Abstract
Background
Lower levels of sun exposure in childhood have been suggested to be associated with increased risk of multiple sclerosis (MS). In this paper we extend previous work, using two novel analytical strategies.
Methods
Data collected in the Environmental risk factors In MS (EnvIMS) study, a case-control study with MS cases and population-based controls from Canada, Italy and Norway, were used. Participants reported on sun exposure behaviours for 5-year age intervals from birth; we focused on the first three age intervals (≤15 years). We compared two life course epidemiology conceptual models, the critical period and the accumulation model. We also used latent class analysis to estimate MS risk for different latent sun exposure behaviour groups.
Results
The analyses included 2251 cases and 4028 controls. The accumulation model was found to be the best model, which demonstrated a nearly 50% increased risk of MS comparing lowest reported summer sun exposure with highest [risk ratio (RR) = 1.47 (1.24, 1.74)]. The latent sun exposure behaviour group, characterized by low sun exposure during summer and winter and high sun protection use, had the highest risk of MS; a 76% increased risk as compared with the group with high sun exposure and low sun protection use [RR = 1.76 (1.27, 2.46)].
Conclusions
Our analyses provide novel insights into the link between sun exposure and MS. We demonstrate that more time indoors during childhood and early adolescence is linked with MS risk, and that sun protection behaviours in those who spend most time indoors may play a key role in increasing risk.
Keywords: Multiple sclerosis, aetiology, sun exposure, case-control, epidemiology
Key Messages
Low levels of sun exposure, throughout childhood, are associated with increased risk of MS.
MS risk is greatest among individuals who, throughout childhood, spent most time indoors, and in their limited time outdoors used sun protection frequently.
Among individuals who spent a lot of time outdoors during summer and a moderate amount of time during winter, sun protection did not impact on MS risk, supporting current recommendations to use sun protection when outdoors.
The findings highlight the importance of providing balanced sun exposure messages, tailored to specific populations, given geographical differences in weather patterns, sun behaviours, distribution of skin pigmentation and cultural practices.
Background
Multiple sclerosis (MS) is a chronic immune-mediated disease affecting the central nervous system. MS is believed to have a long latent period;1,2 the aetiologically relevant period is suggested to be during childhood or early adolescence.3–6 An important goal of aetiological research is to identify modifiable factors that we can intervene on to reduce the burden of disease. Sun exposure is a modifiable factor that has been the focus of public health, due to risk of skin cancer associated with over-exposure.
Several ecological studies have shown that MS prevalence is positively correlated with latitude7–9 and inversely correlated with ambient ultraviolet radiation.10–13 Case-control studies have also demonstrated an inverse association between frequency of time outdoors in the sun and risk of MS.14–23
Some case-control studies measured sun exposure generally over the lifetime (i.e. time spent outdoors), and others have examined MS risk across a number of different age periods,16,18,19,21 consistent with assessing a critical period model. The critical period and accumulation models are two conceptual life course epidemiology models that can be used to explain disease aetiology.24–26 Two case-control studies performed in Australia examined both the critical period model and cumulative exposure over longer periods of time, which is referred to as the accumulation model.19,21 These studies suggested that accumulation of exposure or lack thereof, in the case of sun exposure and MS risk, is an aetiologically relevant model to consider—although the two life course epidemiology models were not directly compared.
We extended previous work16 and used two novel analytical approaches to further explore the link between sun exposure and risk of MS. The first objective of this study was to directly compare two different aetiological models: critical period and accumulation. The second objective considered three sun behaviours simultaneously (i.e. summer sun exposure, winter sun exposure and sun protection use), to understand how these sun-related behaviours, taken together, contribute to MS risk.
Methods
Study design
The analyses presented build on those previously reported by our research group.16 A detailed description of the study design and methodology of the Environmental risk factors In MS study (EnvIMS) is presented elsewhere.27 Briefly, EnvIMS is a case-control study, conducted from 2009 to 2014, that included MS cases and population-based controls in five countries. Participants were required to be 18 years of age or older at the time of sampling, and two to four controls were frequency-matched to cases on year of birth (within 5 years), sex and area of residence. In Europe, cases were sampled from national or regional MS registries or databases, and controls from population-based registries (e.g. Statistics Norway). In Canada national or regional registers do not exist; cases were sampled from MS clinics, and controls through random digit dialling, in three major Canadian cities. Data collected in Canada, Italy and Norway are used here, as these countries enrolled the largest number of study participants. A self-administered questionnaire, the EnvIMS-Q,28 was mailed to participants’ homes. The EnvIMS-Q was translated into each language, and country-specific questionnaires had similar content and aesthetic.
Variables
Exposures variables
The sun exposure-related questions were similar to those used in previous studies;18,19,21 and had been shown to have test-retest reliability.28,29 Frequency of time spent outdoors in summer (summer was not strictly defined) was the main exposure for objective 1. Participants completed a matrix with four response options (i.e. ‘not that often’, ‘reasonably often’, ‘quite often’ and ‘virtually all the time’) for each age interval. In Canada and Italy, 5-year age intervals were used (i.e. 0–5, 6–10 and 11–15 years); whereas in Norway, age intervals were based on the schooling system, with the view that it would aid with recall (i.e. 0–6 years, 7–12 years, and 13–15 years). Although intervals were slightly different they were combined, given the substantial overlap in age (e.g. exposure between 0–5 years in Canada and Italy was combined with 0–6 years in Norway). For objective 2, in addition to frequency of summer sun exposure, exposures representing frequency of sun exposure during winter (same response options as summer exposure) and of sun protection use (‘almost always’, ‘quite often’, ‘sometimes’ and ‘seldom/never’) were used.
Outcome variable
MS diagnosis was defined based on McDonald30 or Poser et al.31 criteria for clinically and laboratory-supported definite or probable MS. Cases were required to have clinical disease onset within 10 years of the time of sampling.
Potential confounding variables
Age and sex were included in all models. Several other potential confounders were assessed. Infant sibling exposure was defined as the number of years with siblings under age 2 years, before age 6 years.32 Physical activity, between ages 13–19 years, was defined as either light and heavy physical activity, light physical activity only or no/minimal physical activity. Body size/shape for each age interval was measured using nine body shape silhouettes, characterizing increasing body size.33 Environmental tobacco smoke exposure in childhood, from mother and/or father, was considered. Other sun-related variables including frequency of winter sun exposure and sun protection, and phenotypic-related variables such as skin [10-point skin colour scale, lighter (1) to darker (10)34,35], eye and hair colour36 and tanning reaction to sun.37 Parent’s highest level of education and the participants’ ethnicity were also assessed. We included variables for self-reported history of mononucleosis infection, indoor allergies, outdoor allergies and other autoimmune disease(s) during or preceding the age interval being considered in the analysis.
Statistical methods
Objective 1 analyses
We used an analytical framework to compare life course epidemiology conceptual models, to examine how consistent the models are with the data.26 A series of regression models were created to represent the different conceptual models, which were then compared with a saturated regression model, using the Bayesian information criterion (BIC) and likelihood ratio test (LRT). The saturated model includes all possible parameters; and the goal was to identify a more parsimonious regression model, with model fit similar to the saturated regression model. The BIC and LRT are both metrics that are used to assess model fit by comparing the likelihood of nested models. BIC incorporates an adjustment for the number of independent variables, and a lower value suggests better model fit. For the LRT, a non-significant P-value suggests similar model fit.
A generalized linear regression model, with a logit link and binomial family, was used. Sun exposure during summer was dichotomized to compare those with lower levels (‘not that often’ or ‘reasonably often’, coded as 1), with those with higher levels (‘quite often’ or ‘virtually all the time’, coded as 0). Data from all three countries were combined, and fixed effects for country were included in all models; heterogeneity of effects by country was examined using an interaction term in the regression model. The frequency-matched variables, age and sex, were also included in all models. A backward deletion approach, using a greater than 10% change in estimate, was used to select a final set of confounding variables.38 As there were only 8% missing data, we used complete case analysis. Analyses were completed in Stata 11.0.39
The following two models were examined: (i) the critical period model and (ii) the accumulation model.24,26 A description of the model parameters is presented in online supplementary material, available as Supplementary data at IJE online. The critical period model suggests that there is a time period during which an individual is susceptible to exposures that determine disease risk, such as a certain age, age period, a developmental process (e.g. puberty) or other distinct event (e.g. pregnancy). We estimated the risk of MS associated with having low levels of outdoor sun exposure during summer compared with having higher levels, for three 5-year age intervals from birth. The accumulation model suggests that the longer the length of time an individual is exposed, the greater the risk of disease irrespective of when exposure occurs. We estimated the risk of MS associated with the sum of the number of the age intervals (0, 1, 2 or 3) for which an individual reported lower levels of sun exposure. Accumulation of exposure was modelled in two ways, as an ordinal variable and using indicator variables.
Objective 2 analyses
Latent class analysis was used to identify latent classes, which we call sun exposure behaviour groups, using indicator variables for frequency of summer sun exposure, winter sun exposure and sun protection use. We estimated the risk of MS across the sun exposure behaviour groups. Analyses were completed in Latent Gold 5.0 using the Step3 procedure.40
The Step3 procedure is completed in three steps. A cluster model was first used to determine the number of latent classes that fit the data best. Given the large dataset, the number of latent classes that were tested ranged from one to seven, and BIC was used to select the best model. Age and sex were included as covariates. Participants were then proportionally assigned to sun exposure behaviour groups, and finally group membership was regressed on MS status, using logistic regression. Relative risk was estimated two ways: (i) using effects coding, which compares risk in each group with the average risk across all groups; and (ii) using dummy coding, to estimate risk in the highest risk group, relative to the other groups.
Results
Demographic characteristics of the 2251 cases and 4028 controls are presented in Table 1. Cases had mean age of 42 years and 69% were female, and controls had mean age of 44 years and 70% were female. Average disease duration in cases was 6.5 years. Characteristics of sun exposure behaviours are presented in Table 2. The greatest proportion of participants reported being outdoors quite often during the summer, and either reasonably often or quite often in the winter, for all three age intervals. The proportion of almost always using sun protection decreased from birth to age 15 years; and the majority of participants reported seldom/never or sometimes using sun protection. Cases and controls had similar distributions for phenotypic characteristics, such as skin, hair and eye colour and tanning reaction to the sun.
Table 1.
Characteristics of study participants (cases and controls) enrolled in the Environmental risk factors In MS (EnvIMS) Study
| Participant characteristics | Cases (n = 2251) | Controls (n = 4028) |
|---|---|---|
| Country of residence, % (n) | ||
| Canada | 26.1 (587) | 24.3 (978) |
| Italy | 31.4 (707) | 33.1 (1333) |
| Norway | 42.5 (957) | 42.6 (1717) |
| Age at study (years), mean (SD, range) | 41.9 (10.7, 18–80) | 44.4 (11.5, 18–86) |
| MS disease duration (years) mean (SD, range) | 6.5 (2.8, 0–11) | n/a |
| Sex, % female (n) | 69.4 (1563) | 70.3 (2832) |
| Ethnicity (Canada and Norway only), % (n) | ||
| At least one parent is European | 94.0 (1451) | 93.0 (2507) |
| Both parents non-European | 3.2 (50) | 4.9 (132) |
| Missing | 2.8 (43) | 2.1 (56) |
| Participant education, % (n) | ||
| Less than high school | 12.0 (271) | 8.9 (357) |
| Completed high school | 29.5 (663) | 27.0 (1088) |
| Post-secondary education | 51.4 (1158) | 54.5 (2195) |
| Missing | 7.1 (159) | 9.6 (388) |
| Highest level of education of parents, % (n) | ||
| Less than high school | 52.1 (1172) | 54.5 (2195) |
| Completed high school | 19.1 (429) | 18.7 (755) |
| Post-secondary education | 20.0 (451) | 17.4 (700) |
| Missing | 8.8 (199) | 9.4 (378) |
| Number of siblings, % (n) | ||
| Only child | 5.0 (203) | 6.4 (143) |
| 1 | 28.3 (1139) | 30.0 (675) |
| 2 | 27.5 (1109) | 29.0 (652) |
| 3 | 16.4 (662) | 15.7 (353) |
| 4 | 8.8 (353) | 7.7 (173) |
| 5 | 5.1 (206) | 3.6 (80) |
| 6+ | 8.0 (324) | 6.1 (138) |
| Missing | 1.6 (37) | 0.8 (32) |
| Physical activity age 13–19 years, % (n) | ||
| Light and heavy physical activity | 84.3 (1897) | 85.3 (3441) |
| Light physical activity only | 8.4 (189) | 7.4 (296) |
| No physical activity | 2.8 (63) | 2.6 (103) |
| Missing | 4.5 (102) | 4.7 (188) |
| Mother smoked during childhood, % (n) | ||
| Yes | 44.6 (1004) | 41.2 (1660) |
| No | 52.2 (1175) | 56.3 (2266) |
| Missing | 3.2 (72) | 2.5 (102) |
| Father smoked during childhood, % (n) | ||
| Yes | 59.6 (1342) | 59.2 (2386) |
| No | 36.8 (829) | 37.9 (1525) |
| Missing | 3.6 (80) | 2.9 (117) |
| Body shape at age 5 years | ||
| Mean (SD, range) | 2.4 (1.5, 1–9) | 2.3 (1.5, 1–8) |
| Missing, % (n) | 7.3 (164) | 7, 1 (287) |
| Body shape at age 10 years | ||
| Mean (SD, range) | 2.6 (1.5, 1–9) | 2.4 (1.5, 1–9) |
| Missing, % (n) | 7.0 (158) | 6.5 (262) |
| Body shape at age 15 years | ||
| Mean (SD, range) | 2.9 (1.4, 1–9) | 2.7 (1.4, 1–8) |
| Missing, % (n) | 6.3 (142) | 6.0 (242) |
| Mononucleosis infection before age 15 years, % (n) | ||
| 0–5 years | 0.4 (10) | 0.2 (7) |
| 6–10 years | 1.4 (31) | 0.6 (25) |
| 11–15 years | 5.4 (121) | 2.2 (90) |
| Outdoor allergies before age 15 years, % (n) | ||
| 0–5 years | 2.2 (49) | 2.0 (79) |
| 6–10 years | 5.3 (119) | 4.6 (185) |
| 11–15 years | 8.3 (186) | 7.0 (280) |
| Indoor allergies before age 15 years, % (n) | ||
| 0–5 years | 1.9 (43) | 1.6 (64) |
| 6–10 years | 4.0 (89) | 3.3 (131) |
| 11–15 years | 5.5 (124) | 4.8 (192) |
| Autoimmune disease before age 15 years (not including MS), % (n) | ||
| 0–5 years | 1.0 (22) | 0.7 (27) |
| 6–10 years | 1.5 (34) | 1.3 (54) |
| 11–15 years | 2.0 (45) | 1.8 (74) |
Table 2.
Sun-related behaviours and phenotypic characteristics of study participants (cases and controls) enrolled in the Environmental risk factors In MS (EnvIMS) Study
| Characteristic, % (n) | Cases (n = 2251) | Controls (n = 4028) |
|---|---|---|
| Sun-related behaviours | ||
| Outdoor activities during summer | ||
| 0–5 years of age | ||
| Not that often | 5.8 (131) | 4.5 (183) |
| Reasonably often | 21.1 (475) | 18.7 (755) |
| Quite often | 41.1 (924) | 42.0 (1692) |
| Virtually all the time | 25.0 (562) | 28.8 (1158) |
| Missing | 7.1 (159) | 6.0 (240) |
| 6–10 years of age | ||
| Not that often | 1.6 (35) | 1.5 (60) |
| Reasonably often | 13.8 (310) | 11.6 (467) |
| Quite often | 43.9 (989) | 43 (1733) |
| Virtually all the time | 37 (832) | 40.3 (1623) |
| Missing | 3.8 (85) | 3.6 (145) |
| 11–15 years of age | ||
| Not that often | 2.6 (58) | 2.9 (88) |
| Reasonably often | 20.5 (461) | 17.1 (687) |
| Quite often | 45.5 (1024) | 46.8 (1886) |
| Virtually all the time | 27.9 (629) | 30.6 (1231) |
| Missing | 3.5 (79) | 3.4 (136) |
| Outdoor activities during winter | ||
| 0-5 years of age | ||
| Not that often | 17.6 (397) | 15.9 (641) |
| Reasonably often | 32 (721) | 33.6 (1354) |
| Quite often | 30.8 (694) | 30.4 (1226) |
| Virtually all the time | 10.1 (228) | 11 (442) |
| Missing | 9.4 (211) | 9.1 (365) |
| 6–10 years of age | ||
| Not that often | 8.2 (185) | 7.7 (310) |
| Reasonably often | 29.2 (658) | 27.9 (1124) |
| Quite often | 40.9 (921) | 41.6 (1676) |
| Virtually all the time | 15.3 (345) | 16 (646) |
| Missing | 6.3 (142) | 6.8 (272) |
| 11–15 years of age | ||
| Not that often | 7.9 (177) | 7.8 (314) |
| Reasonably often | 35.7 (804) | 33 (1328) |
| Quite often | 39 (878) | 39.9 (1608) |
| Virtually all the time | 11 (247) | 12.9 (519) |
| Missing | 6.4 (145) | 6.4 (259) |
| Sun protection use | ||
| 0–5 years of age | ||
| Seldom/never | 36.6 (823) | 40.5 (1632) |
| Sometimes | 17.7 (399) | 17 (684) |
| Quite often | 14 (316 | 12.9 (519) |
| Almost always | 18.8 (423) | 17.6 (709) |
| Missing | 12.9 (290) | 12.0 (484) |
| 6–10 years of age | ||
| Seldom/never | 36.4 (820) | 40.1 (1617) |
| Sometimes | 22.5 (507) | 21.2 (855) |
| Quite often | 15.3 (345) | 14.1 (567) |
| Almost always | 15.5 (349) | 15.2 (611) |
| Missing | 10.2 (230) | 9.4 (378) |
| 11–15 years of age | ||
| Seldom/never | 35.7 (804) | 37.8 (1522) |
| Sometimes | 33.7 (759) | 31.7 (1276) |
| Quite often | 15.3 (344) | 14.4 (581) |
| Almost always | 10 (226) | 11 (444) |
| Missing | 5.2 (118) | 5.1 (205) |
| Phenotypic characteristics | ||
| Skin colour | ||
| Mean (SD, range) | 4.0 (1.7, 1–10) | 4.0 (1.7, 1–10) |
| Missing | 3.4 (74) | 3.0 (119) |
| Natural hair colour | ||
| Black or dark brown | 42.3 (951) | 43.3 (1744) |
| Light brown | 33.6 (757) | 34.5 (1389) |
| Blonde or red | 22.7 (510) | 20.9 (843) |
| Missing | 1.5 (33) | 1.3 (52) |
| Natural eye colour | ||
| Darker (black, brown or hazel) | 46.7 (1052) | 46.8 (1884) |
| Lighter (grey/green or blue) | 50.7 (1142) | 51.3 (2068) |
| Missing | 2.5 (57) | 1.9 (76) |
| Tanning reaction to first sun | ||
| Always burns, never tans | 9.8 (221) | 8.4 (339) |
| Usually burns/sometimes tans | 26.7 (600) | 25.1 (1009) |
| Tan average/sometimes mild burn | 39.7 (894) | 42.9 (1727) |
| Rarely burns/more than average tan | 19.5 (438) | 19.7 (795) |
| Missing | 4.3 (98) | 3.9 (158) |
Objective 1 results
There were 5750 (92%) study participants with non-missing data on frequency of summer sun exposure. The majority of cases (59.8%) and controls (65.8%) reported a high frequency of outdoor sun exposure during summer at all three age intervals, whereas 10.7% of cases and 8.9% of controls reported a low frequency at all three age intervals. Compared with the saturated model, the accumulation model was found to be most consistent with the data, although the critical period models for age intervals 0–5 years and 11–15 years also had good fit criteria (Table 3). Based on the LRT, both accumulation models had similar fit to the saturated model; however, the model using an ordinal variable had the lowest BIC. Based on the LRT, the critical period models for age intervals 0–5 years and 11–15 years also had good fit, but the BIC for these models was larger than the ordinal accumulation model. Whereas the LRT for the 6–10 years model did not suggest better fit, the BIC, though larger, was similar in magnitude. Once age, sex and country were accounted for, no other potential confounders had a substantial impact on the main effect estimates. Based on these results, the ordinal accumulation model was selected as the best model. Risk ratio (RR) estimates for the three top models are presented in Table 4. The ordinal accumulation model suggested a 47% increased risk {RR = 1.47 [95% confidence interval (CI): 1.24, 1.74)]} comparing low sun exposure at all three age intervals with high sun exposure at all three age intervals. We did not identify heterogeneity of effects by country.
Table 3.
Bayesian information criterion (BIC) and likelihood ratio test (LRT) for the life course epidemiology models examined
| Model a | BIC | LRT(χ2, P-value) |
|---|---|---|
| Saturated | −42271.19 | – |
| Critical period (age period) | ||
| 0–5 years | −42314.23 | 8.90, 0.18 |
| 6–10 years | −42308.63 | 14.51, 0.03 |
| 11–15 years | −42311.88 | 11.26, 0.08 |
| Accumulation | ||
| Ordinal variable | −42318.59 | 4.55, 0.60 |
| Indicator variables | −42304.23 | 1.59, 0.81 |
Models include exposure variables and adjustment for age, sex and country.
Table 4.
Risk ratio estimates, for the three life course epidemiology models with the best model fit criteria, comparing the risk of MS in individuals reporting the lowest levels of summer sun exposure to those reporting highest levels of summer sun exposure
| Model | Risk ratio a (95% CI) |
|---|---|
| Accumulation model | 1.47b (1.24, 1.74) |
| Ordinal (0 to 3 age intervals) | |
| Critical period model | 1.29 (1.14, 1.45) |
| Age interval 0–5 years | |
| Critical period model | 1.29 (1.13, 1.47) |
| Age interval 11–15 years |
Estimates adjusted for age, sex and country.
Estimate is for a three-unit change in exposure (three age intervals with low summer sun exposure compared with three age intervals with high summer sun exposure).
Objective 2 results
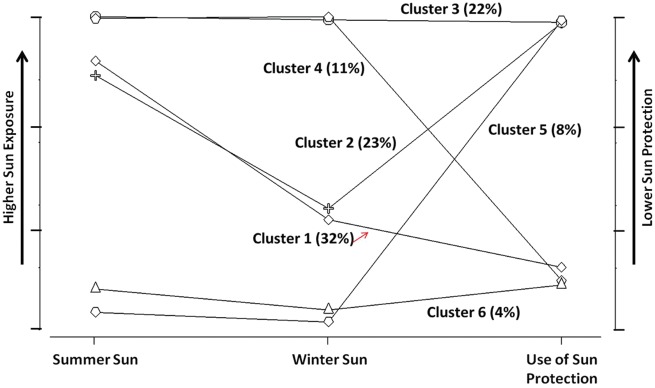
The ordinal accumulation variable was used for objective 2 analyses; accumulation variables for winter sun exposure and for use of sun protection were also used. The cluster model with six latent classes had the lowest BIC, and explained a large amount of variance (R2 >65%). The profile plot (Figure 1) displays the distribution of the six sun exposure behaviour groups; clusters are numbered in order of size (e.g. cluster 1 included the largest proportion of study participants and cluster 6 the smallest). There were two groups with high sun exposure during summer, but lower sun exposure during winter (clusters 1 and 2); two that had high sun exposure in summer and in winter (clusters 3 and 4); and two with low sun exposure in summer and in winter (clusters 5 and 6). Among these pairs, with similar sun exposure profiles, one frequently used sun protection whereas the other rarely used sun protection.
Figure 1.
Profile plot for the cluster model with six latent classes (the proportion of the sample in each cluster).
Sun exposure behaviour groups were found to be associated with MS status. Cases were most likely to be classified into the group characterized by low levels of sun exposure during summer and winter, and high levels of sun protection use (i.e. sun-avoiders). Compared with the average risk across groups, sun-avoiders had 20% increased risk of MS [RR = 1.20 (95% CI: 1.05, 1.37)] (Table 5). Comparing sun-avoiders with all other groups, risk was greatest relative to sun-seekers [i.e. cluster 3: high sun exposure during summer and winter, and low sun protection; RR = 1.76 (95% CI: 1.27, 2.46)]. Risk of MS in sun-avoiders was higher than in all other groups (Table 6). When comparing groups with similar outdoor sun exposure profiles but different levels of sun protection use (e.g. cluster 6 vs 5), there appeared to be an influence of sun protection on risk. Among individuals who spent a lot of time outdoors during summer, and a moderate amount of time during winter (cluster 1 vs 2), sun protection did not impact MS risk [RR = 1.03 (95% CI: 0.86, 1.23)]. Interestingly, when sun-avoiders (cluster 6) were compared with the group characterized by similarly low summer and winter sun exposure, but when outdoors rarely used sun protection ](cluster 5), the risk of MS was 40% greater in sun-avoiders [RR = 1.40 (95% CI: 0.96, 2.04)]. In addition, among individuals who were always outdoors but who used sun protection (cluster 4), there was a 20% increased risk of MS as compared with sun-seekers [cluster 3; RR = 1.21 (95% CI: 0.95–1.52)].
Table 5.
Risk ratio estimates for the six latent sun exposure behaviour groups, relative to average risk across all groups
| Cluster numbera | Risk ratio (95% CI) |
|---|---|
| 1 | 0.95 (0.90, 1.01) |
| 2 | 0.97 (0.90, 1.04) |
| 3 (sun-seekers) | 0.90 (0.84, 0.96) |
| 4 | 0.99 (0.91, 1.08) |
| 5 | 1.01 (0.92, 1.12) |
| 6 (sun-avoiders) | 1.20 (1.05, 1.37) |
Cluster characteristics are displayed in Figure 1, and described in the Results section.
Table 6.
Risk ratio estimates comparing the risk of MS in sun-avoiders (cluster 6), relative to the other latent sun exposure behaviour groups
| Clustera comparisons | Risk ratio (95% CI) |
|---|---|
| 6 vs 1 | 1.57 (1.13, 2.17) |
| 6 vs 2 | 1.53 (1.09, 2.15) |
| 6 vs 3 | 1.76 (1.27, 2.46) |
| 6 vs 4 | 1.46 (1.02, 2.08) |
| 6 vs 5 | 1.40 (0.96, 2.04) |
Cluster characteristics are displayed in Figure 1, and described in the text of the Results section.
Discussion
Our study is the largest case-control study, to date, to examine sun exposure and MS risk, and our analyses include three countries with high MS prevalence (i.e. Canada, Norway, and Sardinia and mainland Italy). We compared two life course epidemiology models and found that accumulation of sun exposure from birth to age 15 years best explained MS risk. Individuals who spent the least amount of time outdoors before the age of 15 years had a nearly 50% greater risk of MS, compared with those who spent the most amount of time outdoors. We also tested the critical period model, and our results suggest that models for age intervals (i) birth to 5 years of age and (ii) 11 to 15 years also had good model fit, perhaps suggesting an increased susceptibility to MS during both these age periods.
Our study was the first to consider all three sun exposure behaviours simultaneously. We identified six sun exposure behaviour groups that had different levels of summer sun exposure, winter sun exposure and sun protection use. Highest risk was found in sun-avoiders (e.g. low summer and winter exposure, and almost always using sun protection for all three age intervals); whereas lowest risk of MS was found in the sun-seeking group (i.e. high summer sun exposure, high winter sun exposure and seldom/never using sun protection for all three age intervals). When comparing sun-avoiders with sun-seekers, we estimated more than 75% increase in the risk of MS. Interestingly, sun protection appeared to influence risk when we compared groups with similar levels of outdoor sun exposure but different levels of sun protection use. The risk of MS was 40% greater in sun-avoiders than in the group that had similarly low summer and winter sun exposure but rarely used sun protection. Among the sun exposure behaviour groups that made up the majority of the sample (clusters 1 and 2 >50%) characterized by intermediate to high sun exposure during summer and lower sun exposure during winter, sun protection use did not appear to impact MS risk. This supports the importance of using sun protection when spending more time in the sun, to avoid negative health effects. However, in those who spend most time indoors, use of sun protection in their limited time outdoors may have negative consequences with respect to increased risk of MS.
Our results are in line with previous studies14–23 that have shown that lower levels of sun exposure during summer,16–19,21–23 during winter,16,18,19,21 and higher levels of sun protection use are associated with increased risk of MS.14–16 Most studies have focused on age-specific effects (i.e. critical period model).16,18,19,21 Two studies also estimated risk using an accumulation model, and indicated that cumulative exposure had a greater impact on MS risk than age-specific effects19,21 although the models were not directly compared (i.e. using appropriate model fit criteria).19,21 Our findings provide evidence to support the observation that risk of MS is determined over childhood, rather than during a specific period. However, collecting information from study participants about sun exposure for several age intervals throughout childhood, is important in order to capture, and model changes in behaviour.
Sun exposure is an important modifiable risk factor, as the ultraviolet rays emitted by the sun play a number of important roles in human physiology. Several of these have been implicated in the aetiology/pathogenesis of MS, such as production of vitamin D and immune system functioning (e.g. T lymphocytes, B lymphocytes, regulatory T cells).41,42 Vitamin D has been the focus of a lot of MS aetiological research, and has been shown to be causally related to MS in a study that used a Mendelian randomization study design.43 In addition to vitamin D production, sun exposure has additional health benefits.44 Both human and animal studies propose that ultraviolet radiation may impact on MS risk, independent of vitamin D.19,45 Childhood is an important time in life during which the immune system develops,46 making children particularly vulnerable to factors that predispose them to disease later in life.
Over time, sun exposure practices have changed as a result of public health promotion programmes to reduce skin cancer risk, and children are more likely to be protected when out in the sun or to be kept indoors during high exposure periods of the day.47 Whereas these measures are essential, a World Health Organization (WHO) report suggests that diseases associated with low sun exposure (e.g. rickets, osteomalacia and osteoporosis) account for greater burden of disease than those associated with high levels of sun exposure.48 It is thus imperative to provide balanced information about the sun’s benefits.49,50 Such messages may include the need to obtain short amounts of daily sun, at certain periods of the day to maximize benefits, while at the same time maintaining the current recommendations to use sun protection and limit excessive exposure during periods of high ultraviolet radiation. Sun safety messages should be tailored to the specific population, given geographical differences in weather patterns, sun behaviours, distribution of skin pigmentation and cultural practices.
Several measures were used to reduce the potential for bias in this study. Data from a population-based case-control study were used to enhance generalizability of results. The EnvIMS study questionnaire was tested for feasibility, applicability and reproducibility,28,29 and the sun-related questions have been shown to work well in previous studies.18,19,21 Nevertheless, non-differential misclassification remains a concern, as sun exposure is difficult to measure using self-report questionnaires and long duration of time from exposure to data collection. Interestingly, effect estimates were stronger in the sensitivity analyses that were restricted to those who reported having help from their mother and/or father, but did not appear to be affected by age at time of study or time since MS onset (see supplementary material, available as Supplementary data at IJE online). Recall bias cannot be dismissed, given the study design. The EnvIMS study was conducted before wide public awareness of a possible link between sun exposure and MS risk, and thus we would not expect a difference in reporting accuracy between cases and controls; however, heat negatively affects MS patients51–53 and current behaviours may impact on reporting of past behaviours: cases may be more likely to report lower levels of sun exposure, than controls.
We show that longer duration of time spent indoors during childhood is linked with increased MS risk. In addition, among children who spend the least amount of time in the sun, lower levels of sun protection may mitigate the negative effects that spending most time indoors has on the risk of developing MS in adulthood. However, among those who spent more time outdoors, sun protection did not appear to have an effect on MS risk. These findings provide support for promoting balanced safe sun practices to reduce disease burden, especially in countries and cultures where children spend a lot of time indoors.
Supplementary Material
Funding
This work was supported through several funding sources. S.M. received doctoral studentship funding from McGill University, the Canadian Institutes of Health Research and the MS Society of Canada. The EnvIMS study was supported by the University of Bergen, Norway, (2007 to T.R.) and the Canadian MS Scientific Research Foundation (2009–2010 to C.W.) for the early planning stages of the study and pilot studies. The full study was supported by the: Italian MS Society/Foundation (Fondazione Italiana Sclerosi Multipla, FISM, grants 2007/R/14, and 2008/R/19 to MP.); Sardinian Autonomous Regional Administration, Italy (Regione Autonoma della Sardegna, Assessorato all’Igiene, Sanità e dell’Assistenza Sociale to M.P.); Fondazione Banco di Sardegna, Italy; Western Norway Regional Health Authority (Helse Vest), Norway (grants 911421/2008 to M.P. and 911474/2009 to K-M.M.); Norwegian MS Society (MS-forbundet i Norge, 2009 to T.R.); Funds for Clinical Research University Hospital Linköping, Sweden (2010–2011 to A.M.L.); and Multiple Sclerosis Society of Canada (2011–2013 to C.W.).
Conflict of interest: M. Pugliatti has participated to Advisory Boards from Bayer Schering, Genzyme, Biogen, Merck Serono, and travel or speaker honoraria from Almirall, Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novartis, Sanofi-Aventis and Teva Neurosciences. K.M. Myhr has received unrestricted research grants to his institution and/or scientific advisory board or speakers honoraria from Almirall, Biogen, Genzyme, Merck, Novartis, Roche and Teva; and has participated in clinical trials organized by Biogen, Merck, Novartis and Roche. All other authors none to declare.
References
- 1. Wolfson C, Wolfson DB, Veri JP, Confavreux C.. Multiple sclerosis: a comparison of the latent periods of different populations. Neuroepidemiology 1993;12:300–06. [DOI] [PubMed] [Google Scholar]
- 2. Wolfson C, Wolfson DB, Zielinski JM.. On the estimation of the distribution of the latent period of multiple sclerosis. Neuroepidemiology 1989;8:239–48. [DOI] [PubMed] [Google Scholar]
- 3. Riise T, Gronning M, Klauber MR, Barrett-Connor E, Nyland H, Albrektsen G.. Clustering of residence of multiple sclerosis patients at age 13 to 20 years in Hordaland, Norway. Am J Epidemiol 1991;133:932. [DOI] [PubMed] [Google Scholar]
- 4. Pugliatti M, Solinas G, Sotgiu S, Castiglia P, Rosati G.. Multiple sclerosis distribution in northern Sardinia: spatial cluster analysis of prevalence. Neurology 2002;58:277–82. [DOI] [PubMed] [Google Scholar]
- 5. Pugliatti M, Riise T, Sotgiu MA. et al. Evidence of early childhood as the susceptibility period in multiple sclerosis: space-time cluster analysis in a Sardinian population. Am J Epidemiol 2006;164:326–33. [DOI] [PubMed] [Google Scholar]
- 6. McLeod JG, Hammond SR, Kurtzke JF.. Migration and multiple sclerosis in immigrants to Australia from United Kingdom and Ireland: a reassessment. I. Risk of MS by age at immigration. J Neurol 2011;258:1140–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alonso A, Hernan MA.. Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology 2008;71:129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zivadinov R, Iona L, Monti-Bragadin L. et al. The use of standardized incidence and prevalence rates in epidemiological studies on multiple sclerosis. A meta-analysis study. Neuroepidemiology 2003;22:65–74. [DOI] [PubMed] [Google Scholar]
- 9. Simpson S Jr, Blizzard L, Otahal P, Van der Mei I, Taylor B.. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry 2011;82:1132–41. [DOI] [PubMed] [Google Scholar]
- 10. Beretich BD, Beretich TM.. Explaining multiple sclerosis prevalence by ultraviolet exposure: a geospatial analysis. Mult Scler 2009;15:891. [DOI] [PubMed] [Google Scholar]
- 11. van der Mei IA, Ponsonby AL, Blizzard L, Dwyer T.. Regional variation in multiple sclerosis prevalence in Australia and its association with ambient ultraviolet radiation. Neuroepidemiology 2001;20:168–74. [DOI] [PubMed] [Google Scholar]
- 12. Sloka JS, Pryse-Phillips WE, Stefanelli M.. The relation of ultraviolet radiation and multiple sclerosis in Newfoundland. Can J Neurol Sci 2008;35:69–74. [DOI] [PubMed] [Google Scholar]
- 13. Sloka S, Silva C, Pryse-Phillips W, Patten S, Metz L, Yong VW.. A quantitative analysis of suspected environmental causes of MS. Can J Neurol Sci 2011;38:98–105. [DOI] [PubMed] [Google Scholar]
- 14. Alonso A, Cook SD, Maghzi AH, Divani AA.. A case-control study of risk factors for multiple sclerosis in Iran. Mult Scler 2011;17:550. [DOI] [PubMed] [Google Scholar]
- 15. Al-Shammri SN, Hanna MG, Chattopadhyay A, Akanji AO.. Sociocultural and demographic risk factors for the development of multiple sclerosis in Kuwait: a case-control study. PLoS One 2015;10:e0132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bjornevik K, Riise T, Casetta I. et al. Sun exposure and multiple sclerosis risk in Norway and Italy: the EnvIMS study. Mult Scler 2014;20:1042–49. [DOI] [PubMed] [Google Scholar]
- 17. Dalmay F, Bhalla D, Nicoletti A. et al. Multiple sclerosis and solar exposure before the age of 15 years: case-control study in Cuba, Martinique and Sicily. Mult Scler 2010;16:899–908. [DOI] [PubMed] [Google Scholar]
- 18. Kampman MT, Wilsgaard T, Mellgren SI.. Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J Neurol 2007;254:471–77. [DOI] [PubMed] [Google Scholar]
- 19. Lucas RM, Ponsonby AL, Dear K. et al. Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology 2011;76:540–48. [DOI] [PubMed] [Google Scholar]
- 20. Mansouri B, Asadollahi S, Heidari K. et al. Risk factors for increased multiple sclerosis susceptibility in the Iranian population. J Clin Neurosci 2014;21:2207–11. [DOI] [PubMed] [Google Scholar]
- 21. van der Mei IA, Ponsonby AL, Dwyer T. et al. Past exposure to sun, skin phenotype, and risk of multiple sclerosis: case-control study. BMJ 2003;327:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tremlett H, Zhu F, Ascherio A, Munger KL.. Sun exposure over the life course and associations with multiple sclerosis. Neurology 2018;90:e1191–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Langer-Gould A, Lucas R, Xiang AH. et al. MS Sunshine Study: sun exposure but not vitamin D is associated with multiple sclerosis risk in Blacks and Hispanics. Nutrients 2018;10:268.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ben-Shlomo Y, Kuh D.. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol 2002;31:285–93. [PubMed] [Google Scholar]
- 25. Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C.. Life course epidemiology. J Epidemiol Community Health 2003;57:778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mishra G, Nitsch D, Black S, De Stavola B, Kuh D, Hardy R.. A structured approach to modelling the effects of binary exposure variables over the life course. Int J Epidemiol 2009;38:528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Magalhaes S, Pugliatti M, Casetta I. et al. The EnvIMS Study: design and methodology of an international case-control study of environmental risk factors in multiple sclerosis. Neuroepidemiology 2015;44:173–81. [DOI] [PubMed] [Google Scholar]
- 28. Pugliatti M, Casetta I, Drulovic J. et al. A questionnaire for multinational case-control studies of environmental risk factors in multiple sclerosis (EnvIMS-Q). Acta Neurol Scand Suppl 2012;126:43–50. [DOI] [PubMed] [Google Scholar]
- 29. van der Mei IA, Blizzard L, Ponsonby AL, Dwyer T.. Validity and reliability of adult recall of past sun exposure in a case-control study of multiple sclerosis. Cancer Epidemiol Biomarkers Prev 2006;15:1538–44. [DOI] [PubMed] [Google Scholar]
- 30. Polman CH, Reingold SC, Banwell B. et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poser CM, Paty DW, Scheinberg L. et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227–31. [DOI] [PubMed] [Google Scholar]
- 32. Ponsonby A, van der Mei I, Dwyer T. et al. Exposure to infant siblings during early life and risk of multiple sclerosis. JAMA 2005;293:463–69. [DOI] [PubMed] [Google Scholar]
- 33. Stunkard AJ, Sørensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis 1983;60:115–20. [PubMed] [Google Scholar]
- 34. Pezic A, Ponsonby AL, Cameron FJ. et al. Constitutive and relative facultative skin pigmentation among Victorian children including comparison of two visual skin charts for determining constitutive melanin density. Photochem Photobiol 2013;89:714–23. [DOI] [PubMed] [Google Scholar]
- 35. Lund E, Dumeaux V, Braaten T. et al. Cohort Profile: The Norwegian Women and Cancer Study—NOWAC. Int J Epidemiol 2008;37:36–41. [DOI] [PubMed] [Google Scholar]
- 36. Ballone E, Passamonti M, Lappa G, Di Blasio G, Fazii P.. Pigmentary traits, nevi and skin phototypes in a youth population of Central Italy. Eur J Epidemiol 1999;15:189–95. [DOI] [PubMed] [Google Scholar]
- 37. Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 1988;124:869–71. [DOI] [PubMed] [Google Scholar]
- 38. Rothman K, Greenland S, Lash T.. Modern Epidemiology. 3rd edn. Philadelphia, PA: Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 39. StataCorp.. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP, 2009. [Google Scholar]
- 40. Vermunt JK, Magidson J.. Latent GOLD 5.0 Upgrade Manual. Belmont, MA: Statistical Innovations Inc, 2013. [Google Scholar]
- 41. Hart PH, Gorman S, Finlay-Jones JJ.. Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat Rev Immunol 2011;11:584–96. [DOI] [PubMed] [Google Scholar]
- 42. Lucas RM, Byrne SN, Correale J, Ilschner S, Hart PH.. Ultraviolet radiation, vitamin D and multiple sclerosis. Neurodegener Dis Manag 2015;5:413–24. [DOI] [PubMed] [Google Scholar]
- 43. Harroud A, Richards JB.. Mendelian randomization in multiple sclerosis: a causal role for vitamin D and obesity? Mult Scler 2018;24:80–85. [DOI] [PubMed] [Google Scholar]
- 44. Juzeniene A, Moan J.. Beneficial effects of UV radiation other than via vitamin D production. Dermatoendocrinology 2012;4:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Becklund BR, Severson KS, Vang SV, DeLuca HF.. UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proc Natl Acad Sci U S A 2010;107:6418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Simon AK, Hollander GA, McMichael A.. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 2015;282:20143085.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization (WHO). Protection Against Exposure to Ultraviolet Radiation. Geneva: WHO, 1995. [Google Scholar]
- 48. Lucas RM, McMichael AJ, Armstrong BK, Smith WT.. Estimating the global disease burden due to ultraviolet radiation exposure. Int J Epidemiol 2008;37:654–67. [DOI] [PubMed] [Google Scholar]
- 49. Lucas RM, Repacholi MH, McMichael AJ.. Is the current public health message on UV exposure correct? Bull World Health Organ 2006;84:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hoel DG, Berwick M, de Gruijl FR, Holick MF.. The risks and benefits of sun exposure 2016. Dermatoendocrinology 2016;8:e1248325.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Romberg A, Ikonen A, Ruutiainen J, Virtanen A, Hamalainen P.. The effects of heat stress on physical functioning in persons with multiple sclerosis. J Neurol Sci 2012;319:42–46. [DOI] [PubMed] [Google Scholar]
- 52. Flensner G, Ek AC, Soderhamn O, Landtblom AM.. Sensitivity to heat in MS patients: a factor strongly influencing symptomology - an explorative survey. BMC Neurol 2011;11:27.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Petrilli S, Durufle A, Nicolas B. et al. Influence of temperature changes on clinical symptoms in multiple sclerosis: an epidemiologic study. Ann Readapt Med Phys 2004;47:204–08. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.