Why was the collaboration set up?
DynaHEALTH is a research consortium funded by the European Commission through the Horizon 2020 research programme. It was established to help solve the societal challenge of an ageing population and the associated burden of non-communicable diseases related to obesity and type 2 diabetes (T2D). The consortium brings together European researchers and datasets to build an empirical model of unhealthy ageing, with a longitudinal perspective, in which causal, bi-directional, mediating and confounding factors operate at a multi-dimensional level (Figure 1).
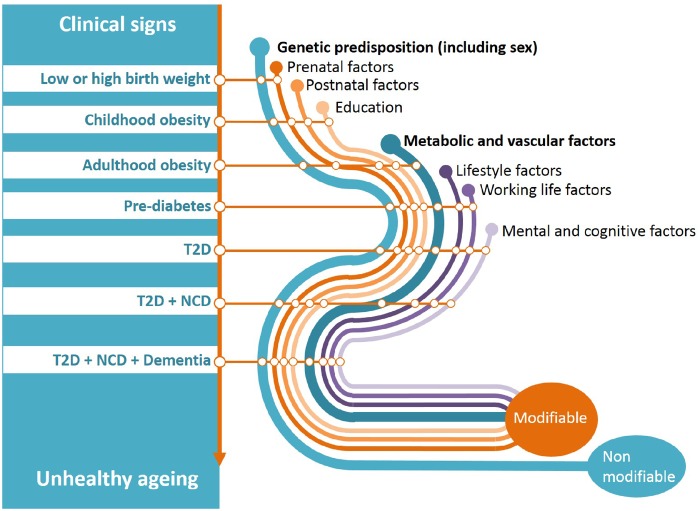
Figure 1.
Illustration of the conceptual framework for unhealthy ageing from early life to old age. From a life-course perspective, it suggests that clinical events accumulate or lead to each other, and co-occur with factors that either can or cannot be modified. (T2D=type 2 diabetes, NCD=non-communicable disease). (Reproduced from https://www.dynahealth.eu/ with permission).
The unhealthy ageing pathways often link altered adiposity in early life, early-onset obesity, T2D and the further accumulation of other chronic physical and mental non-communicable diseases in older ages. At each stage of the life-course there is potential to intervene either on suspected biological causes via classical clinical approaches, or other plausible causative pathways1 via integrated public health interventions (Figure 1). Successful interventions in early life have the potential to reduce subsequent investments in later life. Scientific collaborations in epidemiology and public health such as DynaHEALTH are built on a long-standing tradition of collecting prospective data. Moving on to the era of open sciences policy,2 meta-data analysis, the FAIR data principle (Findable, Accessible, Interoperable and Reusable) and the General Data Protection Regulation (GDPR), we must direct this public legacy in such a way as to better inform both policy makers and practitioners on complex patient and public health issues. Essentially, we must operate data to move beyond association studies and explore how the psychosocial factors, usually classified as confounders in medicine (Figure 2), can be analysed to help their operationalization and integration into healthcare programmes. Despite no apparent consensus in the literature on a single definition of psychosocial health, DynaHEALTH is referring to the following WHO definition3 as a guiding principle: ‘a state of wellbeing in which every individual realises their own potential, can cope with the normal stresses of everyday life, can work productively and fruitfully, and is able to make a contribution to their community’.4,5
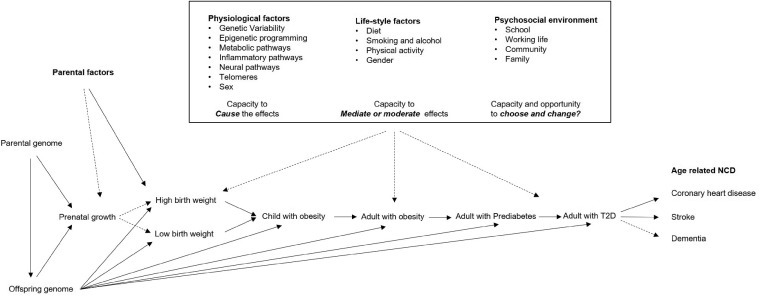
Figure 2.
Physiological, lifestyle and psychosocial factors and stressors acting on life-course pathways linking altered adiposity in early life to non-communicable diseases at older ages.
Conceptual framework of DynaHEALTH: The relationships between psychosocial factors, glycaemic health4,5 and healthy ageing, including a reduced risk of T2D and cardiovascular diseases, may be conceptualized in several different ways. It is an integral part of the DynaHEALTH consortium to develop these concepts and translate them into corresponding analytical study designs compatible with available data (Figure 2).
At the core of DynaHEALTH is the well-established observational and likely causal relationship between stages of disease development: firstly between adiposity and the risk of deteriorating glycaemic health and eventual T2D,6–8 and secondly between T2D and the risk of non-communicable diseases including stroke, coronary heart diseases, dementia and Alzheimer’s disease. All of these (pre-)clinical stages of disease development may share genetic, biological, lifestyle and psychosocial causes, but for both stages considerable knowledge gaps remain concerning action and impact on policies. We lack knowledge on identification of specific causal factors, the pathway(s) they operate through and their life-course aspects (Figure 2).
While considering these relationships, a number of hypotheses may be posed about the role of psychosocial factors throughout the life-course from the foetal period to old age.
Unidirectional causality hypothesis: Adverse psychosocial factors play a causal role in the development and worsening of adiposity, or of particular types of adiposity, with different relationships to glycaemic health and risk of T2D and subsequent morbidities.
Pleiotropy and interaction hypothesis: In the case of causality in such life-course pathways, it can be hypothesized that causal reactions to one or a set of psychosocial factors reflect underlying commonality influencing the clinical outcome in the life-course process. This can be analysed by exploring how the psychosocial factors may modify or contribute to the core relations between adiposity, glycaemic health, risk of T2D and the later onset of cardiovascular diseases, so that each of these relationships becomes stronger when the individuals are exposed to adverse psychosocial factors.
Bi-directional causality hypothesis: The relationships between adiposity, glycaemic health, T2D risk and cardiovascular diseases are worsening one single or set of psychosocial factor(s) in such a way that vicious cycles of deterioration are promoted.
Critical period hypothesis: There are specific time periods within the life-course during which psychosocial factors have a greater impact on these unhealthy ageing pathways.
Biological conversion hypothesis: The effects of psychosocial factors can be ‘transformed’ into causal biological effects. In this case, we want to identify the persisting structural effects during early life and the functional mechanisms, e.g. by epigenetic regulation of gene expression or changes in the metabolite profiles.
Gene–environment hypothesis: The genetic variation between individuals may modify the transformation of psychosocial factors into biological effects.
The impact on future public health recommendations and new technologies strongly depends on the capacity to test this set of hypotheses. This requires large statistical power or the development of a specific study design that is enabled by building sustainable and targeted datasets through consortia such as DynaHEALTH.
Who is in the consortium?
To date, there is no single longitudinal study with a sufficient density of data and long-term follow-up to allow a life-course study of unhealthy ageing via changes in adult glycaemic health. Nonetheless, there is a wealth of somewhat scattered, prospective studies with complementary designs which can be leveraged to study the dynamic determinants of life-long glycaemic health.
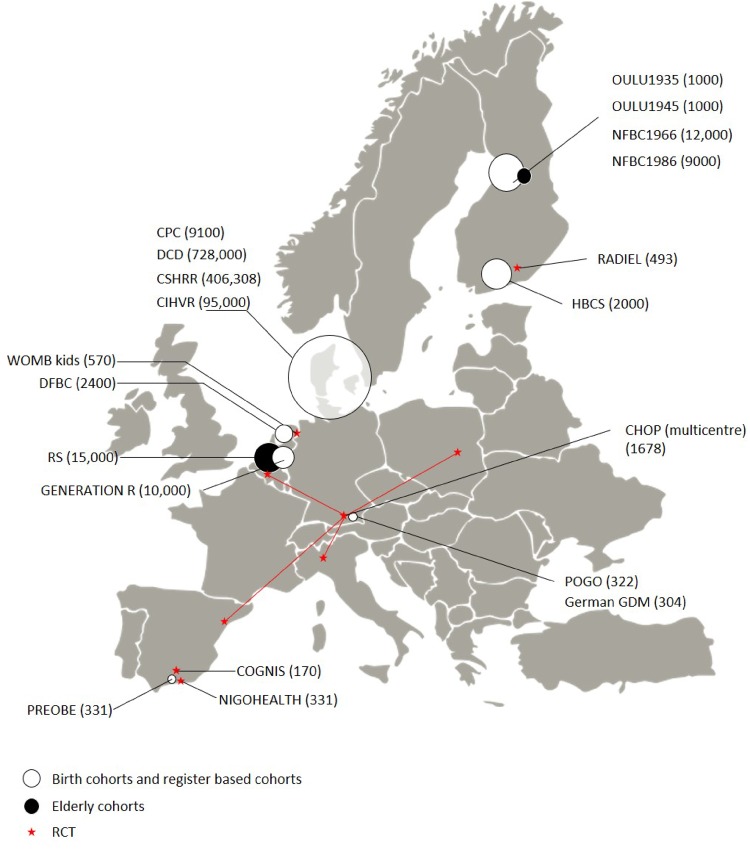
The DynaHEALTH consortium is a repertoire of human studies with longitudinal design where key variables have been inventoried for meta-analysis or triangulation of evidence to test specific epidemiological concepts. The data we are analysing are from two main types of study design: prospective longitudinal surveys and randomized controlled trials (RCT). The consortium currently consists of 20 studies (Table 1) with data from eight European Countries (Figure 3) on up to 1 368 699 participants (Figures 4). The oldest living participants are from the Helsinki Birth Cohort Study (HBCS) born in 1934 in the Finnish city of Helsinki, whereas the youngest were born in April 2018 in the NIGOHEALTH RCT in Granada, Spain. These 20 studies consist of 12 general population cohorts9–20 of which six have a focus on later life and ageing with data beyond 65 years of age.9–11,17–19 Three high-risk cohorts21–23 follow populations of offspring born to mothers with a risk of gestational diabetes (GDM), either via high pre-pregnancy body mass index (BMI), previous history of GDM or other known risk factors. Five RCTs focus24–28 on pre-conception, prenatal and early-life interventions to improve maternal health and child development.
Table 1.
List of cohorts and randomized controlled trials participating in the DynaHEALTH consortium and their contacts
| Abbreviation | Cohort full name | Contact |
|---|---|---|
| HBCS | Helsinki Birth Cohort Study | johan.eriksson@helsinki.fi |
| CSHRR | Copenhagen School Health Record Register | Jennifer.Lyn.Baker@regionh.dk |
| DFBC | Dutch Famine Birth Cohort | t.j.roseboom@amc.uva.nl |
| DCD | Danish conscription database | elme@sund.ku.dk |
| CPC | Copenhagen Perinatal Cohort | elme@sund.ku.dk |
| CIHVR | Copenhagen Infant Health Visitor Records | Jennifer.Lyn.Baker@regionh.dk |
| NFBC1966 | Northern Finland Birth Cohort 1966 | NFBCprojectcenter@oulu.fi |
| NFBC1986 | Northern Finland Birth Cohort 1986 | NFBCprojectcenter@oulu.fi |
| RS | Rotterdam Study | m.a.ikram@erasmusmc.nl |
| OULU1935 | Oulu cohort study | NFBCprojectcenter@oulu.fi |
| OULU1945 | Oulu cohort study | NFBCprojectcenter@oulu.fi |
| POGO German GDM | Postpartum Outcomes in Women with Gestational Diabetes and their Offspring | sandra.hummel@lrz.uni-muenchen.de |
| GENERATION R | Generation R Study | v.jaddoe@erasmusmc.nl or j.felix@erasmusmc.nl |
| PREOBE | Excellence Project PREOBE | ccampoy@ugr.es |
|
| ||
| Abbreviation | Randomized controlled trials full name | Contact |
|
| ||
| CHOP | Childhood Obesity Program | Berthold.Koletzko@med.uni-muenchen.de |
| RADIEL | Finnish Gestational Diabetes Prevention Study | johan.eriksson@helsinki.fi |
| COGNIS | A Neurocognitive and Immunological Study of a New Formula for Healthy Infants | Maria.Rodriguez@ordesa.es |
| WOMB | WOMB kids | t.j.roseboom@amc.uva.nl |
| NIGOHEALTH | Nutritional Intervention during Gestation and Offspring health | Ricardo.Rueda@abbott.com |
This table has been adapted from https://www.dynahealth.eu/ with permission.
Figure 3.
Map of studies participating in DynaHEALTH (sample size in brackets) by country. The size of the circle indicates the relative size of the study. Red arrows and stars show the participating centres of the multinational CHOP study.
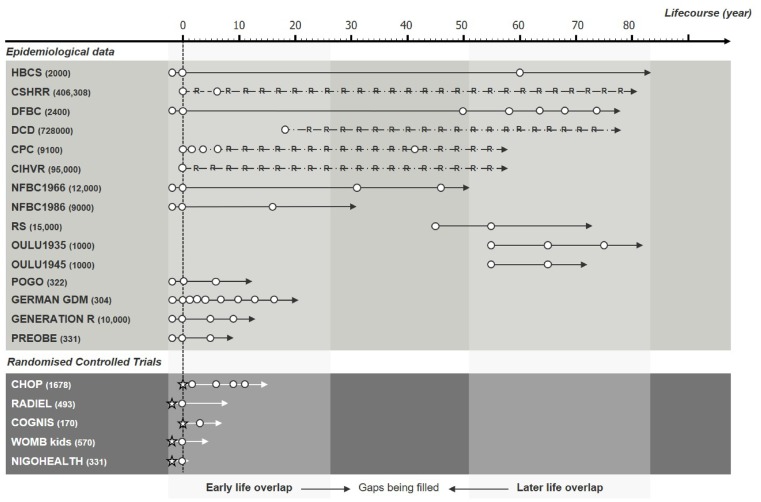
Figure 4.
DynaHEALTH datasets by size (in brackets), type, length and timing across the life-course, from the oldest study (top) to the youngest (bottom), separately for epidemiological longitudinal data and randomized controlled trials. White circles represent follow-up points, stars represent implementation of intervention and ‘R’ represents register-based datasets. Abbreviations are given in Table 1. (Reproduced from https://www.dynahealth.eu/ with permission).
How often have they been followed up?
Altogether there are 17 time periods in which data has been prospectively collected from the pre-conception period until age 80 years of age (see Figure 4 for study timelines). In addition, most follow-up time periods have data from at least two cohorts to allow replication or the use of imputation across studies. The cohort-specific descriptions of follow-ups are provided in Supplementary File 1, available as Supplementary data at IJE online.
What has been measured?
The DynaHEALTH Consortium offers the potential to extend our knowledge about the childhood origin of adult metabolic diseases and focus on key exposures during pregnancy and early life and glycaemic, cardiovascular and metabolic outcomes in later life.
The consortium data harmonization policy is following a research-based focus where only variables defined in template-based analytical plans are being proposed for harmonization. The set of common harmonized data for DynaHEALTH has followed these steps adapted from Rolland et al.29
Identification of the research questions that the harmonized data set is required to answer.
Identification of the high-level data concepts required to answer those questions as described in the conceptual framework of DynaHEALTH.
Assessment of data availability for data concepts.
Inventory of sets of pre-harmonized data due to collaboration in previous consortiums.
Development of analytical plans.
Development of harmonized data following the FAIR data management principles.
Retrospective harmonization of data, especially the psychosocial factors, can be a costly and sometimes an impossible process. In all cases analytical procedures shall account for the source of heterogeneity in inference which is addressed by the consortium via suitable meta-analytical processes and external replication.
In many cases, the cohort studies have been able to link with national, country-specific registers. These include databases such as population, hospital, education and employment registers, health visitor records, hospital and school records. This gives us the opportunity to use objective data alongside self-reported responses. It also enables us to obtain more information than collected from the surveys, such as clinical diagnosis, hospital visits, medication use and cause of death. We also use information from these registers to conduct attrition analyses in the case of participants lost during follow-up. In summary, Tables 2 and 3 provide an overview of data available within each cohort. The co-ordinating team in DynaHEALTH has created, and regularly updates, a detailed inventory showing available data within each cohort, time points and method of collection. This allows researchers to easily identify other data sources they could use to strengthen their study results, test a hypothesis or methodology, take a life-course approach by using data at earlier or later time points or investigate historical or cultural trends. A detailed inventory of what has been measured in each cohort can also be accessed on the DynaHEALTH website (http://dynahealth.eu/).
Table 2.
Data available in general population-based studies (√= data available, x= data not available, - =no data collection at this time point)
| Indicators of interest | HBCS | CSHRR | DFBC | DCD | CPC | CIHVR | NFBC1966 | NFBC1986 | RS | OULU1935 | OULU1945 | POGO | German GDM | GenR | PREOBE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pregnancy | |||||||||||||||
| Anthropometric measures | ✓ | - | ✓ | - | ✓ | × | ✓ | ✓ | - | - | - | ✓ | ✓ | ✓ | ✓ |
| Blood samples | × | - | × | - | × | × | ✓ | ✓ | - | - | - | ✓ | ✓ | ||
| Gestational diabetes | × | - | ✓ | - | × | × | × | ✓ | - | - | - | ✓ | ✓ | ✓ | ✓ |
| Socio-economic indicators | ✓ | - | ✓ | - | ✓ | ✓ | ✓ | ✓ | - | - | - | ✓ | ✓ | ✓ | ✓ |
| Health behaviours | × | - | × | - | ✓ | × | ✓ | ✓ | - | - | - | ✓ | × | ✓ | ✓ |
| Childhood (birth to 12 y) | |||||||||||||||
| Anthropometric measures | ✓ | ✓ | ✓ | - | ✓ | ✓ | ✓ | ✓ | - | - | - | ✓ | ✓ | ✓ | ✓ |
| Growth modelling | × | × | × | - | × | × | ✓ | ✓ | - | - | - | ✓ | ✓ | ✓ | ✓ |
| Blood samples | × | × | × | - | × | × | × | × | - | - | - | ✓ | ✓ | ✓ | ✓ |
| Developmental milestones | × | × | × | - | ✓ | ✓ | ✓ | ✓ | - | - | - | × | × | ✓ | ✓ |
| Early nutrition | ✓ | × | ✓ | - | ✓ | ✓ | ✓ | ✓ | - | - | - | ✓ | ✓ | ✓ | ✓ |
| Family lifestyle information | ✓ | ✓ | × | - | ✓ | × | ✓ | ✓ | - | - | - | × | × | ✓ | ✓ |
| Adolescence (13–18 y) | |||||||||||||||
| Anthropometric measurements | - | ✓ | × | - | ✓ | - | ✓ | ✓ | - | - | - | ✓ | ✓ | ✓ | - |
| Blood samples | - | × | × | - | × | - | × | ✓ | - | - | - | ✓ | ✓ | ✓ | - |
| Socio-economic indicators | - | ✓ | × | - | ✓ | - | ✓ | ✓ | - | - | - | ✓ | ✓ | ✓ | - |
| Health behaviours | - | × | × | - | × | - | × | ✓ | - | - | - | ✓ | × | ✓ | - |
| Adulthood (18y +) | |||||||||||||||
| Anthropometric measurements | ✓ | - | ✓ | ✓ | ✓ | - | ✓ | ✓ | ✓ | ✓ | ✓ | × | × | - | - |
| Blood samples | ✓ | - | ✓ | - | × | - | ✓ | ✓ | ✓ | ✓ | ✓ | × | × | - | - |
| Socio-economic indicators | ✓ | - | ✓ | ✓ | ✓ | - | ✓ | ✓ | ✓ | ✓ | ✓ | × | × | - | - |
| Health behaviours | ✓ | - | ✓ | - | ✓ | - | ✓ | ✓ | ✓ | ✓ | ✓ | × | × | - | - |
Table 3.
Data available or being collected for clinical trials in DynaHEALTH (√= data available, x= data not available, - =no data collection at this time point)
| Indicator of interest | CHOP | RADIEL | COGNIS | WOMB | NIGOHEALTH |
|---|---|---|---|---|---|
| Pregnancy | |||||
| Anthropometric measures | ✓ | ✓ | ✓ | ✓ | ✓ |
| Blood samples | × | ✓ | ✓ | ||
| Gestational diabetes | × | ✓ | ✓ | ✓ | ✓ |
| Socio-economic indicators | ✓ | ✓ | ✓ | ✓ | ✓ |
| Health behaviours | ✓ | ✓ | ✓ | ✓ | ✓ |
| Childhood (birth to 12 y) | |||||
| Anthropometric measures | ✓ | ✓ | ✓ | ✓ | ✓ |
| Growth modelling | ✓ | × | ✓ | × | ✓ |
| Blood samples | ✓ | ✓ | × | ✓ | ✓ |
| Developmental milestones | ✓ | ✓ | ✓ | ✓ | ✓ |
| Early nutrition | ✓ | ✓ | ✓ | ✓ | ✓ |
| Family lifestyle information | ✓ | × | ✓ | ✓ | ✓ |
| Adolescence (13–18 y) | |||||
| Anthropometric measurements | - | - | × | - | - |
| Blood samples | - | - | × | - | - |
| Socio-economic indicators | - | - | ✓ | - | - |
| Health behaviours | - | - | ✓ | - | - |
Pregnancy
A wealth of data is available from early life, as 16 of the study populations were established during pregnancy or even during the pre-conception period, following a cohort design or randomized clinical intervention (Figure 4). Maternal anthropometric measures such as height and weight have been recorded at various time points throughout pregnancy and in some studies at delivery, allowing the calculation of gestational weight gain. Blood samples have been taken from the mother during pregnancy and from the umbilical cord at birth enabling measures such as glucose, insulin or cardiovascular markers to be obtained. Additionally, extraction of DNA has enabled genotyping and DNA methylation arrays. Nuclear magnetic resonance (NMR)- or mass spectrometry-based metabolomics are now also available in a number of cohorts. Questionnaires were administered in some cohorts during pregnancy to collect social and demographic data such as work-related and household information. Health behaviours included smoking and alcohol use during pregnancy.
Childhood
Anthropometric information such as weight and height is particularly dense throughout early childhood as this was collected as part of routine practice within the health and welfare clinics in many European countries. Calculation of the BMI has allowed modelling of growth curves and enables subsequent extraction of growth traits such as age at adiposity peak and rebound, and peak weight and height velocity. In addition, the health visitor records are particularly beneficial in obtaining early-life exposures such as age of achievement of common motor and cognitive developmental milestones and breastfeeding duration. Some cohorts have also collected biological samples during this time period and more recently established studies include detailed body composition measurements such as body fat percentage and skinfold thickness. Many studies include questionnaire responses in relation to a host of lifestyle information such as dietary intake, physical condition and activity, sleep duration and quality, parental smoking and alcohol use, parental occupations and maternity leave.
Adolescence
Height, weight and BMI are readily available for participants during adolescence across the majority of cohorts, although measurements are less regular than in childhood. Some cohorts have collected biological samples that have allowed the inclusion of epigenetic and metabolomics data in collaborative analyses. Social information has been collected by questionnaire, primarily related to the social status of the parents. However, questions have been asked of the participants about their own smoking, alcohol and drug use, how they spend their leisure time and their typical diet. In addition, some cohorts are linked to registered data on school performance.
Adulthood and old age
Anthropometric measures are readily available at many stages of adulthood, as well as blood samples. These have been used to derive a host of indicators including common cardio-metabolic biomarkers such as glucose, insulin and lipid levels and have been used to derive epigenetic and metabolomics information. Almost all cohorts have a vast array of psychosocial variables from questionnaires and national registers. Extensive information is available on employment and work-related information such as type of occupation, hours of work, employment history and income. Educational level and occupational training of the participant and their parents is also reported in many cohorts. We can obtain a wealth of information relating to family life, such as marital status, number of children, housing situation and living environment and their changes over time. Many cohorts have collected a range of background information relating to lifestyle and health behaviours including diet, physical activity, smoking and alcohol consumption. For old-age populations especially, measures of metabolic traits and cognition have been collected.
What has DynaHEALTH found? Key findings and publications
A full list of publications arising from the DynaHEALTH action can be found on the project website (https://www.dynahealth.eu/publications-map). DynaHEALTH aims to operationalize a data-driven approach to provide evidence for the bio-psychosocial pathways of unhealthy ageing associated with alteration of glycaemic health. This should also be examined in the context of strong health inequalities and complex transgenerational issues operating from the pre-conceptional period onwards. In support of others, we have identified several metabolic alterations in mothers with obesity and GDM in comparison with normal weight mothers, associated with offspring health, including changes in DNA methylation,30 gene expression31 and/or later metabolic outcomes.32,33 From an age-related perspective, DynaHEALTH research has also contributed evidence to support links between glucose metabolism, psychosocial factors and the ageing process.34,35
As described in the above sections it is now essential to use and model the data to explore the nature of observed associations as a pre-requisite of informed decisions for prevention, intervention and policy making.36 For example, we have found little evidence for causality between maternal BMI during pregnancy and the child’s risk of obesity. Rather, it is explained by genetic transmission of BMI variants interacting with a stressful, obesogenic environment.37 This is supported by a further study showing that risk scores based on genetic variants linked to specific biological pathways influence body fat development from early life onwards. This study found an association between a genetic risk score based on adult BMI, and BMI at adiposity peak during infancy and abdominal fat measures at the age of 6 years.38
Ongoing research by the consortium is supporting the joint effects of bio-psychosocial factors on glucose metabolism.4 We have also established an opportunity to change the trajectory of an individual from childhood adiposity to T2D in later life.39
Strengths and weaknesses: how does DynaHEALTH offer a unique opportunity and what are the main challenges faced by the consortium?
DynaHEALTH exemplifies the wealth of prospective data collected in Europe that can be harnessed to enhance our understanding of healthy ageing by modelling the relationship between glycaemic health and psychosocial factors throughout the life-course. When combined, these data offer immense potential to inform future health policy in Europe. The data are organized to enable direct replication under collaborative agreements within the consortium and a number of observations can also be meta-analysed. While sample size allowing statistical power is deemed essential for robust evidence-based strategies, it is also important to combine study designs to validate findings under different statistical assumptions. DynaHEALTH’s additional strength is to include data from RCTs. Finally, the DynaHEALTH consortium includes both longitudinal birth cohorts and ageing cohorts from the same geographical location. This is the case in Northern Finland (NFBC1966/86 and Oulu 1935/45), the Rotterdam area (Rotterdam Study and Generation R Study) and in Copenhagen (CSHRR).
The critical mass of data, expertise and long-term collaboration brought together in DynaHEALTH offers a significant resource. However, the main challenge faced by the consortium is how best to combine the characteristics of the cohorts involved. The cohorts were established for their own individual purposes before being brought together under this project and the methods of data collection have thus not been standardized a priori across the consortium. Therefore, some consideration is required for the transferability of the statistical models and there are similar challenges in harmonizing the data. In addition, this is an international project and therefore there are differences between studies and countries in technology, questionnaire data and bio-specimen collection methods, terminology and diagnosis definitions, country-specific measurement techniques and ethical requirements.
However, the consortium has made significant progress in overcoming these challenges and the overarching opportunity for DynaHEALTH is that all studies provide rich data on similar key exposures and the outcome measures of interest.
Currently, there is no single cohort with data available from pre-conception to old age including information relating to both biological and psychosocial measures in relation to health outcomes. This consortium provides unique access to a number of studies ranging over different time periods encompassing different life stages, that will enable us to use a life-course approach to model the risk of poor glycaemic health and T2D, and to better understand the dynamics of how this will change in response to other factors throughout the life stages.
The collaborating cohorts include participants from eight European countries representing general, high-risk, obese and diabetic populations. This broad range of populations enables evaluation of the consistency of results and thus provides greater generalizability of consortium findings. We also have access to expert collaborators from academia and industry with expertise in life-course epidemiology, developmental biology, genetics and epigenetics, metabolomics, biostatistics, clinical nutrition, health care, brain imaging, econometrics and European policy and knowledge management.
The key value of DynaHEALTH is that we foresee the well-established, strong collaboration being used as a platform for many further efforts and continuing beyond the Horizon 2020 programme. Thus, this consortium may provide valuable assistance to investigators planning new cohort studies in terms of study design and the addition of new ideas, providing advice about data collection and management. DynaHEALTH will also offer an example of assumption-based modelling of the dynamic relations of the psychosocial and metabolic factors in the pathways of adiposity -> glycaemic health –> T2D risk –> risk of cardiovascular disease –> unhealthy ageing throughout the life course.
Can I get hold of the data? Where can I find out more?
The project is co-ordinated by the Centre for Life Course Health Research and the Northern Finland Cohort Project Centre at the University of Oulu in Finland. Further details on DynaHEALTH are available from the website: www.dynahealth.eu. The studies are approved by the local institutional review boards. Written informed consent has been obtained for participants. There is no central repository for the data and each participating cohort has its own policy for data sharing.
The DynaHEALTH project legacy will be a collaborative action that will invite researchers with longitudinal life-course data to engage with us via the website (https://www.dynahealth.eu/contact). To ensure continuity, the relevant section of the website and a light governance will remain to bring sustainability to the consortium and support the testing of its scientific concept.
DynaHEALTH profile in a nutshell
DynaHEALTH exemplifies the wealth of prospective data collected in Europe that can be harnessed to enhance our understanding of healthy ageing by modelling the relationship between glycaemic health and psychosocial factors throughout the life-course.
DynaHEALTH includes data on approximately 1.3 million subjects within 20 cohorts in eight European countries. Collectively, data spans the life-course from pre-conception through childhood and adulthood into older age.
Each individual study will adhere to its own protocol but generally we have repeated data collections during pregnancy and very early childhood. A number of studies have continued follow-up visits through to middle or old age. Other studies beginning in middle age have repeated measures relating to ageing.
Data have been collected via clinical examinations including biological samples, brain scan images and questionnaire data relating to psychosocial variables. Many studies also include linkage to national registers.
Further details are available from the DynaHEALTH website: www.dynahealth.eu
Funding
This project received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 633595.
Supplementary Material
Acknowledgements
Cohort-specific acknowledgements are listed in Supplement 2, available as Supplementary data at IJE online.
Conflict of interest: None declared.
References
- 1. Vandenbroucke JP, Broadbent A, Pearce N.. Causality and causal inference in epidemiology: the need for a pluralistic approach. Int J Epidemiol 2016;45:1776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Open Science Policy Platform. https://ec.europa.eu/research/openscience/index.cfm? pg=open-science-policy-platform (7 May 2018, date last accessed).
- 3.WHO Mental Health 2014. http://www.who.int/features/factfiles/mental_health/en/ (18 December 2017, date last accessed).
- 4. Lowry E, Rautio N, Karhunen V, Miettunen J, Ala-Mursula L, Auvinen J. Understanding the complexity of glycaemic health—systematic bio-psychosocial modelling of fasting glucose in middle-age adults; a DynaHEALTH study. Int J Obes (Lond) 2018; doi: 10.1038/s41366-018-0175-1. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 5. Danaei G, Finucane M, Lu Y, Singh G, Cowan M, Paciorek C.. National, regional and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 2011;378:31–40. [DOI] [PubMed] [Google Scholar]
- 6. Horikoshi M, Beaumont R, Day FR. et al. Genome-wide associations for birth weight and correlations with adult disease. Nature 2016;538:248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freathy RM, Mook-Kanamori DO, Sovio U. et al. Variants in ADCY5 and near CCNL1 are associated with fetal growth and birth weight. Nat Genet 2010;42:430–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sovio U, Kaakinen M, Tzoulaki I. et al. How do changes in body mass index in infancy and childhood associate with cardiometabolic profile in adulthood? Findings from the Northern Finland Birth Cohort 1966 Study. Int J Obes 2014;38:53–59. [DOI] [PubMed] [Google Scholar]
- 9. Osmond C, Kajantie E, Forsén T, Eriksson J, Barker D.. Infant growth and stroke in adult life: the Helsinki birth cohort study. Stroke 2007;38:264–70. [DOI] [PubMed] [Google Scholar]
- 10. Baker J, Olsen L, Andersen I, Pearson S, Hansen B, Sørensen T.. Cohort profile: The Copenhagen school health records register. Int J Epid 2009;23:656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Rooij SR, Roseboom TJ.. The developmental origins of ageing: study protocol for the Dutch famine birth cohort study on ageing. BMJ Open 2013;3:e003167.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christensen GT, Molbo D, Ängquist LH. et al. Cohort profile: The Danish Conscription Database(DCD): a cohort of 728, 160 men born from 1939 through 1959. Int J Epidemiol 2015;44:432–40. [DOI] [PubMed] [Google Scholar]
- 13. Bjerregaard LG, Rasmussen KM, Michaelsen KF. et al. Effects of body size and change in body size from infancy through childhood on body mass index in adulthood. Int J Obes (Lond) 2014;38:1305–11. [DOI] [PubMed] [Google Scholar]
- 14. Andersen LG, Holst C, Michaelsen KF, Baker JL, Sørensen TIA.. Weight and weight gain during early infancy predict childhood obesity: a case-cohort study. Int J Obes 2012;36:1306–11. [DOI] [PubMed] [Google Scholar]
- 15. Rantakallio P. The longitudinal study of the northern Finland birth cohort of 1966. Paediatr Perinat Epidemiol 1988;2:59–88. [DOI] [PubMed] [Google Scholar]
- 16. Järvelin M-R, Hartikainen-Sorri A-L, Rantakallio P.. Labour induction policy in hospitals of different levels of specialisation. Br J Obstet Gynaecol 1993;100:310–15. [DOI] [PubMed] [Google Scholar]
- 17. Ikram MA, Brusselle GGO, Murad SD, van Duijn CM, Franco OH, Goedegebure A.. The Rotterdam study: 2018 update on objectives, design and main results. Eur J Epidemiol 2017;32:807–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rajala U, Kein�Nen-Kiukaanniemi S, Kivel� S-L.. Non-insulin-dependent diabetes mellitus and depression in a middle-aged Finnish population. Soc Psychiatry Psychiatr Epidemiol 1997;32:363–67. [DOI] [PubMed] [Google Scholar]
- 19. Hirsso P, Rajala U, Hiltunen L. et al. Association of low-insulin sensitivity measured by quantitative insulin sensitivity check index with hair loss in 55-year-old men. A Finnish population-based study. Diabetes Obes Metab 2006;8:466–68. [DOI] [PubMed] [Google Scholar]
- 20. Kooijman MN, Kruithof CJ, van Duijn CM. et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol 2016;31:1243–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hummel S, Much D, Rossbauer M, Ziegler AG, Beyerlein A.. Postpartum outcomes in women with gestational diabetes and their offspring: POGO study design and first-year results. Rev Diabet Stud 2013;10:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boerschmann H, Pflüger M, Henneberger L, Ziegler AG, Hummel S.. Prevalence and predictors of overweight and insulin resistance in offspring of mothers with gestational diabetes mellitus. Diabetes Care 2010;33: 1845–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berglund S, García-Valdés L, Torres-Espinola FJ. et al. Maternal, fetal and perinatal alterations associated with obesity, overweight and gestational diabetes: an observational cohort study (PREOBE). BMC Public Health 2016;16:207.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koletzko B, von Kries R, Closa R. et al. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr 2009;89:1836–45. [DOI] [PubMed] [Google Scholar]
- 25. Rönö K, Stach-Lempinen B, Klemetti MM. et al. Prevention of gestational diabetes through lifestyle intervention: study design and methods of a Finnish randomized controlled multicenter trial (RADIEL). BMC Pregnancy Childbirth 2014;14:70.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laboratorios Ordesa. A Neurocognitive and Immunological Study of a New Formula for Healthy Infants (COGNIS) https://clinicaltrials.gov/ct2/show/NCT02094547 (7 May 2018, date last accessed).
- 27. van de Beek C, Hoek A, Painter RC. et al. Women, their Offspring and iMproving lifestyle for Better cardiovascular health of both (WOMB project): a protocol of the follow-up of a multicentre randomized controlled trial. BMJ Open 2018;8:e016579.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbott Nutrition. Nutritional Intervention During Gestation and Offspring Health. https://clinicaltrials.gov/ct2/show/NCT02285764.
- 29. Rolland B, Reid S, Stelling D. et al. Toward rigorous data harmonization in cancer epidemiology research: one approach. Am J Epidemiol 2015;182:1033–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharp GC, Salas LA, Monnereau C. et al. Maternal BMI at the start of pregnancy and offspring epigenome-wide DNA methylation: findings from the pregnancy and childhood epigenetics (PACE) consortium. Hum Mol Genet 2017;26:4067–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martino J, Sebert S, Segura MT. et al. Maternal body weight and gestational diabetes differentially influence placental and pregnancy outcomes. J Clin Endocrinol Metab 2016;101:59–68. doi: 10.1210/jc.2015-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santos Ferreira DL, Williams DM, Kangas AJ. et al. Association of pre-pregnancy body mass index with offspring metabolic profile: Analyses of 3 European prospective birth cohorts. PLoS Med 2017;14:e1002376.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Würtz P, Wang Q, Niironen M. et al. Metabolic signatures of birthweight in 18 288 adolescents and adults. Int J Epidemiol 2016;45:1539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rautio N, Varanka-Ruuska T, Vaaramo E. et al. Accumulated exposure to unemployment is related to impaired glucose metabolism in middle-aged men: A follow-up of the Northern Finland Birth Cohort 1966. Prim Care Diabetes 2017;11:365–72. [DOI] [PubMed] [Google Scholar]
- 35. von Bonsdorff MB, von Bonsdorff ME, Haanpää M. et al. Work-loss years among people diagnosed with diabetes: a reappraisal from a life course perspective. Acta Diabetol 2018;55:485–91. doi: 10.1007/s00592-018-1119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Swanson SA, Tiemeier H, Ikram MA, Hernán MA.. Nature as a Trialist?: deconstructing the analogy between mendelian randomization and randomized trials. Epidemiology 2017;28:653–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Richmond R, Timpson N, Felix J. et al. Using genetic variation to explore the causal effect of maternal pregnancy adiposity: a Mendelian randomisation study. PLOS Med 2017;2414:e1002221.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Monnereau C, Vogelezang S, Kruithof CJ, Jaddoe V, Felix J.. Associations of genetic risk scores based on adult adiposity pathways with childhood growth and adiposity measures. BMC Genetics 2016;17:120.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bjerregaard LG, Jensen BW, Ängquist L, Osler M, Sørensen TIA, Baker JL.. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N Engl J Med 2018;378:1302–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.