Abstract
Background
This retrospective study had two main aims: (1) to document possible correlations between plasma Amino Acids (AAs) and circulating Albumin (Alb) and Haemoglobin (Hb); and (2) to identify which AAs were predictors of Alb and Hb.
Methods
The study considered 125 stroke subjects (ST) (61.6% males; 65.6 +/- 14.9 years) who met the eligibility criteria (absence of co morbidities associated with altered plasma AAs and presence of plasma AAs determined after overnight fasting). Fifteen matched healthy subjects with measured plasma AAs served as controls.
Results
The best correlations of Alb were with tryptophan (Trp) and histidine (His) (r = + 0.53; p < 0.0001), and those of Hb were with histidine (r = +0.47) and Essential AAs (r = +0.47) (both p<0.0001). In multivariate analysis, Trp (p< 0.0001) and His (p = 0.01) were shown to be the best positive predictors of Alb, whereas glutamine (p = 0.006) was the best positive predictor of Hb.
Conclusions
The study shows that the majority of plasma AAs were positively correlated with Alb and Hb. The best predictors of circulating Alb and Hb were the levels of tryptophan and glutamine, respectively.
Introduction
Stroke is an important cause of disability and the third cause of death in Western Societies [1,2]. In Italy, about one third of stroke survivors are totally dependent on other people one year after a stroke [3]. The first 30 days after the acute event are crucial for both patient survival and functional prognosis, as 20% of mortality and 80% of neurological recovery occur in this time [4,5].
It is therefore essential to make every effort to achieve the best rehabilitative outcomes during the first month after a stroke.
Considering the lack of availability of drugs to repair the brain area damaged by vascular accidents, nutritional interventions seem to be a promising tool to enhance post-stroke neurocognitive retrieval. Nutrition appears to influence the repair process of damaged brain tissue. An observational study demonstrated that spontaneous neurocognitive recovery in stroke subjects was associated with protein-calorie intakes [6]. Moreover, when stroke patients were supplemented with proteins [7], zinc [8] and Essential Amino Acids (EAAs) [9], their neurocognitive retrieval improved.
Improving circulating Albumin (Alb) and Haemoglobin (Hb) levels may be a way of influencing neurocognitive recovery.
Alb therapy has been shown to be neuroprotective in animal models of transient focal ischemia [10–12], global ischemia [13] and traumatic brain injury[14].
In subjects with subacute stroke (<3 months from the acute event), the gain in serum Alb correlated with the gain in functional independence [15].
With respect to Hb, the circulating protein levels were significantly linked to the functional recovery of stroke subjects during the rehabilitation phase of the disease [16].
The crucial problem, however, is to determine whether and how is it possible to increase Alb and Hb levels in subacute stroke subjects, i.e. in a phase of the disease during which systemic inflammation, even though it is declining, may persist [16], and to maintain alterations of the two circulating protein levels [17]. It is not feasible to act on the extra vascular redistribution and degradation rate of Alb in order to increase its circulating levels, given the scarce information about these processes. In addition, providing patients with Alb as a nutrition support agent is not appropriate [18]. In subjects with mild anaemia, it is not appropriate to administer transfusion or erythropoietin.
At present, the only potential way of increasing Alb and Hb levels is to use nutritional interventions to increase their synthesis. In healthy individuals, Alb synthesis is acutely stimulated after ingestion of a complete meal [19–21] or of carbohydrates and fats without protein [21]. The ingestion of protein alone is equally as effective as the ingestion of a complete meal in stimulating Alb synthesis [22]. This indicates that Amino Acids (AAs) play an important role in regulating Alb synthesis. Indeed, AAs influence Alb synthesis both directly and indirectly [23–27] by stimulating the release of insulin [21]. A third of the AAs in a daily dietary intake are used in the synthesis of Alb and other plasma proteins [28]. In inflamed elderly subjects who had been operated for hip fractures, supplemented EAAs increased circulating Alb and Hb levels [17]. Early in vitro and in vivo experiments had previously documented the importance of AAs in stimulating liver Alb (and other protein) synthesis [24–26, 29].
In addition, Hb synthesis is sensitive to AA availability as these substrates influence erythropoietin production [30]. Amino aciduria negatively correlated with plasma Hb levels [31]. More recently, a study reported that Hb synthesis in chickens is influenced by energy intake [32].
On the basis of these studies, we wanted to develop our understanding of the relationship between circulating AAs and circulating Alb/Hb levels in subacute stroke subjects under overnight fasting conditions instead of after meal stimulation. Therefore, this retrospective observational study had two aims: to document any associations between circulating AAs and circulating Alb and Hb and to highlight the best AA predictors of Alb and Hb.
To this end, we formulated two hypotheses. Firstly, that plasma AAs and circulating Alb/Hb may be positively associated given that (a) during overnight fasting, the synthesis of Alb continues even though at a reduced rate [33]; (b) during overnight fasting, the liver is continuously exposed to circulating AAs whose availability is important for Alb synthesis [22,24]; (c) AA availability is essential to haemopoiesis [30]. AA deficiency reduces protein synthesis in a number of cell types including reticulocytes [34].
The second hypothesis we put forward was that plasma AA Tryptophan (Trp) and isoleucine could predict circulating Alb and Hb levels, respectively. This hypothesis relied on previous studies, which had documented that Alb synthesis in fasting rabbits [35] was stimulated by Trp and, to a lesser extent, isoleucine, but not by a mixture of other AAs. Isoleucine was essential for haemopoiesis in experimental anaemic rats [36].
If we were correct, the findings of the study would provide a useful framework for future investigations into how to increase Alb/Hb during short fasting periods in stroke subjects who often have insufficient nutrition intakes [37,38].
Subjects and methods
Population
This is a retrospective study carried out on stroke patients who were admitted to our Rehabilitation Centre (Rehab) from January 2004 to January 2014.
The patients were selected from our database and were deemed eligible if (1) admission occurred within 35 days after the acute event; (2) they were not affected by diseases that were potentially associated with alterations in plasma amino acids (chronic heart failure, cancer, chronic obstructive pulmonary disease, steroid therapy, diabetes) or by diseases that led to hypoalbuminemia/anaemia (albumin losses from nephrotic syndrome, ascitis, protein–losing enteropathy, overdegradation following trauma or surgery, but not neurosurgery due to stroke). Moreover, patients were only included if their plasma AAs, anthropometrics and routine biohumoral variables had been measured, their nutritional intakes had been analysed, and if there was computerised tomography documentation of vascular brain damage.
Out of a total of 236 subjects in the database, 125 (approximately 53% patients) were selected for this study. The patients came from neurosurgery settings (12%), stroke units (36%) and neurological wards (52%). Computerised tomography documented that 60.8% had brain ischemic damage and 39.2% had brain haemorrhage damage. On admission to Rehab, 58.4% (n = 73) were fed via percutaneous endoscopy gastrostomy (PEG) and 41.6% (n = 52) were self-feeding.
Description and analysed variables
Anthropometric characteristics
Body weight (BW) (kg) had been measured using a mechanical weight lifter; height (m) had been calculated from knee height [39].
Body Mass Index (BMI) was calculated as kg x m-2.
For this study a loss of actual BW in relation to habitual (pre-event) BW>5% (i.e. actual/habitual BW<95%) was considered as an index of significant undernutrition [40].
Biohumoral variables
Routine variables, including serum protein electrophoresis.
- Markers of body inflammation:
- C-Reactive Protein (CRP, normal value<0.3 mg dl-1) determined by an immune-turbidimetric method.
- Acute phase reactant proteins (fibrinogen, normal values 350–495 mg / dl; α1-globulin system, normal values 210–350 mg dl-1) and non-reactant proteins (albumin, normal values 4.02–4.76 g/dl; prealbumin, normal values 18–30 mg/dl; transferrin, normal values 202–364 mg/dl).
Plasma amino acids
The following protocol had been used to measure plasma AA levels. At 8:am after overnight fasting, the patients underwent venipuncture from a basilic vein in the unaffected arm to sample blood to determine the concentrations of plasma AAs. Concentrations of free AAs in the plasma were measured using an Amino Quant II amino acid analyser, based on the HP 1090 HPLC system, with fully automated precolumn derivatisation, using both orthophthal-aldehyde and 9-fluorenyl-methyl-chloformate reaction chemistries according to the manufacturer’s protocol.
The measurements of the plasma amino acids had been carried out in triplicate by the same laboratory.
The mean of the three measurements had been calculated and adopted. The characteristics of the method were based both on precision and standardisation properties:
Precision (Relative Standard Deviation (RSD) was 1.13%
Reliability (bias) was 10.37%
The lower limit of quantitation was 0.18ng/ml
The limit of detection was 0.6ng/ml
For the measurements in triplicate, the intra-day variability (RSD) was 3.21% and the inter-variability was 4.17%.
The results were obtained by injecting 1μL of the derivatised mixture and measuring absorbance simultaneously at 338 and 262nm. Plasma concentrations were expressed as μmol/L.
As a comparison we considered the amino acid measurements carried out in 15 healthy sedentary subjects (no history of disease, no clinical or instrumental signs of disease) during the period 2004–2014 with a similar age (69.3±4.7 years), sex distribution (60% female) and body mass index (23.1±3.6 Kg/m2). The venous blood samples had been drawn at 8:am, after overnight fasting.
Nutritional intakes
These had been measured following our Standard Protocol [40].
In brief, for self-feeding patients (n = 52) a 3-day food diary was kept by the rehabilitation nurses, who had previously been taught how to do so. The nurses recorded the type and weight of cooked or uncooked food selected by patients from the hospital catering menu on a diet sheet for 3 days both before and after the patients’ meals. The amount of food that was actually ingested was converted to its raw equivalent when necessary, using appropriate tables [41].
For this study, we re-analysed the nutritional intakes using a computer Program Food Database DR3 (Dieta ragionata 3. Sintesi informatica, University of Pavia, Italy), in order to calculate ingested calories and macro-/micronutrients.
Nutritional intakes from the pharmaceutical formula given to patients on enteral nutrition (n = 73 of whom 51.6% were dysphagic) were calculated from the nutritional composition reported in the formula labels.
Daily calories and protein intakes were considered adequate to body requirements if they were ≥25 Kcal/Kg and, respectively ≥1g/Kg [42].
Functional status
Evaluated using the functional independence measure (FIM) [15].
The study was approved by the local Institutional Review Board and by the Istituti Clinici Scientifici Maugeri Ethics Committee (approval date 16/05/2017, protocol number 2113 CE). All patients or, where the case, their relatives or appointed caregivers, had signed an informed written consent prior to the participation to the study and provided written consent for the scientific treatment of their data in an anonymous form. The decision whether a patient had capacity to consent (i.e. the ability to use and understand information to make a decision and communicate any decision made) or consent had to be given by relatives or appointed caregivers was based on judgement by appropriately trained and experienced health professionals.
Statistical analysis
Descriptive statistics of all collected data were reported as mean ± SD for continuous variables and as percentage frequency for categorical variables. The Shapiro–Wilk statistic supported by visual inspection was used to test the normality of the distribution of all variables.
Between-group comparisons for continuous variables were carried out by the unpaired Student T-test or by the Mann–Whitney U-test, if appropriate. The Chi-squared test was used for categorical variables.
The association between circulating Alb and Hb levels and plasma AAs was assessed by the linear Pearson correlation coefficient (Pearson r) or by the Spearman's rank correlation coefficient (Spearman r), as appropriate.
Multivariable linear regression analysis was used to assess the association between AAs, considered as explanatory variables, and Albumin and Haemoglobin (dependent variables), respectively, in stroke patients.
A p-value of <0.05 was considered statistically significant. When appropriate, false discovery rate (FDR) was controlled at 5% using the Benjamini-Hochberg method and FDR adjusted p-values were also reported. [43]. All analyses were carried out using the SAS/STAT statistical package, release 9.4 (SAS Institute Inc., Cary, NC, U.S.A.).
Results
Patient characteristics
Table 1 reports the baseline characteristics of the stroke population at admission to the Rehabilitation Institute. The patients were slightly overweight, with an average of 3% loss of habitual (pre-event) body weight (BW), and systemic inflammatory state (increased values of C-Reactive Protein (CRP) and Erytrocyte Sedimentation rate (ESR). Calorie-protein intakes were lower than recommended. Serum total protein levels were normal but Alb concentration was lower than normal values. 69 subjects (55.2%) had Alb<3.5 g/dl. Anaemia was present in 42 patients (33.6%). The entire patient population displayed severe physical disability (Functional Independence Measure (FIM) -52.4% of normal values).
Table 1. Demographic, clinical, anthropometric, biohumoral, functional and nutritional variables of the analysed stroke population in the study.
| Variables | Stroke population (n = 125) | Normal values | %RDA |
|---|---|---|---|
| Demographic | |||
| Male/Female | 77/48 | - | |
| Age (years) | 65.6±14.9 | - | |
| Clinical | |||
| Aetiology: | |||
| Ischemic | 82 (65.6%) | - | |
| Haemorrhagic | 43 (34.4%) | - | |
| Anthropometric | |||
| Actual body weight (kg) | 71.96±15.38 | - | |
| Body mass index (BMI) (Kg/m2) | 25.62±4.56 | ||
| Pre-event body weight (kg) | 74.91±15.89 | ||
| Actual/pre-event BW (%) | 97±7 | - | |
| Blood | |||
| ESR 1st hr (mm) | 37±30.25 | <20 | |
| Haemoglobin (g/dl) | 13±1.93 | 12–15 | |
| Blood urea (mg/dl) | 40.38±20.88 | 20–40 | |
| Serum creatinine (mg/dl) | 0.97±0.28 | 0.7–1.2 | |
| Plasma glucose (mg/dl) | 110±36.5 | 80–110 | |
| α1 globulin (g/dl) | 0.22±0.05 | 0.21–0.35 | |
| Fibrinogen (mg/dl) | 389.67±97.34 | 350–500 | |
| Serum albumin (g/dl) | 3.34±0.56 | 4.02–4.76 | |
| Serum prealbumin (mg/dl) | 21.1±5.8 | 18–30 | |
| Serum transferrin (mg/dl) | 204.7±48.38 | 202–364 | |
| Serum C-reactive protein (CRP) (mg/dl) | 1.38±2.44 | <0.3 | |
| Total proteins (g/dl) | 7.09±0.63 | 6.2–8 | |
| White cell counts (n°/mm3) | 6669.28±1957.01 | 4000–9000 | |
| Neutrophils (%) | 60±10.25 | 45–75 | |
| Lymphocytes (%) | 27.54±9.54 | 20–47 | |
| Function | |||
| FIM (score) | 59.49±28.03 | 126 | |
| Daily nutritional intake | %RDA | ||
| Energy | |||
| Kcal/d | 1561±186 | - | - |
| Kcal/Kg | 21.8±2.01 | ≥25 | 87.2 |
| Proteins | |||
| g/d | 66.2±11.7 | - | - |
| g/Kg | 0.92±0.16 | ≥1.0 | 92 |
| Lipids | |||
| g/d | 52.4±10.1 | - | - |
| g/Kg | 0.73±0.14 | ≤1 | 100 |
| Carbohydrates | |||
| g/d | 206±45.1 | ||
| g/Kg | 2.86±0.57 | - | - |
| Calcium (mg) | 749±90 | 1000 | 74.9 |
| Phosphorus (mg) | 1324±177 | 1000 | 132.4 |
| Potassium (mg) | 3650±767 | 3100 | 118 |
| Sodium (mg) | 1541±139 | - | - |
| Iron (mg) | 16.1±3.2 | 10 | 161 |
| Copper (mg) | 1.46±0.58 | 1.2 | 121.7 |
| Thiamin (mg) | 1.2±0.3 | 1.1 | 109 |
| Riboflavin (mg) | 1.83±0.4 | 1.1 | 166 |
| Niacin (mg) | 19.1±7.6 | 16 | 119 |
| Vitamin C (mg) | 159.5±68.8 | 60 | 266 |
| Folic Acid (μg) | 171±38 | 400 | 42.8 |
| Vitamin B12 (μg) | 2.1±0.85 | 2.4 | 87.5 |
Data are expressed as mean ±standard deviation (SD)
FIM = functional independence measure; ESR: erythrocyte sedimentation rate; RDA: recommended daily allowance; nd: not defined
Compared to the healthy controls, the stroke subjects showed profound alterations of plasma AA concentrations (Table 2), with 71.4% of AAs higher than in healthy controls histidine (His), serine, citrulline, ornithine, glycin, threonine, alanine, phenylalanine, tyrosine, methionine, lysine and Branched Chain AAs (BCAAs: leucine, valine, isoleucine). In contrast, aspartic acid, glutamic acid, asparagine and taurine concentrations were lower in stroke subjects than in healthy controls.
Table 2. Differences in plasma Amino Acid concentrations (μmol/L) and Amino Acid ratios between stroke patients and healthy subjects.
| Variables | Healthy subjects (n = 15) | Stroke patients (n = 125) | p value | FDR-adjusted p value |
|---|---|---|---|---|
| Aspartic Acid | 60.42±52.11 | 11.38±2.26 | 0.053 | 0.0622 |
| Glutamic Acid | 157.90±55.61 | 71.78±23.53 | <0.0001 | <0.0001 |
| Histidine | 53.71±11.05 | 72.02±15.45 | 0.0001 | 0.0002 |
| Asparagine: | 54.43±11.87 | 49.14±11.91 | 0.033 | 0.0405 |
| Serine | 68.77±23.30 | 113.46±29.36 | <0.0001 | <0.0001 |
| Glutamine | 570±70 | 576.45±116.81 | 0.8 | 0.8 |
| Arginine | 60.49±9.62 | 59.27±19.21 | 0.40 | 0.45 |
| Citrulline | 24.57±3.66 | 32.83±9.78 | 0.009 | 0.0128 |
| Glycine | 208.37±76.81 | 269.35±68.01 | 0.023 | 0.0296 |
| Threonine | 104.39±22.60 | 137.96±41.78 | 0.001 | 0.0018 |
| Alanine | 284.41±51.22 | 345.04±92.03 | 0.009 | 0.0128 |
| Taurine | 133.25±23.03 | 94.68±29.73 | 0.0008 | 0.0015 |
| Tyrosine | 49.40±10.66 | 65.08±17.50 | 0.0005 | 0.0010 |
| Valine | 152.62±19.59 | 247.77±68.66 | <0.0001 | <0.0001 |
| Methionine | 15.17±7.42 | 28.58±8.09 | <0.0001 | <0.0001 |
| Tryptophan | 43.31±11.19 | 42.44±11.56 | 0.53 | 0.5504 |
| Phenylalanine | 41.61±7.28 | 61.69±16.43 | <0.0001 | <0.0001 |
| Leucine | 78.52±13.69 | 136.15±38.38 | <0.0001 | <0.0001 |
| Ornithine | 56.38±6.39 | 87.29±31.16 | 0.002 | 0.0034 |
| Lysine | 103.41±24.95 | 217.77±55.76 | <0.0001 | <0.0001 |
| Isoleucine | 44.15±7.45 | 71.06±18.29 | <0.0001 | <0.0001 |
| Total Amino Acids (TAAs) | 2791±404.8 | 2574.55±404.80 | <0.0001 | <0.0001 |
| Branched Chain Amino Acids (BCAAs) | 275.28±37.72 | 454.98±117.74 | <0.0001 | <0.0001 |
| Essential Amino Acids (EAAs) | 583.17±74.78 | 941.55±191.13 | <0.0001 | <0.0001 |
| EAAs /TAAs (%) | 32±6 | 33.7±3.2 | 0.003 | 0.0048 |
| BCAAs /TAAs (%) | 15±4 | 16.3±2.7 | 0.020 | 0.0270 |
| BCAAs /EAAs (%) | 47±4 | 48±5 | 0.46 | 0.50 |
Data are expressed as mean ±standard deviation (SD)
p-values are taken from the Mann-Whitney U test; FDR-adjusted p value: p values for false discovery rate controlled at 5% using the Benjamini-Hochberg method
The only AAs that were similar in patients and controls were Trp, arginine and glutamine (Gln).
As a consequence of AA perturbations, plasma levels of Total AAs (TAAs), EAAs, BCAAs in addition to EAA/TAA and BCAA/TAA ratios were higher in stroke subjects than in controls.
Relationships between plasma AA and circulating Alb/Hb
Table 3 reports the correlations between plasma AAs and circulating Alb/Hb in post absorptive stroke subjects.
Table 3. Correlations between circulating albumin and haemoglobin levels and plasma amino acid concentrations in post absorptive patients.
| Variables | Albumin | P value | FDR-adjusted p value | Variables | Haemoglobin | P value | FDR-adjusted p value |
|---|---|---|---|---|---|---|---|
| Tryptophan | 0.533 | <0.0001 | <0.0001 | Histidine | 0.47 | <0.0001 | <0.0001 |
| Histidine | 0.53 | <0.0001 | <0.0001 | Essential Amino Acids (EAAs) | 0.47 | <0.0001 | <0.0001 |
| Total Amino Acids (TAAs) | 0.33 | 0.0002 | 0.0011 | Branched Chain Amino Acids (BCAAs) | 0.42 | <0.0001 | <0.0001 |
| Serine | 0.31 | 0.0004 | 0.0015 | Total Amino Acids (TAAs) | 0.39 | <0.0001 | <0.0001 |
| Alanine | 0.31 | 0.0004 | 0.0015 | Leucine | 0.36 | <0.0001 | <0.0001 |
| Essential Amino Acids (EAAs) | 0.29 | 0.0009 | 0.0028 | 0.34 | <0.0001 | <0.0001 | <0.0001 |
| Glutamine | 0.27 | 0.003 | 0.0083 | Valine | 0.32 | 0.0002 | 0.0006 |
| Leucine | 0.26 | <0.0001 | 0.0001 | Lysine | 0.32 | 0.0002 | 0.0006 |
| Asparagine | 0.24 | 0.01 | 0.015 | Glutamine | 0.29 | 0.0009 | 0.0022 |
| Threonine | 0.24 | 0.006 | 0.013 | Alanine | 0.26 | 0.003 | 0.0066 |
| Valine | 0.24 | 0.008 | 0.015 | Cytrulline | 0.23 | 0.009 | 0.018 |
| Lysine | 0.24 | 0.006 | 0.013 | Ornithine | 0.22 | 0.01 | 0.018 |
| Branched Chain Amino Acids (BCAAs) | 0.24 | 0.008 | 0.015 | Serine | 0.21 | 0.02 | 0.034 |
| Arginine | 0.22 | 0.01 | 0.015 | Aspartic Acid | 0.19 | 0.03 | 0.044 |
| Cytrulline | 0.22 | 0.01 | 0.015 | Glutamic Acid | 0.19 | 0.03 | 0.044 |
| Isoleucine | 0.18 | 0.04 | 0.055 | Asparagine | 0.18 | 0.05 | 0.065 |
| Aspartic Acid | 0.15 | 0.09 | 0.14 | Threonine | 0.17 | 0.06 | 0.07 |
| Methionine | 0.15 | 0.1 | 0.12 | Tyrosine | 0.17 | 0.06 | 0.07 |
| Ornithine | 0.14 | 0.12 | 0.14 | Methionine | 0.17 | 0.05 | 0.06 |
| Tyrosine | 0.11 | 0.21 | 0.23 | Glycine | 0.16 | 0.07 | 0.08 |
| Glutamic Acid | 0.09 | 0.3 | 0.31 | Arginine | 0.05 | 0.6 | 0.63 |
| Phenylalanine | -0.08 | 0.36 | 0.3600 | Phenylalanine | -0.01 | 0.9 | 0.9 |
Data are expressed as mean ±standard deviation (SD)
p-values are from the Mann-Whitney U test; FDR-adjusted p value: p values for false discovery rate controlled at 5% using the Benjamini-Hochberg method
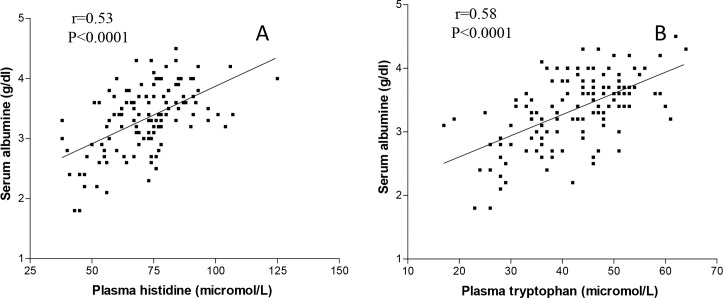
Alb was positively, significantly correlated with all the measured AAs except for aspartic acid, methionine, ornhitine, tyrosine, glutamic acid and phenylalanine. The most important correlations of Alb were with Trp (r = +0.533, p<0.0001) and His (r = +0.53, p<0.0001) (Fig 1 panel A and B). The magnitude of the correlations increased when the two AAs were combined (r = +0.60, p<0.0001).
Fig 1.
Correlations between plasma tryptophan (panel A), plasma histidine (panel B) and serum albumin.
The correlation between Trp and Alb remained significant even when the levels of Alb were normalised for the levels of CRP (Alb/CRP) (r = +0.426, p<0.0001).
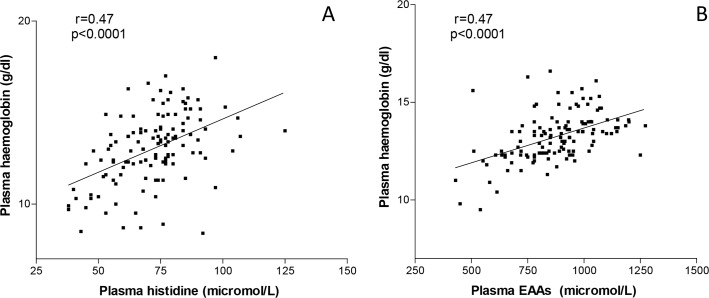
Circulating Hb was positively, significantly associated with 77.4% of plasma AAs and was not correlated with Threonine, tyrosine, glycin, arginine or phenylalanine. The best correlations were with His (r = +0.47, p<0.0001) and EAAs (r = +0.42, p<0.0001) (Fig 2 panel A and B).
Fig 2.
Correlations between plasma histidine (panel A), plasma essential amino acids (panel B) and plasma haemoglobin.
In multivariate analysis (Table 4), the AA Trp, His (positive association) and phenylalanine (inverse association) were found to be independent predictors of Alb (p<0.0001, p = 0.016 and p = 0.0001, respectively) while Glutamine (p = 0.006, direct association), asparagine (p = 0.006, negative association), Glycine (p = 0.009, negative association) and Aspartic acid (p = 0.03, positive association) were found to be the only independent predictors of Hb.
Table 4. Multivariate analysis showing the best amino acid positive and negative predictors of albumin and respectively haemoglobin in the stroke patients.
| Variables | Albumin t value |
p value | Variables | Haemoglobin t value |
p value |
|---|---|---|---|---|---|
| Tryptophan | 5.08 | <0.0001 | Glutamine | 2.8 | 0.006 |
| Histidine | 2.46 | 0.01 | Asparagine | -2.81 | 0.006 |
| Phenylalanine | -4.01 | 0.0001 | Glycine | -2.68 | 0.009 |
| Aspartic Acid | 2.23 | 0.03 |
To highlight the distributions of patient characteristics in relation to the most relevant positive predictors, we stratified the population for Trp≤35μmol/L (Trp ≤35) and Trp >35μmol/L (Trp >35) and respectively for Gln≤500 μmol/L (Gln ≤500) and Gln>500 μmol/L (Gln >500), Trp = 35 and Gln = 500 being thresholds obtained by rounding the values of the lower quartiles.
Table 5 reports the distribution of circulating Alb and Hb in relation to the Trp and respectively Gln cut-off points.
Table 5. Distribution of normoalbuminemic and hypoalbuminemic stroke patients in relation to plasma tryptophan (Trp) levels, and of anaemic patients in relation to plasma glutamine (Gln) concentrations.
The concentrations of the amino acids are expressed as μmol/L.
| Albumin ≥3.5 |
Albumin <3.5 |
|
|---|---|---|
| *Tryptophan >35 | 51 (92.7%) | 42 (60.9%) |
| Tryptophan ≤35 | 4 (7.3%) | 27 (39.1) |
| No anaemia | Anaemia | |
| °Glutamine >500 | 68 (81.9%) | 29 (69%) |
| Glutamine ≤500 | 15 (18.1%) | 13 (31%) |
* χ 2 test p<0.0001
°χ 2 test: p = 0.1
After stratification (Table 6), subjects with Trp ≤35 (25% of the population), in comparison to subjects with Trp >35, showed significant loss (7%; p = 0.002) of pre-event body weight, lower protein-energy intakes, lower serum levels of negative proteins of the acute phase response (Alb, preralbumin, transferrin) but a higher inflammatory state (CRP, p = 0.002; α1 globulin, p = 0.004) and physical disability.
Table 6. Differences in anthropometric, biohumoral, functional status and nutritional intakes of stroke patients after stratifications in the groups with plasma tryptophan levels >35 and respectively ≤35 μmol/L.
| Variables | Tryptophan >35 (n = 94) | Tryptophan ≤ 35 (n = 31) | p value | FDR-adjusted p value |
|---|---|---|---|---|
| Anthropometric | ||||
| Body mass index (BMI) (Kg/m2) | 26.02±4.30 | 24.42±5.17 | 0.09 | 0.15 |
| Actual/pre-event BW (%) | 98±0.6 | 93±0.1 | 0.002 | 0.007 |
| Blood | ||||
| ESR 1st hr (mm) | 32.05±26.13 | 52±36.85 | 0.001 | 0.007 |
| Haemoglobin (g/dl) | 13.32±1.81 | 12.10±2.04 | 0.002 | 0.007 |
| Blood urea (mg/dl) | 40.05±18.62 | 41.35±26.96 | 0.76 | 0.80 |
| Serum creatinine (mg/dl) | 0.98±0.27 | 0.95±0.33 | 0.70 | 0.80 |
| Plasma glucose (mg/dl) | 109.85±36.35 | 110.26±37.32 | 0.96 | 0.9600 |
| α1 globulin (g/dl) | 0.21±0.05 | 0.24±0.05 | 0.004 | 0.012 |
| Fibrinogen (mg/dl) | 383.25±93.91 | 408.28±106.19 | 0.23 | 0.35 |
| Serum albumin (g/dl) | 3.49±0.48 | 2.88±0.51 | <0.0001 | 0.0002 |
| Serum prealbumin (mg/dl) | 21.77±5.47 | 19±6.26 | 0.022 | 0.046 |
| Serum transferrin (mg/dl) | 213.73±47.39 | 178.17±41.57 | 0.0004 | 0.004 |
| Serum C-reactive protein (CRP) (mg/dl) | 0.99±1.47 | 2.54±3.99 | 0.002 | 0.007 |
| White cell counts (mm3) | 6732.77±1951.1 | 6476.77±1994.25 | 0.53 | 0.70 |
| Neutrophils (%) | 59.51±9.96 | 61.55±11.10 | 0.34 | 0.48 |
| Lymphocytes (%) | 28.26±9.29 | 25.36±10.09 | 0.74 | 0.80 |
| Function | ||||
| FIM (score) | 62.85±28.30 | 49.42±24.98 | 0.020 | 0.046 |
| Daily nutritional intake | ||||
| Energy | ||||
| Kcal/d | 1760±275 | |||
| Kcal/Kg | 23.9±3.72 | 19.5±2.05 | <0.05 | 0.076 |
| Proteins | ||||
| g/d | 78.2±12.1 | 54.5±9.8 | ||
| g/Kg | 1.06±0.15 | 0.78±0.13 | <0.02 | 0.026 |
| Lipids | ||||
| g/d | 53.9±15.9 | |||
| g/Kg | 0.8±0.22 | 0.65±0.09 | 0.6 | 0.74 |
| Carbohydrates | ||||
| g/d | 228.2±56.7 | 183.9±33.5 | ||
| g/Kg | 3.08±1.1 | 2.64±0.14 | 0.07 | 0.12 |
Data are expressed as mean ±standard deviation (SD)
p-values are from the unpaired Student t-test; FDR-adjusted p value: p values for false discovery rate controlled at 5% using the Benjamini-Hochberg method
FIM = functional independence measure; ESR: erythrocyte sedimentation rate
Subjects with Gln ≤500 (Table 7, 22.4% of the population), compared to those with Gln >500 displayed lower Hb, Alb and transferrin concentrations but a higher inflammatory state (high CRP, ESR, α1 globulin, fibrinogen, white cell counts, neutrophils %). Lymphocytes % were lower in the Gln<500 group (p = 0.04). The rates of lost pre-event body weight (in 22.4% of the patients) and nutrient intakes (Table 7A) did not vary between the two groups.
Table 7. Differences in anthropometric, biohumoral, functional status and nutritional intakes of stroke patients after stratifications in the groups with plasma glutamine levels >500 and respectively ≤500 μmol/L.
| Variables | Glutamine >500 (n = 97) | Glutamine ≤ 500 (n = 28) | p value | FDR-adjusted p value |
|---|---|---|---|---|
| Anthropometric | ||||
| Body mass index (BMI) (Kg/m2) | 25.62±4.42 | 25.62±5.12 | 0.09 | 0.12 |
| Actual/pre-event BW (%) | 97±0.8 | 96±0.6 | 0.49 | 0.54 |
| Blood | ||||
| ESR 1st hr (mm) | 30.45±25.11 | 58.75±35.76 | <0.0001 | 0.0002 |
| Haemoglobin (g/dl) | 13.27±1.86 | 12.14±1.94 | 0.006 | 0.031 |
| Blood urea (mg/dl) | 39.74±18.04 | 42.57±28.98 | 0.53 | 0.56 |
| Serum creatinine (mg/dl) | 0.97±0.28 | 0.98±0.29 | 0.83 | 0.83 |
| Plasma glucose (mg/dl) | 108.45±36.40 | 115.04±36.82 | 0.40 | 0.47 |
| α1 globulin (g/dl) | 0.21±0.04 | 0.24±0.06 | 0.008 | 0.033 |
| Fibrinogen (mg/dl) | 376.2±89.3 | 437.1±110.8 | 0.005 | 0.031 |
| Serum albumin (g/dl) | 3.41±0.53 | 3.08±0.57 | 0.005 | 0.031 |
| Serum prealbumin (mg/dl) | 21.57±5.60 | 19.41±6.16 | 0.09 | 0.12 |
| Serum transferrin (mg/dl) | 209.56±50.04 | 188.26±38.73 | 0.044 | 0.09 |
| Serum C-reactive protein (CRP) (mg/dl) | 1.12±2.35 | 2.25±2.58 | 0.031 | 0.08 |
| White cell counts (mm3) | 6431.55±1915.17 | 7492.86±1908.19 | 0.011 | 0.038 |
| Neutrophils (%) | 58.95±10.37 | 63.71±9.04 | 0.030 | 0.08 |
| Lymphocytes (%) | 28.47±9.68 | 24.30±8.39 | 0.041 | 0.09 |
| Function | ||||
| FIM (score) | 61.96±27.37 | 51.04±29.10 | 0.069 | 0.11 |
| Daily nutritional intake | ||||
| Energy | ||||
| Kcal/d | 1670±177 | 1460±195 | ||
| Kcal/Kg | 22.9±3.51 | 20.3±2.69 | 0.08 | 0.11 |
| Proteins | ||||
| g/d | 70.5±12.5 | 61.9±10.9 | ||
| g/Kg | 0.97±0.18 | 0.86±0.15 | 0.09 | 0.12 |
| Lipids | ||||
| g/d | 60.3±11.6 | 44.5±8.6 | ||
| g/Kg | 0.83±0.16 | 0.62±0.12 | 0.1 | 0.12 |
| Carbohydrates | ||||
| g/d | 219.3±48 | 192.7±42.2 | ||
| g/Kg | 3.07±0.66 | 2.68±0.55 | 0.07 | 0.11 |
Data are expressed as mean ±standard deviation (SD)
p-values are from the unpaired Student t-test; FDR-adjusted p value: p values for false discovery rate controlled at 5% using the Benjamini-Hochberg method
FIM = functional independence measure; ESR: erythrocyte sedimentation rate
In summary, the patients with low Trp and those with low Gln compared to their respective counterparts shared low circulating Hb, Alb, Transferrin and higher inflammation. In addition, we observed that impaired nutrition and neurocognitive dysfunction were only present in subjects with low Trp, whereas an unbalanced immune response consisting of high innate immune activity and a low adaptive response characterised stroke subjects with low Gln.
Discussion
The study shows that fasting stroke patients may have an increased amount of plasma AAs whose concentrations (for the majority) were correlated with circulating Alb and Hb levels. The study also found that plasma Trp and Gln were the best positive predictors of Alb and Hb, respectively, but rejects our original hypothesis that isoleucine could predict Hb.
Patient characteristics
Increased plasma levels of 71.4% of AAs in stroke patients may seem paradoxical given their persisting inflammation and inadequate nutritional intakes [37,38], and even more so when considering the high EAA concentrations, including BCAAs, which is the main fuel for skeletal muscles. However, plasma AA may actually reflect an increased AA release from hypercatabolic skeletal muscle tissue. The following considerations may suggest the presence of muscle hypercatabolism. Firstly, loss of body weight, insufficient nutrient intakes, inflammation and hypoalbuminemia are all highly suggestive of general body hypercatabolism. Secondly, the patients’ high levels of the AAs that are not metabolised by muscle, such as phenylalanine, tyrosine, lysine and methionine, are indicative of a state of muscle hypercatabolism. A previous investigation showed that the ipsilateral arm (= unaffected) in stroke patients had a net release of phenyalanine [9]. Thirdly, high serum CRP suggests increased Interleukine 6 production [44], which promotes muscle protein breakdown by several mechanisms including both inhibition of muscle protein synthesis, repair, contractility and function [45], and stimulation of the hypothalamus-pitituary-cortical axis leading to increased cortisol production. Lastly, high EAA/TAA and BCAA/TAA ratios observed in the study patients were previously reported in cancer individuals with cachexia [46,47].
The association of hypoalbuminemia and high plasma AAs, as observed in the current study, is a different condition from that of starved subjects in whom serum Alb is spared due to increased AA release from skeletal muscle [28]. This suggests that inflammation in fasting stroke patients may divert the majority of circulating AAs from Alb synthesis to the synthesis of positive proteins of acute-phase response [48] and other body districts, especially immune cells [49].
Low plasma levels of aspartic acid, glutamic acid and asparagine probably reflect their body overconsumption as intermediates of cell tricarboxylic chain acid cycle. Inflammation and oxidative stress [50,51] may be contributing factors to low taurine concentrations.
Inflammation, accelerated Alb distribution from the intravascular space [52] and increased degradation following acute metabolic stress after the vascular event can explain the low level of circulating Alb. In experimental animals, a single injection of the cytokine TNF alfa decreased serum Alb concentrations [53]. In a context of metabolic perturbation, the inadequacy of protein-energy intakes contributes to patient hypoalbuminemia.
The stratification of the patients in terms of Trp levels highlighted the negative effects of inflammation on patient nutritional-, metabolic- and functional variables. The subjects with low Trp, compared to those with normal Trp, had a higher inflammation rate associated with lower serum levels of negative proteins of the acute phase response, lower protein-energy intakes, higher loss of body weight and more severe physical disability. Given that Trp is an EAA, its reduced levels were due to an increased body overconsumption/protein intake ratio.
Hypoalbuminemia is an important risk factor for poorer rehabilitative outcomes given that it is associated with increased serum cortical levels, which potentially stimulate muscle breakdown, particularly in immobilised individuals [54], like the study patients. On the contrary, normal Alb levels favour patient autonomy recovery not only because the protein may enhance neurocognitive recovery [16] but also because it reduces the risk of sarcopenia.
Correlations between plasma AAs and circulating Alb
The study found that the majority of plasma AAs were associated with circulating Alb levels and that Trp and, to a lesser extent, His were predictors of the circulating protein.
The observational nature of the study and correlations do not allow us to infer a cause-effect relationship between AAs and Alb. Yet we postulate that the levels of plasma AAs, primarily Trp and His, could contribute to maintaining Alb synthesis during night fasting. The following factors may support this hypothesis. Firstly, during fasting, Alb synthesis [33] is not abolished, although it occurs at a reduced rate [55–57]. Secondly, Trp availability is essential for Alb synthesis [35]. The biosynthesis of Alb in fasting rabbits [35] was rapidly stimulated by adding Trp to perfusate. In this experiment, the Trp-associated Alb synthesis rate (+138%) was impressive when compared to isoleucine-associated Alb synthesis (+89%), and even more so when compared to a mixture of AAs that did not contain Trp and isoleucine (no increase of Alb synthesis). On the other hand, dietary Trp deficiency in rats inhibits liver synthesis [24]. It is worth noting that the correlation of Trp and Alb remained significant within normal ranges of Trp levels, thus indicating their continuous close connection.
The rate of Trp/Alb associations was higher when Alb was expressed as absolute values rather than when it was normalised for the degree of inflammation (Alb/CRP). This may indicate that the link is even more significant under inflammatory stress that in non-inflammatory conditions. This would confirm that during acute metabolic stress, Alb synthesis increased even though it was at a lower rate than that of the acute phase response [58].
Our hypothesis on Trp contributing to Alb synthesis during nocturnal hours is in contrast to the general belief [22] that during the post absorptive period, Alb catabolism may release EAAs for muscle and other tissue proteins. Bearing in mind that we are in the theoretical field, we think that our hypothesis may be more biologically plausible. Under normal conditions, the increase in Trp levels depends on a decrease in serum Alb and/or an increase in Non-Esterified Fatty Acids (NEFAs) [59], which displace Trp from Alb [29]. In the current study, this was not the case as both low and normal Trp were associated with normal and low Alb.
In addition, if plasma Trp levels in the study were due to Alb dismission of the AA, a negative correlation between the two substances would have been found.
A possible Trp displacement from Alb by NEFAs was unlikely, given that plasma triglyceride and glucose concentrations were normal, thus excluding increases in plasma NEFA levels.
Plasma HIS was shown to be predictive of circulating Alb. Again, there seems to be a cause-effect relationship, given that in the case of a His-deficient diet, whole-body protein turnover decelerates [60] and circulating Alb decreases [61]. This suggests that endogenous [62] synthesis and body store (Hb, carnosine) are not enough to preserve the levels of visceral proteins [61].
The EAA phenyalanine was shown to be a strong negative predictor of Alb. Given that plasma phenyalanine indicates muscle hypercatabolism, the study suggests that muscle hypercatabolism may predict the occurrence of reduced Alb mass. This is in line with the positive relationship between Alb and muscle mass observed in elderly patients, like the study patients [63].
Correlations between plasma AAs and circulating Hb
More than 77% of plasma AAs in post absorptive stroke patients were positively associated with circulating Hb levels. Plasma Gln levels were positive predictors of Hb, whereas asparagine and glycin were negative predictors.
The provision of AAs to bone marrow is essential for haemopoiesis. Optimal AA availability influences haemopoiesis both directly by promoting protein synthesis in reticulocytes, which are supplied with haeme [34,64,65], and indirectly by influencing erythropoietin production [30]. In anaemic rats, haemopoiesis was significantly stimulated by isoleucine [36] and this AA was essential for the recovery of Hb and erythrocytes even though Hb does not contain isoleucine. These metabolic activities of AAs can explain the links between AAs and circulating Hb found in the present study.
Trp also contributed to haemopoiesis in anaemic rats [36] The positive correlation between plasma His and circulating Hb indirectly confirms previous investigations that documented the importance of His for haemopoiesis. Indeed, His-deficient regimen brings about decreases in Hb concentrations haematocrit, red blood cells [60] by causing the reduction in Hb synthesis [66] and/or increase in Hb degradation [67].
The rate of correlations between EAAs and Hb was higher than the rate of correlations between EAAs and Alb. The huge processes of protein synthesis in bone marrow that are necessary for the formation, replication and differentiation of progenitor cells of erythrocytes as well as the haemoglobinisation of red cells are likely to account for the great need for EAAs.
The results reject our hypothesis of isoleucine as a predictor of Hb. The profound differences between previous experimental studies and the current investigation carried out in patients with metabolic alterations may explain the discrepancy. Glutamine, on the other hand, was shown to be the best positive predictor for circulating Hb. The predictive value of Gln may rely on its vast array of metabolic activities, including the nitrogen provision for the other AAs [68], the essential role in gluconeogenesis providing energy [68], the correction of intracellular redox by enhancing the NAD/NADH ratios [69–71] which are particularly relevant to haemopoiesis.
A mixture of Gln + BCAA + arginine increased Hb, erythrocyte count, haematocrit and serum iron [72]. Gln can protect mammalian erythrocytes against oxidative stress and apoptosis [73]. The metabolites of Gln such as alanine, citrulline and proline effectively protect erythrocytes by suppressing the generation of free radicals, release of cytokine C, activation of caspase-3, caspase-8 and caspase-9. Gln normalises red cell morphology and reduces red cell adhesion in subjects with sickle cell disease [74]. Low erythrocytes contents in Gln and glutathione contribute to an altered redox state of the cell and to haemolysis [75].
Low plasma Gln in stroke subjects (22.4%) was likely due to stroke-primed metabolic stress and not to insufficient protein intakes, given that patients with low and normal Gln levels were similar in daily protein intakes.
The study suggests that in the case of high plasma asparagine and/or glycin, the Hb levels could be impaired. The negative roles of these AAs on Hb are not clear. An excess of asparagine could react with glucose and fructose, leading to Hb glycation end products and post-translational modification of Hb [76]. In the present study, however, asparagine levels were lower in stroke subjects than in controls. This may be due to body overconsumption and/or reduced asparagine synthase expression, the enzyme responsible for the biosynthesis of asparagine from aspartate and glutamine [77].
With respect to glycin, it is of interest that while glutamine increases protein synthesis, an isonitrogenous amount of glycine decreases protein turnover [78]. This may contribute to explaining the inverse correlation between glycin and Hb. The high value of plasma glycin found in stroke subjects was probably due to muscle release following protein overdegradation [9].
Inflammation was likely to have been the main reason for the anaemia that was encountered in one third of the study patients. Indeed, TNF alfa and IL-6 can suppress erythropoiesis and the action of erythropoietin [79], and impair the mobilisation of iron from macrophages [80]. Deficiencies in dietary intakes of iron, vitamin B and phosphorus, as observed in subjects with low Gln, could have contributed to anaemia. Even though it was not measured in the study, low serum vitamin D may contribute to anaemia, particularly during inflammation [81,82].
It is of interest that in the study, low plasma Trp was associated with patient nutrition decline, whereas low Gln was linked to immunological alterations. This confirms that Trp is an important anabolic AA [35,36] and that Gln is essential to immune cell functions [83,84].
Conclusion
The study shows that subacute stroke subjects in a post absorptive state may have altered levels of plasma amino acids, the majority of which are positively associated with circulating Alb and Hb levels. Plasma Trp and glutamine were the best positive amino acid predictors of circulating Alb and Hb, respectively. isoleucine levels are not predictors of circulating Hb.
Future studies
The current study is a hypothesis-generating work.
A well-planned investigation could test the hypothesis of improving circulating Alb and Hb in stroke subjects during nocturnal hours by selectively elevating the plasma concentrations of Trp, His and Gln concentrations. This may be of particular importance for stroke individuals with hypoalbuminemia and/or anaemia, particularly within 30 days of the acute event, for at least three reasons. Firstly, during the time in which the majority of neurocognitive recovery occurs, elevating circulating Alb and Hb may be an additive value for the brain dysfunction recovery [10,16]. Indeed, adequate nutrition support takes weeks or months to elevate serum Alb concentration [18]. Secondly, frequently stroke subjects with an oral diet have deficient nutrition intakes [37,38], leading to a reduction of meal-stimulated Alb/Hb synthesis. Thirdly, Trp and His are precursors of the brain neurotransmitters serotonin and histamine, respectively, which are important for motor, cognitive and mood recoveries in stroke patients.
Given the clinical importance of normal Alb and Hb values, there is a need to investigate the relationships between the proteins and circulating amino acids in diseases other than stroke.
Limits of the study
In this study, we excluded patients with diabetes, chronic kidney disease, chronic obstructive pulmonary disease, hepatic cirrhosis, cancer and all diseases associated with important plasma AA abnormalities. Given the high prevalence of some of these diseases, future studies should address the relationship between plasma AAs and Alb in a context of pre-stroke plasma AA alterations.
Patients ingestions of the AAs were not calculated because not all the enteral formulas that were used in our study reported the AA composition.
The study was carried out in a single Rehabilitation Centre. A polycentric investigation may provide more information, particularly in relation to the setting of provenience of the patients (neuro intensive care unit, stroke unit, neurological departments). Moreover, this led to a relatively small sample size of the healthy control group since only a few subjects underwent the complete set of assays defined in our protocol. However, only type II error rate should be affected, with a potential reduction of statistical power.
Evaluating patients’ body composition would have strengthened the discussion, especially in relation to plasma AAs.
Suggestions for clinical practice
Even though it is of observational nature, the study provides some information which may improve nutritional/metabolic treatments of subacute stroke subjects. Keeping in mind that the optimisation of the nutrition of stroke patients within 30 days from the acute event is of paramount importance for neurocognitive recovery, it is necessary to calculate the adequacy of nutrient intakes in all stroke subjects, particularly in self-feeding subjects. Moreover, one should consider that actual nutritional adequacy might not be synonymous with normal plasma AAs, particularly in inflamed subjects. On the basis of this study and given that plasma AAs are not routinely measured, it may be convenient to suspect low Trp in stroke individuals with inflammation and >5% loss of pre-event body weight.
The corrections of inadequate nutrition are relevant to subjects suffering from stroke, not only to enhance neurocognitive retrieval early during the post-acute period, but also to continuously improve overtime rehabilitation outcomes. Indeed, these patients 6 months after a stroke may have substantial energy and protein deficits [38].
Supporting information
(PDF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Abbott R.D.; Donahue R.P.; MacMahon S.W.; Reed D.M.; Yano K. Diabetes and the risk of stroke. The Honolulu Heart Program. JAMA.1987, February 20; 257(7):949–52. [PubMed] [Google Scholar]
- 2.Mathers C.D.; Vos E.T.; Stevenson C.E.; Begg S.J. The Australian Burden of Disease Study: measuring the loss of health from diseases, injuries and risk factors. Med J Aust. 2000. June 19;172(12):592–6. [DOI] [PubMed] [Google Scholar]
- 3.Scholte O.P.; Reimer W.J.; de Haan R.J.; Rijnders P.T.; Limburg M.; van den Bos G.A. The burden of caregiving in partners of long-term stroke survivors. Stroke 1998. August;29(8):1605–11. 10.1161/01.str.29.8.1605 [DOI] [PubMed] [Google Scholar]
- 4.Hata R.; Maeda K.; Hermann D.; Mies G.; Hossmann K.A. Dynamics of regional brain metabolism and gene expression after middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000. February;20(2):306–15. 10.1097/00004647-200002000-00012 [DOI] [PubMed] [Google Scholar]
- 5.Lee J.M.; Zipfel G.J.; Park K.H.; He Y.Y.; Hsu C.Y.; Choi DW. Zinc translocation accelerates infarction after mild transient focal ischemia. Neuroscience 2002;115(3):871–8. 10.1016/s0306-4522(02)00513-4 [DOI] [PubMed] [Google Scholar]
- 6.Aquilani R.; Scocchi M.; Iadarola P.; Viglio S.; Pasini E.; Condello S.; et al. Spontaneous neurocognitive retrieval of patients with sub-acute ischemic stroke is associated with dietary protein intake. Nutr Neurosci. 2010. June;13(3):129–34. 10.1179/147683010X12611460764002 [DOI] [PubMed] [Google Scholar]
- 7.Aquilani R.; Scocchi M.; Iadarola P.; Franciscone P.; Verri M.; Boschi F.; et al. Protein supplementation may enhance the spontaneous recovery of neurological alterations in patients with ischaemic stroke. Clin Rehabil. 2008. December;22(12):1042–50. 10.1177/0269215508094244 [DOI] [PubMed] [Google Scholar]
- 8.Aquilani R.; Baiardi P.; Scocchi M.; Iadarola P.; Verri M.; Sessarego P.; et al. Normalization of zinc intake enhances neurological retrieval of patients suffering from ischemic strokes. Nutr Neurosci. 2009. Oct;12(5):219–25. [DOI] [PubMed] [Google Scholar]
- 9.Aquilani R.; Boselli M.; D'Antona G.; Baiardi P.; Boschi F.; Viglio S.; et al. Unaffected arm muscle hypercatabolism in dysphagic subacute stroke patients: the effects of essential amino acid supplementation. Biomed Res Int. 2014; 2014:964365 10.1155/2014/964365 Epub 2014. November 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belayev L.; Busto R.; Zhao W.; Clemens J.A.; Ginsberg M.D. Effect of delayed albumin hemodilution on infarction volume and brain edema after transient middle cerebral artery occlusion in rats. J Neurosurg. 1997. October;87(4):595–601. 10.3171/jns.1997.87.4.0595 [DOI] [PubMed] [Google Scholar]
- 11.Belayev L.; Zhao W.; Pattany P.M.; Weaver R.G.; Huh P.W.; Lin B.; et al. Diffusion-weighted magnetic resonance imaging confirms marked neuroprotective efficacy of albumin therapy in focal cerebral ischemia. Stroke. 1998. December;29(12):2587–99. 10.1161/01.str.29.12.2587 [DOI] [PubMed] [Google Scholar]
- 12.Belayev L.; Liu Y.; Zhao W.; Busto R.; Ginsberg M.D. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001. February;32(2):553–60. 10.1161/01.str.32.2.553 [DOI] [PubMed] [Google Scholar]
- 13.Yamasaki Y.; Matsuura N.; Shozuhara H.; Onodera H.; Itoyama Y.; Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995. April;26(4):676–80; discussion 681. 10.1161/01.str.26.4.676 [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg M.D.; Zhao W.; Belayev L.; Alonso O.F.; Liu Y.; Loor J.Y.; et al. Diminution of metabolism/blood flow uncoupling following traumatic brain injury in rats in response to high-dose human albumin treatment. J Neurosurg. 2001. March;94(3):499–509. 10.3171/jns.2001.94.3.0499 [DOI] [PubMed] [Google Scholar]
- 15.Boselli M.; Aquilani R.; Baiardi P.; Dioguardi F.S.; Guarnaschelli C.; Achilli M.P.; et al. Supplementation of essential amino acids may reduce the occurrence of infections in rehabilitation patients with brain injury. Nutr Clin Pract. 2012. February;27(1):99–113. 10.1177/0884533611431068 [DOI] [PubMed] [Google Scholar]
- 16.Aquilani R.; Boselli M.; Baiardi P.; Pasini E.; Iadarola P.; Verri M.; et al. Is stroke rehabilitation a metabolic problem? Brain Inj. 2014;28(2):161–73. 10.3109/02699052.2013.860470 [DOI] [PubMed] [Google Scholar]
- 17.Aquilani R.; Zuccarelli G.C.; Condino A.M.; Catani M.; Rutili C.; Del Vecchio C.; et al. Despite Inflammation, Supplemented Essential Amino Acids May Improve Circulating Levels of Albumin and Haemoglobin in Patients after Hip Fractures. Nutrients. 2017. June 21;9(6). E637 10.3390/nu9060637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendez C.M.; McClain C.J.; Marsano L.S. Albumin therapy in clinical practice. Nutr Clin Pract. 2005. June;20(3):314–20. 10.1177/0115426505020003314 [DOI] [PubMed] [Google Scholar]
- 19.De Feo P.; Horber F.F.; Haymond M.W. Meal stimulation of albumin synthesis: a significant contributor to whole body protein synthesis in humans. Am J Physiol. 1992. October;263(4 Pt 1):E794–9. [DOI] [PubMed] [Google Scholar]
- 20.Hunter K.A.; Ballmer P.E.; Anderson S.E.; Broom J.; Garlick P.J.; McNurlan M.A. Acute stimulation of albumin synthesis rate with oral meal feeding in healthy subjects measured with [ring-2H5]phenylalanine. Clin Sci (Lond). 1995. February;88(2):235–42. [DOI] [PubMed] [Google Scholar]
- 21.Volpi E.; Lucidi P., Cruciani G., Monacchia F., Reboldi G., Brunetti P., et al. Contribution of amino acids and insulin to protein anabolism during meal absorption. Diabetes. 1996. September;45(9):1245–52. 10.2337/diab.45.9.1245 [DOI] [PubMed] [Google Scholar]
- 22.Caso G.; Feiner J.; Mileva I.; Bryan L.J.; Kelly P.; Autio K.; et al. Response of albumin synthesis to oral nutrients in young and elderly subjects. Am J Clin Nutr. 2007. February;85(2):446–51. 10.1093/ajcn/85.2.446 [DOI] [PubMed] [Google Scholar]
- 23.Kirsch R.E.; Saunders S.J.; Frith L.; Wicht S.; Kelman L.; Brock J.F. Plasma amino acid concentration and the regulation of albumin synthesis. Am J Clin Nutr. 1969. December; 22(12):1559–62. 10.1093/ajcn/22.12.1559 [DOI] [PubMed] [Google Scholar]
- 24.Flaim K.E.; Peavy D.E.; Everson W.V.; Jefferson L.S. The role of amino acids in the regulation of protein synthesis in perfused rat liver. I. Reduction in rates of synthesis resulting from amino acid deprivation and recovery during flow-through perfusion. J Biol Chem. 1982. March 25;257(6):2932–8. [PubMed] [Google Scholar]
- 25.Flaim K.E.; Liao W.S.; Peavy D.E.; Taylor J.M.; Jefferson L.S. The role of amino acids in the regulation of protein synthesis in perfused rat liver. II. Effects of amino acid deficiency on peptide chain initiation, polysomal aggregation, and distribution of albumin mRNA. J Biol Chem. 1982. March 25;257(6):2939–46. [PubMed] [Google Scholar]
- 26.John D.W.; Miller L.L. Regulation of net biosynthesis of serum albumin and acute phase plasma proteins. Induction of enhanced net synthesis of fibrinogen, alpha1-acid glycoprotein, alpha2 (acute phase)-globulin, and haptoglobin by amino acids and hormones during perfusion of the isolated normal rat liver. J Biol Chem. 1969. November 25;244(22):6134–42. [PubMed] [Google Scholar]
- 27.Kelman L.; Saunders S.J.; Wicht S.; Frith L.; Corrigall A.; Kirsch R.E.; et al. The effects of amino acids on albumin synthesis by the isolated perfused rat liver. Biochem J. 1972. October;129(4):805–9. 10.1042/bj1290805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumgartner R.N.; Koehler K.M; Romero L.; Garry P.J. Serum albumin is associated with skeletal muscle in elderly men and women. Am J Clin Nutr. 1996. October;64(4):552–8. 10.1093/ajcn/64.4.552 [DOI] [PubMed] [Google Scholar]
- 29.Rothschild M.A.; Oratz M.; Schreiber S.S. Serum albumin. Hepatology. 1988. Mar-Apr;8(2):385–401. [DOI] [PubMed] [Google Scholar]
- 30.Anagnostou A.; Schade S.; Ashkinaz M.; Barone J.; Fried W. Effect of protein deprivation on erythropoiesis. Blood. 1977. December;50(6):1093–7. [PubMed] [Google Scholar]
- 31.Mogoş T.; Popescu C.; Dumitrescu A.; Tănase I.; Mincu I. The relationship between aminoaciduria and plasma hemoglobin levels. Rom J Intern Med. 1993. Jul-Sep;31(3):223–8. [PubMed] [Google Scholar]
- 32.Alabi J .; Ng'ambi J.W.; Mbajiorgu E.F.; Norris D.; Mabelebele M. Growth and haematological response of indigenous Venda chickens aged 8 to 13 weeks to varying dietary lysine to energy ratios. J Anim Physiol Anim Nutr (Berl). 2015. June;99(3):436–41. [DOI] [PubMed] [Google Scholar]
- 33.Yap S.H.; Strair R.K.; Shafritz D.A. Effect of a short term fast on the distribution of cytoplasmic albumin messenger ribonucleic acid in rat liver. Evidence for formation of free albumin messenger ribonucleoprotein particles. J Biol Chem. 1978. July 25;253(14):4944–50. [PubMed] [Google Scholar]
- 34.Austin S.A.; Clemens M.J. Control of the initiation of protein synthesis in mammalian cells. FEBS Lett. 1980. January 28;110(1):1–7. 10.1016/0014-5793(80)80009-3 [DOI] [PubMed] [Google Scholar]
- 35.Rothschild M.A.; Oratz M.; Mongelli J.; Fishman L.; Schreiber S.S. Amino acid regulation of albumin synthesis. J Nutr. 1969. August;98(4):395–403. 10.1093/jn/98.4.395 [DOI] [PubMed] [Google Scholar]
- 36.Chandran K.; Damodaran M. Amino-acids and proteins in haemoglobin formation. 2. Isoleucine. Biochem J. 1951. August;49(3):393–8. 10.1042/bj0490393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aquilani R.; Galli M.; Guarnaschelli C.; Fugazza G.; Lorenzoni M.; Varalda E.; et al. Prevalence of malnutrition and inadequate food intake in self-feeding rehabilitation patients with stroke. Europa Medicophysica 1999; 2:75–81 [Google Scholar]
- 38.Perry L. Eating and dietary intake in communication-impaired stroke survivors: a cohort study from acute-stage hospital admission to 6 months post-stroke. Clin Nutr. 2004. December;23(6):1333–43. 10.1016/j.clnu.2004.04.009 [DOI] [PubMed] [Google Scholar]
- 39.Chumlea W.C., Roche A.F., Steinbaugh M.L. Estimating stature from knee height for persons 60 to 90 years of age. Journal of the American Geriatric Society 1985;33:116–120 [DOI] [PubMed] [Google Scholar]
- 40.Aquilani R.; Tramarin R.; Pedretti R.F; Bertolotti G.; Sommaruga M.; Mariani P.; et al. Despite good compliance, very low fat diet alone does not achieve recommended cholesterol goals in outpatients with coronary heart disease. Eur Heart J. 1999. July;20(14):1020–9 10.1053/euhj.1999.1402 [DOI] [PubMed] [Google Scholar]
- 41.Carnevale, E.; Marletta, L.; and Istituto Nazionale di Ricerca per gli alimenti e la nutrizione. Tabelle di composizione degli alimenti, Istituto Superiore Nazionale della Nutrizione Roma, Italy 1989.
- 42.Quarta revisione dei livelli di assunzione di riferimento di nutrienti (LARN) e di energia per la popolazione italiana.
- 43.Benjamini Y, Yekutieli D. (2001) The control of the false discovery rate in multiple testing under dependency. The Annals of Statistics. 2001. 29(4), 1165–1188. [Google Scholar]
- 44.Deodhar S.D. C-reactive protein: the best laboratory indicator available for monitoring disease activity. Cleve Clin J Med. 1989. Mar-Apr;56(2):126–30. [DOI] [PubMed] [Google Scholar]
- 45.Zoico E.; Roubenoff R. The role of cytokines in regulating protein metabolism and muscle function. Nutr Rev. 2002. February;60(2):39–51. 10.1301/00296640260085949 [DOI] [PubMed] [Google Scholar]
- 46.Norton J.A.; Gorschboth C.M.; Wesley R.A.; Burt M.E.; Brennan M.F. Fasting plasma amino acid levels in cancer patients. Cancer. 1985. September 1;56(5):1181–6. [DOI] [PubMed] [Google Scholar]
- 47.Kitagawa M.; Haji S.; Amagai T. High Serum Essential Amino Acids as a Predictor of Skeletal Muscle Depletion in Patients With Cachexia and Advanced Gastrointestinal Cancers. Nutr Clin Pract. 2017. October;32(5):645–651. 10.1177/0884533617724742 [DOI] [PubMed] [Google Scholar]
- 48.Gabay C.; Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999. February 11;340(6):448–54. 10.1056/NEJM199902113400607 [DOI] [PubMed] [Google Scholar]
- 49.Roth E. Immune and cell modulation by amino acids. Clin Nutr. 2007. October;26(5):535–44. Epub 2007 Jun 27. 10.1016/j.clnu.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 50.Grimble R.F.; Jackson A.A.; Persaud C.; Wride M.J.; Delers F.; Engler R. Cysteine and glycine supplementation modulate the metabolic response to tumor necrosis factor alpha in rats fed a low protein diet. J Nutr. 1992. November;122(11):2066–73. 10.1093/jn/122.11.2066 [DOI] [PubMed] [Google Scholar]
- 51.Carubelli V.; Castrini A.I.; Lazzarini V.; Gheorghiade M.; Metra M.; Lombardi C. Amino acids and derivatives, a new treatment of chronic heart failure? Heart Fail Rev. 2015. January;20(1):39–51. 10.1007/s10741-014-9436-9 [DOI] [PubMed] [Google Scholar]
- 52.Rothschild M.A.; Oratz M.; Schreiber S.S. Extravascular albumin. N Engl J Med. 1979. August 30;301(9):497–8. 10.1056/NEJM197908303010909 [DOI] [PubMed] [Google Scholar]
- 53.Hennig B.; Honchel R.; Goldblum S.E.; McClain C.J. Tumor necrosis factor-mediated hypoalbuminemia in rabbits. J Nutr. 1988. December;118(12):1586–90. 10.1093/jn/118.12.1586 [DOI] [PubMed] [Google Scholar]
- 54.Ferrando A.A.; Stuart C.A.; Sheffield-Moore M.; Wolfe R.R. Inactivity amplifies the catabolic response of skeletal muscle to cortisol. J Clin Endocrinol Metab. 1999. October;84(10):3515–21. 10.1210/jcem.84.10.6046 [DOI] [PubMed] [Google Scholar]
- 55.Glass D.J. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol. 2003. February;5(2):87–90. 10.1038/ncb0203-87 [DOI] [PubMed] [Google Scholar]
- 56.Kirsch R.; Frith L.; Black E.; Hoffenberg R. Regulation of albumin synthesis and catabolism by alteration of dietary protein. Nature. 1968. February 10;217(5128):578–9. 10.1038/217578a0 [DOI] [PubMed] [Google Scholar]
- 57.Rothschild M.A.; Oratz M.; Mongelli J.; Schreiber S.S. Effects of a short-term fast on albumin synthesis studied in vivo, in the perfused liver, and on amino acid incorporation by hepatic microsomes. J Clin Invest. 1968. December;47(12):2591–9. 10.1172/JCI105941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Norberg Å.; Rooyackers O.; Segersvärd R.; Wernerman J. Leakage of albumin in major abdominal surgery. Crit Care. 2016. April 26;20(1):113 10.1186/s13054-016-1283-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Curzon G.; Friedel J.; Knott P.J. The effect of fatty acids on the binding of tryptophan to plasma protein. Nature. 1973. March 16;242(5394):198–200. 10.1038/242198a0 [DOI] [PubMed] [Google Scholar]
- 60.Young V.R.; Marchini J.S. Mechanisms and nutritional significance of metabolic responses to altered intakes of protein and amino acids, with reference to nutritional adaptation in humans. Am J Clin Nutr. 1990. February;51(2):270–89. 10.1093/ajcn/51.2.270 [DOI] [PubMed] [Google Scholar]
- 61.Kriengsinyos W.; Rafii M.; Wykes L.J.; Ball R.O.; Pencharz P.B. Long-term effects of histidine depletion on whole-body protein metabolism in healthy adults. J Nutr. 2002. November; 132(11):3340–8. 10.1093/jn/132.11.3340 [DOI] [PubMed] [Google Scholar]
- 62.Sheng Y.B.; Badger T.M.; Asplund J.M.; Wixom R.L. Incorporation of 15NH4Cl into histidine in adult man. J Nutr. 1977. April;107(4):621–30. 10.1093/jn/107.4.621 [DOI] [PubMed] [Google Scholar]
- 63.Visser M.; Kritchevsky S.B.; Newman A.B.; Goodpaster B.H.; Tylavsky F.A.; Nevitt M.C.; et al. Lower serum albumin concentration and change in muscle mass: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005. September;82(3):531–7. 10.1093/ajcn.82.3.531 [DOI] [PubMed] [Google Scholar]
- 64.Bruns G.P.; London I.M. The evìffect of hemin on the synthesis of globin. Biochem Biophys Res Commun. 1965. January 18;18:236–42. 10.1016/0006-291x(65)90746-1 [DOI] [PubMed] [Google Scholar]
- 65.Grayzel A.I.; Hörchner P., London I.M. The stimulation of globin synthesis by heme. Proc Natl Acad Sci U S A. 1966. March;55(3):650–5. 10.1073/pnas.55.3.650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nasset E.S.; Gatewood V.H. Nitrogen balance and hemoglobin of adult rats fed amino acid diets low in L- and D-histidine. J Nutr. 1954. June 10;53(2):163–76. 10.1093/jn/53.2.163 [DOI] [PubMed] [Google Scholar]
- 67.Kopple J.D.; Swendseid M.E. Evidence that histidine is an essential amino acid in normal and chronically uremic man. J Clin Invest. 1975. May;55(5):881–91. 10.1172/JCI108016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gündoğdu R.H.; Temel H.; Bozkırlı B.O.; Ersoy E.; Yazgan A.; Yıldırım Z. Mixture of Arginine, Glutamine, and β-hydroxy-β-methyl Butyrate Enhances the Healing of Ischemic Wounds in Rats. JPEN J Parenter Enteral Nutr. 2017. August;41(6):1045–1050. 10.1177/0148607115625221 [DOI] [PubMed] [Google Scholar]
- 69.Wilmore D.W. Food and Drug Administration Approval of Glutamine for Sickle Cell Disease: Success and Precautions in Glutamine Research. JPEN J Parenter Enteral Nutr. 2017. August;41(6):912–917. 10.1177/0148607117727271 [DOI] [PubMed] [Google Scholar]
- 70.Curi R.; Lagranha C.J.; Doi S.Q.; Sellitti D.F.; Procopio J.; Pithon-Curi T.C.; et al. Molecular mechanisms of glutamine action. J Cell Physiol. 2005. August;204(2):392–401. 10.1002/jcp.20339 [DOI] [PubMed] [Google Scholar]
- 71.Yang R.; Tan X.; Thomas A.M.; Steppacher R.; Qureshi N.; Morrison D.C.; Van Way C.W. 3rd. Alanine-glutamine dipeptide (AGD) inhibits expression of inflammation-related genes in hemorrhagic shock. JPEN J Parenter Enteral Nutr. 2007. Jan-Feb;31(1):32–6. 10.1177/014860710703100132 [DOI] [PubMed] [Google Scholar]
- 72.Ohtani M.; Maruyama K.; Sugita M.; Kobayashi K. Amino acid supplementation affects hematological and biochemical parameters in elite rugby players. Biosci Biotechnol Biochem. 2001. September;65(9):1970–6. 10.1271/bbb.65.1970 [DOI] [PubMed] [Google Scholar]
- 73.Li H.; Jiang W.; Liu Y.; Jiang J.; Zhang Y.; Wu P.; et al. The metabolites of glutamine prevent hydroxyl radical-induced apoptosis through inhibiting mitochondria and calcium ion involved pathways in fish erythrocytes. Free Radic Biol Med. 2016. March;92:126–140. 10.1016/j.freeradbiomed.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 74.Niihara Y.; Macan H.; Eckman J.R. L-glutamine therapy reduces hospitalization for sickle cell anemia and sickle ß-thalassemia patients at six months a phase II randomized trial. Clin Pharmacol Biopham 2014; 3:1–5. [Google Scholar]
- 75.Morris C.R.; Suh J.H.; Haga r W.; Larkin S.; Bland D.A.; Steinberg M.H.; et al. Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease. Blood. 2008. January 1;111(1):402–10. Epub 2007 Sep 11. 10.1182/blood-2007-04-081703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ioannou A.; Varotsis C. Modifications of hemoglobin and myoglobin by Maillard reaction products (MRPs). PLoS One. 2017. November 14;12(11):e0188095 10.1371/journal.pone.0188095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andrulis I.L., Chen J., Ray P.N. Isolation of human cDNAs for asparagine synthetase and expression in Jensen rat sarcoma cells. Mol Cell Biol. 1987. July;7(7):2435–43. 10.1128/mcb.7.7.2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hankard R.G.; Haymond M.W.; Darmaun D. Effect of glutamine on leucine metabolism in humans. Am J Physiol. 1996. October;271(4 Pt 1):E748–54. [DOI] [PubMed] [Google Scholar]
- 79.Soeters P.B. Rationale for albumin infusions. Curr Opin Clin Nutr Metab Care. 2009. May;12(3):258–64. Review. 10.1097/MCO.0b013e32832a3e1a [DOI] [PubMed] [Google Scholar]
- 80.Means R.T. Jr. Pathogenesis of the anemia of chronic disease: a cytokine-mediated anemia. Stem Cells.1995. January;13(1):32–7. 10.1002/stem.5530130105 [DOI] [PubMed] [Google Scholar]
- 81.Bacchetta J.; Zaritsky J.J., Sea J.L.; Chun R.F.; Lisse T.S.; Zavala K.; et al. Suppression of iron-regulatory hepcidin by vitamin D. J Am Soc Nephrol. 2014. March;25(3):564–72. 10.1681/ASN.2013040355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zughaier S.M.; Alvarez J.A.; Sloan J.H.; Konrad R.J.; Tangpricha V. The role of vitamin D in regulating the iron-hepcidin-ferroportin axis in monocytes J Clin Transl Endocrinol. 2014. Mar 21;1(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wischmeyer P.E. Glutamine: role in critical illness and ongoing clinical trials. Curr Opin Gastroenterol. 2008. March;24(2):190–7. 10.1097/MOG.0b013e3282f4db94 [DOI] [PubMed] [Google Scholar]
- 84.Hu Y.M.; Hsiung Y.C.; Pai M.H.; Yeh S.L. Glutamine administration in early or late septic phase. Downregulates lymphocyte PD-1/PD-L1 expression and the inflammatory response in mice with polymicrobial sepsis. JPEN J Parenter Enteral Nutr. 2017. March 1:148607117695245 10.1177/0148607117695245 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.