To better understand treatment patterns and outcomes for patients with advanced gastrointestinal neuroendocrine tumors, a retrospective patient chart review was performed and treatment patterns were assessed for patients at four major tertiary care centers in the U.S. Results of the study are reported here.
Keywords: Gastrointestinal neuroendocrine tumor, Treatment patterns, Somatostatin analogs, Real‐world analysis
Abstract
Background.
We assessed treatment patterns and outcomes of patients with advanced gastrointestinal (GI) neuroendocrine tumors (NET) at four large tertiary referral centers in the U.S.
Patients and Methods.
We performed a retrospective chart review of patients aged ≥18 years at advanced GI NET diagnosis, treated between July 2011 and December 2014. Index date was the histologically confirmed diagnosis date of locally advanced/metastatic GI NET. Data included baseline characteristics, treatment patterns, progression, death, and GI NET‐related health care resource utilization from index date through last contact or death. Time‐to‐event analyses, including treatment discontinuation, progression, and overall survival (OS), were performed using Kaplan‐Meier analysis.
Results.
We identified 273 patients; 156 (57%) had primary ileum NET, and 174 (64%) had functional NET. First‐line treatments included somatostatin analog (SSA) alone (89%) or in combination (2%), liver‐directed therapy (LDT; 8%), and cytotoxic chemotherapy or interferon (2%). One hundred fifty‐five patients continued with second‐line therapy, including SSA alone (17%) or in combination (75%, with 3% combined with peptide receptor radionuclide therapy), LDT (4%), and other treatments (3%). Median time (months) to first‐line discontinuation was 154.0 for SSAs and 3.8 for cytotoxic chemotherapy. Overall median time to investigator‐assessed progression following treatment initiation was 30.3 months. Median OS (months) following first‐line initiation was 151.8 for all patients and 178.9 for first‐line SSA.
Conclusion.
Our study illustrates the common use of SSAs in both first‐line and subsequent treatment of patients with GI NETs, as well as the relatively long survival durations and multiple additional treatments received by patients with this condition. Treatment pattern assessment at later times, following approval of newer treatments, is warranted.
Implications for Practice.
This study, assessing treatment patterns over a period of up to 30 years, showed that SSAs, LDT, cytotoxic chemotherapy, and interferon are common treatments for advanced GI NETs. SSAs alone or in combination with other treatments were the most frequent therapy in first and subsequent lines. Patients in this study remained on SSAs long‐term, with median treatment duration of 12.8 years in first line. Treatment patterns should be assessed beyond this study's time period, given recent U.S. Food and Drug Administration approvals for additional treatments for GI NET, which will likely be incorporated in the continuum of care of patients.
Introduction
Neuroendocrine tumors (NETs) are generally slow‐growing malignancies arising from neuroendocrine cells found throughout the body [1]. Although NETs have been considered rare, increasing incidence has been demonstrated using Surveillance, Epidemiology and End Results (SEER) cancer registry data in the U.S. From 1973 to 2012, incidence in the U.S. rose from 1.09 per 100,000 to 6.98 per 100,000 persons [2]. Prevalence also increased, which might be due to improved detection through endoscopic and radiologic imaging and increasing survival time of people with the disease [2], [3]. Adjusting for age, the estimated 20‐year limited‐duration prevalence of NETs in the U.S. was 171,321 as of January 1, 2014 [2]. Among gastrointestinal (GI) NETs, incidence of NETs in the small intestine and rectum has grown faster than those in the stomach and colon. In 2008, the age‐adjusted incidence rates of NETs per 100,000 persons were 1.2 for the small intestine, 1.1 for the rectum, 0.4 for the stomach, and 0.3 for the colon [3].
Functional NET tumors cause distinct syndromes such as carcinoid syndrome (CS) because of secreted peptides and neuroamines [4]. Patients with symptoms of hormone secretion often benefit from treatment with somatostatin analogs (SSAs) [2], including octreotide, lanreotide, and pasireotide; recently telotristat ethyl has also become available as a treatment for CS diarrhea [5]. A range of treatments are also available to control tumor growth. These also include SSAs [6], [7], [8], as well as targeted therapy such as everolimus [9], peptide receptor radionuclide therapy (PRRT), interferon alfa, cytotoxic chemotherapy, and liver‐directed therapy (LDT) for hepatic‐predominant disease [10]. SSAs are generally recommended as a first‐line treatment for the majority of patients, but no particular treatment sequence has been defined for subsequent lines of therapies [10]. Additionally, the value of continued SSA treatment in subsequent lines of therapy in patients with nonfunctional tumors is unknown. To better understand treatment patterns and outcomes for patients with advanced GI NETs, we performed a retrospective chart review of patients and assessed treatment patterns for patients with GI NETs treated at four major tertiary care centers in the U.S.
Materials and Methods
Study Design and Study Population
This study was a multicenter, noninterventional, retrospective chart review among patients with GI NET, conducted at the following cancer centers: Dana‐Farber Cancer Institute (DFCI) in Boston, MA; MD Anderson Cancer Center (MDACC) in Houston, TX; Helen Diller Family Comprehensive Cancer Center at University of California, San Francisco (UCSF); and Robert H. Lurie Comprehensive Cancer Center at Northwestern University in Chicago, IL. These cancer centers were selected for inclusion in the study because they have sizeable populations of patients with NET with long duration of follow‐up, allowing for the assessment of long‐term outcomes such as progression and survival.
Eligible patients included those with locally advanced or metastatic GI NET, were at least 18 years of age at time of diagnosis, and had a histologic diagnosis of a well‐differentiated or moderately differentiated NET. Those with tumors of unknown primary site were eligible, provided that the treating physician did not suspect medullary thyroid cancer, pancreatic NET, paraganglioma, or pheochromocytoma. Patients were required to have been treated with SSAs, targeted therapy (e.g., everolimus, sunitinib, bevacizumab), cytotoxic chemotherapy, PRRT, LDT, or interferon alfa between July 2011 and December 2014 (i.e., identification period) and while under care at the institution; patients were permitted to have initiated therapy prior to July 2011. Eligible patients may have received some of their care outside of the institution provided that their advanced GI NET treatment and clinical outcome information were available, they received comprehensive care at the institution, and they had at least two visits in the 14 months prior to the last visit at the institution. Patients with poorly differentiated histology, pancreatic NET, or mixed tumor types (e.g., NET plus other histology, goblet cell carcinoid, composite carcinoid, adenocarcinoid) were excluded.
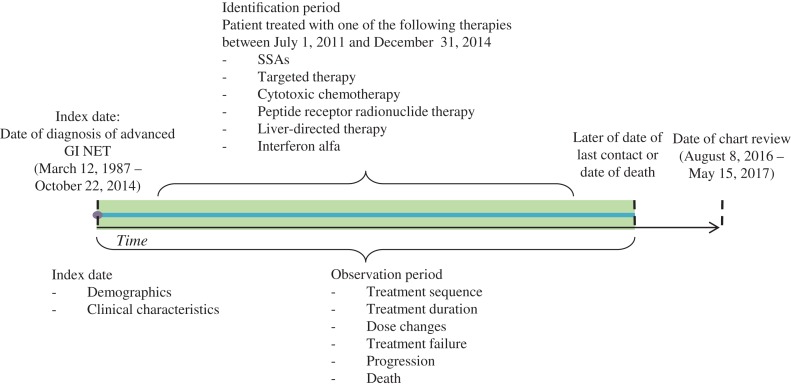
The observation period for a given patient was the time from the date of diagnosis of advanced GI NET (index date) until the later of date of last contact or death (Fig. 1). Baseline patient characteristic data at index date, including demographics, comorbidities, treatment history, and disease characteristics, were collected.
Figure 1.
Study design.
Abbreviations: GI, gastrointestinal; NET, neuroendocrine tumor; SSA, somatostatin analog.
Treatment data, including types of treatment (i.e., pharmacological, surgical, LDT, and radiotherapy), doses, dose modifications, and dates of treatment initiation, termination or discontinuation as recorded in medical charts, and reasons for discontinuation, were collected for the observation period. For the treatment pattern analysis, only pharmacological therapies, LDT, and radiotherapy were considered. Surgeries, such as debulking procedures, were not included in the treatment pattern analysis; surgery as a first‐line treatment for metastatic GI NET is only recommended when a large portion of disease burden can be safely resected, and few patients meet this criterion [11]. In determining treatment sequence, treatment discontinuation was defined as the first 1‐month gap between treatments for the same therapy, with the exception of LDT, for which the gap was 6 months between LDT treatments. Time to treatment discontinuation was defined as the time from initiation of a therapy to its discontinuation for any reason. Overlap of individual pharmacological or medical procedures longer than 14 days was classified as a combination treatment regimen. Multiple LDT procedures occurring within a 6‐month period were considered as one LDT regimen. Addition of a new agent demarcated the line of treatment (e.g., first line and second line of therapy). Data on treatment at progression and treatment after progression were collected.
Clinical outcome data included tumor progression and death. For tumor progression, physicians could review the radiologists’ notes in the medical charts, to assess whether a patient's status improved (responded), stayed the same (stabilized), or worsened (progressed).
GI NET‐related health care resource utilization (HRU) data were also collected and included number and length of inpatient stays, emergency room visits, and outpatient/medical specialist visits.
Clinical research coordinators (CRCs) at the hospitals screened patient records and identified the records of eligible patients based on inclusion criteria. CRCs then entered information from the patient charts related to patient demographic and clinical characteristics, treatment patterns, clinical outcomes, and HRU into an electronic case report form via a secure Web site; data abstraction was conducted between August 8, 2016 and May 15, 2017.
Data were deidentified and complied with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act. All study materials were approved by local institutional review boards at each of the four institutions.
Statistical Analysis
Data collected from all centers were pooled for the analysis. Descriptive statistics were calculated using frequencies and proportions for categorical variables and means, standard deviations, and medians for continuous variables. A Sankey treatment sequence flow chart was developed to show specific treatments by line of therapy over time. A GRAPHx chart was developed, in which each colored segment indicates a treatment and the multicolored line segments reflect treatment durations and sequences over time for individual patients.
In the time‐to‐event analyses, the time origin was set at the initiation of pharmacological therapies, LDT, or radiotherapy. Median and 95% confidence intervals (CIs) for time to treatment discontinuation, time to first physician‐assessed progression, time from first physician‐assessed progression to second physician‐assessed progression, and overall survival from time of first‐line treatment initiation were estimated using Kaplan‐Meier analysis in which patients who did not have the event were censored. Incidence rates were calculated to summarize GI NET‐related hospitalizations, emergency room visits, and outpatient visits from diagnosis of advanced GI NET to the later of the date of last contact or death. A Poisson probability density function was used to calculate 95% CIs of incidence rates.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Two hundred and seventy‐three eligible patients were included in this study (80 at DFCI, 80 at MDACC, 59 at UCSF, and 54 at Northwestern).
Demographic and Clinical Characteristics at Baseline
Among the 273 patients included in this study, half were female, and the majority were white (83%), with a mean age of 59 years at advanced NET diagnosis. The earliest recorded advanced NET diagnosis was in March 1987, and the latest recorded date of contact was in May 2017. Patients were followed for a median of 4.7 years (range, 0.1–29.7) since diagnosis of advanced GI NET. Seventy percent of patients had comorbidities, the most common being hypertension (42%), other nonpancreatic cancers (13%), diabetes without end‐organ damage (12%), and hyperlipidemia (11%). The most common primary site of NET was ileum (57%), and the most common sites of metastases were liver (86%) and lymph nodes (45%). The mean number of metastasis sites at advanced NET diagnosis was 1.4. Ki‐67 proliferation index was available for 45% of patients with a median proliferation index of 2% (interquartile range, 1%–5%). Mitotic rate was available for 50% of patients with a median rate of 1 (interquartile range, 1–2) mitosis per ten high‐power fields (HPFs). Among the 137 patients for whom the mitotic rate was known, 62% had fewer than two mitotic figures per ten HPFs, and 38% had at least two mitotic figures per ten HPFs. Neither Ki‐67 proliferation index nor mitotic rate was available for 34% of the patients, but histologic differentiation was specified in the chart. Three percent of patients had confirmed or suspected hereditary cancer syndrome or family history of NET. The majority of patients were diagnosed with functional NET (64%); of these, 99% had CS and 1% had gastrinoma. The most common CS symptoms were diarrhea (87%) and flushing (73%). Table 1 summarizes the baseline demographic and clinical characteristics of the study population.
Table 1. Demographic and clinical characteristics at baseline.
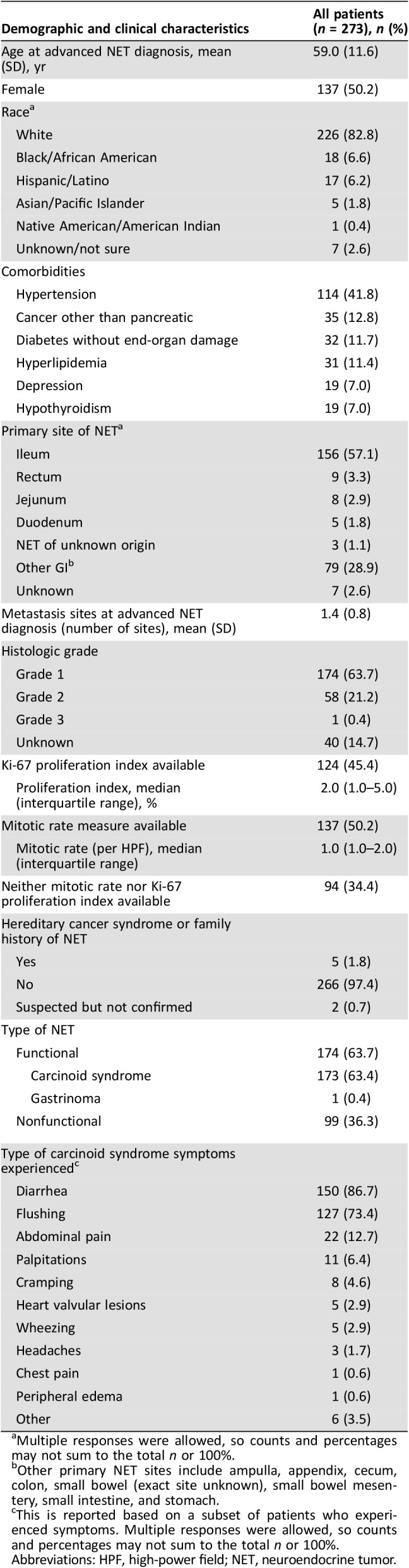
Multiple responses were allowed, so counts and percentages may not sum to the total n or 100%.
Other primary NET sites include ampulla, appendix, cecum, colon, small bowel (exact site unknown), small bowel mesentery, small intestine, and stomach.
This is reported based on a subset of patients who experienced symptoms. Multiple responses were allowed, so counts and percentages may not sum to the total n or 100%.
Abbreviations: HPF, high‐power field; NET, neuroendocrine tumor.
Treatment Patterns
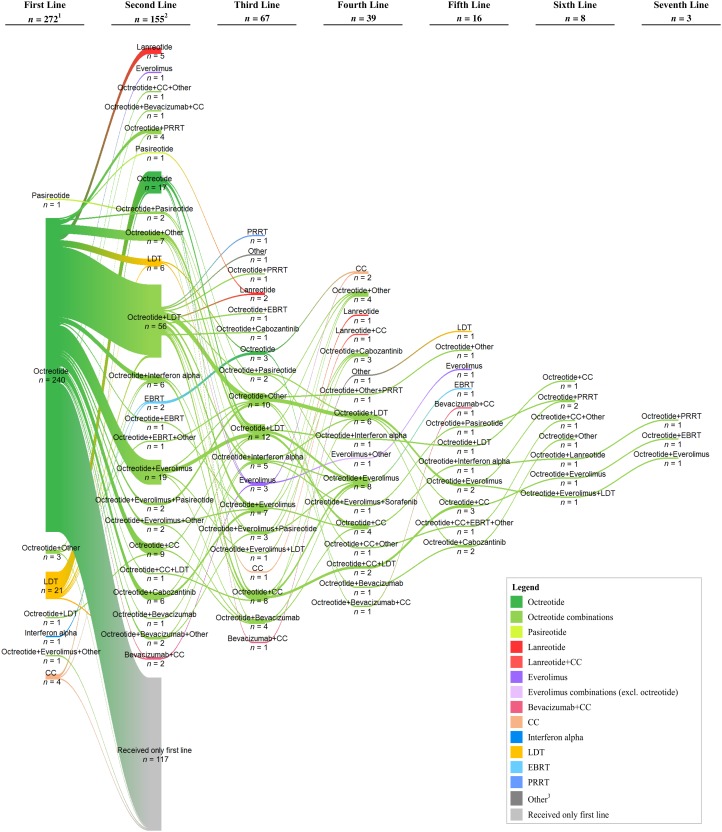
Table 2 displays the first‐ and second‐line treatment regimens for the study population. The majority of patients were treated with SSA: 240 (88%) of patients were treated with octreotide alone and 5 (2%) in combination with LDT, everolimus, or other treatment, as first‐line therapy; 1 (<1%) patient was treated with pasireotide, and none was treated with lanreotide. Twenty‐one (8%) underwent treatment with LDT, and 5 (2%) were treated with cytotoxic chemotherapy (agents include capecitabine, carboplatin, etoposide, gemcitabine, and temozolomide) or interferon as first‐line therapy. LDT included bland embolization, hepatic arterial embolization, liver ablation, liver resection, microwave ablation, radioembolization, radiofrequency ablation, transarterial embolization, and transarterial chemoembolization. In total, 155 (57%) of all patients received second‐line therapy; of these, 144 (93%) continued treatment with SSAs, most commonly with the addition of a second agent. Figure 2 illustrates the switches in therapy from first‐line to second‐line and up to seven lines of therapy. SSAs were a common treatment across lines of therapy. In second‐line and subsequent lines of therapy, there were instances in which PRRT was combined with other treatment or given alone.
Table 2. Treatment regimens for the first two lines of treatment.
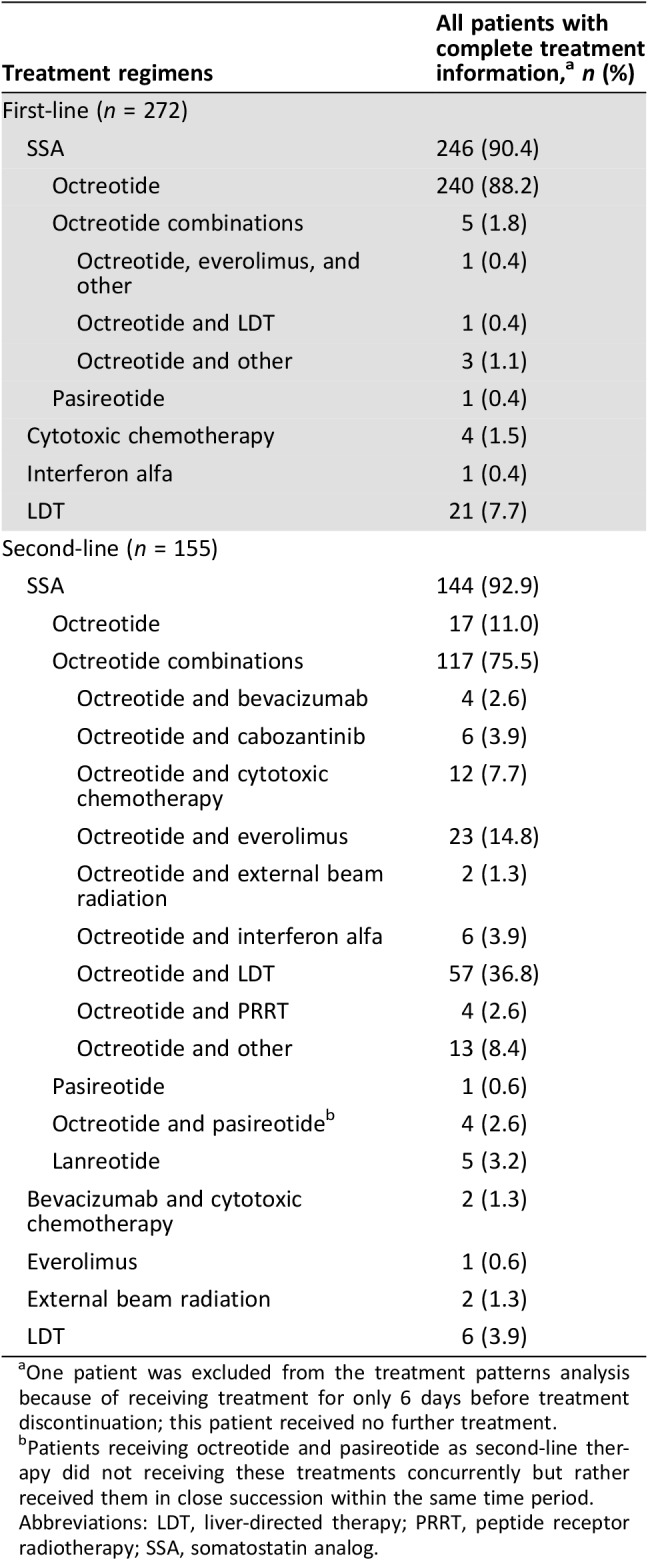
One patient was excluded from the treatment patterns analysis because of receiving treatment for only 6 days before treatment discontinuation; this patient received no further treatment.
Patients receiving octreotide and pasireotide as second‐line therapy did not receiving these treatments concurrently but rather received them in close succession within the same time period.
Abbreviations: LDT, liver‐directed therapy; PRRT, peptide receptor radiotherapy; SSA, somatostatin analog.
Figure 2.
Sankey diagram of treatment sequences. Notes: 1, One patient was excluded from the treatment patterns analysis because of receiving treatment for only 6 days before treatment discontinuation; this patient received no further treatment. 2, The count of patients for the second‐line treatment does not include the 117 patients shown in the diagram who continued their first‐line treatment. 3, Other treatments include aflibercept, alimta, axitinib, cixutumumab, DNA‐PK and TOR kinase inhibitor, denosumab, endostatin, ganitumab, ipilimumab, octreotide implant, panzem, pazopanib, pembrolizumab, pemetrexed, remicirumab, stereotactic radiation therapy, telotristat etiprate, VB‐11, and zoledronic acid (Zometa).
Abbreviations: CC, cytotoxic chemotherapy; EBRT, external beam radiation therapy; LDT, liver‐directed therapy; PRRT, peptide receptor radionuclide therapy.
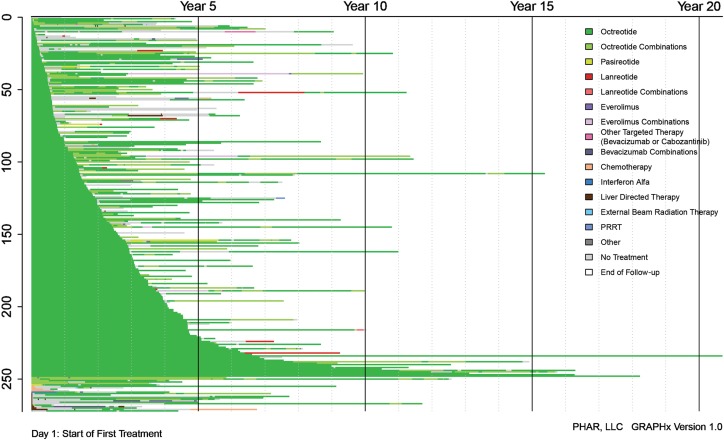
Figure 3 illustrates treatment duration and sequences. All therapies received over the entire course of treatment are shown, each with a different color. SSAs, specifically octreotide, appear as the most frequently used therapy. Median time to first‐line discontinuation was 154.0 (95% CI, 95.1–not reached) months for SSAs and was 144.5 (95% CI, 83.0–177.4) months for octreotide alone, specifically. Those with first‐line octreotide treatment and functional NET had a median time to discontinuation of 144.5 months, and those with nonfunctional NET had a median of 117.1 months. Median time to first‐line discontinuation was 3.8 (95% CI, 2.0–9.2) months for cytotoxic chemotherapy.
Figure 3.
GRAPHx diagram of treatment sequences and durations of treatment by patient.
Abbreviation: PRRT, peptide receptor radionuclide therapy.
Only ten patients (4%) in the study population were treated with lanreotide with doses ranging from 90 mg every 4 weeks (n = 1) to 120 mg every 4 weeks (n = 5); four patients received unknown dosage. In evaluating octreotide doses administered during the observation period, most patients (86%) initiated octreotide with a dose of 30 mg every 4 weeks or less (supplemental online Fig. 1). Dose modification occurred in 103 patients (38%), with a maximum of six instances of modification (supplemental online Table 1). The median (interquartile range) dose ever administered was 30 mg/4 weeks (20–30). Supplemental online Figure 2 displays the patterns of octreotide dose changes observed in at least 1% of patients among those with known octreotide doses.
Clinical Outcomes
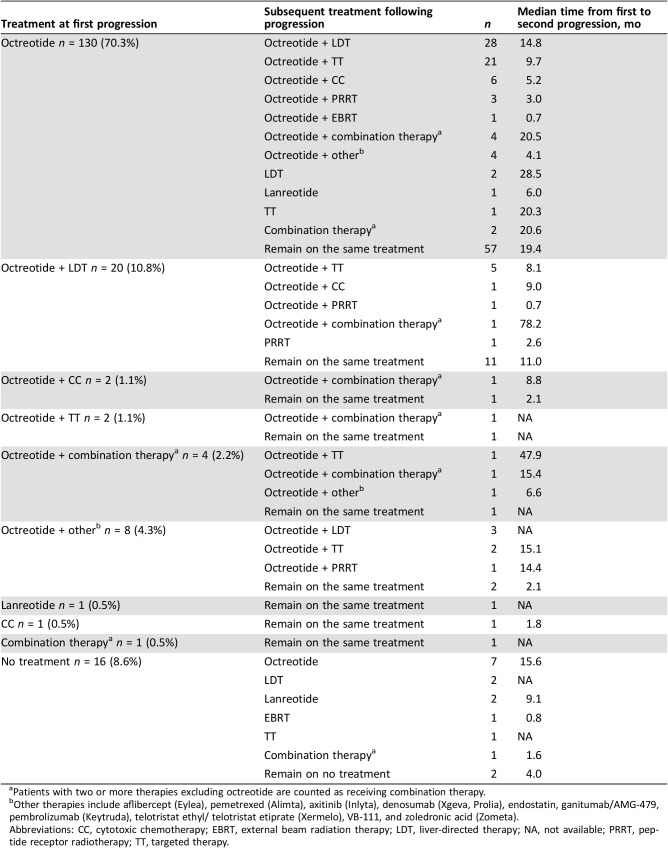
Median time to first progression following treatment initiation was 27.9 (95% CI, 22.0–35.1) months for all patients. For the 185 patients who initiated treatment for advanced GI NET and progressed, Table 3 shows the treatment received at time of first physician‐assessed progression, the next treatment received, and the median time from first to second progression. Among the 167 (90%) patients treated with SSA at the time of first progression, 166 were treated with an octreotide‐based regimen, and one was treated with lanreotide. Half of the patients (52%) treated with octreotide alone at time of first progression were then treated with octreotide and another therapy after progression. The patient treated with lanreotide continued treatment with lanreotide after progression. One patient received cytotoxic chemotherapy (temozolomide and capecitabine) at the time of first progression and continued treatment with cytotoxic chemotherapy; another patient was treated with a combination of non‐SSA therapies (bevacizumab and docetaxel) and continued after progression. Among the 16 patients without treatment at time of progression, 14 (88%) patients initiated a treatment subsequent to progression, and 9 (56%) patients were treated with an SSA‐based treatment. Median time from first to second progression was 19.4 months for patients who continued treatment with octreotide alone, 14.8 months for patients who added LDT to their octreotide regimen, and 9.7 months for patients who added targeted therapy to their octreotide regimen (Table 3). For patients treated with cytotoxic chemotherapy at the time of first progression, median time from first to second progression was 1.8 months.
Table 3. Treatment sequence from first physician‐assessed tumor progression and analysis of time to next progression (n = 185).
Patients with two or more therapies excluding octreotide are counted as receiving combination therapy.
Other therapies include aflibercept (Eylea), pemetrexed (Alimta), axitinib (Inlyta), denosumab (Xgeva, Prolia), endostatin, ganitumab/AMG‐479, pembrolizumab (Keytruda), telotristat ethyl/ telotristat etiprate (Xermelo), VB‐111, and zoledronic acid (Zometa).
Abbreviations: CC, cytotoxic chemotherapy; EBRT, external beam radiation therapy; LDT, liver‐directed therapy; NA, not available; PRRT, peptide receptor radiotherapy; TT, targeted therapy.
Median overall survival among patients with advanced GI NET after initiating first‐line therapy was 151.8 (95% CI, 95.5–188.9) months; 28% of patients died. Patients with first‐line treatment with SSAs had median overall survival of 178.9 (95% CI, 95.5–188.9) months. Those treated with first‐line octreotide also had median overall survival of 178.9 (95% CI, 95.5–188.9) months (functional NET: 178.9 [95% CI, 94.6–not estimable]; nonfunctional NET: 115.4 [95% CI, 87.2–not estimable]).
Health Care Resource Utilization
Patients with advanced GI NET had the following rates of HRU (per person‐year): 0.10 (95% CI, 0.08–0.11) emergency room visits, 0.23 (95% CI, 0.21–026) hospitalizations, and 6.93 (95% CI, 6.80–7.07) outpatient visits.
Discussion
This study, which evaluated treatment patterns and health care utilization of patients with advanced GI NET at four tertiary academic medical centers during the study period, demonstrated that SSAs were a primary treatment choice for most patients. Ninety percent of patients were treated with an SSA alone or in combination with other treatments as first‐line therapy, and a significant number of patients remained on SSA mono‐ or combination therapy during subsequent lines of treatment. Octreotide was the most frequently used SSA treatment. There was limited use of lanreotide because the study evaluated patients receiving treatment between July 2011 and December 2014, and the U.S. Food and Drug Administration (FDA) approved lanreotide for gastroenteropancreatic (GEP) NET in December 2014 [7]. There also was minimal use of targeted therapies such as everolimus as first‐line treatment in the current study because the study period was prior to the February 2016 FDA approval of everolimus for GI NET [9]. Similarly, few patients received PRRT in the current study because 177Lu‐dotatate was FDA approved in January 2018 as the first radiopharmaceutical for GEP NET [12]. All treatments with 177Lu‐dotatate were noted as given in a clinical trial setting. In total, 23% of patients observed in this study received various NET treatments as part of clinical trials.
The high rate of utilization of SSA was consistent with National Comprehensive Cancer Network (NCCN) treatment guidelines during the study time period. The guidelines from NCCN in 2012 for metastatic GI NET listed octreotide with category of evidence and consensus 2A, LDT (category 2B), targeted therapy (category 3), and cytotoxic chemotherapy (category 3) [13]. The guidelines from NCCN have expanded since the time period of the current study; the 2018 guidelines include the SSAs octreotide and lanreotide (both category 2A), and for disease progression include everolimus (category 2A), PRRT with 177Lu‐dotatate (category 1 for midgut tumors), specific LDTs (category 2B), cytotoxic chemotherapy (category 3), and interferon alfa‐2b (category 3) [14]. Dose data for octreotide, the most commonly used SSA in this study, were analyzed, and the analysis showed most octreotide was prescribed at a standard dose of 30 mg every 4 weeks, with most patients remaining at or below this dose for their entire course of treatment. Furthermore, this study adds to the literature by showing GI NET‐related resource utilization following advanced GI NET diagnosis, whereas prior studies on resource utilization for patients with NET have focused on all‐cause utilization [15], [16].
Other observational studies have shown SSA is common as first‐line treatment but report varying proportions of patients receiving it in the first line. This variation may be due to different study populations and study time periods. Benson et al. reported that 63% of patients with GI NET were treated with SSAs, 33% with cytotoxic chemotherapy monotherapy, and 12% with LDT during first‐line pharmacologic therapy [17]. The Benson et al. study was conducted with claims data among a commercially insured population over the time period 2009–2014 [17]. Using physician‐reported data from academic and community settings for patients who may have had surgery as their only treatment, Strosberg et al. reported 77% of patients with lung or GI NET were treated with SSAs [15]. Chuang et al. used claims data from a commercially insured population over the time period 2007–2010 and reported that among those treated with SSAs or chemotherapy following carcinoid tumor or pancreatic islet‐cell tumor diagnosis, more than 90% were treated with long‐acting octreotide, 27% were treated with short‐acting octreotide, and 1% were treated with lanreotide depot during the 12 months following diagnosis [16]. Similar to other studies, the current study also showed that patients continued to use SSA after first‐line therapy. Benson et al. reported that among first‐line SSA users, 70% added treatment such as cytotoxic chemotherapy or targeted therapy as second‐line therapy, and many of those who were not treated with SSA in the first line received it in their second line [17].
This study is novel because it assessed treatment patterns over a period of up to 30 years for a cohort of patients who were regularly followed in cancer centers that captured data on treatments and long‐term clinical outcomes. The long follow‐up duration in this study allowed us to report treatment patterns observed over the entirety of patients’ courses of treatment. The current study showed the flow of patients moving from first‐line therapy to subsequent lines of therapies. Median treatment duration in the current study, as assessed by Kaplan‐Meier analysis, was 12 years for SSAs. Because of small sample sizes, durations assessed for other classes of therapy lacked certainty. Similarly, Benson et al. reported longer treatment duration for SSAs versus cytotoxic chemotherapy and targeted therapy; however, the median times to discontinuation for first‐line therapies were lower. In that study, median (95% CI) treatment durations were 1.61 (1.36–1.80) years for SSA, 0.50 (0.50–0.51) years for cytotoxic chemotherapy, and 0.47 (0.27–0.90) years for targeted therapy [17]. Benson et al. also reported in a claims database study that less than 10% of patients had a second‐line pharmacotherapy. It should be noted that the current study used medical records at academic medical centers rather than claims data as used by Benson et al., the latter of which do not contain detailed clinical data, have much shorter follow‐up time, and reflect a different population of clinicians and patients compared with the data source for the current study [17]. With the detailed medical records and long observation period of the current study, treatments at disease progression and time to subsequent progressions could also be analyzed. This information, gathered from a real‐world clinical setting, is important in light of another observational study that has shown that longer time to disease progression is associated with improved overall survival [18]. Notably, the current study describes treatment duration and time to progression for patients with different treatments but does not adjust for differences in patient characteristics; it is plausible that patients who received combination therapies versus one therapy, for example, may have more severe disease, and thus a shorter time to progression would be expected for these patients based on clinical characteristics.
In the current study, a relatively high proportion of patients (64%) had CS at the time of GI NET diagnosis. Prior studies have reported a wide range of proportions of patients with NET and CS, ranging from 3% to 74% across studies [19], [20]. Halperin et al. reported that 19% of those diagnosed with NET between 2000 and 2011 in SEER‐Medicare had CS within 6 months of NET diagnosis, with the percentage increasing over time [19]. In that study, higher tumor grade and stage were significantly associated with CS at NET diagnosis, and patients with primary NET sites of small bowel, respiratory organs, colon, and rectum were more likely to have CS at diagnosis than those with NET of other origin [19]. In that study, CS was also shown to be associated with advanced disease [19], which was an eligibility requirement for patients in the current study. Using a U.S. cancer database, Fisher et al. reported 43% of patients with metastatic NET had some CS symptoms [20]. Pulgar et al. reported 57% of patients with metastatic GEP NET had CS symptoms in an analysis of U.S. oncology electronic health records [21]. The current study did not ascertain whether treatment decisions were made based on the antiproliferative or antisecretory characteristics of treatment or both.
Median time to progression after treatment initiation was 2.3 years for all patients in the current study and 2.5 years for those initiating treatment with SSAs. Ter‐Minassian et al. conducted an institutional database study of patients with NET in the U.S. treated with SSAs and reported progression‐free survival of 2.2 years (95% CI, 1.8–2.9) for patients with NET of small bowel origin [18]. In the current study, patients with functional GI NET had median overall survival (OS) of 14.9 years, whereas those with nonfunctional GI NET had median OS of 9.6 years after starting first‐line treatment with long‐acting octreotide. In an analysis of SEER‐Medicare data with a higher average age than the current study, Halperin et al. showed that among patients with metastatic grade I–II small‐bowel NETs, median OS from time of NET diagnosis was 4.7 (95% CI, 4.0–5.4) years in patients with CS and 7.1 (95% CI, 5.2–8.1) years in patients without CS [19].
Despite this study using a large database containing detailed clinical information collected directly from medical charts at these cancer centers, there were some limitations in this study. First, lack of information about patient care at outside institutions may have resulted in some underreporting of treatment and health care resource utilization (i.e., patients may have had additional therapy and medical procedures at another institution that were not captured at institutions participating in this study); information in this study was collected only for GI NET‐related health care resource utilization, as noted in the medical records, and may be underestimated. We attempted to minimize such underreporting by requiring that patients frequently sought care at the participating academic cancer center (i.e., 2 visits in the 14 months prior to the last visit at the institution). Second, short‐ and long‐acting octreotide could not be distinguished in the data, and so they were reported in aggregate. However, by examining the dosage data and assuming that units reported in micrograms and doses <10 mg every 4 weeks referred to short‐acting octreotide, we found that <5% of patients were treated with short‐acting octreotide; thus, conclusions here are most likely applicable to long‐acting octreotide. Third, as with all retrospective observational studies, tumor progression was based on radiologist assessment or physician notes; this process is subject to physician's assessment, as RECIST criteria are rarely used in real‐world clinical settings. In the current study, RECIST criteria were available for only 21% of all scans. Radiographic scans may occur at different time intervals (e.g., patients with more aggressive disease may receive more frequent scans) resulting in detection bias. Furthermore, results reported in this study are based on data collected at four cancer referral centers and may not be reflective of practice patterns observed in other institutions. Lastly, this study is descriptive, and no statistical comparisons were performed. Despite these limitations, the current study used detailed clinical data, including information on the functional status of the NETs, covering up to 30 years from four academic institutions.
Conclusion
This study showed that SSAs, at recommended dose, are commonly used as an initial treatment for patients with GI NETs and are often continued, in combination with other therapies for long‐term treatment. We further demonstrated relatively long overall survival for patients with advanced GI NET treated at tertiary referral centers and the common use of multiple lines of therapy for these patients. Follow‐up assessments of treatment patterns are needed to understand the impact of newer treatments for GI NET.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgements
The authors would like to thank Caroline Korves, Sc.D., of Analysis Group, Inc., for her assistance with developing this manuscript. This research was funded by Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA.
Editor's Note: See the companion paper, “Treatment Patterns and Clinical Outcomes in Advanced Lung Neuroendocrine Tumors in Real‐World Settings: A Multicenter Retrospective Chart Review Study” by Arvind Dasari, Emily K. Bergsland, Al B. Benson et al., on page 1066 of this issue.
Author Contributions
Conception/design: Matthew H. Kulke, Al B. Benson, Arvind Dasari, Lynn Huynh, Beilei Cai, Todor Totev, Mei Sheng Duh, Maureen P. Neary, Victoria E. Maurer, Cecile G. Dagohoy, Jennifer Chan, Emily K. Bergsland
Collection and/or assembly of data: Matthew H. Kulke, Al B. Benson, Arvind Dasari, Lynn Huynh, Beilei Cai, Todor Totev, Nina Roesner, Mei Sheng Duh, Maureen P. Neary, Victoria E. Maurer, Brandon E. Shih, Cecile G. Dagohoy, Jennifer Chan, Emily K. Bergsland
Data analysis and interpretation: Matthew H. Kulke, Al B. Benson, Arvind Dasari, Lynn Huynh, Beilei Cai, Todor Totev, Nina Roesner, Mei Sheng Duh, Maureen P. Neary, Victoria E. Maurer, Brandon E. Shih, Cecile G. Dagohoy, Jennifer Chan, Emily K. Bergsland
Manuscript writing: Matthew H. Kulke, Al B. Benson, Arvind Dasari, Lynn Huynh, Beilei Cai, Todor Totev, Nina Roesner, Mei Sheng Duh, Maureen P. Neary, Victoria E. Maurer, Brandon E. Shih, Cecile G. Dagohoy, Jennifer Chan, Emily K. Bergsland
Final approval of manuscript: Matthew H. Kulke, Al B. Benson, Arvind Dasari, Lynn Huynh, Beilei Cai, Todor Totev, Nina Roesner, Mei Sheng Duh, Maureen P. Neary, Victoria E. Maurer, Brandon E. Shih, Cecile G. Dagohoy, Jennifer Chan, Emily K. Bergsland
Disclosures
Al B. Benson: Bristol‐Myers Squibb, Guardant Health, Eli Lilly & Co., Exelixis, Purdue Pharma, Harborside, Xcenda, National Comprehensive Cancer Network, Emron, inVentiv Health Inc., Axio, Genentech, Bayer, Merck, Rafael Pharmaceuticals, Astellas Pharma, Terumo (C/A), Novartis, Acerta, Celegene, Advanced Accelerator Applications, Infinity Pharmaceuticals DMC, Merck Sharp & Dohme, Taiho Pharmaceutical, Bristol‐Myers Squibb, Medimmune/AstraZeneca, Xencor, Bristol‐Myers Squibb DMC (RF); Lynn Huynh: Analysis Group, Inc. (E), Novartis Pharmaceuticals Corporation (RF); Beilei Cai: Novartis (E, OI); Todor Totev: Analysis Group, Inc. (E), Novartis Pharmaceuticals Corporation (RF); Mei Sheng Duh: Analysis Group, Inc. (E), Novartis Pharmaceuticals Corporation (RF); Maureen P. Neary: Novartis Pharmaceuticals Corporation (E); Jennifer Chan: Novartis, Ipsen (SAB), Ipsen (H), Sanofi, Eli Lilly & Co. (RF); Emily K. Bergsland: UpToDate (H), Novartis (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Yao JC, Hassan M, Phan A et al. One hundred years after ‘carcinoid’: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 2.Dasari A, Shen C, Halperin D et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsikitis VL, Wertheim BC, Guerrero MA. Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: A SEER analysis. J Cancer 2012;3:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev 2004;25:458–511. [DOI] [PubMed] [Google Scholar]
- 5.Xermelo (telotristat ethyl) [package insert]. The Woodlands, TX: Lexicon Pharmaceuticals; 2017. https://www.xermelo.com/Media/Default/pdfs/Product_Info_telotristat_ethyl.pdf. Accessed August 15, 2018.
- 6.Sandostatin LAR Depot (octreotide) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016. http://www.pharma.us.novartis.com/product/pi/pdf/sandostatin_lar.pdf. Accessed October 17, 2018.
- 7.Somatuline Depot (lanreotide) [package insert]. Basking Ridge, NJ: Ipsen Biopharmaceuticals, Inc.; 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022074s011lbl.pdf.
- 8.Ipsen Biopharmaceuticals Inc. U.S. FDA approves new indication for Ipsen's Somatuline Depot (lanreotide) injection for the treatment of carcinoid syndrome [press release]. https://www.ipsen.com/websites/IPSENCOM‐PROD/wp‐content/uploads/2017/09/16000129/18‐09‐2017‐Approval‐Somatuline‐US‐carcinoid‐syndrom‐FINAL.pdf. Published September 18, 2017. Accessed February 19, 2018.
- 9.Novartis Pharmaceuticals Corporation . FDA approves new indication for Novartis drug Afinitor for progressive, nonfunctional GI and lung neuroendocrine tumors (NET) [press release]. https://www.novartis.com/news/media‐releases/fda‐approves‐new‐indication‐novartis‐drug‐afinitor‐progressive‐nonfunctional‐gi‐and‐lung‐neuroendocrine‐tumors‐net. Published February 26, 2016. Accessed September 14, 2017.
- 10.Shah MH, Goldner WS, Halfdanarson TR et al. NCCN Guidelines Insights: Neuroendocrine and Adrenal Tumors, Version 2.2018. J Natl Compr Canc Netw 2018;16:693–702. [DOI] [PubMed] [Google Scholar]
- 11.Neychev V, Kebebew E. Management options for advanced low or intermediate grade gastroenteropancreatic neuroendocrine tumors: Review of recent literature. Int J Surg Oncol 2017;2017:6424812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration. FDA approves new treatment for certain digestive tract cancers [press release]. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm594043.htm. Published January 26, 2018. Accessed March 20, 2018.
- 13.Kulke MH, Benson AB 3rd, Bergsland E et al. Neuroendocrine tumors. J Natl Compr Canc Netw 2012;10:724–764. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Neuroendocrine and Adrenal Tumors. Version 2.2018. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2018. [DOI] [PubMed]
- 15.Strosberg J, Casciano R, Stern L et al. United States‐based practice patterns and resource utilization in advanced neuroendocrine tumor treatment. World J Gastroenterol 2013;19:2348–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuang CC, Bhurke S, Chen SY et al. Clinical characteristics, treatment patterns, and economic burden in patients treated for neuroendocrine tumors in the United States: A retrospective cohort study. J Med Econ 2015;18:126–136. [DOI] [PubMed] [Google Scholar]
- 17.Benson AB III, Broder MS, Cai B et al. Real‐world treatment patterns of gastrointestinal neuroendocrine tumors: A claims database analysis. World J Gastroenterol 2017;23:6128–6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ter‐Minassian M, Zhang S, Brooks NV et al. Association between tumor progression endpoints and overall survival in patients with advanced neuroendocrine tumors. The Oncologist 2017;22:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halperin DM, Shen C, Dasari A et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: A population‐based study. Lancet Oncol 2017;18:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher MD, Pulgar S, Kulke MH et al. Treatment patterns and outcomes in metastatic neuroendocrine tumors: results from a retrospective community oncology database. Paper presented at: North American Neuroendocrine Tumor Society Symposium; 2015; Austin, TX; C16.
- 21.Pulgar S, Jiao X, Mirkhur B et al. Treatment patterns, clinical outcomes, and health care resources utilization in patients with metastatic gastroenteropancreatic neuroendocrine tumors (mGEP‐NETs). Paper presented at: North American Neuroendocrine Tumor Society Conference; 2016; Jackson, WY; P13.