Abstract
Dynamic assessment of preoperative exercise capacity may be a useful predictor of postoperative prognosis. We aimed to clarify whether perioperative exercise capacity was related to long-term survival in hepatocellular carcinoma patients with chronic liver injury undergoing hepatectomy. One hundred-six patients with hepatocellular carcinoma underwent pre- and postoperative cardiopulmonary exercise testing to determine their anaerobic threshold, defined as the point between carbon dioxide production and oxygen consumption per unit of time. Testing involved 35 items including blood biochemistry analysis, in-vivo component analysis, dual-energy X-ray absorptiometry, and cardiopulmonary exercise testing preoperatively and 6 months postoperatively. We classified patients with anaerobic threshold ≥ 90% 6 months postoperatively compared with the preoperative level as the maintenance group (n = 78) and patients with anaerobic threshold < 90% as the decrease group (n = 28). Five-year recurrence-free survival rates were 39.9% vs. 9.9% (maintenance vs. decrease group) (hazard ratio: 1.87 [95% confidence interval: 1.12–3.13]; P = 0.018). Five-year overall survival rates were maintenance: 81.9%, and decrease: 61.7% (hazard ratio: 2.95 [95% confidence interval: 1.37–6.33]; P = 0.006). Multivariable Cox proportional hazards models showed that perioperative maintenance of anaerobic threshold was an independent prognostic indicator for both recurrence-free- and overall survival. Although the mean anaerobic threshold from preoperative to postoperative month 6 decreased in the exercise-not-implemented group, the exercise-implemented group experienced increased anaerobic threshold, on average, at postoperative month 6. The significant prognostic factor affecting postoperative survival for chronic liver injury patients with HCC undergoing hepatectomy was maintenance of anaerobic threshold up to 6 months postoperatively.
Introduction
Major surgery increases oxygen demand by approximately 40%, which may place severe stress on cardiopulmonary reserve [1]. Patients with high cardiopulmonary risk have traditionally been assessed using tests such as transthoracic echocardiography, dobutamine stress echocardiography, radionuclide ventriculography, and spirometry. However, these assessments have not been validated as preoperative screening tests, and provide primarily static measurements of cardiopulmonary performance [2–4]. Walking distance and ability to climb stairs are subjective measurements of exercise tolerance, and have been shown to predict perioperative complications [5,6]. However, these measurements lack objectivity and do not detect silent cardiopulmonary abnormalities. Dynamic assessment of preoperative exercise capacity may be a useful predictor of short- and long-term postoperative prognosis. Cardiopulmonary exercise (CPX) testing measures oxygen uptake at increasing levels of work and predicts cardiopulmonary performance under stress, such as after surgery. In older patients undergoing major abdominal surgical procedures, the majority of deaths from cardiopulmonary complications occur in patients with an anaerobic threshold (AT) < 11 ml/min/kg [7,8].
Hepatocellular carcinoma (HCC) is the fifth-most-common cancer worldwide [9]. Maintenance of good perioperative nutrition and metabolism may improve the prognosis of patients with HCC undergoing hepatectomy [10, 11]. To date, few studies have examined the usefulness of pre- and postoperative CPX testing in patients undergoing hepatectomy. In the present study, we aimed to clarify whether perioperative exercise capacity was related to long-term survival in HCC patients with chronic liver injury undergoing hepatectomy.
Materials and methods
Patients
HCC patients with chronic hepatitis or cirrhosis who were scheduled for liver resection at Hirakata Hospital of Kansai Medical University (Osaka, Japan) between March 2010 and September 2015 were screened for inclusion in this study. A total of 111 patients with HCC underwent curative resection (defined as macroscopic removal of all tumor). No in-patient mortality occurred, and we analyzed data for 106/111 patients. We excluded data for the remaining five patients because these patients were followed at other hospitals. All patients gave written informed consent to participate in this study, and the study protocol was approved by the institutional ethics committee of Kansai Medical University (reference number: KMU 2018082).
Cardiopulmonary exercise testing
Patients underwent preoperative CPX testing using a bicycle ergometer with an incremental protocol (5.0, 7.5, and 10 W/min). Twelve-lead electrocardiography was used to monitor heart rate, ST segment deviation, and arrhythmias, at rest and continuously during exercise and recovery periods. Blood pressure was recorded at rest and every 2 min during exercise and recovery periods. Peak oxygen consumption per unit of time (VO2) was obtained from breath-by-breath analysis of expired air. Peak VO2 was defined as the highest mean value during exercise when the subject could no longer continue pedaling at 60 rpm. AT, indicating the onset of metabolic acidosis, was defined as the break point between carbon dioxide production and VO2 [12], or the point at which the ventilatory equivalent for oxygen and end-tidal oxygen partial pressure curves reached their respective nadirs before beginning to increase again [13]. Thus, AT was set at the time of maximum fat combustion [14]. The respiratory compensation point was set at the point at which the ventilatory equivalent for carbon dioxide was lowest before a systemic increase, and when the end-tidal carbon dioxide partial pressure reached a maximum and began to decrease [15]. Exercise was stopped when the patient requested because of fatigue, pain, or headache, or if there was failure to maintain a speed greater than 40 rpm for more than 30 seconds despite encouragement.
For included patients wishing to begin exercise therapy, the following protocol was performed perioperatively, tailored to each patient: [16] Exercise was started as soon as possible after diagnosis, up to 1 month preoperatively, and was resumed from 1 week postoperatively, and continued for 6 months (> 3 times a week). The program consisted of three 60-minute exercise sessions per week, and each session included 5 minutes of stretching exercises, 30 minutes of walking at an intensity based on the AT of each patient, 20 minutes of targeted stretching exercises, and 5 minutes of cooling down with stretching. Once or twice a month postoperatively, a medical doctor and exercise trainer confirmed the frequency and quantity of exercise each patient undertook. Fifty-nine patients (exercise-implemented group) engaged in exercise therapy perioperatively and 47 did not (exercise not-implemented group).
CPX measurements were performed preoperatively and 6 months postoperatively to assess postoperative changes. The median (interquartile range) 6-month postoperative AT VO2 level was 101.5% (89.3%–113.7%). Patients were classified based on whether they had an AT VO2 level of 90% (25th percentile of the AT VO2 level) 6 months postoperatively compared with their preoperative level. Patients who had an AT VO2 level ≥ 90% 6 months postoperatively compared with their preoperative level were classified as the maintenance group (n = 78), and patients with AT VO2 < 90% for the same comparison were classified as the decrease group (n = 28).
Clinical variables and surgery
Before surgery, each patient underwent peripheral blood count, general biochemical blood laboratory testing, conventional liver function testing, and measurement of indocyanine green retention rate at 15 min. Hepatitis virus infection screening was performed by testing for hepatitis B surface antigen and hepatitis C virus antibody (HCVAb). Alpha-fetoprotein and protein induced by vitamin K absence/antagonism-II (PIVKA-II) levels were measured in all patients. We used two methods to determine body composition: dual-energy X-ray absorptiometry (DEXA) [17] and bioelectrical impedance analysis (BIA) [18] preoperatively and 6 months postoperatively. Total weight, nonfat content, and fat content were measured by whole-body DEXA. BIA was performed using the whole-body eight-electrode approach with a 5–500 kHz multifrequency impedance analyzer (InBody720, Biospace Co., Ltd, Tokyo, Japan). Skeletal muscle content, fat content, and body mass index were also measured.
Surgical procedures were classified according to the Brisbane terminology proposed by Strasberg et al. [19]. Anatomical resection was defined as resection of the tumor together with the related portal vein branches and corresponding hepatic territory, and was classified as hemihepatectomy (resection of half of the liver), extended hemihepatectomy (right trisectionectomy, or similar procedures on the left or for smaller resections), sectionectomy (resection of two Couinaud subsegments [20]), or segmentectomy (resection of one Couinaud subsegment). All other procedures were classified as nonanatomical resection, which was frequently performed for peripheral or central tumors. Peripheral tumors and those with extrahepatic growth were treated by partial hepatectomy because this procedure achieved sufficient surgical margins. Central tumors located near the hepatic hilum or major vessels were treated by enucleation only because it was too difficult and/or dangerous to remove enough liver tissue to obtain adequate margins. One consultant pathologist reviewed all specimens for histological confirmation of the diagnosis. The width of the surgical margin was measured as the distance from the tumor edge to the resection line.
Follow-up
Peri- and postoperative complications and deaths were recorded to determine morbidity and mortality following hepatectomy. All surviving patients were followed at least every 3 months after discharge. Follow-up included physical examination, liver function testing, chest radiographs to check for pulmonary metastases, and ultrasonography, computed tomography, or magnetic resonance imaging to check for intrahepatic recurrence. Chest computed tomography was performed if the chest radiograph showed any abnormalities, and bone metastases were diagnosed by bone scintigraphy.
When HCC recurrence was detected by changes in tumor markers or on imaging, recurrence limited to the remnant liver was treated by transarterial chemoembolization, lipiodolization, re-resection, or percutaneous local ablative therapy such as radiofrequency ablation. When extrahepatic metastases were detected, active treatment and/or molecular target drugs such as sorafenib was prescribed in patients with good hepatic functional reserve (Child–Pugh class A or B) and good performance status (0 or 1), while other patients received radiation therapy, alone, to relieve symptoms of bone metastases. Surgical resection was performed in patients with a solitary extrahepatic metastasis and no intrahepatic recurrence.
Statistical analysis
We compared patients’ clinical characteristics in the two groups using either Wilcoxon’s rank-sum test, chi-square test, or Fisher’s exact test. Probabilities for recurrence-free survival (RFS) and overall survival (OS) by treatment type were calculated by the Kaplan–Meier method. Hazard ratios for RFS and OS and their 95% confidence intervals (CI) were estimated using the univariate Cox model. Multivariate analysis was performed using the Cox proportional hazards model. The following variables were examined as potential prognostic predictors: age, sex, diabetes mellitus, HCVAb status, serum albumin, alanine aminotransferase, total bilirubin, prothrombin activity, platelet count, alfa-fetoprotein and PIVKA-II concentrations, tumor number, maximum tumor size, portal vein and or hepatic vein invasion, tumor stage, volume of hepatectomy, operative blood loss, and the rate of change from preoperative to 6-months-postoperative values including total weight and nonfat content by DEXA, and peak VO2 and AT VO2 by CPX. Differences between groups in perioperative changes in AT VO2 were assessed by two-way repeated measures ANOVA (analysis of variance) and Student's t-test post hoc test. A P-value <0.05 (two-sided) was considered statistically significant. All statistical analyses were performed with R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria) with the survival and matching packages.
Results
Table 1 summarizes the preoperative characteristics of both groups. Patients' age and number differed significantly between the two perioperative exercise groups. No difference was detected between groups regarding sex, American Society of Anesthesiologists physical status classification, alcohol intake, diabetes mellitus, hypertension, esophageal and/or gastric varices, hepatitis B surface antigen, HCVAb, Child–Pugh class, indocyanine green retention rate at 15 min, peripheral blood count, general biochemical blood laboratory testing, conventional liver function testing, or serum alfa-fetoprotein and PIVKA-II concentrations.
Table 1. Preoperative characteristics of HCC patients classified according to AT VO2 change.
| Variables | Maintenance (n = 78) |
Decrease (n = 28) |
P | ||
|---|---|---|---|---|---|
| Age, yr | 70 | (63.0, 73.0) | 73 | (67.8, 75.0) | 0.023 |
| Sex | 0.604 | ||||
| Male | 61 | (78%) | 20 | (71%) | |
| Female | 17 | (22%) | 8 | (29%) | |
| ASA-PS | 0.227 | ||||
| 1 | 8 | (10%) | 0 | (0%) | |
| 2 | 65 | (83%) | 26 | (93%) | |
| 3 | 5 | (6%) | 2 | (7%) | |
| Alcohol intake | 0.112 | ||||
| None | 53 | (68%) | 14 | (50%) | |
| Positive | 25 | (32%) | 14 | (50%) | |
| Exercise | 0.004 | ||||
| Implemented | 50 | (64%) | 9 | (32%) | |
| Not-implemented | 28 | (36%) | 19 | (68%) | |
| Diabetes | 0.805 | ||||
| Absent | 58 | (74%) | 20 | (71%) | |
| Present | 20 | (26%) | 8 | (29%) | |
| Hypertension | 0.260 | ||||
| Absent | 50 | (64%) | 14 | (50%) | |
| Present | 28 | (36%) | 14 | (50%) | |
| Esophageal and/or gastric varices | 1.000 | ||||
| Absent | 74 | (95%) | 27 | (96%) | |
| Present | 4 | (5%) | 1 | (4%) | |
| HBsAg | 0.389 | ||||
| Negative | 63 | (81%) | 25 | (89%) | |
| Positive | 15 | (19%) | 3 | (11%) | |
| HCVAb | 0.825 | ||||
| Negative | 45 | (58%) | 15 | (54%) | |
| Positive | 33 | (42%) | 13 | (46%) | |
| Child–Pugh class | 1.000 | ||||
| A | 76 | (97%) | 27 | (96%) | |
| B | 2 | (3%) | 1 | (4%) | |
| ICGR15, % | 13.2 | (9.7, 19.0) | 12.5 | (9.7, 20.1) | 0.848 |
| WBC, 102/μL | 50.5 | (41.0, 60.0) | 50.5 | (41.0, 64.3) | 0.739 |
| Hemoglobin, g/dL | 13.1 | (12.4, 14.2) | 12.7 | (11.5, 14.0) | 0.060 |
| Hematocrit, % | 37.2 | (35.4, 39.9) | 37.2 | (34.4, 40.1) | 0.571 |
| Platelet count, ×104/mm3 | 14.6 | (11.5, 18.7) | 16.5 | (13.3, 19.4) | 0.337 |
| Albumin, g/dL | 4.1 | (3.8, 4.3) | 3.9 | (3.7, 4.3) | 0.371 |
| AST, U/L | 35.5 | (23.5, 50.8) | 38.0 | (28.5, 66.5) | 0.488 |
| ALT, U/L | 35.0 | (23.0, 53.8) | 38.0 | (20.5, 59.5) | 0.761 |
| Prothrombin activity, % | 88.6 | (81.7, 96.1) | 84.0 | (76.0, 92.7) | 0.121 |
| ALP, U/L | 248.0 | (187.8, 346.0) | 298.0 | (256.8, 347.5) | 0.050 |
| γGTP, U/L | 58.5 | (32.8, 104.3) | 48.0 | (31.0, 88.0) | 0.495 |
| Cholinesterase, U/L | 254.0 | (197.0, 275.0) | 221.0 | (175.0, 287.5) | 0.231 |
| Total bilirubin, mg/dL | 0.8 | (0.6, 0.9) | 0.6 | (0.5, 0.9) | 0.252 |
| Creatinine, mg/dL | 0.8 | (0.6, 0.9) | 0.8 | (0.7, 1.0) | 0.099 |
| CRP, mg/dL | 0.1 | (0.0, 0.2) | 0.1 | (0.0, 0.2) | 0.830 |
| Triglyceride, mg/dL | 104.0 | (75.5, 133.0) | 91.0 | (72.0, 120.0) | 0.241 |
| Total cholesterol, mg/dL | 163.5 | (141.3, 179.0) | 171.0 | (141.0, 190.0) | 0.467 |
| Glucose, mg/dL | 106.0 | (94.0, 140.3) | 102.0 | (94.0, 131.0) | 0.685 |
| Insulin, μIU/mL | 12.6 | (6.3, 19.2) | 13.0 | (8.3, 26.8) | 0.520 |
| HbA1c | 5.6 | (5.3, 6.7) | 5.5 | (5.0, 5.8) | 0.095 |
| Alpha-fetoprotein, ng/mL | 7.2 | (3.7, 15.6) | 11.0 | (4.7, 64.4) | 0.313 |
| PIVKA-II | 39.5 | (18.0, 151.0) | 109.5 | (22.5, 1246.3) | 0.126 |
Data are shown as median (5th percentile, 95th percentile) or n (%).
AT, anaerobic threshold; VO2, oxygen consumption; ASA-PS, The American Society of Anesthesiologists physical status; HBsAg, hepatitis B surface antigen; HCVAb, hepatitis C virus antibody; ICGR15, indocyanine green retention rate at 15 min; WBC, white blood cell count; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; γGTP, γ-glutamyltranspeptidase; CRP, C-reactive protein; HbA1c, glycated hemoglobin; PIVKA-II, protein induced by vitamin K absence-II.
Preoperative patient characteristics evaluating body composition, and the related indices of CPX including AT VO2, AT watt, peak heart rate, and peak VO2 were significantly higher in the maintenance group (Table 2). In contrast, total body weight, muscle content, and fat content measured by both BIA and DEXA did not differ between the two groups.
Table 2. Preoperative body composition characteristics of HCC patients classified according to AT VO2 change.
| Variables | Maintenance (n = 78) |
Decrease (n = 28) |
P | ||
|---|---|---|---|---|---|
| Total weight by BIA (rate of change) | 97.1 | (94.8, 100.4) | 98.8 | (95.1, 103.0) | 0.425 |
| Skeletal muscle content by BIA | 99.6 | (98.1, 101.2) | 100.3 | (97.8, 101.9) | 0.464 |
| Fat content by BIA | 93.6 | (82.4, 103.3) | 97.2 | (84.5, 103.5) | 0.910 |
| BMI by BIA | 97.3 | (94.8, 100.4) | 98.9 | (95.1, 103.7) | 0.422 |
| Total weight by DEXA | 97.8 | (95.5, 100.1) | 98.5 | (96.0, 101.3) | 0.437 |
| Fat content by DEXA | 90.4 | (82.6, 102.2) | 99.9 | (88.8, 102.7) | 0.184 |
| Non-fat content by DEXA | 100.7 | (97.9, 103.3) | 99.4 | (97.4, 102.3) | 0.262 |
| AT VO2 | 105.8 | (98.5, 117.1) | 81.2 | (76.5, 87.8) | <0.001 |
| AT W | 109.4 | (98.4, 126.3) | 92.9 | (78.8, 104.4) | <0.001 |
| AT VE.VCO2 | 97.4 | (92.5, 103.2) | 104.9 | (99.2, 108.8) | <0.001 |
| Peak HR | 103.5 | (96.2, 110.1) | 97.5 | (88.3, 104.0) | 0.014 |
| Peak VO2 | 110.0 | (96.8, 122.0) | 97.4 | (84.6, 103.1) | <0.001 |
Data are shown as median (5th percentile, 95th percentile).
AT, anaerobic threshold; VO2, oxygen consumption; BMI, body mass index; BIA, bioelectrical impedance analysis; DEXA, dual-energy X-ray absorptiometry; W, Watt; VE, minute ventilation; VCO2, carbon dioxide output; HR, heart rate.
No difference was detected between the two groups for operative, pathological features, and postoperative characteristics (Table 3). Complications attributable to surgery were noted in eight (10%) patients in the maintenance group and two (7%) patients in the decrease group.
Table 3. Operative and postoperative characteristics of HCC patients classified according to AT VO2 change.
| Variables | Maintenance (n = 78) |
Decrease (n = 28) |
P | ||
|---|---|---|---|---|---|
| Tumor number | 0.266 | ||||
| 1 | 54 | (69%) | 23 | (82%) | |
| 2 | 16 | (21%) | 2 | (7%) | |
| ≥3 | 8 | (10%) | 3 | (11%) | |
| Tumor size, cm | 3.0 | (2.0, 4.0) | 3.1 | (2.2, 5.3) | 0.680 |
| Degree of differentiation | 0.217 | ||||
| Well | 20 | (26%) | 11 | (39%) | |
| Moderately | 47 | (60%) | 16 | (57%) | |
| Poorly | 0 | (0%) | 0 | (0%) | |
| Necrosis or unknown | 11 | (14%) | 1 | (4%) | |
| fc | 0.810 | ||||
| Absent | 22 | (28%) | 7 | (25%) | |
| Present | 56 | (72%) | 21 | (75%) | |
| vp and/or vv | 0.821 | ||||
| Negative | 28 | (36%) | 11 | (39%) | |
| Positive | 50 | (64%) | 17 | (61%) | |
| sm | 0.377 | ||||
| Negative | 74 | (95%) | 25 | (89%) | |
| Positive | 4 | (5%) | 3 | (11%) | |
| Underlying liver disease | 0.778 | ||||
| Chronic hepatitis | 41 | (53%) | 17 | (61%) | |
| Cirrhosis | 27 | (35%) | 8 | (29%) | |
| Normal | 10 | (13%) | 3 | (11%) | |
| Tumor stage | 0.484 | ||||
| I | 11 | (14%) | 6 | (21%) | |
| II | 37 | (47%) | 9 | (32%) | |
| III | 24 | (31%) | 10 | (36%) | |
| IVa | 6 | (8%) | 3 | (11%) | |
| Operative procedure | 0.250 | ||||
| Anatomical resection | 53 | (68%) | 15 | (54%) | |
| Non-anatomical resection | 25 | (32%) | 13 | (46%) | |
| Amount of hepatic resection | 0.810 | ||||
| Less than hemihepatectomy | 56 | (72%) | 21 | (75%) | |
| More than hemihepatectomy | 22 | (28%) | 7 | (25%) | |
| Operating time, min | 353 | (270, 408) | 305 | (230, 416) | 0.162 |
| Operative blood loss, ml | 712 | (378, 1137) | 569 | (265, 1061) | 0.396 |
| Postoperative hospital stay, days | 12 | (11, 15) | 12 | (10, 15) | 0.484 |
| Blood transfusion | 0.741 | ||||
| Absent | 69 | (88%) | 24 | (86%) | |
| Present | 9 | (12%) | 4 | (14%) | |
| Complications | 1.000 | ||||
| Absent | 70 | (90%) | 26 | (93%) | |
| Present | 8 | (10%) | 2 | (7%) | |
| Mortality | - | ||||
| Absent | 78 | (100%) | 28 | (100%) | |
| Present | 0 | (0%) | 0 | (0%) | |
Data are shown as median (5th percentile, 95th percentile) or n (%).
AT, anaerobic threshold; VO2, oxygen consumption; fc, capsule formation; vp, portal vein invasion; vv, hepatic vein invasion; sm, surgical margin.
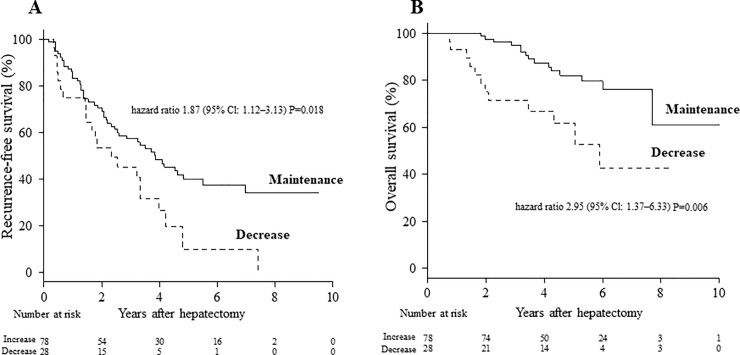
Fig 1 shows a comparison of the long-term outcomes between the two groups. The median follow-up period was 57.0 months in the maintenance group and 46.3 months in the decrease group. The 5-year RFS rates were 39.9% for the maintenance group and 9.9% for the decrease group (hazard ratio: 1.87 [95% CI: 1.12–3.13]; P = 0.018; Fig 1A). The 5-year OS rates were 81.9% for the maintenance group and 61.7% for the decrease group (hazard ratio: 2.95 [95% CI: 1.37–6.33]; P = 0.006; Fig 1B).
Fig 1. Survival outcomes between the maintenance and decrease groups.
A, Recurrence-free survival. B, Overall survival. CI: confidence interval.
Table 4 shows results of a multivariate statistical model incorporating several covariates. Multivariable Cox proportional hazards model identified six independent prognostic predictors for RFS: HCVAb-positive status (hazard ratio, 2.43; 95% CI: 1.19–4.97; P = 0.015), prothrombin activity ≥ 87% (hazard ratio, 2.78; 95% CI: 1.31–5.88; P = 0.008), PIVKA-II ≥ 46 mAU/mL (hazard ratio, 3.10; 95% CI: 1.36–7.09; P = 0.007), tumor number ≥ 2 (hazard ratio, 2.61; 95% CI: 1.13–6.01; P = 0.024), peak VO2 (rate of change) ≥ 90% (hazard ratio, 2.47; 95% CI: 1.03–5.94; P = 0.043), and AT VO2 (rate of change) ≥ 90% (hazard ratio, 2.58; 95% CI: 1.22–5.46; P = 0.013). Cox models also identified one independent prognostic predictor for OS: AT VO2 (rate of change) ≥ 90% (hazard ratio, 5.03; 95% CI: 1.40–18.02; P = 0.013; Table 4).
Table 4. Hazard ratios for recurrence-free survival and overall survival in HCC patients who underwent hepatic resection: Multivariable cox regression analysis.
| Variable | RFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | P | HR | (95% CI) | P | |
| Age ≥ 70 years (vs. < 70 years) | 1.26 | (0.63–2.49) | 0.514 | 2.31 | (0.73–7.32) | 0.156 |
| Sex female (vs. male) | 1.89 | (0.81–4.43) | 0.143 | 0.59 | (0.12–2.95) | 0.517 |
| DM present (vs. absent) | 0.71 | (0.33–1.53) | 0.383 | 2.40 | (0.66–8.75) | 0.186 |
| HCVAb positive (vs. negative) | 2.43 | (1.19–4.97) | 0.015 | 0.67 | (0.19–2.35) | 0.530 |
| Platelet count ≥ 15.7×104/mm3 (vs. < 15.7×104/mm3) | 1.47 | (0.77–2.80) | 0.238 | 0.69 | (0.24–1.96) | 0.480 |
| Prothrombin activity ≥ 87% (vs. < 87%) | 2.78 | (1.31–5.88) | 0.008 | 1.46 | (0.39–5.46) | 0.571 |
| Serum albumin ≥ 4.0 g/dL (vs. < 4.0 g/dL) | 0.74 | (0.35–1.53) | 0.416 | 0.58 | (0.18–1.88) | 0.360 |
| ALT ≥ 40 (vs. < 40) | 1.44 | (0.74–2.82) | 0.285 | 0.71 | (0.21–2.46) | 0.592 |
| Serum total bilirubin ≥ 0.7 mg/dL (vs. < 0.7 mg/dL) | 1.50 | (0.66–3.42) | 0.338 | 1.49 | (0.32–6.84) | 0.610 |
| Alpha-fetoprotein ≥ 8.0 ng/mL (vs. < 8.0 ng/mL) | 0.67 | (0.34–1.32) | 0.248 | 1.88 | (0.48–7.32) | 0.361 |
| PIVKA-II ≥ 46 mAU/mL (vs. < 46 mAU/mL) | 3.10 | (1.36–7.09) | 0.007 | 3.37 | (0.73–15.64) | 0.120 |
| Tumor number ≥ 2 (vs. single) | 2.61 | (1.13–6.01) | 0.024 | 1.78 | (0.47–6.71) | 0.394 |
| Tumor size ≥ 30 mm (vs. < 30 mm) | 0.71 | (0.35–1.47) | 0.358 | 0.55 | (0.18–1.76) | 0.316 |
| vp and/or vv positive (vs. negative) | 0.84 | (0.33–2.15) | 0.723 | 0.53 | (0.12–2.31) | 0.395 |
| Tumor stage III or IVa (vs. I or II) | 1.55 | (0.60–3.99) | 0.366 | 0.56 | (0.12–2.60) | 0.461 |
| More than hemihepatectomy (vs. less than hemihepatectomy) | 0.87 | (0.36–2.10) | 0.761 | 0.33 | (0.05–2.27) | 0.260 |
| Operative blood loss ≥ 500 ml (vs. < 500 ml) | 1.20 | (0.61–2.38) | 0.601 | 1.62 | (0.51–5.20) | 0.413 |
| Total weight by DEXA (rate of change) ≥ 100% (vs. < 100%) | 0.76 | (0.32–1.79) | 0.530 | 0.25 | (0.05–1.15) | 0.074 |
| Non-fat content by DEXA (rate of change) ≥ 100% (vs. < 100%) | 1.42 | (0.74–2.75) | 0.291 | 2.65 | (0.93–7.58) | 0.068 |
| Peak VO2 (rate of change) ≥ 90% (vs. < 90%) | 2.47 | (1.03–5.94) | 0.043 | 1.36 | (0.31–6.02) | 0.687 |
| AT VO2 (rate of change) ≥ 90% (vs. < 90%) | 2.58 | (1.22–5.46) | 0.013 | 5.03 | (1.40–18.02) | 0.013 |
RFS, recurrence-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; DM, diabetes mellitus; HCVAb, hepatitis C virus antibody; ALT, alanine aminotransferase; PIVKA-II, protein induced by vitamin K absence-II; vp, portal vein invasion; vv, hepatic vein invasion; DEXA, dual-energy X-ray absorptiometry; AT, anaerobic threshold; VO2, oxygen consumption.
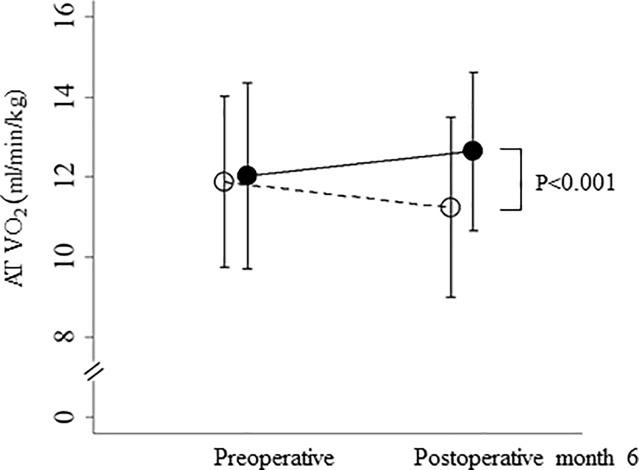
In the exercise not-implemented group, mean AT VO2 from preoperative to 6 months postoperatively decreased from 11.9 ml/min/kg to 11.2 ml/min/kg, on average, compared with the exercise-implemented group, in which values increased from 12.0 ml/min/kg to 12.6 ml/min/kg, on average, and the difference was significant (p <0.001) (Fig 2).
Fig 2. Perioperative changes in AT VO2 in the two groups.
AT VO2 in the maintenance (closed circles) and decrease (open circles) groups after liver resection. Data are shown as the mean ± standard deviation. AT VO2: anaerobic threshold as the point between carbon dioxide production and oxygen consumption per unit of time.
Discussion
We examined the relationships between perioperative CPX parameters and long-term survival in 106 patients undergoing hepatectomy. To the best of our knowledge, this retrospective study is the first to investigate the relationship between perioperative cardiopulmonary function related to exercise load and log-term survival in patients undergoing first hepatectomy for HCC. The variables derived from CPX testing included peak VO2, which is the maximum oxygen uptake at peak exercise. Previous studies indicated that peak VO2 was the most useful predictor of postoperative cardiopulmonary complications in patients undergoing radical esophagectomy with three-field lymphadenectomy [21] and patients undergoing surgical procedures for lung cancer [22–26]. AT is defined as the point during exercise at which oxygen demand outstrips oxygen delivery and metabolism starts to become anaerobic. AT is a measure of the ability of the cardiopulmonary system to deliver adequate oxygen to tissues, and has the advantage of being independent of patient motivation. Reaching AT does not require high levels of physical stress and occurs well before peak VO2 [27]. The usefulness of measuring AT has been assessed predominantly in older patients undergoing major surgical procedures, allowing the development of an operative risk grading and treatment protocol [7,8]. An earlier prospective study of preoperative CPX testing in 187 patients undergoing major intra-abdominal surgery demonstrated an association between AT < 11 ml O2/kg/min and postoperative cardiovascular mortality [21]. Postoperative complications are associated with reduced fitness, and AT is a prognosticator of postoperative complications [7, 15]; CPX testing has been used in risk stratification. West et al. reported that CPX testing is associated with postoperative morbidity, and a multivariable model including VO2 at estimated lactate threshold and gender discriminated those with complications after colonic surgery [28]. Junejo et al. reported that an AT of 9.9 ml O2/kg/min predicted inhospital death and subsequent survival in their patients undergoing hepatic resection [29]. In contrast, Dunne et al. reported that when CPX testing is used to delineate perioperative management, a low relative oxygen uptake [VO2 (ml/kg/min)] at does not place patients at significantly higher risk of postoperative complications. This suggested that CPX testing-assessed “poor” fitness should not be used as a barrier to surgical intervention [30]. Although we evaluated preoperative AT VO2 value, alone, in our study, preoperative AT VO2 was not identified as an independent prognostic predictor for RFS and OS. CPX measurements were performed preoperatively and 6 months postoperatively to evaluate postoperative changes. Therefore, as a future study, we evaluated the rate of change of AT VO2 from the preoperative value to 6 months postoperatively as an indicator of prognosis.
Sarcopenia is a strong predictor of outcome after liver resection and orthotopic liver transplantation in HCC patients [31–33]. Therefore, it is important to identify sarcopenia, preoperatively. However, changes in muscle content measured using DEXA in our HCC patients were not associated with long-term survival in this study (Table 4). Although we evaluated preoperative muscle content using DEXA in a multivariable Cox proportional hazards model for RFS and OS, preoperative muscle content was not identified as a significant prognostic indicator (data not shown). However, we did identify perioperative maintenance of AT VO2 as an independent prognostic indicator for RFS and OS in our 106 HCC patients (Table 4). Both RFS and OS rates were significantly higher for patients who maintained AT VO2 than for patients with decreased AT VO2 (Fig 1). Although maintenance of AT VO2 correlated with both RFS and OS, the direct mechanism of the association is unclear. Our previous study demonstrated preoperative exercise capacity as an independent prognostic indicator of event-free survival and maintenance of Child–Pugh class in HCC patients with chronic liver injury undergoing hepatectomy [34]. We identified AT VO2 < 11.5 ml/min/kg and branched-chain amino acid/tyrosine ratio as independent prognostic indicators of maintenance of Child–Pugh class in 61 HCC patients undergoing hepatectomy. We also found a significant correlation between branched-chain amino acid/tyrosine ratio and AT VO2 in HCC patients in our previous study. CPX testing may be useful to predict postoperative recurrence of either HCC or liver dysfunction.
Recently, we found that in patients with HCC and hepatic impairment undergoing liver resection, exercise significantly decreased body mass and fat mass, as well as insulin resistance, 6 months postoperatively [16]. Maintenance of postoperative physical strength and earlier resumption of daily activities could be possible by intensifying perioperative and postoperative exercise. The exercise program we implemented was tailored to each patient who wished to participate. Although mean AT VO2 from preoperative to 6 months postoperatively decreased in the exercise not-implemented group, the exercise-implemented group experienced increased AT VO2, on average, 6 months postoperatively (Fig 2). Fifty patients were able to exercise perioperatively in the maintenance group and nine were able in the decrease group; a significantly higher number in the maintenance group (Table 1). Our findings suggest that perioperative exercise is important to maintain postoperative AT VO2 after hepatectomy for HCC.
In conclusion, the most significant prognostic factor affecting postoperative survival for chronic liver injury patients with HCC undergoing hepatectomy was maintenance of AT VO2 up to 6 months postoperatively, as measured by CPX testing. Our results suggest that exercise involving > 5000 steps per day is necessary for postoperative walking to maintain AT VO2, and indicate the importance of introducing perioperative exercise to HCC patients with chronic liver injury.
Supporting information
(ZIP)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Older P, Smith R: Experience with the preoperative invasive measurement of haemodynamic, respiratory and renal function in 100 elderly patients scheduled for major abdominal surgery. Anaesth Intensive Care 1988, 16:389–395. 10.1177/0310057X8801600402 [DOI] [PubMed] [Google Scholar]
- 2.Halm EA, Browner WS, Tubau JF, Tateo IM, Mangano DT. Echocardiography for assessing cardiac risk in patients having noncardiac surgery. Study of Perioperative Ischemia Reserch Group. Ann Intern Med. 1996; 125: 433–41. 10.7326/0003-4819-125-6-199609150-00001 [DOI] [PubMed] [Google Scholar]
- 3.Dunselman PH, Kuntze CE, van Bruggen A, Beekhuis H, Piers B, Scaf AH, et al. Value of New York Herat Association classification, radionuclide ventriculography, and cardiopulmonary exercise tests for selection of patients for congestive heart failure studies. Am Heart J. 1988; 116: 1475–82. 10.1016/0002-8703(88)90731-4 [DOI] [PubMed] [Google Scholar]
- 4.Mangano DT, London MJ, Tubau JF, Browner WS, Hollenberg M, Krupski W, et al. Dipyridamole thallium-201scintigraphy as a preoperative screening test. A reexamination of its predictive potential. Study of Perioperative Ischemia Reserch Group. Circulation. 1991; 84: 493–502. 10.1161/01.cir.84.2.493 [DOI] [PubMed] [Google Scholar]
- 5.Reilly DF, McNeely MJ, Doerner D, Greenberg DL, Staiger TO, Geist MJ, et al. Self-reported exercise tolerance and the risk of serious perioperative complications. Arch Intern Med. 1999; 159: 2185–92. [DOI] [PubMed] [Google Scholar]
- 6.Girish M, Trayner E Jr, Dammann O, Pinto-Plata V, Celli B. Symptom-limited stair climbing as a predictor of postoperative cardiopulmonary complications after high-risk surgery. Chest. 2001; 120: 1147–51. 10.1378/chest.120.4.1147 [DOI] [PubMed] [Google Scholar]
- 7.Older P, Hall A, Hader R. Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest. 1999; 116: 355–62. 10.1378/chest.116.2.355 [DOI] [PubMed] [Google Scholar]
- 8.Older P, Smith R, Courtney P, Hone R. Preoperative evaluation of cardiac failure and ischemia in elderly patients by cardiopulmonary exercise testing. Chest. 1993; 104: 701–4. 10.1378/chest.104.3.701 [DOI] [PubMed] [Google Scholar]
- 9.Bosch X, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999; 19: 271–285. 10.1055/s-2007-1007117 [DOI] [PubMed] [Google Scholar]
- 10.Ziegler TR. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. JPEN J Parenter Enteral Nutr. 1996; 20: 91–2. 10.1177/014860719602000191 [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa Y, Yoshida H, Mamada Y, Taniai N, Matsumoto S, Bando K, et al. Prospective randomized controlled study of short-term perioperative oral nutrition with branched chain amino acids in patients undergoing liver surgery. Hepatogastroenterology. 2010; 57(99–100):583–90. [PubMed] [Google Scholar]
- 12.Beaver WL, Wasserman K, Whipp BJ. Bicarbonate buffering of lactic acid generated during exercise. J Appl Phsiol. 1986; 60: 472–8. [DOI] [PubMed] [Google Scholar]
- 13.Whipp BJ, Wasserman K. Oxygen uptake kinetics for various intensities of constant-load work. J Appl Physiol. 1972; 33: 351–6. 10.1152/jappl.1972.33.3.351 [DOI] [PubMed] [Google Scholar]
- 14.Wasserman K, Hansen JE, Sue DY. Principles of exercise testing and interpretation In: Wasserman K, Hansen JE, Sue DY, Whipp BJ, eds. Measurement of the Physiological Response to Exercise. Philadelphia, PA: Lea and Febiger; 1987:27–46. [Google Scholar]
- 15.Snowden CP, Prentis JM, Anderson HL, Roberts DR, Randles D, Renton M, et al. Submaximal cardiopulmonary exercise testing predicts complications and hospital length of stay in patients undergoing major elective surgery. Ann Surg. 2010; 251: 535–41. 10.1097/SLA.0b013e3181cf811d [DOI] [PubMed] [Google Scholar]
- 16.Kaibori M, Ishizaki M, Matsui K, Nakatake R, Yoshiuchi S, Kimura Y, et al. Perioperative exercise for chronic liver injury patients with hepatocellular carcinoma undergoing hepatectomy. Am J Surg. 2013; 206: 202–9. 10.1016/j.amjsurg.2012.07.035 [DOI] [PubMed] [Google Scholar]
- 17.Coin A, Sergi G, Minicuci N, Giannini S, Barbiero E, Manzato E, et al. Fat-free mass and fat mass reference values by dual-energy X-ray absorptiometry (DEXA) in a 20–80 year-old Italian population. Clin Nutr. 2008; 27: 87–94. 10.1016/j.clnu.2007.10.008 [DOI] [PubMed] [Google Scholar]
- 18.Lehnert ME, Clarke DD, Gibbons JG Ward LC, Golding SM, Shepherd RW, et al. Estimation ofbody water compartments in cirrhosis by multiplefrequency bioelectrical-impedance analysis. Nutrition. 2001; 17: 31–4. [DOI] [PubMed] [Google Scholar]
- 19.Strasberg SM, Belghiti J, Clavien PA. The Brisbane 2000 terminology of liver anatomy and resection. Terminology Committee of the International Hepato-Pancreato-Biliary Association. HPB. 2000; 2: 333–339. [Google Scholar]
- 20.Couinaud C (ed): “Le Foie: Etudes Anatomiques et Chirurgicales.” Paris: Masson, 1957.
- 21.Nagamatsu Y, Shima I, Yamana H, Fujita H, Shirouzu K, Ishitake T. Preoperative evaluation of cardiopulmonary reserve with the use of expired gas analysis during exercise testing in patients with squamous cell carcinoma of the thoracic esophagus. J Thorac Cardiovasc Surg. 2001; 121: 1064–8. 10.1067/mtc.2001.113596 [DOI] [PubMed] [Google Scholar]
- 22.Win T, Jackson A, Sharples L, Groves AM, Wells FC, Ritchie AJ, et al. Cardiopulmonary exercise tests and lung cancer surgical outcome. Chest. 2005; 127: 1159–65. 10.1378/chest.127.4.1159 [DOI] [PubMed] [Google Scholar]
- 23.Nagamatsu Y, Terazaki Y, Muta F, Yamana H, Shirouzu K, Ishitake T. Expired gas analysis during exercise testing pre-pneumonectomy. Surg Today. 2005; 35: 1021–5. 10.1007/s00595-005-3078-4 [DOI] [PubMed] [Google Scholar]
- 24.Smith TP, Kinasewitz GT, Tucker WY, Spillers WP, George RB. Exercise capacity as a predictor of post-thoracotomy morbidity. Am Rev Respir Dis. 1984; 129:730–4. 10.1164/arrd.1984.129.5.730 [DOI] [PubMed] [Google Scholar]
- 25.Bechard D, Wetstein L. Assessment of exercise oxygen consumption as preoperative criterion for lung resection. Ann Thorac Surg. 1987; 44: 344–9. 10.1016/s0003-4975(10)63787-3 [DOI] [PubMed] [Google Scholar]
- 26.Bolliger CT, Soler M, Stulz P, Grädel E, Müller-Brand J, Elsasser S, et al. Evaluation of high-risk lung resection candidates: pulmonary haemodynamics versus exercise testing. A series of five patients. Respiration. 1994; 61: 181–6. 10.1159/000196334 [DOI] [PubMed] [Google Scholar]
- 27.Weisman IM. Cardiopulmonary exercise testing in the preoperative assessment for lung resection surgery. Semin Thorac Cardiovasc Surg. 2001; 13: 116–25. [DOI] [PubMed] [Google Scholar]
- 28.West MA, Lythgoe D, Barben CP, Noble L, Kemp GJ, Jack S, et al. Cardiopulmonary exercise variables are associated with postoperative morbidity after major colonic surgery: a prospective blinded observational study. Br J Anaesth. 2014; 112: 665–71. 10.1093/bja/aet408 [DOI] [PubMed] [Google Scholar]
- 29.Junejo MA, Mason JM, Sheen AJ, Moore J, Foster P, Atkinson D, et al. Cardiopulmonary exercise testing for preoperative risk assessment before hepatic resection. Br J Surg. 2012; 99: 1097–104. 10.1002/bjs.8773 [DOI] [PubMed] [Google Scholar]
- 30.Dunne DF, Jones RP, Lythgoe DT, Pilkington FJ, Palmer DH, Malik HZ, et al. Cardiopulmonary exercise testing before liver surgery. J Surg Oncol. 2014;110: 439–44. 10.1002/jso.23670 [DOI] [PubMed] [Google Scholar]
- 31.van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN.: Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 2012; 99: 550–57. 10.1002/bjs.7823 [DOI] [PubMed] [Google Scholar]
- 32.Harimoto N, Shirabe Y, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, et al. : Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg 2013; 100: 1523–30. 10.1002/bjs.9258 [DOI] [PubMed] [Google Scholar]
- 33.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, et al. : Sarcopenia and mortality after liver transplantation. J Am Coll Surg 2010; 211: 271–8. 10.1016/j.jamcollsurg.2010.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaibori M, Ishizaki M, Matsui K, Nakatake R, Sakaguchi T, Habu D, et al. : Assessment of preoperative exercise capacity in hepatocellular carcinoma patients with chronic liver injury undergoing hepatectomy. BMC Gastroenterol. 2013; 13: 119 10.1186/1471-230X-13-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.