ABSTRACT
Introduction
Owing to its well-established ergogenic potential, creatine is a highly popular food supplement in sports. As an oral supplement, creatine is considered safe and ethical. However, no data exist on the safety of creatine on lung function in athletes. The aim of this project was to evaluate the effects of a standard course of creatine on the airways of youth elite athletes.
Methods
Nineteen elite soccer players, 16–21 yr old, completed a stratified, randomized, double-blind, placebo-controlled, parallel-group trial. The creatine group (n = 9) ingested 0.3 g·kg−1⋅d−1 of creatine monohydrate (CM) for 1 wk (loading phase) and 5 g·d−1 for 7 wk (maintenance phase), and the placebo group (n = 10) received the same dosages of maltodextrin. Airway inflammation (assessed by exhaled nitric oxide, FENO) and airway responsiveness (to dry air hyperpnoea) were measured pre- and postsupplementation.
Results
Mild, unfavorable changes in FENO were noticed by trend over the supplementation period in the CM group only (P = 0.056 for interaction, η2 = 0.199), with a mean group change of 9 ± 13 ppb in the CM group versus −5 ± 16 ppb in the placebo group (P = 0.056, d = 0.695). Further, the maximum fall in forced expiratory volume in 1 s after dry air hyperpnoea was larger by trend postsupplementation in the CM group compared with the placebo group: 9.7% ± 7.5% vs 4.4% ± 1.4%, respectively (P = 0.070, d = 0.975). These adverse effects were more pronounced when atopic players only (n = 15) were considered.
Conclusion
On the basis of the observed trends and medium to large effect sizes, we cannot exclude that creatine supplementation has an adverse effect on the airways of elite athletes, particularly in those with allergic sensitization. Further safety profiling of the ergogenic food supplement is warranted.
Key Words: CREATINE MONOHYDRATE, FOOTBALL, AIRWAY INFLAMMATION, AIRWAY HYPERRESPONSIVENESS, EXHALED NITRIC OXIDE, EUCAPNIC VOLUNTARY HYPERPNOEA
Creatine is one of the most popular food supplements used by athletes (1); it is used in a wide variety of sports and at all levels (e.g., about a third of English professional soccer players [2] and the same percentage of American children and adolescents [3] declare using the supplement). The popularity of creatine stems from the fact that the ergogenic potential of creatine is supported by strong scientific evidence (1) and its mechanisms of action are well understood. Since the early 1990s (4), studies have consistently shown that creatine supplementation raises intramuscular creatine stores, thereby helping to maintain adenosine triphosphate availability during exercise and enhancing high-intensity (anaerobic) exercise capacity (1). That creatine is not prohibited by the governing bodies of sport (including the World Anti-Doping Agency) and is purported to be safe (1,5) likely contributes to its widespread use.
The recognized potential of creatine to stimulate muscle strength and power, to increase muscle volume/fat-free mass, and to stimulate recovery from exercise (5) makes it particularly attractive in sports with repeated short bursts of high-intensity activity. Evidence of an ergogenic effect of the supplement has repeatedly been reported in team sports, including soccer (6). Further, in semiprofessional soccer players, a standard 8-wk course of creatine monohydrate (CM) supplementation has been reported to be safe (7). However, no study has so far evaluated the possible adverse effect of creatine on lung function in athletes. This is particularly striking considering that, in an animal model of asthma, creatine has been shown to exacerbate allergic-induced lung inflammation, airway remodeling, and airway hyperresponsiveness (AHR) (8). Creatine is thought to initiate an important cellular signaling pathway in the airway epithelium that results, among others, in an increased infiltration of inflammatory cells to the airways, an increased number of mucus-synthesizing cells, and an upregulation of airway remodeling (9). Similar features have been observed in bronchial biopsies of elite athletes (10,11), many of whom suffer from asthma/AHR (10–13).
Asthma is a chronic inflammatory disorder of the airways, with associated allergic sensitization, that is characterized by reversible airway obstruction and AHR to a wide range of stimuli (including exercise). In elite sports, asthma/AHR is the most common chronic medical condition, with an overall prevalence of ~8% (12). The distribution of asthma/AHR in sport is however largely skewed, with the highest prevalence rates observed in endurance sports and/or in disciplines performed in unfavorable environments (e.g., swimming or cross-country skiing) (12–14). Nonetheless, recent reports have highlighted that team sport players, such as soccer (15,16) and rugby players (17,18), may also commonly suffer from asthma/AHR. Given the high prevalence of asthma-related problems and the widespread use of creatine supplements in elite sport, it is important to identify any adverse effects that ergogenic food supplements may have on lung function in this population.
The aim of our study was therefore to evaluate the effects of a standard course of creatine supplementation on the respiratory health of youth elite athletes. Our hypothesis was that ingestion of a standard dose of CM for 8 wk, in combination with intensive soccer-specific training, increases airway inflammation and airway responsiveness.
METHODS
Study Participants
Nineteen under 18 (U18) and nine under 21 (U21), nonsmoking, male, elite soccer players from Watford Football Club (FC) Academy agreed to take part in this project. Participants completed a medical questionnaire before the start of the study to check that none were currently injured or on medication (except for asthma and/or exercise-induced bronchoconstriction [EIB]). On enrolment, one participant disclosed doctor-diagnosed asthma and, based on skin prick testing (cf. methodology below), 19 (68%) had evidence of atopy. The study was approved by the Research Ethics Committee from the School of Sport and Education, Brunel University London (no. RE69-13). All participants gave written informed consent before taking part.
Study Design
A stratified, randomized, double-blind, placebo-controlled, parallel-group trial of creatine supplementation was conducted over 8 wk during the first half of the English competitive season (October–December). Stratified randomization was used to ensure equal distribution between groups of (i) participants with allergic sensitization (atopy) or doctor-diagnosed allergic asthma and (ii) U18 and U21 players. Atopy was assessed at study entry by standard skin prick test (19), with the following allergens tested: house dust mite, timothy grass, silver birch, dog hair, and cat hair. In line with current international recommendations (19), wheal diameters ≥3 mm were considered as positive.
All players abstained from caffeine and alcohol on the day of the tests, from exercise for at least 4 h, and from antihistamines for 72 h. To control for diurnal variation in lung function, all tests were conducted in the morning/early afternoon.
Supplementation
Participants were randomized into groups by an independent third party, using a random number generator. Allergic and nonallergic players as well as U18 and U21 players were allocated, in equal numbers, to the creatine and placebo groups. A typical CM supplementation protocol was followed (1), whereby participants ingested 0.3 g·kg−1⋅d−1 of CM (MyProtein, Informed Sports Range, 100% pure) during a 1-wk loading phase. The placebo group received the same dosage of maltodextrin (MyProtein, Informed Sports Range, 100% pure). The supplement was combined with 90 g of the participant’s usual protein drink (Maxiraw Protein Complex; 60% whey protein concentrate, 20% soy protein isolate, and 20% micellar casein) provided to the players at Watford FC Academy and ingested in four daily 150-mL intakes. This was followed by a 7-wk maintenance phase during which players ingested 5 g·d−1 of CM, or of placebo, mixed up in 30 g of protein drink, and taken at the end of their daily training session or after games (at the weekend). To ensure adherence, the protein with supplement drinks were prepared daily by the Head Academy Sport Scientist (PS) at Watford FC Academy and handed out directly to the players at the required times (except on Sundays, when players were provided with shakers to take home).
The body mass of the participants was measured using a calibrated electronic scale pre- and postsupplementation. Body composition was assessed via skinfold measurement. In line with the International Society for the Advancement of Kinanthropometry, eight sites (i.e., triceps, subscapular, biceps, iliac crest, supraspinal, abdominal, front thigh, and medial calf) were measured. All measurements were taken by a researcher with level 1 qualification from the International Society for the Advancement of Kinanthropometry (with an average technical error of measurement of 1.50% ± 0.31%).
Diet was not standardized, but participants were asked not to change their dietary habits during the study. None of the players declared using creatine supplements in the 3 months before the study.
Daily record of asthma symptoms, upper or lower respiratory tract infection symptoms, use of asthma reliever medication (salbutamol), and other potential side effects (e.g., muscle cramps, musculoskeletal injury, and gastrointestinal discomfort) were logged for all players.
Training Program
This research did not interfere with the training schedule devised by the players’ coaches. The 8-wk training program included ~11 h·wk−1 of soccer-specific training (i.e., technical and tactical exercises), with little strength and conditioning work (approximately one session of 50 min·wk−1). As part of the Football League Youth Alliance, the U18 team had regular match fixtures over the course of the trial. Further, some the U21 players played for Watford FC first team (which, at the time of the study, was competing in the English Football League Championship).
Lung Health Assessment
All the tests below were performed twice: once before and once after the 8-wk supplementation period.
Airway inflammation
Fractional nitric oxide in exhaled breath (FENO) was measured at rest using NIOX VERO (Aerocrine, Solna, Sweden) and was used as a noninvasive marker of airway inflammation (20). Players were asked to refrain from exercising, eating, and drinking for an hour before the FENO measurements (21). As per standard recommendations (21), at least two measurements agreeing within 10% of each other were recorded, and the mean of the two values was calculated and used for analysis.
Lung function
Standard spirometry was performed at rest in all players using the MicroLoop spirometer (Micromedical, Kent, UK) to determine baseline lung volume and expiratory flow rates. In accordance with the international recommendations (22), at least three technically acceptable forced vital capacity (FVC) maneuvers were performed (up to a maximum of eight), with a minimum of two reproducible recordings (difference ≤ 150 m for forced expiratory volume in 1 s [FEV1] and FVC). The highest FEV1 and FVC readings were kept for analysis. Forced expiratory flow between 25% and 75% of FVC was taken from the reproducible maneuver with the highest sum “FEV1 + FVC.” The highest peak expiratory flow from all acceptable maneuvers was kept for analysis. Predicted values were calculated from the GLI-2012 equations (23).
AHR
A standard 6-min eucapnic voluntary hyperpnoea (EVH) test with dry air was used to estimate the degree of airway responsiveness. The test was performed on a commercially available system (EucapSys, SMTEC, Nyon, Swiss) and involved 6 min of heavy breathing of a dry gas mixture containing 5% CO2, 21% O2, and balance nitrogen (24). Presupplementation, the target ventilation (V˙E) was set at 85% of predicted maximum voluntary ventilation (MVV) (24), calculated as resting FEV1 multiplied by 30. Postsupplementation, the target V˙E was set at the achieved level during the initial test. Participants failing to achieve the minimal recommended threshold of 60% MVV (24) were noted and discussed in the Results section. Before and at 2, 5, 10, 15, and 20 min of recovery, FVC maneuvers were performed in duplicate. The best FEV1 values from reproducible measurements were kept for analysis. The maximal fall in FEV1 post-EVH (expressed as % difference from baseline) was calculated as an index of airway responsiveness. A test was considered positive for EIB if FEV1 fell by ≥10% from baseline over two consecutive time points (24).
Data Analysis
Sample size
FENO was the primary outcome measure. The statistical power was set at 80% and the alpha level at 5%. To detect a 10-ppb difference in FENO between the CM and the placebo groups postsupplementation (in noninflamed airways, a change of 10 ppb being considered clinically meaningful [20]), with a within-group SD of 6 ppb and a control-to-experimental participant ratio of 1, we calculated that a sample size of 14 players would be required (i.e., seven players per group). Taking account of an estimated dropout rate of 30%, a minimum of 20 players was required.
Statistical analysis
Normal distribution was examined using the Shapiro–Wilk test. Data were analyzed using unpaired t-tests for between-group comparisons at the start of the trial and for changes over the supplementation period (Δ values). A two-way repeated-measures ANOVA (RM-ANOVA), with “group” and “time” as the main factors, was used to test between- and within-group differences during the trial. FENO data had skewed distribution. As RM-ANOVA is robust and can tolerate data that are nonnormally distributed with only a small effect on the type I error rate, the statistical test was however retained. Spearman correlation coefficient was calculated to determine the relationship between baseline FENO and change in FENO after supplementation with CM. Considering that some previous investigations indicated that the deleterious effects of creatine may be specific to sensitized animals (8), post hoc analyses were conducted (following the same procedures as described above) in atopic players only (n = 7 in the CM group and n = 8 in the placebo group). The significance level was set at P < 0.05. A trend for statistical significance was deemed at P < 0.10. Effect size was calculated via partial eta-squared values (η2) for ANOVA and Cohen’s d for t-tests. The magnitude of effect sizes was classified as small, medium, and large for η2 > 0.01, 0.06, and 0.14, respectively, and for d > 0.2, 0.5, and 0.8, respectively (25). Data were analyzed using the Statistical Package for the Social Sciences for Windows (version 24; SPSS Inc., Chicago, IL). Unless otherwise stated, data are presented as group mean ± SD.
RESULTS
Participant characteristics
Twenty-eight players (19 U18 and 9 U21) were initially recruited, of whom 19 completed the study. Details regarding participant withdrawals are provided in the CONSORT diagram (Fig. 1). The only player with doctor-diagnosed asthma (treated by inhaled corticosteroids and inhaled beta2-agonist at the time of the study) withdrew because of an asthma exacerbation before randomization. Out of the 19 players who completed the full trial (13 U18 and 6 U21), 9 were in the CM group and 10 were in the placebo group.
FIGURE 1.
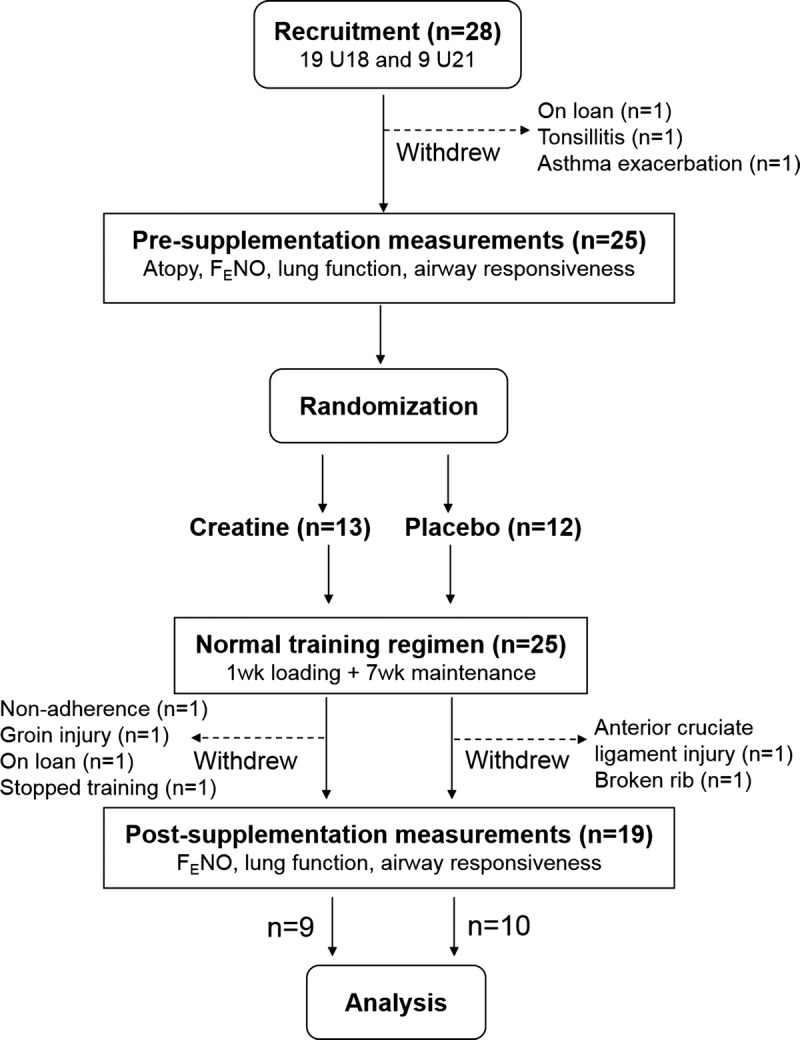
Outline of the study design. FENO, fractional nitric oxide in exhaled air.
At study entry, participants were 17.4 ± 1.6 yr of age, had a stature of 178.3 ± 5.6 cm, and had a body mass of 73.7 ± 5.3 kg. No group differences were noted for age or anthropometry (Table 1). Eight weeks of CM or placebo supplementation did not significantly change the body mass of the players (P = 0.421): in the CM group, 75.7 ± 4.6 kg presupplementation versus 75.7 ± 4.6 kg postsupplementation; in the placebo group, 72.0 ± 5.5 kg presupplementation versus 72.3 ± 5.5 kg postsupplementation. A significant time effect was however noticed for body composition (P = 0.002, η2 = 0.436), with the sum of eight skinfolds reduced from 64 ± 15 to 61 ± 15 mm postsupplementation (and no group or interaction effect). The relative reduction in skinfold thickness was 5.5% ± 5.0% in the CM group and 4.4% ± 6.1% in the placebo group (P = 0.678).
TABLE 1.
Characteristics of study participants.
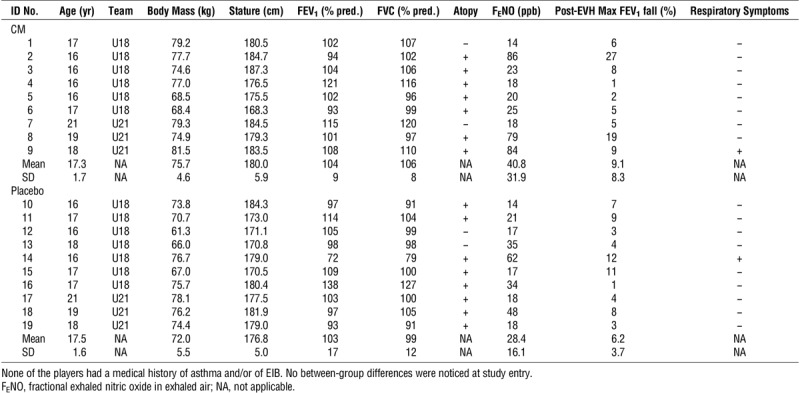
Fifteen (79%) players were atopic (Table 1), with grass being the most common allergen for them. Twelve (63%) players were positive to grass, six (32%) to house dust mite, five (26%) to dog hair, five (26%) to cat hair, and two (11%) to silver birch.
One participant reported minor gastrointestinal discomfort during the supplementation period; he was in the CM group. None of the other players reported any side effects from ingesting the supplements. Two players reported a history of respiratory symptoms on exertion (i.e., chest tightness with/without cough) (Table 1), with one assigned to the CM group; neither of them reported an exacerbation of these symptoms or usage of asthma drugs over the supplementation period.
FENO
The RM-ANOVA revealed no group or time effect for FENO, but there was a trend for an interaction effect, with a P value of 0.056 and an η2 value of 0.199 (the latter indicating a large effect size). Post hoc tests showed that FENO did not significantly change over the supplementation period in the placebo group (P = 0.374), but there was a trend, with a medium effect size, for an increase in the CM group (P = 0.071, d = 0.695) (Fig. 2). A statistical trend, with a large effect size in the direction of an unfavorable change in FENO after CM (Δ FENO = 9 ± 13 ppb) compared with PLA (Δ FENO = −5 ± 16 ppb), was also observed (P = 0.056, d = 0.950) (Fig. 3). A significant positive relationship was noted between baseline FENO and Δ FENO in the CM group (rs2 = 0.460, P = 0.045).
FIGURE 2.
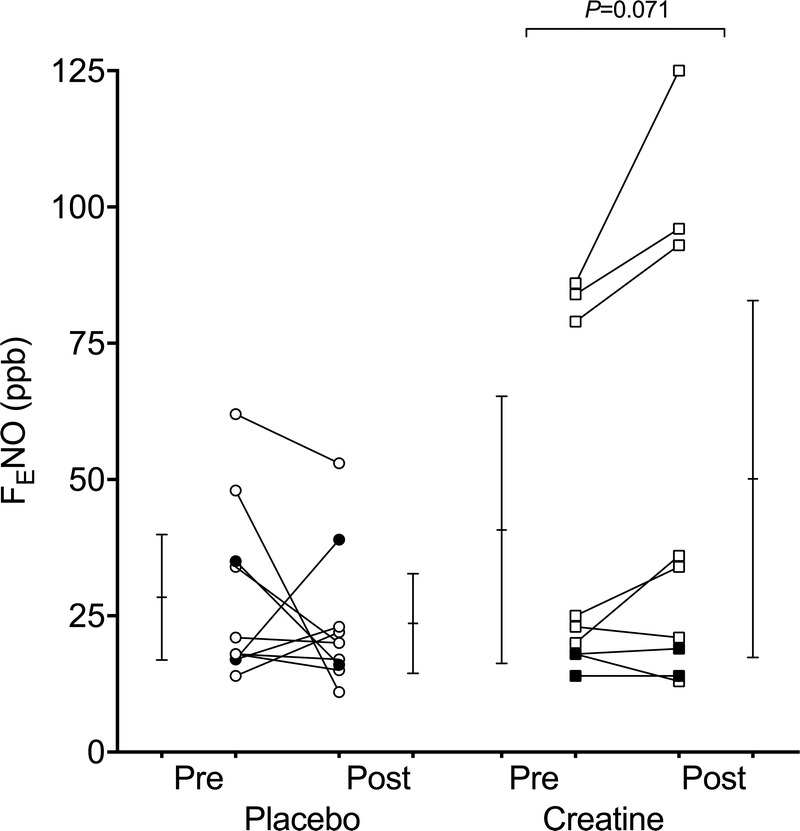
Fractional nitric oxide in exhaled air (FENO) in youth elite soccer players before and after 8 wk of CM (squares) or placebo supplementation (circles). Open symbols represent atopic players, and closed symbols represent nonatopic players. Individual and mean values (with 95% confidence intervals) are shown. When atopic players only were analyzed, the P value for the within-group difference in the creatine group was 0.072.
FIGURE 3.
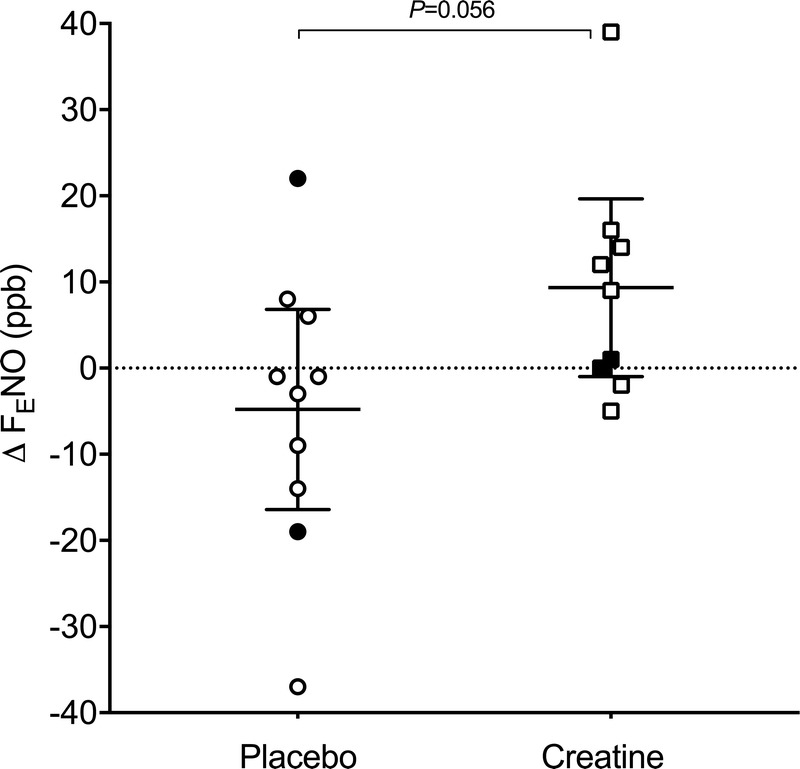
Change in fractional nitric oxide in exhaled air (Δ FENO) in youth elite soccer players over 8 wk of CM (squares) or placebo supplementation (circles). Open symbols represent atopic players, and closed symbols represent nonatopic players. Individual and mean values (with 95% confidence intervals) are shown. When atopic players only were analyzed, the P value for the between-group difference was significant at 0.026.
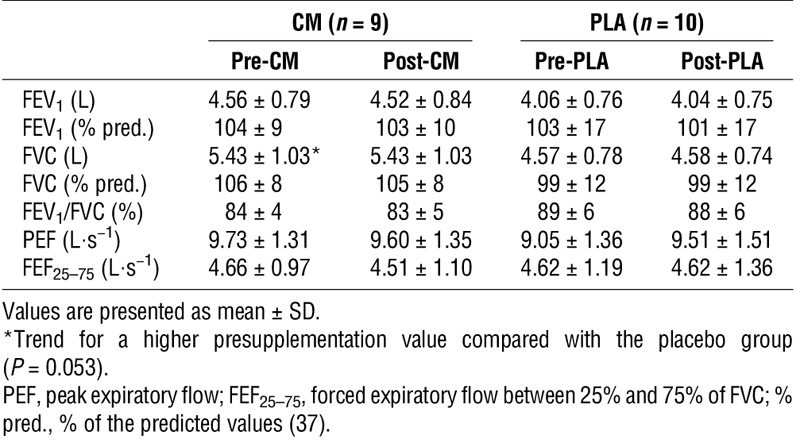
Resting lung function
Resting lung function was normal (i.e., >lower limit of normal) in all the players, except one (Table 1, ID no. 14). This U18 player reported respiratory symptoms during strenuous exercise and had a family history of asthma, but no previous doctor diagnosis of asthma and/or EIB. No statistically significant differences in resting spirometry were observed between groups and/or times (i.e., pre- to postsupplementation) (Table 2). Although there was a trend for athletes in the CM group to have a larger resting FVC (P = 0.053, η2 = 0.203), this trend disappeared when the data were normalized for age, stature, and ethnicity (FVC % predicted) (Table 2).
TABLE 2.
Resting lung function data in youth elite soccer players before and after 8 wk of CM or placebo (PLA) supplementation.
AHR
During the EVH tests, players sustained mean V˙E values of 114 ± 23 L⋅min−1 presupplementation and 115 ± 25 L⋅min−1 postsupplementation in the CM group and 94 ± 18 L⋅min−1 presupplementation and 96 ± 17 L⋅min−1 postsupplementation in the placebo group. The statistical trend in the direction of a greater V˙E in the CM compared with the placebo group (P = 0.063, η2 = 0.188) disappeared when the data were expressed as % of predicted MVV (P = 0.514). V˙E values were 71% ± 10% of predicted MVV presupplementation and 73% ± 11% of predicted MVV postsupplementation for the CM group versus 68% ± 13% of predicted MVV presupplementation and 69% ± 13% of predicted MVV postsupplementation for the placebo group. The supplementation had no effect on V˙E (i.e., no time or interaction effect). Three U18 players were unable to reach the minimum target V˙E of ≥60% of predicted MVV presupplementation (with recorded V˙E at 59%, 58%, and 37% of predicted MVV). Postsupplementation, two of these players reached the minimum threshold, whereas the latter only managed 40% of predicted MVV.
The RM-ANOVA revealed no significant group or time effect on the maximum fall in FEV1 post-EVH, but a trend for an interaction effect was noticed (P = 0.086, η2 = 0.163, large effect size). Post hoc tests showed no significant changes in the fall in FEV1 over the supplementation period in the CM group, but a trend, with a medium effect size, for a reduced fall in FEV1 in the placebo group was found (P = 0.060, d = 0.679) (Fig. 4). Further, the fall in FEV1 recorded postsupplementation was larger by trend, with a large effect size, in the CM group (9.7% ± 7.5%) compared with the placebo group (4.4% ± 1.4%) (P = 0.070, d = 0.975) (Fig. 4).
FIGURE 4.
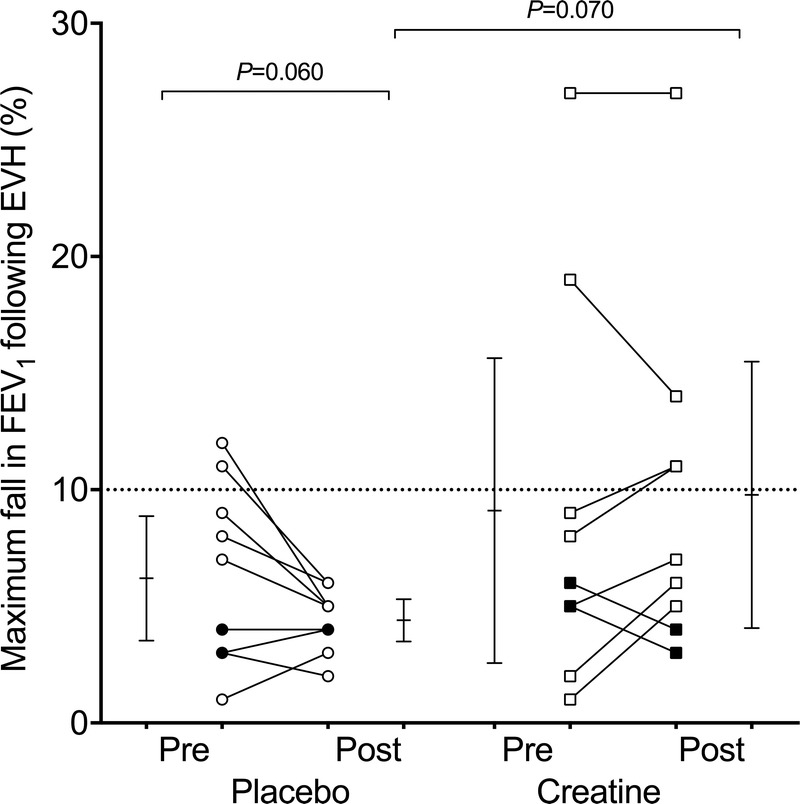
Pre- and postsupplementation fall in FEV1 after 6 min of EVH of dry air in youth elite soccer players supplemented for 8 wk with CM (squares) or a placebo (circles). Open symbols represent atopic players, and closed symbols represent nonatopic players. Individual and mean values (with 95% confidence intervals) are shown. When atopic players only were analyzed, the P value for the within-group difference in the placebo group was significant at 0.041.
At an individual level, three players (Table 1, ID nos. 2, 8, and 14) had a ≥10% fall in FEV1 over at least two consecutive time points after EVH (consistent with a diagnosis of EIB) presupplementation; two of these players (both under CM) had also a sustained fall in FEV1 postsupplementation (Fig. 4). None of the three players with a positive EVH test had a history of asthma and/or EIB, but they all had abnormally high FENO values (Table 1). The player who was positive for EIB presupplementation but negative postsupplementation (Table 1, ID no. 14) was the one who had an abnormally low lung function at rest (at both time points) and who reported respiratory symptoms during exercise; his data were consistent with a diagnosis of mild asthma. Another player in the CM group who reported occasional respiratory symptoms during exercise (Table 1, ID no. 9) had a borderline response to EVH (i.e., 9% fall in FEV1 presupplementation and a nonsustained 11% fall postsupplementation) and markedly increased FENO levels at both time points (84 and 96 ppb pre- and postsupplementation, respectively); his overall profile was therefore suggestive of mild, seasonal EIB. Altogether, this suggests that 4 (21%) of the 19 players had undiagnosed asthma and/or EIB.
Post hoc analysis in atopic participants (n = 15)
Similar to the whole group analysis, an RM-ANOVA conducted on FENO measurements revealed a significant interaction effect in the atopic players (P = 0.029, η2 = 0.317). Post hoc tests showed a trend, with a large effect size, for FENO to increase after supplementation with CM (P = 0.072, d = 0.824) and no change after placebo (P = 0.248) (Fig. 2). The statistical trend for a between-group difference for the change in FENO over the supplementation period was significant in atopic players (P = 0.029), with a large effect size (d = 1.272): Δ FENO = 12 ± 14 ppb in the CM group versus −6 ± 14 ppb in the placebo group (Fig. 3).
Trend for within- and between-group differences in airway responsiveness reached statistical significance when atopic athletes only were entered into the analysis. For the maximum fall in FEV1 post-EVH, the RM-ANOVA revealed a significant interaction effect (P = 0.027, η2 = 0.323). Post hoc tests showed no significant changes in the fall in FEV1 over the supplementation period in the CM group (P = 0.297) but a reduced fall in FEV1 in the placebo group (P = 0.041, d = 0.852) (Fig. 4). Consequently, the change in AHR after the supplementation period was significantly different between the atopic players supplemented with CM (−1.3% ± 3.0%) and placebo (2.3% ± 2.6%) (P = 0.027, d = 1.289). Further, the fall in FEV1 recorded postsupplementation was significantly larger (P = 0.026, d = 1.295) in the CM group (11.4% ± 7.6%) compared with the placebo group (4.5% ± 1.5%) in atopic players (Fig. 4).
DISCUSSION
The aim of this study was to determine whether creatine supplementation in combination with intensive training increased airway inflammation and airway responsiveness in elite athletes. In soccer academy players, trends for unfavorable changes in FENO (an indirect marker of airway inflammation) were noticed after daily ingestion of CM for 8 wk. A statistical trend in the direction of a greater fall in FEV1 after dry air hyperpnoea—indicative of increased airway responsiveness—was also observed after CM supplementation compared with placebo. These effects were more pronounced when atopic individuals only were entered into the statistical analysis. Therefore, our findings highlight possible adverse effects of the popular ergogenic food supplement on the airways of elite athletes, particularly in those with allergic sensitization.
The safety of creatine has widely been investigated in athletes through serum and urinary markers of metabolic, hepatic, renal, and muscular function (e.g., [1,7]). The general consensus is that, at the recommended dosage, creatine is safe (1,5). However, up to now, the effects of creatine supplements on lung function in athletes have not been assessed. In our study, trends (with medium to large effect sizes) for an increase in FENO (a well-established marker for eosinophilic airway inflammation in humans [20]) were observed after 8 wk of CM supplementation in a mixed group of atopic and nonatopic athletes. When atopic athletes (representing ~80% of the study population) were analyzed separately, statistical significance in favor of a larger increase in FENO postsupplementation in the CM compared with the placebo group was noted. This is in keeping with data obtained in a murine model for allergic asthma (i.e., ovalbumin-treated mice) (8) that showed an increase in airway inflammation (as identified by infiltration of eosinophils and of proinflammatory cytokines within the airways) and occurrence of airway remodeling after 32 d of creatine supplementation. In chronically sensitized mice, low-intensity aerobic exercise, however, reduced the exacerbatory effect of creatine (26). The divergence in findings may be explained by the intensity of exercise. Indeed, there is a growing body of evidence of positive effects of regular physical activity on AHR in the general population (27) and on airway inflammation (28) and health-related quality of life in patients with asthma (29), yet a detrimental effect of repeated, intensive physical training on the airways of elite athletes (30,31).
At a cellular level, the proinflammatory effects of creatine could be explained by an upregulation of an inflammatory cascade and by an increased infiltration of cells (including eosinophils) within the airways (9). In sensitized and nonsensitized mice, creatine supplementation has been shown to increase the epithelial expression of inducible nitric oxide synthase (9), i.e., the major determinant of FENO (32). This could therefore explain the trend for an increased FENO in the group supplemented with CM in our study. An upregulation of the airway remodeling process was also noticed after creatine supplementation in both groups of mice (i.e., sensitized and nonsensitized) (9). Signs of airway inflammation and remodeling (including goblet cell hyperplasia and overexpression of mucin) have previously been observed in bronchial biopsies of elite athletes, which were attributed to repeated exposure to high ventilation in unfavorable environments (10,11). Whether an additive inflammatory effect could have arisen because of the use of creatine supplements by some athletes is unknown.
In our study, the mild, unfavorable changes in FENO were accompanied by a trend for increased airway responsiveness (as measured by the fall in FEV1 post-EVH) in the group supplemented with CM compared with the placebo. This trend became significant when atopic athletes only were entered into the analysis. As the maximum fall in FEV1 post-EVH was reduced in the atopic players after placebo but not CM supplementation, creatine could somewhat alter the natural course of airway responsiveness during a sporting season. It is not the first time that fluctuations in airway responsiveness are observed in elite athletes. Seasonal variability in the airway response to exercise has previously been noticed in elite runners (33) and elite swimmers (34), with AHR diminishing when changes in environmental conditions and/or reductions in training load limited the stress to the airways. Although, in our study, the within- and between-group differences in the fall in FEV1 post-EVH were small and did not reach clinical significance (i.e., no athletes under CM became positive for EIB postsupplementation), the duration of the intervention was relatively short (8 wk). We cannot exclude that subclinical changes in airway responsiveness progress toward clinical changes (i.e., asthma or EIB) when atopic athletes ingest creatine chronically and/or do not adhere to the recommended dosage.
Asthma/AHR is the most common chronic medical condition in elite sport (12,35). However, it is largely misdiagnosed in professional (17,36) and recreational sports (37). In our study, we found objective evidence of asthma and/or AHR in about one in five youth players, but none had a medical history of asthma/EIB. In slightly younger (12–14 yr old) elite soccer players, 4 (21%) out of 19 players were positive for EIB when tested with EVH (15). These results therefore highlight the need for implementing screening programs for asthma/AHR in youth elite sport. This is all the more important in that cases of sudden fatal asthma exacerbations have been reported after competitive and recreational sporting activities, particularly in young (10–20 yr old) males (38). Considering that 3 (23%) out of the 13 U18 players in our study were not able to reach the minimum recommended ventilatory threshold of 60% predicted MVV (24) during the initial EVH test (an issue reported elsewhere [15]), there is probably a need to revisit and validate the minimum required VE for EVH when testing youth athletes.
Although it is commonly accepted that creatine promotes muscle mass gains during resistance training (1,5), in our study, skinfold thickness was reduced postsupplementation in both groups (whereas body mass remained unchanged). This suggests that CM supplementation did not promote gains in muscle mass beyond the effects observed with soccer training alone. This may be explained by the fact that (i) creatine does not have a direct anabolic effect on protein synthesis (39,40) and muscle hypertrophy (41), (ii) the benefits of the supplement seem mainly dependent on an increased training workload (42) and (iii) our groups were well matched for training load, and players spent limited time in the gym during the supplementation period (~50 min of strength and conditioning per week). Beyond asking participants to maintain the same diet throughout the supplementation period, we were unfortunately not in a position to control their food intake; this could have influenced muscle creatine content (43) and responses to the creatine supplement (5).
The strength of this study is the use of a rigorous methodological approach (i.e., a stratified, randomized, double-blind, placebo-controlled, parallel-group study design) in a difficult-to-reach sporting population (i.e., elite youth athletes). One limitation that comes with inclusion of elite athletes is the relatively large dropout rate (32%). Despite recruiting the entire U18 and U21 squads, only 9 participants in the CM group and 10 participants in the placebo group completed the full trial. Further, although we met our recruitment target (based on a priori sample size calculation), many of our data were largely spread (including our primary research outcome, FENO). Having asked all our participants to refrain eating and drinking for 1 h before the FENO measurements (based on the recommendations from the ATS/ERS [21]), we do not think that food and beverages could explain the large interindividual differences in FENO. That the three participants with the highest baseline FENO values were atopic and presented the largest increase in FENO after CM supplementation suggest that individuals with underlying atopic airway inflammation may be more responsive to the effects of creatine. However, further studies (based around additional markers of airway inflammation) are now warranted to evaluate the clinical significance of these findings. This is particularly necessary as a 20% increase in FENO is usually recommended as a cutoff (rather than 10 ppb increase used in this study) to establish the effect of an intervention in individuals with raised baseline values (i.e., FENO > 50 ppb [20]).
The large interindividual difference in our primary research outcome (FENO) could also have contributed to several of our P values not quite reaching the level of significance when analyzing our mixed group of atopic and nonatopic athletes. To overcome this limitation, two steps were taken: (i) effect sizes were reported, with results suggesting that our intervention (i.e., CM supplementation) had moderate to large effects on FENO and on the fall in FEV1 post-EVH, and (ii) post hoc analyses were conducted on atopic players only (n = 15), which confirmed (or even reinforced) the original trends. Therefore, in the absence of any other human-based data, we cannot exclude a possible detrimental effect of CM on the airways of elite athletes, especially in the atopic ones.
CONCLUSION
In summary, mild unfavorable changes in FENO were observed in youth elite soccer players, particularly in those with allergic sensitization, after a recommended 8-wk course of CM supplementation. This observation was coupled with a trend for the airways of athletes supplemented with CM to be slightly more responsive to dry air hyperpnoea compared with the placebo group postsupplementation. Until further work is performed on the safety profile of creatine supplements on lung function in humans, caution should prevail, and monitoring of the respiratory health of elite athletes regularly taking creatine supplements is advised.
Acknowledgments
This study was supported by the Union of European Football Associations (UEFA), through its UEFA Research Grant Programme.
The authors thank all the Watford FC Academy players who participated in this study and Mr. Guy Farrier for his help with data collection. Andrew Simpson is currently affiliated with the Department of Sport, Health and Exercise Science, School of Life Sciences, University of Hull.
The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine. The authors have no conflicts of interest to declare.
REFERENCES
- 1.Kreider RB, Kalman DS, Antonio J, et al. International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. J Int Soc Sports Nutr. 2017;14(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waddington I, Malcolm D, Roderick M, Naik R. Drug use in English professional football. Br J Sports Med. 2005;39(4):e18–discussione18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans MW, Ndetan H, Perko M, Williams R, Walker C. Dietary supplement use by children and adolescents in the United States to enhance sport performance: results of the National Health Interview Survey. J Prim Prev. 2012;33(1):3–12. [DOI] [PubMed] [Google Scholar]
- 4.Harris RC, Söderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci. 1992;83(3):367–74. [DOI] [PubMed] [Google Scholar]
- 5.Cooper R, Naclerio F, Allgrove J, Jimenez A. Creatine supplementation with specific view to exercise/sports performance: an update. J Int Soc Sports Nutr. 2012;9(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostojic SM. Creatine supplementation in young soccer players. Int J Sport Nutr Exerc Metab. 2004;14(1):95–103. [DOI] [PubMed] [Google Scholar]
- 7.Cancela P, Ohanian C, Cuitiño E, Hackney AC. Creatine supplementation does not affect clinical health markers in football players. Br J Sports Med. 2008;42(9):731–5. [DOI] [PubMed] [Google Scholar]
- 8.Vieira RP, Duarte AC, Claudino RC, et al. Creatine supplementation exacerbates allergic lung inflammation and airway remodeling in mice. Am J Respir Cell Mol Biol. 2007;37(6):660–7. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira SC, Toledo AC, Hage M, et al. Creatine activates airway epithelium in asthma. Int J Sports Med. 2010;31(12):906–12. [DOI] [PubMed] [Google Scholar]
- 10.Bougault VV, Loubaki LL, Joubert PP, et al. Airway remodeling and inflammation in competitive swimmers training in indoor chlorinated swimming pools. J Allergy Clin Immunol. 2012;129(2):351–1. [DOI] [PubMed] [Google Scholar]
- 11.Karjalainen EM, Laitinen A, Sue-Chu M, Altraja A, Bjermer L, Laitinen LA. Evidence of airway inflammation and remodeling in ski athletes with and without bronchial hyperresponsiveness to methacholine. Am J Respir Crit Care Med. 2000;161(6):2086–91. [DOI] [PubMed] [Google Scholar]
- 12.Fitch KD. An overview of asthma and airway hyper-responsiveness in Olympic athletes. Br J Sports Med. 2012;46(6):413–6. [DOI] [PubMed] [Google Scholar]
- 13.Carlsen K-H, Anderson SD, Bjermer L, et al. Exercise-induced asthma, respiratory and allergic disorders in elite athletes: epidemiology, mechanisms and diagnosis: part I of the report from the Joint Task Force of the European Respiratory Society (ERS) and the European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA 2LEN. Allergy. 2008;63(4):387–403. [DOI] [PubMed] [Google Scholar]
- 14.Levai IK, Hull JH, Loosemore M, Greenwell J, Whyte G, Dickinson JW. Environmental influence on the prevalence and pattern of airway dysfunction in elite athletes. Respirology. 2016;21(8):1391–6. [DOI] [PubMed] [Google Scholar]
- 15.Van der Eycken S, Schelpe A, Marijsse G, et al. Feasibility to apply eucapnic voluntary hyperventilation in young elite athletes. Respir Med. 2016;111:91–3. [DOI] [PubMed] [Google Scholar]
- 16.Jackson AR, Hull J, Hopker JG, Dickinson J. Impact of detecting and treating exercise-induced bronchoconstriction in elite footballers. ERJ Open Res. 2018;4(2):00122–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickinson J, McConnell A, Whyte G. Diagnosis of exercise-induced bronchoconstriction: eucapnic voluntary hyperpnoea challenges identify previously undiagnosed elite athletes with exercise-induced bronchoconstriction. Br J Sports Med. 2011;45(14):1126–31. [DOI] [PubMed] [Google Scholar]
- 18.Falvey EC, McCarthy C, O’Connor TM, Shanahan F, Molloy MG, Plant BJ. Exercise-induced bronchoconstriction and exercise testing in an international rugby union team. Thorax. 2010;65(9):843–4. [DOI] [PubMed] [Google Scholar]
- 19.Bousquet J, Heinzerling L, Bachert C, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012;67(1):18–24. [DOI] [PubMed] [Google Scholar]
- 20.Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–30. [DOI] [PubMed] [Google Scholar]
- 22.Miller MR, Hankinson JL, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. [DOI] [PubMed] [Google Scholar]
- 23.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson SD, Kippelen P. Assessment and prevention of exercise-induced bronchoconstriction. Br J Sports Med. 2012;46(6):391–6. [DOI] [PubMed] [Google Scholar]
- 25.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale (NJ): Lawrence Erlbaum Associates; 1988. pp. 19–74. [Google Scholar]
- 26.Vieira RP, Duarte AC, Santos AB, et al. Exercise reduces effects of creatine on lung. Int J Sports Med. 2009;30(9):684–90. [DOI] [PubMed] [Google Scholar]
- 27.Shaaban R, Leynaert B, Soussan D, et al. Physical activity and bronchial hyperresponsiveness: European Community Respiratory Health Survey II. Thorax. 2007;62(5):403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendes FA, Almeida FM, Cukier A, et al. Effects of aerobic training on airway inflammation in asthmatic patients. Med Sci Sports Exerc. 2011;43(2):197–203. [DOI] [PubMed] [Google Scholar]
- 29.Carson KV, Chandratilleke MG, Picot J, Brinn MP, Esterman AJ, Smith BJ. Physical training for asthma. Cochrane Database Syst Rev. 2013;104(9):CD001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson SD, Kippelen P. Exercise-induced bronchoconstriction: pathogenesis. Curr Allergy Asthma Rep. 2005;5(2):116–22. [DOI] [PubMed] [Google Scholar]
- 31.Kippelen P, Anderson SD. Airway injury during high-level exercise. Br J Sports Med. 2012;46(6):385–90. [DOI] [PubMed] [Google Scholar]
- 32.Lane C, Knight D, Burgess S, et al. Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax. 2004;59(9):757–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helenius IJ, Tikkanen HO, Haahtela T. Occurrence of exercise induced bronchospasm in elite runners: dependence on atopy and exposure to cold air and pollen. Br J Sports Med. 1998;32(2):125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bougault V, Turmel J, Boulet LP. Airway hyperresponsiveness in elite swimmers: is it a transient phenomenon? J Allergy Clin Immunol. 2011;127(4):892–8. [DOI] [PubMed] [Google Scholar]
- 35.Fitch KD, Sue-Chu M, Anderson SD, et al. Asthma and the elite athlete: summary of the International Olympic Committee's consensus conference, Lausanne, Switzerland, January 22–24, 2008. J Allergy Clin Immunol. 2008;122:254–60. [DOI] [PubMed] [Google Scholar]
- 36.Ansley L, Kippelen P, Dickinson J, Hull J. Misdiagnosis of exercise-induced bronchoconstriction in professional soccer players. Allergy. 2012;67(3):390–5. [DOI] [PubMed] [Google Scholar]
- 37.Molphy J, Dickinson J, Hu J, Chester N, Whyte G. Prevalence of bronchoconstriction induced by eucapnic voluntary hyperpnoea in recreationally active individuals. J Asthma. 2014;51(1):44–50. [DOI] [PubMed] [Google Scholar]
- 38.Becker JM, Rogers J, Rossini G, Mirchandani H, D’Alonzo GE., Jr Asthma deaths during sports: report of a 7-year experience. J Allergy Clin Immunol. 2004;113(2):264–7. [DOI] [PubMed] [Google Scholar]
- 39.Parise G, Mihic S, MacLennan D, Yarasheski KE, Tarnopolsky MA. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J Appl Physiol. 2001;91(3):1041–7. [DOI] [PubMed] [Google Scholar]
- 40.Louis M, Poortmans JR, Francaux M, et al. No effect of creatine supplementation on human myofibrillar and sarcoplasmic protein synthesis after resistance exercise. Am J Physiol Endocrinol Metab. 2003;285(5):E1089–94. [DOI] [PubMed] [Google Scholar]
- 41.Dangott B, Schultz E, Mozdziak PE. Dietary creatine monohydrate supplementation increases satellite cell mitotic activity during compensatory hypertrophy. Int J Sports Med. 2000;21(1):13–6. [DOI] [PubMed] [Google Scholar]
- 42.Aguiar AF, de Souza RW, Aguiar DH, Aguiar RC, Vechetti IJ, Dal-Pai-Silva M. Creatine does not promote hypertrophy in skeletal muscle in supplemented compared with nonsupplemented rats subjected to a similar workload. Nutr Res. 2011;31(8):652–7. [DOI] [PubMed] [Google Scholar]
- 43.Brosnan ME, Brosnan JT. The role of dietary creatine. Amino Acids. 2016;48(8):1785–91. [DOI] [PubMed] [Google Scholar]


