further development. Clinical trials are currently ongoing to evaluate the efficacy and safety of GC1118 in combination with cytotoxic chemotherapeutic agents.
Trial Information
- Disease
Advanced cancer/solid tumor only
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
No designated number of regimens
- Type of Study – 1
Phase I
- Type of Study – 2
Adaptive design
- Primary Endpoint
Maximum tolerated dose
- Primary Endpoint
Recommended phase II dose
- Primary Endpoint
Safety
- Secondary Endpoint
Efficacy
- Secondary Endpoint
Pharmacokinetics
- Secondary Endpoint
Immunogenicity
- Secondary Endpoint
Exploration of potential predictive and pharmacodynamic markers
- Additional Details of Endpoints or Study Design
- This study consisted of two parts, a dose escalation part and a cohort expansion part. The study was conducted at two sites and was approved by the institutional review boards of each institution (NCT02352571). The primary objective was to determine the MTD, recommended phase II dose, and safety of GC1118 during once‐weekly administration. Secondary objectives included assessment of efficacy, pharmacokinetics, and immunogenicity of GC1118 as well as exploration of potential predictive and pharmacodynamic markers.
- In the dose escalation part, patients who met the following key criteria were enrolled: (a) histologically confirmed solid tumors refractory to standard therapy or for which there is no standard therapy; (b) Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; (c) adequate organ function. In the dose escalation part, DLT was evaluated. DLT was defined as follows: (a) grade 4 neutropenia lasting for ≥7 days; (b) grade 3 neutropenia with fever or infection; (c) grade 4 thrombocytopenia; (d) grade 3 thrombocytopenia lasting for ≥7 days, or with bleeding or requiring platelet transfusion; (e) grade 3 or 4 nausea/vomiting or diarrhea despite optimal use of antiemetics or antidiarrheal drugs; (f) grade 3 or 4 skin rash despite optimal use of skin care; (g) other grade 3 or 4 nonhematological toxicity (except alopecia); (h) delay of >2 weeks in next cycle administration because of inadequate recovery from toxicity. DLT was evaluated during the first 6 weeks of treatment (4‐week treatment followed by a 2‐week rest period). The highest dose at which DLT was observed at lower than 33% of probability was the MTD.
- In the cohort expansion part, C1 recruited patients with metastatic CRC who received no prior EGFR antibody treatment and who failed on 5‐FU, oxaliplatin, and irinotecan treatment. C2 enrolled patients with metastatic CRC with resistance to prior EGFR antibody therapy. C3 enrolled patients with EGFR over‐expressing (2+ or 3+ by immunohistochemistry [IHC]) metastatic gastric cancer (GC) who failed on standard treatment.
- Key exclusion criteria were as follows: (a) central nervous system metastasis; (b) heart failure, coronary heart disease, or other clinically significant cardiac disease within 6 months prior to enrollment; (c) known KRAS mutation in the dose escalation part or known KRAS or BRAF mutation in the cohort expansion part.
- For bioanalytical and pharmacokinetic analyses, peripheral blood was collected from all patients at the following time points on days 1 and 22: predose; 1 hour after the start of infusion; immediately after the end of infusion; and at 0.5, 1, 2, 4, 8, 12, 24, 72, and 120 hours after the end of infusion. Additional samples were collected on Day 22 at 168, 336, and 504 hours after the end of infusion, and on Days 8 and 15 predosing. A similar set of sample time points was used for the pharmacokinetic analysis in the biweekly cohort. Serum concentrations of GC1118 were determined using a validated enzyme‐linked immunosorbent assay (Invitrogen, Carlsbad, CA) with a lower and upper limit of quantification of 25 and 500 ng/mL, respectively. Pharmacokinetic parameters were estimated using a noncompartmental method implemented in the Phoenix 64 software (WinNonlin 6.4; Pharsight, A Certara Company, Princeton, NJ). Pharmacokinetic linearity was also assessed using a power model.
- Safety was assessed according to the National Cancer Institute‐Common Terminology Criteria for Adverse Events version 4.02. Efficacy was measured according to RECIST version 1.1. All safety analyses were carried out in the population exposed to at least one dose of GC1118.
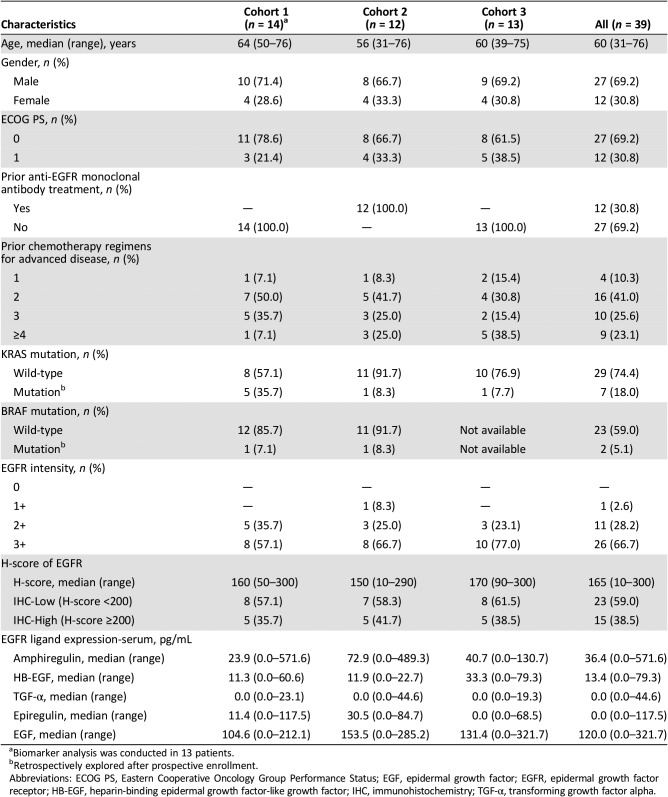
- Biomarker analysis was performed retrospectively. EGFR expression was measured in tumor tissues by IHC and H‐score. KRAS and BRAF mutation status was analyzed. EGFR ligands including amphiregulin, heparin‐binding epidermal growth factor‐like growth factor (HB‐EGF), transforming growth factor alpha, epiregulin, and epidermal growth factor were measured in blood samples.
- Investigator's Analysis
Active and should be pursued further
Drug Information: Dose Escalation
- Drug 1
- Generic/Working Name
GC1118
- Company Name
GC Pharma
- Drug Type
Antibody
- Drug Class
EGFR
- Dose
Weekly (0.3 mg/kg; 1.0 mg/kg; 3.0 mg/kg; 5.0 mg/kg; 4.0 mg/kg) and biweekly (8.0 mg/kg)
- Route
IV
Drug Information: Dose Expansion
- Drug 1
- Generic/Working Name
GC1118
- Company Name
GC Pharma
- Drug Type
Antibody
- Drug Class
EGFR
- Dose
4 mg/kg weekly
- Route
IV
- Schedule of Administration
Dose Escalation Table
Patient Characteristics: Dose Escalation
- Number of Patients, Male
20
- Number of Patients, Female
12
- Stage
Stage IV (100%)
- Age
Median (range): 57.5 (34–72)
- Number of Prior Systemic Therapies
Median (range): 3 (1 to ≥4)
- Performance Status: ECOG
-
0 — 19
1 — 13
2 — 0
3 — 0
Unknown — 0
- Other
Patient characteristics in the dose escalation part (including the biweekly cohort). Detailed descriptions are shown in Table 2.
- Cancer Types or Histologic Subtypes
-
Colorectal cancer, 18
Other, 14
Table 2. Patient characteristics in the dose escalation part (including the biweekly cohort).
Other included breast cancer (1), esophageal cancer (1), tonsil cancer (1), gastric cancer (1), ampulla of Vater cancer (1), gallbladder cancer (1), and appendix cancer (1).
Other included cholangiocarcinoma (3), ampulla of Vater cancer (1), pancreas cancer (1), nasal cavity cancer (1) and nasopharyngeal cancer (1).
Retrospectively explored after prospectively enrolled.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; IHC, immunohistochemistry.
Patient Characteristics: Dose Expansion
- Number of Patients, Male
27
- Number of Patients, Female
12
- Stage
Stage IV (100%)
- Age
Median (range): 60 (31–76)
- Number of Prior Systemic Therapies
Median (range): 2 (1 to ≥4)
- Performance Status: ECOG
-
0 — 27
1 — 12
2 — 0
3 — 0
Unknown — 0
- Other
Patient characteristics in the cohort expansion part. Detailed descriptions are shown in Table 3.
- Cancer Types or Histologic Subtypes
-
Colorectal cancer, 26
Gastric cancer, 13
Table 3. Patient characteristics in the cohort expansion part.
Biomarker analysis was conducted in 13 patients.
Retrospectively explored after prospective enrollment.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; HB‐EGF, heparin‐binding epidermal growth factor‐like growth factor; IHC, immunohistochemistry; TGF‐α, transforming growth factor alpha.
Primary Assessment Method: Dose Escalation
- Title
Efficacy in the dose escalation part (including the biweekly cohort)
- Number of Patients Screened
36
- Number of Patients Enrolled
32
- Number of Patients Evaluable for Toxicity
32
- Number of Patients Evaluated for Efficacy
32
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 0 (0.0%)
- Response Assessment PR
n = 3 (9.4%)
- Response Assessment SD
n = 16 (50%)
- Response Assessment PD
n = 13 (40.6%)
- (Median) Duration Assessments PFS
13.3 weeks, 95% CI: 5.3–25.1
- Outcome Notes
- In the dose escalation part, a total of 24 patients received weekly GC1118. Among these patients, three (12.5%) showed PR. All PR patients had KRAS‐wild type (WT) and BRAF‐WT CRC (response rate: 17.6% among 17 patients with CRC [3/17]). Of 24 patients who had received weekly GC1118 (n = 24), 12 were assessed as having SD (50.0%) and 9 showed progressive disease (PD; 37.5%). Among eight patients who were enrolled into the biweekly cohort, no tumor response was observed, and the best response in the biweekly cohort was SD (n = 4; Table 4).
Table 4. Efficacy in the dose escalation part (including the biweekly cohort).
Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Primary Assessment Method: Dose Expansion
- Title
Efficacy in the cohort expansion part
- Number of Patients Screened
51
- Number of Patients Enrolled
39
- Number of Patients Evaluable for Toxicity
39
- Number of Patients Evaluated for Efficacy
36
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 0 (0.0%)
- Response Assessment PR
n = 3 (8.3%)
- Response Assessment SD
n = 10 (27.8%)
- Response Assessment PD
n = 23 (63.9%)
- (Median) Duration Assessments PFS
7.1 weeks, 95% CI: 6.6–11.0
- Outcome Notes
- In the cohort expansion part, a total of 39 patients were enrolled into three cohorts: C1, patients with CRC who received no prior EGFR antibody treatment and who had treatment failure on 5‐FU, oxaliplatin, and irinotecan treatment (n = 14); C2, patients with CRC with tumors resistant to prior anti‐EGFR therapy (n = 12); and C3, patients with EGFR overexpressing (2+ or 3+ by IHC) metastatic/recurrent GC who had treatment failure on standard treatment (n = 13).
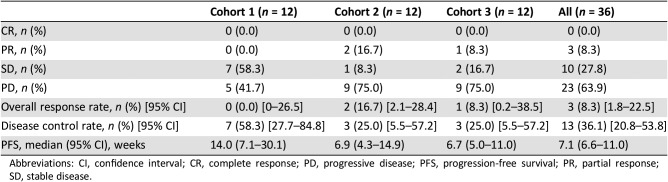
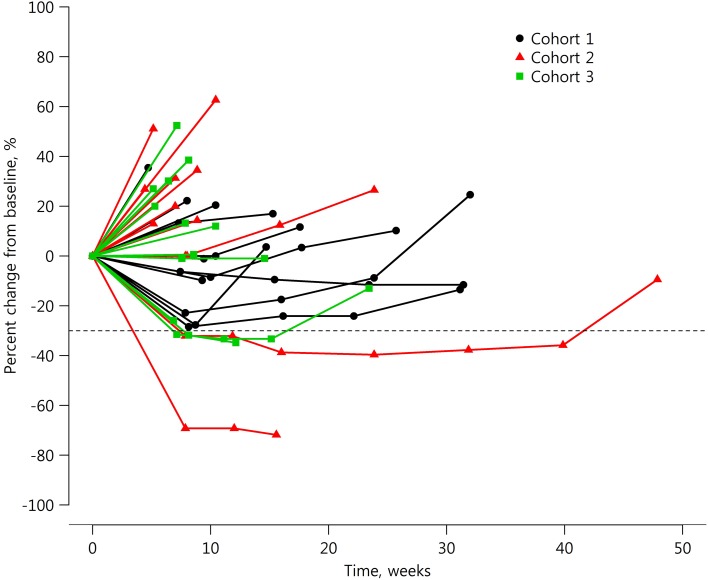
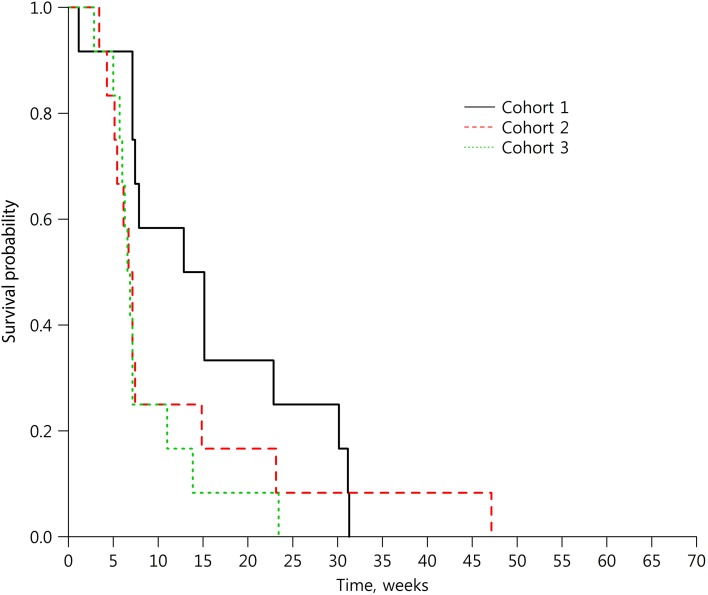
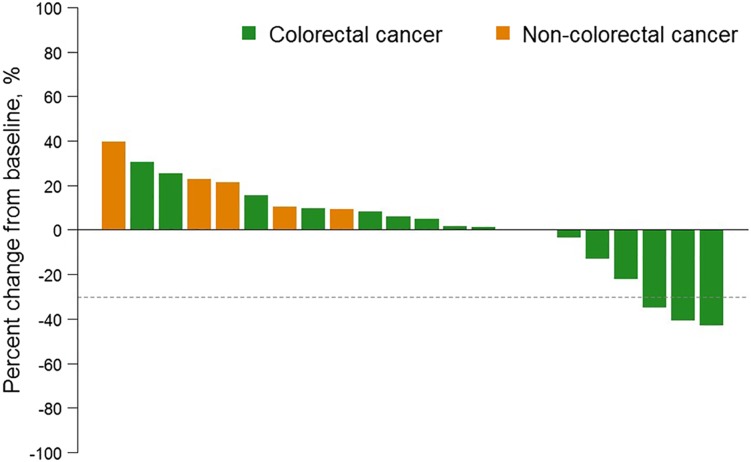
- Response evaluation was completed in 36 patients (12 in each cohort). In C1, SD was observed in 58.3% (7/12) and disease control rate (DCR) was 58.3% (95% confidence interval [CI], 27.7–84.8); median progression‐free survival (PFS) was 14.0 weeks (95% CI, 7.1–30.1). In C2, two patients (16.7%; 2/12) achieved PR and one SD (8.3%; 1/12). DCR was 25.0% (95% CI, 5.5–57.2) and median PFS was 6.9 weeks (95% CI, 4.3–14.9). In C3, PR was 8.3% (1/12) and SD 16.7% (2/12). DCR was 25.0% (95% CI, 5.5–57.2) and median PFS was 6.7 weeks (95% CI, 5.0–11.0; Table 1; Figs. 2, 3, 4, 5, 6).
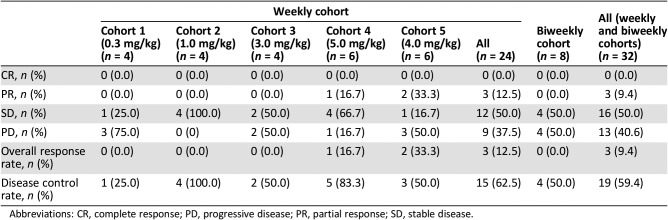
Figure 2.
Waterfall plot of maximum percent change in tumor size from baseline in the cohort expansion part: Cohort 1.
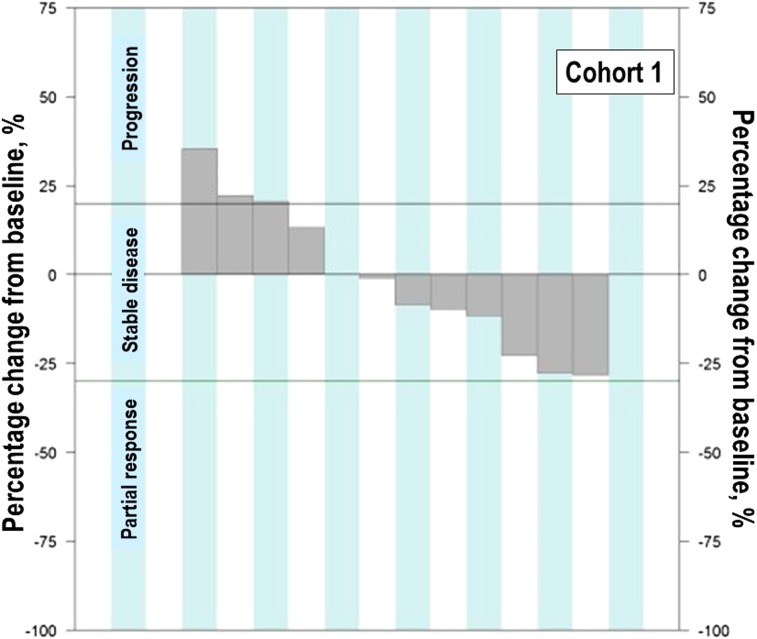
Figure 3.
Waterfall plot of maximum percent change in tumor size from baseline in the cohort expansion part: Cohort 2.
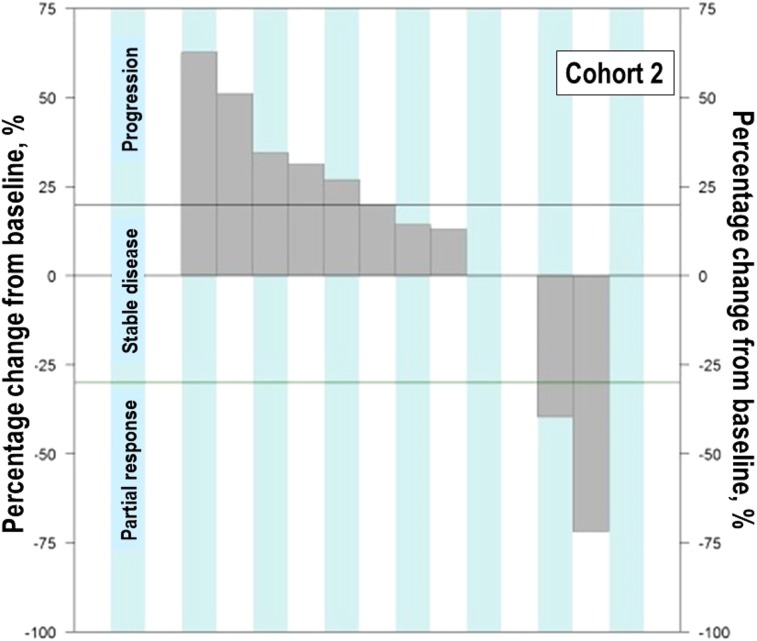
Figure 4.
Waterfall plot of maximum percent change in tumor size from baseline in the cohort expansion part: Cohort 3.
Figure 5.
Spider plot of percent change in tumor size from baseline in the cohort expansion part.
Figure 6.
Progression‐free survival in the cohort expansion part.
Adverse Events
- Treatment‐related adverse events in the dose escalation part (including the biweekly cohort) are shown in Table 5. Treatment‐related adverse events in the cohort expansion part are shown in Table 6.
Table 5. Treatment‐related adverse events occurring in >5% of patients in the dose escalation part (including the biweekly cohort).
Abbreviation: G, grade.
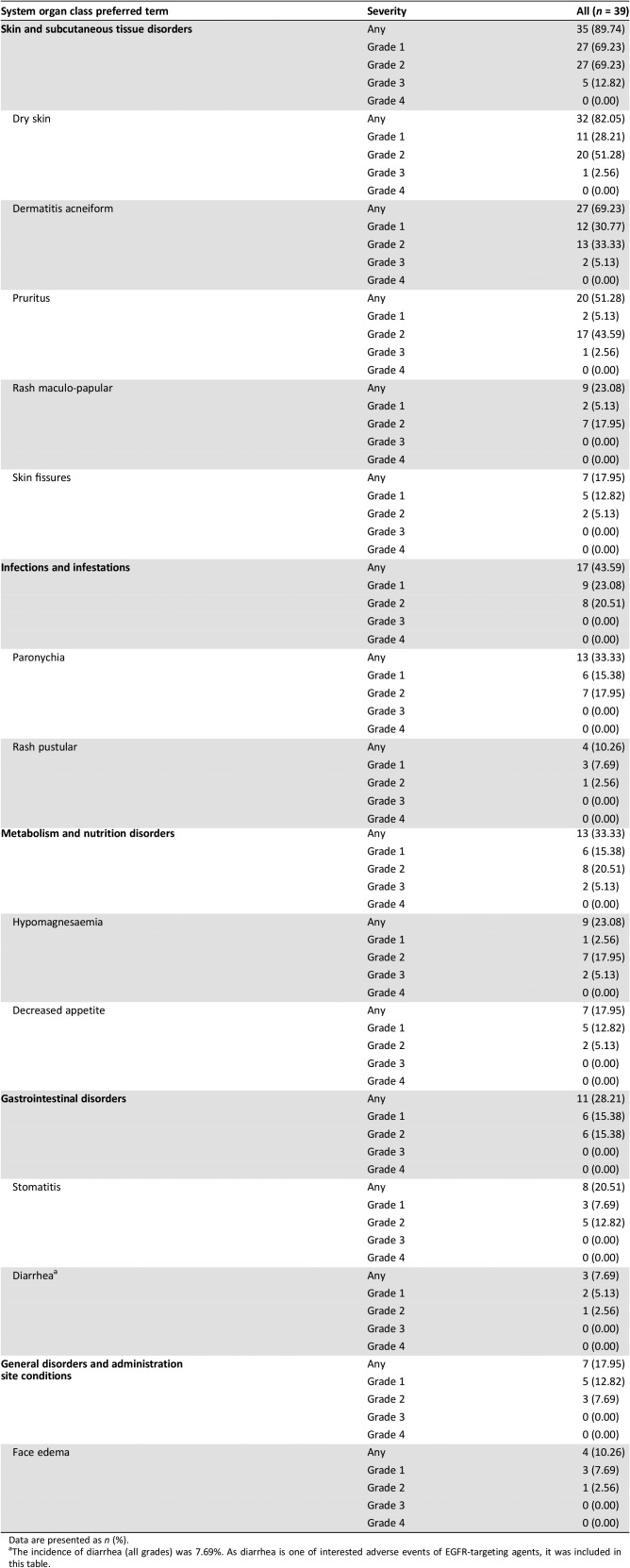
Table 6. Treatment‐related adverse events occurring in >10% of patients in the cohort expansion part.
Data are presented as n (%).
The incidence of diarrhea (all grades) was 7.69%. As diarrhea is one of interested adverse events of EGFR‐targeting agents, it was included in this table.
Dose Limiting Toxicities: Dose Escalation
Pharmacokinetics/Pharmacodynamics
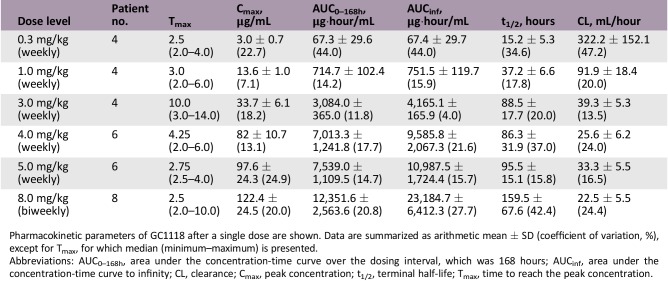
Pharmacokinetic parameters of GC1118 after a single dose are shown. Data are summarized as arithmetic mean ± SD (coefficient of variation, %), except for Tmax, for which median (minimum–maximum) is presented.
Abbreviations: AUC0–168h, area under the concentration‐time curve over the dosing interval, which was 168 hours; AUCinf, area under the concentration‐time curve to infinity; CL, clearance; Cmax, peak concentration; t1/2, terminal half‐life; Tmax, time to reach the peak concentration.
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Active and should be pursued further
The epidermal growth factor receptor (EGFR) is a transmembrane receptor and one of the most commonly overexpressed proteins in solid tumors [1], [2]. The EGFR pathway plays a critical role in carcinogenesis, including in the survival, migration, angiogenesis, and apoptosis of cancer cells. Anti‐EGFR antibodies such as cetuximab and panitumumab have been shown to be effective in several tumor types including colorectal cancer (CRC) and head and neck squamous cell carcinoma [1], [2], [3].
GC1118 is a novel fully human anti‐EGFR antibody with a distinct binding epitope and efficacy [4]. GC1118 binds to the nonoverlapping epitopes of EGFR, distinct from those bound by currently available anti‐EGFR antibodies such as cetuximab and panitumumab [5], [6]. GC1118 exhibited a potent inhibitory effect on both high‐ and low‐affinity ligand‐induced EGFR signaling and proliferation, whereas the suppressive effects of cetuximab and panitumumab were limited to low‐affinity EGFR ligands [4]. In line with in vitro signaling and proliferation assays, GC1118 consistently showed strong antitumor activity on colorectal tumor xenografts with elevated expression of high‐affinity ligands, whereas cetuximab did not [4]. High‐affinity ligands including transforming growth factor alpha (TGF‐α) and/or heparin‐binding epidermal growth factor‐like growth factor (HB‐EGF) have been shown to be highly expressed in mouse CRC xenografts with acquired resistance to cetuximab [7], [8]. In our previous study, GC1118 exerted robust inhibition on tumor progression of both parental SW48 and SW48 xenografts with acquired resistance to cetuximab. HB‐EGF‐induced vascular endothelial growth factor expression was shown to be inhibited by GC1118 but not by cetuximab in SW48 CRC cell line [8]. HB‐EGF is a strong inducer of angiogenesis, and the ligand‐induced EGFR signaling circuit is implicated in tumor‐associated endothelial survival as well as tumor growth [9], [10], [11], [12]. Based on the potential role of high‐affinity ligands in acquired resistance to cetuximab and angiogenesis, we postulated that GC1118 would have beneficial effects in the treatment of cancer in patients with innate or acquired resistance to currently available anti‐EGFR antibodies. These promising preclinical data led to this first‐in‐human, phase I study of GC1118 in patients with advanced solid tumors.
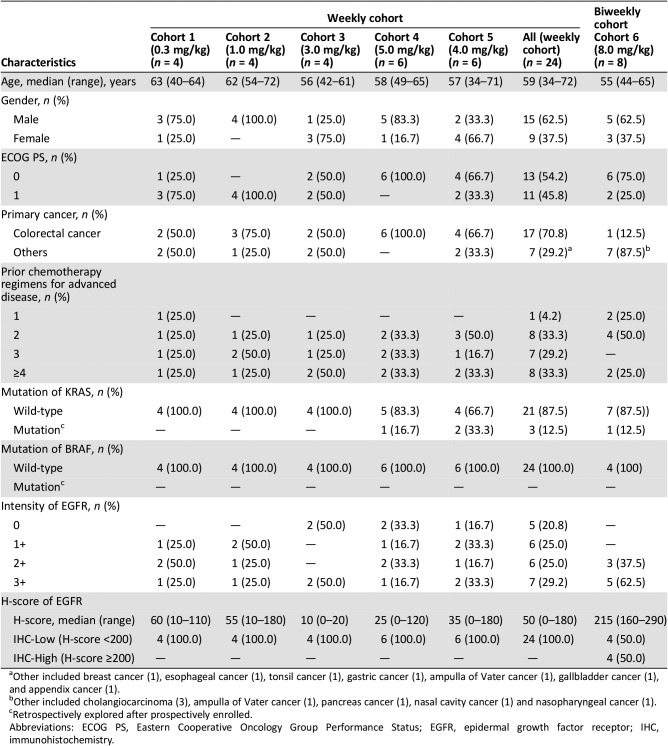
In the dose escalation part, a total of 24 patients received weekly GC1118 (0.3 mg/kg [n = 4], 1.0 mg/kg [n = 4], 3.0 mg/kg [n = 4], 5.0 mg/kg [n = 6], and 4.0 mg/kg [n = 6]; Table 2). GC1118 was administered at doses of 0.3, 1.0, 3.0, and 5.0 mg/kg in consecutive order. Dose‐limiting toxicities (DLTs) were reported in two of six patients in the 5.0 mg/kg cohort and were grade 3 skin toxicities (maculopapular rash and exfoliative dermatitis). Next, an additional six patients received 4.0 mg/kg of GC1118. In this cohort, no DLTs were reported. Therefore, the maximum‐tolerated dose (MTD) of GC1118 was determined as 4.0 mg/kg. The most frequently reported adverse events (AEs) during all treatment periods were skin AEs including pruritus (grade 1 [38%]; grade 2 [25%]), acneiform rash (grade 1 [25%]; grade 2 [21%]), dry skin (grade 1 [13%]; grade 2 [29%]), paronychia (grade 1 [13%]; grade 2 [25%]), maculopapular rash (grade 1 [8%]; grade 2 [13%]; grade 3 [4%]), and exfoliative dermatitis (grade 2 [4%]; grade 3 [4%]). Diarrhea was observed in only two patients (grade 1 [4%]; grade 2 [4%]; Table 5). Among 24 response‐evaluable patients, there were 3 patients (13%) with a partial response (PR). All PR patients had KRAS‐wild type (WT) and BRAF‐WT CRC (response rate: 18% among 17 patients with CRC [3/17]). Twelve patients were assessed as having stable disease (SD; 50%), and nine patients showed progressive disease (38%; Table 4; Fig. 1).
Figure 1.
Waterfall plot of maximum percent change in tumor size from baseline in the dose escalation part.
Systemic exposure to GC1118 increased in a greater‐than‐dose‐proportional manner as the dose was increased. The pharmacokinetic profile of GC1118 was not linear; exposure to GC1118 was increased to a much greater extent than the increase in dose. The average dose‐normalized area under the concentration‐time curve of GC1118 over the dosing interval after repeated administration was markedly increased from 3,976.5 to 49,186.0 ng·hour/mL/mg as the dose increased from 0.3 to 5.0 mg/kg (data not shown). This type of nonlinear pharmacokinetic profile has previously been observed with other monoclonal antibodies. The clearance of GC1118 was saturated at 3.0 mg/kg and beyond, suggesting that EGFR was fully occupied with GC1118. In consideration of the toxicity and pharmacokinetic data, the recommended phase II dose (RP2D) of weekly GC1118 administration was determined as 4.0 mg/kg/week.
To explore the feasibility of biweekly administration of GC1118, which is more convenient to patients than weekly administration, another eight patients were enrolled and received 8.0 mg/kg of GC1118 every other week (the biweekly cohort). In pharmacokinetic analyses, biweekly administration of GC1118 (8.0 mg/kg every other week) showed similar total exposure in the body, but the lowest serum concentration of GC1118 that could affect the anticancer effect was relatively low compared with weekly administration of GC1118 (4.0 mg/kg every week). In addition, no tumor response was observed and the best response in the biweekly cohort was SD (Table 4). Therefore, we proceeded with 4.0 mg/kg every week for further clinical development of GC1118.
In the cohort expansion part, a total of 39 patients were enrolled and treated with weekly GC1118 (4.0 mg/kg: Cohort 1 [C1], n = 14; Cohort 2 [C2], n = 12; Cohort 3 [C3], n = 13). Baseline characteristics of patients are shown in Table 3. Response evaluation was completed in 36 patients (12 in each cohort; Table 1, Figures 2, 3, 4). Overall, GC1118 was well tolerated. Treatment‐related AEs are shown in Table 6. Skin toxicity (all grade) was observed in 90% of patients (35/39), stomatitis in 21% (8/39, all grade 1/2), and diarrhea in 8% (3/39, all grade 1/2). No treatment‐related deaths were reported. The most commonly reported AEs among other EGFR antibodies are gastrointestinal AEs such as diarrhea and stomatitis. Compared with other EGFR antibodies, GC1118 showed markedly less diarrhea and stomatitis, and far more common skin AEs. Therefore, the toxicity profile appears to differ among EGFR antibodies based on differences in the non‐overlapping epitopes of EGFR.
In the cohort expansion part, C1 evaluated GC1118 in patients with metastatic CRC with no prior EGFR antibody treatment who failed on 5‐FU, oxaliplatin, and irinotecan. In this cohort, SD was observed in 58% (7 out of 12) of patients. Although no patients showed tumor response (complete response or PR), the median progression‐free survival was 14 weeks (95% confidence interval [CI], 7.1–30.1; Table 1; Figs. 2, 5, 6).
C2 evaluated GC1118 in patients with metastatic CRC with resistance to prior EGFR antibody treatment. In C2, two patients (17%) achieved PR and one achieved SD (8%); therefore, DCR was 25.0% (95% CI, 5.5–57.2; Table 1; Figs. 3, 5, 6). It is very interesting that, despite having acquired resistance to prior cetuximab treatment, two patients achieved PR. These two PR patients harbored KRAS WT, BRAF WT, and NRAS WT tumors. Intensity of EGFR expression was 2+ in one patient and 3+ in the other, but both had immunohistochemistry (IHC)‐low status based on H‐score. EGFR expression does not therefore appear to be related to the antitumor activity of GC1118 in patients with CRC. In preclinical studies, GC1118 exhibited a potent inhibitory effect on high‐affinity ligand‐induced EGFR signaling and proliferation as well as low‐affinity ligands, whereas the suppressive effects of cetuximab and panitumumab were limited to low‐affinity EGFR ligands. Therefore, one of our hypotheses was that antitumor activity of GC1118 would correlate with EGFR ligand status in the present study. However, the levels of serum EGFR ligands such as amphiregulin, HB‐EGF, TGF‐α, epiregulin, and epidermal growth factor did not show a strong correlation with efficacy of GC1118 (data not shown). Whether serum EGFR ligands and peritumoral EGFR ligand secretion correlate with each other requires further investigation.
In gastric cancer (GC), EGFR overexpression and/or amplification is observed in about 30% of cases and is associated with poor prognosis [13]. However, the anti‐EGFR antibodies cetuximab and panitumumab have failed to improve the overall survival of patients with GC when administered in combination with cytotoxic chemotherapy compared with chemotherapy alone [14], [15]. Nimotuzumab, another EGFR antibody, is currently under investigation in a second‐line setting in advanced GC. In advanced GC, the incidence of KRAS mutation and amplification is about 5% and 9%, respectively [16], [17]. In our preclinical study, GC1118 alone or in combination with cytotoxic chemotherapeutic agents exerted more potent antitumor effects than cetuximab in GC cells, regardless of KRAS status [18]. C3 aimed to explore GC1118 in patients with EGFR overexpressing (2+ or 3+ by IHC) metastatic GC who failed on standard treatment. Among the 13 patients enrolled, the intensity of EGFR expression was IHC 3+ in 10 patients and 2+ in 3 patients. Based on the H‐score, IHC‐high‐status tumors were observed in five patients and IHC‐low‐status in eight patients. Eighty‐five percent of patients were in third‐ or later‐line settings of advanced GC. In C3, PR was 8% (1/12) and SD 17% (2/12; Table 1; Figs. 4, 5, 6). One patient with PR in C3 showed 3+ intensity of EGFR, high status of EGFR H‐score, and KRAS WT.
In conclusion, this study demonstrated the feasibility of a novel EGFR antibody, GC1118, in patients with advanced solid tumors. GC1118 at an MTD and RP2D of 4.0 mg/kg, administered on weekly schedule, was safe and well tolerated. GC1118 showed promising antitumor activity, especially in patients with CRC resistant to prior EGFR antibody treatment, showing a response rate of 17%, even in a heavily treated population. Less frequent diarrhea compared with other anti‐EGFR antibodies might be unique and advantageous for further development. Clinical trials are currently underway to evaluate the efficacy and safety of GC1118 in combination with cytotoxic chemotherapies in patients with metastatic CRC (NCT03454620).
Figures and Tables
Acknowledgments
We thank Clare Cox, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. This study was supported by a research grant from GC Pharma and the Korea Drug Development Fund (KDDF) from MSIP, MOTIE, and MOHW (KDDF201412‐08, Republic of Korea).
Contributed equally
Footnotes
ClinicalTrials.gov Identifier: NCT02352571
Sponsor(s): GC Pharma
Principal Investigator: Yung‐Jue Bang
IRB Approved: Yes
Disclosures
Do‐Youn Oh: AstraZeneca, Novartis, Genentech/Roche, Merck Serono, Bayer, Taiho, ASLAN, Halozyme (C/A), AstraZeneca, Novartis, Array, Eli Lilly, Green Cross (RF); Keun‐Wook Lee: AstraZeneca/MedImmune, Roche, Merck Sharp & Dohme, Merck Serono, Green Cross, Taiho, Daiichi Sankyo, Astellas, Pfizer, MacroGenics, FiverPrime, Ono, ASLAN, LSK Biopharma, Array Biopharma, Pharmacyclics, ALX Oncology (RF); Yung‐Jue Bang: AstraZeneca, Novartis, Genentech/Roche, Merck Sharp & Dohme, Merck Serono, Bayer, Bristol‐Meyers Squibb, Eli Lilly, Taiho, Daiich‐Sankyo, Astellas, BeiGene, GreenCross, Samyang Biopharm, Hanmi, Genexine (C/A), AstraZeneca, Novartis, Genentech/Roche, Merck Sharp & Dohme, Merck Serono, Bayer, Bristol‐Meyers Squibb, GlaxoSmithKline, Pfizer, Eli Lilly, Boeringer‐Ingelheim, MacroGenics, Boston Biomedical, FivePrime, Curis, Taiho, Takeda, Ono, Daiichi Sankyo, Astellas, BeiGene, Green Cross, CKD Pharma, Genexine (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell Mol Life Sci 2008;65:1566–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yarden Y, Pines G. The ERBB network: At last, cancer therapy meets systems biology. Nat Rev Cancer 2012;12:553–563. [DOI] [PubMed] [Google Scholar]
- 3.Dokala A, Thakur SS. Extracellular region of epidermal growth factor receptor: A potential target for anti‐EGFR drug discovery. Oncogene 2017;36:2337–2344. [DOI] [PubMed] [Google Scholar]
- 4.Lim Y, Yoo J, Kim MS et al. GC1118, an anti‐EGFR antibody with a distinct binding epitope and superior inhibitory activity against high‐affinity EGFR ligands. Mol Cancer Ther 2016;15:251–263. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Schmitz KR, Jeffrey PD et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 2005;7:301–311. [DOI] [PubMed] [Google Scholar]
- 6.Voigt M, Braig F, Gothel M et al. Functional dissection of the epidermal growth factor receptor epitopes targeted by panitumumab and cetuximab. Neoplasia 2012;14:1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troiani T, Martinelli E, Napolitano S et al. Increased TGF‐alpha as a mechanism of acquired resistance to the anti‐EGFR inhibitor cetuximab through EGFR‐MET interaction and activation of MET signaling in colon cancer cells. Clin Cancer Res 2013;19:6751–6765. [DOI] [PubMed] [Google Scholar]
- 8.Lee SN, Cho HJ, Lim Y et al. Abstract LB‐114: GC1118, a new anti‐EGFR antibody overcome acquired resistance to cetuximab in colorectal cancer xenograft model. Cancer Res 2016;76(suppl 14):LB‐114a. [Google Scholar]
- 9.Ongusaha PP, Kwak JC, Zwible AJ et al. HB‐EGF is a potent inducer of tumor growth and angiogenesis. Cancer Res 2004;64:5283–5290. [DOI] [PubMed] [Google Scholar]
- 10.Baker CH, Kedar D, McCarty MF et al. Blockade of epidermal growth factor receptor signaling on tumor cells and tumor‐associated endothelial cells for therapy of human carcinomas. Am J Pathol 2002;161:929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SJ, Uehara H, Karashima T et al. Blockade of epidermal growth factor receptor signaling in tumor cells and tumor‐associated endothelial cells for therapy of androgen‐independent human prostate cancer growing in the bone of nude mice. Clin Cancer Res 2003;9:1200–1210. [PubMed] [Google Scholar]
- 12.Amin DN, Hida K, Bielenberg DR et al. Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res 2006;66:2173–2180. [DOI] [PubMed] [Google Scholar]
- 13.Kim MA, Lee HS, Lee HE et al. EGFR in gastric carcinomas: Prognostic significance of protein overexpression and high gene copy number. Histopathology 2008;52:738–746. [DOI] [PubMed] [Google Scholar]
- 14.Lordick F, Kang YK, Chung HC et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): A randomised, open‐label phase 3 trial. Lancet Oncol 2013;14:490–499. [DOI] [PubMed] [Google Scholar]
- 15.Waddell T, Chau I, Cunningham D et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): A randomised, open‐label phase 3 trial. Lancet Oncol 2013;14:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi N, Yamada Y, Taniguchi H et al. Clinicopathological features and prognostic roles of KRAS, BRAF, PIK3CA and NRAS mutations in advanced gastric cancer. BMC Res Notes 2014;7:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng N, Goh LK, Wang H et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co‐occurrence among distinct therapeutic targets. Gut 2012;61:673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JE, Jin MH, Nam AR et al. Abstract 3496: GC1118, a novel anti‐EGFR andtibody, shows more potent antitumor activity regardless of KRAS mutation or high‐affinity lignad stimulation compared with cetuximab in gastric cancer. Cancer Res 2018;78(suppl 13):3496a. [Google Scholar]