This review discusses the currently available regimens for poorly differentiated gastroenteropancreatic neuroendocrine carcinomas, as well as future direction for treatment.
Keywords: Carcinoma, Neuroendocrine, Current treatment
Abstract
Poorly differentiated gastroenteropancreatic neuroendocrine carcinomas (GEPNECs) are a rare neoplasm with a bleak prognosis. Currently there are little prospective data available for optimal treatment. This review discusses the current available regimens and the future direction for the treatment of GEPNECs. Treatment plans for GEPNECs are often adapted from those devised for small cell lung cancer; however, differences in these malignancies exist, and GEPNECs require their own treatment paradigms. As such, current first‐line treatment for GEPNECs is platinum‐based chemotherapy with etoposide. Studies show that response rate and overall survival remain comparable between cisplatin and carboplatin versus etoposide and irinotecan; however, prognosis remains poor, and more efficacious therapy is needed to treat this malignancy. Additional first‐line and second‐line treatment options beyond platinum‐based chemotherapy have also been investigated and may offer further treatment options, but again with suboptimal outcomes. Recent U.S. Food and Drug Administration approval of peptide receptor radionuclide therapy in low‐ and intermediate‐grade neuroendocrine tumors may open the door for further research in its usefulness in GEPNECs. Additionally, the availability of checkpoint inhibitors lends promise to the treatment of GEPNECs. This review highlights the lack of large, prospective studies that focus on the treatment of GEPNECs. There is a need for randomized control trials to elucidate optimal treatment regimens specific to this malignancy.
Implications for Practice.
There are limited data available for the treatment of poorly differentiated gastroenteropancreatic neuroendocrine carcinomas (GEPNECs) because of the rarity of this malignancy. Much of the treatment regimens used in practice today come from research in small cell lung cancer. Given the poor prognosis of GEPNECs, it is necessary to have treatment paradigms specific to this malignancy. The aim of this literature review is to summarize the available first‐ and second‐line GEPNEC therapy, outline future treatments, and highlight the vast gap in the literature.
摘要
低分化胃肠胰神经内分泌癌 (GEPNEC) 是一种罕见的肿瘤,预后较差。目前,几乎没有关于可用的最佳治疗的前瞻性数据。本综述讨论了当前可用的方案和 GEPNEC 治疗的未来方向。GEPNEC 的治疗计划通常从针对小细胞肺癌的治疗计划中进行改进;然而,这些恶性肿瘤中存在差异,并且 GEPNEC 需要自己的治疗规范。因此,目前对 GEPNEC 的一线治疗是基于铂类化疗联合依托泊苷。研究表明,顺铂和卡铂与依托泊苷和伊立替康治疗的反应率和总生存率相当;然而,预后仍然很差,需要更有效的疗法来治疗这种恶性肿瘤。除了铂类化疗之外,其他一线和二线治疗方案也已经过研究,可能提供了进一步的治疗方案,但同样产生了不理想的结果。最近美国食品药品管理局批准在低级别和中级别神经内分泌肿瘤中使用肽受体放射性核素治疗,可能为进一步研究其在 GEPNEC 中的有用性打开了一扇大门。此外,免疫检查点抑制剂的可用性为 GEPNEC 的治疗带来了希望。本综述强调了缺乏研究 GEPNEC 治疗的大型前瞻性研究。需要随机对照试验来阐明针对该恶性肿瘤的最佳治疗方案。
实践意义:由于这种恶性肿瘤的罕见性,可用于治疗低分化胃肠胰神经内分泌癌 (GEPNEC) 的数据有限。如今在实践中使用的许多治疗方案来自于对小细胞肺癌的研究。鉴于 GEPNEC 的预后不良,有必要获得针对该恶性肿瘤的治疗规范。本文献综述的目的是总结现有的一线和二线 GEPNEC 疗法,概述未来的治疗方法,并强调文献中的巨大差距。
Introduction
Neuroendocrine tumors (NETs) are varied solid tumor neoplasms that differ in pathophysiology and clinical presentation depending on the primary site of origin. Currently, NETs are classified into three subcategories depending mainly on morphological features and proliferation rate, which is determined by the mitotic count and Ki‐67 index [1], [2], [3].
According to the 2017 World Health Organization (WHO) classification of NETs, gastroenteropancreatic GEP NETs are classified as grade (G)1, G2, and G3. Grade is determined by both mitotic count and Ki‐67 labeling index. G1 NETs have a mitotic count of <2 and/or Ki‐67 index <3%. G2 NETs have a mitotic count 2–20 and/or Ki‐67 index 3%–20%. G3 tumors have a mitotic count of >20 and/or Ki‐67 index >20%. G3 tumors are high‐grade neoplasms that were further divided under the 2017 WHO classification into well differentiated neuroendocrine tumors (WDNETs) or poorly differentiated neuroendocrine carcinomas (PDNECs) based on morphological appearance [4], [5].
GEPNECs are rare malignancies. GEPNECs represent roughly 55% of all extrapulmonary high grade NETs, the majority being metastatic at the time of diagnosis [6], [7], [8]. GEPNECs generally do not respond to the standard treatments traditionally utilized in G1 and G2 NETs such as somatostatin analogs (SSAs), everolimus, sunitinib, and interferon [9], [10]. Limited evidence supports treatment recommendations specific to GEPNECs, most likely secondary to the lack of sufficient patient numbers to conduct large phase II or III clinical trials.
Limited evidence supports treatment recommendations specific to GEPNECs, most likely secondary to the lack of sufficient patient numbers to conduct large phase II or III clinical trials.
Much of the information is derived from limited retrospective studies and scarce noncontrolled clinical trials [11]. However, small cell lung cancer (SCLC) and GEPNECs share similar histologic and clinical patterns and are often treated similarly. Both SCLC and GEPNECs have high Ki‐67 indices, stain for neuron‐specific enolase and chromogranin A, and have a similar clinical course [12]. Most treatment options for GEPNECs are based on therapy responses reported in SCLC studies. First‐line treatment for GEPNECs consists of etoposide and platinum‐based chemotherapy, but despite treatment, prognosis is bleak, with a median survival of 19 months [12], [13], [14], [15], [16].
Although systemic platinum‐based treatment is the standard of care in GEPNECs, there is a paucity of data about other first‐line therapies. Additionally, limited data exist on appropriate second‐line therapies for GEPNECs. Peptide receptor radionuclide therapy (PRRT) also shows promise as an alternative treatment paradigm for GEPNECs [17], [18], [19]. Immunotherapy has changed the treatment landscape in multiple tumor types, including non‐small cell lung cancer (NSCLC), melanoma, and renal cell carcinoma, but it is not clear how this will translate to GEPNECs [20].
The objective of this review is to evaluate first‐ and second‐line chemotherapy regimens, as well as to explore the use of immunotherapy and PRRT in the treatment of GEPNECs.
First‐Line Chemotherapy in GEPNECs
Cisplatin/Carboplatin + Etoposide
Grounded in their recognized role in treating metastatic SCLC, cisplatin/carboplatin and etoposide have been used for decades in the treatment of GEPNECs [15], [16], [21], [22], [23], [24], [25].
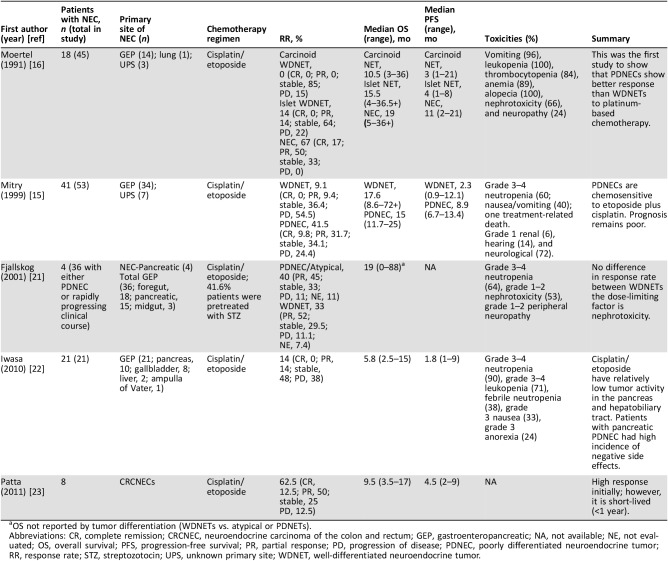
The effectiveness of cisplatin and etoposide in tumor regression and prolonged survival in SCLC led Moertel et al. (1991) to conduct the first study in examining this treatment protocol in G3 NETs [16]. In this study, 45 patients with metastatic NETs were treated with etoposide (130 mg/m2/day intravenously [IV] 3 days) and cisplatin (45 mg/m2/day IV on days 2 and 3). Of these patients, 67% of those with PDNECs had complete or partial regression, in contrast to only 7% of patients with WDNET. Toxicity was a major problem, as bone marrow suppression (100%), alopecia (100%), and gastrointestinal (GI) symptoms (96%) were common. Additionally, neuropathy occurred in 24% of patients, and 66% of patients experienced nephrotoxicity most likely secondary to the cisplatin exposure (Table 1). The high dose of etoposide (130 mg/m2) may account for these negative side effects. At the time of the study, tissue was not routinely tested for Ki‐67 index, nor were guidelines available on grading HGNETs. Regardless, this was the first study to show that PDNECs show better response than WDNETs to platinum‐based chemotherapy.
Table 1. Studies evaluating platinum‐based chemotherapy.
OS not reported by tumor differentiation (WDNETs vs. atypical or PDNETs).
Abbreviations: CR, complete remission; CRCNEC, neuroendocrine carcinoma of the colon and rectum; GEP, gastroenteropancreatic; NA, not available; NE, not evaluated; OS, overall survival; PFS, progression‐free survival; PR, partial response; PD, progression of disease; PDNEC, poorly differentiated neuroendocrine tumor; RR, response rate; STZ, streptozotocin; UPS, unknown primary site; WDNET, well‐differentiated neuroendocrine tumor.
Mitry et al. aimed to confirm results outlined by the Moertel et al. study [15], [26]. This group conducted a retrospective analysis of 53 patients, 41 with PDNECs and 12 with WDNETs, to determine the efficacy of treating NETs with etoposide (100 mg/m2/day IV for 3 days) and cisplatin (100 mg/m2 IV on day 1) every 3 weeks. Among the 12 patients with WDNETs, only 9.1% achieved a partial response. Those with PDNECs achieved a response in 41.5% of cases (Table 1). Although these results were not statistically significant (p = 0.09), the trend was in line with the results demonstrated by Moertel et al. Overall, this study provided further support for the use of cisplatin and etoposide in PDNECs. The side effects endured by patients in this study were similar to those experienced in the predecessor study; however, the peripheral neuropathy, bone marrow suppression, and GI dysfunction were less severe. The etoposide regimen was less than that used by Moertel et al. (100 mg/m2 vs. 130 mg/m2), which may account for the decrease in side effects.
These two studies advocated for differentiation status to predict response to chemotherapy. A study by Fjallskog et al. (2001), however, showed that clinical behavior may be a greater prognosticator of chemotherapy response [21]. This study examined 36 patients with PDNECs (n = 4) or NETs with a rapidly progressive clinical course (n = 32). Of these, the origin was foregut in 18, midgut in 3, and pancreas in 15. Treatment consisted of etoposide (100 mg/m2/day IV for 3 days by continuous infusion) and cisplatin (45 mg/m2/day IV on days 2 and 3 by continuous infusion) every 4 weeks (Table 1). Unlike Moertel et al. and Mitry et al., Fjallskog et al. found no difference in response rate between WDNET and PDNECs. This finding may be due to small sample size, and drawing significant conclusions may be difficult. Toxicity was found to be significant in this study, with 19 patients (53%) showing grade 1–2 nephrotoxicity, 23 patients (64%) developing grade 3–4 neutropenia, and 17% suffering from grade 1 and grade 2 peripheral neuropathy; however, many of these patients were pretreated with streptozotocin, which is known to be nephrotoxic. Additionally, Mitry et al. reported pointedly less kidney damage (6%), potentially owing to the infusion being given over a 2‐hour period in contrast to continuous infusion. Nephrotoxicity was the most common dose‐limiting factor in the study conducted by Fjallskog et al., leading to a dose reduction.
Although the aforementioned regimen has been considered the default for all GEPNECs, the literature demonstrates that response may be site specific. Iwasa et al. (2010) investigated the impact of cisplatin and etoposide on carcinoma arising from the hepatobiliary tract and pancreas [22]. This retrospective study examined 21 patients treated with this regimen (etoposide 100 mg/m2/day IV on days 1–3 and cisplatin 80 mg/m2 IV on the first day every 3–4 weeks). This study found that 14% of patients had a partial response, the median progression‐free survival (PFS) was 1.8 months, and the median overall survival (OS) was 5.8 months (Table 1). These are dismal results when compared with previous studies; Moertel and Mitry described partial or complete response in 67% and 41.5% of patients, respectively [15], [16]. The discrepancy in survival is most likely secondary to the primary site of the tumor. Moertel and Mitry reported on extrapulmonary G3 NETs; their study included not only hepatobiliary and pancreatic tumors (as in the study conducted by Iwasa et al.) but also GI, head and neck, and tracheal carcinomas. Hepatobiliary and pancreatic G3 NETs frequently metastasize to the liver, which is a well‐recognized poor prognostic indicator [27], [28], [29], [30]. Eighty‐one percent of patients in the study conducted by Iwasa et al. (2010) developed liver metastasis. Thus, the anatomic behavior of these hepatobiliary and pancreatic cancers may be partially responsible for the poor prognosis reported. Additionally, grade 3–4 neutropenia occurred in 90% of patients, followed by grade 3 nausea and anorexia in 33% and 24% of patients, respectively [22]. Taken together, these results demonstrate that perhaps cisplatin and etoposide have relatively low tumor activity in the pancreas and hepatobiliary tract, with a high incidence of negative side effects.
Patta conducted a retrospective analysis of eight patients with PDNECs of the colon and rectum treated with cisplatin and etoposide [23]. One patient had a complete response, whereas four had a partial response. This translates to a 62.5% response rate, which is similar to that reported by Moertel and colleagues [15], [16]. The response was short lived, however, with a median PFS of 4.5 months (range, 2–9 months) and a median OS of 9.5 months (range, 3.5 to 17 months; Table 1). An OS of 9.5 months is lower than the median OS reported by Moertel et al. and Mitry et al. (19 months and 15 months, respectively). Unlike the response reported in the hepatobiliary tract and pancreas, PDNECs originating in the colon or rectum seem to respond well initially to cisplatin and etoposide; however, survival of this disease is <1 year according to the study by Patta and colleagues.
Cisplatin Versus Carboplatin: Which Platinum‐Based Chemotherapy Should Be Used in GEPNECs?
A 2012 meta‐analysis of four randomized controlled trials using cisplatin and carboplatin to treat SCLC showed no disparity in OS or PFS rates but different toxicity profiles: carboplatin was associated with more grade 3–4 hematologic toxicities, whereas cisplatin‐based therapies exhibited more nonhematological toxicities of any grade [31].
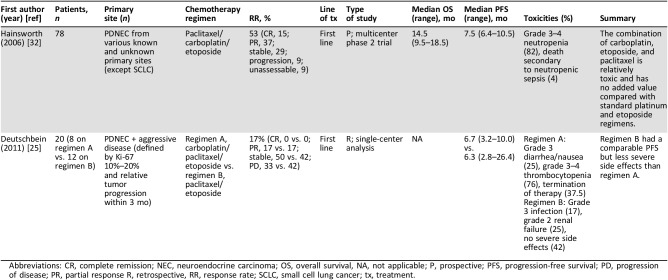
Hainsworth et al. (1997) demonstrated that patients with SCLC responded well to the combination of carboplatin, etoposide, and paclitaxel, and, given the side effect burden associated with cisplatin and etoposide, this research group aimed to reduce toxicity and improve outcomes by extrapolating these results to the treatment of PDNECs [32], [33], [34]. In a multicenter phase II trial, 78 patients with metastatic PDNECs without previous treatment received four cycles of carboplatin, etoposide, and paclitaxel every 3 weeks. Paclitaxel was given for three additional cycles to patients who demonstrated a response or had stable disease. A response rate of 53% and a median PFS of 7.5 months were achieved [32]. Toxicities were high, with 82% of patients experiencing grade 3 or 4 neutropenia, and 4% of patients died secondary to neutropenic sepsis (Table 2). Although this study did not directly compare platinum‐based chemotherapy, the similarity in efficacy but difference in toxicity profile between carboplatin and cisplatin can be inferred when comparing the results of this study with the cisplatin‐based studies cited above.
Table 2. Studies evaluating treatment with carboplatin.
Abbreviations: CR, complete remission; NEC, neuroendocrine carcinoma; OS, overall survival, NA, not applicable; P, prospective; PFS, progression‐free survival; PD, progression of disease; PR, partial response R, retrospective, RR, response rate; SCLC, small cell lung cancer; tx, treatment.
A retrospective study in 2011 contrasted platinum‐based chemotherapy to elucidate differences in efficacy and toxicities [25]. Deutschbein et al. (2011) examined the effect of two treatment regimens on 20 patients with PDNECs and those with aggressive disease (defined by a Ki‐67 index 10%–20% and relative tumor progression within 3 months). Of the 20 patients, 8 received carboplatin, etoposide, and paclitaxel, and 12 patients received cisplatin and etoposide. No statistically significant results were found between regimens with respect to complete response (0% vs. 0%, respectively), partial response (17% vs. 17%), stable disease (50% vs. 42%), progressive disease (33% vs. 42%), and median PFS (6.7 vs. 6.3 months; Table 2). Initially, eight patients were assigned to receive the carboplatin‐containing regimen; however, three had their treatment terminated prematurely, and two patients terminated prior to the completion of the first course. This result contrasts with cisplatin and etoposide arm, in which none of the 12 patients stopped the treatment because of side effects.
The results of this study support those of Mitry et al. that showed relatively decent tolerability of cisplatin and etoposide [15]. Conversely, Moertel and colleagues (1991) demonstrated severe intolerable side effects in their study examining the use of cisplatin and etoposide, mainly consisting of bone marrow suppression and GI distress [16]. Interestingly, Deutschbein et al. (2011) and Mitry et al. (1999) used a reduced dose of etoposide (100 mg/m2), compared with the dose utilized by Moertel (1991; 130 mg/m2), which may account for the relatively tolerable side effect profile [15], [16], [25]. Hainsworth et al. (2006) also administered etoposide at similar dosing (along with paclitaxel and carboplatin) [32]; however, side effects were severe as evident by the increased mortality rate secondary to sepsis. Deutschbein and colleagues concluded that the response rate is similar between regimens; however, the combination of cisplatin and etoposide may have a more tolerable side effect profile.
Taken together, these studies support the use of either carboplatin or cisplatin; however, the choice between therapies should be grounded in the toxicity profile.
Lastly, Sorbye et al. indicate no difference in efficacy between platinum‐based chemotherapy regimens [35]. In their retrospective analysis of epidemiological, tumor, and treatment data of 305 patients treated in Nordic hospitals in 2000–2009, cisplatin/etoposide (n = 129), carboplatin/etoposide (n = 67), and carboplatin/etoposide/vincristine (n = 28) were used as first‐line chemotherapy and showed comparable effectiveness in response rates, PFS, and survival. Taken together, these studies support the use of either carboplatin or cisplatin; however, the choice between therapies should be grounded in the toxicity profile.
Is Irinotecan as Effective as Etoposide in GEPNECs?
Although cisplatin and etoposide have been commonly adopted throughout the world, in Japan, the combination of irinotecan and cisplatin is used to treat SCLC and GEPNECs [24].
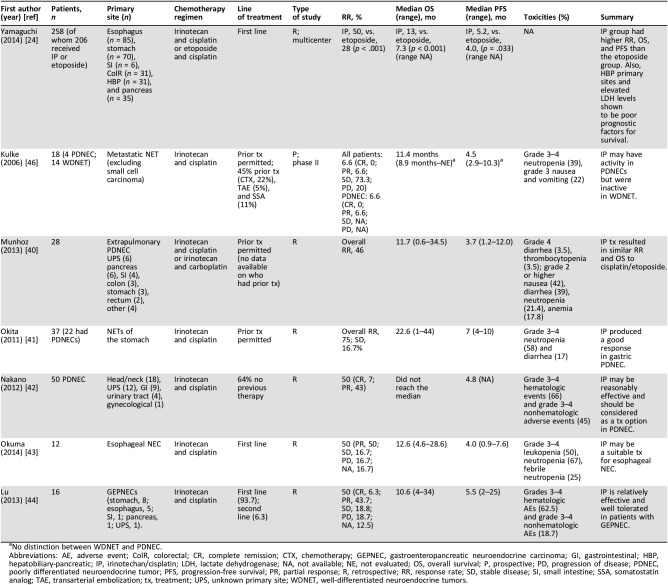
A Japanese study found that cisplatin and irinotecan were more effective than cisplatin and etoposide in treating SCLC. This prospective, phase II trial conducted by Noda et al. reported a median OS of 12.8 months and 9.4 months for cisplatin/irinotecan and cisplatin/etoposide, respectively (p = 0.002). The cisplatin/etoposide group experienced more myelosuppression than the cisplatin/irinotecan group, whereas the cisplatin/irinotecan group had more frequent diarrhea than the cisplatin/etoposide group [36]. These survival results, however, have not been duplicated. Hanna et al. conducted a phase III clinical trial and found no significant difference in OS between the cisplatin/irinotecan group and the cisplatin/etoposide group (9.3 months vs. 10.2 months, respectively; p = 0.74) or PFS (4.1 vs. 4.6 months, respectively; p = 0.37) [37]. Patients receiving cisplatin/irinotecan had less grade 3–4 myelosuppression and febrile neutropenia than patients receiving cisplatin/etoposide but more diarrhea and vomiting than patients receiving cisplatin/etoposide. A phase III clinical trial by Lara et al. examined 651 North American patients and randomly assigned them to receive cisplatin/irinotecan or cisplatin/etoposide; this study also failed to duplicate the survival results reported by Noda et al. [36], [38]. Lara et al. reported a median PFS in patients treated with cisplatin/irinotecan or with cisplatin/etoposide of 5.8 and 5.2 months, respectively (p = .07) and a median OS for the cisplatin/irinotecan and cisplatin/etoposide treatment groups of 9.9 and 9.1 months, respectively (p = .71). Although the Lara et al. and Noda et al. studies share the same research design, the populations in which the studies were conducted were racially and geographically different.
Building on the aforementioned SCLC studies, the use of irinotecan to treat GEPNECs has been investigated. Yamaguchi et al. conducted a multicenter, retrospective study in 258 patients to determine the effectiveness of cisplatin/irinotecan versus cisplatin/etoposide as first‐line chemotherapy in unresectable or recurrent GEPNECs [24]. Patients treated with cisplatin/irinotecan had a better response rate than patients treated with cisplatin/etoposide (50% vs. 28%, respectively; p < .001). The cisplatin/irinotecan and cisplatin/etoposide treatment groups had a median OS of 13 months and 7.3 months, respectively (p < .001; Table 3). As in the study conducted by Noda et al., the patient population in the Yamaguchi et al. study was Japanese, raising the question as to whether these results can be duplicated in a different study population. When the results were analyzed by primary site, patients with hepatobiliary‐pancreatic (HBP) NECs had a significantly better response rate to cisplatin/irinotecan than to cisplatin/etoposide (39% vs. 12%; p = 0.034). The Yamaguchi et al. study may act as a segue to further explore the use of cisplatin/irinotecan in GEPNECs treatment, especially for those with HBP sites of origin.
Table 3. Studies evaluating irinotecan based chemotherapy.
No distinction between WDNET and PDNEC.
Abbreviations: AE, adverse event; ColR, colorectal; CR, complete remission; CTX, chemotherapy; GEPNEC, gastroenteropancreatic neuroendocrine carcinoma; GI, gastrointestinal; HBP, hepatobiliary‐pancreatic; IP, irinotechan/cisplatin; LDH, lactate dehydrogenase; NA, not available; NE, not evaluated; OS, overall survival; P, prospective; PD, progression of disease; PDNEC, poorly differentiated neuroendocrine tumor; PFS, progression‐free survival; PR, partial response; R, retrospective; RR, response rate; SD, stable disease; SI, small intestine; SSA, somatostatin analog; TAE, transarterial embolization; tx, treatment; UPS, unknown primary site; WDNET, well‐differentiated neuroendocrine tumors.
Kulke et al. reported in a 2006 paper that although cisplatin and irinotecan may be effective in treating tumors, the combination of the two is inactive in WDNETs [39]. This phase II study included 18 patients with metastatic neuroendocrine tumors treated with irinotecan and cisplatin. Four patients had PDNECs; among these patients, one partial response was achieved. No responses were observed in patients with WDNETs. The low power of this study makes extrapolating the results to make general comments about the regimen difficult. Additionally, RR and OS were reported as one number for the study population (Table 3). A subset analysis of regimen response in PDNECs would have been beneficial. Last, in this paper, PDNECs were classified as having more than two mitoses per high‐powered field, which is generally classified as being a G2 NET or G3 WDNET [1]. Including less‐aggressive tumors may have skewed the results of this study, as lower grade tumors traditionally respond poorly to chemotherapy. Despite these limitations, additional smaller, retrospective studies have validated the substitution of irinotecan for etoposide and demonstrated equivalence in efficacy when compared with data in the literature (Table 3) [40], [41], [42], [43], [44].
Concomitantly, it appears that irinotecan and cisplatin may be considered for patients with GEPNECs, even though the PFS and OS are of short duration. These studies indicate that the toxicity profile of irinotecan appears to include less grade 4 toxicities than etoposide with no treatment‐induced deaths, and these considerations may play a role in choosing irinotecan over etoposide. Further phase II prospective studies are needed to make appropriate treatment recommendations.
These studies indicate that the toxicity profile of irinotecan appears to include less grade 4 toxicities than etoposide with no treatment‐induced deaths, and these considerations may play a role in choosing irinotecan over etoposide.
Alternative First‐Line Treatments
Recently, focus has shifted to the oral alkylating agent temozolomide as a single or combination therapy [45], [46], [47], [48], [49]. Several studies have demonstrated the effectiveness of capecitabine and temozolomide (CAPTEM) in WDNETs; in fact, this therapy has shown a response rate of up to 70% in G1 and G2 tumors [50], [51], [52]. Data supporting the use of this chemotherapy regimen in patients with GEPNECs are deficient.
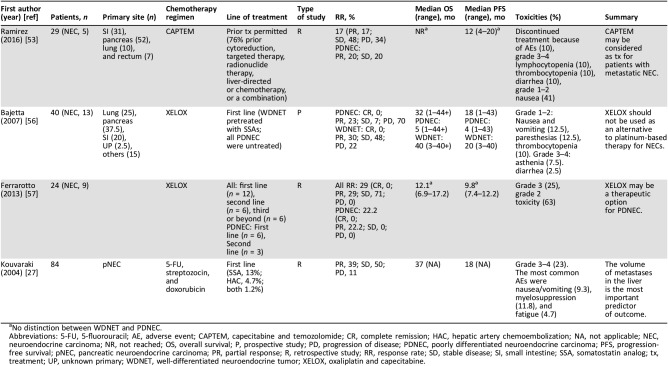
Our group conducted a retrospective study examining all patients with NETs who received at least one cycle of CAPTEM [53]. Although only 17% had a Ki‐67 index >20%, this study found that 20% of those patients demonstrated a partial response to CAPTEM and 20% had stability of disease (Table 4). Additionally, CAPTEM was also well tolerated in this study, with the majority of toxicities being grade 1 or 2 (Table 4). This study included a small number of G3 NETs patients and was retrospective in design, and a majority of the participants had undergone previous treatment. Nevertheless, these data indicate that CAPTEM may be beneficial for G3 NETs treatment and call for larger, prospective studies to be implemented. In fact, Eads and colleagues are currently conducting the first prospective study to investigate the use of CAPTEM or platinum and etoposide therapy in the treatment of GEPNECs (ClinicalTrials.gov identifier: NCT02595424) [54]. Results from this study will address gaps in the current literature [55].
Table 4. Studies evaluating alternative first‐line treatments for GEPNECs.
No distinction between WDNET and PDNEC.
Abbreviations: 5‐FU, 5‐fluorouracil; AE, adverse event; CAPTEM, capecitabine and temozolomide; CR, complete remission; HAC, hepatic artery chemoembolization; NA, not applicable; NEC, neuroendocrine carcinoma; NR, not reached; OS, overall survival; P, prospective study; PD, progression of disease; PDNEC, poorly differentiated neuroendocrine carcinoma; PFS, progression‐free survival; pNEC, pancreatic neuroendocrine carcinoma; PR, partial response; R, retrospective study; RR, response rate; SD, stable disease; SI, small intestine; SSA, somatostatin analog; tx, treatment; UP, unknown primary; WDNET, well‐differentiated neuroendocrine tumor; XELOX, oxaliplatin and capecitabine.
The use of capecitabine and oxaliplatin (XELOX) as first‐line therapy for patients with G3 NETs has also been investigated. Bajetta et al. conducted a phase II study in which 40 patients with NETs received oxaliplatin and capecitabine [56]. Of the 13 patients with PDNECs in this study, 3 patients (23%) demonstrated a partial response, 1 patient had stabilization of disease (7%), and 9 patients (70%) had disease progression. The median OS was 5 months, and the median PFS was 4 months (Table 4). As previously discussed, WDNETs have a worse response to chemotherapy than PDNECs. In this study, however, WDNETs showed promising results, with a median OS of 40 months and a median PFS of 20 months. As such, the data suggest that an oxaliplatin and capecitabine regimen may be considered as first‐line therapy for WDNETs. Conversely, this study suggests oxaliplatin and capecitabine should not be used as an alternative to conventional cisplatin/carboplatin‐based therapy for patients with PDNECs. These results conflict with the known chemosensitivity to cisplatin, indicating that PDNECs may have specific characteristics, making them insensitive to certain platinum‐based chemotherapies such as oxaliplatin but not to cisplatin or carboplatin.
Ferrarotto and colleagues also examined the use of XELOX in NETs, regardless of grade [56], [57]. Of the 24 patients, 9 patients had PDNECs. Of these, six patients received capecitabine and oxaliplatin as first‐line therapy, and three received the drug combination as at least second‐line therapy. Partial response was achieved in two patients, and two more patients had at least a 25% shrinkage in tumor (Table 4). The literature reports wide variation in survival between cisplatin and etoposide, from 14%–67% [16], [46]. The results reported by Ferrarotto and colleagues are within this range, indicating that capecitabine and oxaliplatin may be a viable alternative therapy option after all. The studies by both Bajetta et al. (2007) and Ferrarotto et al. (2013) are limited by the small number of patients with PDNECs included in their cohort (n = 13 and n = 9, respectively). Further prospective studies are needed to examine the effect of XELOX as an alternative treatment for PDNECs.
Although chemotherapy is not generally considered first‐line therapy for patients with WDNETs, streptozocin and 5‐fluorouracil (5‐FU) or doxorubicin have shown promising results as first‐line therapy or in disease progression while patients are taking SSA [26], [58], [59], [60], [61]. The use of this regimen in GEPNECs has been controversial because of the mixed responses [26], [62], [63]. Kouvaraki et al. (2004) examined the combination of fluorouracil, doxorubicin, and streptozocin in 84 patients with pancreatic G3 NETs [27]. This study found that the metastatic burden in the liver is the most vital predictor of patient outcome; greater than 75% disease burden was associated with a worse PFS and OS (Table 4). This finding has been shown in several other studies, highlighting the importance of maintaining a disease‐free state in the liver [64], [65], [66]. Toxicity profile was low, with GI issues and myelosuppression being the most common reported side effects. These side effects, however, did not significantly compromise treatment course [27]. For patients who cannot be surgically resected because of metastatic disease or local extension, 5FU/doxorubicin/streptozocin may offer a promising regimen. Again, further research of this treatment is necessary.
Finally, Du et al. (2013) conducted a retrospective review of 11 patients with GEPNECs who received the combination of 5‐FU, leucovorin, and irinotecan (FOLFIRI) as first‐line therapy [67]. This regimen yielded partial responses in seven patients with a median PFS of 6.5 months and a median OS of 13 months. FOLFIRI could be considered in the first‐line treatment of GEPNECs for those patients not felt to be a candidate for platinum.
Second‐Line Chemotherapy in GEPNECs
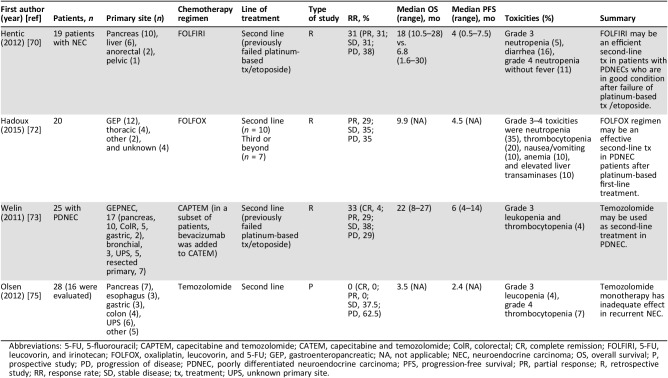
An established second‐line chemotherapy regimen does not currently exist for GEPNECs. Sorbye et al. (2012) noted in the NORDIC NEC study that of 100 patients who received second‐line therapy, 51% achieved disease stabilization [35]. This suggests that many patients with GEPNECs would benefit from subsequent lines of chemotherapy. Topoisomerase 1 inhibitors have shown promising results in SCLC and are currently the second‐line therapy of choice for this malignancy [68], [69]. Hentic et al. (2012) conducted the first series to suggest the effectiveness and tolerability of FOLFIRI as a second‐line therapy in PDNECs [70]. This retrospective, single‐institution study examined the use of FOLFIRI in 19 patients with PDNECs who failed platinum plus etoposide in first‐line treatment. The median OS was greater in patients who received FOLFIRI, compared with patients who failed first‐line therapy but were considered ineligible for FOLFIRI (18 vs. 6.8 months). The PFS was 4 months (Table 5). More than half of the patients in this series were not eligible for second‐line treatment with FOLFIRI, secondary to debility or major liver involvement. Irinotecan is metabolically active in the liver; thus, liver dysfunction is a limitation to this therapy [71].
Table 5. Studies evaluating second‐line therapy for GEPNECs.
Abbreviations: 5‐FU, 5‐fluorouracil; CAPTEM, capecitabine and temozolomide; CATEM, capecitabine and temozolomide; ColR, colorectal; CR, complete remission; FOLFIRI, 5‐FU, leucovorin, and irinotecan; FOLFOX, oxaliplatin, leucovorin, and 5‐FU; GEP, gastroenteropancreatic; NA, not applicable; NEC, neuroendocrine carcinoma; OS, overall survival; P, prospective study; PD, progression of disease; PDNEC, poorly differentiated neuroendocrine carcinoma; PFS, progression‐free survival; PR, partial response; R, retrospective study; RR, response rate; SD, stable disease; tx, treatment; UPS, unknown primary site.
To further evaluate the efficacy of oxaliplatin‐based therapy, Hadoux et al. (2015) retrospectively examined 5‐FU and oxaliplatin (FOLFOX) as second‐line therapy in 20 patients with PDNECs [72]. Of those who received therapy, 29% had partial response, 35% had stable disease, and 35% experienced disease progression. Median PFS was 4.5 months, and the median OS was 9.9 months (Table 5). As do the results by Bajetta et al. (2007; discussed above), this study advocates for the use of an oxaliplatin‐based therapy as second‐line treatment in patients with PDNECs [56].
Promising results using temozolomide‐based chemotherapy have been published. Welin et al. published the first study examining the effect of temozolomide with CAPTEM or temozolomide alone as second‐line therapy in patients with PDNECs [73]. All 25 patients had PDNECs (17 were GEPNEC in origin) that had progressed on platinum‐based first‐line treatment. In a subset of seven patients, bevacizumab was added to the treatment regimen. Median PFS was 6 months, median OS was 22 months, and 71% of patients achieved response or stabilization of disease after progression on first‐line therapy (Table 5). The benefit of adding capecitabine or bevacizumab to temozolomide was not proven in this study. Of interest, Welin et al. showed that patients with a Ki‐67 index <60%, with strong uptake of somatostatin receptors and positive staining for chromogranin A, showed more response to temozolomide. However, the small sample size of this study prevents any strong conclusions to be drawn regarding foretelling factors for temozolomide sensitivity. Last, silencing of MGMT (O6 methylguanine‐DNA methyltransferase), a DNA repair gene, has been shown to be a predictor of treatment success with temozolomide in brain glioblastoma [74]. The lack of expression predicts a greater response to temozolomide. Interestingly, in the present study, only one patient displayed methylation of the MGMT gene, potentially highlighting the lack of predictive value this test holds for PDNEC specifically. In contrast, a study by Olsen et al. that looked at the effect of temozolomide alone after progression on a platinum‐containing chemotherapy regimen in 28 patients with G3 NETs found no objective response to treatment [75]. This study found that patients with a Ki‐67 index ≥50% had a shorter median OS compared with patients with a Ki‐67 index <50% (2.7 months vs. 10.9 months; p < .0001; Table 5). These findings lend further support to the heterogeneity of G3 NETs and indicate that focus should be placed on the use of temozolomide in patients with a Ki‐67 index <50%. Olsen et al. did not include WDNETs and Welin et al. did not differentiate G3 NETs based on morphology. Some participants in the Welin et al. study likely had WDNETs, which may account for the increased response to temozolomide. Ultimately, conflicting results in temozolomide‐based chemotherapy highlight the need for further prospective research using a greater number of patients.
Immunotherapy in GEPNECs
Although systemic platinum‐based therapy remains the mainstay for first‐line treatment in GEPNECs, limited data exist on appropriate second‐line therapy for these rare tumors. The use of immunotherapy has shown benefit in multiple malignancies [20]. Currently, the use of immunotherapy is recommended as second‐line therapy for SCLC; however, limited studies have evaluated the use of immunotherapy in GEPNECs.
The CheckMate 032 trial demonstrated the efficacy of both monotherapy with a PD‐1 inhibitor (nivolumab, 10% response rate) and a combination of PD‐1 and CTLA‐4 inhibitor (nivolumab and ipilimumab, 19% response rate) in SCLC [76]. As a result, the National Comprehensive Cancer Network now recommends nivolumab and ipilimumab as an option for second‐line treatment for SCLC [77]. Multiple studies are currently underway to examine the effect of immunotherapy as first‐line (ClinicalTrials.gov identifiers: NCT02763579, NCT02046733), second‐line (ClinicalTrials.gov identifiers: NCT02701400, NCT02734004, NCT02551432, NCT02628067, NCT02481830), and maintenance therapy (ClinicalTrials.gov identifier: NCT02538666) in SCLC [78], [79], [80], [81], [82], [83], [84], [85].
Trials are currently underway to investigate the use of a PD‐1 inhibitor (pembrolizumab) in patients with G3 NETs who have previously failed first‐line therapy (NCT02939651, NCT03190213, NCT03136055) [86], [87], [88]. Also, in a design similar to CheckMate 032, patients are being recruited for a study using nivolumab and ipilimumab in the treatment of rare tumors, including GEPNECs (NCT02834013) [89]. The combination of checkpoint inhibitors with chemotherapy may also be of benefit, as demonstrated in the recent press release noting that interim results from IMpower 133 show an improvement in OS and PFS in patients with extensive‐stage SCLC [90]. Given that SCLC has shown promising responses to immunotherapy, patients with GEPNECs may benefit from this therapy. Further research is necessary to elucidate the role that immunotherapy plays in the treatment of neuroendocrine tumors.
In addition to targeting PD‐L1 and PD‐1 for treatment, PD‐L1 expression may serve as an indicator for prognosis and treatment response in GEPNECs. The association between PD‐L1 expression and overall survival was demonstrated in KEYNOTE 010, a study that evaluated the effect of pembrolizumab in patients with NSCLC. Patients with a PD‐L1 expression >50% did better with pembrolizumab at 2 mg/kg (median OS 14.9 vs. 8.2 months; p = .0002) and 10 mg/kg (median OS 17.3 vs. 8.2 months; p < .0001) than patients with a PD‐L1 expression <50% [91]. Mixed findings have been reported in SCLC. Miao et al. (2016) demonstrated that the expression of PD‐L1 was correlated with limited disease and may indicate better OS in SCLC, compared with patients having PD‐L1‐negative tumors (17.0 vs. 9.0 months; p = .018) [92]. Contrary to these findings, CheckMate 032 noted no association between PD‐L1 expression and response rate in patients with SCLC receiving immunotherapy [76].
The role of PD‐L1 as a prognostic indicator in GEPNECs is also uncertain. Recent studies have shown that high grade tumors express increased levels of PD‐L1 on their tumor surface and within their microenvironment [93], [94]. A single‐institution, retrospective analysis was conducted in 32 patients to determine impact of PD‐L1 expression on survival and response rate in G3 NETs. This study found that a PD‐L1 positive tumors were associated with a higher tumor grade (p = 0.008), a significant decrease in OS (16 vs. 24.8 months; p = .037), and a significantly increased response to first‐line therapy (75% vs. 11.8%; p = .02) [93]. These results are in line with previous studies showing that patients with PDNECs respond better to first‐line chemotherapy than patients with WDNETs [3], [35], [95], [96]. The results suggest that the hyperproliferative and aggressive features of PDNECs may be due to aberrant expression of PD‐L1, which increases immune system invasion. Although associated with decreased survival, high expression of PD‐L1 may serve as a potential target for immunotherapies in the treatment of GEPNECs. Anecdotal cases do exist indicating potential benefit, but these promising results highlight the need for further studies to determine the effect of PD‐L1 as a predictor of prognosis and treatment response in GEPNECs, specifically.
There are also data to suggest that tumors with microsatellite instability‐high (MSI‐H) and deficient DNA mismatch repair (dMMR) are more responsive to PD‐1 inhibitors. Several clinical trials (KEYNOTE016, KEYNOTE 158, KEYNOTE 164) have confirmed the benefit of pembrolizumab for the treatment of MSI‐H and dMMR tumors that have progressed on first‐line therapy [97]. As such, patients with GEPNECs who have failed first‐line therapy should consider microsatellite instability testing to determine the utility of pembrolizumab in their treatment plan.
PRRT in GEPNECs
In recent years, promising results have been generated by using radionuclide therapy with SSAs, or PRRT, in patients with NETs. PRRT works by radiopeptides, most commonly lutetium‐177 (177Lu), dotatate, yttrium‐90 (90Y), or indium‐111 (111In) connected to an SSA, binding to overexpressed somatostatin receptors in WDNETs. This molecule is then internalized to deliver direct intercellular radiotherapy [2]. Normal cells express significantly less somatostatin receptors compared with malignant cells; thus, PRRT has little effect on healthy tissues. The more somatostatin receptors a tumor cell expresses, the more successful PRRT will be. As such, somatostatin scintigraphy and gallium‐68 positron emission tomography and computed tomography have been used to predict the effectiveness of this therapy in individuals with NETs [98].
PRRT is better tolerated when paralleled to chemotherapy [19]. In a recent study evaluating 504 patients with NETs, 177Lu produced digestive side effects in 25% of patients and hematologic side effects in 3.6%. Serious adverse effects, including myelodysplastic syndrome, acute leukemia, and liver toxicity, occurred in roughly 1% of patients [99].
Recently, the U.S. Food and Drug Administration (FDA) approved 177Lu in the treatment of somatostatin receptor positive GEPNETs, making it the first FDA‐approved PRRT in the U.S. The approval of this therapy is based on the NETTER‐1 trial, a phase III study that contrasted treatment with 177Lu plus octreotide long‐acting repeatable (LAR) 30 mg every 4 weeks with 60 mg of octreotide LAR alone every 4 weeks in patients with midgut WDNETs who expressed high amounts of somatostatin receptors. This study demonstrated that 177Lu led to a 79% reduction in risk of disease progression or death compared with the 60 mg octreotide LAR arm (p < .0001). The RR was 13% for patients who received 177Lu plus octreotide LAR 30 mg and 4% in the octreotide LAR 60 mg group (p < .0148) [100].
To date, however, no prospective research studies are assessing the impact of PRRT on GEPNECs. Thus, PRRT is not currently recommended for GEPNECs, and chemotherapy remains the mainstay therapy.
Two case reports and one retrospective study, however, investigated the utility of PRRT in G3 NETs [17], [18], [19]. Garske et al. (2012) discussed a patient with an PDNEC of unknown primary with liver metastasis who had a successful response to PRRT after progression of disease on two chemotherapies [17]. Interestingly, the patient had a high uptake on somatostatin scintigraphy, despite Ki‐67 proliferation rates that ranged from 10% to 50% on liver metastases (Table 7). Previous research groups have demonstrated this inverse relationship between proliferation rate and expression of somatostatin receptors [101], [102], [103]. In fact, NET guidelines state that somatostatin receptors are commonly negative in PDNEC [104]. Contrarily, studies have found somatostatin receptor scintigraphy (SRS) to be positive in high numbers of patients with PDNECs [73]. For example, Welin et al. found that 62% of patients with PDNECs had a positive SRS, and Binderup et al. reported that 69% of patients with PDNECs were SRS positive at their original visit [73], [105]. As such, it has been argued that the lack of somatostatin receptor expression in GEPNECs may not completely account for the reported ineffectiveness of PRRT in this subgroup [18].
Ezziddin et al. (2011) conducted a retrospective review of 81 patients with GEPNET (7 of whom had GEPNECs) who were treated with 177Lu [18]. All patients were screened for adequate somatostatin receptor expression and treated with PRRT. In the patients with a Ki‐67 index ≤20%, 55% demonstrated a partial or minor response, 34% showed stable disease, and 11% had progression of disease. In the seven patients with a Ki‐67 index >20%, significant progression was demonstrated in 71.4% despite having satisfactory receptor expression to initiate therapy (Table 7). Although patients with GEPNECs were a small percentage of the study population (n = 7; 8.6%), this study provides further evidence that another unknown mechanism besides receptor expressivity may account for the ineffectiveness of PRRT in GEPNECs.
It has been proposed that cellular differentiation and proliferation index may play a role in predicting the effectiveness of PRRT in patients with GEPNECs. G3 NETs have proven to be a heterogeneous group that should be categorized based on morphology and proliferation index because of their varying degrees of treatment response [3], [35], [95], [96], [106]. When comparing subcategories of G3 NETs, based on morphology Vélayoudom‐Céphise et al. found that 88% of WDNETs had positive somatostatin receptor imaging (vs. 50% in PDNECs), perhaps indicating that the presence of these receptors, rather than a cutoff of Ki‐67 > 20%, is more indicative of PRRT response [3].
A 2017 case report describes the use of PRRT in a patient with a pancreatic NET with liver metastasis who experienced complete remission for more than 3 years after the initiation of therapy [19]. Interestingly, the subject had a mitotic count that was low (classified as a G2 NET) but a high Ki‐67 index of 45%–70%. The authors of this paper concluded that PRRT may be recommended for proliferative‐discordant G3 NETs before conventional treatment, whereas chemotherapy may be initiated first in proliferative‐concordant PDNECs. As such, more attention needs to be placed on the discordance between proliferative markers, as discordance may predict a more favorable prognosis and a positive response to PRRT therapy.
In summary, high proliferation rate alone should not exclude the use of PRRT. Rather, somatostatin receptor expression, discordance between proliferative markers, and morphologic analysis should be considered when recommending the use of PRRT. Further research in the form of randomized controlled trials and prospective studies is required to assess the effect of PRRT versus chemotherapy in G3 NETs with discordant proliferative markers in which malignant cells display significant somatostatin receptors. Future researchers may also wish to focus on how tumors with a Ki‐67 index >20% but a varying degree of cell differentiation on histology respond to PRRT. Although the need for specific (and arguably new) treatment protocols for GEPNECs is obvious, the solution is far from obvious. A main limitation in the progression of research and development is the small number of patients included in each study. Currently, no large randomized controlled trials are examining various treatment modalities because of the rarity of this neoplasm.
Conclusion
Current first‐line GEPNECs therapy is platinum‐based chemotherapy with etoposide. Responses and survival remain the same between cisplatin and carboplatin; however, the side effect profiles are different. Second‐line therapies are urgently needed for GEPNECs. FOLFIRI and FOLFOX have shown promising results. When determining the usefulness of PRRT in GEPNECs, focus should be placed on somatostatin receptor expression, discordance between proliferative markers, and morphologic analysis rather than merely on proliferation index. Immunotherapy with or without chemotherapy or PRRT may also play a role; however, studies are needed for this often aggressive and universally fatal disease.
Overall, the limited data from small, nonrandomized studies make it difficult to draw definite conclusions. Larger prospective studies and, ideally, randomized controlled trials are necessary to enhance and expand the current data. This will allow for the establishment of accurate GEPNECs categorization and ultimately optimal treatment regimens.
Footnotes
For Further Reading: Andrew E. Hendifar, Deepti Dhall, Jonathan R. Strosberg. The Evolving Treatment Algorithm for Advanced Neuroendocrine Neoplasms: Diversity and Commonalities Across Tumor Types. The Oncologist 2019;24:54–61.
Implications for Practice: This review raises awareness of the evolution of the treatment algorithm for advanced neuroendocrine neoplasms (NEN) from one that is directed by primary tumor site–specific classification to one that is directed by biologic classification. In addition, this review promotes understanding of the new pathologic category of well‐differentiated G3 pancreatic neuroendocrine tumors and highlights the need for prospective trials in this patient population, for whom there is currently no standard of care. This review further provides a conceptual treatment schematic that categorizes the recommendations for systemic treatments for advanced disease by biologic classification, including the new and established categories of NEN.
Author Contributions
Conception/design: Katharine E.H. Thomas, Brianne A. Voros, J. Philip Boudreaux, Ramcharan Thiagarajan, Eugene A. Woltering, Robert A. Ramirez
Provision of study material or patients: Katharine E.H. Thomas, Brianne A. Voros, Robert A. Ramirez
Collection and/or assembly of data: Katharine E.H. Thomas, Brianne A. Voros, Robert A. Ramirez
Data analysis and interpretation: Katharine E.H. Thomas, Brianne A. Voros, J. Philip Boudreaux, Ramcharan Thiagarajan, Eugene A. Woltering, Robert A. Ramirez
Manuscript writing: Katharine E.H. Thomas, Brianne A. Voros, J. Philip Boudreaux, Ramcharan Thiagarajan, Eugene A. Woltering, Robert A. Ramirez
Final approval of manuscript: Katharine E.H. Thomas, Brianne A. Voros, J. Philip Boudreaux, Ramcharan Thiagarajan, Eugene A. Woltering, Robert A. Ramirez
Disclosures
J. Philip Boudreaux: Ipsen Pharmaceuticals, Inc., Lexicon Pharmaceuticals, Inc. (C/A), Ipsen Pharmaceuticals, Inc., Lexicon Pharmaceuticals, Inc., Novartis Pharmaceuticals Corporation (other—speakers bureau); Eugene A. Woltering: Inter Science Institute (C/A), Gastrointestinal Council (other—chairman); Robert A. Ramirez: Ipsen Biopharmaceuticals, Inc., Advanced Accelerator Applications, Biotheranostics, Inc. (C/A), Merck & Co., Inc., Genentech, AstraZeneca, Guardant, Ipsen Biopharmaceuticals, Inc. (other—speakers bureau). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Kruljac I, Pape U. The classification of neuroendocrine neoplasms: “Neuroendocrine carcinomas” revisited–a 2017 update and future perspectives. Endocr Oncol Metab 2017;3:37–42. [Google Scholar]
- 2.Mahjoub AR, O'Reilly EM. Emerging therapies for pancreas neuroendocrine cancers. Chin Clin Oncol 2013;2:23. [DOI] [PubMed] [Google Scholar]
- 3.Vélayoudom‐Céphise F, Duvillard P, Foucan L et al. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr Relat Cancer 2013;20:649–657. [DOI] [PubMed] [Google Scholar]
- 4.Kim JY, Hong S, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol 2017;29:11–16. [DOI] [PubMed] [Google Scholar]
- 5.Strosberg JR, Halfdanarson TR, Bellizzi AM et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas 2017;46:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klöppel G, Heitz PU, Capella C et al. Endocrine tumours of the pancreas In: Solcia E, Klöppel G, Sobin LH, eds. Histological Typing of Endocrine Tumours. Berlin: Springer‐Verlag, 2000:56–60. [Google Scholar]
- 7.Lee SS, Lee J, Ryu MH et al. Extrapulmonary small cell carcinoma: Single center experience with 61 patients. Acta Oncol 2007;46:846–851. [DOI] [PubMed] [Google Scholar]
- 8.Galanis E, Frytak S, Lloyd RV. Extrapulmonary small cell carcinoma. Cancer 1997;79:1729–1736. [DOI] [PubMed] [Google Scholar]
- 9.Ahlman H, Nilsson O, McNicol AM et al. Poorly‐differentiated endocrine carcinomas of midgut and hindgut origin. Neuroendocrinology 2008;87:40–46. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson O, Van Cutsem E, Delle Fave G et al. Poorly differentiated carcinomas of the foregut (gastric, duodenal and pancreatic). Neuroendocrinology 2006;84:212–215. [DOI] [PubMed] [Google Scholar]
- 11.Garcia‐Carbonero R, Sorbye H, Baudin E et al. ENETS consensus guidelines for high‐grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology 2016;103:186–194. [DOI] [PubMed] [Google Scholar]
- 12.Fazio N, Spada F, Giovannini M. Chemotherapy in gastroenteropancreatic (GEP) neuroendocrine carcinomas (NEC): A critical view. Cancer Treat Rev 2013;39:270–274. [DOI] [PubMed] [Google Scholar]
- 13.Spada F, Antonuzzo L, Marconcini R et al. Chemotherapy with capecitabine plus temozolomide (CAP‐TEM) in patients with advanced neuroendocrine neoplasms (NENs): An Italian multicenter retrospective analysis. J Clin Oncol 2015;33:e15174. [Google Scholar]
- 14.Johnson LA, Lavin P, Moertel CG et al. Carcinoids: The association of histologic growth pattern and survival. Cancer 1983;51:882–889. [DOI] [PubMed] [Google Scholar]
- 15.Mitry E, Baudin E, Ducreux M et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer 1999;81:1351–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moertel CG, Kvols LK, O'Connell MJ et al. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer 1991;68:227–232. [DOI] [PubMed] [Google Scholar]
- 17.Garske U, Sandström M, Johansson S et al. Lessons on tumour response: Imaging during therapy with 177Lu‐DOTA‐octreotate. A case report on a patient with a large volume of poorly differentiated neuroendocrine carcinoma. Theranostics 2012;2:459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezziddin S, Opitz M, Attassi M et al. Impact of the ki‐67 proliferation index on response to peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging 2011;38:459–466. [DOI] [PubMed] [Google Scholar]
- 19.Montanier N, Joubert‐Zakeyh J, Pétorin C et al. The prognostic influence of the proliferative discordance in metastatic pancreatic neuroendocrine carcinoma revealed by peptide receptor radionuclide therapy: Case report and review of literature. Medicine (Baltimore) 2017;96:e6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehman JM, Gwin ME, Massion PP. Immunotherapy and targeted therapy for small cell lung cancer: There is hope. Curr Oncol Rep 2017;19:49. [DOI] [PubMed] [Google Scholar]
- 21.Fjällskog MH, Granberg D, Welin SL et al. Treatment with cisplatin and etoposide in patients with neuroendocrine tumors. Cancer 2001;92:1101–1107. [DOI] [PubMed] [Google Scholar]
- 22.Iwasa S, Morizane C, Okusaka T et al. Cisplatin and etoposide as first‐line chemotherapy for poorly differentiated neuroendocrine carcinoma of the hepatobiliary tract and pancreas. Jpn J Clin Oncol 2010;40:313–318. [DOI] [PubMed] [Google Scholar]
- 23.Patta A, Fakih M. First‐line cisplatin plus etoposide in high‐grade metastatic neuroendocrine tumors of colon and rectum (MCRC NET): Review of 8 cases. Anticancer Res 2011;31:975–978. [PubMed] [Google Scholar]
- 24.Yamaguchi T, Machida N, Morizane C et al. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci 2014;105:1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deutschbein T, Unger N, Yuece A et al. Chemotherapy in patients with progressive, undifferentiated neuroendocrine tumors: A single‐center experience. Horm Metab Res 2011;43:838–843. [DOI] [PubMed] [Google Scholar]
- 26.Moertel CG, Lefkopoulo M, Lipsitz S et al. Streptozocin–doxorubicin, streptozocin–fluorouracil, or chlorozotocin in the treatment of advanced islet‐cell carcinoma. N Engl J Med 1992;326:519–523. [DOI] [PubMed] [Google Scholar]
- 27.Kouvaraki MA, Ajani JA, Hoff P et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 2004;22:4762–4771. [DOI] [PubMed] [Google Scholar]
- 28.Madeira I, Terris B, Voss M et al. Prognostic factors in patients with endocrine tumours of the duodenopancreatic area. Gut 1998;43:422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panzuto F, Nasoni S, Falconi M et al. Prognostic factors and survival in endocrine tumor patients: Comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer 2005;12:1083–1092. [DOI] [PubMed] [Google Scholar]
- 30.Bettini R, Boninsegna L, Mantovani W et al. Prognostic factors at diagnosis and value of WHO classification in a mono‐institutional series of 180 non‐functioning pancreatic endocrine tumours. Ann Oncol 2008;19:903–908. [DOI] [PubMed] [Google Scholar]
- 31.Rossi A, Di Maio M, Chiodini P et al. Carboplatin‐or cisplatin‐based chemotherapy in first‐line treatment of small‐cell lung cancer: The COCIS meta‐analysis of individual patient data. J Clin Oncol 2012;30:1692–1698. [DOI] [PubMed] [Google Scholar]
- 32.Hainsworth JD, Spigel DR, Litchy S et al. Phase II trial of paclitaxel, carboplatin, and etoposide in advanced poorly differentiated neuroendocrine carcinoma: A Minnie Pearl Cancer Research Network study. J Clin Oncol 2006;24:3548–3554. [DOI] [PubMed] [Google Scholar]
- 33.Hainsworth JD, Gray JR, Stroup SL et al. Paclitaxel, carboplatin, and extended‐schedule etoposide in the treatment of small‐cell lung cancer: Comparison of sequential phase II trials using different dose‐intensities. J Clin Oncol 1997;15:3464–3470. [DOI] [PubMed] [Google Scholar]
- 34.Hainsworth JD, Erland JB, Kalman LA et al. Carcinoma of unknown primary site: Treatment with 1‐hour paclitaxel, carboplatin, and extended‐schedule etoposide. J Clin Oncol 1997;15:2385–2393. [DOI] [PubMed] [Google Scholar]
- 35.Sorbye H, Welin S, Langer SW et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann Oncol 2012;24:152–160. [DOI] [PubMed] [Google Scholar]
- 36.Noda K, Nishiwaki Y, Kawahara M et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small‐cell lung cancer. N Engl J Med 2002;346:85–91. [DOI] [PubMed] [Google Scholar]
- 37.Hanna N, Bunn PA Jr, Langer C et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive‐stage disease small‐cell lung cancer. J Clin Oncol 2006;24:2038–2043. [DOI] [PubMed] [Google Scholar]
- 38.Lara PN Jr, Natale R, Crowley J et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive‐stage small‐cell lung cancer: Clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009;27:2530–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulke MH, Wu B, Ryan DP et al. A phase II trial of irinotecan and cisplatin in patients with metastatic neuroendocrine tumors. Dig Dis Sci 2006;51:1033–1038. [DOI] [PubMed] [Google Scholar]
- 40.Ramella Munhoz R1, de Mendonça Rego JF, de Celis Ferrari AR et al. Combination of irinotecan and a platinum agent for poorly differentiated neuroendocrine carcinomas. Rare Tumors 2013;5:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okita NT, Kato K, Takahari D et al. Neuroendocrine tumors of the stomach: Chemotherapy with cisplatin plus irinotecan is effective for gastric poorly‐differentiated neuroendocrine carcinoma. Gastric Cancer 2011;14:161–165. [DOI] [PubMed] [Google Scholar]
- 42.Nakano K, Takahashi S, Yuasa T et al. Feasibility and efficacy of combined cisplatin and irinotecan chemotherapy for poorly differentiated neuroendocrine carcinomas. Jpn J Clin Oncol 2012;42:697–703. [DOI] [PubMed] [Google Scholar]
- 43.Okuma HS, Iwasa S, Shoji H et al. Irinotecan plus cisplatin in patients with extensive‐disease poorly differentiated neuroendocrine carcinoma of the esophagus. Anticancer Res 2014;34:5037–5041. [PubMed] [Google Scholar]
- 44.Lu ZH, Li J, Lu M et al. Feasibility and efficacy of combined cisplatin plus irinotecan chemotherapy for gastroenteropancreatic neuroendocrine carcinomas. Med Oncol 2013;30:664. [DOI] [PubMed] [Google Scholar]
- 45.Ekeblad S, Sundin A, Janson ET et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res 2007;13:2986–2991. [DOI] [PubMed] [Google Scholar]
- 46.Kulke MH, Stuart K, Enzinger PC et al. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol 2006;24:401–406. [DOI] [PubMed] [Google Scholar]
- 47.Chan JA, Stuart K, Earle CC et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J Clin Oncol 2012;30:2963–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fine RL, Fogelman DR, Schreibman SM. Effective treatment of neuroendocrine tumors with temozolomide and capecitabine. J Clin Oncol 2005;23(16 suppl):4216. [Google Scholar]
- 49.Lyons JM 3rd, Abergel J, Thomson JL et al. In vitro chemoresistance testing in well‐differentiated carcinoid tumors. Ann Surg Oncol 2009;16:649–655. [DOI] [PubMed] [Google Scholar]
- 50.Chan JA, Kulke MH. New treatment options for patients with advanced neuroendocrine tumors. Curr Treat Options Oncol 2011;12:136–148. [DOI] [PubMed] [Google Scholar]
- 51.Strosberg JR, Fine RL, Choi J et al. First‐line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011;117:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Auernhammer CJ, Göke B. Therapeutic strategies for advanced neuroendocrine carcinomas of jejunum/ileum and pancreatic origin. Gut 2011;60:1009–1021. [DOI] [PubMed] [Google Scholar]
- 53.Ramirez RA, Beyer DT, Chauhan A et al. The role of capecitabine/temozolomide in metastatic neuroendocrine tumors. The Oncologist 2016;21:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cisplatin, carboplatin and etoposide or temozolomide and capecitabine in treating patients with neuroendocrine carcinoma of the gastrointestinal tract or pancreas that is metastatic or cannot be removed by surgery. ClinicalTrials.gov identifier NCT02595424. Bethesda, MD: National Library of Medicine; November 3, 2013. Available at https://clinicaltrials.gov/ct2/show/NCT02595424. Accessed March 7, 2018.
- 55.Eads JR, Catalano PJ, Fisher GA et al. Randomized phase II study of cisplatin and etoposide versus temozolomide and capecitabine in patients (pts) with advanced G3 non‐small cell gastroenteropancreatic neuroendocrine carcinomas (GEPNEC): A trial of the ECOG‐ACRIN cancer research group (EA2142). J Clin Oncol 2016;34:TPS4149. [Google Scholar]
- 56.Bajetta E, Catena L, Procopio G et al. Are capecitabine and oxaliplatin (XELOX) suitable treatments for progressing low‐grade and high‐grade neuroendocrine tumours? Cancer Chemother Pharmacol 2007;59:637–642. [DOI] [PubMed] [Google Scholar]
- 57.Ferrarotto R, Testa L, Riechelmann RP et al. Combination of capecitabine and oxaliplatin is an effective treatment option for advanced neuroendocrine tumors. Rare Tumors 2013;5:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rivera E, Ajani JA. Doxorubicin, streptozocin, and 5‐fluorouracil chemotherapy for patients with metastatic islet‐cell carcinoma. Am J Clin Oncol 1998;21:36–38. [DOI] [PubMed] [Google Scholar]
- 59.Bajetta E, Ferrari L, Procopio G et al. Efficacy of a chemotherapy combination for the treatment of metastatic neuroendocrine tumours. Ann Oncol 2002;13:614–621. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez MA, Biswas S, Clifton L et al. Treatment of neuroendocrine tumours with infusional 5‐fluorouracil, folinic acid and streptozocin. Br J Cancer 2003;89:455–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ridolfi R, Amaducci L, Derni S et al. Chemotherapy with 5‐fluorouracil and streptozotocin in carcinoid tumors of gastrointestinal origin: Experiences with 13 patients. J Chemother 1991;3:328–331. [DOI] [PubMed] [Google Scholar]
- 62.Moertel CG, Hanley JA, Johnson LA. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet‐cell carcinoma. N Engl J Med 1980;303:1189–1194. [DOI] [PubMed] [Google Scholar]
- 63.Cheng PN, Saltz LB. Failure to confirm major objective antitumor activity for streptozocin and doxorubicin in the treatment of patients with advanced islet cell carcinoma. Cancer 1999;86:944–948. [PubMed] [Google Scholar]
- 64.Solorzano CC, Lee JE, Pisters PW et al. Nonfunctioning islet cell carcinoma of the pancreas: Survival results in a contemporary series of 163 patients. Surgery 2001;130:1078–1085. [DOI] [PubMed] [Google Scholar]
- 65.Yu F, Venzon DJ, Serrano J et al. Prospective study of the clinical course, prognostic factors, causes of death, and survival in patients with long‐standing zollinger‐ellison syndrome. J Clin Oncol 1999;17:615–630. [DOI] [PubMed] [Google Scholar]
- 66.Chu QD, Hill HC, Douglass HO et al. Predictive factors associated with long‐term survival in patients with neuroendocrine tumors of the pancreas. Ann Surg Oncol 2002;9:855–862. [DOI] [PubMed] [Google Scholar]
- 67.Du Z, Wang Y, Zhou Y et al. First‐line irinotecan combined with 5‐fluorouracil and leucovorin for high‐grade metastatic gastrointestinal neuroendocrine carcinoma. Tumori 2013;99:57–60. [DOI] [PubMed] [Google Scholar]
- 68.Langer CJ. Treatment of non‐small‐cell lung cancer in North America: The emerging role of irinotecan. Oncology (Williston Park) 2001;15(1 suppl 1):19–24. [PubMed] [Google Scholar]
- 69.O'Brien M, Eckardt J, Ramlau R. Recent advances with topotecan in the treatment of lung cancer. The Oncologist 2007;12:1194–1204. [DOI] [PubMed] [Google Scholar]
- 70.Hentic O, Hammel P, Couvelard A et al. FOLFIRI regimen: An effective second‐line chemotherapy after failure of etoposide–platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr Relat Cancer 2012;19:751–757. [DOI] [PubMed] [Google Scholar]
- 71.Raymond E, Boige V, Faivre S et al. Dosage adjustment and pharmacokinetic profile of irinotecan in cancer patients with hepatic dysfunction. J Clin Oncol 2002;20:4303–4312. [DOI] [PubMed] [Google Scholar]
- 72.Hadoux J, Malka D, Planchard D et al. Post‐first‐line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocr Relat Cancer 2015;22:289–298. [DOI] [PubMed] [Google Scholar]
- 73.Welin S, Sorbye H, Sebjornsen S et al. Clinical effect of temozolomide‐based chemotherapy in poorly differentiated endocrine carcinoma after progression on first‐line chemotherapy. Cancer 2011;117:4617–4622. [DOI] [PubMed] [Google Scholar]
- 74.Hegi ME, Diserens A, Gorlia T et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352:997–1003. [DOI] [PubMed] [Google Scholar]
- 75.Olsen IH, Sørensen JB, Federspiel B et al. Temozolomide as second or third line treatment of patients with neuroendocrine carcinomas. Scientific World Journal 2012;2012:170496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Antonia SJ, López‐Martin JA, Bendell J et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small‐cell lung cancer (CheckMate 032): A multicentre, open‐label, phase 1/2 trial. Lancet Oncol 2016;17:883–895. [DOI] [PubMed] [Google Scholar]
- 77.National Comprehensive Cancer Network . Bone cancer (version 2.2017). https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accessed November 7, 2017.
- 78.A study of carboplatin plus etoposide with or without atezolizumab in participants with untreated extensive‐stage (ES) small cell lung cancer (SCLC) (IMpower133). ClinicalTrials.gov identifier NCT02763579. Bethesda, MD: National Library of Medicine; May 5, 2016. Available at https://clinicaltrials.gov/ct2/show/NCT02763579. Accessed March 7, 2018.
- 79.An investigational immuno‐therapy study of nivolumab, or nivolumab in combination with ipilimumab, or placebo in patients with extensive‐stage disease small cell lung cancer (ED‐SCLC) after completion of platinum‐based chemotherapy (CheckMate 451). ClinicalTrials.gov identifier NCT02538666. Bethesda, MD: National Library of Medicine; September 2, 2015. Available at https://clinicaltrials.gov/ct2/show/NCT02538666. Accessed March 7, 2018.
- 80.Effectiveness study of nivolumab compared to chemotherapy in patients with relapsed small‐cell lung cancer (CheckMate331). ClinicalTrials.gov identifier NCT02481830. Bethesda, MD: National Library of Medicine; June 25, 2015. Available at https://clinicaltrials.gov/ct2/show/NCT02481830. Accessed March 7, 2018.
- 81.Pembrolizumab and paclitaxel in refractory small cell lung cancer (MISP‐MK3475). ClinicalTrials.gov identifier NCT02551432. Bethesda, MD: National Library of Medicine; September 16, 2015. Available at https://clinicaltrials.gov/ct2/show/NCT02551432. Accessed March 7, 2018.
- 82.Small cell lung carcinoma trial with nivolumab and IpiliMUmab in LImited disease (STIMULI). ClinicalTrials.gov identifier NCT02046733. Bethesda, MD: National Library of Medicine (US); January 28, 2014. Available at https://clinicaltrials.gov/ct2/show/NCT02046733. Accessed March 7, 2018.
- 83.Study of pembrolizumab (MK‐3475) in participants with advanced solid tumors (MK‐3475‐158/KEYNOTE‐158). ClinicalTrials.gov identifier NCT02628067. Bethesda, MD: National Library of Medicine; December 11, 2015. Available at https://clinicaltrials.gov/ct2/show/NCT02628067. Accessed March 7, 2018.
- 84.Tremelimumab and durvalumab with or without radiation therapy in patients with relapsed small cell lung cancer. ClinicalTrials.gov identifier NCT02701400. Bethesda, MD: National Library of Medicine; March 8, 2016. Available at https://clinicaltrials.gov/ct2/show/NCT02701400. Accessed March 7, 2018.
- 85.A phase I/II study of MEDI4736 in combination with olaparib in patients with advanced solid tumors. (MEDIOLA). ClinicalTrials.gov identifier NCT02734004. Bethesda, MD: National Library of Medicine; April 12, 2016. Available at https://clinicaltrials.gov/ct2/show/NCT02734004. Accessed March 7, 2018.
- 86.Pembrolizumab‐based therapy in previously treated high grade neuroendocrine carcinomas. ClinicalTrials.gov identifier NCT03136055. Bethesda, MD: National Library of Medicine; May 2, 2017. Available at https://clinicaltrials.gov/ct2/show/NCT03136055. Accessed March 7, 2018.
- 87.Pembrolizumab for the treatment of recurrent high grade neuroendocrine carcinoma (pembro NEC). ClinicalTrials.gov identifier NCT03190213. Bethesda, MD: National Library of Medicine; June 16, 2017. Available at https://clinicaltrials.gov/ct2/show/NCT03190213. Accessed March 7, 2018.
- 88.A study of pembrolizumab in patients with neuroendocrine tumors. ClinicalTrials.gov identifier NCT02939651. Bethesda, MD: National Library of Medicine; October 20, 2016. Available at https://clinicaltrials.gov/ct2/show/NCT02939651. Accessed March 7, 2018.
- 89.DART: Dual anti‐CTLA‐4 and anti‐PD‐1 blockade in rare tumors. ClinicalTrials.gov identifier NCT02834013. Bethesda, MD: National Library of Medicine; July 15, 2016. Available at https://clinicaltrials.gov/ct2/show/NCT02834013. Accessed March 7, 2018.
- 90.The ASCO Post . Mpower133: Atezolizumab in combination with chemotherapy in previously untreated, extensive‐stage small cell lung cancer. http://www.ascopost.com/News/59020. Updated 2018. Accessed August 9, 2018.
- 91.Herbst RS, Baas P, Kim D et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 92.Miao L, Lu Y, Xu Y et al. PD‐L1 and c‐MET expression and survival in patients with small cell lung cancer. Oncotarget 2017;8:53978–53988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim ST, Ha SY, Lee S et al. The impact of PD‐L1 expression in patients with metastatic GEP‐NETs. J Cancer 2016;7:484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schultheis AM, Scheel AH, Ozretić L et al. PD‐L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer 2015;51:421–426. [DOI] [PubMed] [Google Scholar]
- 95.Heetfeld M, Chougnet CN, Olsen IH et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer 2015;22:657–664. [DOI] [PubMed] [Google Scholar]
- 96.Milione M, Maisonneuve P, Spada F et al. The clinicopathologic heterogeneity of grade 3 gastroenteropancreatic neuroendocrine neoplasms: Morphological differentiation and proliferation identify different prognostic categories. Neuroendocrinology 2017;104:85–93. [DOI] [PubMed] [Google Scholar]
- 97.Yan L, Zhang W. Precision medicine becomes reality‐tumor type‐agnostic therapy. Cancer Commun. 2018;38:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Demirkan BHM, Eriksson B. Systemic treatment of neuroendocrine tumors with hepatic metastases. Turk J Gastroenterol 2012;23:427–437. [DOI] [PubMed] [Google Scholar]
- 99.Kwekkeboom DJ, de Herder WW, Kam BL et al. Treatment with the radiolabeled somatostatin analog [177Lu‐DOTA0, Tyr3] octreotate: Toxicity, efficacy, and survival. J Clin Oncol 2008;26:2124–2130. [DOI] [PubMed] [Google Scholar]
- 100.Strosberg J, El‐Haddad G, Wolin E et al. Phase 3 trial of 177Lu‐dotatate for midgut neuroendocrine tumors. N Engl J Med 2017;376:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ezziddin S, Khalaf F, Vanezi M et al. Outcome of peptide receptor radionuclide therapy with 177Lu‐octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2014;41:925–933. [DOI] [PubMed] [Google Scholar]
- 102.Ezziddin S, Logvinski T, Yong‐Hing C et al. Factors predicting tracer uptake in somatostatin receptor and MIBG scintigraphy of metastatic gastroenteropancreatic neuroendocrine tumors. J Nucl Med 2006;47:223–233. [PubMed] [Google Scholar]
- 103.Kayani I, Bomanji JB, Groves A et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga‐DOTATATE (DOTA‐DPhe1, Tyr3‐octreotate) and 18F‐FDG. Cancer 2008;112:2447–2455. [DOI] [PubMed] [Google Scholar]
- 104.Janson ET, Sørbye H, Welin S et al. Nordic guidelines 2010 for diagnosis and treatment of gastroenteropancreatic neuroendocrine tumours. Acta Oncol 2010;49:740–756. [DOI] [PubMed] [Google Scholar]
- 105.Binderup T, Knigge U, Loft A et al. Functional imaging of neuroendocrine tumors: A head‐to‐head comparison of somatostatin receptor scintigraphy, 123I‐MIBG scintigraphy, and 18F‐FDG PET. J Nucl Med 2010;51:704–712. [DOI] [PubMed] [Google Scholar]
- 106.Basturk O, Yang Z, Tang LH et al. The high‐grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol 2015;39:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]