Abstract
Genetic information is constantly being attacked by intrinsic and extrinsic damaging agents, such as reactive oxygen species, atmospheric radiation, environmental chemicals, and chemotherapeutics. If DNA modifications persist, they can adversely affect the polymerization of DNA or RNA, leading to replication fork collapse or transcription arrest, or can serve as mutagenic templates during nucleic acid synthesis reactions. To combat the deleterious consequences of DNA damage, organisms have developed complex repair networks that remove chemical modifications or aberrant base arrangements and restore the genome to its original state. Not surprisingly, inherited or sporadic defects in DNA repair mechanisms can give rise to cellular outcomes that underlie disease and aging, such as transformation, apoptosis, and senescence. In the review here, we discuss several genetic disorders linked to DNA repair defects, attempting to draw correlations between the nature of the accumulating DNA damage and the pathological endpoints, namely cancer, neurological disease, and premature aging.
Main Text
Introduction
Several essential biochemical processes generate reactive oxygen species (ROS) or other intracellular damaging agents that challenge the genome integrity of an aerobic organism. These processes include, most notably, mitochondrial respiration, which produces chemical energy in the form of ATP that fuels many cellular operations. When considering spontaneous hydrolytic decay, the different endogenous metabolic genotoxins, the many environmental chemical agents, and background radiation, it has been estimated that approximately 104–105 DNA lesions are generated in each mammalian genome per day. In light of the adverse consequences of unwanted DNA modifications, our genetic material is a pivotal macromolecule that needs to be maintained and preserved in a timely and efficient manner. In particular, DNA damage can stall the replication or transcription machinery, leading to mutagenic or stress responses. For example, cells might erroneously bypass a miscoding lesion or transiently deploy low-fidelity DNA polymerases to overcome persistent DNA damage, choosing mutagenesis over cell death. Such mutational events can lead to either inactivation of tumor suppressor genes or activation of oncogenes, seeding the carcinogenic process. Alternatively, lesion-induced replication or transcription arrest can perturb cellular homeostasis, often promoting the activation of cell death responses, such as p53 (MIM: 191170)-dependent apoptosis. Given the importance of removing DNA damage from the genome and sustaining genomic integrity, organisms have evolved intricate interdependent systems that involve cell cycle check point pathways, the DNA-damage response (DDR), and DNA repair mechanisms. Deficiency in many members of these processes is associated with a range of diseases, including cancer predisposition, neurodegeneration, and premature aging.
Overview of the Formation and Consequences of DNA Damage
Intrinsic Damaging Agents
Sources of DNA damage can be intrinsic or extrinsic. Intrinsic sources of DNA damage include spontaneous hydrolysis, replication mistakes, replicative stress, and reactions with endogenous chemicals, such as the ROS noted earlier (Table 1). As a chemical itself within an aqueous environment, DNA is subject to reactions with water, resulting in, for example, the spontaneous formation of an apurinic/apyrimidinic (AP) site (via hydrolysis of the glycosidic bond) or uracil from cytosine (via deamination).1 AP sites, which lack the instructional information of the base, are non-coding templates that can cause mutagenic end-points or serve as blocks to replicative DNA or RNA polymerases.2, 3 Moreover, abasic sites have the potential to chemically react with guanine residues in the strand opposite, forming a more severe form of DNA damage, i.e., a covalent interstrand crosslink (ICL).4 Uracil in DNA base-pairs with adenine when copied, thereby changing the coding properties of the original deaminated cytosine, a feature that seemingly underlies the common cancer-associated C→T mutational signature.5, 6 Additionally, even with a highly evolved replication apparatus that includes proofreading activity, approximately 1 nucleotide misincorporation occurs in every 108 insertion events.7 The accidental incorporation of a ribonucleotide is even more frequent, given that they are present at concentrations of 30- to 200-fold higher than their corresponding dNTPs.8 Inappropriate base-pairs can drive mutagenic outcomes, and ribonucleotides can alter the coding properties of the genome, as well as protein binding platforms (such as used by a transcription factor), epigenetic landscapes, etc. Strand-slippage errors can also occur during DNA replication, particularly in microsatellite sequences (stretches of 2–6 nucleotide repeats), since nearby duplicate bases can stabilize the incorrect pairing and permit chromosome duplication to proceed, creating an insertion/deletion (indel) precursor.9, 10 A subsequent round of replication would result in an expansion or retraction event, such as commonly seen in diseases of trinucleotide repeats, like Huntington disease (MIM: 143100). Lastly, replication through either microsatellite sequences, which are often found in fragile sites within the chromosome, or transcription-derived R-loops (i.e., RNA-DNA hybrids), can cause replicative stress, leading to fork collapse, the formation of double-strand breaks (DSBs), and genomic instability.11, 12, 13, 14
Table 1.
Intrinsic and Extrinsic DNA-Damaging Agents, and the Types and Consequences of Associated DNA Damage
| Source | Damaging Agent or Event | Major Form(s) of Damage | Primary Consequence on DNA Structure and Transactions | |
|---|---|---|---|---|
| Intrinsic | spontaneous hydrolysis | uracil | little structural impact; causes C→T mutations | |
| AP sites | increased phosphodiester backbone flexibility, possible structural distortion; non-coding, mutagenic; polymerase block | |||
| replication mistakes | mispair | some DNA conformational effects, depending on mispair; mutagenic | ||
| indel | conformational effect (hairpin loop); MSI | |||
| replicative stress | DSBs | fork collapse, genomic instability | ||
| reactive endogenous chemicals | ROS | base modifications, AP sites, SSBs | depending on the damage, mutagenic or polymerase block (see text for examples) | |
| SAM | methylated bases, e.g., O6-methylguanine | little structural impact; causes G→A mutations | ||
| aldehydes (e.g., malondialdehyde) | base adducts, ICLs | helix-distortion, covalent bridge; polymerase block; possible error-prone bypass | ||
| Extrinsic | radiation | UV | CPDs, 6-4PPs | helix-distorting; polymerase block; possible error-prone bypass |
| IR | base modifications, SSBs and DSBs | depending on the damage, mutagenic or polymerase block; genomic instability | ||
| environmental chemicals | B[a]P | base adduct | helix-distorting; polymerase block; possible G→T mutations | |
| AFB1 | base adducts | helix-distortion; polymerase block; possible error-prone bypass | ||
| chemotherapeutic agents | cisplatin | base adducts, intra and interstrand crosslinks | helix-distortion; polymerase block | |
| etoposide, doxorubicin | DSBs, protein-DNA adduct | replication and transcription block | ||
Another prominent intrinsic source of DNA damage is ROS, which are generated during mitochondrial respiration or through metal-based Fenton reactions. ROS, including superoxide, hydrogen peroxide, hydroxyl radicals, and singlet oxygen, can directly produce oxidatively damaged bases, abasic (AP) sites, and single-strand breaks (SSBs)15, 16, 17 (Table 1). Some of the more common oxidative base modifications include 8-oxoguanine (8-oxoG), thymine glycol, 8-hydroxyadenine, 2-hydroxyadenine, 2,6-diamino-4-hydroxy-5-formamidopyrimidine and 4,6-diamino-5-formamidopyrimidine (the so-called FaPys), and cyclopurines, with each serving as a miscoding template or obstruction to a DNA or RNA polymerase depending on their chemical make-up. For example, 8-oxoG, one of the most frequent oxidative base lesions and a biomarker of oxidative stress, causes little change to the DNA helical structure, yet frequently mispairs with adenine, causing G→T transversions.18 At the other end of the spectrum, 8,5′-cyclopurine-2′-deoxynucleosides, which are generated by oxidation and covalent intramolecular cyclization of the C5′- and C8-positions of the purine nucleoside, induce unusual puckering of the sugar moiety and distortion of the DNA helix.15, 19 Cyclopurines are considered “bulky” adducts, more adept at blocking DNA or RNA polymerase progression than driving mutagenic events. As noted earlier, AP sites, whether generated via ROS-induced mechanisms or as repair intermediates (see later), are non-coding modifications that can lead to mutations or polymerase arrest. ROS attack of the sugar ring in DNA can also lead to the direct formation of SSBs, many of which harbor non-conventional termini, such as 3′-phosphate, 3′-phosphoglycolate, or 5′-hydroxyl groups. SSBs represent interruptions in the phosphodiester backbone and thus can collapse a progressing DNA or RNA polymerase complex or drive recombination events when encountered during replication.20
Damage to other cellular macromolecules (besides DNA) by endogenous ROS can produce additional forms of intracellular genotoxins. For example, lipid peroxidation can yield malondialdehyde, which can react with DNA bases to generate bulky adducts, such as pyrimido[1,2α]purin-10(3H)-one (M1G), or promote the formation of ICLs that link the two strands of DNA. Both modifications can create structural alterations in DNA and have the ability to inhibit the replication or transcription machinery.21 In addition, peroxidation of polyunsaturated fatty acids in cellular membranes generates 4-hydroxy-2-nonenals (4-HNE), a major lipid peroxidation-derived aldehyde that can react with all four DNA bases, albeit most efficiently with guanine. Notably, 4-HNE-dG adducts are biomarkers associated with p53 mutation-related cancers.22 Finally, various endogenous alkylating compounds, mainly S-adenosyl-L-methionine (SAM), a common co-substrate involved in methyl group transfers, can modify bases to form pre-mutagenic lesions, such as O6-methylguanine, or blocking modifications, such as 3-methyladenine.23 During replication, O6-methylguanine mispairs with T, resulting in G→A transition mutations.
Extrinsic Damaging Agents
Some of the major extrinsic DNA-damaging threats include ultraviolet (UV) or ionizing (IR) radiation, numerous environmental chemicals, and intended-use chemotherapeutic agents (Table 1). An almost unavoidable environmental exposure is atmospheric radiation. UV radiation is indeed one of the most significant health hazards to humans, causing mild skin burns or, in extreme cases, skin cancer.24 Since UV-C is thought to be mostly absorbed by the earth’s atmosphere, UV-A and UV-B comprise the primary damaging component of the solar UV spectrum, producing 105 DNA photolesions per hour in an exposed cell.25 Cyclobutene pyrimidine dimers (CPDs) and pyrimidine-(6,4)-pyrimidone photoproducts (6-4PPs) are the predominant UV-induced DNA modifications,26 with the latter making up around 1/3rd of the total lesion number yet representing a greater mutagenic threat.25 Photolesions cause mild structural alterations to the DNA helix that can impede polymerase progression or promote C→T transition mutations that underlie melanoma through the activity of translesion DNA polymerases. IR produces a wide spectrum of DNA damage, either through direct ionization or indirectly through the production of ROS via the radiolysis of water. The array of lesions generated include base modifications, SSBs, and DSBs, depending upon the type and dose of exposure. For example, low linear energy transfer γ-radiation produces approximately 450–850 base lesions, 1,000 SSBs, and 20–40 DSBs/cell/Gy, with many of the lesions being clustered (within a helical turn) due to the nature of the IR track.27, 28, 29
Other extrinsic DNA-damaging agents are part of either our lifestyle choices or everyday routines (e.g., driving). For instance, cigarette smoke contains nicotine, the polycyclic aromatic hydrocarbon (PAH), benzo[a]pyrene (B[a]P), heterocyclic compounds (furan), N-nitrosamines (N-nitrosodimethylamine), aldehydes (formaldehyde), volatile hydrocarbons (benzene), metals, and inorganic compounds (arsenic), many of which are known human carcinogens.30 In addition, PAHs, like naphthalene, benz[a]anthracene, and B[a]P, are naturally occurring chemicals in various daily-use energy sources, such as coal, crude oil, and gasoline, with the ability to either directly or indirectly induce DNA damage.31 Indeed, many PAH-related mutation signatures are observed in the tumor suppressor gene p53, which regulates cell proliferation, differentiation, apoptosis, and DNA repair.32 B[a]P, one of the most highly distributed mutagenic chemical agents found in the environment and foods, is metabolized by cytochrome P450 and epoxide hydrolase to form (+)benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide (BPDE), a major carcinogenic metabolite that covalently binds to DNA to form BPDE-N2-dG adducts.33 This adduct promotes G→T transversion mutations and adversely affects replication. Alkylators, such as sulfur and nitrogen mustards, which are both environmental contaminants and chemotherapeutic compounds (see below), mostly react with the O6 and N7 position of guanine, along with the N1, N3, and N7 position of adenine and the N1 position of cytosine.34 Moreover, bifunctional alkylating agents can lead to bridging of adjacent bases to form intrastrand or interstrand crosslinks, DNA products that adversely affect replication or transcription. Aromatic agents (e.g., 2-aminofluorene), often found in synthetic chemical insecticides, form C8-guanine lesions and can lead to base substitutions.35 Finally, a number of agricultural products like peanuts and corn are contaminated with aflatoxins (B1, B2, G1, G2, and M1), which are produced by pathogenic fungi such as Aspergillus flavus and Aspergillus parasiticus. Biotransformation of the most prevalent aflatoxin B1 (AFB1), a potent hepatocarcinogen following dietary or inhalation exposure,36 generates AFB1-exo-8,9-epoxide, which can alkylate guanine to form an AFB1-N7-guanine adduct. This adduct is further hydrolyzed to form AFB1-formamidopyrimidine (AFB1-FaPy), which is less distortive to the DNA helix, but still a strong replication block with a greater G→T transversion potential.36
The final DNA-damaging agents covered are those that are employed intentionally, often in clinical settings to treat cancer-affected individuals. Although not without their own harmful side effects, particularly to areas involving rapidly dividing cells such as hair follicles, nails, the mouth, digestive tract, and bone marrow, cytotoxins remain a major part of the front-line arsenal to eradicate cancer. A few of the most commonly employed anti-cancer drugs that elicit their effects through the induction of lethal DNA damage include cisplatin or cis-diamminedichloroplatinum(II), carboplatin, oxaliplatin, temozolomide, 5-fluorouracil (5-FU), methotrexate, etoposide, and doxorubicin.37 Due to the low chlorine concentration of the intracellular environment relative to blood, cisplatin is hydrolyzed upon entry into a cell to an electrophile that reacts with nucleophilic centers, such as those found in DNA, to form intrastrand diadducts, e.g., Pt-d(GpG), Pt-d(ApG), and Pt-d(GpXpG), and to a lesser extent Pt-G-G ICLs. These cisplatin-DNA lesions, which are similar in nature to those formed by the other platinum-based analogs, have great propensity to stall DNA replication and transcription, activating cell death responses.38, 39, 40 Additional alkylators, such as temozolomide, react with DNA to form O6 methylated guanine adducts that have the potential to cause cytotoxic outcomes.41 Antimetabolites, such as 5-FU and methotrexate, represent a distinct class of anticancer drugs that interfere with DNA replication either by substituting for natural nucleotides during DNA or RNA copying or by promoting nucleotide pool imbalances that arrest chromosome duplication.37 Agents such as etoposide and doxorubicin are part of a class of drugs called topoisomerase poisons, as they inhibit the superhelical relaxation activity of topoisomerases, leading to stable protein-DNA adduct formation, DSBs, and subsequent cell death.37, 42 Collectively, modifications to the genome, however introduced, have the potential to change the coding properties of DNA or to block DNA transactions, leading to mutagenesis, genomic instability, or the activation of various cellular fates, such apoptosis, necrosis, or senescence.
DNA Repair Mechanisms
The repair response initiated for DNA lesions that do not break the phosphodiester backbone, namely base and sugar damage, is in part dictated by the structural consequences of the modification on the duplex or their ability to affect the progression of DNA replication or RNA transcription. SSBs and DSBs, which sever one or both strands of DNA, respectively, and offer an impediment to ongoing polymerization events, are resolved by specialized pathways that can be influenced by cell cycle phase or replicative status of the cell. For the remainder of the review, we define DNA modifications that do not overtly alter the helical structure or interfere with polymerase progression as “idle,” whereas lesions that distort the helical structure or at least impede polymerase activity are classified as “active.” While idle lesions may appear innocuous to the cell, they are potentially harmful in that they can serve as miscoding templates. Active damage can stimulate mutagenic translesion synthesis (tolerance) mechanisms or more generalized responses that result in cell death or senescence. Further discussion on the nature and consequences of DNA damage is incorporated throughout. We provide next an overview of the major DNA repair mechanisms and their role in resolving specific forms of DNA damage.
Repair of Idle Base Damage
Mismatched nucleotides or small indels, which arise due to DNA polymerase mistakes as noted earlier, have the potential to alter the helical structure of DNA depending on their specific composition, yet are unlikely to cause significant interference to the transcription or replication machinery, classifying them as idle lesions. Such replication errors are typically rectified by the DNA mismatch repair (MMR) system (Figure 1A), prior to becoming permanent genetic alterations following another round of chromosome duplication.43, 44 Mispair or indel recognition is performed by heterodimer complexes of Escherichia coli MutS homologs (MSH), i.e., MutSα (composed of MSH2 [MIM: 609309] and MSH6 [MIM: 600678]), which detects base mismatches and indels of 1–2 nucleotides, or MutSβ (composed of MSH2 and MSH3 [MIM: 600887]), which resolves larger indels. While the mechanism by which DNA repair proteins recognize DNA perturbations requires further elucidation, upon mismatch detection, MutSα or MutSβ is converted into a sliding clamp through the exchange of ADP→ATP in its nucleotide binding site, facilitating damage verification.45 The clamp then diffuses along the DNA and interacts with MutLα, which consists of MutL homolog 1 (MLH1 [MIM: 120436]) and post-meiotic segregation2 (PMS2 [MIM: 600259]).46 Proliferating cell nuclear antigen (PCNA [MIM: 176740]), a key replicative sliding clamp for long-range DNA polymerization, is vital for recruitment of the MMR assembly, including MSH2, MSH3, and/or MSH6, MLH1, exonuclease 1 (EXO1 [MIM: 606063]) and the DNA polymerases. Hemi-methylation (dGATC site) status and pre-existing nicks help in strand discrimination during MMR and serve as a potential PCNA loading site, an event that is likely assisted by replication factor C (RFC).47 In situations involving a 5′ nick, MutSα activates the 5′ to 3′ exonuclease activity of EXO1 to remove the mispair. While 3′-directed excision is not well understood, the endonuclease activity of MutLα is essential for this process;48 MutLα is activated upon interacting with either MSH complex and PCNA.49 Presumably in cooperation with MutLα, meiotic recombination 11 (MRE11 [MIM: 600814]), DNA polymerase (POL) δ (MIM: 174761), and ε (MIM: 174762) take part in the 3′→5′ exonuclease processing.50, 51 The remaining gap in either 5′- or 3′-directed MMR is filled by POLδ after mismatch removal, and DNA ligase 1 (LIG1 [MIM: 126391]) completes the response by sealing the remaining nick.44 As discussed later, defects in MMR are associated with genomic instability and cancer proneness.
Figure 1.
MMR and BER
(A) Replication infidelity leads to nucleotide mismatches (red star) or indels (not shown). MutS homologs (MSH) and MutL homologs (MLH) interact to carry out mismatch recognition. Pre-existing nicks in the newly synthesized strand or generated either 5′ (depicted) or 3′ (not shown) facilitate recruitment of an excision nuclease, such as EXO1. Following mismatch excision, the gap is filled, and the remaining nick sealed to complete the MMR response. See text for further details.
(B) Classic BER is initiated by base damage (yellow star) removal, creating an AP site (yellow circle). AP sites can be processed by multiple enzymes but are the main substrate of APE1. Following incision at the AP damage, the break termini are processed and the gap filled. Depending on the nature of the substrate and the cellular environment, repair synthesis involves either short-patch or long-patch events. The final nick is sealed to complete the response. See text for further details. Figures were created using artwork of Servier Medical Art and Chemdraw.
Small, mostly idle, base lesions arising from oxidation (e.g., 8-oxoG), deamination (i.e., uracil), or alkylation (e.g., 3-methyladenine) are primarily repaired by BER (Figure 1B). To initiate the classic form of the pathway, a DNA glycosylase scans the genome and selectively recognizes a damaged or inappropriate substrate base by stably flipping the base into the compatible enzyme active site before excising it from the DNA backbone and leaving behind an abasic site product. In mammals, there are 11 different damage-specific DNA glycosylases, several of which have both nuclear and mitochondrial isoforms: uracil-DNA glycosylase (UNG [MIM: 191525]), single-strand selective monofunctional uracil glycosylase (SMUG1 [MIM: 607753]), thymine DNA glycosylase (TDG [MIM: 601423]), methylpurine glycosylase (MPG [MIM: 156565]), methyl binding domain 4 protein (MBD4 [MIM: 603574]), E. coli MutY homolog (MUTYH [MIM: 604933]), E. coli nth endonuclease III-like 1 (NTHL1 [MIM: 602656]), E. coli nei endonuclease VIII-like 1/2/3 (NEIL1/2/3 [MIM: 608844]/[MIM: 608933]/[MIM: 608934]), and 8-oxoguanine DNA glycosylase (OGG1 [MIM: 601982]).52, 53 Some glycosylases are monofunctional in nature (UNG, SMUG1, TDG, MPG, MBD4), i.e., they only excise substrate bases, whereas the others are bifunctional (OGG1, NEIL1/2/3), meaning that they not only remove the target base, but have the ability to cleave the DNA backbone at the resulting AP site via a β- or β,δ-lyase activity. In the latter situation, bifunctional DNA glycosylases generate an SSB that needs to be processed by a 3′-damage SSB repair (SSBR) enzyme prior to gap filling and ligation (see later). In situations where the abasic site product is left intact, presumed to be the major repair response, an AP endonuclease recognizes the damage and cleaves the DNA backbone 5′ to the AP site to create a strand break with a priming 3′-hydroxyl group and a 5′-abasic fragment (deoxyribose phosphate). In mammals, the major, if not sole, AP endonuclease is APE1 (MIM: 107748).54 Following AP site cleavage by APE1, BER would proceed via either a “short-patch” (or single-nucleotide) or “long-patch” repair reaction. Short-patch BER, which is presumably the favored pathway, involves the replacement of only the damaged nucleotide, typically by the major gap filling DNA polymerase, POLβ (MIM: 174760).55 POLβ also carries out excision of the remaining 5′-abasic fragment, permitting nick ligation by the X-ray cross-complementing protein 1 (XRCC1 [MIM: 194360])/DNA ligase 3α (LIG3α [MIM: 600940]) complex.56 Long-path BER, as the name suggests, involves incorporation of multiple (2–10) nucleotides and strand-displacement synthesis. This mechanism of BER is thought to be driven by the relative BER protein concentrations, the cell cycle phase, and ATP abundance (a co-factor for the ligation reaction), and is likely carried out by the action of the replicative polymerases, POLε or POLδ, in concert with PCNA. The created 5′-flap is removed by the flap structure-specific endonuclease 1 (FEN1 [MIM: 600393]), and the remaining nick is sealed by LIG1, giving the final steps of long-patch BER a distinct replicative feel. While many of the enzymes are similar in nuclear and mitochondrial BER, our description above is most relevant to the former process.57 Notably, only a few genetic disorders are associated with a defect in BER, specifically in a DNA glycosylase function (discussed later), resulting in either cancer predisposition or immunodysfunction.
Repair of Active Base Damage
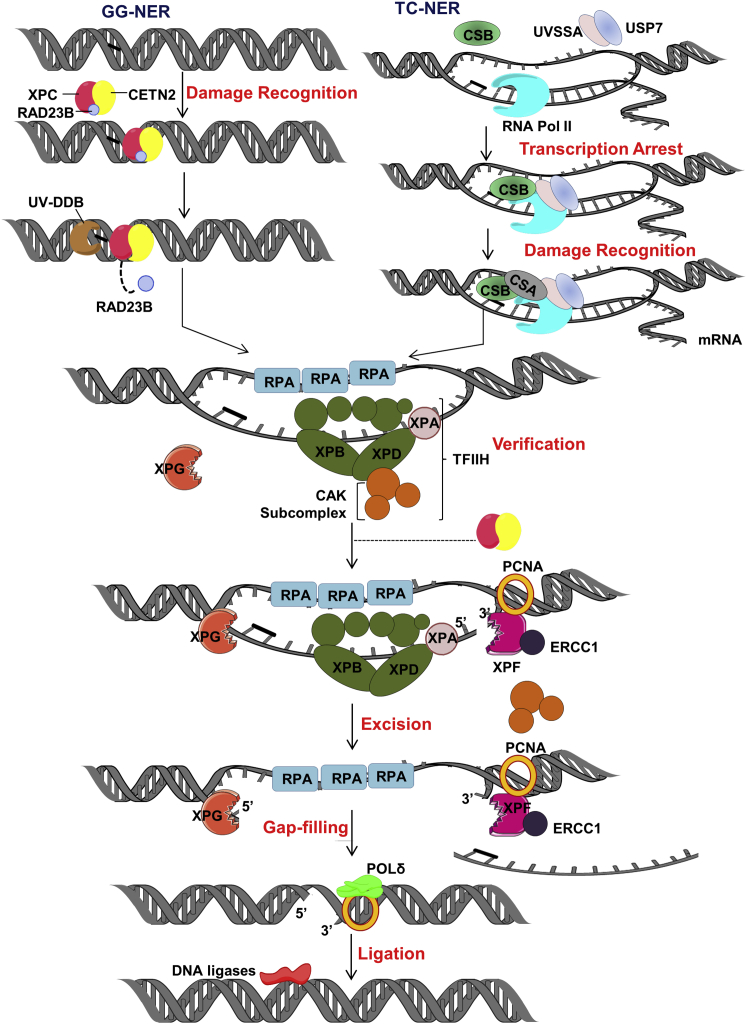
Active DNA modifications, which induce alterations to the helical structure and typically interfere with the progression of RNA polymerases, such as cyclopurines, UV photoproducts, or cis-platinum monoadducts, are primarily repaired by one of two sub-pathways of nucleotide excision repair (NER): global-genome (GG-NER) or transcription-coupled (TC-NER).58 Each of the NER sub-pathways can be divided into five major molecular events: (1) damage recognition, (2) damage verification, (3) incision of the DNA backbone on either side of the damage to excise a lesion-containing oligonucleotide, (4) refilling of the nucleotide gap, and (5) ligation of the remaining nick to complete the process. As will be discussed in more detail below, GG-NER is initiated by specific recognition of a helix-distorting lesion within the broader genome, whereas TC-NER is engaged upon arrest of RNA polymerase II (RNAPII) at the site of the damage in an actively transcribed gene. Studies indicate that TC-NER acts rapidly (in minutes) to resume transcription, while GG-NER requires hours to remove substrate lesions.59
In GG-NER (Figure 2, left), xeroderma pigmentosum complementation group C (XPC [MIM: 613208, 278720]) is the main DNA damage sensor, forming a complex with the UV excision repair protein RAD23B (MIM: 600062) and centrin 2 (CETN2 [MIM: 300006]).60, 61 The recognition mechanism of this protein complex allows GG-NER to target a broad range of helix-distorting lesions, as such modifications introduce an important single-stranded DNA (ssDNA) binding platform for XPC.62 For those lesions that have a more subtle DNA helix destabilizing effect, such as UV photoproducts, and do not show strong specificity to the XPC-RAD23B (MIM: 600062) complex,63, 64, 65 there exists another damage-recognition factor known as ultraviolet radiation-DNA damage binding protein (UV-DDB) complex, which consists of two proteins, UV-DDB1 (MIM: 600045) and 2 (MIM: 600811) (a.k.a. XPE [MIM: 278740]).66 UV-DDB acts upstream of XPC-RAD23B complex as a specialized detection complex to enhance identification of modest helix-destabilizing lesions. DDB2/XPE also facilitates recruitment of the DDB1-cullin 4A (CUL4A [MIM: 603137])DDB2/XPE complex to the site of the lesion, resulting in ubiquitination of XPC and DDB2/XPE itself. This ubiquitination stabilizes XPC, but leads to DDB2/XPE degradation, which increases XPC affinity for the substrate and propels the repair reaction.61, 67 In addition to serving as a major recognition factor for GG-NER, the XPC-RAD23B complex is essential for assembly of the downstream NER proteins and efficient execution of the repair response (discussed below).68 Significantly, failure of GG-NER to repair photolesion damage is associated with the sun-sensitive genetic disorder, xeroderma pigmentosum (XP). XP, as discussed in greater detail later, is characterized by cancer predisposition, particularly UV-related basal cell and squamous cell carcinomas, as well as an increase in internal cancers, and in some cases, neurological disease.69, 70
Figure 2.
NER
NER is divided into two sub-pathways: GG-NER (left) and TC-NER (right). In GG-NER, DNA helix-distorting lesions are recognized by the collaborative effort of XPC, RAD23B, and CENT2, sometimes with the assistance of UV-DDB. In TC-NER, blocking lesions are revealed by arrest of an RNA polymerase at the site of damage. Persistent RNA polymerase stalling leads to recruitment of the TC-repair factors, namely CSB and CSA. Following recognition, lesion verification, damage excision, repair synthesis, and nick ligation are shared biochemical processes. See text for further details.
Despite the continual action of the GG-NER system to identify and remove substrate lesions from the entire genome, a subset of DNA modifications will be revealed by the transcription machinery. In situations when RNAPII arrests at an active lesion, TC-NER is called upon to remove the damage and restore RNA synthesis (Figure 2, right). A stalled RNAPII spans roughly 35 nucleotides on the transcribed strand,71 thereby preventing the incision machinery from gaining access to the damage site. Though much of the molecular mechanics of TC-NER is still being worked out, studies indicate that upon stalling of RNAPII, an early step in the repair reaction is recruitment of the key TC-NER factor, Cockayne syndrome protein B (CSB; also known as excision repair cross-complementing rodent repair deficiency, complementation group 6, ERCC6 [MIM: 133540]).72 CSB is a SWI/SNF family member with DNA-dependent ATPase activity and presumably an important chromatin remodeling or motor function. Recent evidence argues that the forward translocation action of CSB leads to RNAPII removal from the damage site, facilitating the repair response.73 However, another view argues that RNAPII is not removed, but rather reverse translocated (i.e., backtracked), creating enough space for the TC-NER incision complex to assemble and operate.74 Proteasomal degradation of RNAPII would occur only as a last-gasp escape route to resolve a persistently stalled RNAPII, where RNAPII is monoubiquitylated by the neural precursor cell expressed developmentally downregulated protein 4 (NEDD4 [MIM: 602278]) E3 ubiquitin ligase at lysine-48, followed by its polyubiquitylation by Elongin A (MIM: 600786)/B (MIM: 600787)/C (MIM: 600788) and Cullin5 (CUL5 [MIM: 601741]-RBX2 [MIM: 603863].75, 76 Regardless of the fate of the polymerase, CSB recruitment to the lesion site leads to organization of the Cockayne syndrome A (CSA; also known as ERCC8 [MIM: 216400])-E3 ubiquitin ligase complex (CRL4) comprised of Cullin 4A (MIM: 603137), DDB1, and RBX1/regulator of cullins 1 (ROC1 [MIM: 603814]); as well as other specific TC-NER proteins, including UV-stimulated scaffold protein A (UVSSA [MIM: 614632]), ubiquitin-specific processing protease 7 (USP7 [MIM: 602519]), XPA-binding protein 2 (XAB2; also known as pre-mRNA-splicing factor, SYF1 [MIM: 610850]) and high mobility group nucleosome-binding domain-containing protein 1 (HMGN1 [MIM: 163920]).77, 78 Following the initial action of the TC-NER factors, the remaining steps are conserved between the two sub-pathways of NER as discussed below. Notably, defects in TC-NER give rise to the recessive genetic disorder, Cockayne syndrome (CS), a disease characterized by sun sensitivity, developmental defects, neurological abnormalities, and premature aging features, without cancer predisposition.79
After damage recognition (Figure 2), the next step in either NER pathway is DNA opening and lesion verification directed upon the arrival of transcription initiation factor IIH (TFIIH), which consists of ten subunits: XPB (MIM: 610651, 133510), p52 (MIM: 601760), p8 (MIM: 608780), p62 (MIM: 189972), p34 (MIM: 601750), and p44 (MIM: 601748) make up the core complex; cyclin-activated kinase (CAK), comprised of cyclin-dependent kinase 7 (CDK7 [MIM: 601955]), cyclin H (MIM: 601953), and menage A trois 1 (MAT1 [MIM: 602659]); and XPD (MIM: 278730, 126340).80, 81, 82 The responsibility of DNA unwinding lies mainly with the two helicase subunits, XPB and XPD, which exhibit opposite directional polarity.83, 84 During translocation, bulky lesions on the target ssDNA stall the progression of XPD, allowing damage verification, whereas undamaged ssDNA passes through the protein, resulting in collapse of the repair reaction.85, 86 Within the TFIIH complex, p52 stimulates the activity of XPB, whereas p44 promotes the helicase activity of XPD.87 p8 is the smallest TFIIH subunit and is genetically linked to trichothiodystrophy (TTD [MIM: 601675]) complementation group A, an autosomal-recessive genetic disorder associated with photosensitivity, brittle hair, small stature, and intellectual impairment.88, 89 Though p8/TTD-A is dispensable for transcription, it is essential for TFIIH-mediated DNA melting and XPA (MIM: 278700, 611153) recruitment during the core NER response.90, 91 XPD engagement with DNA damage permits final assembly of the pre-incision complex, which involves recruitment of XPA, the XPG nuclease (MIM: 133530, 278780), and the ssDNA binding protein, replication protein A (RPA).80, 82, 92 XPA then coordinates with TFIIH, RPA, XPG, and PCNA to direct the 5′ incision event by the nuclease complex XPF (MIM: 278760, 133520)-ERCC1 (MIM: 126380) (Figure 2). Following XPF-ERCC1 incision, which initiates repair synthesis, 3′ incision by XPG generates the 5′ phosphate group utilized at the ligation step, releasing a damage-containing oligonucleotide in the process. Gap filling and ligation are performed by DNA polymerases δ and ε, PCNA, RFC, and DNA LIG1 or 3α.61, 93, 94
Repair of DNA Strand Break Interruptions
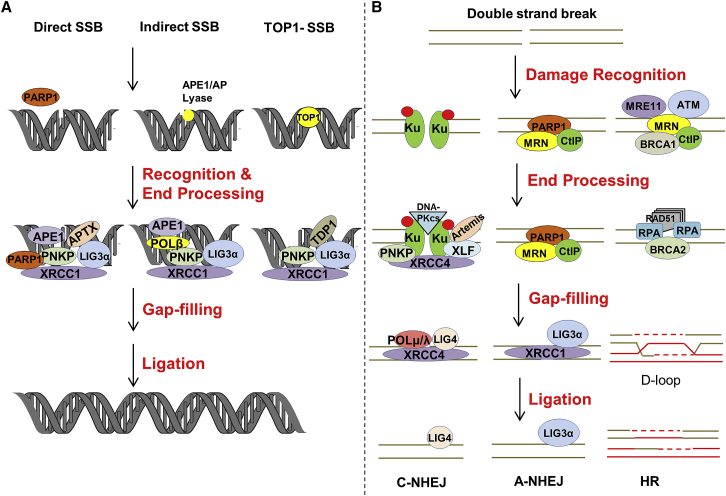
SSBs are among the most common DNA lesions generated through processes like (1) reactions of DNA with naturally produced or environmentally induced chemical species (namely ROS), (2) enzymatic processing intermediates of a repair response, or (3) incomplete catalytic activities of DNA binding proteins such as topoisomerase 1 (TOP1 [MIM: 126420]), which generates a strand break during replication and transcription to resolve supercoiling. As strand interruptions, SSBs can cause replication or transcription breakdown, although they are likely to have only minor effects on overall DNA structure.95 Poly(ADP-ribose) polymerase 1 (PARP1 [MIM: 173870]) and the non-enzymatic scaffold protein XRCC1 are key organizers of the SSBR response.96 PARP1, a major cellular nick sensor, is likely the main initiator of repair at direct SSBs, playing a central role in the recruitment of XRCC1 to sites of DNA damage and assembly of the complete repair machinery through its ability to carry out DNA-activated poly(ADP-ribose)ylation. XRCC1 also has the ability to bind SSBs, but most significantly engages in physical interactions with many of the required processing enzymes, including polynucleotide kinase 3′-phosphatase (PNKP [MIM: 605610]), Aprataxin (APTX [MIM: 606350]), POLβ, and LIG3α.20, 96 APTX and PNKP interact with phosphorylated XRCC1 through a forkhead-associated (FHA) domain.97 Both chemical- and enzyme-derived strand breaks commonly harbor non-conventional termini, such as a 5′-AMP, 3′-phosphate, 3′-phosphoglycolate, or 3′-protein adducts. Such SSBs demand DNA end processing to convert them to the necessary 5′-phosphate and 3′-hydroxyl ends for gap filling and nick ligation, the final steps of a SSBR response (Figure 3A). Among the common types of damaged termini, 3′-phosphates are the principal substrate of PNKP, 3′-α,β-unsaturated aldehydes and 3′-phosphoglycolates are handled by APE1, TOP1-DNA intermediates are processed by tyrosyl-DNA phosphodiesterase 1 (TDP1 [MIM: 607198]), and 5′-AMP groups are resolved by APTX.98 After end resolution occurs, DNA gap filling is typically performed by POLβ, before ligation by LIG3α. Although often considered a specialized sub-pathway of BER since it engages an overlapping set of proteins (e.g., APE1, POLβ, and LIG3α), SSBR is probably better thought of as a unique repair system, as germline defects in SSBR give rise to strictly neurological disease, without cancer predisposition, as seen in situations of BER deficiency.95
Figure 3.
SSBR and DSBR
(A) DNA SSBs can be generated through the action of ROS (direct) or by intended (indirect) or failed (TOP1) enzymatic processing events. PARP1 plays an important role in the resolution of direct SSBs, but may also participate in the response to other forms of DNA SSBs. Following recognition by the appropriate protein(s), specific processing enzymes are called upon to resolve abnormal 5′ or 3′ ends to permit repair synthesis and nick ligation. See text for further details.
(B) DSBs can arise via several mechanisms, with two-ended DSBs being depicted. Depending on cell cycle and other factors not fully understood, DSB recognition is carried out by the Ku complex (left), PARP1 (center), or HR factors, such as ATM and the MRN complex. End processing prepares the DSB for realignment, potential gap filling, and ligation in either NHEJ event (classic or alternative), or for strand exchange, the formation of a D-loop, recombination, and eventual resolution in the case of HR. See text for further details.
DSBs arise due to the nearby breakage of both DNA strands, either directly (such as in the case of IR) or through independent processing events, and in both situations, are known as two-ended DSBs. Additionally, one-ended DSBs can be formed as DNA intermediates when a replication fork encounters a persistent SSB or another form of obstruction, such as a bulky, blocking lesion, a repetitive sequence, or an R-loop. Depending on the cell cycle phase and the broader cellular make-up, DSBs are resolved by either non-homologous end-joining (NHEJ) or homologous recombination (HR) (Figure 3B).99 Unlike HR, NHEJ works independently of a homologous template and involves direct ligation of the broken strands, typically following appropriate processing (see more below). Thus, NHEJ is not restricted to any stage of the cell cycle, whereas HR is active only during S and G2 phases due to the requirement of a homologous partner, typically a sister chromatid.
There are two sub-types of NHEJ, processes that are primarily designed to deal with two-ended DSBs: classic (C-NHEJ)100 and alternative (A-NHEJ).101 C-NHEJ is more rapid and efficient than A-NHEJ, but both tend to be error prone as they are likely to involve some processing prior to ligation, leading to insertions or deletions. In C-NHEJ, DNA ends are recognized by the Ku heterodimer (Ku70 [MIM: 152690] and Ku80 [MIM: 194364]), which prevents promiscuous end resection. The Ku complex acts as scaffold to recruit other NHEJ proteins, specifically leading to the assembly of DNA-dependent protein kinase catalytic subunit (DNA-PKcs [MIM: 600899]), X-ray cross complementing protein 4 (XRCC4 [MIM: 194363]), XRCC4-like factor (XLF [MIM: 611290]), DNA ligase 4 (LIG4 [MIM: 601837]), and Aprataxin-and-PNKP-like factor (APLF [MIM: 611035]).102 DNA-PKcs helps to hold the two ends of the broken DNA in close proximity through synaptic complex formation. In addition, the XRCC4-XLF filamentous complex stabilizes the broken ends by bridging them. Like SSBs, DSBs also often harbor non-ligatable termini, such as 5′-hydroxyl, 3′-phosphate, and 5′-deoxyribose-5-phosphate groups, which require end processing by enzymes such as Artemis (a.k.a. DNA cross-link repair protein 1C, DCLRE1C [MIM: 605988]), PNKP, APTX, APLF, DNA polymerases μ and λ (POLμ [MIM: 606344] and POLλ [MIM: 606343]), and Werner helicase/exonuclease protein (WRN [MIM: 277700, 604611]). This processing, which often uncovers needed micro-homology, can lead to the loss or gain of nucleotide information. Once cleanup and alignment of the two DSB ends is complete, gap filling is performed if necessary, by POLμ or POLλ, while nick sealing is carried out by LIG4.103 A-NHEJ is mostly active in situations where C-NHEJ is deficient and tends to be more error prone than the classic pathway. Indeed, A-NHEJ is often associated with tumorigenesis and cancer aggressiveness, typically due to mutation or expression alterations associated with a particular cancer type.104 While C-NHEJ depends on Ku, LIG4, and other associated factors, A-NHEJ works in the absence of these proteins and engages the MRN complex—comprised of MRE11, DNA repair protein RAD50 (MIM: 604040), and Nibrin (Nijmegen breakage syndrome 1, NBS1 [MIM: 602667])—CtIP (MIM: 604124), XRCC1, PARP1, and LIG1 or 3. The MRN complex and CtIP initiate end resection, with MRN generating ssDNA regions through its endonuclease and exonucleolytic activities. This end resection allows loading of nucleases like EXO1 or DNA replication ATP-dependent helicase/nuclease 2 (DNA2 [MIM: 601810]) to generate longer stretches of ssDNA, likely contributing to the larger deletion events associated with A-NHEJ. PARP1 plays a role in DNA end-bridging and alignment, at which time noncomplementary 3′ tails are removed by not clearly identified nucleases and gap filling synthesis is performed by most probably DNA polymerase θ (POLθ [MIM: 604419]).105, 106 Finally, the nick is sealed by either LIG1 or 3.100, 101
Unlike the end-joining processes, HR is an error-free repair mechanism that utilizes a homologous copy of DNA (sister chromatid) to resolve identified DSBs.107 In cells, as noted above, DSBs can exist as either one-ended or two-ended. While HR, like NHEJ, can cope with the latter form of DSB, depending on cell cycle phase and initial recognition event, homology-directed repair has likely evolved to resolve mostly one-ended DSBs that arise due to replication fork collapse.108 HR is initiated by recognition of a DSB by ataxia telangiectasia mutated (ATM [MIM: 607585]). ATM is key protein kinase that phosphorylates critical players in cell cycle checkpoint regulation, such as p53, breast cancer 1 (BRCA1 [MIM: 113705]), checkpoint kinase 2 (CHK2 [MIM: 604373]), structural maintenance of chromosomes 1 (SMC1 [MIM: 300040]), and NBS1, activating G1, S, and G2/M checkpoints.109 Following DSB identification, HR entails the resection of the 5′ end to create a 3′ ssDNA stretch through the action of the C-terminal binding protein 1 (CtBP1 [MIM: 602618]) nuclease in collaboration with the MRN complex. This ssDNA is protected from degradation by RPA and is employed to mitigate the exchange of genetic information. Specifically, in collaboration with the BRCA1/partner and localizer of BRCA2 (PALB2 [MIM: 610355])/breast cancer 2 (BRCA2 [MIM: 600185]) protein complex, RAD51 recombinase displaces RPA, executing the pre-synapsis phase of HR.110 The RAD51-ssDNA complex then promotes strand invasion into a homologous duplex in the synapsis phase, leading to the formation of heteroduplex DNA (D-loop); this synaptic complex is formed through identification of homology between the invading and template DNA strands. In a calcium-dependent manner during the post-synapsis stage, RAD51 stimulates the ATPase activity of RAD54 to promote intertwining of the 3′ invading strand and the complementary template strand to generate a primer-template junction suitable for DNA synthesis.111, 112 At the D-loop stage, the intermediate can be processed in three different manners. (1) The invading strand gets displaced from the D-loop after DNA synthesis and anneals with its original complementary strand, setting the stage for DSB resolution. This pathway represents the synthesis-dependent strand-annealing mode of HR, where strands are ultimately disengaged via non-crossover. (2) If both DSB ends are engaged in D-loop stabilization during HR, this can lead to the formation of a double-Holliday junction,113 which is either resolved by resolvase into non-crossover or crossover products, or processed by the Bloom syndrome DNA helicase (BLM [MIM: 604610])-topoisomerase (TOPIIIα [MIM: 601243]) (BLM-TOPIIIα) complex to generate non-crossover products.114, 115 (3) In the break-induced replication pathway of HR, one end of the DSB is involved in strand exchange, creating a replication fork that facilitates duplication of the chromosome along the remainder of the sister chromatid. Since the second end of the DSB is not involved in this reaction, the portion of chromosome contained within that segment of DNA is lost, resulting in loss of heterozygosity.116 Failure to successfully execute DSB resolution by one of the HR-mediated pathways can lead to complex DNA structural intermediates, which have the potential to drive chromosomal aberrations or cell death outcomes.
Clinical Phenotypes Stemming from Defective DNA Repair
The broader importance of DNA repair is perhaps best exemplified by the fact that several inherited genetic disorders that give rise to disease and accelerated aging (some noted above) stem from defects in DNA repair factors (Table 2). Instead of looking at each disorder independently, we look, in the discussion below, at the major clinical phenotypes, i.e., cancer predisposition, neurological disease, and premature aging, and attempt to find a common mechanism underlying the different clinical outcomes.
Table 2.
Major DNA Repair Pathways and Associated Inherited Genetic Defects
| DNA Repair Pathway (Sub-Pathway) | Inherited Genetic Defects (Syndrome) | Major Clinical Phenotypes | |
|---|---|---|---|
| MMR | MSH2, MSH6, MLH1, PMS1, PMS2 (LS or MMRCS) | cancer predisposition | |
| BER | MUTYH (MAP), NTHL1 (colorectal cancer), UNG (HIGM-V) | cancer predisposition, immunological defects | |
| NER | GG-NER | XPA, XPB, XPC, XPD, XPE, XPF, XPG, ERCC1 (XP) | cancer predisposition (particularly UV-induced skin melanoma), some instances of neurological disease |
| TC-NER | CSA, CSB, XPB, XPD, XPF, XPG, ERCC1, TTD-A (CS, combined XP/CS, TTD) | developmental defects, premature aging, neurological abnormalities, no cancer predisposition | |
| SSBR | APTX (AOA1), TDP1 (SCAN1), PNKP (AOA4), XRCC1 (AOA5) | neurological disease, no cancer predisposition | |
| DSBR | NHEJ | LIG4 (LIG4 syndrome, MPD), XLF/Cernunnos (SCID), Artemis (SCID), DNA-PKcs (SCID), XRCC4 (MPD), ATM (AT), MRE11 (ATLD), NBS1 (NBS) | cancer predisposition, immunodeficiency, neurological disease |
| HR | BRCA1/2 (breast cancer), RAD50, RAD51, PALB2 (ovarian cancer), FANCA, FANCC, and FANCG (FA) | cancer predisposition, aplastic anemia | |
Specific syndrome linked to the repair defect is indicated in parentheses. Predominant clinical features are listed. See text for further information
Cancer
Cancer is a disease that involves a “multi-hit” process in which selective genetic mutations lead to loss of control over cell proliferation and unregulated cell growth, and eventually metastasis. Events such as point mutations, indels, and larger chromosome rearrangements are a few of the genetic changes that can compromise the functionality of tumor suppressor or oncogenes, driving cellular transformation. Consistent with genetic instability underlying carcinogenesis, higher mutation frequencies have been associated with both inherited and sporadic cases of disease arising from increased infidelity in the replication machinery.7, 117 As DNA damage is a primary contributor to genetic change, it is not surprising that defects in DNA repair processes are often associated with cancer predisposition as well (Table 2). This connection was initially made in the context of NER (see below) but was more broadly appreciated with the discovery that MMR defects are genetically linked to colorectal cancer.
The link between defects in DNA repair and cancer gained wide-spread notoriety when it was reported that hereditary non-polyposis colorectal cancer (HNPCC), a genetically heterogenous, autosomal-dominant disorder, also known as Lynch syndrome (LS [MIM: 120435]), originates from germline mutations in MLH1, MSH2, MLH3, MSH6, or PMS2;118, 119 mutations in MLH1 or MSH2 account for more than 3/4th of the cases.120 The connection between MMR defects and cancer risk has since expanded to include “MMR cancer syndrome” (MMRCS [MIM: 276300]), which involves inherited recessive mutations and sporadic cases of disease that stem from somatic mutations in or epigenetic silencing of MMR genes.121, 122 Regardless of the underlying genetic mechanism or disease nomenclature, disorders stemming from a MMR defect exhibit almost exclusively an increase in cancer development, not only of the colon, but also of the stomach, endometrium, biliary and pancreatic system, and urinary tract. The signature mutational event in cells deficient in MMR is microsatellite instability (MSI), a characteristic that is consistent with the role of MMR in resolving small indel loops that arise due to polymerase slippage in repeat sequences. Such MSI (or repeat length changes) is revealed via loci-specific PCR coupled with capillary gel electrophoresis and alignment with the Bethesda reference panel.123 Whereas inactivation of MMR results in carcinogenesis, it would appear that the substrates of MMR, i.e., mismatches or small indel loops, or the resulting genomic instability, are not significant contributors to other pathological outcomes, suggesting that these lesions exist in an idle state, not interfering with other DNA transactions, such as transcription.
To date, inherited mutations in BER have been linked to only cancer predisposition and immunodysfunction and have been reported specifically in DNA glycosylases that resolve primarily idle base lesions: MUTYH, NTHL1, and UNG (Table 2). In the first two instances, the related disorders are characterized by increased cancer risk, particularly of the colon, without any additional prominent phenotypes. MUTYH is a specialized DNA glycosylase that copes with idle 8oxoG:A base pairing, specifically excising the adenine commonly inserted opposite 8oxoG during chromosome duplication. Such a repair function permits a gap filling polymerase (e.g., POLβ) to re-insert C opposite the base lesion, setting up conventional BER of 8oxoG via OGG1 and the reestablishment of the normal G:C base-pair. Consistent with its repair activity, tumors from MUTYH-associated polyposis (MAP)-affected individuals display a G:C to T:A mutational signature, reflective of failed 8oxoG:A resolution.124 In situations involving germline mutations in NTHL1, affected individuals similarly exhibit increased cancer predisposition, particularly of the colon, with a C:G to T:A mutational signature that likely reflects ineffective repair of idle base lesions such as oxidized pyrimidines and ring-opened purines (FaPy).125 Finally, genetic missense mutations in the uracil DNA glycoylase (UNG)—i.e., R88C and G143R—are associated with hyper-IgM syndrome type V (HIGM-V [MIM: 608106]), a disorder that is characterized by elevated serum IgM levels and the absence of other immunoglobulins.126 UNG plays a key role in a specialized process, i.e., somatic hypermutation, which involves uracil intermediates formed by activation-induced cytidine deaminase (AID) to generate antibody diversity. It’s interesting that HIGM-V-affected individuals do not appear to exhibit increased cancer predisposition (unlike UNG knockout mice, which develop lymphoma127), since as noted earlier, spontaneous deamination of cytosine can lead to C→T transitions, although the lack of increased carcinogenesis could be explained by the presence of backup uracil DNA glycosylases, such as SMUG1, or other poorly defined compensatory mechanisms. Although not linked to a known inherited disorder, a good deal of epidemiological evidence suggests that defects in the repair of the idle base modification 8oxoG due to alterations in OGG1 can lead to increased cancer risk, particularly of the lung.128
The lack of a clear disease association with defects in the central BER proteins, namely APE1 and POLβ, likely stems from these enzymes being essential to normal embryonic development, perhaps reflective of the harmful nature of their substrates, AP sites and SSBs, respectively. In the case of APE1, germline deletion leads to embryonic lethality in mice,129 whereas inactivation of POLβ results in neonatal inviability after birth due to failed neurogenesis.130 These findings would imply that extreme loss-of-function alleles in APE1 or POLβ would not support life and thus persist in the general population, although subtle reduced-function alleles might be maintained and affect disease susceptibility.131 Notably, recent studies in mice, using Cre recombinase-mediated gene deletion techniques, reveal that whole-body post-natal inactivation of APE1 can lead to cellular senescence and premature aging characteristics, i.e., hair loss and impaired wound healing,52 while brain-specific deletion of APE1 can promote degeneration of the developing central and peripheral nervous systems after birth, as well as tumorigenesis in the form of medulloblastoma and glioblastoma.132 Although there have been many genetic variants recorded in APE1 in humans, their involvement in disease remains unclear, though a tumor-associated variant (R237C) has been reported to exhibit reduced repair activity.133 While further investigation is necessary to assess whether APE1 deficiency associates with broader disease susceptibility in relevant human cohorts, the animal studies reveal that APE1 substrates (most likely AP sites) have the potential to be both lethal and mutagenic to the cell and organism.54, 132 As for POLβ, the major gap filling DNA polymerase, nearly 30% of solid cancers carry mutations in this gene, primarily in cases of colorectal and gastric cancer.134 Most of the mutations are somatic, although a few of them are germline in nature, such as the P242R substitution that has been shown to induce genomic instability.135 Many of the sporadic mutations occur in the catalytic domain of POLβ, with the I260M variant reported to exhibit impaired processivity and fidelity.136 Thus, studies suggest that mutations in POLβ can, by causing inefficient or inaccurate repair, promote genomic instability and consequent cellular transformation associated with carcinogenesis. Consistent with a prominent role for BER in processes that generate antibody diversity, recent reports implicate a defect in POLβ in lupus-like symptoms that potentially stem from defects in somatic hypermutation.137
XP, literally meaning dry pigmented skin, is an autosomal-recessive disorder comprised of eight complementation groups (XPA-XPG, XPV) with a prevalence of around 1–45 per million across different parts of the world24, 69, 138 (Table 2). The disorder is marked by extreme photosensitivity (in most cases), dynamic skin pigmentation, and an increased skin cancer incidence. Within the US, the median age of diagnosis of non-melanoma skin cancer (NMSC) in XP-affected individuals is 9 years old, as compared to 67 in the general population, translating to an ∼10,000-fold increased risk of disease. Similarly, the median age of diagnosis for melanoma in XP is 22, as compared to 55 in the general population. There is also evidence that XP-affected individuals have a 50-fold increased risk of developing internal tumors, like brain tumors, presumably due to the inefficient removal of endogenous DNA damage.139, 140 The observation in 1968 by James Cleaver that cells from XP-affected individuals fail to efficiently carry out DNA repair synthesis after UV irradiation was the first demonstration that a defect in DNA repair likely underlies cancer predisposition.141 It is now well known that deficiencies in NER lead to damage accumulation arising from both extrinsic (UV irradiation) and intrinsic (ROS) sources.
Out of the eight complementation groups, seven of the gene products (XPA-XPG) are directly involved in the repair of DNA damage. The other complementation group, XPV (MIM: 278750), does not entail a defect in repair; instead, deficiency in XPV, a specialized translesion DNA polymerase (POLη [MIM: 603968]) that faithfully copies UV damage, leads to increased error-prone bypass of UV photoproducts and cancer formation. XPC and XPE operate exclusively in GG-NER, whereas the other factors are part of the shared NER mechanism142, 143 (Figure 2). In cases involving XPC or XPE mutations, where TC-NER is still intact, affected individuals exhibit a distinct form of disease, with moderate sun sensitivity and cancer predisposition at later stages of life. However, we are just learning about the clinical presentation of these individuals now that they are avoiding the complications of UV exposure and skin cancer.
The complexity and severity of XP generally increases with mutations in genes that function as part of the shared NER response, since both sub-pathways are compromised. Classic examples of such proteins are XPD and XPB, the DNA unwinding components of the TFIIH complex that assembles at the site of the lesion during the different NER sub-pathway processes. Individuals with XPD or XPB alterations exhibit severe UV sensitivity, and in some cases, likely dependent on the specific nature of the genetic mutation, will display clinical symptoms that resemble combined XP/CS144, 145, 146 (Table 2). Consistently, mutations affecting CAK, another subunit of TFIIH, also give rise to XP and XP/CS conditions.147 Genetic alterations that lead to complete loss of or a non-functional XPA, a protein that operates in both NER sub-pathways, similarly result in a severe form of XP. Notably, separation of function missense mutations in XPA (i.e., R207G or R207K), which disable its participation in GG-NER but retain its involvement in TC-NER,148 mainly give rise to cancer predisposition, without the underlying premature aging features associated with CS (see later). Finally, defects in the core NER nucleases, XPG or XPF-ERCC1, can lead to combined XP/CS and other severe clinical outcomes, likely reflecting their roles in both NER sub-pathways, but also potentially their contributions to distinct repair mechanisms, such as oxidative DNA damage or ICL resolution.149 Collectively, the findings indicate that failed GG-NER promotes a cancer predisposition phenotype and indicate a role for this pathway in avoiding mutagenesis, while, as discussed later, TC-NER defects lead to progeria-type outcomes.
Whereas the above disorders involve mostly idle, mutagenic base precursors, defects in the processing of recombinogenic DNA DSBs are also directly linked to cancer predisposition. In particular, genetic mutations in genes involved in NHEJ lead to potential lethal lesions that not only cause loss of chromosomal content, but give rise to gross rearrangements.150, 151 In addition, since NHEJ plays a programmed role in class switch recombination and antibody diversity, defects in the pathway components lead to immunological impairment. For example, hypomorphic mutations in LIG4 cause LIG4 syndrome (MIM: 606593), a disorder characterized by microcephaly, abnormal facial features, IR sensitivity, and combined immunodeficiency.152 XRCC4 and XLF (also called Cernunnos) are nonenzymatic partners of LIG4 that stimulate ligase activity and readenylation,153 and defective XLF similarly leads to LIG4 syndrome. In addition, mutations in the Artemis gene, another nuclease involved in NHEJ, cause severe combined immunodeficiency (SCID) (Art-SCID [MIM: 601457]). XLF and Artemis mutated individuals are classified as radiosensitive (RS)-SCID (MIM: 602450), due to defective DSBR and increased IR sensitivity.154 Similarly, defects in DNA-PKcs, which affect autophosphorylation and recruitment of Artemis during V(D)J recombination, result in a phenotype similar to RS-SCID.155 While genetic defects in NHEJ are often associated with cancer predisposition and immunodeficiency (Table 2), recent findings indicate that biallelic mutations in LIG4 or XRCC4 can also lead to microcephalic primordial dwarfism (MPD), a disorder characterized by an extreme prenatal-onset growth failure phenotype.156
As seen with NHEJ, inherited defects in HR, another mechanism involved in the resolution of DSBs, are likewise associated with cancer predisposition. Most notably, mutations in BRCA1 or BRCA2 underlie common hereditary forms of breast and ovarian cancer and give rise to pronounced chromosome instability157 (Table 2). Germline mutations in non-BRCA genes, such as RAD50, RAD51B (MIM: 602948), RAD51C (MIM: 602774), RAD51D (MIM: 602954), MRE11, and PALB2 similarly lead to ovarian cancer.158, 159, 160, 161 BRCA1 colocalizes with BRCA2 and RAD51 during the repair of DSBs, and the BRCA pathway also communicates with the Fanconi anemia (FA) pathway to resolve replication-disrupting lesions, such as ICLs.162, 163 FA is an inherited bone marrow failure syndrome that involves congenital abnormalities, short stature, hypopigmentation, and predisposition to cancer.164 Among the 19 gene products (FANCA to FANCT) that participate in the FA pathway, FANCA (MIM: 607139), FANCC (MIM: 613899), FANCG (MIM: 602956), and FANCD2 (MIM: 613984) are the most frequently mutated genes. Recent studies have revealed that endogenous aldehydes, such as acetaldehyde, contribute directly to the etiology of FA, likely through the production of DNA ICL damage.165, 166 Not surprisingly, mutations in aldehyde detoxifying enzymes like aldehyde dehydrogenase 2 (ALDH2 [MIM: 100650]) also lead to aldehyde-induced genotoxicity, p53-induced cell cycle arrest, and phenotypes similar to FA.167 Thus, complex DNA lesions that cause replication fork collapse have the potential to induce cell death or genome instability outcomes, giving rise to associated clinical pathologies.
Neurological Disease
Neurological complications are observed in many cases of DNA repair deficiency and are particularly prominent in disorders stemming from a defect in resolving DNA strand breaks. Indeed, inherited defects in MMR or classic BER, which deal with idle base damage, have not been reported to be genetically linked to central nervous system complications, although there exist many proposed associations in the literature. Conversely, in situations involving poor repair of SSBs, several inherited disorders exhibit mostly neurological abnormalities, without cancer predisposition.95 The thought here is that non-dividing, post-mitotic cells, such as neurons, which lack backup faithful repair mechanisms such as HR, rely almost exclusively on SSBR processes to resolve strand break interruptions that might otherwise adversely affect transcription and activate cell death responses. Neuronal cells would be particularly susceptible to SSB formation due to their high demand for energy, and thus, high level of ROS generation and consequent DNA damage. The initial observation that SSBR defects give rise to neurological disease was reported for spinocerebellar ataxia axonal neuropathy 1 (SCAN1 [MIM: 607250]), a disorder characterized by late-childhood-onset slowly progressive cerebellar ataxia, followed by areflexia and signs of peripheral neuropathy. SCAN1 was found to arise from inherited mutations in TDP1 (H493R), an enzyme that resolves stalled topoisomerase-DNA complexes as part of the SSBR response.168 Subsequently, germline mutations in APTX were found to give rise to spinocerebellar ataxia syndrome, ataxia ocular motor apraxia (AOA1 [MIM: 208920]),169 a disease where the neuropathophysiology resembles Friedreich ataxia (MIM: 229300) and ataxia telangiectasia (AT [MIM: 208900]; see below). APTX, which possesses a conserved central histidine triad (HIT) domain, was found to specifically act on DNA substrates, excising AMP from the 5′-terminus of failed ligation reactions due to obstructive oxidative termini.170 Pedigree analysis also found that mutations in PNKP (i.e., E326K, L176F, and a homozygous 17-bp duplication in exon 14), which encodes a protein that displays both 3′-DNA phosphatase and 5′-DNA kinase activity, cause the autosomal-recessive disease microcephaly, seizures, and developmental delay (MCSZ [MIM: 613402], now known as AOA4), a disorder characterized by microcephaly, early-onset, intractable seizures and delayed development.171, 172 More recent work has reported that biallelic mutations in exon 12 of the key SSBR scaffold protein XRCC1 (generating a stop codon at amino acid 465 and a K431N missense mutant) led to axonal neuropathy and cerebellar ataxia in the form of AOA5.173
Unlike SSBR defects, which can be compensated for in replicating cells by HR, DSBR deficiencies can lead to harmful outcomes in both cycling and non-cycling cells. Thus, besides the degenerative phenotypes that can arise after development, inherited mutations in DSBR components can adversely affect the maturation of the nervous system as well. AT is an autosomal-recessive neurodegenerative disease stemming from germline mutations in ATM, and likely involves both neurodevelopmental and neurodegenerative pathologies.174, 175 AT is characterized by uncoordinated or ataxic movements, ocular telangiectasia, immunodeficiency, and cancer predisposition. ATM, as introduced above, is a protein kinase that acts at the apex of the signaling cascade that reacts to DSBs, helping to regulate G1, S, and G2/M cell cycle checkpoints as well as coordinate faithful strand-break processing. Dysfunctional ATM leads to DSB accumulation that drives cell death or genomic instability, biological outcomes that underlie the variety of clinical phenotypes noted above. Consistently, hypomorphic mutations in MRE11 or NBS1, proteins that collaborate with ATM in the DSBR response, cause ataxia telangiectasia-like disease (ATLD) and Nijmegen breakage syndrome (NBS [MIM: 251260]), respectively, disorders that phenocopy AT.176, 177, 178 In addition, hypomorphic mutations in ATM and Rad3-related kinase (ATR [MIM: 601215]), another important kinase that participates in the repair of stalled forks created during the replication of damaged DNA, result in ATR-Seckel syndrome, which is characterized by growth and developmental delay and microcephaly.179
In addition to the strand-break repair syndromes noted above, neurological disease is observed in ∼30% of XP-affected individuals, who suffer from impaired NER. The neurological defects, such as areflexia, ataxia, peripheral neuropathy, sensorineural deafness, abnormal gait, and difficulty in walking, occur due to neuronal loss, cortical atrophy, and ventricular dilatations. Those XP-affected individuals that experience severe neurological complications harbor mutations in one of the following genes: XPA, XPB, XPD, XPF, or XPG, with most mutations occurring in XPD.70 Notably, all the encoded proteins operate in both sub-pathways of NER. Since mutations in XPC or XPE, genes that function strictly in GG-NER, do not show early neurological symptoms, the data implicate impaired TC-NER as causative to the pronounced brain pathology in the subset of XP-affected individuals. Consistent with a prominent role for TC-NER in the prevention of neurological disease, CS-affected individuals similarly show brain abnormalities that possibly stem from failed resolution of active base damage (see next section).
Premature Aging
While it has been argued recently that many of the DNA repair syndromes exhibit premature aging phenotypes, one disorder that has been consistently categorized as a segmental progeria is CS (see also Closing Remarks). CS-affected individuals display sensorineural deafness, progressive visual loss, and neurological degeneration early in life, a set of features typically associated with advanced age, as well as severe developmental failings.79, 180 Approximately 70% of CS-affected individuals harbor a genetic mutation in CSB (ERCC6), whereas most of the remaining carry CSA (ERCC8) mutations. Around 78 different mutations in CSB are known, giving rise to a wide range of clinical phenotypes in CS.181 Approximately 30 different mutations have been reported in the CSA gene, and they typically result in a more moderate form of CS. To date, as is the case for most genetic disorders, no clear genotype-phenotype correlations have been established between the different mutations of CSA or CSB and the clinical outcomes seen in the affected individuals.
As discussed earlier, the factors linked to CS maintain specific roles in TC-NER. While much of the historical work has gone into understanding TC-NER for UV photodamage, UV irradiation is not involved in most of the clinical complications seen in CS-affected individuals. Thus, either there are other endogenous transcription-blocking lesions that are substrates for TC-NER, or the CS proteins have additional roles outside of DNA repair. As for the latter possibility, there certainly is evidence that the CS proteins play important roles in regulating transcription, including in promoting RNAPII elongation.182 However, given that mutations in XPB, XPD, and the XPF-ERCC1 and XPG nucleases also give rise to CS-like phenotypes,183 it seems reasonable to conclude that the clinical symptoms of CS, at least in part, stem from a defect in TC-NER. In the case of XPF (ERCC4), mild mutations in the gene cause moderate phenotypes, such as delayed incidence of skin cancer, and mainly associate with XP, whereas severe mutations can lead to early-onset CS, combined XP/CS, and an independently classified segmental progeria (XFE [MIM: 610965]), which features pronounced dwarfism, cachexia, and microcephaly. Notably, cells derived from the XFE-affected individual exhibit exquisite sensitivity to DNA crosslinking agents, implicating a defect in ICL repair in disease etiology,184 a biochemical flaw that has recently been argued to underlie CS as well.185 Mutations in XPF have also been linked to variations of FA, a disorder diagnosed by cellular sensitivity to the DNA crosslinking agent mitomycin C.186 Thus, besides defects in TC-NER, a picture is emerging in which ICL accumulation is particularly relevant to accelerated aging.
An existing model for CS is that due to the defect in removing transcription-blocking lesions, persistently stalled RNAPs would activate cell death responses, namely p53-driven apoptosis, giving rise to UV sensitivity and degenerative phenotypes, without cancer predisposition.187 Of course, other than the UV-induced photoproducts, endogenous lesions, such as AP sites, strand-breaks, cyclopurines, and ICLs (as emphasized above), have been shown to obstruct RNAPII transcription and thus may elicit a TC-NER response.188 The tissue or organ specificity seen in CS may stem from differences in cellular metabolism, replicative status, repair response capacities, and even external exposures. For example, post-mitotic neurons are highly metabolic, generating high levels of oxidative damage, and are non-cycling, thereby lacking the ability to carry out HR, while also having less proficient GG-NER. These features put an enormous burden on TC-NER to resolve pathogenic DNA damage in non-dividing brain cells.189
Closing Remarks
While no single model can easily explain all aspects of disease etiology associated with a DNA repair deficiency, we have attempted here to provide a different perspective on the many repair disorders. Instead of simply reviewing each as a separate entity, we aimed to identify common themes among the various syndromes, starting with the accumulated DNA damage and ending with the clinical outcome in the affected individual. As for cancer predisposition, this phenotype clearly involves mutagenic events, such as point mutations, MSI, and genomic instability. What appears to be true is that the various disorders that give rise to cancer, mostly cases involving MMR, BER, or DSBR defects, involve either idle base lesions or recombinogenic intermediates, namely DSBs. Idle base lesions would not impede transcription or replication processes, but would instead serve as mutagenic templates, seeding mutational outcomes, while DSBs would promote unwanted chromosome exchange and consequent aberrations. In contrast, active base lesions, i.e., those that block transcription or replication (namely the former), appear to be associated with neurological abnormalities and premature aging phenotypes, such as observed in cases of CS. Presumably, active base damage would promote cellular outcomes, like death and senescence, seeding the degenerative or accelerated aging pathologies. Of course, in certain situations, such as seen in instances involving UV damage, error-prone bypass mechanisms can be activated to permit cell survival at the expense of mutagenesis, as seen in XP.
Strikingly, defects in SSBR give rise to almost exclusively neurological disease, as cancer predisposition is not observed in these affected individuals. Such a finding implies that SSBs uniquely affect neuronal cell viability, likely due to their ability to block transcriptional processes and activate cell death responses. In replicating cells, accumulating SSBs would presumably be faithfully resolved by HR mechanisms, reducing the possibility of genetic instability and consequent cellular transformation. DSBs, not surprisingly, are complex, as they can both interfere with transcriptional events as well as promote unwanted chromosome rearrangements. As a result, defects in DSBR are affiliated with a range of pathological outcomes, including cancer predisposition and neurological complications. In Figure 4, we have summarized the main points of our discussion above regarding the relationship between specific DNA modifications and particular pathological end-points. Importantly, in those disorders involving immunodysfunction, stemming from defects in BER or DSBR/NHEJ, the repair mechanism plays a unique and specialized role in processes dedicated to antibody diversification, i.e., somatic hypermutation and V(D)J recombination.
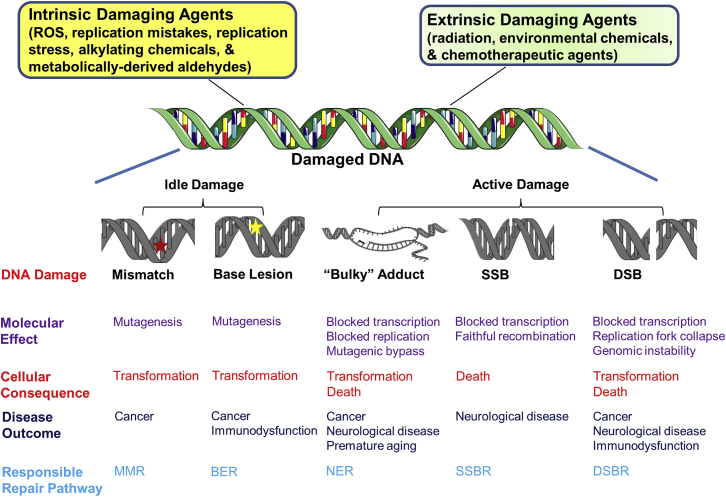
Figure 4.
Sources, Consequences, and Clinical Outcomes of DNA Damage
DNA damage can arise from intrinsic or extrinsic agents or events, some of which are highlighted. DNA damaging agents can generate a variety of lesions, such as mismatched nucleotides, base lesions, bulky (helix-distorting) adducts, SSBs, or DSBs. These damages, depending on their chemical make-up or effect on transcription/replication can cause specific molecular effects, such as mutagenesis, blocked polymerization events, or genomic instability. The molecular outcomes can have specific cellular consequences, including transformation or cell death. Certain cellular events underlie disease outcomes, such as cancer, neurological disease, or premature aging. To prevent these deleterious end-points, cells have evolved a set of DNA repair mechanisms, shown at the bottom. See text for details.
Worth pointing out is a panel of so-called segmental progerias, which prematurely replicate many, albeit not all, aspects of normal aging. Notably, most of these disorders involve some aspect of DNA instability stemming from ineffective genome maintenance systems or aberrant nuclear architecture. Besides CS, two of the most recognized premature aging disorders are Werner syndrome (WS) and Hutchinson-Gilford progeria syndrome (HPGS [MIM: 176670]). WS is characterized by growth retardation, short stature, premature graying of the hair, alopecia, atrophic skin, lipodystrophy, ulcerations, bilateral cataracts, arteriosclerosis, osteoporosis, type II diabetes, and increased malignancies, and it results from autosomal-recessive mutations in the WRN gene. WS cells show limited replicative lifespan in culture, an attribute that likely underlies the premature aging phenotype.190 WRN protein is a member of the RECQ family of DNA helicases, while possessing a unique 3′ to 5′ exonuclease domain, and appears to facilitate the resolution of stalled replication forks, with possible roles in NHEJ and HR.191 These complex biological functions likely explain the profound chromosome instability associated with WS, supporting a fundamental role for genomic alterations, including in telomeric regions, in the aging process.192 HPGS is a rare accelerated aging disorder caused by mutation of the lamin A gene (LMNA [MIM: 150330]), giving rise to a so-called laminopathy.193 It is characterized by failure to thrive early in life, a small and fragile stature, full-body hair loss, low body weight, lipodystrophy, wrinkled skin, scleroderma, and cardiovascular complications. The LMNA gene, through alternative splicing, encodes lamins A and C, proteins that have essential structural roles in the nuclear envelope to establish size and shape. Classic HPGS case subjects carry a sporadic autosomal-dominant mutation in LMNA, with 3/4th of the affected individuals harboring a C1824T (Gly608Gly) mutation.194 The known genetic changes associated with HPGS primarily result in the production and accumulation of a toxic truncated form of lamin A, called progerin. HGPS cells exhibit nuclear architecture abnormalities, altered chromatin, and misregulated gene expression, which collectively contribute to defective DNA repair, particularly DSB repair, DNA damage accumulation, and genomic instability.195 Thus, mounting evidence indicates a causative role for modifications to DNA in driving pathological conditions, with the nature of the damage often dictating the cellular and organismal consequences (Figure 4). We close by noting that while our review has focused primarily on cancer, neurological disease, and premature aging, DNA repair defects have been associated with many other diseases, including macular degeneration, cardiovascular disease, osteoarthritis, diabetes, dementia, and frailty.196, 197, 198 The tissue specificity seen in most monogenic disorders is obviously an area of great interest going forward.
Acknowledgments
We thank Drs. Beverly Baptiste and Anthony Moore (NIA) for their helpful comments on the article. This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
Contributor Information
Vinod Tiwari, Email: vinod.tiwari@nih.gov.
David M. Wilson, III, Email: dmwilson3@outlook.com.
References
- 1.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 2.Loeb L.A., Preston B.D. Mutagenesis by apurinic/apyrimidinic sites. Annu. Rev. Genet. 1986;20:201–230. doi: 10.1146/annurev.ge.20.120186.001221. [DOI] [PubMed] [Google Scholar]
- 3.Wang W., Walmacq C., Chong J., Kashlev M., Wang D. Structural basis of transcriptional stalling and bypass of abasic DNA lesion by RNA polymerase II. Proc. Natl. Acad. Sci. USA. 2018;115:E2538–E2545. doi: 10.1073/pnas.1722050115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalano M.J., Liu S., Andersen N., Yang Z., Johnson K.M., Price N.E., Wang Y., Gates K.S. Chemical structure and properties of interstrand cross-links formed by reaction of guanine residues with abasic sites in duplex DNA. J. Am. Chem. Soc. 2015;137:3933–3945. doi: 10.1021/jacs.5b00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogozin I.B., Pavlov Y.I., Goncearenco A., De S., Lada A.G., Poliakov E., Panchenko A.R., Cooper D.N. Mutational signatures and mutable motifs in cancer genomes. Brief. Bioinform. 2018;19:1085–1101. doi: 10.1093/bib/bbx049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X., Meng F.L. Generation of Genomic Alteration from Cytidine Deamination. Adv. Exp. Med. Biol. 2018;1044:49–64. doi: 10.1007/978-981-13-0593-1_5. [DOI] [PubMed] [Google Scholar]
- 7.Bębenek A., Ziuzia-Graczyk I. Fidelity of DNA replication-a matter of proofreading. Curr. Genet. 2018;64:985–996. doi: 10.1007/s00294-018-0820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams J.S., Lujan S.A., Kunkel T.A. Processing ribonucleotides incorporated during eukaryotic DNA replication. Nat. Rev. Mol. Cell Biol. 2016;17:350–363. doi: 10.1038/nrm.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madireddy A., Gerhardt J. Replication Through Repetitive DNA Elements and Their Role in Human Diseases. Adv. Exp. Med. Biol. 2017;1042:549–581. doi: 10.1007/978-981-10-6955-0_23. [DOI] [PubMed] [Google Scholar]
- 10.Neil A.J., Kim J.C., Mirkin S.M. Precarious maintenance of simple DNA repeats in eukaryotes. BioEssays. 2017;39:39. doi: 10.1002/bies.201700077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushal S., Freudenreich C.H. The role of fork stalling and DNA structures in causing chromosome fragility. Genes Chromosomes Cancer. 2018;58:270–283. doi: 10.1002/gcc.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel D.R., Weiss R.S. A tough row to hoe: when replication forks encounter DNA damage. Biochem. Soc. Trans. 2018;46:1643–1651. doi: 10.1042/BST20180308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richard P., Manley J.L. R Loops and Links to Human Disease. J. Mol. Biol. 2017;429:3168–3180. doi: 10.1016/j.jmb.2016.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos-Pereira J.M., Aguilera A. R loops: new modulators of genome dynamics and function. Nat. Rev. Genet. 2015;16:583–597. doi: 10.1038/nrg3961. [DOI] [PubMed] [Google Scholar]
- 15.Brooks P.J. The cyclopurine deoxynucleosides: DNA repair, biological effects, mechanistic insights, and unanswered questions. Free Radic. Biol. Med. 2017;107:90–100. doi: 10.1016/j.freeradbiomed.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Dizdaroglu M., Jaruga P. Mechanisms of free radical-induced damage to DNA. Free Radic. Res. 2012;46:382–419. doi: 10.3109/10715762.2011.653969. [DOI] [PubMed] [Google Scholar]
- 17.Cadet J., Davies K.J.A., Medeiros M.H., Di Mascio P., Wagner J.R. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Biol. Med. 2017;107:13–34. doi: 10.1016/j.freeradbiomed.2016.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming A.M., Burrows C.J. 8-Oxo-7,8-dihydroguanine, friend and foe: Epigenetic-like regulator versus initiator of mutagenesis. DNA Repair (Amst.) 2017;56:75–83. doi: 10.1016/j.dnarep.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaruga P., Dizdaroglu M. 8,5′-Cyclopurine-2′-deoxynucleosides in DNA: mechanisms of formation, measurement, repair and biological effects. DNA Repair (Amst.) 2008;7:1413–1425. doi: 10.1016/j.dnarep.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Caldecott K.W. Single-strand break repair and genetic disease. Nat. Rev. Genet. 2008;9:619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- 21.Marnett L.J. Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res. 1999;424:83–95. doi: 10.1016/s0027-5107(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 22.Zhong H., Yin H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol. 2015;4:193–199. doi: 10.1016/j.redox.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rydberg B., Lindahl T. Nonenzymatic methylation of DNA by the intracellular methyl group donor S-adenosyl-L-methionine is a potentially mutagenic reaction. EMBO J. 1982;1:211–216. doi: 10.1002/j.1460-2075.1982.tb01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullenders L.H.F. Solar UV damage to cellular DNA: from mechanisms to biological effects. Photochem. Photobiol. Sci. 2018;17:1842–1852. doi: 10.1039/c8pp00182k. [DOI] [PubMed] [Google Scholar]
- 25.Cadet J., Douki T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem. Photobiol. Sci. 2018;17:1816–1841. doi: 10.1039/c7pp00395a. [DOI] [PubMed] [Google Scholar]
- 26.Jackson S.P., Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foray N., Bourguignon M., Hamada N. Individual response to ionizing radiation. Mutat. Res. 2016;770(Pt B):369–386. doi: 10.1016/j.mrrev.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Tiwari V., Kamran M.Z., Ranjan A., Nimesh H., Singh M., Tandon V. Akt1/NFκB signaling pathway activation by a small molecule DMA confers radioprotection to intestinal epithelium in xenograft model. Free Radic. Biol. Med. 2017;108:564–574. doi: 10.1016/j.freeradbiomed.2017.04.029. [DOI] [PubMed] [Google Scholar]
- 29.Reisz J.A., Bansal N., Qian J., Zhao W., Furdui C.M. Effects of ionizing radiation on biological molecules--mechanisms of damage and emerging methods of detection. Antioxid. Redox Signal. 2014;21:260–292. doi: 10.1089/ars.2013.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips D.H., Venitt S. DNA and protein adducts in human tissues resulting from exposure to tobacco smoke. Int. J. Cancer. 2012;131:2733–2753. doi: 10.1002/ijc.27827. [DOI] [PubMed] [Google Scholar]
- 31.Barnes J.L., Zubair M., John K., Poirier M.C., Martin F.L. Carcinogens and DNA damage. Biochem. Soc. Trans. 2018;46:1213–1224. doi: 10.1042/BST20180519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mordukhovich I., Rossner P., Jr., Terry M.B., Santella R., Zhang Y.J., Hibshoosh H., Memeo L., Mansukhani M., Long C.M., Garbowski G. Associations between polycyclic aromatic hydrocarbon-related exposures and p53 mutations in breast tumors. Environ. Health Perspect. 2010;118:511–518. doi: 10.1289/ehp.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozack R., Seo K.Y., Jelinsky S.A., Loechler E.L. Toward an understanding of the role of DNA adduct conformation in defining mutagenic mechanism based on studies of the major adduct (formed at N(2)-dG) of the potent environmental carcinogen, benzo[a]pyrene. Mutat. Res. 2000;450:41–59. doi: 10.1016/s0027-5107(00)00015-4. [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee N., Walker G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017;58:235–263. doi: 10.1002/em.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibutani S., Suzuki N., Tan X., Johnson F., Grollman A.P. Influence of flanking sequence context on the mutagenicity of acetylaminofluorene-derived DNA adducts in mammalian cells. Biochemistry. 2001;40:3717–3722. doi: 10.1021/bi0027581. [DOI] [PubMed] [Google Scholar]
- 36.Smela M.E., Hamm M.L., Henderson P.T., Harris C.M., Harris T.M., Essigmann J.M. The aflatoxin B(1) formamidopyrimidine adduct plays a major role in causing the types of mutations observed in human hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA. 2002;99:6655–6660. doi: 10.1073/pnas.102167699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheung-Ong K., Giaever G., Nislow C. DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem. Biol. 2013;20:648–659. doi: 10.1016/j.chembiol.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Eljack N.D., Ma H.Y., Drucker J., Shen C., Hambley T.W., New E.J., Friedrich T., Clarke R.J. Mechanisms of cell uptake and toxicity of the anticancer drug cisplatin. Metallomics. 2014;6:2126–2133. doi: 10.1039/c4mt00238e. [DOI] [PubMed] [Google Scholar]
- 39.Hu J., Lieb J.D., Sancar A., Adar S. Cisplatin DNA damage and repair maps of the human genome at single-nucleotide resolution. Proc. Natl. Acad. Sci. USA. 2016;113:11507–11512. doi: 10.1073/pnas.1614430113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basu A., Krishnamurthy S. Cellular responses to Cisplatin-induced DNA damage. J. Nucleic Acids. 2010;2010:2010. doi: 10.4061/2010/201367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roos W.P., Kaina B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013;332:237–248. doi: 10.1016/j.canlet.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Delgado J.L., Hsieh C.M., Chan N.L., Hiasa H. Topoisomerases as anticancer targets. Biochem. J. 2018;475:373–398. doi: 10.1042/BCJ20160583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu D., Keijzers G., Rasmussen L.J. DNA mismatch repair and its many roles in eukaryotic cells. Mutat. Res. 2017;773:174–187. doi: 10.1016/j.mrrev.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Fishel R. Mismatch repair. J. Biol. Chem. 2015;290:26395–26403. doi: 10.1074/jbc.R115.660142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim D., Fishel R., Lee J.B. Coordinating Multi-Protein Mismatch Repair by Managing Diffusion Mechanics on the DNA. J. Mol. Biol. 2018;430:4469–4480. doi: 10.1016/j.jmb.2018.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiricny J. The multifaceted mismatch-repair system. Nat. Rev. Mol. Cell Biol. 2006;7:335–346. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- 47.Umar A., Buermeyer A.B., Simon J.A., Thomas D.C., Clark A.B., Liskay R.M., Kunkel T.A. Requirement for PCNA in DNA mismatch repair at a step preceding DNA resynthesis. Cell. 1996;87:65–73. doi: 10.1016/s0092-8674(00)81323-9. [DOI] [PubMed] [Google Scholar]
- 48.Kadyrov F.A., Dzantiev L., Constantin N., Modrich P. Endonucleolytic function of MutLalpha in human mismatch repair. Cell. 2006;126:297–308. doi: 10.1016/j.cell.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 49.Goellner E.M., Smith C.E., Campbell C.S., Hombauer H., Desai A., Putnam C.D., Kolodner R.D. PCNA and Msh2-Msh6 activate an Mlh1-Pms1 endonuclease pathway required for Exo1-independent mismatch repair. Mol. Cell. 2014;55:291–304. doi: 10.1016/j.molcel.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vo A.T., Zhu F., Wu X., Yuan F., Gao Y., Gu L., Li G.M., Lee T.H., Her C. hMRE11 deficiency leads to microsatellite instability and defective DNA mismatch repair. EMBO Rep. 2005;6:438–444. doi: 10.1038/sj.embor.7400392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tran H.T., Gordenin D.A., Resnick M.A. The 3′-->5′ exonucleases of DNA polymerases delta and epsilon and the 5′-->3′ exonuclease Exo1 have major roles in postreplication mutation avoidance in Saccharomyces cerevisiae. Mol. Cell. Biol. 1999;19:2000–2007. doi: 10.1128/mcb.19.3.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krokan H.E., Bjørås M. Base excision repair. Cold Spring Harb. Perspect. Biol. 2013;5:a012583. doi: 10.1101/cshperspect.a012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dizdaroglu M., Coskun E., Jaruga P. Repair of oxidatively induced DNA damage by DNA glycosylases: Mechanisms of action, substrate specificities and excision kinetics. Mutat. Res. 2017;771:99–127. doi: 10.1016/j.mrrev.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li M., Wilson D.M., 3rd Human apurinic/apyrimidinic endonuclease 1. Antioxid. Redox Signal. 2014;20:678–707. doi: 10.1089/ars.2013.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beard W.A., Wilson S.H. Structure and mechanism of DNA polymerase β. Biochemistry. 2014;53:2768–2780. doi: 10.1021/bi500139h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moor N.A., Lavrik O.I. Protein-Protein Interactions in DNA Base Excision Repair. Biochemistry (Mosc.) 2018;83:411–422. doi: 10.1134/S0006297918040120. [DOI] [PubMed] [Google Scholar]
- 57.Robertson A.B., Klungland A., Rognes T., Leiros I. DNA repair in mammalian cells: Base excision repair: the long and short of it. Cell. Mol. Life Sci. 2009;66:981–993. doi: 10.1007/s00018-009-8736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spivak G. Nucleotide excision repair in humans. DNA Repair (Amst.) 2015;36:13–18. doi: 10.1016/j.dnarep.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rapin I. Disorders of nucleotide excision repair. Handb. Clin. Neurol. 2013;113:1637–1650. doi: 10.1016/B978-0-444-59565-2.00032-0. [DOI] [PubMed] [Google Scholar]
- 60.Sugasawa K. Molecular mechanisms of DNA damage recognition for mammalian nucleotide excision repair. DNA Repair (Amst.) 2016;44:110–117. doi: 10.1016/j.dnarep.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 61.Zhu Q., Wani A.A. Nucleotide Excision Repair: Finely Tuned Molecular Orchestra of Early Pre-incision Events. Photochem. Photobiol. 2017;93:166–177. doi: 10.1111/php.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang J.C., Hsu D.S., Kazantsev A., Sancar A. Substrate spectrum of human excinuclease: repair of abasic sites, methylated bases, mismatches, and bulky adducts. Proc. Natl. Acad. Sci. USA. 1994;91:12213–12217. doi: 10.1073/pnas.91.25.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sugasawa K., Okamoto T., Shimizu Y., Masutani C., Iwai S., Hanaoka F. A multistep damage recognition mechanism for global genomic nucleotide excision repair. Genes Dev. 2001;15:507–521. doi: 10.1101/gad.866301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reardon J.T., Sancar A. Recognition and repair of the cyclobutane thymine dimer, a major cause of skin cancers, by the human excision nuclease. Genes Dev. 2003;17:2539–2551. doi: 10.1101/gad.1131003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wittschieben B.O., Iwai S., Wood R.D. DDB1-DDB2 (xeroderma pigmentosum group E) protein complex recognizes a cyclobutane pyrimidine dimer, mismatches, apurinic/apyrimidinic sites, and compound lesions in DNA. J. Biol. Chem. 2005;280:39982–39989. doi: 10.1074/jbc.M507854200. [DOI] [PubMed] [Google Scholar]
- 66.Tang J., Chu G. Xeroderma pigmentosum complementation group E and UV-damaged DNA-binding protein. DNA Repair (Amst.) 2002;1:601–616. doi: 10.1016/s1568-7864(02)00052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sugasawa K., Okuda Y., Saijo M., Nishi R., Matsuda N., Chu G., Mori T., Iwai S., Tanaka K., Tanaka K., Hanaoka F. UV-induced ubiquitylation of XPC protein mediated by UV-DDB-ubiquitin ligase complex. Cell. 2005;121:387–400. doi: 10.1016/j.cell.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 68.Schärer O.D. Nucleotide excision repair in eukaryotes. Cold Spring Harb. Perspect. Biol. 2013;5:a012609. doi: 10.1101/cshperspect.a012609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lehmann J., Seebode C., Martens M.C., Emmert S. Xeroderma Pigmentosum - Facts and Perspectives. Anticancer Res. 2018;38:1159–1164. doi: 10.21873/anticanres.12335. [DOI] [PubMed] [Google Scholar]
- 70.Kraemer K.H., Patronas N.J., Schiffmann R., Brooks B.P., Tamura D., DiGiovanna J.J. Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: a complex genotype-phenotype relationship. Neuroscience. 2007;145:1388–1396. doi: 10.1016/j.neuroscience.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kresge N., Simoni R.D., Hill R.L. Discovery and characterization of DNA excision repair pathways: the work of Philip Courtland Hanawalt. J. Biol. Chem. 2010;285:e9–e11. doi: 10.1074/jbc.O110.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boetefuer E.L., Lake R.J., Fan H.Y. Mechanistic insights into the regulation of transcription and transcription-coupled DNA repair by Cockayne syndrome protein B. Nucleic Acids Res. 2018;46:7471–7479. doi: 10.1093/nar/gky660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiou Y.Y., Hu J., Sancar A., Selby C.P. RNA polymerase II is released from the DNA template during transcription-coupled repair in mammalian cells. J. Biol. Chem. 2018;293:2476–2486. doi: 10.1074/jbc.RA117.000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sigurdsson S., Dirac-Svejstrup A.B., Svejstrup J.Q. Evidence that transcript cleavage is essential for RNA polymerase II transcription and cell viability. Mol. Cell. 2010;38:202–210. doi: 10.1016/j.molcel.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anindya R., Aygün O., Svejstrup J.Q. Damage-induced ubiquitylation of human RNA polymerase II by the ubiquitin ligase Nedd4, but not Cockayne syndrome proteins or BRCA1. Mol. Cell. 2007;28:386–397. doi: 10.1016/j.molcel.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 76.Wilson M.D., Harreman M., Svejstrup J.Q. Ubiquitylation and degradation of elongating RNA polymerase II: the last resort. Biochim. Biophys. Acta. 2013;1829:151–157. doi: 10.1016/j.bbagrm.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Fousteri M., Vermeulen W., van Zeeland A.A., Mullenders L.H. Cockayne syndrome A and B proteins differentially regulate recruitment of chromatin remodeling and repair factors to stalled RNA polymerase II in vivo. Mol. Cell. 2006;23:471–482. doi: 10.1016/j.molcel.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 78.Schwertman P., Lagarou A., Dekkers D.H., Raams A., van der Hoek A.C., Laffeber C., Hoeijmakers J.H., Demmers J.A., Fousteri M., Vermeulen W., Marteijn J.A. UV-sensitive syndrome protein UVSSA recruits USP7 to regulate transcription-coupled repair. Nat. Genet. 2012;44:598–602. doi: 10.1038/ng.2230. [DOI] [PubMed] [Google Scholar]
- 79.Karikkineth A.C., Scheibye-Knudsen M., Fivenson E., Croteau D.L., Bohr V.A. Cockayne syndrome: Clinical features, model systems and pathways. Ageing Res. Rev. 2017;33:3–17. doi: 10.1016/j.arr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Volker M., Moné M.J., Karmakar P., van Hoffen A., Schul W., Vermeulen W., Hoeijmakers J.H., van Driel R., van Zeeland A.A., Mullenders L.H. Sequential assembly of the nucleotide excision repair factors in vivo. Mol. Cell. 2001;8:213–224. doi: 10.1016/s1097-2765(01)00281-7. [DOI] [PubMed] [Google Scholar]
- 81.Yokoi M., Masutani C., Maekawa T., Sugasawa K., Ohkuma Y., Hanaoka F. The xeroderma pigmentosum group C protein complex XPC-HR23B plays an important role in the recruitment of transcription factor IIH to damaged DNA. J. Biol. Chem. 2000;275:9870–9875. doi: 10.1074/jbc.275.13.9870. [DOI] [PubMed] [Google Scholar]
- 82.Riedl T., Hanaoka F., Egly J.M. The comings and goings of nucleotide excision repair factors on damaged DNA. EMBO J. 2003;22:5293–5303. doi: 10.1093/emboj/cdg489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tapias A., Auriol J., Forget D., Enzlin J.H., Schärer O.D., Coin F., Coulombe B., Egly J.M. Ordered conformational changes in damaged DNA induced by nucleotide excision repair factors. J. Biol. Chem. 2004;279:19074–19083. doi: 10.1074/jbc.M312611200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Compe E., Egly J.M. TFIIH: when transcription met DNA repair. Nat. Rev. Mol. Cell Biol. 2012;13:343–354. doi: 10.1038/nrm3350. [DOI] [PubMed] [Google Scholar]
- 85.Fan L., Fuss J.O., Cheng Q.J., Arvai A.S., Hammel M., Roberts V.A., Cooper P.K., Tainer J.A. XPD helicase structures and activities: insights into the cancer and aging phenotypes from XPD mutations. Cell. 2008;133:789–800. doi: 10.1016/j.cell.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mathieu N., Kaczmarek N., Rüthemann P., Luch A., Naegeli H. DNA quality control by a lesion sensor pocket of the xeroderma pigmentosum group D helicase subunit of TFIIH. Curr. Biol. 2013;23:204–212. doi: 10.1016/j.cub.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 87.Coin F., Oksenych V., Egly J.M. Distinct roles for the XPB/p52 and XPD/p44 subcomplexes of TFIIH in damaged DNA opening during nucleotide excision repair. Mol. Cell. 2007;26:245–256. doi: 10.1016/j.molcel.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 88.Giglia-Mari G., Coin F., Ranish J.A., Hoogstraten D., Theil A., Wijgers N., Jaspers N.G., Raams A., Argentini M., van der Spek P.J. A new, tenth subunit of TFIIH is responsible for the DNA repair syndrome trichothiodystrophy group A. Nat. Genet. 2004;36:714–719. doi: 10.1038/ng1387. [DOI] [PubMed] [Google Scholar]
- 89.Ranish J.A., Hahn S., Lu Y., Yi E.C., Li X.J., Eng J., Aebersold R. Identification of TFB5, a new component of general transcription and DNA repair factor IIH. Nat. Genet. 2004;36:707–713. doi: 10.1038/ng1385. [DOI] [PubMed] [Google Scholar]
- 90.Theil A.F., Nonnekens J., Steurer B., Mari P.O., de Wit J., Lemaitre C., Marteijn J.A., Raams A., Maas A., Vermeij M. Disruption of TTDA results in complete nucleotide excision repair deficiency and embryonic lethality. PLoS Genet. 2013;9:e1003431. doi: 10.1371/journal.pgen.1003431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Coin F., Proietti De Santis L., Nardo T., Zlobinskaya O., Stefanini M., Egly J.M. p8/TTD-A as a repair-specific TFIIH subunit. Mol. Cell. 2006;21:215–226. doi: 10.1016/j.molcel.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 92.Wakasugi M., Sancar A. Assembly, subunit composition, and footprint of human DNA repair excision nuclease. Proc. Natl. Acad. Sci. USA. 1998;95:6669–6674. doi: 10.1073/pnas.95.12.6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marteijn J.A., Lans H., Vermeulen W., Hoeijmakers J.H. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 2014;15:465–481. doi: 10.1038/nrm3822. [DOI] [PubMed] [Google Scholar]
- 94.Shivji M.K., Podust V.N., Hübscher U., Wood R.D. Nucleotide excision repair DNA synthesis by DNA polymerase epsilon in the presence of PCNA, RFC, and RPA. Biochemistry. 1995;34:5011–5017. doi: 10.1021/bi00015a012. [DOI] [PubMed] [Google Scholar]
- 95.Yoon G., Caldecott K.W. Nonsyndromic cerebellar ataxias associated with disorders of DNA single-strand break repair. Handb. Clin. Neurol. 2018;155:105–115. doi: 10.1016/B978-0-444-64189-2.00007-X. [DOI] [PubMed] [Google Scholar]
- 96.Abbotts R., Wilson D.M., 3rd Coordination of DNA single strand break repair. Free Radic. Biol. Med. 2017;107:228–244. doi: 10.1016/j.freeradbiomed.2016.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caldecott K.W. DNA single-strand break repair and spinocerebellar ataxia. Cell. 2003;112:7–10. doi: 10.1016/s0092-8674(02)01247-3. [DOI] [PubMed] [Google Scholar]
- 98.Andres S.N., Schellenberg M.J., Wallace B.D., Tumbale P., Williams R.S. Recognition and repair of chemically heterogeneous structures at DNA ends. Environ. Mol. Mutagen. 2015;56:1–21. doi: 10.1002/em.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ranjha L., Howard S.M., Cejka P. Main steps in DNA double-strand break repair: an introduction to homologous recombination and related processes. Chromosoma. 2018;127:187–214. doi: 10.1007/s00412-017-0658-1. [DOI] [PubMed] [Google Scholar]
- 100.Pannunzio N.R., Watanabe G., Lieber M.R. Nonhomologous DNA end-joining for repair of DNA double-strand breaks. J. Biol. Chem. 2018;293:10512–10523. doi: 10.1074/jbc.TM117.000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sallmyr A., Tomkinson A.E. Repair of DNA double-strand breaks by mammalian alternative end-joining pathways. J. Biol. Chem. 2018;293:10536–10546. doi: 10.1074/jbc.TM117.000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Williams G.J., Hammel M., Radhakrishnan S.K., Ramsden D., Lees-Miller S.P., Tainer J.A. Structural insights into NHEJ: building up an integrated picture of the dynamic DSB repair super complex, one component and interaction at a time. DNA Repair (Amst.) 2014;17:110–120. doi: 10.1016/j.dnarep.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Menon V., Povirk L.F. End-processing nucleases and phosphodiesterases: An elite supporting cast for the non-homologous end joining pathway of DNA double-strand break repair. DNA Repair (Amst.) 2016;43:57–68. doi: 10.1016/j.dnarep.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 104.Chang H.H.Y., Pannunzio N.R., Adachi N., Lieber M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017;18:495–506. doi: 10.1038/nrm.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Audebert M., Salles B., Calsou P. Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J. Biol. Chem. 2004;279:55117–55126. doi: 10.1074/jbc.M404524200. [DOI] [PubMed] [Google Scholar]
- 106.Mateos-Gomez P.A., Gong F., Nair N., Miller K.M., Lazzerini-Denchi E., Sfeir A. Mammalian polymerase θ promotes alternative NHEJ and suppresses recombination. Nature. 2015;518:254–257. doi: 10.1038/nature14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wright W.D., Shah S.S., Heyer W.D. Homologous recombination and the repair of DNA double-strand breaks. J. Biol. Chem. 2018;293:10524–10535. doi: 10.1074/jbc.TM118.000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ait Saada A., Lambert S.A.E., Carr A.M. Preserving replication fork integrity and competence via the homologous recombination pathway. DNA Repair (Amst.) 2018;71:135–147. doi: 10.1016/j.dnarep.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clouaire T., Marnef A., Legube G. Taming Tricky DSBs: ATM on duty. DNA Repair (Amst.) 2017;56:84–91. doi: 10.1016/j.dnarep.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 110.Jasin M., Rothstein R. Repair of strand breaks by homologous recombination. Cold Spring Harb. Perspect. Biol. 2013;5:a012740. doi: 10.1101/cshperspect.a012740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sung P. Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science. 1994;265:1241–1243. doi: 10.1126/science.8066464. [DOI] [PubMed] [Google Scholar]
- 112.New J.H., Sugiyama T., Zaitseva E., Kowalczykowski S.C. Rad52 protein stimulates DNA strand exchange by Rad51 and replication protein A. Nature. 1998;391:407–410. doi: 10.1038/34950. [DOI] [PubMed] [Google Scholar]
- 113.Szostak J.W., Orr-Weaver T.L., Rothstein R.J., Stahl F.W. The double-strand-break repair model for recombination. Cell. 1983;33:25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- 114.Wu L., Hickson I.D. The Bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- 115.Ira G., Malkova A., Liberi G., Foiani M., Haber J.E. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell. 2003;115:401–411. doi: 10.1016/s0092-8674(03)00886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moynahan M.E., Jasin M. Loss of heterozygosity induced by a chromosomal double-strand break. Proc. Natl. Acad. Sci. USA. 1997;94:8988–8993. doi: 10.1073/pnas.94.17.8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barbari S.R., Shcherbakova P.V. Replicative DNA polymerase defects in human cancers: Consequences, mechanisms, and implications for therapy. DNA Repair (Amst.) 2017;56:16–25. doi: 10.1016/j.dnarep.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fishel R., Lescoe M.K., Rao M.R., Copeland N.G., Jenkins N.A., Garber J., Kane M., Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993;75:1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- 119.Parsons R., Li G.M., Longley M.J., Fang W.H., Papadopoulos N., Jen J., de la Chapelle A., Kinzler K.W., Vogelstein B., Modrich P. Hypermutability and mismatch repair deficiency in RER+ tumor cells. Cell. 1993;75:1227–1236. doi: 10.1016/0092-8674(93)90331-j. [DOI] [PubMed] [Google Scholar]
- 120.Duraturo F., Liccardo R., De Rosa M., Izzo P. Genetics, diagnosis and treatment of Lynch syndrome: Old lessons and current challenges. Oncol. Lett. 2019;17:3048–3054. doi: 10.3892/ol.2019.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Abedalthagafi M. Constitutional mismatch repair-deficiency: current problems and emerging therapeutic strategies. Oncotarget. 2018;9:35458–35469. doi: 10.18632/oncotarget.26249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ryan E., Sheahan K., Creavin B., Mohan H.M., Winter D.C. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit. Rev. Oncol. Hematol. 2017;116:38–57. doi: 10.1016/j.critrevonc.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 123.Zeinalian M., Hashemzadeh-Chaleshtori M., Salehi R., Emami M.H. Clinical Aspects of Microsatellite Instability Testing in Colorectal Cancer. Adv. Biomed. Res. 2018;7:28. doi: 10.4103/abr.abr_185_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Al-Tassan N., Chmiel N.H., Maynard J., Fleming N., Livingston A.L., Williams G.T., Hodges A.K., Davies D.R., David S.S., Sampson J.R., Cheadle J.P. Inherited variants of MYH associated with somatic G:C-->T:A mutations in colorectal tumors. Nat. Genet. 2002;30:227–232. doi: 10.1038/ng828. [DOI] [PubMed] [Google Scholar]
- 125.Weren R.D., Ligtenberg M.J., Kets C.M., de Voer R.M., Verwiel E.T., Spruijt L., van Zelst-Stams W.A., Jongmans M.C., Gilissen C., Hehir-Kwa J.Y. A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat. Genet. 2015;47:668–671. doi: 10.1038/ng.3287. [DOI] [PubMed] [Google Scholar]
- 126.Imai K., Slupphaug G., Lee W.I., Revy P., Nonoyama S., Catalan N., Yel L., Forveille M., Kavli B., Krokan H.E. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat. Immunol. 2003;4:1023–1028. doi: 10.1038/ni974. [DOI] [PubMed] [Google Scholar]
- 127.Nilsen H., Stamp G., Andersen S., Hrivnak G., Krokan H.E., Lindahl T., Barnes D.E. Gene-targeted mice lacking the Ung uracil-DNA glycosylase develop B-cell lymphomas. Oncogene. 2003;22:5381–5386. doi: 10.1038/sj.onc.1206860. [DOI] [PubMed] [Google Scholar]
- 128.Vlahopoulos S., Adamaki M., Khoury N., Zoumpourlis V., Boldogh I. Roles of DNA repair enzyme OGG1 in innate immunity and its significance for lung cancer. Pharmacol. Ther. 2019;194:59–72. doi: 10.1016/j.pharmthera.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xanthoudakis S., Smeyne R.J., Wallace J.D., Curran T. The redox/DNA repair protein, Ref-1, is essential for early embryonic development in mice. Proc. Natl. Acad. Sci. USA. 1996;93:8919–8923. doi: 10.1073/pnas.93.17.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sugo N., Aratani Y., Nagashima Y., Kubota Y., Koyama H. Neonatal lethality with abnormal neurogenesis in mice deficient in DNA polymerase beta. EMBO J. 2000;19:1397–1404. doi: 10.1093/emboj/19.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brenerman B.M., Illuzzi J.L., Wilson D.M., 3rd Base excision repair capacity in informing healthspan. Carcinogenesis. 2014;35:2643–2652. doi: 10.1093/carcin/bgu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li M., Yang X., Lu X., Dai N., Zhang S., Cheng Y., Zhang L., Yang Y., Liu Y., Yang Z. APE1 deficiency promotes cellular senescence and premature aging features. Nucleic Acids Res. 2018;46:5664–5677. doi: 10.1093/nar/gky326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Illuzzi J.L., McNeill D.R., Bastian P., Brenerman B., Wersto R., Russell H.R., Bunz F., McKinnon P.J., Becker K.G., Wilson D.M., 3rd Tumor-associated APE1 variant exhibits reduced complementation efficiency but does not promote cancer cell phenotypes. Environ. Mol. Mutagen. 2017;58:84–98. doi: 10.1002/em.22074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wallace S.S., Murphy D.L., Sweasy J.B. Base excision repair and cancer. Cancer Lett. 2012;327:73–89. doi: 10.1016/j.canlet.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yamtich J., Nemec A.A., Keh A., Sweasy J.B. A germline polymorphism of DNA polymerase beta induces genomic instability and cellular transformation. PLoS Genet. 2012;8:e1003052. doi: 10.1371/journal.pgen.1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alnajjar K.S., Negahbani A., Nakhjiri M., Krylov I.S., Kashemirov B.A., McKenna C.E., Goodman M.F., Sweasy J.B. DNA Polymerase β Cancer-Associated Variant I260M Exhibits Nonspecific Selectivity toward the β-γ Bridging Group of the Incoming dNTP. Biochemistry. 2017;56:5449–5456. doi: 10.1021/acs.biochem.7b00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Senejani A.G., Liu Y., Kidane D., Maher S.E., Zeiss C.J., Park H.J., Kashgarian M., McNiff J.M., Zelterman D., Bothwell A.L., Sweasy J.B. Mutation of POLB causes lupus in mice. Cell Rep. 2014;6:1–8. doi: 10.1016/j.celrep.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Abeti R., Zeitlberger A., Peelo C., Fassihi H., Sarkany R.P.E., Lehmann A.R., Giunti P. Xeroderma pigmentosum: overview of pharmacology and novel therapeutic strategies for neurological symptoms. Br. J. Pharmacol. 2018 doi: 10.1111/bph.14557. Published online November 30, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kraemer K.H., Lee M.M., Scotto J. DNA repair protects against cutaneous and internal neoplasia: evidence from xeroderma pigmentosum. Carcinogenesis. 1984;5:511–514. doi: 10.1093/carcin/5.4.511. [DOI] [PubMed] [Google Scholar]
- 140.DiGiovanna J.J., Kraemer K.H. Shining a light on xeroderma pigmentosum. J. Invest. Dermatol. 2012;132:785–796. doi: 10.1038/jid.2011.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cleaver J.E. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968;218:652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- 142.Mu H., Geacintov N.E., Broyde S., Yeo J.E., Schärer O.D. Molecular basis for damage recognition and verification by XPC-RAD23B and TFIIH in nucleotide excision repair. DNA Repair (Amst.) 2018;71:33–42. doi: 10.1016/j.dnarep.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bennett D., Itoh T. The XPE gene of xeroderma pigmentosum, its product and biological roles. Adv. Exp. Med. Biol. 2008;637:57–64. doi: 10.1007/978-0-387-09599-8_7. [DOI] [PubMed] [Google Scholar]
- 144.Kolesnikova O., Radu L., Poterszman A. TFIIH: A multi-subunit complex at the cross-roads of transcription and DNA repair. Adv. Protein Chem. Struct. Biol. 2019;115:21–67. doi: 10.1016/bs.apcsb.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 145.Rimel J.K., Taatjes D.J. The essential and multifunctional TFIIH complex. Protein Sci. 2018;27:1018–1037. doi: 10.1002/pro.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Natale V., Raquer H. Xeroderma pigmentosum-Cockayne syndrome complex. Orphanet J. Rare Dis. 2017;12:65. doi: 10.1186/s13023-017-0616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dubaele S., Proietti De Santis L., Bienstock R.J., Keriel A., Stefanini M., Van Houten B., Egly J.M. Basal transcription defect discriminates between xeroderma pigmentosum and trichothiodystrophy in XPD patients. Mol. Cell. 2003;11:1635–1646. doi: 10.1016/s1097-2765(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 148.McDowell M.L., Nguyen T., Cleaver J.E. A single-site mutation in the XPAC gene alters photoproduct recognition. Mutagenesis. 1993;8:155–161. doi: 10.1093/mutage/8.2.155. [DOI] [PubMed] [Google Scholar]
- 149.Dehé P.M., Gaillard P.H. Control of structure-specific endonucleases to maintain genome stability. Nat. Rev. Mol. Cell Biol. 2017;18:315–330. doi: 10.1038/nrm.2016.177. [DOI] [PubMed] [Google Scholar]
- 150.Difilippantonio M.J., Zhu J., Chen H.T., Meffre E., Nussenzweig M.C., Max E.E., Ried T., Nussenzweig A. DNA repair protein Ku80 suppresses chromosomal aberrations and malignant transformation. Nature. 2000;404:510–514. doi: 10.1038/35006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bunting S.F., Nussenzweig A. End-joining, translocations and cancer. Nat. Rev. Cancer. 2013;13:443–454. doi: 10.1038/nrc3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chistiakov D.A. Ligase IV syndrome. Adv. Exp. Med. Biol. 2010;685:175–185. doi: 10.1007/978-1-4419-6448-9_16. [DOI] [PubMed] [Google Scholar]
- 153.Riballo E., Woodbine L., Stiff T., Walker S.A., Goodarzi A.A., Jeggo P.A. XLF-Cernunnos promotes DNA ligase IV-XRCC4 re-adenylation following ligation. Nucleic Acids Res. 2009;37:482–492. doi: 10.1093/nar/gkn957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Morio T. Recent advances in the study of immunodeficiency and DNA damage response. Int. J. Hematol. 2017;106:357–365. doi: 10.1007/s12185-017-2263-8. [DOI] [PubMed] [Google Scholar]
- 155.O’Driscoll M. Diseases associated with defective responses to DNA damage. Cold Spring Harb. Perspect. Biol. 2012;4:4. doi: 10.1101/cshperspect.a012773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Murray J.E., van der Burg M., IJspeert H., Carroll P., Wu Q., Ochi T., Leitch A., Miller E.S., Kysela B., Jawad A. Mutations in the NHEJ component XRCC4 cause primordial dwarfism. Am. J. Hum. Genet. 2015;96:412–424. doi: 10.1016/j.ajhg.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Katsuki Y., Takata M. Defects in homologous recombination repair behind the human diseases: FA and HBOC. Endocr. Relat. Cancer. 2016;23:T19–T37. doi: 10.1530/ERC-16-0221. [DOI] [PubMed] [Google Scholar]
- 158.Pennington K.P., Walsh T., Harrell M.I., Lee M.K., Pennil C.C., Rendi M.H., Thornton A., Norquist B.M., Casadei S., Nord A.S. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin. Cancer Res. 2014;20:764–775. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cunningham J.M., Cicek M.S., Larson N.B., Davila J., Wang C., Larson M.C., Song H., Dicks E.M., Harrington P., Wick M. Clinical characteristics of ovarian cancer classified by BRCA1, BRCA2, and RAD51C status. Sci. Rep. 2014;4:4026. doi: 10.1038/srep04026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Song H., Dicks E., Ramus S.J., Tyrer J.P., Intermaggio M.P., Hayward J., Edlund C.K., Conti D., Harrington P., Fraser L. Contribution of Germline Mutations in the RAD51B, RAD51C, and RAD51D Genes to Ovarian Cancer in the Population. J. Clin. Oncol. 2015;33:2901–2907. doi: 10.1200/JCO.2015.61.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ramus S.J., Song H., Dicks E., Tyrer J.P., Rosenthal A.N., Intermaggio M.P., Fraser L., Gentry-Maharaj A., Hayward J., Philpott S., AOCS Study Group. Ovarian Cancer Association Consortium Germline Mutations in the BRIP1, BARD1, PALB2, and NBN Genes in Women With Ovarian Cancer. J. Natl. Cancer Inst. 2015;107:107. doi: 10.1093/jnci/djv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen J., Silver D.P., Walpita D., Cantor S.B., Gazdar A.F., Tomlinson G., Couch F.J., Weber B.L., Ashley T., Livingston D.M., Scully R. Stable interaction between the products of the BRCA1 and BRCA2 tumor suppressor genes in mitotic and meiotic cells. Mol. Cell. 1998;2:317–328. doi: 10.1016/s1097-2765(00)80276-2. [DOI] [PubMed] [Google Scholar]
- 163.Scully R., Chen J., Plug A., Xiao Y., Weaver D., Feunteun J., Ashley T., Livingston D.M. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88:265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- 164.Niraj J., Färkkilä A., D’Andrea A.D. The Fanconi Anemia Pathway in Cancer. Annu Rev Cancer Biol. 2019;3:457–478. doi: 10.1146/annurev-cancerbio-030617-050422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Langevin F., Crossan G.P., Rosado I.V., Arends M.J., Patel K.J. Fancd2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature. 2011;475:53–58. doi: 10.1038/nature10192. [DOI] [PubMed] [Google Scholar]
- 166.Garaycoechea J.I., Crossan G.P., Langevin F., Daly M., Arends M.J., Patel K.J. Genotoxic consequences of endogenous aldehydes on mouse haematopoietic stem cell function. Nature. 2012;489:571–575. doi: 10.1038/nature11368. [DOI] [PubMed] [Google Scholar]
- 167.Van Wassenhove L.D., Mochly-Rosen D., Weinberg K.I. Aldehyde dehydrogenase 2 in aplastic anemia, Fanconi anemia and hematopoietic stem cells. Mol. Genet. Metab. 2016;119:28–36. doi: 10.1016/j.ymgme.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Takashima H., Boerkoel C.F., John J., Saifi G.M., Salih M.A., Armstrong D., Mao Y., Quiocho F.A., Roa B.B., Nakagawa M. Mutation of TDP1, encoding a topoisomerase I-dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nat. Genet. 2002;32:267–272. doi: 10.1038/ng987. [DOI] [PubMed] [Google Scholar]
- 169.Moreira M.C., Barbot C., Tachi N., Kozuka N., Uchida E., Gibson T., Mendonça P., Costa M., Barros J., Yanagisawa T. The gene mutated in ataxia-ocular apraxia 1 encodes the new HIT/Zn-finger protein aprataxin. Nat. Genet. 2001;29:189–193. doi: 10.1038/ng1001-189. [DOI] [PubMed] [Google Scholar]
- 170.Ahel I., Rass U., El-Khamisy S.F., Katyal S., Clements P.M., McKinnon P.J., Caldecott K.W., West S.C. The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates. Nature. 2006;443:713–716. doi: 10.1038/nature05164. [DOI] [PubMed] [Google Scholar]
- 171.Dumitrache L.C., McKinnon P.J. Polynucleotide kinase-phosphatase (PNKP) mutations and neurologic disease. Mech. Ageing Dev. 2017;161(Pt A):121–129. doi: 10.1016/j.mad.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shen J., Gilmore E.C., Marshall C.A., Haddadin M., Reynolds J.J., Eyaid W., Bodell A., Barry B., Gleason D., Allen K. Mutations in PNKP cause microcephaly, seizures and defects in DNA repair. Nat. Genet. 2010;42:245–249. doi: 10.1038/ng.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hoch N.C., Hanzlikova H., Rulten S.L., Tétreault M., Komulainen E., Ju L., Hornyak P., Zeng Z., Gittens W., Rey S.A., Care4Rare Canada Consortium XRCC1 mutation is associated with PARP1 hyperactivation and cerebellar ataxia. Nature. 2017;541:87–91. doi: 10.1038/nature20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McKinnon P.J. Genome integrity and disease prevention in the nervous system. Genes Dev. 2017;31:1180–1194. doi: 10.1101/gad.301325.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shiloh Y., Lederman H.M. Ataxia-telangiectasia (A-T): An emerging dimension of premature ageing. Ageing Res. Rev. 2017;33:76–88. doi: 10.1016/j.arr.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 176.Stewart G.S., Maser R.S., Stankovic T., Bressan D.A., Kaplan M.I., Jaspers N.G., Raams A., Byrd P.J., Petrini J.H., Taylor A.M. The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell. 1999;99:577–587. doi: 10.1016/s0092-8674(00)81547-0. [DOI] [PubMed] [Google Scholar]
- 177.Carney J.P., Maser R.S., Olivares H., Davis E.M., Le Beau M., Yates J.R., 3rd, Hays L., Morgan W.F., Petrini J.H. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell. 1998;93:477–486. doi: 10.1016/s0092-8674(00)81175-7. [DOI] [PubMed] [Google Scholar]
- 178.Matsuura S., Tauchi H., Nakamura A., Kondo N., Sakamoto S., Endo S., Smeets D., Solder B., Belohradsky B.H., Der Kaloustian V.M. Positional cloning of the gene for Nijmegen breakage syndrome. Nat. Genet. 1998;19:179–181. doi: 10.1038/549. [DOI] [PubMed] [Google Scholar]
- 179.O’Driscoll M., Ruiz-Perez V.L., Woods C.G., Jeggo P.A., Goodship J.A. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat. Genet. 2003;33:497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- 180.Natale V. A comprehensive description of the severity groups in Cockayne syndrome. Am. J. Med. Genet. A. 2011;155A:1081–1095. doi: 10.1002/ajmg.a.33933. [DOI] [PubMed] [Google Scholar]
- 181.Laugel V. Cockayne syndrome: the expanding clinical and mutational spectrum. Mech. Ageing Dev. 2013;134:161–170. doi: 10.1016/j.mad.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 182.Vélez-Cruz R., Egly J.M. Cockayne syndrome group B (CSB) protein: at the crossroads of transcriptional networks. Mech. Ageing Dev. 2013;134:234–242. doi: 10.1016/j.mad.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 183.Ferri D., Orioli D., Botta E. Heterogeneity and overlaps in nucleotide excision repair disorders. Clin. Genet. 2019 doi: 10.1111/cge.13545. Published online March 28, 2019. [DOI] [PubMed] [Google Scholar]
- 184.Niedernhofer L.J., Garinis G.A., Raams A., Lalai A.S., Robinson A.R., Appeldoorn E., Odijk H., Oostendorp R., Ahmad A., van Leeuwen W. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 185.Iyama T., Lee S.Y., Berquist B.R., Gileadi O., Bohr V.A., Seidman M.M., McHugh P.J., Wilson D.M., 3rd CSB interacts with SNM1A and promotes DNA interstrand crosslink processing. Nucleic Acids Res. 2015;43:247–258. doi: 10.1093/nar/gku1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Marín M., Ramírez M.J., Carmona M.A., Jia N., Ogi T., Bogliolo M., Surrallés J. Functional Comparison of XPF Missense Mutations Associated to Multiple DNA Repair Disorders. Genes (Basel) 2019;10:10. doi: 10.3390/genes10010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fousteri M., Mullenders L.H. Transcription-coupled nucleotide excision repair in mammalian cells: molecular mechanisms and biological effects. Cell Res. 2008;18:73–84. doi: 10.1038/cr.2008.6. [DOI] [PubMed] [Google Scholar]
- 188.Saxowsky T.T., Doetsch P.W. RNA polymerase encounters with DNA damage: transcription-coupled repair or transcriptional mutagenesis? Chem. Rev. 2006;106:474–488. doi: 10.1021/cr040466q. [DOI] [PubMed] [Google Scholar]
- 189.Iyama T., Wilson D.M., 3rd DNA repair mechanisms in dividing and non-dividing cells. DNA Repair (Amst.) 2013;12:620–636. doi: 10.1016/j.dnarep.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Oshima J., Sidorova J.M., Monnat R.J., Jr. Werner syndrome: Clinical features, pathogenesis and potential therapeutic interventions. Ageing Res. Rev. 2017;33:105–114. doi: 10.1016/j.arr.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mukherjee S., Sinha D., Bhattacharya S., Srinivasan K., Abdisalaam S., Asaithamby A. Werner Syndrome Protein and DNA Replication. Int. J. Mol. Sci. 2018;19:19. doi: 10.3390/ijms19113442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang L., Vijg J. Somatic Mutagenesis in Mammals and Its Implications for Human Disease and Aging. Annu. Rev. Genet. 2018;52:397–419. doi: 10.1146/annurev-genet-120417-031501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Harhouri K., Frankel D., Bartoli C., Roll P., De Sandre-Giovannoli A., Lévy N. An overview of treatment strategies for Hutchinson-Gilford Progeria syndrome. Nucleus. 2018;9:246–257. doi: 10.1080/19491034.2018.1460045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hennekam R.C. Hutchinson-Gilford progeria syndrome: review of the phenotype. Am. J. Med. Genet. A. 2006;140:2603–2624. doi: 10.1002/ajmg.a.31346. [DOI] [PubMed] [Google Scholar]
- 195.Gonzalo S., Kreienkamp R., Askjaer P. Hutchinson-Gilford Progeria Syndrome: A premature aging disease caused by LMNA gene mutations. Ageing Res. Rev. 2017;33:18–29. doi: 10.1016/j.arr.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jaul E., Barron J. Age-Related Diseases and Clinical and Public Health Implications for the 85cYears Old and Over Population. Front. Public Health. 2017;5:335. doi: 10.3389/fpubh.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.De S., Rabin D.M., Salero E., Lederman P.L., Temple S., Stern J.H. Human retinal pigment epithelium cell changes and expression of alphaB-crystallin: a biomarker for retinal pigment epithelium cell change in age-related macular degeneration. Arch. Ophthalmol. 2007;125:641–645. doi: 10.1001/archopht.125.5.641. [DOI] [PubMed] [Google Scholar]
- 198.Wilson D.M., 3rd, Bohr V.A., McKinnon P.J. DNA damage, DNA repair, ageing and age-related disease. Mech. Ageing Dev. 2008;129:349–352. doi: 10.1016/j.mad.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]