Data resource basics
Acute respiratory infections (ARI) are a major cause of morbidity worldwide.1 Some of the earliest studies to describe the epidemiology of these infections were household cohort studies.2–7 The largest of these were conducted in Seattle, Washington and Tecumseh, Michigan in the 1960s and 1970s6,7 and they provided information on the relative frequency, seasonality and symptomatic characteristics of ARI shortly after many respiratory viruses were first identified.7 Given that the role of household structure in seasonal incidence and transmission of respiratory viruses was the primary objective, an individual’s longitudinal history of infection was not a major focus. Indeed, during the first phase of the Tecumseh Study of Respiratory Illness, households were maintained on report for only 1 year, and then gradually replaced so that the entire community could be represented over time.8
These historical studies relied on cell culture for virus identification, a method that requires specimens to be processed quickly and is considerably less sensitive than current molecular methods.9 For this reason, the Tecumseh study and others relied extensively on serodiagnosis of influenza infection using twice yearly blood specimens.10 It was not possible to time infections in those who only were serologically positive, limiting the ability to do robust analyses of transmission patterns.11
With current molecular techniques, it has become much easier to identify a broad range of agents of respiratory infection. This allows documentation of infection, co-infection and subsequent re-infection. Collecting specimens within a short time from the onset of symptoms still maximizes the likelihood of accurate and timely identification of viruses associated with a respiratory illness for studies of transmission and vaccine effectiveness. Collection of regular blood specimens continues to be valuable as well, for both virus identification and for analysis of serologic correlates of protection.
The Household Influenza Vaccine Evaluation (HIVE) Study is an ongoing prospective household cohort study that began in 2010. The HIVE Study was based on the original Tecumseh Study of Respiratory Illness6 with several key modifications to illness surveillance and to laboratory methods for identification of respiratory viruses. While respiratory virus infections in general could be studied, the primary objective was to estimate the effectiveness of influenza vaccines using a cohort design for comparison with studies using the test-negative design (TND). Under the TND, specimens are collected from participants who meet a respiratory illness case definition, and vaccination frequency is compared between those who test positive for influenza and those who test negative.12 The TND is focused on those individuals with illnesses severe enough to seek care, which reduces bias due to differential care-seeking behaviour but misses milder presentations of respiratory virus illnesses.13–15 The use of a prospective cohort design has allowed inclusion of these mild illnesses in evaluations of vaccine effectiveness. Here we report on the first phase of the HIVE Study, conducted from 2010 through 2016.
Data collected
Who is included?
From 2010–2011 through 2013–2014, eligible households were those who lived within 30 miles of the study clinic in Ann Arbor MI, and who had at least four individuals who received primary care from the University of Michigan Health System (UMHS) and at least two children <18 years old; in 2014–2015 the eligibility criteria were changed to allow three-person households. We recruited or re-recruited households each summer or autumn. Previously participating households were invited to re-enroll provided that they continued to meet the eligibility criteria. We supplemented this recruitment with direct mail or email invitations to households in the source population. In total, we have collected data and specimens with active ARI surveillance for 2850 individuals from 413 households (Table 1). The majority of these individuals were children <18 years of age, 73% were white (non-Hispanic), and 51% were female. The study cohort size has ranged from 943 to 1441 participants in each year (213 to 340 households), and 1822 (64%) individuals have been followed longitudinally over multiple years.
Table 1.
Baseline demographics and health history of HIVE participants
| Overall | Young children (< 9 years) | Older children (9–17 years) | Adults (18+ years) | |
|---|---|---|---|---|
| Total | 2850 | 995 | 691 | 1164 |
| Sex – n (%) | ||||
| Female | 1465 (51.4) | 470 (47.2) | 324 (46.9) | 671 (57.6) |
| Male | 1385 (48.6) | 525 (52.8) | 367 (53.1) | 493 (42.4) |
| Race/Ethnicity – n (%) | ||||
| White | 2071 (73) | 707 (71) | 482 (70) | 882 (76) |
| Black/African American | 237 (8.3) | 83 (8.3) | 77 (11.1) | 77 (6.6) |
| Asian | 248 (8.7) | 85 (8.5) | 56 (8.1) | 107 (9.2) |
| Multi-racial | 32 (1.1) | 20 (2.0) | 9 (1.3) | 3 (0.3) |
| Hispanic | 30 (1.1) | 9 (0.9) | 9 (1.3) | 12 (1.0) |
| Other | 23 (0.8) | 9 (0.9) | 8 (1.2) | 6 (0.5) |
| Unknown | 122 (4.3) | 46 (4.6) | 24 (3.5) | 52 (4.5) |
| ACIP-defined high-risk condition – n (%) | 471 (16.5) | 111 (11.2) | 126 (18.2) | 234 (20.1) |
How often have they been followed up?
Active surveillance for ARI meeting a case definition was conducted seasonally (October–May) from 2010–2014. Beginning in October 2014, year round surveillance was initiated. HIVE Study participants were contacted weekly by email or phone to ascertain new ARI meeting the study case definition. For participants ≥3 years of age, the case definition consisted of two or more of the following symptoms: cough, fever/feverishness, nasal congestion, sore throat, body aches, chills, headache. For participants <3 years of age, the case definition consisted of two or more of the following symptoms: cough, fever/feverishness, nasal congestion/runny nose, trouble breathing, fussiness/irritability, decreased appetite. Each household received a calendar at enrollment to assist with recording illness onset dates. When illnesses were reported, the household was contacted by study staff to schedule a clinic visit for sample collection. At illness visits for ARI meeting the case definition, trained research staff collected nasal and throat swabs (or nasal swabs only for participants <3 years of age) for detection of influenza and other respiratory viruses. Since October 2014, an additional, self-collected or parent-collected specimen was also collected in the home on the day of symptom onset.
Beginning in December 2011, all household members ≥13 years of age were asked to contribute a blood specimen at the initial enrollment visit and at scheduled visits twice annually thereafter. Each year, autumn blood specimens (pre-season) were collected after the majority of study participants had received influenza vaccine and prior to the start of the influenza season (beginning in November). Spring (post-season) specimens were collected after local influenza circulation ended each year (May–June).
Data and specimens collected
At the annual enrollment visits, individual participants were queried about household and demographic characteristics and Advisory Committee on Immunization Practices (ACIP)-defined high-risk conditions. A detailed clinical history for these participants is extracted from the UMHS electronic medical record (EMR), or from their regular primary care provider if they are not part of the health system.
Influenza vaccination information, including date, lot number, vaccine type, dose and manufacturer, was confirmed using multiple sources. Information from the Michigan Care Improvement Registry (a state-maintained repository of vaccination records that requires reporting of all childhood vaccines in Michigan) was requested for all study participants. Provider-based vaccine records were obtained directly from the UMHS EMR or through medical record requests for individuals with outside-system care providers. These records were supplemented by vaccine diary cards that were filled out by participants at the time of vaccination. There has historically been a high level of vaccine uptake in the HIVE Study population, ranging from 59–69% (Table 2).
Table 2.
Detection of influenza among HIVE participants and vaccination by influenza season
| 2010–2011 | 2011–2012 | 2012–2013 | 2013–2014 | 2014–2015a | 2015–2016b | |
|---|---|---|---|---|---|---|
| Households | 328 | 213 | 321 | 232 | 340 | 227 |
| Participants | 1441 | 943 | 1426 | 1049 | 1431 | 996 |
| Influenza-positive individuals | 125 | 32 | 111 | 50 | 202 | 38 |
| Influenza-positive specimens | 130 | 32 | 117 | 52 | 210 | 40 |
| Strain | ||||||
| Ac | 86 | 23 | 69 | 48 | 166 | 30 |
| H1N1pdm09 | 27 | 1 | 3 | 47 | 0 | 28 |
| H3N2 | 59 | 22 | 66 | 1 | 166 | 1 |
| Bc | 45 | 7 | 49 | 4 | 44 | 10 |
| Yamagata | 1 | 3 | 38 | 4 | 34 | 5 |
| Victoria | 37 | 0 | 10 | 0 | 10 | 5 |
| Current vaccination, n (%) | ||||||
| Self-report | 783 (54)d | 477 (51) | 855 (60) | 680 (65) | 785 (55) | 547 (55) |
| Documentede | 866 (60) | 554 (59) | 850 (60) | 661 (63) | 935 (65) | 641 (64) |
| Self-report or documented | 934 (65) | 554 (59) | 942 (66) | 722 (69) | 992 (69) | 681 (68) |
Year-round surveillance began in October 2014.
Home specimens not included in flu testing.
Includes un-subtypable and not repeatable specimens.
Includes ‘unknown’ vaccination status in percentage for years 2010–2011.
Documented vaccination includes individuals with evidence of vaccine receipt in either the Michigan Care Improvement Registry or the subject’s medical record.
Respiratory specimens collected at illness visits were tested by real-time reverse transcriptase polymerase chain reaction (RT-PCR) for laboratory confirmation of influenza, using primers and probes from the Centers for Disease Control and Prevention. Influenza subtype was determined for influenza A specimens and lineage was determined for influenza B specimens. From 2010 through 2016, a total of 581 influenza infections were identified. Specimens are also tested by PCR for non-influenza respiratory viruses including respiratory syncytial virus, human metapneumovirus, parainfluenza, coronavirus and rhinovirus. Symptoms present since illness onset were recorded and participants completed a follow-up survey reporting illness outcomes (e.g. duration of symptoms, care seeking behaviour).
Blood specimens were centrifuged, and the serum was separated and stored in 1 mL aliquots at –20°C until processing in serologic assays. Collected sera were tested in parallel using hemagglutinin inhibition (HAI) assays. HAI assays use inactivated influenza vaccine subunit material (Sanofi-Pasteur) from relevant A/H3N2, A/H1N1 and B vaccine strains. Anti-neuraminidase antibody (NAI) was measured by an enzyme-linked lectin assay (ELLA) using inactivated, reassortant viral targets containing contemporary neuraminidase segments and a mismatched H6 hemagglutinin HA.16 Antibody titres measured from blood specimens collected in the autumn have been analysed as both pre-season titres for the coming influenza season as well as post-vaccination titres for those individuals who were vaccinated prior to collection. Similarly, antibody titres measured from blood specimens collected in the spring have served as post-season titres as well as pre-vaccination titres in analyses for the following study year. A full description of data collected throughout study follow-up can be found in Table 3 and the timeline for key study activities can be found in Figure 1.
Table 3.
Description of data collected by phase
| Phase | Measurements |
|---|---|
| Enrollment (baseline) |
|
| Electronic health records |
|
| ARI surveillance |
|
| Serologic studies |
|
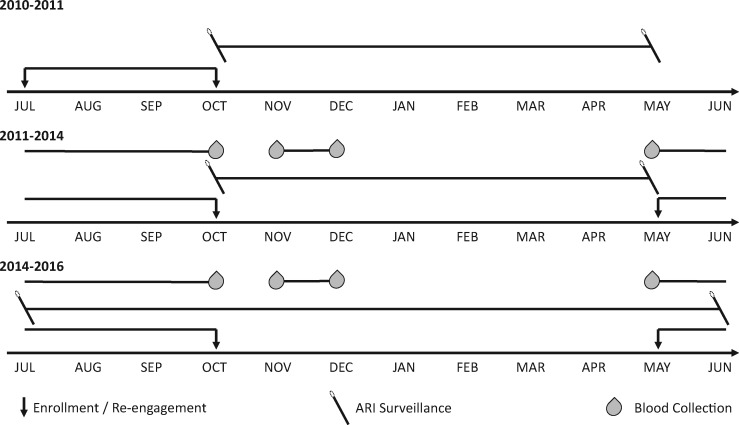
Figure 1.
Timeline of study enrollment, acute respiratory illness (ARI) surveillance and blood collection activities. Blood collection for antibody studies began in the autumn of 2011. ARI surveillance has been carried out continuously on a year-round basis since the autumn of 2014; ARI surveillance was limited to the typical influenza season prior to that.
Data resource use
The original aims of this study were to estimate influenza vaccine effectiveness annually and to compare those estimates with those from TND studies conducted in outpatient clinics during the same seasons. With additional funding, we have expanded on these original aims by collecting blood specimens for studies of antibody-mediated immunity, extending ARI surveillance year-round, and incorporating laboratory testing for other respiratory viruses.
Influenza vaccine effectiveness and effect of prior vaccination
Statistical analyses to estimate vaccine effectiveness were performed annually. Adjusted hazard ratios (aHR) were estimated by Cox proportional hazard models with robust variance using a sandwich estimator to account for household clustering. Adjusted models included age, high-risk health status and vaccination status. Vaccination status was modelled as a time-varying covariate, with subjects considered vaccinated 14 days after vaccine receipt. Vaccine effectiveness was calculated as 100 x [1 – aHR].
Vaccine effectiveness (VE) has varied markedly by year and age group. In 2010–2011 and 2012–2013, for example, we observed VE against any influenza infection of approximately 30% in two of three age groups. VE was lowest among adults (≥18 years) in 2010–2011, however, young children (6 months to 8 years) had the lowest VE in 2012–2013.17,18 In 2013–2014, when influenza A/H1N1 predominated, we observed VE against community acquired influenza A/H1N1 infection of 54% (95% confidence interval –4 to 80).19 During the 2014–2015 season, we observed no effect of vaccine on the risk of infection with antigenically drifted influenza A/H3N2, but point estimates against B Yamagata were near 50%. The estimated VE against B Yamagata appeared to be primarily driven by high effectiveness in young children.20 In general our annual estimates of influenza VE have been consistent with those from the US Flu VE network.21–26
During the 2010–2011 influenza season, we found that those who had been vaccinated in the previous and current year were less protected than those who received the vaccine in the current year only.17 This was the first time in the recent era that such a reduction was documented, thus continuing historical debates on whether repeat vaccination resulted in reduced protection.27–30 These original findings of a repeat vaccination effect prompted a furthering of the hypothesis that antibody response to vaccination was decreased after multiple annual administration of vaccines of similar antigenic make-up.
In order to evaluate this hypothesis, we began collecting blood specimens in December 2011. In the 2012–13 season, the predominant circulating influenza virus was A(H3N2). Relative to those unvaccinated in both years, VE was higher in those vaccinated in the current year, lower in those vaccinated in the current and previous year, and lowest in those vaccinated in the previous year only. With the addition of blood collection in the cohort, we found that HAI titres correlated with the observed VE for the type A viruses. Lower post-vaccination titres were found among those vaccinated in both years relative to those vaccinated only in the current season. A similar difference in post-vaccination titres was not observed for influenza B in 2012–2013.
Importantly, the effects of prior season vaccination on VE and serologic susceptibility to infection have not been observed in all years. During the 2013–2014 season, a year in which influenza A(H1N1)pdm09 predominated, we found similar levels of effectiveness among those vaccinated in both the current and previous seasons compared with those vaccinated only in the current season.19 Consistent with this finding, HAI and NAI titres against influenza A(H1N1)pdm09 were similar comparing those vaccinated in both the current and prior season to those vaccinated only in the current season.
Correlates of protection
Antibody titres measured in twice annual blood specimens have enabled additional evaluations of the relationship between vaccination and antibody-mediated protection. The fact that participants were observed over time allowed us to demonstrate that in this highly vaccinated cohort, pre-vaccination antibody titres were generally high and a 4-fold rise in titre following vaccination was less frequent than expected.19,20 Finally, measuring antibody titres both against viruses similar to those that circulated locally and against those in the vaccine, we observed increased susceptibility among those with high vaccine-strain-specific antibody titres but low circulating-strain-specific antibody titres.31
Transmission of influenza viruses
Interrupting transmission is a major goal of influenza prevention strategies and could be key to controlling the burden of disease during a pandemic. The household may be an effective place to accomplish this goal due to the high proportion of transmission estimated to occur in this setting. We have used standard epidemiologic methods to estimate serial intervals by virus type and to identify household and individual level characteristics associated with secondary infection risk.32 These methods remain susceptible to misclassification of a transmission event, as infections are generally linked retrospectively based on the time between onset of each illness and viral type and subtype despite continued risk of infection from the community. We have attempted to address this potential for misclassification in two ways. First, it is now possible using next-generation sequencing to differentiate the two types of transmission on the basis of genetic similarity as we have demonstrated with influenza A viruses.33 Second, we have adapted individual-based transmission models to account for risk of infection from both the community and the household and to allow for chains of transmission.34
Frequency and seasonality of respiratory viruses
The HIVE Study from the start has identified viruses other than influenza, and, for one season, bacterial agents associated with respiratory illnesses.35 Surveillance initially extended through much of the respiratory season and has been conducted year-round since October 2014; thus it has been possible to determine seasonality of these respiratory viruses.36 In addition, the detection of multiple respiratory viruses has allowed us to describe the frequency of co-infection in different age groups.36
Future research and data collection plans
The HIVE Study has recently received continued core funding and is now recruiting existing and new households into a second phase of the cohort. A commitment to 5 years of follow-up with year-round surveillance for ARI is now required of those entering the study. Blood specimen collection, which in the past was limited to those ≥13 years of age, is now extended to younger children and includes collection of peripheral blood mononuclear cells (PBMCs) for studies of cell-mediated immunity. In addition, targeted recruitment strategies are now focused on children <36 months old and their households in an effort to study infections and immune response in early life.
Strengths and weaknesses
The main strength of the HIVE Study is the longitudinal, prospective study design with intensive data collection. Active surveillance for symptomatic illness with collection of respiratory specimens for laboratory-confirmation of influenza and other viruses allows for calculations of the incidence and determinants of infection over time, and builds on previous cohorts in the region that defined illness based on serology alone. Using multiple sources, including participant medical records, to document influenza vaccination status and regular serologic sampling are major strengths of the cohort. The number of participants that have been prospectively followed for multiple years has enabled multi-year studies of immune response to infection and vaccination, studies that are increasingly important for understanding how individual influenza immunity evolves over time.37 The illness sampling strategy requires participant symptom reporting. It is therefore important to note that subclinical infections are not captured by illness sampling, as this would require routine swabs in symptom-free individuals. However, the availability of end-of-season serologic specimens allows for analysis for uncaptured influenza virus infections in unvaccinated individuals.
The intensive data collection increases the burden on study participants. It is therefore necessary to build relationships with the community to ensure enrollment and long-term participation. As a result the study is resource intensive and the study population is limited in terms of both sample size and generalizability. In particular the source population from which participants is drawn is limited geographically to households who are able to travel to the University of Michigan study site for enrollment visits, blood draws and illness specimen collection. The HIVE Study population consists of suburban residents and largely reflects the high education level and vaccination uptake in our region. One approach to increase sample size and generalizability would be to establish multi-site cohorts, presenting logistical considerations that might be challenging to unify under a common protocol. Indeed, other longitudinal cohorts of respiratory illness have used varied recruitment strategies or follow-up methods tailored to their unique communities.38,39 Our history of involvement in this community, stretching back to the 1960s, has given us the opportunity to optimize the cohort for long-term success.
Data resource access
The investigators regularly collaborate with others to address key questions in respiratory virus epidemiology and immune correlates of protection. Proposals for future collaborations using HIVE Study specimens and data can be submitted to the study investigators for consideration.
Profile in a nutshell
The HIVE Study is a prospective cohort of households with children originally established to estimate influenza vaccine effectiveness on an annual basis. It now examines the incidence, etiology and determinants of influenza and other respiratory pathogens.
We recruited 2850 individuals from 652 households who have participated in at least one year of active surveillance from June 2010–May 2016. Of these, 1686 (59%) individuals were children <18 years old at enrollment. Additional recruitment and study follow-up is currently ongoing.
Households were contacted weekly to ascertain incident ARI seasonally (approximately October–May) from 2010–2014 and year-round beginning in October 2014. Blood specimens were collected from participants ≥13 years of age at up to two time points each year. A total of 417 (64%) households participated for more than one season. Illnesses are now followed year round.
We collected longitudinal data on demographics, health history, influenza vaccination status and ARI incidence. We collected specimens to detect occurrence by RT-PCR of influenza and other respiratory viruses and serum to determine antibody titres to hemagglutinin and neuraminidase. Specimens are archived.
Investigators interested in learning more about the HIVE Study as well as data and specimen availability are welcome to email the study investigators.
Funding
The HIVE Study was supported by the Centers for Disease Control and Prevention (U01 IP000170, U01 IP000474) and the National Institute of Allergy and Infectious Diseases (R01 AI097150, R56 AI097150).
Conflict of interest: E.T.M has received grant support from Merck and Pfizer for work unrelated to this report. A.S.M. has received consultancy fees from Sanofi, Seqirus and Novavax for work unrelated to this report. A.S.L. has received consultancy fees from Sanofi for work unrelated to this report. All other authors declare no conflict of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. HIVE Study Research Staff: Barbara Aaron; Amy P. Callear; Rachel Truscon; Emileigh Johnson; Caroline K. Cheng; Anne Kaniclides; Natalie Williams; Casey Martens.
Contributor Information
HIVE Study Research Staff:
Barbara Aaron, Amy P Callear, Rachel Truscon, Emileigh Johnson, Caroline K Cheng, Anne Kaniclides, Natalie Williams, and Casey Martens
References
- 1. Troeger C, Forouzanfar M, Rao PC. et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 2017;17:1133–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van Volkenburgh VA, Frost WH.. Acute Minor Respiratory Diseases prevailing in a Group of Families residing in Baltimore, Maryland, 1928-1930. Prevalence, distribution and clinical description of observed cases. Am J Hyg 1933;17:122–53. [Google Scholar]
- 3. Dingle JH, Badger GF, Feller A, Hodges RG, Jordan WS, Rammelkamp CH.. A study of illness in a group of Cleveland families I. Plan of study and certain general observations. Am J Epidemiol 1953;58:16–30. [DOI] [PubMed] [Google Scholar]
- 4. Buck C. Acute upper respiratory infections in families. Am J Epidemiol 1956;63:1–12. [DOI] [PubMed] [Google Scholar]
- 5. Katz AH. Illness in the home: a study of 25, 000 illnesses in a group of Cleveland families. Am J Public Health Nations Health 1966;56:683–4. [Google Scholar]
- 6. Monto AS, Napier JA, Metzner HL.. The Tecumseh study of respiratory illness: I. Plan of study and observations on syndromes of acute respiratory disease. Am J Epidemiol 1971;94:269–79. [DOI] [PubMed] [Google Scholar]
- 7. Fox JP, Hall CE, Cooney MK, Luce RE, Kronmal RA.. The Seattle virus watch. II. Objectives, study population and its observation, data processing and summary of illnesses. Am J Epidemiol 1972;96:270–85. [DOI] [PubMed] [Google Scholar]
- 8. Monto AS, Ullman BM.. Acute respiratory illness in an American community. The Tecumseh study. JAMA 1974;227:164–9. [PubMed] [Google Scholar]
- 9. Templeton KE, Scheltinga SA, Beersma MFC, Kroes ACM, Claas E.. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol 2004;42:1564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Monto AS, Kioumehr F.. The Tecumseh study of respiratory illness. IX. Occurence of influenza in the community, 1966-1971. Am J Epidemiol 1975;102:553–63. [DOI] [PubMed] [Google Scholar]
- 11. Monto AS, Koopman JS, Longini IM.. Tecumseh study of illness. XIII. Influenza infection and disease, 1976-1981. Am J Epidemiol 1985;121:811–22. [DOI] [PubMed] [Google Scholar]
- 12. Serres GD, Skowronski DM, Wu XW, Ambrose CS.. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Eurosurveillance 2013;18:20585.. [DOI] [PubMed] [Google Scholar]
- 13. Foppa IM, Haber M, Ferdinands JM, Shay DK.. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine 2013;31:3104–9. [DOI] [PubMed] [Google Scholar]
- 14. Jackson ML, Nelson JC.. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013;31:2165–8. [DOI] [PubMed] [Google Scholar]
- 15. Sullivan SG, Tchetgen TJE, Cowling BJ.. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am J Epidemiol 2016;184:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Couzens L, Gao J, Westgeest K. et al. An optimized enzyme-linked lectin assay to measure influenza A virus neuraminidase inhibition antibody titers in human sera. J Virol Methods 2014;210C:7–14. [DOI] [PubMed] [Google Scholar]
- 17. Ohmit SE, Petrie JG, Malosh RE. et al. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis 2013;56:1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ohmit SE, Petrie JG, Malosh RE, Fry AM, Thompson MG, Monto AS.. Influenza vaccine effectiveness in households with children during the 2012-2013 season: assessments of prior vaccination and serologic susceptibility. J Infect Dis 2015;211:1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohmit SE, Petrie JG, Malosh RE. et al. Substantial influenza vaccine effectiveness in households with children during the 2013-2014 influenza season, when 2009 pandemic influenza A(H1N1) virus predominated. J Infect Dis 2016;213:1229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petrie JG, Malosh RE, Cheng CK. et al. The household influenza vaccine effectiveness study: lack of antibody response and protection following receipt of 2014-2015 influenza vaccine. Clin Infect Dis 2017;65:1644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Treanor JJ, Talbot HK, Ohmit SE. et al. Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis 2012;55:951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ohmit SE, Thompson MG, Petrie JG. et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014;58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McLean HQ, Thompson MG, Sundaram ME. et al. Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type. J Infect Dis 2015;211:1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaglani M, Pruszynski J, Murthy K. et al. Influenza vaccine effectiveness against 2009 pandemic influenza A(H1N1) virus differed by vaccine type during 2013–2014 in the United States. J Infect Dis 2016;213:1546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zimmerman RK, Nowalk MP, Chung J. et al. 2014–2015 influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis 2016;63:1564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackson ML, Chung JR, Jackson LA. et al. Influenza vaccine effectiveness in the United States during the 2015–2016 season. N Engl J Med 2017;377:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Monto AS, Malosh RE, Petrie JG, Martin ET.. The doctrine of original antigenic sin: separating good from evil. J Infect Dis 2017;215:1782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoskins TW, Davies JR, Smith AJ, Miller CL, Allchin A.. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ’s Hospital. Lancet 1979;1:33–5. [DOI] [PubMed] [Google Scholar]
- 29. Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR.. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 1997;15:1114–22. [DOI] [PubMed] [Google Scholar]
- 30. Smith DJ, Forrest S, Ackley DH, Perelson AS.. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci USA 1999;96:14001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petrie JG, Parkhouse K, Ohmit SE, Malosh RE, Monto AS, Hensley SE.. Antibodies against the current influenza A(H1N1) vaccine strain do not protect some individuals from infection with contemporary circulating influenza A(H1N1) virus strains. J Infect Dis 2016;214:1947–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petrie JG, Ohmit SE, Cowling BJ. et al. Influenza transmission in a cohort of households with children: 2010-2011. PLoS One 2013;8:e75339.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCrone JT, Woods RJ, Martin ET, Malosh RE, Monto AS, Lauring AS.. Stochastic processes constrain the within and between host evolution of influenza virus. ELife 2018;7. doi: 10.7554/eLife.35962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petrie JG, Eisenberg MC, Ng S. et al. Application of an individual-based transmission hazard model for estimation of influenza vaccine effectiveness in a household cohort. Am J Epidemiol 2017;186:1380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DE Lastours V, Malosh R, Ramadugu K. et al. Co-colonization by Streptococcus pneumoniae and Staphylococcus aureus in the throat during acute respiratory illnesses. Epidemiol Infect 2016;144:3507–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Monto AS, Malosh RE, Petrie JG, Thompson MG, Ohmit SE.. Frequency of acute respiratory illnesses and circulation of respiratory viruses in households with children over 3 surveillance seasons. J Infect Dis 2014;210:1792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Erbelding EJ, Post DJ, Stemmy EJ. et al. A universal influenza vaccine: the strategic plan for the national institute of allergy and infectious diseases. J Infect Dis 2018;218:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stockwell MS, Reed C, Vargas CY. et al. MoSAIC: mobile surveillance for acute respiratory infections and influenza-like illness in the community. Am J Epidemiol 2014;180:1196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Byington CL, Ampofo K, Stockmann C. et al. Community surveillance of respiratory viruses among families in the Utah better identification of germs-longitudinal viral epidemiology (BIG-LoVE) study. Clin Infect Dis 2015;61:1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]