Abstract
We exploit a reversible acid–base triggered molecular shuttling process to switch an appropriately designed rotaxane between prochiral and mechanically planar chiral forms. The mechanically planar enantiomers and their interconversion, arising from ring shuttling, have been characterized by NMR spectroscopy. We also show that the supramolecular interaction of the positively charged rotaxane with optically active anions causes an imbalance in the population of the two enantiomeric coconformations. This result represents an unprecedented example of chiral molecular recognition and can disclose innovative approaches to enantioselective sensing and catalysis.
Mechanically interlocked molecules (MIMs)1,2 such as catenanes and rotaxanes may exhibit large amplitude motion of their interlocked components that renders them ideal candidates for the construction of molecular machines.2−4 While the absence of covalent bonds between the components enables facile relative movements, the mechanical constriction limits the possibilities for their mutual arrangement, with interesting outcomes from a stereochemical viewpoint.
In fact, chiral MIMs can be obtained by interlocking molecular components which are themselves achiral.5,6 This happens, for example, when an axle with C∞v symmetry (i.e., having a principal axis and mirror planes aligned along the axle length) is surrounded by a macrocycle with a Cs symmetry7 (i.e., having only one mirror plane coinciding with the plane of the ring (Figure 1a)).8 When the ring and axle are interlocked, the improper symmetry operations of the separated components are not symmetry operations of the rotaxane, which therefore becomes chiral (Figure 1b).6 The synthesis of mechanically planar (MP) chiral rotaxanes was pioneered by Vögtle and co-workers,9 and further investigated in more recent times,10−13 when efficient and stereoselective methodologies have enabled the synthesis of highly enantiopure samples.14,15 The exploitation of MP stereogenic elements of MIMs for the development of novel chiroptical materials, enantioselective sensors and asymmetric catalysis, is a fascinating research topic with development opportunities.6,16
Figure 1.
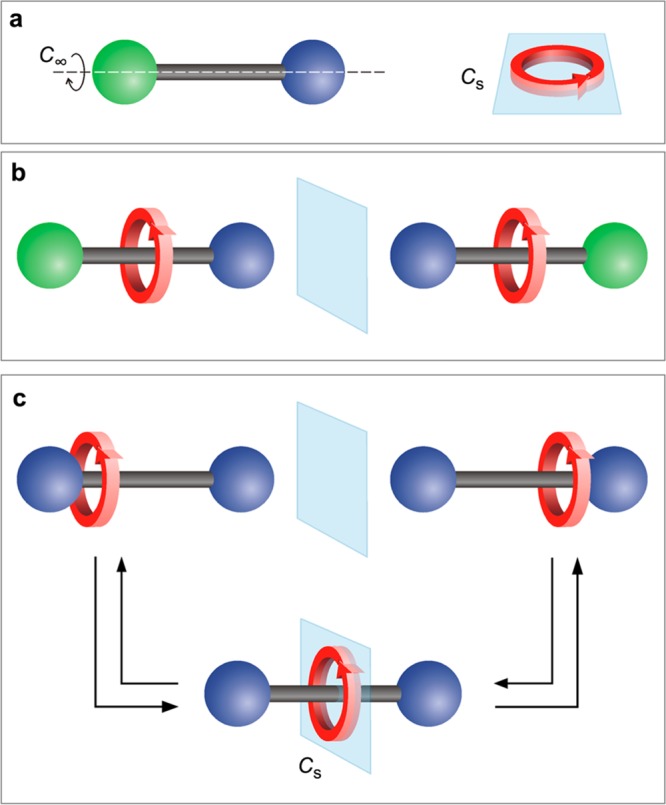
Schematic representation of a C∞v symmetric axle and a Cs symmetric ring (a); the two enantiomers of a mechanically planar (MP) chiral rotaxane (b); the two enantiomers of a coconformationally MP chiral rotaxane and their interconversion by ring shuttling through an achiral coconformation that features a mirror plane (c).
MP Chiral rotaxanes can also be obtained by interlocking a Cs symmetric macrocycle with an axle that has identical extremities, provided that the ring is located on either side of the mirror plane at the center of the axle (Figure 1c).6,17 In other words, it is the position of the oriented macrocycle that desymmetrizes the axle component, yielding a MP chiral [2]rotaxane. In systems of this kind, ring shuttling along the axle leads to interconversion of the two enantiomers by passing through an achiral coconformation in which the ring is located in the center of the axle (Figure 1c). Only one coconformationally mechanically planar chiral rotaxane has been reported to date, whose enantiomers were separated and their racemization rate was determined.17 However, in this case the position of the ring along the axle could not be controlled because of the absence of any recognition site.
The relation between coconformational dynamics and chirality13 in systems such as those shown in Figure 1c prompted us to investigate the possibility to exploit the stimuli-controlled switching of a molecular shuttle to enable MP chirality. Here we describe a [2]rotaxane that can be reversibly switched between prochiral and chiral states upon chemical stimulation. The presence of two enantiomers in the chiral state was probed experimentally, and the inversion of the MP stereogenic element via thermally activated ring shuttling was investigated. Finally, we report on the effect of optically active counteranions on the coconformational behavior and stereochemical properties of the positively charged rotaxane.
We based our design on a crown ether macrocycle, and on dibenzylammonium and triazolium recognition sites located along the axle (Scheme 1) to exploit acid–base stimulation of the molecular shuttle18−20 A dibenzo[24]crown-8 (DB24C8)-type ring encircles preferentially the ammonium center because of strong hydrogen bonding, and can be moved on the triazolium station upon deprotonation of the ammonium.
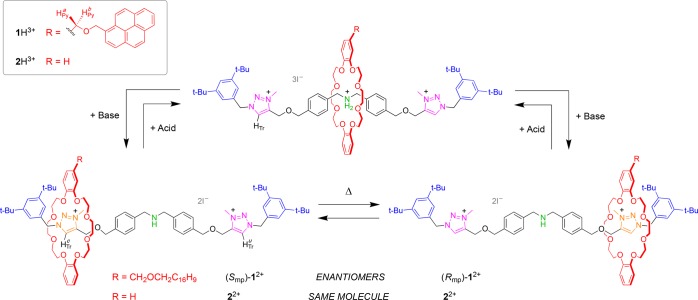
Scheme 1. Rotaxanes 1H3+ and 2H3+ (top), and Their Base-Triggered Switching to 12+ and 22+ (bottom).
The latter species can exist in two interconverting co-conformations, which constitute an enantiomeric pair for 12+ (see ref (6) for the assignment of the absolute configurations) while they are the same molecule in the case of 22+. The starting rotaxanes are regenerated upon addition of an acid.
Rotaxanes 1H3+ and 2H3+, equipped respectively with an oriented (Cs) and a nonoriented (D2h) macrocycle (Scheme 1, top), were synthesized by stoppering of the corresponding pseudorotaxanes via CuAAC. In rotaxane 1H3+ the DB24C8 skeleton is desymmetrized by placing a substituent in the 4-position of one of its 1,2-dioxybenzene moieties. A pyrenyl tether was chosen as the ring orienting substituent, with the aim of (i) enhancing the transfer of chiral information with a large aromatic moiety, and (ii) having a fluorescent reporter for the switching process. In the symmetric rotaxane 2H3+ the ring is plain DB24C8.
In both 1H3+ and 2H3+ the ring encircles the ammonium center, in line with literature data.18−20 We treated 2H3+ with a polymer-bound phosphazene base in CD2Cl2 to afford rotaxane 22+ (Scheme 1, bottom). The 1H NMR signal of HTr in 2H3+ (9.14 ppm) splits at low temperature into two, HTrc and HTru, associated respectively with the complexed and uncomplexed triazolium station in slow exchange on the NMR time scale. Total line-shape analysis of HTr and HTru at various temperatures (Figure 2a) allowed us to estimate the shuttling activation parameters (see the SI). These results confirm that the crown ether encircles one of the two equivalent triazolium sites, and moves between them. Similar results were obtained upon deprotonation of 1H3+ to yield 12+ (Figure 2b), showing that the pyrenyl tether of the macrocycle does not affect the kinetics of the coconformational equilibrium.
Figure 2.
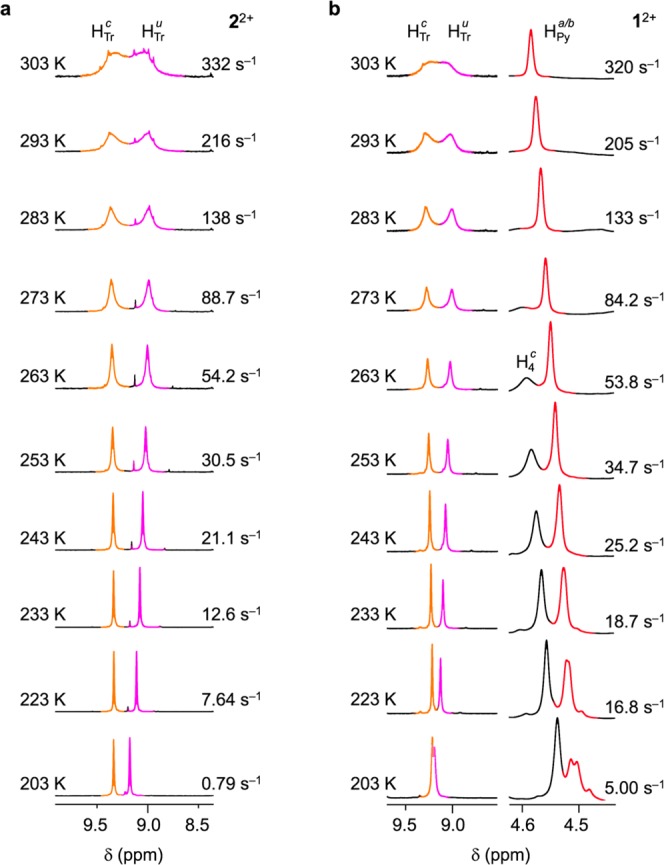
(a) Variable temperature (VT) 1H NMR spectra (500 MHz, CD2Cl2) of 22+ in the region of the triazolium protons (HTru, HTrc). (b) VT 1H NMR spectra (500 MHz, CD2Cl2) of 12+ in the regions of the triazolium protons (HTru, HTrc; left) and of the methylene protons in the pyrenyl tether of the macrocycle, adjacent to the dioxybenzene unit (HPya, HPyb; right). See Scheme 1 and SI for proton labeling.
In contrast with 22+, however, ring shuttling in 12+ generates a 50:50 population of two mirror image coconformations—that is, a racemic mixture of two enantiomers (Rmp)-12+ and (Smp)-12+.21 In this regard, 12+ is an example of a degenerate molecular shuttle22 whose coconformations are energetically equivalent but not superimposable (Scheme 1, bottom). The presence of the MP enantiomers of 12+ in the racemate was confirmed by analyzing the NMR signals of the two methylene protons in the pyrenyl tether of the macrocycle, adjacent to the dioxybenzene unit (HPy, Scheme 1). These protons are enantiotopic—and thus isochronous—in 1H3+, while they become diastereotopic in 12+. We therefore envisioned that in the deprotonated rotaxane they should resonate at different frequencies and form a coupled spin system.23
The 1H NMR spectra of 12+ recorded at 223 and 203 K showed that the signal at 4.60 ppm, associated with HPy, consistently splits into a couple of two almost overlapped doublets (Figure 2b).24 Additionally, analysis of the signals corresponding to HPya/b and HTr in CD2Cl2 revealed that the rate constants for shuttling (ksh) and racemization (krac) are approximately the same (see the SI). This observation confirms that in 12+ ring shuttling and inversion of the MP chiral configuration are two aspects of the same phenomenon (Scheme 1) which, interestingly, can be monitored separately. In fact, while the exchange of HTru and HTr (Figure 2b, left) yields information on the ring shuttling rate–an observation that can also be made for 22+ (Figure 2a) – the exchange of HPya and HPy (Figure 2b, right) is related to the racemization rate. This set of results is consistent with the emergence of two enantiomers of 12+ upon deprotonation.
The switching of 1H3+/12+ can also be followed by absorption and luminescence spectroscopy (Figure 3). The spectrum of 1H3+ shows an absorption tail in the 280–430 nm region assigned to a charge-transfer interaction between the pyrenyl electron donor and a triazolium electron acceptor. Such a tail disappears in 12+, presumably because the pyrenyl unit cannot undergo efficient electronic interactions with either triazolium unit (the complexed one is surrounded by the crown ether, and the free one is relatively distant). Consistently, in 1H3+ the pyrenyl fluorescence is strongly quenched with respect to the free macrocycle,19d,19g and it is 5-fold enhanced upon addition of base. Such a luminescence turn-on behavior provides a useful signal to monitor the occurrence of the chiral state, even by the naked eye (see the SI).
Figure 3.
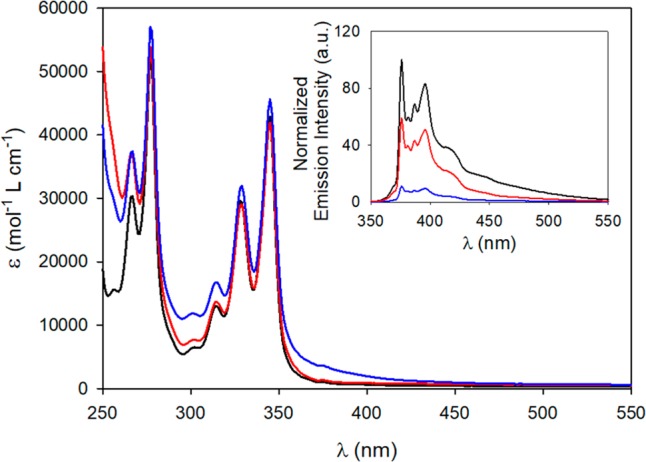
Absorption and fluorescence (inset, λexc = 328 nm) spectra of the free macrocycle (black), 1H3+ (blue) and 12+ (red). Air equilibrated CH2Cl2, 20 °C.
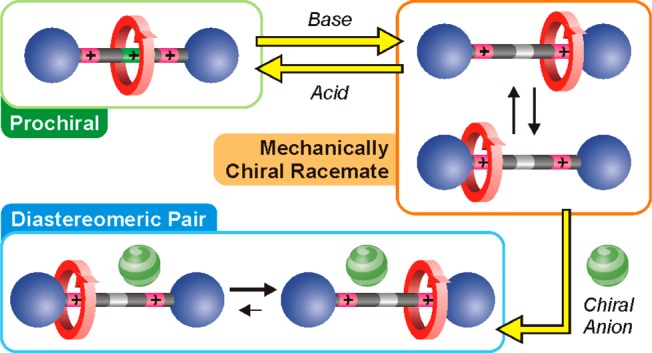
Having confirmed that 12+ exists as a dynamic racemic mixture of (Smp) and (Rmp) forms, we investigated the possibility to induce an enantiomeric excess. Since the triazolium stations are positively charged, an interesting option is ion pairing with an optically active anion.25 In such a case, two diastereomeric salts would be formed, which can have different energies and thus exhibit unbalanced populations of the macrocycles on the stations (Figure 4a).
Figure 4.
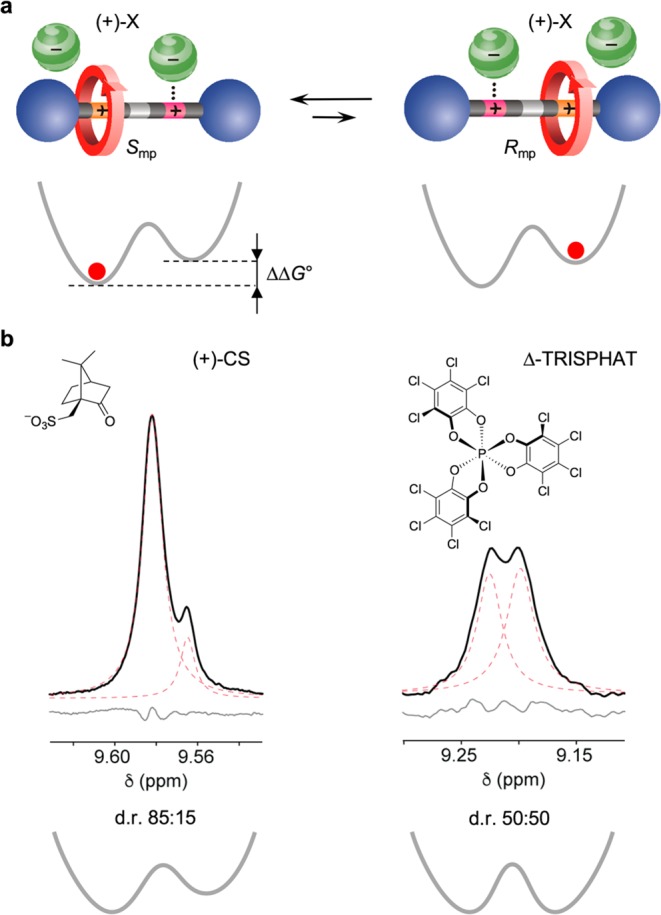
(a) Interconversion between two diastereomeric ion pairs composed of a coconformationally MP chiral rotaxane dication, such as 12+, and a chiral monoanion. In the proposed structures, one anion is coordinated to the unencircled triazolium, while another is weakly paired with the encircled site. Simplified potential energy curves for the location of the ring along the axle are also shown. As the two ion pairs can have different stabilities [ΔΔG° ≠ 0], the ring distribution between the two identical stations can become unbalanced. (b) Partial 1H NMR spectra (500 MHz) of the HTruresonance in 12+ after the addition of 8 equiv of the tetrabutylammonium salt of (1S)-(+)-10-camphorsulfonate (CD2Cl2, 223 K; left) or Δ-TRISPHAT (toluene-d8, 243 K; right). Black, red, and gray traces show respectively the experimental spectrum, the deconvoluted peaks, and the fitting residuals.
Upon addition of the enantiopure anion (1S)-(+)-10-camphorsulfonate [(+)-CS] (tetrabutylammonium salt) to 12+ in CD2Cl2 at 223 K, the NMR signal of the HTru proton—that appears as a singlet at 9.14 ppm in the iodide salt—splits into two singlets with different intensities (Δδ = 0.02 ppm; Figure 4b, left), assigned to the two different diastereomeric ion pairs (analysis of other resonances also supports this interpretation; see the SI). Deconvolution of these peaks affords a diastereomeric ratio of 85:15, which corresponds to a difference in stability of the two diastereoisomers of 3.2 kJ mol–1. Titration data show that the diastereomeric ratio does not depend on the CS/12+ stoichiometry. Moreover, the spectra recorded upon addition of the opposite enantiomer [(−)-CS] display identical resonances and integral ratio, in full agreement with the formation of a diastereomeric pair that is enantiomerically related to that observed upon addition of (+)-CS (see the SI). In all cases the signal of HTr shifts downfield from 9.14 to 9.58 ppm (major diastereoisomer), confirming that the sulfonate anion is coordinated by the free triazolium unit of the rotaxane.25 Conversely, the fact that the signal of HTrc is almost unaffected by the presence of the anion indicates that the macrocycle wrapped around the triazolium prevents a tight ion pairing.
Taken together, these observations (see also the SI) suggest that the ring-axle arrangement in 12+ creates a nonsymmetric environment around the unencircled triazolium such that enantioselective anion recognition can take place. The encircled triazolium site does not effectively compete for anion binding and it does not contribute to the stereodifferentiation. The fact that the recognition occurs relatively far away from the site of the mechanical entanglement—where the stereogenic unit is formally located—is quite remarkable.26 A possible explanation is that the molecule folds to create a “chiral pocket” similar to that of an enzyme, suggesting that such MIMs can have significant potential in chiral sensing.
The addition of tetrabutylammonium Δ-TRISPHAT27 to 12+ in toluene-d8 at 243 K28 also causes a splitting of the NMR singlet corresponding to the HTru proton into two overlapping singlets (Δδ = 0.02 ppm; Figure 4b, right). Integration of these signals, however, revealed that the two diastereoisomers have the same concentration within errors. Thus, Δ-TRISPHAT plays the role of a chiral shift reagent26 by ion-pairing with 12+ in an apolar solvent, but enantioselective molecular recognition does not occur. Presumably, the large and soft TRISPHAT anion, being loosely bound to the triazolium site, is unable to “read” the mechanical chirality of 12+ and determine an imbalance of its two coconformations.
In summary, we have described a three-station molecular shuttle that can be switched reversibly between symmetric prochiral and desymmetrized mechanically planar chiral states. The two enantiomers in the chiral state have been observed, and their interconversion—caused by thermally driven shuttling between two identical stations—has been quantitatively characterized. We have established a clear connection between the stimuli-controlled dynamic behavior of rotaxanes (i.e., their molecular machine aspect) and the unique stereochemical features arising from the mechanical bond. Furthermore, we have induced a difference in the population of the stations by interaction with an optically active anion, which is of interest for, e.g., enantioselective sensing and catalysis,16a,16b,29 or activating molecular machines with a chiral trigger. Considering the central role of chirality in chemistry, and the fact that mechanical chirality of MIMs is often overlooked,30 studies of this kind have not only exciting implications for basic science but also open new avenues for the development of molecular devices and materials for practical applications.
Acknowledgments
We thank Prof. Steve Goldup for fruitful discussions and Dr. Massimo Capobianco for assistance in the MS experiments. This work was supported by the European Research Council (H2020 AdG no. 692981) and the Ministero dell’Istruzione, Università e Ricerca (FARE grant no. R16S9XXKX3).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b00941.
General methods and experimental procedures, synthesis, NMR and CD spectra (PDF)
Author Contributions
# S.C. and C.d.V. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Sauvage J.-P.; Dietrich-Buchecker C.. Molecular Catenanes, Rotaxanes and Knots; Wiley: New York, 1999. [Google Scholar]
- Bruns C. J.; Stoddart J. F.. The Nature of the Mechanical Bond: From Molecules to Machines; Wiley: Hoboken, 2016. [Google Scholar]
- Balzani V.; Credi A.; Venturi M.. Molecular Devices and Machines - Concepts and Perspectives for the Nanoworld; Wiley-VCH: Weinheim, 2008. [Google Scholar]
- Erbas-Cakmak S.; Leigh D. A.; McTernan C. T.; Nussbaumer A. L. Artificial Molecular Machines. Chem. Rev. 2015, 115, 10081–10206. 10.1021/acs.chemrev.5b00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans N. H. Chiral Catenanes and Rotaxanes: Fundamentals and Emerging Applications. Chem. - Eur. J. 2018, 24, 3101–3112. 10.1002/chem.201704149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson E. M. G.; Modicom F.; Goldup S. M. Chirality in Rotaxanes and Catenanes. Chem. Soc. Rev. 2018, 47, 5266–5311. 10.1039/C8CS00097B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A molecular ring with Cs (or C1h) symmetry is often said to be “oriented”, because it is usually obtained by placing substituents in appropriate positions around the macrocycle skeleton.
- Schill G.Catenanes, Rotaxanes and Knots; Academic Press: New York, 1971. [Google Scholar]
- Yamamoto C.; Okamoto Y.; Schmidt T.; Jäger R.; Vögtle F. Enantiomeric Resolution of Cycloenantiomeric Rotaxane, Topologically Chiral Catenane, and Pretzel-Shaped Molecules:? Observation of Pronounced Circular Dichroism. J. Am. Chem. Soc. 1997, 119, 10547–10548. 10.1021/ja971764q. [DOI] [Google Scholar]
- Schalley C. A.; Beizai K.; Vögtle F. On the Way to Rotaxane-Based Molecular Motors: Studies in Molecular Mobility and Topological Chirality. Acc. Chem. Res. 2001, 34, 465–476. 10.1021/ar000179i. [DOI] [PubMed] [Google Scholar]
- Kameta N.; Hiratani K.; Nagawa Y. A Novel Synthesis of Chiral Rotaxanes via Covalent Bond Formation. Chem. Commun. 2004, 466–467. 10.1039/b314744d. [DOI] [PubMed] [Google Scholar]
- Makita Y.; Kihara N.; Nakakoji N.; Takata T.; Inagaki S.; Yamamoto C.; Okamoto Y. Catalytic Asymmetric Synthesis and Optical Resolution of Planar Chiral Rotaxane. Chem. Lett. 2007, 36, 162–163. 10.1246/cl.2007.162. [DOI] [Google Scholar]
- Gell C. E.; Mcardle-Ismaguilov T. A.; Evans N. H. Modulating the Expression of Chirality in a Mechanically Chiral Rotaxane. Chem. Commun. 2019, 55, 1576–1579. 10.1039/C8CC10044F. [DOI] [PubMed] [Google Scholar]
- Bordoli R. J.; Goldup S. M. An Efficient Approach to Mechanically Planar Chiral Rotaxanes. J. Am. Chem. Soc. 2014, 136, 4817–4820. 10.1021/ja412715m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks M. A.; de Juan A.; Denis M.; Fletcher C. J.; Galli M.; Jamieson E. M. G.; Modicom F.; Zhang Z.; Goldup S. M. Stereoselective Synthesis of Mechanically Planar Chiral Rotaxanes. Angew. Chem., Int. Ed. 2018, 57, 14806–18410. 10.1002/anie.201808990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kameta N.; Nagawa Y.; Karikomi M.; Hiratani K. Chiral Sensing for Amino Acid Derivative Based on a [2]Rotaxane Composed of an Asymmetric Rotor and an Asymmetric Axle. Chem. Commun. 2006, 3714–3716. 10.1039/b607251h. [DOI] [PubMed] [Google Scholar]; b Ishiwari F.; Nakazono K.; Koyama Y.; Takata T. Induction of Single-Handed Helicity of Polyacetylenes Using Mechanically Chiral Rotaxanes as Chiral Sources. Angew. Chem., Int. Ed. 2017, 56, 14858–14862. 10.1002/anie.201707926. [DOI] [PubMed] [Google Scholar]; c Hirose K.; Ukimi M.; Ueda S.; Onoda C.; Kano R.; Tsuda K.; Hinohara Y.; Tobe Y. The Asymmetry is Derived from Mechanical Interlocking of Achiral Axle and Achiral Ring Components - Syntheses and Properties of Optically Pure [2]Rotaxanes. Symmetry 2018, 10, 20. 10.3390/sym10010020. [DOI] [Google Scholar]
- Mochizuki Y.; Ikeyatsu K.; Mutoh Y.; Hosoya S.; Saito S. Synthesis of Mechanically Planar Chiral rac-[2]Rotaxanes by Partitioning of an Achiral [2]Rotaxane: Stereoinversion Induced by Shuttling. Org. Lett. 2017, 19, 4347–4350. 10.1021/acs.orglett.7b02043. [DOI] [PubMed] [Google Scholar]
- a Coutrot F.; Busseron E. A New Glycorotaxane Molecular Machine Based on an Anilinium and a Triazolium Station. Chem. - Eur. J. 2008, 14, 4784–4787. 10.1002/chem.200800480. [DOI] [PubMed] [Google Scholar]; b Coutrot F. A Focus on Triazolium as a Multipurpose Molecular Station for pH-Sensitive Interlocked Crown-Ether-Based Molecular Machines. ChemistryOpen 2015, 4, 556–576. 10.1002/open.201500088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recent examples of rotaxanes:; a Yang W.; Li Y.; Zhang J.; Yu Y.; Liu T.; Liu H.; Li Y. Synthesis of a [2]Rotaxane Operated in Basic Environment. Org. Biomol. Chem. 2011, 9, 6022–6026. 10.1039/c1ob05726j. [DOI] [PubMed] [Google Scholar]; b Blanco V.; Leigh D. A.; Marcos V.; Morales-Serna J. A.; Nussbaumer A. L. A Switchable [2]Rotaxane Asymmetric Organocatalyst That Utilizes an Acyclic Chiral Secondary Amine. J. Am. Chem. Soc. 2014, 136, 4905–4908. 10.1021/ja501561c. [DOI] [PubMed] [Google Scholar]; c Meng Z.; Xiang J.-F.; Chen C.-F. Directional Molecular Transportation Based on a Catalytic Stopper-Leaving Rotaxane System. J. Am. Chem. Soc. 2016, 138, 5652–5658. 10.1021/jacs.6b01852. [DOI] [PubMed] [Google Scholar]; d Ragazzon G.; Credi A.; Colasson B. Thermodynamic Insights on a Bistable Acid-Base Switchable Molecular Shuttle with Strongly Shifted Co-conformational Equilibria. Chem. - Eur. J. 2017, 23, 2149–2156. 10.1002/chem.201604783. [DOI] [PubMed] [Google Scholar]; e Erbas-Cakmak S.; Fielden S. D. P.; Karaca U.; Leigh D. A.; McTernan C. T.; Tetlow D. J.; Wilson M. R. Rotary and Linear Molecular Motors Driven by Pulses of a Chemical Fuel. Science 2017, 358, 340–343. 10.1126/science.aao1377. [DOI] [PubMed] [Google Scholar]; f Waeles P.; Fournel-Marotte K.; Coutrot F. Distinguishing Two Ammonium and Triazolium Sites of Interaction in a Three-Station [2]Rotaxane Molecular Shuttle. Chem. - Eur. J. 2017, 23, 11529–11539. 10.1002/chem.201701912. [DOI] [PubMed] [Google Scholar]; g Ghosh A.; Paul I.; Adlung M.; Wickleder C.; Schmittel M. Oscillating Emission of [2]Rotaxane Driven by Chemical Fuel. Org. Lett. 2018, 20, 1046–1049. 10.1021/acs.orglett.7b03996. [DOI] [PubMed] [Google Scholar]; h Zhu K.; Baggi G.; Loeb S. J. Ring-Through-Ring Molecular Shuttling in a Saturated [3]Rotaxane. Nat. Chem. 2018, 10, 625–630. 10.1038/s41557-018-0040-9. [DOI] [PubMed] [Google Scholar]; i Chen S.; Wang Y.; Nie T.; Bao C.; Wang C.; Xu T.; Lin Q.; Qu D.-H.; Gong X.; Yang Y.; Zhu L.; Tian H. An Artificial Molecular Shuttle Operates in Lipid Bilayers for Ion Transport. J. Am. Chem. Soc. 2018, 140, 17992–17998. 10.1021/jacs.8b09580. [DOI] [PubMed] [Google Scholar]
- Recent examples of other MIMs:; a Meng Z.; Han Y.; Wang L.-N.; Xiang J.-F.; He S.-G.; Chen C.-F. Stepwise Motion in a Multivalent [2](3)Catenane. J. Am. Chem. Soc. 2015, 137, 9739–9745. 10.1021/jacs.5b05758. [DOI] [PubMed] [Google Scholar]; b Goujon A.; Lang T.; Mariani G.; Moulin E.; Fuks G.; Raya J.; Buhler E.; Giuseppone N. Bistable [c2] Daisy Chain Rotaxanes as Reversible Muscle-like Actuators in Mechanically Active Gels. J. Am. Chem. Soc. 2017, 139, 14825–14828. 10.1021/jacs.7b06710. [DOI] [PubMed] [Google Scholar]; c Zhang Q.; Rao S.-J.; Xie T.; Li X.; Xu T.-Y.; Li D.-W.; Qu D.-H.; Long Y.-T.; Tian H. Muscle-like Artificial Molecular Actuators for Nanoparticles. Chem. 2018, 4, 2670–2684. 10.1016/j.chempr.2018.08.030. [DOI] [Google Scholar]
- For a discussion on the assignment of absolute stereochemistry of mechanically planar chiral rotaxanes, see ref (6) and:; Reuter C.; Mohry A.; Sobanski A.; Vögtle F. [1]Rotaxanes and Pretzelanes: Synthesis, Chirality, and Absolute Configuration. Chem. - Eur. J. 2000, 6, 1674–1682. . [DOI] [PubMed] [Google Scholar]
- Anelli P. L.; Spencer N.; Stoddart J. F. A Molecular Shuttle. J. Am. Chem. Soc. 1991, 113, 5131–5133. 10.1021/ja00013a096. [DOI] [PubMed] [Google Scholar]
- Jennings W. B. Chemical Shift Nonequivalence in Prochiral Groups. Chem. Rev. 1975, 75, 307–322. 10.1021/cr60295a003. [DOI] [Google Scholar]
- Similar molecular examples:; a Egan W.; Tang R.; Zon G.; Mislow K. Low Barrier to Pyramidal Inversion in Phospholes. Measure of Aromaticity. J. Am. Chem. Soc. 1970, 92, 1442–1444. 10.1021/ja00708a077. [DOI] [Google Scholar]; b Anet F. A. L.; Jochims J. C.; Bradley C. H. Energy Barrier of Racemization in Diisopropylcarbodiimide. J. Am. Chem. Soc. 1970, 92, 2557–2558. 10.1021/ja00711a064. [DOI] [Google Scholar]
- a Hua Y.; Flood A. H. Click Chemistry Generates Privileged CH Hydrogen-bonding Triazoles: The Latest Addition to Anion Supramolecular Chemistry. Chem. Soc. Rev. 2010, 39, 1262–1271. 10.1039/b818033b. [DOI] [PubMed] [Google Scholar]; b Evans N. H.; Beer P. D. Advances in Anion Supramolecular Chemistry: From Recognition to Chemical Applications. Angew. Chem., Int. Ed. 2014, 53, 11716–11754. 10.1002/anie.201309937. [DOI] [PubMed] [Google Scholar]
- Morrow S. M.; Bissette A. J.; Fletcher S. P. Transmission of Chirality Through Space and Across Length Scales. Nat. Nanotechnol. 2017, 12, 410–419. 10.1038/nnano.2017.62. [DOI] [PubMed] [Google Scholar]
- TRISPHAT = [Tris(tetrachlorobenzenediolato) phosphate(V)]. For its use as a chiral shift reagent, see:; Lacour J.; Ginglinger C.; Favarger F.; Torche-Haldimann S. Application of TRISPHAT anion as NMR chiral shift reagent. Chem. Commun. 1997, 2285–2286. 10.1039/a706916b. [DOI] [Google Scholar]
- The solvent was changed from CD2Cl2 to toluene-d8 in order to favor ion pairing and thus enhance chiral shift effects on the signals. The 1H NMR spectra of 12+·2I– in toluene-d8 are consistent with those in CD2Cl2.
- See, e.g.:; a Cakmak Y.; Erbas-Cakmak S.; Leigh D. A. Asymmetric Catalysis with a Mechanically Point-Chiral Rotaxane. J. Am. Chem. Soc. 2016, 138, 1749–1751. 10.1021/jacs.6b00303. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Eichstaedt K.; Jaramillo-Garcia J.; Leigh D. A.; Marcos V.; Pisano S.; Singleton T. A. Switching between Anion-Binding Catalysis and Aminocatalysis with a Rotaxane Dual-Function Catalyst. J. Am. Chem. Soc. 2017, 139, 9376–9381. 10.1021/jacs.7b04955. [DOI] [PubMed] [Google Scholar]; c Lim J. Y. C.; Marques I.; Felix V.; Beer P. D. Enantioselective Anion Recognition by Chiral Halogen-Bonding [2]Rotaxanes. J. Am. Chem. Soc. 2017, 139, 12228–12239. 10.1021/jacs.7b06144. [DOI] [PubMed] [Google Scholar]
- See, e.g., ref (19i) and:; a Li J.; Li Y.; Guo Y.; Xu J.; Lv J.; Li Y.; Liu H.; Wang S.; Zhu D. A Novel Supramolecular System: Combination of Two Switchable Processes in a [2]Rotaxane. Chem. - Asian J. 2008, 3, 2091–2096. 10.1002/asia.200800217. [DOI] [PubMed] [Google Scholar]; b Iwamoto H.; Yawata Y.; Fukazawa Y.; Haino T. Highly Efficient Synthesis of [3]Rotaxane Assisted by Preorganisation of Pseudorotaxane Using bis(Crown Ether)s. Supramol. Chem. 2010, 22, 815–826. 10.1080/10610278.2010.514611. [DOI] [Google Scholar]; c Wang X.-Y.; Han J.-M.; Pei J. Energy Trabfer and Concentration-Dependent Conformational Modulation: A Porpyrin-Containing [3]Rotaxane. Chem. - Asian J. 2012, 7, 2429–2437. 10.1002/asia.201200443. [DOI] [PubMed] [Google Scholar]; d Bleve V.; Schaefer C.; Franchi P.; Silvi S.; Mezzina E.; Credi A.; Lucarini M. Reversible Mechanical Switching of Magnetic Interactions in a Molecular Shuttle. ChemistryOpen 2015, 4, 18–21. 10.1002/open.201402073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.