Abstract
Background
Earlier age at menopause has been associated with increased risk of coronary heart disease (CHD), but the shape of association and role of established cardiovascular risk factors remain unclear. Therefore, we examined the associations between menopausal characteristics and CHD risk; the shape of the association between age at menopause and CHD risk; and the extent to which these associations are explained by established cardiovascular risk factors.
Methods
We used data from EPIC-CVD, a case–cohort study, which includes data from 23 centres from 10 European countries. We included only women, of whom 10 880 comprise the randomly selected sub-cohort, supplemented with 4522 cases outside the sub-cohort. We conducted Prentice-weighted Cox proportional hazards regressions with age as the underlying time scale, stratified by country and adjusted for relevant confounders.
Results
After confounder and intermediate adjustment, post-menopausal women were not at higher CHD risk compared with pre-menopausal women. Among post-menopausal women, earlier menopause was linearly associated with higher CHD risk [HRconfounder and intermediate adjusted per-year decrease = 1.02, 95% confidence interval (CI) = 1.01–1.03, p = 0.001]. Women with a surgical menopause were at higher risk of CHD compared with those with natural menopause (HRconfounder-adjusted = 1.25, 95% CI = 1.10–1.42, p < 0.001), but this attenuated after additional adjustment for age at menopause and intermediates (HR = 1.12, 95% CI = 0.96–1.29, p = 0.15). A proportion of the association was explained by cardiovascular risk factors.
Conclusions
Earlier and surgical menopause were associated with higher CHD risk. These associations could partially be explained by differences in conventional cardiovascular risk factors. These women might benefit from close monitoring of cardiovascular risk factors and disease.
Keywords: Menopause, coronary disease, ageing, Epidemiology, women, risk factors
Key Messages
Age at menopause has an inverse dose–response relationship with coronary heart disease (CHD) risk.
Surgical menopause is associated with an increased CHD risk, even after accounting for age at menopause.
A proportion of the risk appears to be explained by cardiovascular risk factors.
As a residual association between menopausal characteristics and CHD remains and the mechanism is not fully understood, this merits further research.
Introduction
Cardiovascular disease (CVD) is the leading cause of death in men and women from Western countries, with 17.5 million deaths worldwide in 2012, representing 31% of all global deaths.1 Approximately 7.4 million of these were due to coronary heart disease (CHD). CHD risk increases in women after the age of 50 years, leading to suggestions that menopause may be a contributing factor.2–4 A recent meta-analysis suggested that women who had early menopause (before age 45 years) are at 50% higher CHD risk compared with those with later menopause.5 However, that analysis was not able to include 9 studies out of the 14 studies they found examining the association between age at menopause and CHD, nor was it able to examine whether there is a (non-)linear dose–response relationship or threshold effect or whether type of menopause (surgical or natural) was associated with CHD risk.
The biological mechanisms through which menopause might influence CHD risk are postulated to include reductions in oestrogen levels, but rises in conventional cardiovascular risk factors (e.g. major lipids and blood pressure) around the time of menopause may also play a role.6–8 However, the extent to which the association between menopausal characteristics and CHD can be explained by such factors remains unclear.
We conducted a large pan-European prospective case–cohort study (EPIC-CVD) with an average of 11 years of follow-up to quantify the associations of menopausal status, age at menopause and type of menopause with risk of CHD; we also examined the shape of the relationship between age at menopause (as a continuous exposure) and risk of CHD; and we assessed the extent to which the associations of menopausal characteristics with risk of CHD could be explained by established cardiovascular risk factors.
Methods
Participants
We used data from female participants in the EPIC-CVD study—a case–cohort study nested within the European Prospective Investigation into Cancer and Nutrition (EPIC) study.9 EPIC consists of 519 978 adults (366 521 women), aged between 35 and 70 years at baseline, and recruited from 23 centres across 10 European countries (Denmark, France, Germany, Greece, Italy, The Netherlands, Norway, Spain, Sweden and the UK) between 1992 and 2000. Baseline questionnaires included questions on diet, lifestyle, reproductive and medical factors. Blood samples were collected for approximately 70% of the participants and stored in liquid nitrogen at –196°C. For EPIC-CVD, a representative sub-cohort of 18 249 participants was selected by simple random sampling, stratified by centre, from participants who had available stored blood and buffy coat samples.10,11 After exclusion of 609 participants with a prior history of myocardial infarction or stroke at baseline, 17 640 sub-cohort members remained. After subsequent exclusion of the 6760 men, a sub-cohort of 10 880 women remained, of whom 231 had a CHD event. Subsequently, incident CHD cases in women outside the sub-cohort were added to the study sample using the same exclusion criteria (N = 4522).
EPIC complies with the Declaration of Helsinki, and all participants gave written informed consent before participating in this study. The study was approved by the local ethics committees of the participating centres and the Institutional Review Board of the International Agency for Research on Cancer (IARC, Lyon).
Menopausal status, timing and type of menopause
Menopause was assessed by questionnaire at baseline. Women were categorized as pre-menopausal if they had experienced menses over the past 12 months before recruitment and by design, for women with missing or incomplete questionnaires, if they were 54 years or younger at recruitment. The pre-menopausal group also includes the peri-menopausal women, since numbers were too small to analyse them as a separate group. Women were categorized as post-menopausal if they had experienced no menses for 12 months or longer due to natural or surgical menopause and by design, for women with missing or incomplete questionnaire data, if they were 55 years or older at recruitment.12
Post-menopausal women were classified as having had a natural or surgical menopause, where surgical menopause was defined as having had a hysterectomy, unilateral or bilateral oophorectomy, only when age at surgery preceded or was equal to age at menopause. In the Malmö centre, since the age at removal of a woman’s womb and/or one or both ovaries was not recorded, women were classified as having had a surgical menopause regardless of age at surgery and age at menopause was then imputed (see below). For naturally post-menopausal women, age at menopause was defined as the age at which they had their last menstruation. For surgically post-menopausal women, their age at surgery was used instead. Since most other studies compare early menopause with late menopause, we present risk associations for decreases (rather than increases) in age at menopause, by multiplying age at menopause by –1.
Covariate measurement
Baseline questionnaires included questions on age, smoking status (current, former, never), highest education level (no schooling/primary school, secondary school, vocational education/university), age at menarche (≤10, 11, 12, 13, 14, 15, 16, ≥17 years), full-term pregnancy (yes/no) and whether participants had ever used post-menopausal hormones (yes/no). All centres used trained professionals to measure height and weight except the French centre, for which self-reported measures were used for a subset of participants, and Oxford, for which recalibrated self-reported measures were used based on a comparison between self-reported and measured data in a subset of participants. Both height and weight were adjusted for clothing worn.9,13 Body mass index (BMI) was calculated as weight divided by the square of height in metres and was categorized (≤20, >20 to <25, ≥25 to <30, ≥30 kg/m2). Physical activity was categorized using the Cambridge Physical Activity Index into inactive, moderately inactive, moderately active and active.14 Baseline systolic and diastolic blood pressure measurements were available in 62% of participants.11 Therefore, to maximize the availability of information, we used a composite variable (‘high blood pressure’, available in 98% of participants) defined as any of self-reported hypertension, self-reported use of anti-hypertensive medication, systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg.
Serum biomarkers were measured in baseline non-fasted samples at Stichting Huisartsen Laboratorium (Etten-Leur, The Netherlands) and included high-sensitivity C-reactive protein (hsCRP), total cholesterol, high-density lipoprotein cholesterol (HDL-c) and triglycerides. Erythrocyte haemoglobin A1c (HbA1c) was measured using the Tosoh-G8 HPLC analyser (Tosoh Bioscience, Japan); all other biomarkers were measured using a Cobas enzymatic assay (Roche Diagnostics, Mannheim, Germany) on a Roche HitachiModular P analyser.
First fatal or non-fatal CHD event
First fatal or non-fatal CHD events were defined by codes 410–414 of the International classification of diseases Ninth Edition (ICD-9) and codes I20–I25 of the Tenth Edition (ICD-10). Methods used in the recruitment centres to determine first non-fatal CHD events included self-report and linkage with morbidity or hospital registries. Non-fatal CHD events were further validated by a review of medical records and/or linkage with registries. Fatal CHD events were generally determined through mortality registries.11 The final year of follow-up for CHD events varied between centres from 2003 to 2010 and median follow-up time was 11 years.
Statistical analyses
Missing values in the exposures and covariates were imputed with multiple imputation using the package MICE in R15 with 10 imputations and 50 iterations (Supplementary Appendix 1, available as Supplementary data at IJE online). Women from Norway were excluded prior to imputation due to high levels of missing data. Hazard ratios were estimated using Prentice-weighted Cox proportional hazards regression, with age as the underlying time scale and with country-stratified baseline hazards.16 Robust standard errors were used to construct 95% confidence intervals (CIs). In order to study the association between menopausal characteristics and CHD, three levels of covariate adjustment were applied: adjustment for age at baseline only (age-adjusted model), further adjustment for CHD and reproductive risk factors: smoking status, BMI, HbA1c, education level, physical activity, age at menarche, full-term pregnancy and ever hormone use (confounder-adjusted model). The third model—the confounder- and intermediate-adjusted model—additionally includes the established cardiovascular risk factors that might mediate the association between menopausal characteristics and CHD (total cholesterol, HDL-c, triglycerides, high blood pressure and C-reactive protein). Since the association between menopausal age and CHD may vary depending on smoking and obesity status,17 we also assessed effect-modification by including interaction terms between menopausal age and smoking status and between menopausal age and obesity status, respectively, in the confounder-adjusted model. Surgically post-menopausal women tend to have an earlier age at menopause.18–20 Thus, the analysis of type of menopause was also adjusted for age at menopause (Model 3b).
To verify the expected linear relationship between age at menopause and CHD, we used floating absolute risks to display the hazard ratios (HRs) for age at menopause categories [<40, 40–44, 45–49, 50–54, >55 years (reference)] and CHD risk in the confounder-adjusted model. Instead of using a fixed value for the reference group, floating absolute risks redistribute the overall variance across the groups, which results in a reference category with a CI and narrower CIs for the other categories.21
To estimate the proportion of the association between menopause and CHD risk that could be explained by potential mediators that were also CVD risk factors, we used the difference method22,23 for which two regression coefficients of the exposure–outcome association are required: the direct effect (i.e. with adjustment for the possible mediators or established CVD risk factors) and the total effect (without adjustment). First, the total effect of each menopausal characteristic on CHD was estimated based on Model 1 (adjusted for age). Subsequently, for each model of adjustment separately, we estimated the direct effect when removing the indirect via the added risk factors. The proportion of the effect explained (PE) by the mediators was then calculated as: PE = (total effect – direct effect)/total effect, where effects were considered on the logarithmic scale, i.e. log(HR). Thereafter, we performed the same analyses for each separate risk factor. Bootstrap re-sampling (1000 bootstrap samples) was used to obtain 95% CIs around the PE (Supplementary Appendix 1, available as Supplementary data at IJE online).
We performed three sensitivity analyses: (i) restricting to women who had never used hormone therapy, since age at menopause may be difficult to determine under hormone use and the effects of surgical menopause on CHD are attenuated in women using hormone therapy (HT)24–26; (ii) excluding the first 2 years of follow-up to reduce the likelihood of reverse causality; (iii) excluding women with unilateral oophorectomy or hysterectomy from the surgical menopause category to reflect alternative definitions of surgical menopause used previously. We also conducted a complete case analysis and compared results with the multiple imputation approach. All analyses were performed on each imputed dataset separately and the estimates were pooled using Rubin’s rules,27 with R version 3.2.0.28
Results
After exclusions, there were 10 880 women in the sub-cohort and 4753 incident CHD cases (231 of whom were also in the sub-cohort) comprising a total of 15 402 participants, of whom 5486 were pre-menopausal and 9916 were post-menopausal. Compared with pre-menopausal women, post-menopausal women in the sub-cohort were older, less likely to be smokers, less educated, more likely to have a history of high blood pressure and had higher total cholesterol levels and BMI (Table 1). Mean age at menopause was 49.2 years [standard deviation (SD) 4.5] for women with a natural menopause and 45.1 years (SD 5.8) for women with a surgical menopause. Within post-menopausal women, natural post-menopausal women more often had a high blood pressure, less often used HT and they had a higher age at menopause compared with surgical post-menopausal women (Supplementary Appendix Table 1, available as Supplementary data at IJE online).
Table 1.
Baseline characteristics of women in the sub-cohort of the EPIC-CVD case–cohort study
| Pre-menopausal (N = 5486) | Post-menopausal (N = 9916) | |
|---|---|---|
| CHD risk factors | ||
| Age at baseline (years) | 44.8 ± 6.5 | 59.7 ± 6.8 |
| Smoking status | ||
| Never | 2788 (51.7%) | 5386 (55.0%) |
| Former | 1133 (21.0%) | 2141 (21.9%) |
| Current | 1474 (27.3%) | 2266 (23.1%) |
| Body mass index (kg/m2)a | ||
| ≤20 | 390 (7.2%) | 418 (4.2%) |
| >20 to <25 | 2543 (46.7%) | 3653 (37.0%) |
| ≥25 to <30 | 1679 (30.9%) | 3739 (37.9%) |
| ≥30 | 828 (15.2%) | 2057 (20.8%) |
| Physical activity | ||
| Inactive | 1448 (26.8%) | 3155 (32.2%) |
| Moderately inactive | 1871 (34.6%) | 3313 (33.9%) |
| Moderately active | 1188 (22.0%) | 1798 (18.4%) |
| Active | 894 (16.6%) | 1517 (15.5%) |
| Education level | ||
| No schooling/primary school | 1926 (35.9%) | 4926 (52.0%) |
| Secondary school | 1065 (19.8%) | 1174 (12.4%) |
| Vocational/university | 2376 (44.3%) | 3380 (35.7%) |
| High blood pressure (history) | 1249 (23.0%) | 5201 (53.4%) |
| HbA1c (%)b | 5.4 (5.1-5.5) | 5.6 (5.4-5.8) |
| hsCRP (mg/L)b | 1.0 (0.4-2.2) | 1.5 (0.7-3.3) |
| Total cholesterol (mmol/L) | 5.6 ± 1.0 | 6.4 ± 1.2 |
| HDL cholesterol (mmol/L) | 1.6 ± 0.4 | 1.5 ± 0.4 |
| Triglycerides (mmol/L)b | 0.9 (0.7-1.3) | 1.2 (0.9-1.8) |
| Reproductive factors | ||
| Age at menopause (years)c | – | 47.6 ± 5.6 |
| Full-term pregnancy (Yes) | 4415 (87.0%) | 8562 (88.5%) |
| Ever hormone use (Yes) | 444 (9.4%) | 3006 (33.8%) |
| Age at menarche (years) | ||
| ≤10 | 209 (4.1%) | 271 (2.8%) |
| 11 | 698 (13.6%) | 1006 (10.4%) |
| 12 | 1270 (24.7%) | 1733 (18.0%) |
| 13 | 1330 (25.8%) | 2228 (23.1%) |
| 14 | 1051 (20.4%) | 2235 (23.2%) |
| 15 | 375 (7.3%) | 1208 (12.5%) |
| 16 | 156 (3.0%) | 595 (6.2%) |
| ≥17 | 57 (1.1%) | 365 (3.8%) |
| Follow-up | ||
| Number of events | 679 (12.4%) | 4074 (41.1%) |
| Age at event | 56.4 ± 7.1 | 69.8 ± 7.0 |
Mean ± standard deviation.
Adjusted for clothing.
Median (Q1–Q3).
Only post-menopausal women.
Post-menopausal women had a higher CHD risk compared with pre-menopausal women (age-adjusted model HR = 1.23, 95% CI: 1.08–1.40, p-value = 0.002) (Table 2), but this attenuated in the confounder- and intermediate-adjusted model (HR = 1.08, 95% CI: 0.93–1.26, p-value = 0.29).
Table 2.
Hazard ratio (HR) and 95% confidence intervals (CIs) for the association between menopausal status and any first CHD event
| Post-menopausal vs pre-menopausal |
|||
|---|---|---|---|
| Model | HR (95% CI) | p-value | PE% (95% CI)a |
| Age-adjusted model | 1.23 (1.08–1.40) | 0.002 | / |
| Confounder-adjusted modelb | 1.13 (0.98–1.30) | 0.09 | 40.5 (30.4–54.6) |
| Confounder- and intermediate-adjusted modelc | 1.08 (0.93–1.26) | 0.29 | 60.7 (54.4–80.6) |
N (N of events): post-menopausal 9916 (4074), pre-menopausal 5486 (679).
PE, proportion explained.
Adjusted for baseline age, smoking status, BMI, HbA1c, education level, physical activity, full-term pregnancy, age at menarche and ever hormone use.
Additionally adjusted for high-sensitivity C-reactive protein, total cholesterol, HDL-cholesterol, triglycerides and high blood pressure.
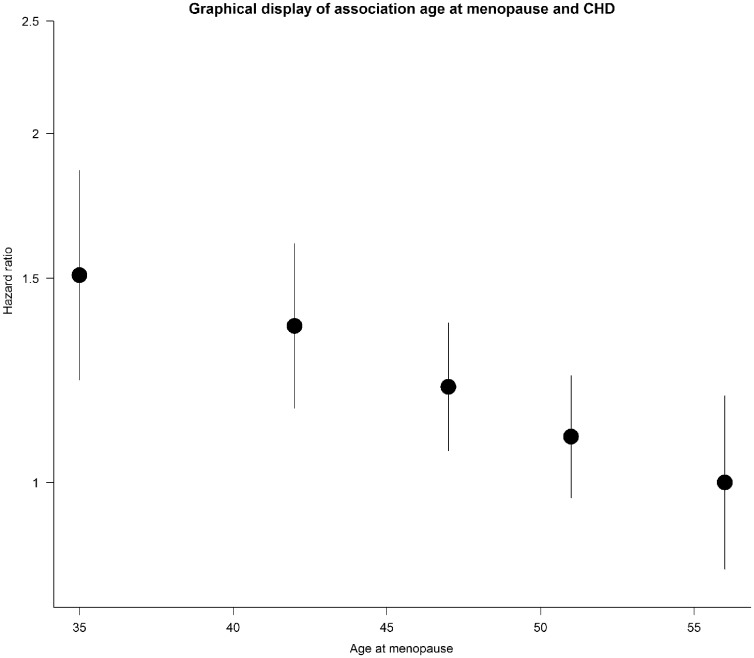
Age at menopause had an approximately linear association with CHD risk, with women in the lowest category (menopausal age <40 years) having a 51% (confounder-adjusted model HR = 1.51, 95% CI: 1.15–1.98), p = 0.003) higher risk than those in the highest category (menopausal age ≥55 years) (Figure 1). In addition, Table 3 showed that, for each 1-year decrease in age at menopause, CHD risk was 2% higher (HRconfounder and intermediate adjusted = 1.02, 95% CI: 1.01–1.03, p < 0.001) and, for each SD decrease (7.9 years) in age at menopause, risk was 14% higher (HRconfounder and intermediate adjusted = 1.14, 95% CI: 1.05–1.22, p = 0.001).
Figure 1.
Graphical display of the linear relationship between age at menopause and CHD using floating absolute risks to display the hazard ratios (HRs) for age at menopause categories [<40, 40–44, 45–49, 50–54, ≥55 years (reference), with 364, 551, 1284, 1563, 312 CHD events, respectively]. HRs were plotted against the mean age at menopause of each category, which are 34, 42, 47, 51 and 57 accordingly.
Table 3.
Hazard ratio (HR) and 95% confidence intervals (CIs) for the association between age at menopause and any first CHD event in post-menopausal women
| HR per-year decrease in age at menopause |
HR per SD decrease in age at menopause |
||||
|---|---|---|---|---|---|
| Model | HR (95% CI) | p-value | HR (95% CI) | p-value | PE% (95% CI)a |
| Age-adjusted model | 1.03 (1.02–1.04) | <0.001 | 1.23 (1.15–1.33) | <0.001 | – |
| Confounder-adjusted modelb | 1.02 (1.01–1.03) | <0.001 | 1.16 (1.08–1.25) | <0.001 | 28.6 (23.2–34.5) |
| Confounder- and intermediate- adjusted modelc | 1.02 (1.01–1.03) | 0.001 | 1.14 (1.05–1.23) | 0.001 | 38.7 (30.4–44.6) |
N (N of events): 9916 (4074).
PE, proportion explained.
Adjusted for baseline age, smoking status, BMI, HbA1c, education level, physical activity, full-term pregnancy, age at menarche and ever hormone use.
Additionally adjusted for high-sensitivity C-reactive protein, total cholesterol, HDL-cholesterol, triglycerides and high blood pressure.
Post-menopausal women with a surgical menopause had a higher risk of CHD compared with women with a natural menopause (HRconfounder-adjusted = 1.25, 95% CI: 1.10–1.42, p < 0.001), which attenuated on further adjustment for age at menopause (HRconfounder-adjusted(b) = 1.15, 95% CI: 1.00–1.33, p = 0.05) and intermediates (HRconfounder and intermediate adjusted = 1.12, 95% CI: 0.96–1.29, p = 0.15) (Table 4).
Table 4.
Hazard ratio (HR) and 95% confidence intervals (CIs) for the association between type of menopause and any first CHD event in post-menopausal women
| Surgical vs natural menopause | |||
|---|---|---|---|
| Model | HR (95% CI) | p-value | PE% (95% CI)a |
| Age-adjusted model | 1.31 (1.16–1.47) | <0.001 | / |
| Confounder-adjusted modelb | 1.25 (1.10–1.42) | <0.001 | 17.6 (10.4–25.4) |
| Confounder-adjusted model(b)c | 1.15 (1.00–1.33) | 0.05 | 47.2 (37.3–59.1) |
| Confounder- and intermediate-adjusted modeld | 1.12 (0.96–1.29) | 0.15 | 59.2 (46.6–73.5) |
N (N of events): surgical 2206 (935), natural 7710 (3139).
PE, proportion explained.
Adjusted for baseline age, smoking status, BMI, HbA1c, education level, physical activity, full-term pregnancy, age at menarche and ever hormone use.
Additionally adjusted for age at menopause.
Additionally adjusted for high-sensitivity C-reactive protein, total cholesterol, HDL-cholesterol, triglycerides and high blood pressure.
In the association between age at menopause and CHD, BMI was an effect modifier (p = 0.003) where smoking was not (p = 0.56). The BMI stratified results (Supplementary Appendix Table 2, available as Supplementary data at IJE online) showed that, in women with a BMI of 25 or higher, each 1-year decrease in age at menopause resulted in a 2 or 4% higher CHD risk (HRconfounder and intermediate adjusted[BMI ≥ 25–<30] 1.02, 95% CI: 1.01–1.04, p-value = 0.01; HRconfounder and intermediate adjusted[BMI ≥ 30] 1.04, 95% CI: 1.02–1.06, p-value < 0.001). Women with a BMI between >20 and <25 had no increased CHD risk (HRconfounder and intermediate adjusted = 1.00, 95% CI: 0.99–1.02, p = 0.66) and women with a BMI ≤20 had a 1% increase in CHD risk for each 1-year decrease in age at menopause (HRconfounder and intermediate adjusted = 1.01, 95% CI: 0.97–1.07, p-value = 0.56).
Finally, in all analyses, we added possible mediators for the associations in Model 4. For post-menopausal compared with pre-menopausal women, we found that adding the established risk factors in Model 4 explains an additional 20% of the association compared with the confounder-adjusted Model 3 (Table 2). In the association with age at menopause, the possible mediators or established risk factors explained an additional 10% of the association compared with the confounder-adjusted model, although the HR only slightly changes (Table 4). Finally, for types of menopause, the possible mediators explained an additional part of the association of approximately 10% compared with model confounder and age at menopause adjusted model. However, in this case, it seemed that age at menopause explained the largest part of the association (Table 3). Furthermore, Supplementary Appendix Table 3, available as Supplementary data at IJE online, shows the proportion explained of all the risk factors separately.
Sensitivity analyses
Similar results were obtained in analyses that were restricted to women who never used HT (Supplementary Appendix Tables 4–6, available as Supplementary data at IJE online) and that excluded the first 2 years of follow-up (Supplementary Appendix Tables 7–9, available as Supplementary data at IJE online). When surgical menopause was defined as bilateral oophorectomy only, the risk estimates for menopausal status attenuated compared with the main analyses (HRconfounder and intermediate adjusted = 0.95, 95% CI: 0.82–1.10, p = 0.50) as did the results for type of menopause (HRconfounder and intermediate adjusted = 0.92, 95% CI = 0.74–1.15, p = 0.47) (Supplementary Appendix Tables 10 and 11, available as Supplementary data at IJE online). The complete case analysis (data not shown) gave similar results to those from the multiple imputation approach.
Discussion
Our study has shown that age at menopause has an inverse dose–response relationship with risk of CHD. Surgical menopause is also associated with an increased CHD risk, even once the earlier age at menopause is accounted for. A proportion of the risk appears to be explained by cardiovascular risk factors that have been postulated to mediate the associations of menopausal characteristics with risk of CHD.
Our finding that the higher risk of CHD in post-menopausal women attenuated upon adjustment for conventional cardiovascular risk factors and reproductive factors is in line with a previous meta-analysis29 that also found an increased risk for post-menopausal women. These analyses could be challenging, since one might expect both pre- and post-menopausal women to have their events around the same age in their post-menopausal period. However, the age at event in our study was 56.4 ± 7.1 years for pre-menopausal women and 69.8 ± 7.0 years for post-menopausal women, indicating enough dispersion to show a robust effect. Similarly, our finding that earlier menopause is associated with a higher CHD risk is also consistent with a recent meta-analysis5 that showed a higher CHD risk for women with an age at menopause before 45 years. However, our access to individual participant data (rather than literature-based summary results) meant that we were able to amplify previous findings by showing that the relationship is continuous and approximately linear across the range in age at menopause. Hence, there is no clear age threshold below which early menopause appears to be of intrinsic concern, within the approximate mean ages of the earliest (34 years) and latest (56 years) categories of menopausal age. Age at menopause might be harder to recall when women used HT, but the results of the sensitivity analysis excluding women using HT barely changed, indicating that this did not influence our results. We identified BMI as an effect modifier and the stratified results appeared similar to the findings of a smaller study,18 which suggested that age at menopause has a stronger association with CHD in obese women compared with non-obese women.
Previous evidence on the associations of surgical and natural menopause with CHD is conflicting.2,3,24,30,31 Comparison of these studies is difficult, since the definition of surgical menopause and inclusion of women using HT differs by study. Notably, none of the studies on surgical menopause adjusted for age at menopause in their analysis, notwithstanding the fact that a surgical menopause occurs consistently earlier than a natural menopause. Our study shows that the association between surgical menopause and CHD risk is largely explained by the earlier age at menopause, but residual risk remains. Excluding women using HT only slightly altered the results. However, when we defined surgical menopause as bilateral oophorectomy only, the results attenuated towards the null, suggesting that the effect of surgical menopause might be smaller than previously thought.
As conventional cardiovascular risk factors such as blood pressure, lipids and C-reactive protein (CRP) rise around the age of menopause, we specifically examined the extent to which these potential mediators explained the associations we observed in Model 4. Our analyses suggested that these factors can explain part of the association between menopausal characteristics and higher risk of CHD, because the greatest difference in the percentage of proportion explained was found between Model 3 and Model 4 in each association. This concurs with the findings of several other studies, which showed the greatest changes in lipid levels around the time of menopausal transition.7,8,32–36 Our results should be interpreted with caution, as measurement error might exist in the mediators that could distort the adjustment.37 Furthermore, as EPIC-CVD has only a single measure of these risk factors at baseline (i.e. after the menopause in post-menopausal women), it is not possible to reliably distinguish whether the attenuations seen are due to mediation or confounding.38
Our study has several strengths. We used data from a large prospective study encompassing diverse European populations with a long duration of follow-up and a substantial number of validated incident CHD events. The availability of a wide range of cardiovascular and reproductive risk factors allowed us to systematically examine the effects of accounting for these factors. We were also able to examine the impact of HT use, which has not been possible in many previous studies. Potential limitations include missing or incomplete menopause data, which may have led to non-differential misclassification resulting in under-estimation of the true associations39; self-reported menopausal characteristics, although studies show that the validity is rather good for menopausal status and age and varies for surgical menopause40–42; the possibility of residual confounding; and measurement error in the intermediates. As EPIC-CVD did not have measures of sex hormones, we were not able to evaluate the contribution of oestrogen. The fact that there are HT users among pre-menopausal women can be explained by the inclusion of peri-menopausal women in this category. Finally, a substantial number of the pre-menopausal women would likely have become post-menopausal during the follow-up period. Therefore, our associations may have been slightly underestimated.
In conclusion, earlier age at menopause and surgical menopause are both associated with higher risk of CHD, which might suggest that these women need close monitoring in clinical practice. The excess risk is, in part, explained by conventional cardiovascular risk factors. Therefore, these risk factors should play an important role in the monitoring of these women. However, there is still a residual association between menopausal characteristics and CHD, of which the mechanism is not fully understood and which merits further research.
Funding
This work was supported by the European Union Framework 7 (HEALTH-F2-2012–279233), the European Research Council (268834), the UK Medical Research Council (G0800270, MR/L003120/1), the British Heart Foundation (SP/09/002, RG/08/014, RG13/13/30194) and the UK National Institute of Health Research (to EPIC-CVD). The national cohorts are supported by the Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); Deutsche Krebshilfe, Deutsches Krebsforschungszentrum and Federal Ministry of Education and Research (Germany); Ministry of Health and Social Solidarity, Stavros Niarchos Foundation and Hellenic Health Foundation (Greece); Italian Association for Research on Cancer (AIRC) and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), ERC-2009-AdG 232997 and Nordforsk, Nordic Centre of Excellence programme on Food, Nutrition and Health (Norway); Health Research Fund (FIS), Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, ISCIII RETIC (RD06/0020) (Spain); Swedish Cancer Society, Swedish Scientific Council and Regional Government of Skåne and Västerbotten (Sweden); Cancer Research UK, Medical Research Council (UK). This work is supported by the Dutch Heart Foundation (2013T083 to V.D.). This work was supported by a UK Medical Research Council Skills Development Fellowship (MR/P014550/1 to S.A.E.P.). None of the funding sources had a role in the collection, analysis and interpretation of the data, nor in the decision to submit the article for publication.
Supplementary Material
Acknowledgements
We thank all EPIC participants and staff for their contribution to the study. We also thank staff from the EPIC-CVD coordinating centers for sample preparation and data handling. Statistics Netherlands is acknowledged for providing causes of death for the Dutch contribution to EPIC-CVD.
Conflict of interest: None.
References
- 1.World Health Organisation (WHO). Cardiovascular Diseases (CVDs) Fact Sheet Number 137 2017. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- 2. Kannel WB, Hjortland MC, McNamara PM, Gordon T.. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med 1976;85:447–52. [DOI] [PubMed] [Google Scholar]
- 3. Gordon T, Kannel WB, Hjortland MC, McNamara PM.. Menopause and coronary heart disease. Ann Intern Med 1978;89:157–61. [DOI] [PubMed] [Google Scholar]
- 4. Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH.. Menopause and the risk of coronary heart disease in women. N Engl J Med 1987;316:1105–10. [DOI] [PubMed] [Google Scholar]
- 5. Muka T, Oliver-Williams C, Kunutsor S. et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol 2016;1:767.. [DOI] [PubMed] [Google Scholar]
- 6. Lindquist O. Intraindividual changes of blood pressure, serum lipids, and body weight in relation to menstrual status: results from a prospective population study of women in Goteborg, Sweden. Prev Med 1982;11:162–72. [DOI] [PubMed] [Google Scholar]
- 7. Matthews KA, Crawford SL, Chae CU. et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol 2009;54:2366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peters HW, Westendorp IC, Hak AE. et al. Menopausal status and risk factors for cardiovascular disease. J Intern Med 1999;246:521–28. [DOI] [PubMed] [Google Scholar]
- 9. Riboli E, Hunt KJ, Slimani N. et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr 2002;5:1113–24. [DOI] [PubMed] [Google Scholar]
- 10. Langenberg C, Sharp S, Forouhi NG. et al. Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC Study. Diabetologia 2011;54:2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Danesh J, Saracci R, Berglund G. et al. EPIC-Heart: the cardiovascular component of a prospective study of nutritional, lifestyle and biological factors in 520,000 middle-aged participants from 10 European countries. Eur J Epidemiol 2007;22:129–41. [DOI] [PubMed] [Google Scholar]
- 12. Clavel-Chapelon F. Differential effects of reproductive factors on the risk of pre- and postmenopausal breast cancer: results from a large cohort of French women. Br J Cancer 2002;86:723–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haftenberger M, Lahmann PH, Panico S. et al. Overweight, obesity and fat distribution in 50- to 64-year-old participants in the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr 2002;5:1147–62. [DOI] [PubMed] [Google Scholar]
- 14. Wareham NJ, Jakes RW, Rennie KL. et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr 2003;6:407–13. [DOI] [PubMed] [Google Scholar]
- 15. van Buuren S, Groothuis-Oudshoorn K.. Mice: Multivariate Imputation by Chained Equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- 16. Prentice RL. A case–cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika 1986;73:1–11. [Google Scholar]
- 17. Morris DH, Jones ME, Schoemaker MJ, McFadden E, Ashworth A, Swerdlow AJ.. Body mass index, exercise, and other lifestyle factors in relation to age at natural menopause: analyses from the breakthrough generations study. Am J Epidemiol 2012;175:998–1005. [DOI] [PubMed] [Google Scholar]
- 18. Ossewaarde ME, Bots ML, Verbeek AL. et al. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology 2005;16:556–62. [DOI] [PubMed] [Google Scholar]
- 19. Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D.. Early menopause predicts future coronary heart disease and stroke: the multi-ethnic study of atherosclerosis. Menopause 2012;19:1081–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matthews KA, Gibson CJ, El Khoudary SR, Thurston RC.. Changes in cardiovascular risk factors by hysterectomy status with and without oophorectomy: Study of Women’s Health Across the Nation. J Am Coll Cardiol 2013;62:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Easton DF, Peto J, Babiker AG.. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med 1991;10:1025–35. [DOI] [PubMed] [Google Scholar]
- 22. Judd CM, Kenny DA.. Process analysis: estimating mediation in treatment evaluations. Eval Rev 1981;5:602–19. [Google Scholar]
- 23. Baron RM, Kenny DA.. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173–82. [DOI] [PubMed] [Google Scholar]
- 24. Rivera CM, Grossardt BR, Rhodes DJ. et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 2009;16:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stampfer MJ, Colditz GA.. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev Med 1991;20:47–63. [DOI] [PubMed] [Google Scholar]
- 26. Grodstein F, Manson JE, Stampfer MJ.. Postmenopausal hormone use and secondary prevention of coronary events in the nurses’ health study: a prospective, observational study. Ann Intern Med 2001;135:01–08. [DOI] [PubMed] [Google Scholar]
- 27. Rubin DB. Multiple Imputation for Non Response in Surveys. New York: Wiley, 1987. [Google Scholar]
- 28.Team RC. R: A Language and Environment for Statistical Computing, 3.2.0 ed. Vienna: R Foundation for Statistical Computing, 2015. [Google Scholar]
- 29. Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT.. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause 2006;13:265–79. [DOI] [PubMed] [Google Scholar]
- 30. Falkeborn M, Schairer C, Naessen T, Persson I.. Risk of myocardial infarction after oophorectomy and hysterectomy. J Clin Epidemiol 2000;53:832–37. [DOI] [PubMed] [Google Scholar]
- 31. Peters SA, Woodward M.. Women’s reproductive factors and incident cardiovascular disease in the UK Biobank. Heart 2018;104:1069–75. [DOI] [PubMed] [Google Scholar]
- 32. Do KA, Green A, Guthrie JR, Dudley EC, Burger HG, Dennerstein L.. Longitudinal study of risk factors for coronary heart disease across the menopausal transition. Am J Epidemiol 2000;151:584–93. [DOI] [PubMed] [Google Scholar]
- 33. Matthews KA, Kuller LH, Sutton-Tyrrell K, Chang YF.. Changes in cardiovascular risk factors during the perimenopause and postmenopause and carotid artery atherosclerosis in healthy women. Stroke 2001;32:1104–11. [DOI] [PubMed] [Google Scholar]
- 34. Torng P-L, Su T-C, Sung FC. et al. Effects of menopause on intraindividual changes in serum lipids, blood pressure, and body weight—the Chin-Shan Community Cardiovascular Cohort study. Atherosclerosis 2002;161:409–15. [DOI] [PubMed] [Google Scholar]
- 35. Schubert CM, Rogers NL, Remsberg KE. et al. Lipids, lipoproteins, lifestyle, adiposity and fat-free mass during middle age: the fels longitudinal study. Int J Obes 2006;30:251–60. [DOI] [PubMed] [Google Scholar]
- 36. de Kat AC, Dam V, Onland-Moret NC, Eijkemans MJ, Broekmans FJ, van der Schouw YT.. Unraveling the associations of age and menopause with cardiovascular risk factors in a large population-based study. BMC Med 2017;15:2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Le Cessie S, Debeij J, Rosendaal FR, Cannegieter SC, Vandenbroucke JP.. Quantification of bias in direct effects estimates due to different types of measurement error in the mediator. Epidemiology 2012;23:551–60. [DOI] [PubMed] [Google Scholar]
- 38. Kok HS, van Asselt KM, van der Schouw YT. et al. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol 2006;47:1976–83. [DOI] [PubMed] [Google Scholar]
- 39. Rothman KJ. Epidemiology, an Introduction. Oxford: Oxford University Press, 2012. [Google Scholar]
- 40. Clavel-Chapelon F, Dormoy-Mortier N.. A validation study on status and age of natural menopause reported in the E3N cohort. Maturitas 1998;29:99–103. [DOI] [PubMed] [Google Scholar]
- 41. Colditz GA, Stampfer MJ, Willett WC. et al. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol 1987;126:319–25. [DOI] [PubMed] [Google Scholar]
- 42. Phipps AI, Buist DS.. Validation of self-reported history of hysterectomy and oophorectomy among women in an integrated group practice setting. Menopause 2009;16:576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.