Abstract
Background
Epidemiologic studies suggest a strong link between poor habitual sleep quality and increased cardiovascular disease risk. However, the underlying mechanisms are not entirely clear. Metabolomic profiling may elucidate systemic differences associated with sleep quality that influence cardiometabolic health.
Methods
We explored cross-sectional associations between sleep quality and plasma metabolites in a nested case–control study of coronary heart disease (CHD) in the Women’s Health Initiative (WHI; n = 1956) and attempted to replicate the results in an independent sample from the Nurses’ Health Study II (NHSII; n = 209). A sleep-quality score (SQS) was derived from self-reported sleep problems asked in both populations. Plasma metabolomics were assayed using LC–MS with 347 known metabolites. General linear regression was used to identify individual metabolites associated with continuous SQS (false-discovery rate <0.05). Using least absolute shrinkage and selection operator (LASSO) algorithms, a metabolite score was created from replicated metabolites and evaluated with CHD risk in the WHI.
Results
After adjusting for age, race/ethnicity, body mass index (BMI) and smoking, we identified 69 metabolites associated with SQS in the WHI (59 were lipids). Of these, 16 were replicated in NHSII (15 were lipids), including 6 triglycerides (TAGs), 4 phosphatidylethanolamines (PEs), 3 phosphatidylcholines (PCs), 1 diglyceride (DAG), 1 lysophosphatidylcholine and N6-acetyl-L-lysine (a product of histone acetylation). These metabolites were consistently higher among women with poorer sleep quality. The LASSO selection resulted in a nine-metabolite score (TAGs 45: 1, 48: 1, 50: 4; DAG 32: 1; PEs 36: 4, 38: 5; PCs 30: 1, 40: 6; N6-acetyl-L-lysine), which was positively associated with CHD risk (odds ratio per SD increase in the score: 1.16; 95% confidence interval: 1.05, 1.28; p = 0.0003) in the WHI after adjustment for matching factors and conventional CHD risk factors.
Conclusions
Differences in lipid metabolites may be an important pathogenic pathway linking poor habitual sleep quality and CHD risk.
Keywords: coronary heart disease, epidemiology, metabolomics, sleep, women
Key Messages
We identified and replicated differences in lipid profiles associated with sleep quality in two independent samples of post-menopausal women.
Sleep-related metabolomic signature was strongly predictive of future coronary heart disease (CHD) risk.
Potential epigenetic changes associated with sleep, particularly histone acetylation and its relevance to CHD development, warrant additional studies.
More studies are needed to confirm our results in other populations.
Introduction
Women have a substantially increased risk of developing coronary heart disease (CHD) after menopause.1 In addition to a reduction in sex hormones that may be cardioprotective,2 a growing body of evidence suggests that women, compared with men, are particularly vulnerable to the consequences of metabolic abnormalities that lead to cardiovascular morbidity and mortality.3,4 However, the behavioural and biologic factors contributing to such sex differences are not fully understood.
Interestingly, women are also more likely to report poorer sleep quality and insomnia symptoms than men, with even greater differences in older populations.5 Previous studies provide consistent evidence linking insomnia with increased risk of CHD,6,7 with women being more likely to have unfavourable biologic responses to sleep disturbances, such as increased inflammation and insulin resistance.8,9 Given that sleep plays a central role in energy homeostasis and metabolism, a comprehensive profiling of the systemic metabolic differences associated with variations in sleep quality may provide important insights into the underlying pathways through which poor sleep quality increases CHD risk.
Several small experimental studies, predominantly in men, reported alterations in plasma metabolites after total or partial sleep deprivation,10–12 including changes in different species of lipids and fatty acids (e.g. phosphatidylcholine and diacylglycerol), metabolites related to neurotransmitter synthesis and metabolism (e.g. tryptophan and serotonin), glycolate metabolites (e.g. oxalic acid) and gut microbial metabolites (e.g. trihydroxypyrazine). Yet, it remains unclear whether these findings can be translated to the effects of habitual sleep patterns, which have greater relevance towards chronic CHD development, or applied to post-menopausal women, who are susceptible to both insomnia and cardiovascular consequences of metabolic dysregulation. Therefore, we conducted the present study to identify a metabolomic signature associated with self-reported habitual sleep quality in two independent samples of post-menopausal women. We further explored the relationships of sleep-related metabolomic profiles with development of CHD.
Methods
Study population
The discovery sample was a nested case–control study of metabolomics and incident CHD in the Women’s Health Initiative (WHI), which has observational study and clinical trial components.13 CHD was defined as acute myocardial infarction (confirmed by medical record review) or death due to CHD (ascertained by death certificate). Briefly, 1153 participants who developed CHD after baseline screening (cases) and 1153 participants who did not develop CHD (controls) were frequency-matched on age (5-year interval), race/ethnicity, hysterectomy status and 2-year enrolment windows (Supplementary Figure 1, available as Supplementary data at IJE online).14 Of these, 472 cases and 472 controls were drawn from the Women’s Health Initiative-Observational Study (WHI-OS), in which 93 676 post-menopausal women (age: 50–79) ineligible or unwilling to participate in the randomized trials were recruited from 40 clinical centres across the USA between 1994 and 1998. The remaining 681 case–control pairs were sampled from the Women Health Initiative-Hormone Therapy Trials (WHI-HT), in which 27 347 post-menopausal women (age: 50–79) were randomized to an oestrogen plus progesterone vs placebo arm, or oestrogen only vs placebo arm, according to hysterectomy status. At baseline screening (prior to randomization), all 2306 women were free of CHD, completed a clinic visit and provided a fasting blood sample for an initial metabolomics assay and extensive questionnaire information. Of these, 1072 women in the WHI-HT completed another metabolomics assay using blood samples collected at 1-year follow-up.
Replication was conducted among a subset of post-menopausal women from the Nurses’ Health Study II (NHSII). NHSII is a large, ongoing, prospective cohort study of 116 429 US female registered nurses initiated in 1989. At baseline, all participants completed a questionnaire regarding their lifestyle and medical history and have been followed by biennial questionnaires to update information on exposure data and disease diagnoses. In 2013, 233 women (age: 49–67) participated in a substudy that aimed to identify biologic predictors for psychosocial stress, providing multiple biospecimens (including fasting blood) and completing a comprehensive psychosocial assessment online, including habitual sleep quality (Supplementary Figure 1, available as Supplementary data at IJE online).15
In both cohorts, the analysis included women who had complete data on sleep assessment and excluded women with prevalent diabetes or extreme BMI (women with BMI > 50 kg/m2, all of whom were diabetic) to minimize the complex influences of the resulting glucose dysregulation on metabolomic profiles, leaving 1956 women in the WHI (889 with repeated metabolomics assays) and 209 in the NHSII.
Sleep assessment
Habitual sleep patterns were assessed from the baseline 10-item sleep questionnaire in the WHI16 and from the Pittsburgh Sleep Quality Index (PSQI) in the NHSII substudy.17 To facilitate comparison, we derived a sleep-quality score (SQS) by harmonizing questions that have been asked in both cohorts concerning four major areas related to insomnia symptoms (Supplementary Table 1, available as Supplementary data at IJE online), including (i) difficulty in initiating sleep, (ii) difficulty in maintaining sleep and early awakening, (iii) use of sleep medication and (iv) subjective sleep quality. Each component was scored 0–3 according to frequency or severity of sleep problems. The overall SQS ranged from 0 to 12, with higher scores suggesting poorer habitual sleep quality. The internal consistency for the SQS was acceptable (Cronbach’s alpha = 0.65). In the WHI, the SQS had a correlation of 0.87 with the five-item WHI Insomnia Rating Scale, which is based on a similar set of questions and has been validated in prior studies showing good reliability, internal consistency and correlations with objective sleep measures by actigraphy.18 In the NHSII, the SQS had a correlation of 0.83 with the 19-item overall PSQI score, which has been extensively validated across different populations.19 Further, among 200 NHSII participants who completed a second sleep assessment 1 year after the baseline questionnaire, the SQS was highly reproducible [intra-class correlation coefficient (ICC) = 0.68], suggesting that this score captures long-term habitual sleep patterns.
Metabolomics analyses
Plasma samples from WHI and NHSII participants were analysed using three liquid chromatography–tandem mass spectrometry (LC–MS)-based metabolite profiling methods: hydrophilic interaction liquid chromatography (HILIC) analyses of water-soluble metabolites in the positive ionization mode (HILIC-pos), C8 chromatography with positive ion mode analyses of polar and non-polar plasma lipids (C8-pos) and C18 chromatography with negative ion mode analyses of free fatty acids and bile acids (C18-neg). Assays were performed as described previously.20 Briefly, internal standard peak areas were monitored for quality control and to ensure system performance throughout analyses. Pooled plasma reference samples were also inserted every 20 samples as an additional quality control. Raw LC–MS data were processed using TraceFinder software (Thermo Fisher Scientific; Waltham, MA) and Progenesis QI (Nonlinear Dynamics; Newcastle upon Tyne, UK). After excluding 26 metabolites with a coefficient of variation (CV) ≥ 20%, 347 shared known metabolites were identified across the two cohorts (Supplementary Table 2, available as Supplementary data at IJE online). In our pilot testing of the metabolomics platform, 92% of metabolites had acceptable assay reproducibility (CV < 20%) and nearly 90% of metabolites were stable over 1–2 years within women (Spearman correlation or ICC ≥ 0.4).21
Statistical analysis
We calculated means (SD) for continuous variables and percentages for categorical variables across quartiles of SQS in WHI and NHSII separately. All metabolite values were natural log-transformed to reduce right skewness; within each cohort, the distribution for each log-transformed metabolite was converted to a z-score with a mean of 0 and a SD of 1. Multivariable linear-regression models were used to assess the associations between habitual sleep quality and individual plasma metabolites, with the standardized z-score metabolite level as the outcome and the continuous SQS as the predictor. We adjusted for factors likely influencing metabolites including age (continuous), race/ethnicity (White, non-White), BMI (continuous) and current smoking status (yes, no) in the primary model. To evaluate the robustness of the results, we additionally adjusted for known CHD risk factors and other factors that may be consequences of poor habitual sleep (Supplementary Figure 2, available as Supplementary data at IJE online), including alcohol use, caffeine intake, dietary quality (measured by Health Eating Index), physical activity (measured by MET-hours/week), sleep duration, prevalent hypertension, depressive symptoms (measured by short-form Center for Epidemiologic Studies—Depression scale), current hormone therapy, aspirin use, statin use and other lipid-lowering medications. We also repeated the analysis restricting to controls or excluded women reporting sleep-medication use. To deal with multiple comparisons, a false-discovery rate (FDR) <0.05 was used as the threshold to identify sleep-related metabolites.22 Metabolites identified in WHI were further replicated in NHSII using the same FDR threshold. Effect estimates from each cohort were pooled using random-effects meta-analysis. Secondarily, similar analyses were conducted for each component of the SQS.
For replicated plasma metabolites, we examined whether baseline SQS was associated with changes in their levels among 889 WHI women who had a second metabolomic measurement at 1-year follow-up, as a way to assess potential reverse causation due to the cross-sectional nature of the primary analysis. In addition, we evaluated the pairwise relationships among replicated metabolites using the Spearman partial correlation coefficient (r). As metabolites were expected to be correlated, we used LASSO (least absolute shrinkage and selection operator) regression to select a parsimonious model that was most representative of the metabolic correlates of habitual sleep quality.23 A sleep-related metabolite score (SMS) was created for each participant using , where βk is the corresponding regression coefficient for metabolite k, metabolitek is the z-score for metabolite k and n is the total number of LASSO-selected metabolites. The relationship between SQS and SMS was visualized in a histogram.
Next, we examined the associations between SMS and several CHD risk biomarkers, including total cholesterol, high-density lipoprotein (HDL) cholesterol, total triglycerides, C-reactive protein (CRP) and fasting glucose. Log-transformed biomarker concentrations were regressed on SMS using the general linear model. With similar covariate adjustment as described above, we calculated the adjusted least-squares geometric biomarker means for each SMS quintile and estimated the adjusted percentage difference in biomarker levels for every SD increase in SMS.
Further, we evaluated the association between SMS and CHD risk in the WHI. Unconditional logistic regression was used, with the score evaluated both continuously and in quintiles (based on controls). The first model adjusted for frequency matching factors, including age, race/ethnicity, hysterectomy status and enrolment window. The second model accounted for additional CHD risk factors listed above. The third model further adjusted for several known CHD biomarkers. Secondarily, we evaluated the associations between individual replicated metabolites and CHD risk.
Finally, to evaluate to what extent sleep-disrupted metabolites (continuous SMS) may explain the association between habitual sleep quality and CHD risk, we used the approach nested in the counterfactual theory to decompose the total effect into direct (i.e. independent of SMS) and indirect effects (i.e. mediated through SMS), allowing for an exposure–mediator interaction and adjusted for demographic, lifestyle and health-related factors listed above.24 Habitual sleep quality was modelled as a binary exposure (SQS ≥ 6 vs <6). To further explore potential residual confounding, we performed a sensitivity analysis to estimate the mediating effect of SMS with additional adjustment for known CHD risk biomarkers. Analyses were conducted using SAS version 9.4 and R statistical packages version 3.2.5.
Results
The mean age was 67 years (range: 50–79) in the WHI sample and 61 years (range: 49–67) in the NHSII sample; all women were post-menopausal. In both samples, compared with women with good sleep quality (i.e. the bottom quartile of SQS), those with poor sleep quality (i.e. the top quartile of SQS) had higher BMI, shorter sleep duration, higher depressive symptoms and were more likely to be a habitual snorer, have hypertension, consume more caffeine and use aspirin, statins, other lipid-lowering drugs or sleep medications (Table 1).
Table 1.
Age-standardized characteristics of the study population by sleep-quality score
| The Women’s Health Initiative (1994) |
The Nurses’ Health Study II (2013) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Quartiles of sleep-quality score |
||||||||
| 0 to 1 | 2 to 3 | 4 to 5 | 6 to 12 | 0 to 2 | 3 to 4 | 5 to 6 | 7 to 12 | |
| N | 395 | 604 | 451 | 506 | 51 | 63 | 42 | 53 |
| Age, years | 67.2 (7.5) | 67.4 (6.4) | 67.5 (6.7) | 66.2 (7.1) | 60.8 (4.2) | 60.4 (4.4) | 61.2 (3.6) | 60.2 (3.8) |
| Non-White, % | 21 | 21 | 17 | 15 | 2 | 2 | 5 | 6 |
| BMI, kg/m2 | 27.5 (5.9) | 28.0 (5.8) | 28.4 (5.7) | 28.6 (5.9) | 25.8 (7.0) | 25.1 (4.6) | 27.4 (6.4) | 26.8 (5.4) |
| Current smokers, % | 12 | 12 | 9 | 13 | 2 | 7 | 0 | 2 |
| Physical activity, MET-hrs/week | 11.9 (12.8) | 12.0 (13.6) | 10.4 (12.1) | 10.0 (11.5) | 26.7 (21.9) | 32.6 (29.4) | 33.6 (28.0) | 21.1 (19.4) |
| Hypertension, % | 37 | 40 | 42 | 48 | 28 | 32 | 31 | 38 |
| Elevated depressive symptoms, % | 3 | 9 | 16 | 29 | 0 | 9 | 25 | 47 |
| Diet quality score | 67.9 (10.6) | 67.5 (11.0) | 66.4 (11.7) | 65.7 (10.8) | 67.6 (10.7) | 71.6 (12.8) | 67.0 (14.1) | 69.2 (13.2) |
| Caffeine intake, mg/day | 174 (140) | 166 (148) | 161 (132) | 178 (146) | 179 (112) | 165 (129) | 166 (135) | 200 (188) |
| Alcohol drinking, g/day | 4.6 (10.2) | 5.1 (11.3) | 4.7 (9.6) | 5.6 (13.9) | 9.7 (14.5) | 8.1 (10.6) | 8.5 (14.1) | 6.6 (8.2) |
| Current statin use, % | 8 | 10 | 10 | 13 | 16 | 18 | 29 | 34 |
| Other lipid-lowering drug use, % | 1 | 1 | 2 | 3 | 0 | 4 | 0 | 9 |
| Current aspirin use, % | 23 | 24 | 26 | 31 | 26 | 33 | 29 | 41 |
| Current hormone therapy, % | 1 | 1 | 2 | 1 | 20 | 26 | 24 | 30 |
| Sleep-medication use, % | 1 | 8 | 29 | 67 | 9 | 33 | 47 | 84 |
| Sleep duration, hours | 7.1 (1.1) | 7.0 (1.0) | 6.7 (1.0) | 6.2 (1.2) | 7.1 (0.8) | 7.1 (1.0) | 6.9 (0.8) | 6.3 (1.1) |
| Habitual snoring, % | 33 | 37 | 41 | 51 | 20 | 17 | 33 | 41 |
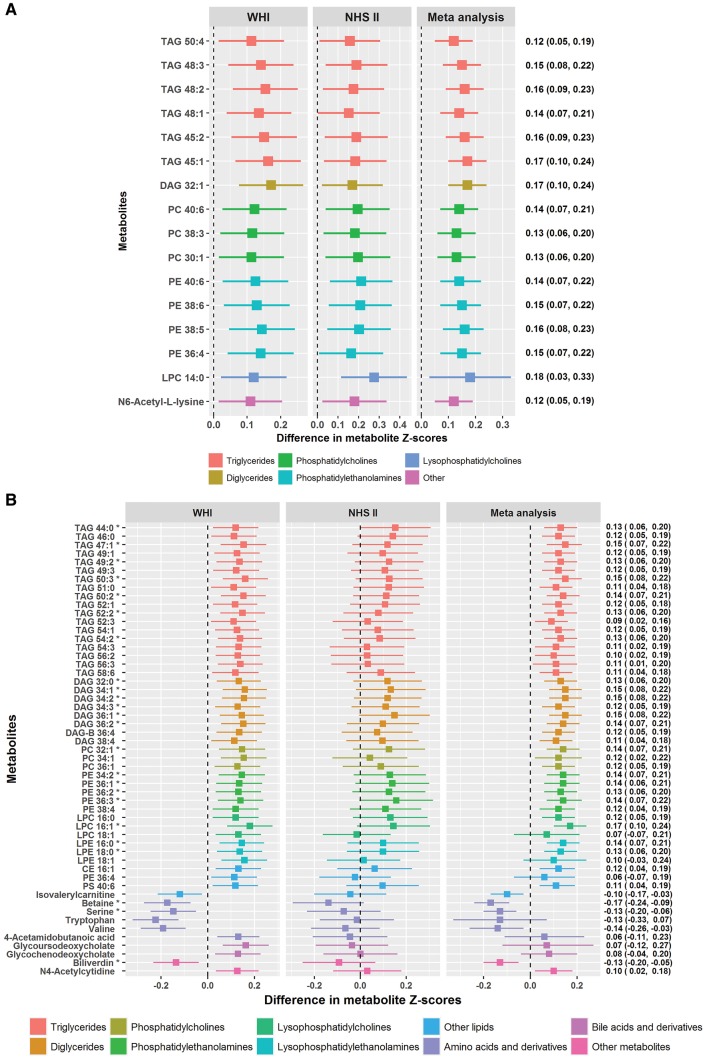
After adjusting for age, race/ethnicity, BMI and smoking, we identified 69 metabolites associated with continuous SQS in WHI (FDR < 0.05; nominal p < 0.01; Figure 1). Of these, 59 were lipids-related species, including 24 triglycerides (TAG), 9 diglycerides (DAG), 9 phosphatidylethanolamines (PE), 6 phosphatidylcholines (PC), 4 lysophosphatidylcholines (LPC), 3 lysophosphatidylethanolamines (LPE) and 4 other lipid derivatives. All of these lipid metabolites were higher with poorer sleep quality, except for isovalerylcarnitine, which had an inverse association. Most of these lipids (particularly TAG, DAG, PC, LPC and LPE) were characterized by short- or medium-chain fatty acids with a low number of double bonds. For example, of the 24 TAGs, 18 had ≤52 acyl chain carbons and 22 had 0–3 double bonds. Poor sleep quality was also associated with 10 non-lipid metabolites, including lower levels of tryptophan, betaine, serine, valine and biliverdin, and higher levels of N6-acetyl-L-lysine, 4-acetamidobutanoic acid, N4-acetylcytidine, glycoursodeoxycholate and glycochenodeoxycholate.
Figure 1.
(A) Differences in metabolite z-score for every SD increase in the sleep-quality score. Metabolites presented in the figure were those discovered in the WHI (FDR < 0.05; nominal p < 0.01) and replicated in the NHSII (FDR < 0.05; nominal p < 0.03). All estimates were adjusted for age, race/ethnicity, BMI and current smoking status. (B) Differences in metabolite z-score for every SD increase in the sleep-quality score. Metabolites presented in the figure were those discovered in the WHI (FDR < 0.05; nominal p < 0.01) but not replicated in the NHSII (FDR > 0.05). All estimates were adjusted for age, race/ethnicity, BMI and current smoking status. *Nominal p < 0.05/69 = 0.0007 for pooled-effect estimates (i.e. after Bonferroni correction).
Of the 69 metabolites discovered in the WHI, 16 were replicated in NHSII (FDR < 0.05; nominal p < 0.03; Figure 1A); 15 were classed as lipids, including 6 TAGs (45: 1, 45: 2, 48: 1, 48: 2, 48: 3, 50: 4), 4 PEs (36: 4, 38: 5, 38: 6, 40: 6), 3 PCs (30: 1, 38: 3, 40: 6), 1 DAG (32: 1) and 1 LPC (14: 0) and 1 was a non-lipid metabolite (N6-acetyl-L-lysine). Metabolite levels were consistently elevated among women with poorer sleep quality. In meta-analysis, the standardized z-score difference in metabolite levels for every SD increase (SD: 3) in SQS ranged from 0.12 (nominal p = 0.0007) for TAG 50: 4 to 0.18 (nominal p = 6.16E-6) for LPC 14: 0. Several sensitivity analyses resulted in similar effect estimates for most associations, including (i) adjusting for a number of potentially relevant lifestyle factors (i.e. alcohol use, caffeine intake, dietary quality, physical activity, sleep duration and depressive symptoms) and medication use (i.e. aspirin, statin and other lipid-lowering medications), (ii) restricting the analysis to controls or (iii) excluding women reporting sleep-medication use (Supplementary Table 3, available as Supplementary data at IJE online). Notably, among 889 WHI women with a repeated 1-year post-baseline metabolomic assessment, poor baseline sleep quality was associated with an increase in 11 of 16 replicated metabolites (Supplementary Table 4, available as Supplementary data at IJE online). For the 53 metabolites that were not replicated in the NHSII, 24 showed associations in the same direction, resulting in strong estimates in meta-analysis (nominal p < 0.05/69 = 0.0007 by the more conservative Bonferroni correction for these metabolites; Figure 1B). These metabolites included 7 TAGs, 6 DAGs, 4 PEs, 2 LPEs, 1 PC, 1 LPC, betaine, serine and biliverdin.
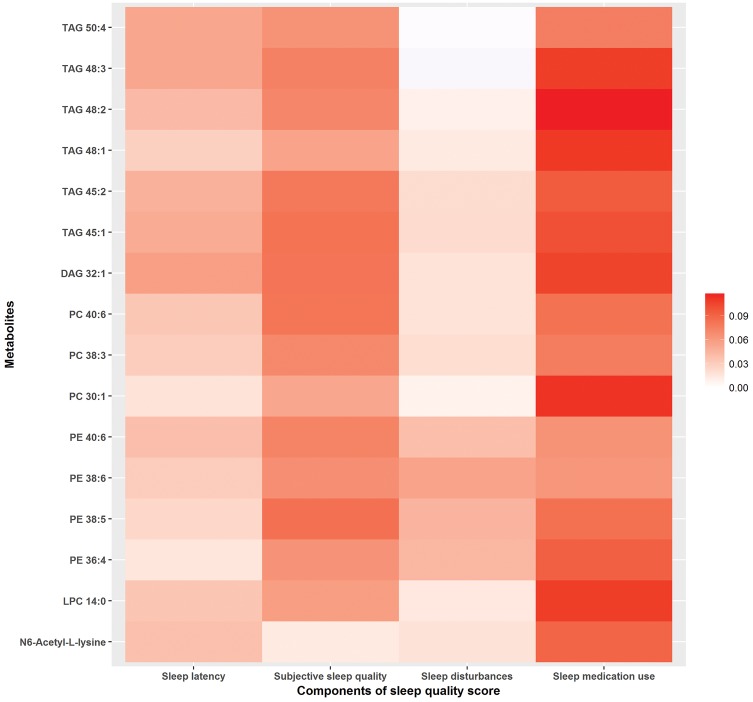
In the pooled sample, we further examined the 16 replicated metabolites in relation to each SQS component (Figure 2). Use of sleep medication and subjective sleep quality were more strongly associated with the replicated metabolites compared with sleep latency and sleep disturbances. Similar differences in the association patterns by SQS components were also observed for the remaining 53 metabolites (Supplementary Figure 3, available as Supplementary data at IJE online). Sleep-medication use had the strongest positive associations with lipid metabolites, whereas better reported sleep quality showed the strongest inverse associations with several amino acids (e.g. serine, tryptophan, valine).
Figure 2.
Differences in metabolite z-score for every 1-point increase in the sleep-quality score (SQS) component. Metabolites presented in the figure include 16 metabolites replicated across WHI and NHSII. The colour indicates the magnitude of the beta coefficients for each SQS component from linear-regression models adjusted for age, race/ethnicity, BMI and current smoking status.
In general, replicated lipid metabolites were positively correlated, with stronger correlations observed for the same lipid species with a similar number of carbon and double bonds (Supplementary Figure 4, available as Supplementary data at IJE online). Individual lipid metabolites were moderately correlated with conventional CHD risk factors, particularly total triglycerides. N6-acetyl-L-lysine was not correlated with lipid metabolites or CHD risk factors except age. The LASSO algorithm selected a parsimonious model of 9 metabolites (TAGs 45: 1 48: 1 50: 4, DAG 32: 1, PEs 36: 4 38: 5, PCs 30: 1 40: 6, N6-acetyl-L-lysine) from the 16 replicated lipid metabolites. A SMS comprising these nine metabolites was not only strongly correlated with SQS (Supplementary Figure 5, available as Supplementary data at IJE online), but also associated with several CHD risk biomarkers (Table 2). For every SD increase in SMS, the adjusted percentage difference [95% confidence interval (CI)] in the biomarker levels was 4.1 (3.2, 5.0) for total cholesterol, –7.3 (–8.3, –6.2) for HDL cholesterol, 35.1 (32.4, 37.9) for total triglycerides, 22.7 (17.2, 28.5) for CRP and 1.1 (0.1, 2.1) for fasting glucose.
Table 2.
Associations of the sleep-associated plasma metabolite score with conventional coronary heart disease biomarkers among post-menopausal women in the Women’s Health Initiative
| Sleep-associated plasma metabolite scorea |
Percent difference (95% CI) per SD increase in SQS | P-trend | |||||
|---|---|---|---|---|---|---|---|
| Quintile 1 |
Quintile 2 |
Quintile 3 |
Quintile 4 |
Quintile 5 |
|||
| Adjusted least-squares geometric means (95% CI) (mg/dL) | |||||||
| Total cholesterol | |||||||
| Model 1b | 217 (214, 221) | 224 (220, 228) | 234 (230, 238) | 238 (234, 242) | 245 (241, 250) | 3.9 (3.0, 4.7) | 2.8E-20 |
| Model 2c | 217 (213, 221) | 224 (220, 228) | 234 (230, 238) | 239 (234, 243) | 247 (242, 251) | 4.1 (3.2, 5.0) | 6.5E-21 |
| HDL cholesterol | |||||||
| Model 1 | 57 (56, 58) | 52 (51, 53) | 51 (50, 53) | 48 (47, 49) | 44 (43, 46) | −8.4 (−9.4, −7.4) | 1.9E-50 |
| Model 2 | 56 (55, 57) | 51 (50, 53) | 51 (50, 52) | 49 (48, 50) | 45 (44, 46) | −7.3 (−8.3, −6.2) | 2.0E-38 |
| Total triglycerides | |||||||
| Model 1 | 89 (85, 93) | 111 (106, 116) | 129 (124, 135) | 162 (155, 169) | 215 (206, 225) | 35.8 (33.2, 38.6) | 4.7E-155 |
| Model 2 | 89 (86, 93) | 111 (107, 116) | 129 (123, 134) | 161 (154, 168) | 213 (204, 224) | 35.1 (32.4, 37.9) | 3.5E-142 |
| C-reactive protein | |||||||
| Model 1 | 1.7 (1.5, 1.9) | 2.3 (2.1, 2.6) | 2.5 (2.3, 2.8) | 3.2 (2.9, 3.6) | 3.6 (3.2, 4.0) | 31.3 (25.3, 37.7) | 7.6E-29 |
| Model 2 | 1.9 (1.7, 2.1) | 2.4 (2.2, 2.6) | 2.6 (2.3, 2.8) | 3.0 (2.7, 3.3) | 3.3 (3.0, 3.6) | 22.7 (17.2, 28.5) | 4.8E-18 |
| Fasting glucose | |||||||
| Model 1 | 95 (93, 97) | 95 (93, 97) | 96 (94, 98) | 98 (96, 100) | 101 (99, 104) | 2.0 (1.0, 3.0) | 3.9E-5 |
| Model 2 | 97 (95, 99) | 95 (93, 97) | 96 (94, 98) | 97 (95, 99) | 100 (98, 103) | 1.1 (0.1, 2.1) | 0.025 |
The metabolite score included nine sleep-related metabolites (TAGs 45: 1 48: 1 50: 4, DAG 32: 1, PEs 36: 4 38: 5, PCs 30: 1 40: 6, N6-acetyl-L-lysine).
Model 1 adjusted for age.
Model 2 adjusted for age, race/ethnicity, BMI, smoking, alcohol use, caffeine intake, dietary quality, physical activity, prevalent hypertension, depressive symptoms, current hormone therapy, aspirin use, statin use and other lipid-lowering medications.
This SMS was positively associated with CHD risk (Table 3). Compared with the bottom SMS quintile, the multivariable-adjusted odds ratio (95% CI) for developing CHD was 1.79 (1.31, 2.45) for the top quintile (p-trend = 0.0003). When evaluating the score continuously, every SD increase in SMS was associated with a 16% higher odds of developing CHD (95% CI: 1.05, 1.28) after multivariable adjustment. Further adjustment for CHD biomarkers only slightly attenuated the association. When examining 16 replicated metabolites individually (Supplementary Table 5, available as Supplementary data at IJE online), three metabolites (TAG 45: 1, DAG 32: 1, N6-acetyl-L-lysine; all selected in SMS) showed a linear positive association with CHD risk. However, these individual associations were weaker compared with the association with the composite SMS.
Table 3.
Associations of the sleep-associated plasma metabolite score with risk of coronary heart disease among post-menopausal women in the Women’s Health Initiative
| Sleep-related metabolite score (SMS)a |
Per SD increase in SMS | P-trend | |||||
|---|---|---|---|---|---|---|---|
| Quintile 1 |
Quintile 2 |
Quintile 3 |
Quintile 4 |
Quintile 5 |
|||
| Odds ratio (95% CI) | |||||||
| Cases/controls | 109/207 | 162/212 | 186/209 | 220/210 | 231/210 | ||
| Model 1b | 1.00 (ref) | 1.46 (1.08, 1.98) | 1.70 (1.26, 2.29) | 1.93 (1.44, 2.60) | 2.08 (1.55, 2.80) | 1.24 (1.13, 1.36) | 4.411E-06 |
| Model 2c | 1.00 (ref) | 1.48 (1.08, 2.03) | 1.59 (1.17, 2.16) | 1.67 (1.23, 2.28) | 1.79 (1.31, 2.45) | 1.16 (1.05, 1.28) | 0.0003 |
| BMI < 25 kg/m2 | 1.00 (ref) | 1.83 (1.11, 3.03) | 1.67 (1.02, 2.75) | 1.91 (1.13, 3.23) | 1.83 (1.05, 3.17) | 1.19 (1.01, 1.41) | 0.04 |
| BMI ≥ 25 kg/m2 | 1.00 (ref) | 1.26 (0.83, 1.91) | 1.48 (0.99, 2.22) | 1.50 (1.00, 2.23) | 1.67 (1.12, 2.50) | 1.14 (1.01, 1.29) | 0.03 |
| Model 3d | 1.00 (ref) | 1.44 (0.97, 2.14) | 1.65 (1.10, 2.46) | 1.89 (1.23, 2.90) | 1.48 (0.91, 2.41) | 1.17 (0.99, 1.37) | 0.06 |
The metabolite score included nine sleep-related metabolites (TAGs 45: 1 48: 1 50: 4, DAG 32: 1, PEs 36: 4 38: 5, PCs 30: 1 40: 6, N6-acetyl-L-lysine).
Model 1: adjusted for matching factors, including age, race/ethnicity, hysterectomy status and enrolment window.
Model 2: Model 1 + adjusted for BMI, smoking, alcohol use, caffeine intake, dietary quality, physical activity, prevalent hypertension, depressive symptoms, current hormone therapy, aspirin use, statin use and other lipid-lowering medications.
Model 3: Model 2 + adjusted for total cholesterol, HDL cholesterol, total triglycerides, fasting glucose and C-reactive protein. As total triglycerides and fasting glucose were not measured on every participant, Model 3 was based on a subset of 1287 participants (629 cases and 658 controls).
Compared with women with SQS < 6, those with SQS ≥ 6 (poorer sleep quality) had a 36% higher CHD risk (95% CI: 1.02, 1.81; Table 4). About 20.2% of the association was mediated by SMS (indirect effect OR: 1.06, 95% CI: 0.97, 1.15). Although additional adjustment for known CHD biomarkers attenuated the association between SQS and CHD risk (OR: 1.18, 95% CI: 0.88, 1.58), SMS explained a similar amount of the association (21.3%) in the alternative model.
Table 4.
Association of habitual sleep quality with risk of coronary heart disease overall (total), mediated by SMS (indirect) and independent of SMS (direct)a
| Total effect |
Direct effect |
Indirect effect |
% mediated | |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Model 1b | 1.36 (1.02, 1.81) | 1.28 (0.95, 1.73) | 1.06 (0.97, 1.15) | 20.2 |
| Model 2c | 1.18 (0.88, 1.58) | 1.14 (0.85, 1.52) | 1.03 (0.95, 1.11) | 21.3 |
The sleep-related metabolite score (SMS) included nine sleep-related metabolites (TAGs 45: 1 48: 1 50: 4, DAG 32: 1, PEs 36: 4 38: 5, PCs 30: 1 40: 6, N6-acetyl-L-lysine). Habitual sleep quality was evaluated as a binary variable (sleep-quality score ≥6 vs <6).
Model 1: adjusted for matching factors (age, race/ethnicity, hysterectomy status and enrolment window), BMI, smoking, dietary quality, physical activity, prevalent hypertension, current hormone therapy, aspirin use, statin use and other lipid-lowering medications.
Model 2: Model 1 + adjusted for total cholesterol, HDL cholesterol, total triglycerides, fasting glucose and C-reactive protein. As total triglycerides and fasting glucose were not measured on every participant, Model 3 were based on a subset of 1287 participants (629 cases and 658 controls).
Discussion
In two independent samples of post-menopausal women, we found systematic differences in plasma metabolite profiles with poor habitual sleep quality, particularly elevations in several lipid species (TAG, DAG, PE, PC, LPC) and N6-acetyl-L-lysine, a product of histone acetylation. A SMS based on nine representative metabolites (eight lipids and N6-acetyl-L-lysine) was associated with CHD risk and CHD-related risk biomarkers, and was estimated to partially mediate the association between habitual sleep quality and CHD risk. These observational data provide important mechanistic insights that poor sleep quality may increase CHD risk through dysregulation of lipid metabolism.
Several prior studies have evaluated the impact of acute sleep deprivation on plasma metabolites under a controlled, experimental setting. A cross-species investigation in rat and human models revealed changes in the blood metabolome after sleep restriction, mostly of lipid species (18 of 28 in rats and 32 of 37 in humans).11 Similarly to our findings, lipids with low carbon number and low double-bond content across multiple sub-classes (e.g. TAG 46: 0, LPC 16: 1, PC 32: 1, PE 36: 1) were elevated after sleep restriction in humans. Particularly, experimental sleep restriction was related to decreases in two metabolites (DAG 36: 3 and oxalic acid) and was quantitatively replicated across species;11 these markers were not related to habitual sleep patterns in our study. Another study of 12 healthy men reported elevations in 24 plasma metabolites during total sleep deprivation (i.e. 24 hours of wakefulness), 21 of which were related to lipids or fatty acids, including acylcarnitines, glycerophospholipids and sphingolipids.10 However, TAG, DAG, PE, PC and LPC were not measured in that study. A similar study exclusively focusing on lipidomics observed that more than one-third of all measured lipid metabolites changed during total sleep deprivation for 40 hours.12 Both TAGs and PCs substantially increased with time awake, although the TAGs observed in that study generally had longer poly-unsaturated fatty acid chains compared with our findings. A recent study in Chinese adults found that sleep timing, but not sleep duration, was associated with multiple metabolites involved in lipid and amino acid metabolism.25 In another study that combined blood transcriptome and metabolome, altered cholesterol and inflammatory pathways were observed in the context of both experimental and habitual sleep restriction.26 Notably, our focus on a clinically relevant sleep rating score (sleep satisfaction, sleep latency, sleep awakenings and hypnotic use), as described in a recent conceptual model of sleep health,27 differs from the sleep-duration and sleep-timing measures studied in prior metabolomic studies. Further, the associations persisted after adjusting for sleep duration, possibly reflecting the physiological impact of disrupted sleep on metabolism—a mechanism supported by several studies reporting stronger associations of cardiometabolic outcomes with short sleep occurring with symptoms of sleep disruption compared with short sleep without such symptoms.28,29 We also showed that sleep-medication use and sleep satisfaction were more strongly associated with lipid metabolites than other sleep-quality components. This may be due to more severe, multifaceted sleep problems captured by these two components. Although exclusion of women with sleep-medication use did not appreciably alter our results, whether certain sleep medications directly influence lipid profiles requires further investigation. Collectively, our findings, coupled with these experimental studies, strongly support the paradigm that both acute and chronic disturbances in sleep contribute to systemic dysregulation of lipid metabolism.
The lipid signature identified for poor habitual sleep quality in this study, namely lipids of lower carbon number and double-bond content, has previously been demonstrated to strongly predict risk of cardiovascular disease (CVD), diabetes and insulin resistance. In the Bruneck Study,30 such lipids (e.g. TAG with 50–54 carbons and 1–3 double bonds) were positively associated with CVD risk. The top three lipid species most predictive of CVD risk were TAG 54: 2, PE 36: 5 and CE 16: 1. In the Framingham Heart Study,31 lipids characterized by lower carbon number and double-bond content, including TAG, PC, LPE and LPC, were consistently associated with diabetes risk. Consistently with these studies, our results show that a SMS based on four lipid species (TAG, DAG, PE, PC) most reflective of poor habitual sleep quality was associated with CHD risk and established biomarkers of cardiometabolic risk. The role of the sleep-related metabolomic alterations in CHD development was further implicated by the results of the mediation analysis, in which SMS explained about 20% of the association between SQS and CHD risk. Taken together, these data suggest that alterations in lipid profiles represent a pathogenic pathway through which habitual sleep quality may influence development of cardiometabolic disease, including CHD.
N6-acetyl-L-lysine, an amino acid derivative that plays an important role in post-translational modification and epigenetic regulation,32–34 was the only non-lipid metabolite associated with SQS in both studies. Whereas it is known that acetylation of lysine residues on the N-terminal of histone via histone acetyltransferases (HATs) reduces the binding affinity of histone to DNA and could activate/increase gene transcription and expression,32–34 whether circulating N6-acetyl-L-lysine is directly indicative of histone acetylation levels or gene transcriptional activity is less clear. Interestingly, the protein CLOCK, a key player in circadian physiology, possesses HAT activity with specific histone acetylation essential for circadian regulation.35 Further, histone acetylation/deacetylation exhibits circadian rhythmicity in mouse liver and controls hepatic lipid homeostasis; persistent histone acetylation up-regulates genes involved in lipid metabolism, leading to ∼10-fold increase in liver triglyceride and a fatty liver phenotype.36 Given the emerging evidence for the epigenetic influence by sleep37 and the role of epigenetic mechanisms in CVD development,38 further research is needed to determine whether histone acetylation may be a novel pathway linking sleep, lipid dysregulation and CVD risk.
Our primary analysis in WHI also identified other non-lipid metabolites associated with poor sleep quality, including lower levels of betaine, serine and biliverdin, which have previously been implicated in the pathogenesis of cardiometabolic disease. Betaine and serine, both involved in choline metabolism, are inversely associated with insulin resistance and inflammation.39–41 In a randomized–controlled trial evaluating lifestyle vs metformin intervention in diabetes prevention,42 baseline betaine and serine were significant predictors for diabetes risk. Particularly, lifestyle intervention, which was most effective in preventing diabetes, resulted in increased betaine levels and this increase was also associated with lower incidence of diabetes. Biliverdin and its reduction product, bilirubin, are bile pigments with potent antioxidant properties that inhibit lipid oxidation and atherosclerotic development.43,44 A number of studies reported an inverse association between bilirubin levels and risk of hypertension, diabetes and CHD.45–47 These suggestive associations should be explored further in other independent studies and, if confirmed, could support the hypothesis that elevated oxidative stress and inflammation are important mechanisms through which sleep influences cardiometabolic health.
The limitations of our study should be acknowledged. First, as our study of habitual sleep and metabolites was cross-sectional, we cannot establish the directionality of the associations. Although the possibility that metabolic dysregulation reciprocally precipitates sleep disturbances cannot be excluded, we showed that poor baseline sleep quality was associated with unfavourable changes of most replicated metabolites, suggesting that the observed associations reflect the systemic effects of sleep on metabolism. This argument is also supported by the consistency of our findings with prior experimental studies. Similarly, given emerging evidence suggesting bidirectional relationships of sleep with lifestyle factors, mood and co-morbidities,48–50 our assumptions regarding the temporal relationships of exposure, mediator and covariates (Supplementary Figure 2, available as Supplementary data at IJE online) may not be accurately reflected by the cross-sectional data. Second, our assessment of habitual sleep quality was based on self-reported symptoms using two slightly different scales/questions, which may introduce differential measurement errors influenced by certain participant characteristics (e.g. depression). We did not have objective information on sleep architecture or sleep apnoea to explore the potential influence of these factors on our findings.51–53 However, self-reported symptoms are routinely assessed in clinical diagnostic interviews for sleep disorders and reflect the subjective experience of sleep, an essential aspect of sleep disorders. In future studies, elucidating the associations of objective measures of sleep quality with plasma metabolites will provide complementary evidence to our results. Third, some notable differences between the discovery and replication samples (e.g. age, socio-demographics, medication use, etc.) may undermine our ability to identify certain metabolites influenced by these factors. The small size of the NHSII substudy limits the capacity for replication. Nevertheless, the consistent signals we observed across the two divergent samples highlight some common metabolic correlates of habitual sleep quality. Our composite SMS that integrated multiple risk metabolites yielded stronger associations than individual metabolites, suggesting that this approach may capture co-altered metabolic dysregulation in disease development. In addition, since our study focused on post-menopausal women, whether differences by sex or menopausal status exist requires further investigation. Finally, it is noteworthy that our metabolomics did not include carbohydrates and the technical impact of long-term blood storage in WHI on metabolomic profiles or specific metabolite categories should be identified in further studies.
Conclusions
Across two samples of post-menopausal women, poor habitual sleep quality is associated with unfavourable metabolomic profiles that may lead to higher risk of CHD. Potential epigenetic changes associated with sleep, particularly histone acetylation and its relevance to CHD development, warrant additional studies. Further investigations are also needed to determine whether interventions to improve sleep can improve metabolic profiles and modify pathways that reduce cardiometabolic disease risk.
Supplementary Material
Acknowledgements
We acknowledge the contributions of the WHI staff and participants for enabling this research. Metabolomic analyses in the WHI were funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through contract HHSN268201300008C. The WHI programme is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C and HHSN268201600004C. We thank the participants and the staff of the Nurses’ Health Study II for their valuable contributions. This work was supported by the National Institute of Health (R01 CA163451, U54 CA155626, UM1 CA176726). T.H. is a recipient of the American Heart Association post-doctoral fellowship (Founders Affiliate) award (16POST27480007). S.R. is supported by NHLBI R35HL135818. T.H. and K.M.R. assume full responsibility for analyses and interpretation of these data.
Conflict of interest: None declared.
References
- 1. Gordon T, Kannel WB, Hjortland MC, McNamara PM.. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med 1978;89:157–61. [DOI] [PubMed] [Google Scholar]
- 2. Vitale C, Mendelsohn ME, Rosano GM.. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol 2009;6:532–42. [DOI] [PubMed] [Google Scholar]
- 3. Regensteiner JG, Golden S, Huebschmann AG.. Sex differences in the cardiovascular consequences of diabetes mellitus: a scientific statement from the American Heart Association. Circulation 2015;132:2424–47. [DOI] [PubMed] [Google Scholar]
- 4. Peters SA, Huxley RR, Woodward M.. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014;383:1973–80. [DOI] [PubMed] [Google Scholar]
- 5. Zhang B, Wing YK.. Sex differences in insomnia: a meta-analysis. Sleep 2006;29:85–93. [DOI] [PubMed] [Google Scholar]
- 6. Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF.. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol 2014;21:57–64. [DOI] [PubMed] [Google Scholar]
- 7. Li M, Zhang XW, Hou WS, Tang ZY.. Insomnia and risk of cardiovascular disease: a meta-analysis of cohort studies. Int J Cardiol 2014;176:1044–47. [DOI] [PubMed] [Google Scholar]
- 8. Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immun 2008;22:960–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller MA, Kandala NB, Kivimaki M. et al. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep 2009;32:857–64. [PMC free article] [PubMed] [Google Scholar]
- 10. Davies SK, Ang JE, Revell VL. et al. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci USA 2014;111:10761–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weljie AM, Meerlo P, Goel N. et al. Oxalic acid and diacylglycerol 36: 3 are cross-species markers of sleep debt. Proc Natl Acad Sci USA 2015;112:2569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chua EC, Shui G, Cazenave-Gassiot A, Wenk MR, Gooley JJ.. Changes in plasma lipids during exposure to total sleep deprivation. Sleep 2015;38:1683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anderson GL, Manson J, Wallace R. et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol 2003;13:S5–17. [DOI] [PubMed] [Google Scholar]
- 14. Paynter NP, Balasubramanian R, Giulianini F. et al. Metabolic predictors of incident coronary heart disease in women. Circulation 2018;137:841–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang T, Poole EM, Vetter C. et al. Habitual sleep quality and diurnal rhythms of salivary cortisol and dehydroepiandrosterone in postmenopausal women. Psychoneuroendocrinology 2017;84:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luo J, Sands M, Wactawski-Wende J, Song Y, Margolis KL.. Sleep disturbance and incidence of thyroid cancer in postmenopausal women the Women’s Health Initiative. Am J Epidemiol 2013;177:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ.. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 18. Levine DW, Kripke DF, Kaplan RM. et al. Reliability and validity of the Women’s Health Initiative insomnia rating scale. Psychol Assess 2003;15:137–48. [DOI] [PubMed] [Google Scholar]
- 19. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A.. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev 2016;25:52–73. [DOI] [PubMed] [Google Scholar]
- 20. Mascanfroni ID, Takenaka MC, Yeste A. et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat Med 2015;21:638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Townsend MK, Clish CB, Kraft P. et al. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem 2013;59:1657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Storey JD. A direct approach to false discovery rates. J R Stat Soc B Stat Methodol 2002;64:479–98. [Google Scholar]
- 23. Efron B, Hastie T, Johnstone I, Tibshirani R.. Least angle regression. Ann Stat 2004;32:407–99. [Google Scholar]
- 24. Valeri L, Vanderweele TJ.. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 2013;18:137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xiao Q, Derkach A, Moore SC. et al. Habitual sleep and human plasma metabolomics. Metabolomics 2017;13:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aho V, Ollila HM, Kronholm E. et al. Prolonged sleep restriction induces changes in pathways involved in cholesterol metabolism and inflammatory responses. Sci Rep 2016;6:24828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buysse DJ. Sleep health: can we define it? Does it matter? Sleep 2014;37:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bertisch SM, Pollock BD, Mittleman MA. et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep 2018;41. doi: 10.1093/sleep/zsy047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO.. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care 2009;32:1980–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stegemann C, Pechlaner R, Willeit P. et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 2014;129:1821–31. [DOI] [PubMed] [Google Scholar]
- 31. Rhee EP, Cheng S, Larson MG. et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 2011;121:1402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grunstein M. Histone acetylation in chromatin structure and transcription. Nature 1997;389:349–52. [DOI] [PubMed] [Google Scholar]
- 33. Rice JC, Allis CD.. Histone methylation versus histone acetylation: new insights into epigenetic regulation. Curr Opin Cell Biol 2001;13:263–73. [DOI] [PubMed] [Google Scholar]
- 34. Hebbes TR, Thorne AW, Crane-Robinson C.. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J 1988;7:1395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Doi M, Hirayama J, Sassone-Corsi P.. Circadian regulator CLOCK is a histone acetyltransferase. Cell 2006;125:497–508. [DOI] [PubMed] [Google Scholar]
- 36. Feng D, Liu T, Sun Z. et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science 2011;331:1315–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nilsson EK, Bostrom AE, Mwinyi J, Schioth HB.. Epigenomics of total acute sleep deprivation in relation to genome-wide DNA methylation profiles and RNA expression. OMICS 2016;20:334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ordovás JM, Smith CE.. Epigenetics and cardiovascular disease. Nat Rev Cardiol 2010;7:510–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Detopoulou P, Panagiotakos DB, Antonopoulou S, Pitsavos C, Stefanadis C.. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr 2008;87:424–30. [DOI] [PubMed] [Google Scholar]
- 40. Konstantinova SV, Tell GS, Vollset SE, Nygård O, Bleie Ø, Ueland PM.. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr 2008;138:914–20. [DOI] [PubMed] [Google Scholar]
- 41. Ejaz A, Martinez-Guino L, Goldfine AB. et al. Dietary betaine supplementation increases Fgf21 levels to improve glucose homeostasis and reduce hepatic lipid accumulation in mice. Diabetes 2016;65:902–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walford GA, Ma Y, Clish C, Florez JC, Wang TJ, Gerszten RE.. Metabolite profiles of diabetes incidence and intervention response in the diabetes prevention program. Diabetes 2016;65:1424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN.. Bilirubin is an antioxidant of possible physiological importance. Science 1987;235:1043–46. [DOI] [PubMed] [Google Scholar]
- 44. Stocker R, McDonagh AF, Glazer AN, Ames BN.. Antioxidant activities of bile pigments: biliverdin and bilirubin. Methods Enzymol 1990;186:301–09. [DOI] [PubMed] [Google Scholar]
- 45. Kunutsor SK, Bakker SJ, Gansevoort RT, Chowdhury R, Dullaart RP.. Circulating total bilirubin and risk of incident cardiovascular disease in the general population. Arterioscler Thromb Vasc Biol 2015;35:716–24. [DOI] [PubMed] [Google Scholar]
- 46. Abbasi A, Deetman PE, Corpeleijn E. et al. Bilirubin as a potential causal factor in type 2 diabetes risk: a Mendelian randomization study. Diabetes 2015;64:1459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang L, Bautista LE.. Serum bilirubin and the risk of hypertension. Int J Epidemiol 2015;44:142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kahn M, Sheppes G, Sadeh A.. Sleep and emotions: bidirectional links and underlying mechanisms. Int J Psychophysiol 2013;89:218–28. [DOI] [PubMed] [Google Scholar]
- 49. Vgontzas AN, Bixler EO, Basta M.. Obesity and sleep: a bidirectional association? Sleep 2010;33:573–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang T, Lin BM, Stampfer MJ, Tworoger SS, Hu FB, Redline S.. A population-based study of the bidirectional association between obstructive sleep apnea and type 2 diabetes in three prospective U.S. cohorts. Diabetes Care 2018;41:2111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Campos-Rodriguez F, Gonzalez-Martinez M, Sanchez-Armengol A. et al. Effect of continuous positive airway pressure on blood pressure and metabolic profile in women with sleep apnoea. Eur Respir J 2017;50. doi: 10.1183/13993003.00257-2017. [DOI] [PubMed] [Google Scholar]
- 52. Xu H, Zheng X, Qian Y. et al. Metabolomics profiling for obstructive sleep apnea and simple snorers. Sci Rep 2016;6:30958.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ferrarini A, Ruperez FJ, Erazo M. et al. Fingerprinting-based metabolomic approach with LC-MS to sleep apnea and hypopnea syndrome: a pilot study. Electrophoresis 2013;34:2873–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.