Abstract
Background:
The long-term use of opioids for analgesia carries significant risk for tolerance, addiction, and diversion. These adverse effects are largely mediated by mu-opioid receptors (MORs) in the CNS. Based on our prior observation that morphine and delta-opioid receptor (DOR) agonists synergize in spinal cord in a PKCε-dependent manner, we predicted that this MOR-DOR synergy would take place in the central terminals of nociceptive afferent fibers and generalize to their peripheral terminals. Therefore, we hypothesized that loperamide, a highly efficacious MOR agonist that is excluded from the CNS, and oxymorphindole, a DOR agonist that was shown to synergize with morphine spinally, would synergistically reverse CFA-induced hyperalgesia.
Methods:
Using the Hargreaves assay for thermal nociception, the von Frey assay for mechanical nociception and the CFA-induced model of inflammatory pain, we tested the antinociceptive and anti-hyperalgesic effect of loperamide, oxymorphindole, or the loperamide-oxymorphindole combination. Animals (I.C.R. mice, n=511) received drug by either systemic injection, intraplantar injection to the injured paw, or a transdermal solution on the injured paw. Dose-response curves for each route of administration and each nociceptive test were generated, and analgesic synergy was assessed by isobolographic analysis.
Results:
In naïve animals, the loperamide-oxymorphindole combination ED50 value was 10 times lower than the theoretical additive ED50 value whether given systemically or locally. In inflamed animals, the combination was 150 times more potent systemically, and 84 times more potent locally. All combinations showed statistically significant synergy when compared to the theoretical additive values, as verified by isobolographic analysis. The anti-hyperalgesia was ablated by a peripherally-restricted opioid antagonist.
Conclusions:
From these data we conclude that the loperamide-oxymorphindole combination synergistically reverses CFA-induced inflammatory hyperalgesia. We also conclude that this interaction is mediated by opioid receptors located in the peripheral nervous system.
Introduction
Chronic pain is generally thought of as pain without apparent biological value, persisting beyond normal tissue healing time, and is not amenable to treatments based on specific remedies1. The gold standard for the treatment of chronic pain has been opioids (e.g. morphine, oxycodone etc.) that exert their analgesic effect through their interaction with the μ-opioid receptor (MOR), primarily in the CNS. Long-term use of centrally active opioids carries substantial risk for undesirable effects, namely tolerance, addiction, hyperalgesia, and respiratory depression. In the peripheral nervous system, MORs are expressed by nociceptive dorsal root ganglion neurons2, as well as the peripheral terminals of primary afferent fibers3, and are known to modulate nociceptive signaling4–7. These observations motivated development of opioid therapeutics with chemical properties either restricting their access to the CNS8,9 or optimizing their activity in inflamed peripheral tissues10. Loperamide, a mu-opioid receptor (MOR) agonist that is a substrate for the transport molecule P-glycoprotein (Pgp) and therefore unable to cross the blood-brain barrier, has been shown to have weak analgesic and anti-hyperalgesic properties on its own9,11, thought to be mediated at the peripheral terminals of afferent fibers. Loperamide is available over the counter for control of diarrhea and manifests a therapeutic index as an analgesic relative to constipation of about unity, rendering it ineffective as a pain therapeutic in humans. Fortunately, μ-opioid agonists synergize with select other analgesics when co-administered12. In combination they exhibit a statistically significant shift in potency, allowing sub-therapeutic doses of two drugs to be given to achieve full antinociception or anti-hyperalgesia. Importantly, interactions between analgesics have been shown not to cause a parallel synergy in side effects such as sedation and cardiovascular effects13. In theory, therefore, analgesic synergy may provide peripheral opioid-mediated antinociception without adverse effects in the CNS or the gut.
Our laboratory recently published that the MOR agonist morphine synergized with the δ-opioid receptor (DOR) agonist oxymorphindole14 when administered intrathecally15. It is known that opioids exert their analgesic effect partly by binding opioid receptors on the central terminals of primary afferent fibers in the spinal dorsal horn. Importantly, the synergism in this study was dependent on the epsilon isoform of protein kinase C (PKCε), an intracellular signaling molecule present in 95% of primary afferent fibers15. We reasoned that, because the synergistic interaction depended on intracellular actions, MORs and DORs must co-localize in individual neuronal compartments, possibly the central terminals of primary afferent neurons. Because primary afferents are pseudo-unipolar neurons, with terminals in both the CNS and PNS, it follows that MORs and DORs might also co-localize on the peripheral terminals of these neurons. Therefore, we investigated whether MOR-DOR synergy between loperamide and oxymorphindole could be i) localized electrophysiologically to the central terminals of nociceptors and ii) demonstrated at the peripheral terminals of nociceptors by measuring thermal withdrawal latencies using the Complete Freund’s Adjuvant (CFA)-induced mouse model of inflammatory pain. We hypothesized that the combination of loperamide and oxymorphindole would synergistically reverse CFA-induced hyperalgesia.
Materials and Methods
Animals:
For behavioral experiments, adult male I.C.R. CD1 mice (25–35 g, n=511) were housed four to a cage and maintained on a 12h light/dark cycle, with ad libitum access to food and water. Testing was performed during the light phase. The University of Minnesota Institutional Animal Care and Use Committee approved all protocols employing animals. Immediately following experiments, animals underwent CO2 euthanasia.
For electrophysiological experiments, Nav1.8-ChR2+ mice were created as described previously16. Conditional expression of channelrhodopsin-2 (ChR2) was targeted to Nav1.8+ sensory neurons by crossing heterozygous Nav1.8-ChR2 mice with wild-type C57BL/6 mice purchased from Jackson Laboratories. This cross yielded approximately 10% of offspring expressing ChR2, and Nav1.8-ChR2+ mice were identified by phenotyping for a nocifensive reaction to illumination of hind paws with 470 nm light (5 mW/mm2) from a light-emitting diode (LED, Plexon) with an attached fiber optic cable.
Spinal cord slicing procedure:
Nav1.8-ChR2+ mice (N=8) were anesthetized using an overdose of isoflurane. Transcardial perfusion was performed using oxygenated (5% CO2, 95% O2), high sucrose/kynurenic acid aCSF containing (in mM): NaCl 95, KCl 1.8, KH2PO4 1.2, CaCl2 0.5, MgSO4 7, NaHCO3 26, glucose 15, sucrose 50, and kynurenic acid 1. Spinal cords were removed via dorsal laminectomy, and the ventral and dorsal spinal roots were removed. The ventral side of the spinal cord was glued to an agar block and sliced into 400 micron sections using a vibrating microtome (Leica VT1200S). Slices were incubated at 37°C for 1 hour in oxygenated aCSF containing (in mM): NaCl 127, KCl 1.8, KH2PO4 1.2, CaCl2 2.4, MgSO4 1.3, NaHCO3 26, and glucose 15. Slices were then moved to a chamber with oxygenated aCSF at room temperature until recordings were perfomed17.
Electrophysiological recordings:
Slices were placed in a recording chamber and perfused with oxygenated aCSF at 30°C. Glass patch pipettes were filled with a solution containing (in mM): K-gluconate 135, KCl 5, MgCl2 2, CaCl2 0.5, HEPES 5, EGTA 5, ATP-Mg 5, and GTP-Na 0.5. Lamina I/II neurons were visualized using DIC optics on an Olympus BX50WI microscope, and whole cell patch clamp configuration was obtained. An Axopatch 200b amplifier (Molecular Devices, Sunnyvale CA) was used to record membrane currents at a holding potential of −65 mV. Miniature excitatory post-synaptic currents (mEPSCs) were acquired using a Digidata 1322A and PClamp 8.0 software (Molecular Devices), and mEPSC data were analyzed using MiniAnalysis 6.0.7 (Synaptosoft, Fort Lee, NJ). AMPA-mediated mEPSCs were isolated by perfusing the slice with aCSF containing 1 μM tetrodotoxin, 100 μM picrotoxin, 100 μM DL-APV, and 5 μM strychnine. To evoke glutamate release from Nav1.8-expressing primary afferent terminals, 470 nm light (0.75 mW/mm2) was continuously shone on the slice through the 40x objective. Data obtained in our lab not shown here indicate that continuous illumination of tissue results in a consistent and static increase of mEPSC frequency over a 20 minute period. Next, increasing concentrations of drug were included in the bath at 3-minute intervals to yield cumulative concentration-response curves.
Drug Preparation & Administration:
The compounds used were: loperamide HCl (Sigma, St. Louis, MO), oxymorphindole HCl14, β-funaltrexamine18, naltrindole19, and naloxone methiodide (Sigma, St. Louis, MO). Stock solutions were prepared with 5% Cremophor EL and 5% DMSO in H2O. All drugs were diluted to testing concentrations with 0.9% sterile saline (or aCSF for electrophysiology) such that final Cremophor and DMSO concentrations were <1%; the topical vehicle was further diluted 1:1 with 95% ethanol. The routes and volumes of administration were: intrathecal (i.t.), 5 μL; intraplantar (i.pl.), 30 μL; subcutaneous (s.c.), 10 μL per gram; and topical, 20 μL. Intrathecal injections were performed as previously described20. For i.pl. injections and topical administration, animals were lightly anesthetized with 2.5% isoflurane and the drugs were administered to the left hindpaw.
Behavioral Measures:
Central thermal antinociception was assessed using i.t. injections20 and the warm water (52.5°C) tail immersion assay as described previously21. Briefly, each animal was gently held wrapped in a cloth and the tail dipped into a controlled temperature water bath. Withdrawal latency was recorded as the amount of time that passed before a rapid movement of the tail; cutoff was set to 12 seconds. Baseline latency was recorded before drug administration, and subsequent latencies were recorded 7 min after each dose, immediately before the next dose. Each agonist or combination was administered sequentially approximately every 7 min in increasing doses to generate a cumulative dose–response curve; each mouse received no less than three and no more than four doses22.
Peripheral thermal nociception was assessed using the Hargreaves assay as described previously23. Briefly, animals were placed on a heated glass floor (30°C) and a small plastic box restricted their movement. After allowing the animals to acclimate to the testing environment for a minimum of 15 minutes, a radiant heat lamp was shone on the left hind paw until the animal withdrew the paw. Paw withdrawal latencies (PWLs) were measured by an IITC plantar stimulator antinociception meter, and a cutoff time of 20 seconds was used to prevent tissue damage. An average of 3 PWLs was taken, with a minimum of 30 seconds between tests. Baseline latencies were recorded before drug administration, and subsequent latencies were recorded 15 minutes after injection for i.pl. and topical experiments, and 45–60 minutes after injection for s.c. experiments. Initial dose-ranging studies were not blinded; subsequent replicate experiments with an experimenter blinded to treatment yielded similar results.
Mechanical hypersensitivity was assessed using the von Frey assay. Briefly, mice were placed on a wire mesh grid under a glass enclosure and allowed to acclimate for 30 minutes before testing. Hypersensitivity was tested by using an electronic von Frey device (Life Sciences, IITC). The tip of the stimulator was pressed to the plantar surface of both the left and right hind paws until the animal withdrew its paw from the tip, typically with a flinching behavior. The amount of force required for the response was recorded in grams by the IITC stimulator.
Respiratory depression was measured using a STARR MouseOx Plus. Briefly, animals were shaved on the back and sides of the neck, and recording collars were placed on the exposed skin. Animals were placed in beakers, where they were allowed free movement while readings were collected. Each animal was recorded for 1 hour of baseline measurements before s.c. drug or vehicle injection. Following injection, O2 saturation was measured for 1 hour24,25.
Gastrointestinal motility was measured by counting the number of fecal pellets produced. Animals were given an s.c. injection of drug or vehicle and placed in a beaker for 1 hour. After 1 hour, animals were returned to their home cages, and the number of fecal pellets was manually counted26
Freund’s Complete Adjuvant (CFA)-induced Hyperalgesia:
After determining naive PWLs, animals were lightly anesthetized using 2.5% isoflurane and an emulsion of CFA in saline (1:1, 30 μL final volume) was administered by i.pl. injection into the left hindpaw. 3–5 days after injection, a robust, inflammatory hyperalgesia was present, and hyperalgesic PWLs were determined27.
Topical tolerance paradigm:
Animals were baselined on the Hargreaves assay, and subsequently given a unilateral injection of CFA into the left hindpaw. 3 days following CFA administration, animals were randomized to two groups, receiving twice daily topical administration of vehicle or drug. After 4 days, all animals received sequential topical administrations of drug to generate cumulative concentration-response curves.
Data Analysis:
For electrophysiological experiments, data were analyzed as a % inhibition of blue-light-evoked responses, given by the equation: % inhibition = ((blue light frequency – baseline frequency) – (experimental frequency – baseline frequency))/(blue light frequency – baseline frequency)*100. For behavioral experiments with non-inflamed subjects, data were analyzed as a % of maximum possible effect (% MPE), given by the equation: % MPE = (experimental value - baseline)/(cutoff - baseline)*100. For injury models, data were analyzed as a % of anti-hyperalgesia (% AH), given by the equation: % AH = ((post-injury value - baseline)-(experimental value - baseline))/(post-injury value - baseline)*100. The ED50 of all agonists and combinations was calculated using the graded dose-response curve method28. Dose ratios for drug combinations were estimated based on comparison of previously determined single drug ED50 values. Two-tailed isobolographic analyses were performed using the numerical method29,30, as implemented by the JFlashCalc Pharmacological Calculations Program software package (http://u.arizona.edu/~michaelo/jflashcalc.html). P-values for isobolographic analysis were determined by the JFlashCalc software program. For all isobolograms, error bars for theoretical additive and observed combination ED50 values represent the vector sum of vertical and horizontal confidence limits. Error bars in isobolograms are presented as 95% confidence intervals, and error bars for all other data are presented as standard deviation (SD). For analysis between multiple groups, either an ordinary one-way ANOVA or a repeated measures two-way ANOVA was performed. ANOVA calculations were performed using GraphPad Prism 7.0, and used the Shapiro-Wilk normality test, and Bonferroni’s multiple comparisons test where applicable. For all data, p-values <0.05 were considered statistically significant. No statistical power calculation was conducted prior to the study, and the sample sizes for all experiments were based on our extensive previous experience with this design. No randomization methods were used to assign subjects to treatment groups, and animals were tested in sequential order. No animals were excluded from the study, and the data were monitored for statistical outliers, but no data were excluded from the analyses presented here.
Results
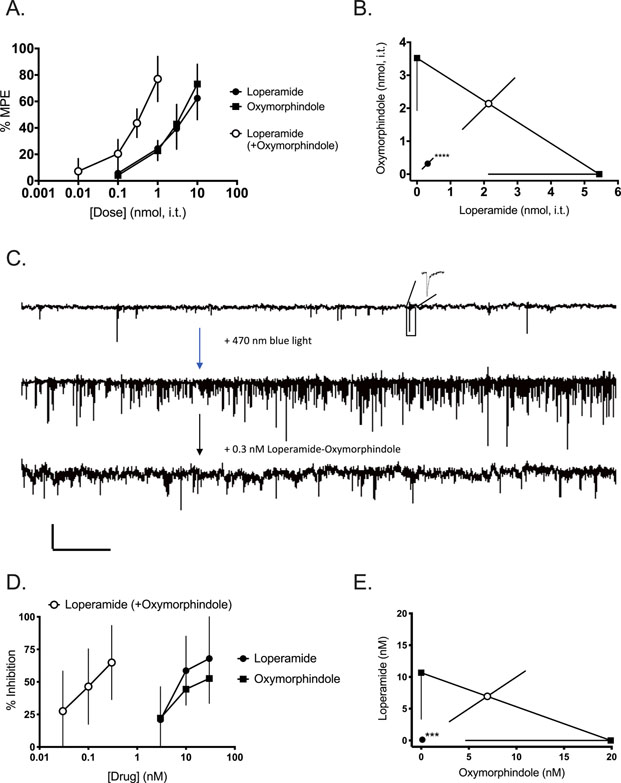
Having previously shown that morphine and oxymorphindole synergize spinally15, we first sought to assess whether loperamide would also synergize with oxymorphindole in an antinociceptive assay when given spinally. Figure 1A shows cumulative dose-response curves in naïve mice following an intrathecal injection. Either loperamide or oxymorphindole (0.1 – 10 nmol) given alone produced antinociception in the hot water tail flick assay; the 1:1 combination (0.01 – 1 nmol) was similarly effective. The ED50s of the individual drugs were 5.4 nmol (loperamide) and 3.5 nmol (oxymorphindole), and the ED50 of the combination was 0.6 nmol (n=6 per group). This measured ED50 for the combination differed significantly from the expected additive ED50 (p<0.0001), indicating that loperamide and oxymorphindole synergize when delivered spinally. This interaction is represented graphically by the isobologram in Figure 1B.
Figure 1: Loperamide and oxymorphindole synergize on primary afferents in the spinal cord.
A) Dose-response curves showing the ability of loperamide, oxymorphindole, or their 1:1 combination to block nocifensive responses in the hot water tail flick assay (n=6 animals per group). Data are shown as % maximum possible effect (MPE) ± SD. B) Isobolographic analysis of data from A, demonstrating a synergistic interaction. C) Representative traces of mEPSC frequency at baseline and following application of 470 nm blue light. Scale bars 20 pA by 2.1 s. Inset shows a representative mEPSC. D) Concentration-response curves showing the ability of loperamide, oxymorphindole, or their combination (1:1) to inhibit mEPSC frequency (n=3–6 cells per group). Data are shown as a % inhibition. E) Isobolographic analysis of the data from D, demonstrating a synergistic interaction.
*** p<0.001, **** p<0.0001.
Having demonstrated that loperamide and oxymorphindole synergized spinally, we sought to determine whether this interaction was mediated in the central terminals of primary afferent nociceptive fibers in the dorsal horn. Therefore, we conducted whole cell patch clamp recordings in spinal cord neurons located in the superficial laminae of the lumbar dorsal horn. For these recordings we used a transgenic mouse line bred to express channelrhodopsin-2 (ChR2), a light-activated cation channel, under the control of the promoter for the Nav1.8 isoform of voltage-gated sodium channels. Nav1.8 is primarily expressed by nociceptive afferents, and the majority of light-responsive fibers in this mouse line had been shown to be C polymodal nociceptors31. Therefore, we measured the frequency and amplitude of miniature excitatory post-synaptic currents (mEPSCs) driven by 470 nm light illuminating the field around the neuron being recorded through the 40x objective as a measure of presynaptic nociceptive afferent activity. Figure 1C shows representative traces of the baseline mEPSC frequency, the frequency in the presence of blue light stimulation and the frequency in the presence of blue light and the loperamide-oxymorphindole combination (0.3 nM). Figure 1D shows the cumulative concentration-response curves for loperamide, oxymorphindole or a 1:1 combination to inhibit the mEPSC frequency driven by blue light (n=3–6 cells per drug or combination, N=8). Either loperamide or oxymorphindole given alone inhibited mEPSC frequency in a concentration-dependent manner, and the combination was 100-fold more potent than either drug given alone. This shift in potency was confirmed to be synergistic by isobolographic analysis (p=0.002, Figure 1E). We interpret these data to indicate that loperamide and oxymorphindole are binding their respective receptors on the presynaptic terminals of primary afferents and inhibiting the release of glutamate from these central terminals. By the same token, we take the combination’s shift in potency to indicate that the synergy between loperamide and oxymorphindole is also mediated within these central terminals.
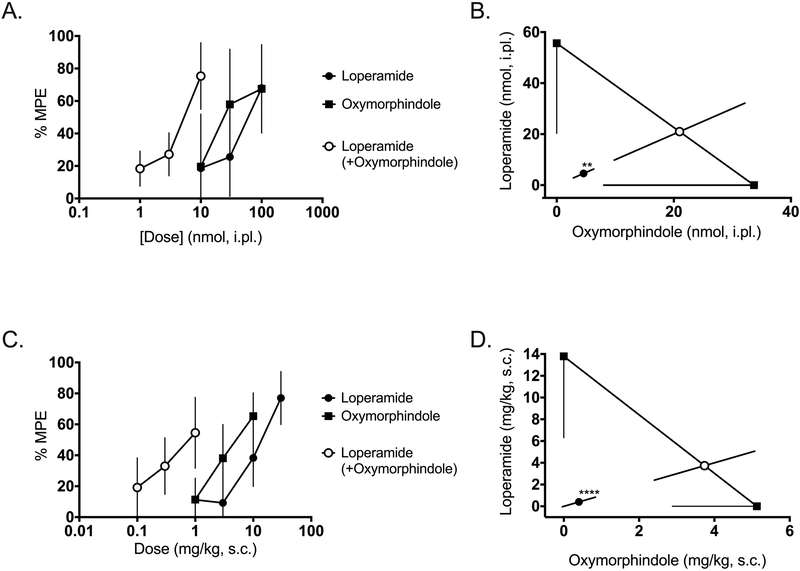
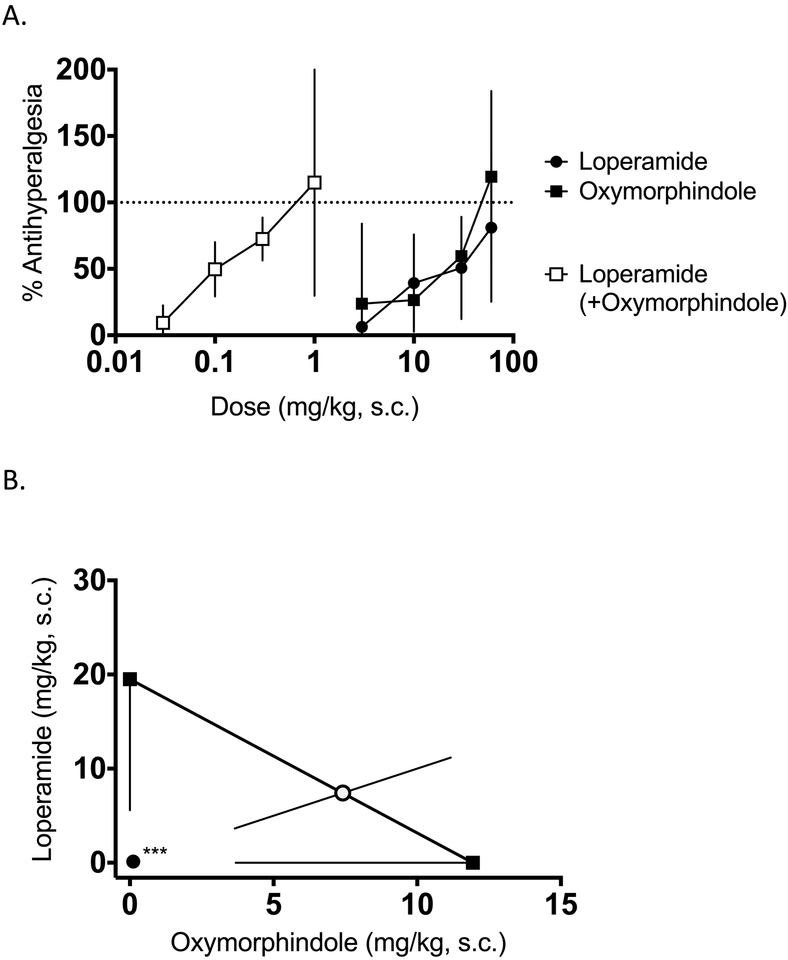
Next, we tested the hypothesis that loperamide and oxymorphindole would display antinociceptive synergy when administered in the periphery, i.e. on the peripheral terminals of nociceptors in the skin. To test this hypothesis, either drug or the 1:1 combination was given as an intraplantar injection in the hindpaw of mice, and thermal nociceptive responses were tested on the Hargreaves assay 15 minutes later. Figure 2A shows the dose-response curves for loperamide, oxymorphindole, and their 1:1 combination in naïve mice. Consistent with the interaction observed spinally, the combination ED50 is approximately 10-fold less than either drug alone: combination ED50 value 4.6 nmol vs. 57 nmol for loperamide and 34 nmol for oxymorphindole (n=6 per dose). This shift in potency was significantly synergistic (p=0.002), as demonstrated in Figure 2B. Next, we assessed the ability of systemically administered loperamide and oxymorphindole to synergize in naïve animals. Drugs were given as a subcutaneous injection, and nociceptive responses were measured on the Hargreaves assay 45 minutes later. The dose-response curves are shown in Figure 2C. With the systemic route of administration, loperamide’s and oxymorphindole’s ED50 values were 14 and 5.1 mg/kg, respectively, while the combination ED50 was 0.8 mg/kg (n=5 per dose). This interaction was statistically validated as a synergistic interaction (p<0.0001), as shown in Figure 2D, with the shift in potency remaining at approximately 10-fold.
Figure 2: Locally and systemically administered loperamide-oxymorphindole synergizes in naive animals.
A) Dose-response curves showing the ability of intraplantar loperamide, oxymorphindole or their combination (1:1) to inhibit nociceptive responses in the Hargreaves assay (n=6 per dose). Data are shown as % maximum possible effect (MPE) ± SD. B) Isobolographic analysis of the data from A, showing a synergistic interaction. C) Dose-response curves for subcutaneous loperamide, oxymorphindole, or their combination (1:1) (n=5 per dose). Data are shown as %MPE. D) Isobolographic analysis of the data from C, demonstrating a synergistic interaction.
** p<0.01, ****p<0.0001
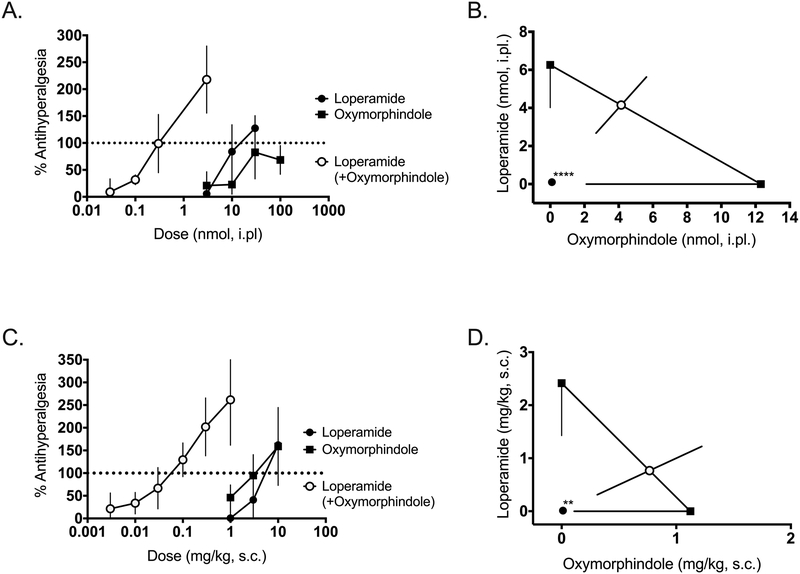
With the antinociceptive effects of the loperamide-oxymorphindole combination verified in central and peripheral terminals, and after systemic administration, we evaluated the ability of loperamide, oxymorphindole, or their combination to reverse an established inflammatory pain state. Three to five days before testing, animals were given an intraplantar injection of complete Freund’s adjuvant (CFA) in the left hindpaw, resulting in a robust inflammatory state and hyperalgesic thermal withdrawal latencies on the Hargreaves assay. Following the confirmation of hyperalgesia, animals were treated with intraplantar drug or combination as previously described. Figure 3A shows the dose-response curves for intraplantar loperamide, oxymorphindole or their combination in CFA-inflamed hindpaws. The ED50 values were 12 nmol for loperamide, 6.4 nmol for oxymorphindole and 0.1 nmol for their 1:1 combination (n=5–6 per dose). Again, this approximately 100-fold shift in potency observed for the combination was significantly different from the theoretical additive combination ED50 (Figure 3B, p<0.0001). Following the paradigm of the naive study, we next repeated this experiment with a systemic route of administration. After subcutaneous injection, the observed ED50 values for loperamide, oxymorphindole and their combination were 2.4, 1.1, and 0.01 mg/kg, respectively (n=4–6 per dose). These dose-response curves are shown in Figure 3C. The isobologram in Figure 3D demonstrates that the interaction between systemically administered loperamide and oxymorphindole in inflamed mice is also synergistic (p=0.0013), with an apparent leftward shift of 150-fold.
Figure 3: Locally and systemically administered loperamide-oxymorphindole synergizes in inflamed animals.
A) Peripherally mediated thermal nociceptive responses in the Hargreaves assay were assessed. Following CFA-induced inflammation in the hindpaw, subjects were given an intraplantar injection of loperamide, oxymorphindole or combination and post-drug nociceptive responses were shown as % anti-hyperalgesia, which was used to generate dose-response curves. B) Isobolographic analysis of the data from A, showing a synergistic interaction compared to the theoretical additive ED50 value. C) Dose-response curves for subcutaneous loperamide, oxymorphindole or combination following CFA-induced inflammation in the hindpaw. Data are shown as % anti-hyperalgesia. D) Isobolographic analysis of the data from C, demonstrating a synergistic interaction compared to the theoretical additive ED50 value.
** p<0.01, *** p<0.001
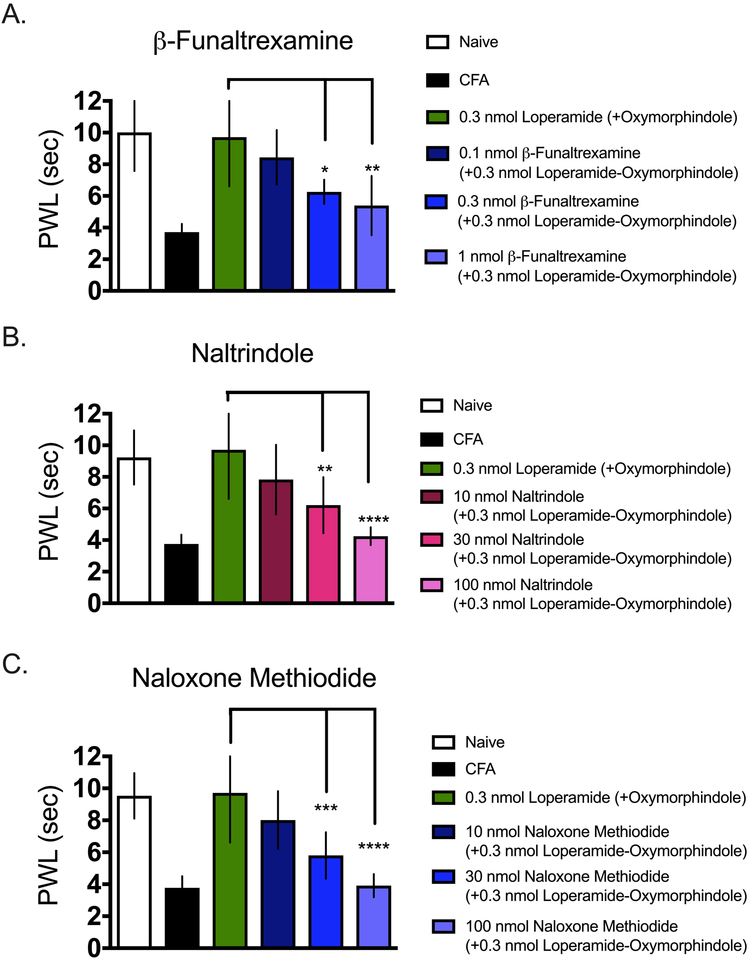
To confirm that the antinociceptive and anti-hyperalgesic effects observed in the previous experiments were being mediated by action at MORs and DORs, we tested the ability of a panel of opioid antagonists to block the synergism in in CFA-inflamed mice. We chose naloxone methiodide, a pan-opioid antagonist that is peripherally restricted; naltrindole, a DOR-selective antagonist; and β-funaltrexamine, a MOR-selective antagonist (n=5–6 per dose). Naltrindole and naloxone methiodide were co-administered as an intraplantar injection with 0.3 nmol of the loperamide-oxymorphindole combination. β-funaltrexamine was administered as an intraplantar injection 24 hours before the combination. Animals were tested on the Hargreaves assay 15 minutes after receiving the loperamide-oxymorphindole combination. When naloxone methiodide was co-administered with loperamide-oxymorphindole, there was a statistically significant reduction in anti-hyperalgesia, as calculated by ordinary one-way ANOVA (p<0.0001, F(5, 44 = 30.91)). When we tested loperamide-oxymorphindole with naltrindole, we observed similar results. Co-administration of the combination with naltrindole resulted in a statistically significant reduction in anti-hyperalgesia (ordinary one-way ANOVA, p<0.0001, F(5, 44) = 23.04). Finally we pretreated animals with β-funaltrexamine 24-h before loperamide-oxymorphindole administration, and in keeping with the other antagonists, we observed a statistically significant reduction in anti-hyperalgesia (ordinary one-way ANOVA, p<0.0001, F(5, 44) = 20.59). The degree to which all the antagonists reversed loperamide-oxymorphindole’s anti-hyperalgesia was dose-dependent. These data are presented in Figure 4. We interpret these data to indicate that the antinociceptive and anti-hyperalgesic effects of the loperamide-oxymorphindole combination are mediated by peripheral MORs and DORs.
Figure 4: Antagonism of locally-administered loperamide-oxymorphindole.
Paw withdrawal thresholds using the Hargreaves assay were measured for naïve animals, inflamed animals, and animals treated with an intraplantar injection of 0.3 nmol loperamide and oxymorphindole. A) Ability of β-funaltrexamine, an irreversible MOR antagonist, to inhibit loperamide-oxymorphindole anti-hyperalgesia. Three different doses of β-funaltrexamine were given 24 hours before loperamide-oxymorphindole as an intraplantar injection. B) Ability of naltrindole, a DOR antagonist, to inhibit loperamide-oxymorphindole anti-hyperalgesia. Increasing doses of naltrindole were given concurrently with 0.3 nmol of loperamide-oxymorphindole as an intraplantar injection. C) Ability of naloxone methiodide, a peripherally restricted opioid antagonist, to inhibit loperamide-oxymorphindole anti-hyperalgesia. Increasing doses of naloxone methiodide were given concurrently with 0.3 nmol loperamide-oxymorphindole as an intraplantar injection. A,B,C) Paw withdrawal thresholds were measured using the Hargreaves assay and antagonist data were compared to 0.3 nmol loperamide-oxymorphindole using ordinary one-way ANOVA with Bonferroni’s test for multiple comparisons.
* p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
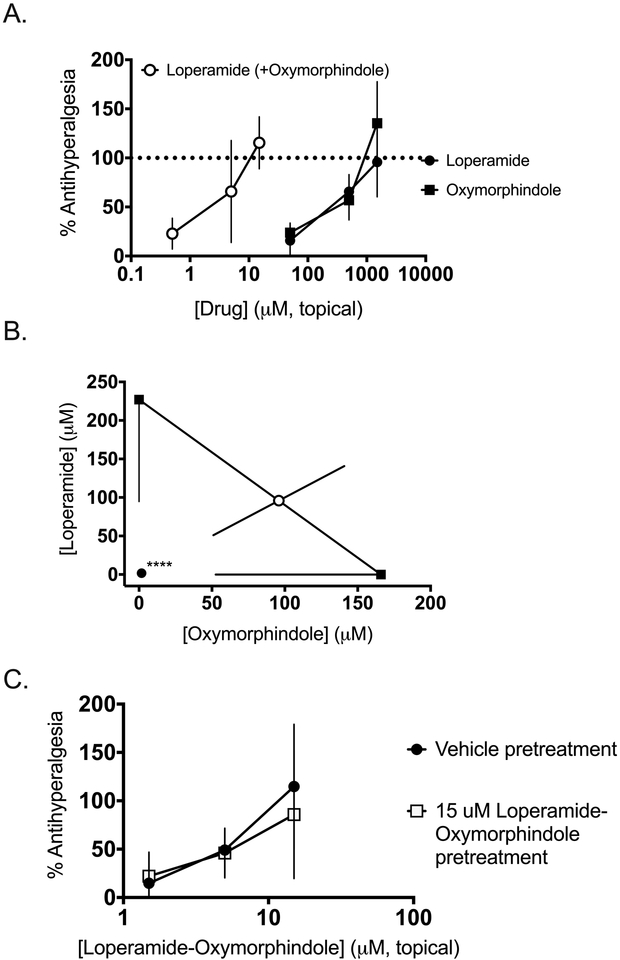
In order to refine the localization of the synergy between loperamide and oxymorphindole, we administered the drugs alone or in combination as topical solutions in CFA-inflamed animals. As shown in Figure 5A, loperamide and oxymorphindole showed similar potencies as topical solutions (in a 50% ethanol:50% water mixture), with EC50 values in this preparation of 230 and 170 μM, respectively. When combined, the shift in potency was comparable to that with intraplantar administration, with a combination EC50 of 3.4 μM, which corresponds to an approximately 50-fold shift in potency. This synergistic interaction was validated by isobolographic analysis (n=6 per dose, p<0.0001), as shown in Figure 5B. Taken together, these data strongly suggest a cutaneous site of action for the combination, potentially in the epidermis.
Figure 5: Topically administered loperamide-oxymorphindole synergizes in inflamed animals.
A) Peripherally mediated thermal nociceptive responses in the Hargreaves assay were assessed. Subjects were given a topical solution of loperamide, oxymorphindole or combination on the inflamed hindpaw and post-drug responses are shown as % anti-hyperalgesia, which was used to generate concentration-response curves. B) Isobolographic analysis of the data from A, showing a synergistic interaction compared to the theoretical additive value. **** p<0.0001. C) Anti-hyperalgesic tolerance to repeated administrations of topical loperamide-oxymorphindole was assessed. Subjects were given twice daily topical treatments of either vehicle or 15 μM loperamide-oxymorphindole for 3 days. On the fourth day, all animals received increasing concentrations of loperamide-oxymorphindole to generate cumulative dose-response curves.
Given that peripheral MORs have been recently implicated in the development of analgesic tolerance to classical opioids such as mophine32, we wanted to test whether the topically-delivered combination of loperamide-oxymorphindole would induce analgesic tolerance. Three days before testing, animals (n=6 per group) were given an intraplantar injection of complete Freund’s adjuvant (CFA) in the left hindpaw, resulting in a robust inflammatory state and hyperalgesic thermal withdrawal latencies on the Hargreaves assay. Then animals received twice daily topical administrations of vehicle or 15 μM loperamide-oxymorphindole for four days. 24 hours after the last administration, all animals received increasing concentrations of topically-administered loperamide-oxymorphindole and cumulative concentration-response curves were generated (Figure 5C). Data from this experiment were analyzed by repeated measures two-way ANOVA, and animals that received twice daily loperamide-oxymorphindole did not show a statistically significant difference from animals that received twice daily vehicle (F(1, 5) = 0.4582, p=0.53 for treatment). Therefore, we conclude that repeated topical administration of loperamide-oxymorphindole does not induce analgesic tolerance in animals with inflammatory pain.
To rule out the possibility that the observed synergy in the above experiments was unique to radiant heat stimulation, we tested the ability of loperamide, oxymorphindole, and the loperamide-oxymorphindole combination to reverse CFA-induced hypersensitivity as measured by the von Frey mechanical sensitivity assay. Baseline measurements of mechanical withdrawal thresholds were taken before CFA administration, and three to five days before testing, animals were given an intraplantar injection of complete Freund’s adjuvant (CFA) in the left hindpaw. Post-injury measurements confirmed that CFA administration reduced mechanical withdrawal thresholds only on the ipsilateral hindpaw. Following the confirmation of hyperalgesia, animals were treated with subcutaneous drug or combination as previously described, and post-treatment von Frey measurements were recorded. Figure 6A shows the dose-response curves for loperamide, oxymorphindole, or their combination (1:1) in CFA-inflamed hindpaws. In this assay, the observed ED50 values were 20 mg/kg for loperamide, 12 mg/kg for oxymorphindole, and 0.1 mg/kg for the combination. This interaction was confirmed to be statistically significant synergy, and the resulting isobologram is shown in Figure 6B (p=0.0002). The magnitude of the potency shift mirrored what was observed using the Hargreaves assay, with the combination being approximately 100 times more potent than either drug alone. Therefore, the synergistic effect following loperamide-oxymorphindole administration is not specific to heat hyperalgesia, but generalizes to mechanical hypersensitivity as well.
Figure 6: Systemically administered loperamide-oxymorphindole synergistically reverses mechanical hypersensitivity in inflamed animals.
A) Peripherally mediated mechanical nociceptive responses in the von Frey assay were assessed. Following CFA-induced inflammation in the left hindpaw, subjects were given a subcutaneous injection of loperamide, oxymorphindole or combination and post-drug mechanical sensitivity responses were analyzed as % anti-hyperalgesia, which was used to generate dose-response curves. B) Isobolographic analysis of the data from A, showing a synergistic interaction compared to the theoretical additive ED50 value.
*** p<0.001
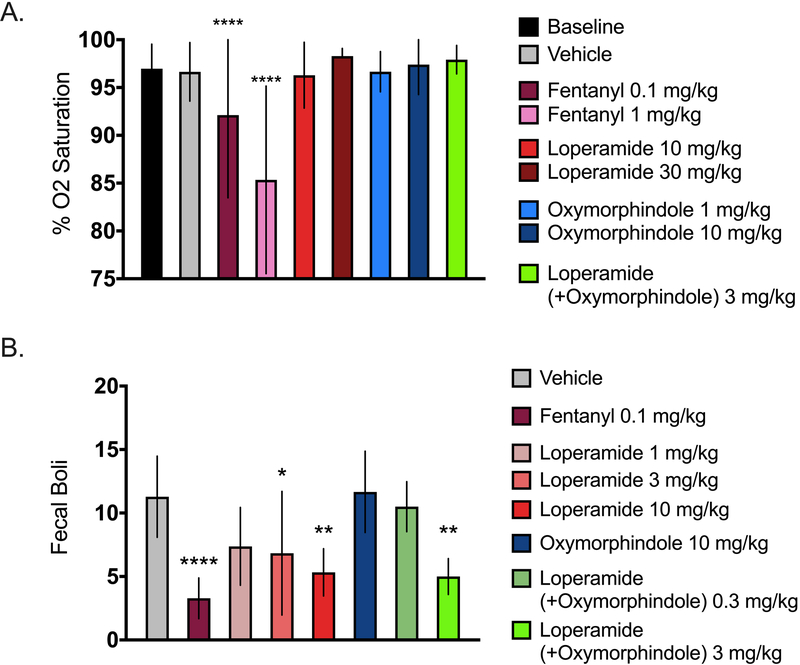
Finally, we measured whether the combination of loperamide and oxymorphindole demonstrated typical acute opioid-induced side effects, namely respiratory depression and constipation. To test for respiratory depression, mice were given a subcutaneous injection of vehicle, fentanyl, or the loperamide-oxymorphindole combination, and vital signs were monitored using a pulse oximeter. As a positive control, fentanyl reduced arterial oxygen saturation in a dose-dependent and statistically significant manner (ordinary one-way ANOVA with Bonferroni’s test for multiple comparisons, p=0.004, F(8, 63) = 3.268). Neither loperamide, oxymorphindole, nor their combination showed any statistically significant reduction of arterial oxygen saturation from baseline measurements (Figure 7A, n=8 per group). Therefore, we conclude that the loperamide-oxymorphindole combination has a significantly lower risk for centrally-mediated adverse effects. We also assessed the loperamide-oxymorphindole combination for reduction of GI transit, which is an expected effect for loperamide as well as other MOR agonists. Following subcutaneous injection of vehicle, fentanyl, loperamide, oxymorphindole, or the loperamide-oxymorphindole combination, fecal boli were counted for 1 hour (n=6 per group). The data are summarized in Figure 7B and were analyzed by ordinary one-way ANOVA with Bonferroni’s test for multiple comparisons (p<0.0001, F(7, 44) = 7.869). Whereas a high dose of loperamide or the combination did show a constipating effect, an anti-hyperalgesic dose of the combination did not; this result suggests a therapeutic window of the combination with respect to constipation of 3–10. Loperamide and fentanyl predictably caused constipation, but oxymorphindole alone had no effect, consistent with previous work showing that MORs, but not DORs, contribute to inhibition of GI transit33.
Figure 7: Acute safety profile of loperamide-oxymorphindole.
A) Arterial blood oxygenation was continuously monitored using a STARR MouseOx Plus to assess respiratory effects. After acclimation and baseline readings, animals were administered either saline, or increasing doses of fentanyl, loperamide, oxymorphindole, or loperamide-oxymorphindole, and SpO2 readings were recorded. Data were analyzed by ordinary one-way ANOVA with Bonferroni’s test for multiple comparisons. **** p<0.0001 vs. baseline. B) Constipation was assessed by counting fecal boli produced for 1 hour after drug or saline administration. Data were analyzed by ordinary one-way ANOVA with Bonferroni’s test for multiple comparisons.
* p<0.05, ** p<0.01, **** p<0.0001 vs. vehicle.
Discussion
The results presented here clearly demonstrate that analgesic synergy between opioid agonists can be mediated by the peripheral nervous system without the involvement of spinal cord circuitry. By using a MOR agonist that is unable to penetrate the CNS together with local routes of administration, we have shown that the involvement of central opioid receptors is not necessary to achieve either robust antinociception in naïve animals, or anti-hyperalgesia in a mouse model of inflammatory pain. It is well known that the analgesic effect of opioids is enhanced following tissue injury6,7,34,35, MORs are upregulated36 , and the binding efficiency of peripheral MORs increases37. Similar results have also been reported concerning the up-regulation and trafficking of the DOR following inflammation38. In humans, a meta-analysis of peripherally-delivered opioids for post-operative pain found that preoperative inflammation was a key factor in determining post-operative analgesic outcomes39. Accordingly, we report here that the potencies of both loperamide and oxymorphindole were increased following inflammation, such that ED50 values following CFA are approximately 3 to 6-fold lower in inflamed than in naïve mice. This holds true for both drugs, as well as for both intraplantar and subcutaneous routes of administration. Therefore, the present results confirm that inflammation potentiates the peripheral analgesic action of both MOR and DOR agonists.
Interestingly, inflammation also magnified the synergism observed following co-administration of loperamide and oxymorphindole. For example, when the combination was administered as an intraplantar injection in naïve mice, the shift in potency from the single drug ED50 value to the combination ED50 value was approximately 10-fold. By contrast, in animals that had been previously inflamed with CFA, the intraplantar synergy was approximately 100-fold. When the drugs were given subcutaneously, the shifts in naïve and inflamed animals were approximately 10-fold and 150-fold, respectively. Because the synergistic shifts are relative to the single drugs in either injury state, the increased potency of the loperamide and/or oxymorphindole alone following inflammation is not sufficient to explain this additional potentiation.
One potential mechanism for this increased synergism is that the loperamide-oxymorphindole combination exerts its pharmacodynamic action through MOR-DOR heteromers, and the increased expression of MORs and DORs at the cell membrane following inflammation allows for the formation of more heterodimers. In naïve animals, anywhere from 29–38% of unmyelinated sensory axons at the dermal-epidermal junction express either MORs or DORs40, and it follows that the increases in expression of MORs and DORs after inflammation would provide more opportunities for heterodimerization. There is in vivo evidence indicating that co-expressing MORs and DORs allows for interactions between co-administered agonists, and that MOR-DOR heteromers activate different downstream signaling pathways41–43. These findings are supported by pharmacological studies examining the ability of bivalent ligands—molecules consisting of two pharmacophores connected by a chemical linker—to selectively activate putative heteromers. For example, Daniels et al. showed that a series of bivalent ligands with a MOR agonist and a DOR antagonist pharmacophore separated by different linker lengths caused different pharmacologic effects depending on the spacer length44. There was a specific spacer length that resulted in a 10-fold increase in potency and mitigated the development of tolerance, which is suggestive of a receptor pair that is able to stabilize in multiple conformations and utilize different signaling pathways. This idea is borne out by the finding that co-administration of the two monovalent pharmacophores did not recapitulate the analgesic effect of the bivalent ligand, as well as a recent finding from our laboratories that showed that different DOR agonists co-delivered with morphine activate different signaling cascades15,44. In rhesus monkeys, the co-administration of naltrindole with classical MOR-selective agonists causes a rightward shift in potency, suggesting that the MOR agonists are at least partially exerting their analgesic effect through MOR-DOR heteromers45. In contrast to the above studies, Scherrer et al. have shown that MORs and DORs are not expressed in the same sensory neurons, and differentially modulated mechanical and heat pain46, which is in direct conflict with studies asserting the co-expression of MOR and DOR in the same cell47. Therefore, additional work is necessary to fully understand the mechanism underlying inflammation’s role in enhancing loperamide-oxymorphindole synergy.
With regard to the antinociceptive mechanism of action of the loperamide-oxymorphindole combination, the data presented above reinforce the idea that binding to opioid receptors located on primary afferent neurons is required for antinociception. Either β-FNA, a MOR antagonist, or naltrindole, a DOR antagonist, was able to reverse the anti-hyperalgesic effect of the loperamide-oxymorphindole combination in a dose-dependent manner. Importantly, because the peripherally-restricted antagonist, naloxone methiodide, completely ablated the anti-hyperalgesia, we interpret these data to confirm that the synergistic interaction between loperamide and oxymorphindole is being mediated by MORs and DORs in the peripheral nervous system, and not in the spinal cord or other supraspinal opioid-targeting regions. The action at primary afferents is supported by our electrophysiological data, which suggest that loperamide and oxymorphindole both act and synergize at presynaptic terminals of Nav1.8-expressing neurons innervating the dorsal horn. A report published in 2017 on the mouse line used for those experiments examined the in vivo response properties of cutaneous nociceptors that were responsive to blue light. The authors found that 77% of C-fibers responsive to blue light were also activated by mechanical and thermal stimuli, suggesting that most Nav1.8-expressing primary afferents are C polymodal nociceptors31. That the profound synergy observed after intraplantar delivery generalized to the topical route indicates that the anti-hyperalgesic action of the combination is manifest superficially in the skin and suggests that local topical application to painful areas could prove to be clinically useful. The experimental characterization of topical opioids has precedent48, and we have extended that logic to incorporate synergism between topically applied opioids. Taking these data into account, we conclude that MOR and DOR action at the level of primary afferent nociceptors mediates the analgesic effect of the loperamide-oxymorphindole combination.
Finally, recent efforts to develop new opioid therapeutics have focused on reducing or eliminating classic opioid adverse effects, for example, tolerance, respiratory depression and addiction. One popular method for mitigating opioid toxicity is to attempt to synthesize agonists at the MOR that preferentially activate G protein-mediated signaling pathways. The hypothesis underlying that research is that β-arrestin signaling is responsible for the non-analgesic effects of MOR agonists. For example, a 2016 paper in Nature reported the structure-based optimization of PZM21, a MOR agonist that robustly activated Gi, but not β-arrestin49. In that report, the authors support the conclusion that biased agonism will reduce opioid toxicity with data showing that PZM21 is an effective analgesic that is devoid of both respiratory depression and morphine-like reinforcing activity in mice at analgesic doses. Alternatively, we have recently shown that analgesic synergy also represents an effective method of limiting side effects. Stone et al. published data establishing that morphine and clonidine, an α2A adrenergic receptor agonist, syngergized in their analgesic effect, but not in sedative or cardiovascular effects13. The data presented here corroborate that finding, demonstrating that the loperamide-oxymorphindole combination, in contrast to the prescription opioid fentanyl, does not cause respiratory depression or constipation at therapeutic doses (e.g. 0.3 mg/kg). Additionally, the loperamide-oxymorphindole combination did not cause respiratory depression at a supratherapeutic dose (e.g. 3 mg/kg). The same supratherapeutic dose did cause some constipation due to the dose of loperamide in the combination being of the same order of magnitude as its constipatory dose alone50. Overall, because loperamide is restricted from the CNS, and we are able to drastically lower the doses given to achieve antinociception with synergy, these data uphold the idea that peripherally mediated opioid synergy is a safe and effective strategy for further development. There are currently no peripherally restricted opioid analgesics on the market for treatment of acute or chronic pain. The current report may represent an initial step toward the development of peripherally restricted, synergistic analgesic combination pharmacotherapies for moderate to severe inflammatory pain.
Table 1.
ED50 Values of Loperamide, Oxymorphindole, and Their Combination Compared with the Theoretical Additive ED50 Values by Three Routes of Administration in Naive and Inflamed States.
| Systemic ED50, mg/kg (Mean, 95% CL) |
Intraplantar ED50, nmol (Mean, 95% CL) |
Topical EC50, μM (Mean, 95% CL) |
||||
|---|---|---|---|---|---|---|
| Naïve | Inflamed | Naïve | Inflamed | Naïve | Inflamed | |
| Loperamide | 14 (6.3 – 22) |
2.4 (1.4 – 3.4) |
57 (15 −99) |
6.4 (4 – 8.7) |
n.d. | 227 (94.7 – 359) |
| Oxymorphindole | 5.1 (2.9 – 7.4) |
1.1 (0.1 – 2.1) |
34 (8 – 59) |
12 (2.1 – 23) |
n.d. | 166 (52.6 – 279) |
| Loperamide + Oxymorphindole (1:1 ratio) | 0.8* (−0.08 – 1.7) |
0.01*
(0.004 – 0.02) |
4.6*
(2.9 – 6.3) |
0.1*
(0.06 – 0.1) |
n.d. | 3.4*
(0.5 – 6.4) |
| Theoretical additive | 7.5 (4.8 – 10) |
1.5 (0.6 – 2.4) |
42 (19 – 65) |
8.4 (5.4 – 11) |
n.d. | 192 (102 – 282) |
Statistically significant difference between experimentally observed combination ED50 and the theoretical additive value, p<0.05 by JFlashCalc isobolographic analysis. n.d., not done.
Acknowledgements:
We thank Dr. Michael Ossipov for providing access to the JFlashCalc software and Dr. Philippe Seguela for providing Nav1.8-ChR2 breeder mice.
Funding Statement: The University of Minnesota PharmacoNeuroImmunology Training Grant, T32 DA007097–32, supported DJB. NIDA R01 DA 015438–10 to G.L.W. supported initial studies. We also acknowledge preclinical development grants from the University of Minnesota Committee on Pharmaceutical Development (CPD) and MN-REACH Program (to GLW). GLW holds the R.W. Goltz Professorship in Dermatology, which also provided support.
Footnotes
Prior Presentations: University of Minnesota PharmacoNeuroImmunology Seminar, May 2015; International Narcotics Research Conference (INRC), July 2016, Bath, UK; International Association for the Study of Pain (IASP) Symposium, September 2016, Yokohama, Japan; Society for Neuroscience annual meeting, November 12, 2016, San Diego, CA; Society for Neuroscience annual meeting, November 11, 2017 Washington, DC; Montagna Symposium on the Biology of Skin, October 22, 2016, Gleneden Beach, OR; Winter Conference on Brain Research, January 2017, Big Sky MT; Virginia Commonwealth University, Department of Pharmacology and Toxicology Seminar, April 2017, Richmond VA; American Pain Society Symposium, May 2017, Pittsburgh PA; IASP Special Meeting Symposium, October 2017, Santa Cruz, Bolivia; Pain Mechanisms and Therapeutics Conference, June 5, 2016 and June 2, 2018, Taormina, Sicily, Italy.
Clinical trial number and registry URL: Not applicable
Conflicts of Interest: D.J. Bruce, E. Akgün, P.S. Portoghese, C.A. Fairbanks, and G.L. Wilcox have a related international patent pending. An International Patent Application was published under the Patent Cooperation Treaty (PCT) on 28 September 2017: International Publication Number WO 2017/165558 A1. Combination for treating pain. The invention provides compositions and methods that can be used to treat pain with reduced CNS side effects like addiction liability and respiratory depression. The remaining authors declare no competing interests.
References
- 1.Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl 1986; 3: S1–226 [PubMed] [Google Scholar]
- 2.Werz MA, MacDonald RL: Opioid peptides selective for mu- and delta-opiate receptors reduce calcium-dependent action potential duration by increasing potassium conductance. Neurosci Lett 1983; 42: 173–8 [DOI] [PubMed] [Google Scholar]
- 3.Parsons CG, Czlonkowski A, Stein C, Herz A: Peripheral opioid receptors mediating antinociception in inflammation. Activation by endogenous opioids and role of the pituitary-adrenal axis. Pain 1990; 41: 81–93 [DOI] [PubMed] [Google Scholar]
- 4.Schafer M, Imai Y, Uhl GR, Stein C: Inflammation enhances peripheral mu-opioid receptor-mediated analgesia, but not mu-opioid receptor transcription in dorsal root ganglia. Eur J Pharmacol 1995; 279: 165–9 [DOI] [PubMed] [Google Scholar]
- 5.Stein C, Clark JD, Oh U, Vasko MR, Wilcox GL, Overland AC, Vanderah TW, Spencer RH: Peripheral mechanisms of pain and analgesia. Brain Res Rev 2009; 60: 90–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenk HN, Brederson JD, Honda CN: Morphine directly inhibits nociceptors in inflamed skin. J Neurophysiol 2006; 95: 2083–97 [DOI] [PubMed] [Google Scholar]
- 7.Brederson JD, Honda CN: Primary afferent neurons express functional delta opioid receptors in inflamed skin. Brain Res 2015; 1614: 105–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bileviciute-Ljungar I, Spetea M, Guo Y, Schutz J, Windisch P, Schmidhammer H: Peripherally mediated antinociception of the mu-opioid receptor agonist 2-[(4,5alpha-epoxy-3-hydroxy-14beta-methoxy-17-methylmorphinan-6beta-yl)amino]ace tic acid (HS-731) after subcutaneous and oral administration in rats with carrageenan-induced hindpaw inflammation. J Pharmacol Exp Ther 2006; 317: 220–7 [DOI] [PubMed] [Google Scholar]
- 9.DeHaven-Hudkins DL, Cowan A, Cortes Burgos L, Daubert JD, Cassel JA, DeHaven RN, Kehner GB, Kumar V: Antipruritic and antihyperalgesic actions of loperamide and analogs. Life Sci 2002; 71: 2787–96 [DOI] [PubMed] [Google Scholar]
- 10.Spahn V, Del Vecchio G, Labuz D, Rodriguez-Gaztelumendi A, Massaly N, Temp J, Durmaz V, Sabri P, Reidelbach M, Machelska H, Weber M, Stein C: A nontoxic pain killer designed by modeling of pathological receptor conformations. Science 2017; 355: 966–969 [DOI] [PubMed] [Google Scholar]
- 11.Nozaki-Taguchi N, Yaksh TL: Characterization of the antihyperalgesic action of a novel peripheral mu-opioid receptor agonist--loperamide. Anesthesiology 1999; 90: 225–34 [DOI] [PubMed] [Google Scholar]
- 12.Chabot-Dore AJ, Schuster DJ, Stone LS, Wilcox GL: Analgesic synergy between opioid and alpha2 -adrenoceptors. Br J Pharmacol 2015; 172: 388–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone LS, German JP, Kitto KF, Fairbanks CA, Wilcox GL: Morphine and clonidine combination therapy improves therapeutic window in mice: synergy in antinociceptive but not in sedative or cardiovascular effects. PLoS One 2014; 9: e109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Portoghese PS, Sultana M, Nagase H, Takemori AE: Application of the message-address concept in the design of highly potent and selective non-peptide delta opioid receptor antagonists. J Med Chem 1988; 31: 281–2 [DOI] [PubMed] [Google Scholar]
- 15.Schuster DJ, Metcalf MD, Kitto KF, Messing RO, Fairbanks CA, Wilcox GL: Ligand requirements for involvement of PKCepsilon in synergistic analgesic interactions between spinal mu and delta opioid receptors. Br J Pharmacol 2015; 172: 642–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daou I, Tuttle AH, Longo G, Wieskopf JS, Bonin RP, Ase AR, Wood JN, De Koninck Y, Ribeiro-da-Silva A, Mogil JS, Seguela P: Remote optogenetic activation and sensitization of pain pathways in freely moving mice. J Neurosci 2013; 33: 18631–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doolen S, Blake CB, Smith BN, Taylor BK: Peripheral nerve injury increases glutamate-evoked calcium mobilization in adult spinal cord neurons. Mol Pain 2012; 8: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Portoghese PS, Larson DL, Sayre LM, Fries DS, Takemori AE: A novel opioid receptor site directed alkylating agent with irreversible narcotic antagonistic and reversible agonistic activities. J Med Chem 1980; 23: 233–4 [DOI] [PubMed] [Google Scholar]
- 19.Portoghese PS, Sultana M, Takemori AE: Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol 1988; 146: 185–6 [DOI] [PubMed] [Google Scholar]
- 20.Hylden JL, Wilcox GL: Intrathecal morphine in mice: a new technique. Eur J Pharmacol 1980; 67: 313–6 [DOI] [PubMed] [Google Scholar]
- 21.D’Amour FE, Smith DL: A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther 1941; 72: 74 [Google Scholar]
- 22.Shin SW, Eisenach JC: Intrathecal morphine reduces the visceromotor response to acute uterine cervical distension in an estrogen-independent manner. Anesthesiology 2003; 98: 1467–71; discussion 6A [DOI] [PubMed] [Google Scholar]
- 23.Hargreaves K, Dubner R, Brown F, Flores C, Joris J: A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88 [DOI] [PubMed] [Google Scholar]
- 24.Early MA, Lishnevsky M, Gilchrist JM, Higgins DM, Orme IM, Muller WA, Gonzalez-Juarerro M, Schenkel AR: Non-invasive diagnosis of early pulmonary disease in PECAM-deficient mice using infrared pulse oximetry. Exp Mol Pathol 2009; 87: 152–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daijo H, Kai S, Tanaka T, Wakamatsu T, Kishimoto S, Suzuki K, Harada H, Takabuchi S, Adachi T, Fukuda K, Hirota K: Fentanyl activates hypoxia-inducible factor 1 in neuronal SH-SY5Y cells and mice under non-hypoxic conditions in a mu-opioid receptor-dependent manner. Eur J Pharmacol 2011; 667: 144–52 [DOI] [PubMed] [Google Scholar]
- 26.Wade PR, Palmer JM, McKenney S, Kenigs V, Chevalier K, Moore BA, Mabus JR, Saunders PR, Wallace NH, Schneider CR, Kimball ES, Breslin HJ, He W, Hornby PJ: Modulation of gastrointestinal function by MuDelta, a mixed micro opioid receptor agonist/ micro opioid receptor antagonist. Br J Pharmacol 2012; 167: 1111–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newbould BB: Chemotherapy of Arthritis Induced in Rats by Mycobacterial Adjuvant. Br J Pharmacol Chemother 1963; 21: 127–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tallarida RJ, Murray RB : Graded Dose-response. New York, Springer, 1987 [Google Scholar]
- 29.Tallarida RJ: Statistical analysis of drug combinations for synergism. Pain 1992; 49: 93–7 [DOI] [PubMed] [Google Scholar]
- 30.Ossipov MH, Lopez Y, Bian D, Nichols ML, Porreca F: Synergistic antinociceptive interactions of morphine and clonidine in rats with nerve-ligation injury. Anesthesiology 1997; 86: 196–204 [DOI] [PubMed] [Google Scholar]
- 31.Uhelski ML, Bruce DJ, Seguela P, Wilcox GL, Simone DA: In vivo optogenetic activation of Nav1.8+ cutaneous nociceptors and their responses to natural stimuli. J Neurophysiol 2017; 117: 2218–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, Sotoudeh C, Clark JD, Barres BA, Bohlen CJ, Scherrer G: Loss of mu opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat Med 2017; 23: 164–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shook JE, Lemcke PK, Gehrig CA, Hruby VJ, Burks TF: Antidiarrheal properties of supraspinal mu and delta and peripheral mu, delta and kappa opioid receptors: inhibition of diarrhea without constipation. J Pharmacol Exp Ther 1989; 249: 83–90 [PubMed] [Google Scholar]
- 34.Stein C, Machelska H, Schafer M: Peripheral analgesic and antiinflammatory effects of opioids. Z Rheumatol 2001; 60: 416–24 [DOI] [PubMed] [Google Scholar]
- 35.Stein C, Zollner C: Opioids and sensory nerves. Handb Exp Pharmacol 2009: 495–518 [DOI] [PubMed] [Google Scholar]
- 36.Ji RR, Zhang Q, Law PY, Low HH, Elde R, Hokfelt T: Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J Neurosci 1995; 15: 8156–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zollner C, Shaqura MA, Bopaiah CP, Mousa S, Stein C, Schafer M: Painful inflammation-induced increase in mu-opioid receptor binding and G-protein coupling in primary afferent neurons. Mol Pharmacol 2003; 64: 202–10 [DOI] [PubMed] [Google Scholar]
- 38.Cahill CM, Morinville A, Hoffert C, O’Donnell D, Beaudet A: Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain 2003; 101: 199–208 [DOI] [PubMed] [Google Scholar]
- 39.Nielsen BN, Henneberg SW, Schmiegelow K, Friis SM, Romsing J: Peripherally applied opioids for postoperative pain: evidence of an analgesic effect? A systematic review and meta-analysis. Acta Anaesthesiol Scand 2015; 59: 830–45 [DOI] [PubMed] [Google Scholar]
- 40.Coggeshall RE, Zhou S, Carlton SM: Opioid receptors on peripheral sensory axons. Brain Res 1997; 764: 126–32 [DOI] [PubMed] [Google Scholar]
- 41.Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA: A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A 2004; 101: 5135–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O’Dowd BF: Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem 2000; 275: 26128–35 [DOI] [PubMed] [Google Scholar]
- 43.Rozenfeld R, Devi LA: Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J 2007; 21: 2455–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS: Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci U S A 2005; 102: 19208–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yekkirala AS, Banks ML, Lunzer MM, Negus SS, Rice KC, Portoghese PS: Clinically employed opioid analgesics produce antinociception via mu-delta opioid receptor heteromers in Rhesus monkeys. ACS Chem Neurosci 2012; 3: 720–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI: Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 2009; 137: 1148–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, Wu SX, Hokfelt TG, Bao L, Zhang X: Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A 2010; 107: 13117–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolesnikov Y, Pasternak GW: Topical opioids in mice: analgesia and reversal of tolerance by a topical N-methyl-D-aspartate antagonist. J Pharmacol Exp Ther 1999; 290: 247–52 [PubMed] [Google Scholar]
- 49.Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hubner H, Huang XP, Sassano MF, Giguere PM, Lober S, Da D, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, Shoichet BK: Structure-based discovery of opioid analgesics with reduced side effects. Nature 2016; 537: 185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li C, Nie SP, Zhu KX, Xiong T, Li C, Gong J, Xie MY: Effect of Lactobacillus plantarum NCU116 on loperamide-induced constipation in mice. Int J Food Sci Nutr 2015; 66: 533–8 [DOI] [PubMed] [Google Scholar]