Abstract
Background:
Abdominal pain is the most common symptom in chronic pancreatitis (CP) and has an extensive impact on patients’ lives. Quantitative sensory testing (QST) provides information on sensitivity to pain and mechanisms that can help quantify pain and guide treatment. The aims of this study were (1) to explore sensitivity to pain in patients with CP using QST and (2) to associate patient and disease characteristics with QST results.
Methods:
Ninety-one patients with painful CP and 28 healthy control participants completed a QST paradigm using static tests (muscle pressure stimulation and electrical skin stimulations) to unravel segmental and widespread hyperalgesia as a consequence of visceral pain. A dynamic conditioned pain modulation (CPM) paradigm was used as a proxy of pain modulation from the brainstem to inhibit incoming nociceptive barrage, and questionnaires were used to gather information on pain experience and quality of life.
Results:
Patients had impaired CPM compared with controls (18.0±29.3% vs. 30.9±29.3%, P=0.04) and were hypersensitive to pressure stimulation, specifically in the pancreatic (Th10) dermatome (P<0.001). The capacity of CPM was associated with clinical pain intensity (P=0.01) and (in the univariate analysis only) the use of opioids was associated with hyperalgesia to pressure stimulation (P<0.05).
Conclusions:
Sensitivity to pain in CP patients can be characterized by a simple bedside QST. Severe clinical pain in CP was associated with reduced CPM function and should be targeted in management.
Key Words: chronic pancreatitis, pain, pain measurement, hyperalgesia
Abdominal pain is the most common symptom in chronic pancreatitis (CP) and is present during the clinical course of the disease in up to 90% of patients.1,2 The pain is often intense and long-lasting, and it is frequently associated with malnutrition, opioid addiction, physical and emotional disability, and major socioeconomic problems.3 Pain is a symptom that has an extensive influence on patients’ wellbeing and functional ability. Treatment of pancreatic pain can, however, be difficult with unpredictable outcome.4
Studies on pancreatic pain are difficult to interpret, as visceral pain is a highly individual perception, which is difficult to quantify.5 In the clinic, pain is affected by several confounders such as comorbidities, anxiety, and side effects to medications, which complicates the assessment of pain. To enable health care providers to better characterize sensitivity to pain, experimental models based on quantitative sensory testing (QST) can be used. These techniques are based on the rationale that different neural pathways and networks can be explored using standardized stimulation with simultaneous recording of the evoked pain response by psychophysical and/or objective methods.6–9
Because of convergence between visceral and somatic afferent nerves at the spinal level, somatic QST can be used indirectly to obtain information on pain sensitivity including segmental (spinal) and central sensitization in the context of visceral pain. In addition, QST may also provide knowledge on the dynamic function of the pain system using, for example, the CPM paradigm. This is an experimental paradigm designed to activate endogenous pain inhibitory systems, whereby centers in the brainstem gate incoming stimuli at the spinal cord. Both static and dynamic QST paradigms have previously been used in patients with CP and provided evidence for a malfunctioning pain system with signs of central sensitization and deficient inhibitory pain modulation.3,4,6,9 However, the association between QST assessment parameters and patients’ and disease characteristics remains unexplored.
In recent studies, QST has been used to characterize pain sensitivity in different diseases including neuropathy, chronic pelvic pain, irritable bowel syndrome, and functional dyspepsia.10–13 In these studies, “transetiological” patterns of sensory symptoms and deficits have been observed, thus emphasizing that abnormal pain sensitivity is universally observed across chronic pain conditions.14 Moreover, past studies have shown correlations between QST parameters and clinical pain characteristics,15 and QST has been used in predictive contexts, wherein pretreatment QST profiles were able to predict the outcome of surgery16 and treatment with analgesics.6,17
We hypothesized that somatic QST can be used to characterize pain sensitivity in CP and that putative changes would associate with clinical pain characteristics. The aims of the study were as follows: (1) to show that a simple somatic QST paradigm reveals significant and plausible differences in pain sensitivity between patients with painful CP and controls and (2) to investigate associations between clinical characteristics of pain and pain sensitivity in CP patients.
MATERIALS AND METHODS
Study Oversight
This was a cross-sectional study including patients with painful CP and healthy, pain-free volunteers as controls. All participants provided written informed consent before the examinations, which were conducted at the Centre for Pancreatic Diseases at Aalborg University Hospital, Denmark, and the Department of Surgery at Radboud University Nijmegen Medical Centre, Netherlands. The protocol was approved by the local ethical committees and medical agencies.
Patients
CP Patients
A total of 91 patients with CP from chronic abdominal pain were included in the study. Inclusion criteria included a minimum age of 18 years, CP diagnosis based on the Lünenburg criteria with a score of ≥4,18,19 and chronic abdominal pain typical for CP (ie, dull epigastric pain at least 3 days a week). Exclusion criteria were generalized chronic painful conditions other than CP and cognitive impairment hindering their ability to follow instructions. Patients were instructed to continue their usual analgesic treatment on the day of the examination.
A flowchart of the patient inclusion process is provided in Figure 1.
FIGURE 1.
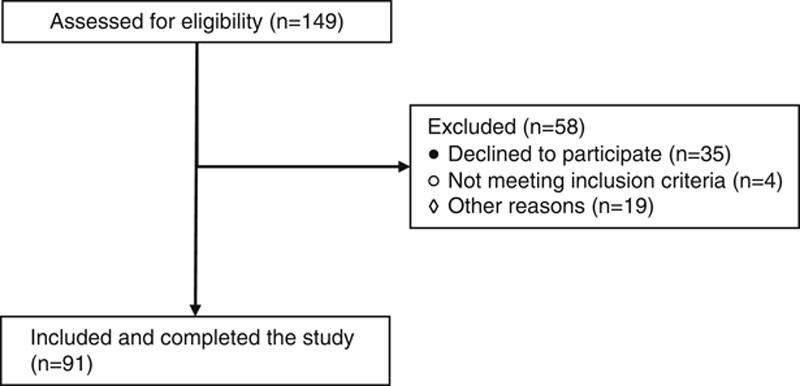
Flowchart of the inclusion process.
Healthy Controls
Twenty-eight healthy, pain-free adults were included as control group. Inclusion criteria included a minimum age of 18 years. Exclusion criteria included chronic or acutely painful conditions, regular use of any kind of analgesics, and pregnancy.
Procedures
Screening
Initially, all patients were screened according to the inclusion and exclusion criteria; this included a detailed patient history in the CP group to determine pain localization and characterization, comorbidities, alcohol and tobacco use, and medications. Opioid doses were converted to morphine equivalents in milligrams.20 All patients completed a pain questionnaire (Brief Pain Inventory—short form)21 and a quality of life questionnaire (EORTC QLQ-C3022).
The patients were instructed to keep a pain diary record on an 11-point Numeric Rating Scale every day for 1 week before examination. The patients reported the highest pain score and the average pain score experienced during the last 24 hours.
QST
QST was performed on all study participants using a standardized test sequence. Three investigators were trained in QST and performed the examinations.
Static QST Assessments
The static QST paradigm included pressure stimulation and electrical stimulation. Pressure stimulation thresholds were tested 1 time using an algometer with a 1.0 cm2 probe (Somedic AB, Stockholm, Sweden). The pressure thresholds were examined on the participant’s right side at 5 different sites: below the midline of the clavicula (C5 dermatome), pancreatic abdominal area, above the umbilicus (abdominal Th10 dermatome), pancreatic site (just lateral of the spine in the dorsal Th10 dermatome), hip region on the anterior superior iliac spine (L1 dermatome), and on the quadriceps muscle 5 cm proximal to the patella (L4 dermatome). Two thresholds were measured: pressure pain detection threshold and pressure pain tolerance threshold (pPTT).6,7,23
Thresholds to electric constant current skin stimulation (Digistim; Biometer A/S, Copenhagen, Denmark) with tetanic stimulation at 100 Hz were measured in the same dermatomes, using 2 electrodes placed 3 cm apart.24 The equivalents of the 2 thresholds were determined (electrical pain detection threshold and electrical pain tolerance threshold). These methods have previously been described in detail.25
Dynamic QST Assessment
CPM is a clinically measurable proxy of endogenous pain modulation. It can be experimentally induced using a conditioning stimulus (eg, the cold pressor test) and quantified by applying a test stimulation before and after the conditioning stimulus.26 In this study, the conditioning stimulus consisted of immersion of the dominant hand in cold water (2.0±0.3°C, continuously stirred) for 2 minutes. If the pain became intolerable before this point, the participants were allowed to remove their hand from the water. The duration of cold pressor stimulation was noted. The test stimulus was pressure stimulation (pPTT) measured on the L4 dermatome on the nondominant side, before the cold pressor test and immediately after its completion. The CPM capacity was quantified as the absolute and relative changes (%) in pPTT before and after the conditioning stimulation.
Comprehensive QST batteries have previously been recommended when examining pain sensitivity in chronic pain patients.27 These batteries consisted of up to 13 examination modalities and were very time consuming. In this study, we have focused on developing a QST paradigm that was possible to perform at the bedside in a limited time, consisting only of 4 elements designed to evaluate the most important pain mechanisms. The need for specialized equipment in this paradigm has also been kept at a minimum.
Patient and Disease Characteristics
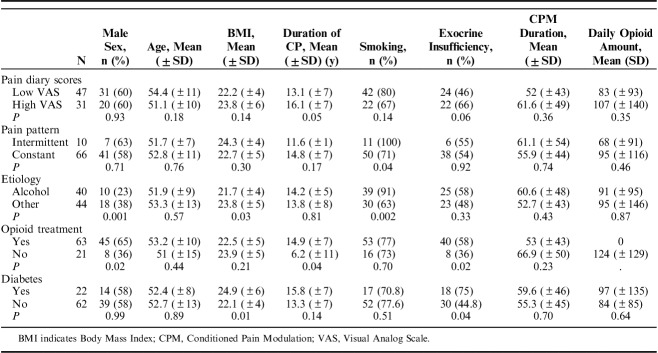
To examine whether clinical characteristics were associated with QST results, the patient group was subdivided according to pain intensity (mild to moderate: mean Visual Analog Scale score ≤5/severe: >5 in pain diary),28 presence of diabetes mellitus,29 opioid consumption (yes/no),30 and pain pattern (continuous/intermittent).31 Pain patterns were based on the pain diaries; constant pain was defined as persistent (daily) pain and intermittent pain as short periods of pain separated by pain-free days.
Statistical Analysis
All data are presented as mean±SD or number (%) unless otherwise indicated. Demographics, clinical data, and CPM parameters of CP patients and controls were compared by the Fisher exact test, Student t test, and 1-way analysis of variance, as appropriate. The 1-way analysis of variance tests were Bonferroni-corrected post hoc. Electrical and pressure stimulation data were log transformed to obtain a secondary normal distribution, and a mixed effects model was used with CP/healthy participants as fixed effects and stimulation site as a random effect, allowing us to obtain an analysis of the overall difference on pressure/electrical stimulation independent of the stimulation site. A mixed effect model was also used for subanalysis of electrical and pressure stimulation data, with clinical subgroups as fixed effects and stimulation site as a random effect. Univariate and multivariate regression analyses were performed to investigate the association of clinical subgroups with QST parameters. Correlations between electrical and pressure stimulation data were analyzed using the Pearson correlation coefficients.
A P-value ≤0.05 was considered statistically significant. The software package STATA, version 15.1 (StataCorp LP, College Station, TX) was used for statistical calculations.
RESULTS
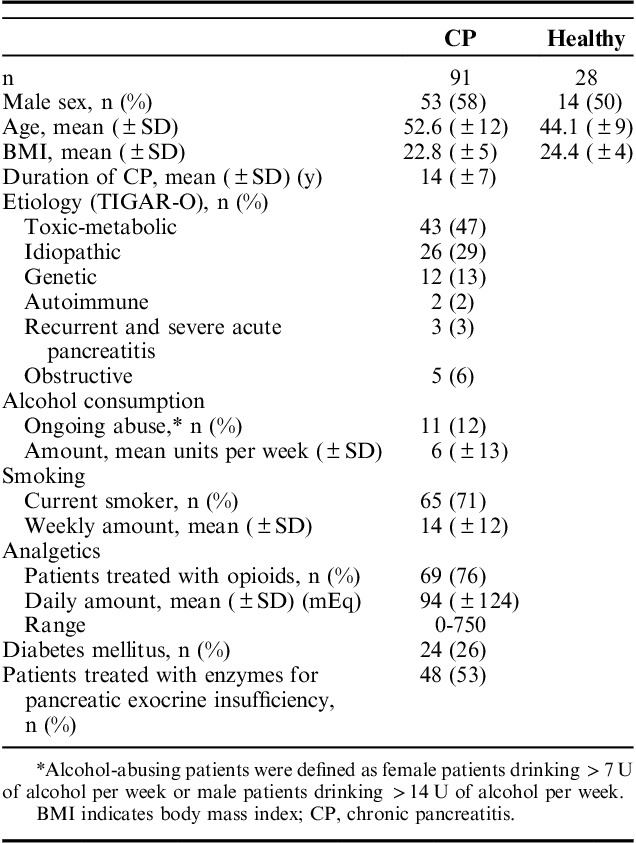
A total of 91 CP patients and 28 controls completed the study. Clinical and demographic characteristics of the 2 groups are presented in Table 1. The mean age of patients was 52.6±11.5 years compared with 44.1±9.0 years in controls (P<0.001). The distribution of sex was proportionate between patients and controls (male patients: 58% vs. 50%; P=0.44).
TABLE 1.
Clinical and Demographic Characteristics
Sensitivity to Pain in Patients With CP and in Healthy Controls
Pressure Stimulation
Overall, patients were hypersensitive to pressure stimulation compared with healthy controls (P<0.001). When comparing the examined dermatomes separately, patients had lower pressure pain threshold at all examined sites, albeit significant differences were only seen for the dorsal pancreatic dermatome (P=0.01), abdominal pancreatic dermatome (P<0.001), and L4-control dermatome (P=0.045) (Fig. 2).
FIGURE 2.
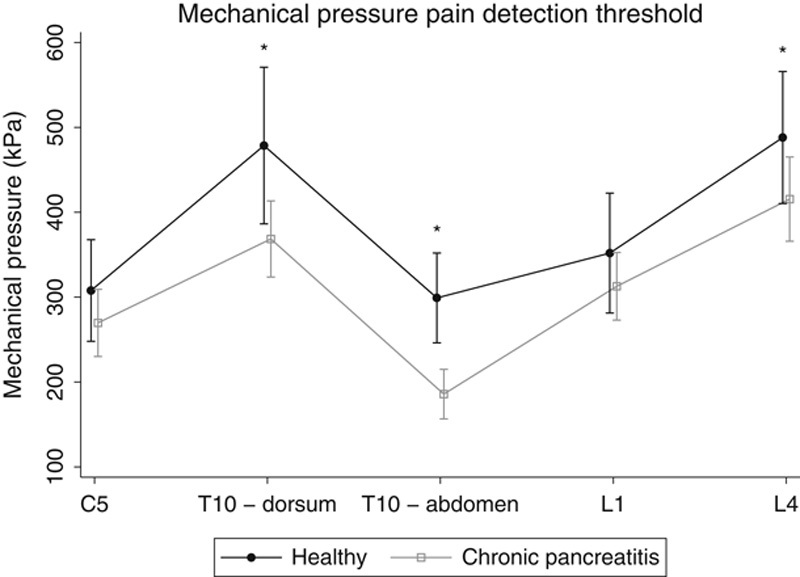
Pressure pain detection threshold in patients and healthy control participants. *P<0.05.
Electrical Stimulation
There were no significant overall differences in electrical pain thresholds between controls and patients (P=0.13), but an interaction between group and stimulation site was seen (P=0.002). Hence, differences in electrical pain thresholds were confined to specific dermatomes, and, when comparing the examined dermatomes separately, patients had lower electrical pain threshold in the dorsal pancreatic dermatome (P=0.01) and L1-control dermatome (P=0.045) (Fig. 3).
FIGURE 3.
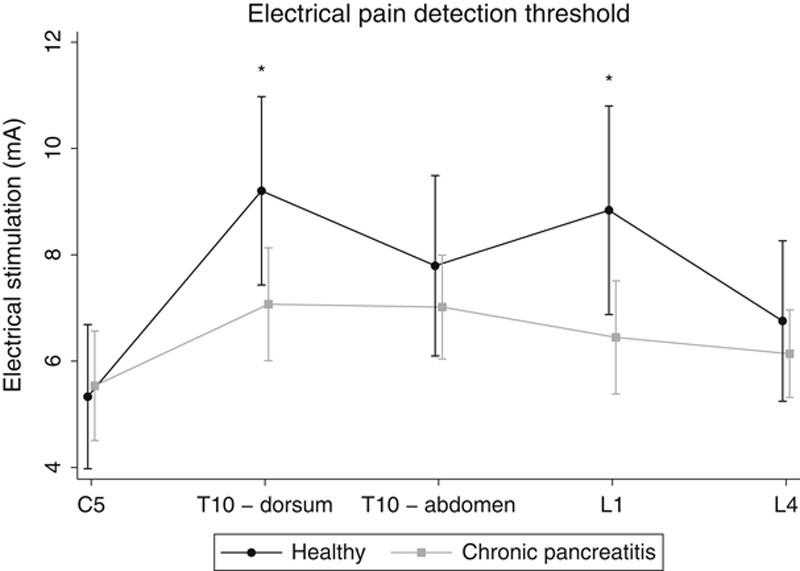
Electrical pain detection threshold in patients and healthy control participants. *P<0.05.
CPM
The mean duration of the cold pressor test was 56.4 seconds (±41.6) for patients and 118.9 seconds (±5.7) for the healthy controls (P<0.001). In controls, pPTT was 712±240 kPa at baseline and 922±327 kPa after the conditioning stimulation (P<0.001), and the absolute increase was 210±187 kPa. In patients, pPTT was 693±276 kPa at baseline and 794±307 kPa after the conditioning stimulus (P<0.001), and the absolute increase was 102±161 kPa. The relative increase in pPTT (CPM capacity) was 30.9±29.3% in the control group compared with 18.0±29.3% in the patient group (P=0.04). In the patients, there was no significant correlation between the duration of the cold pressor test and the CPM response (ρ=0.168, P=0.130).
To test whether age difference between patients and controls influenced the results, a regression analysis was performed and showed that neither the results of the pressure test (P=0.889), the electrical test (P=0.411), or CPM function (P=0.602) were dependent on age.
Sensitivity to Pain in Patients With CP by Patient and Disease Characteristics
The clinical and demographic characteristics of the different subgroups are presented in Table 2.
TABLE 2.
Demographic and Clinical Characteristics of the Patient Subgroups
Sensitivity to Pain by Pain Intensity
Patients with severe clinical pain had impaired mean CPM capacity compared with patients with mild to moderate pain and with controls (9.7±23.2% vs. 22.7±28.1% vs. 30.9±29.3%, P=0.01) (Fig. 4). There was no difference in the pain experienced during the cold pressor test in the subgroups (P=1.00). No other differences in QST parameters were observed between mild to moderate clinical pain and severe clinical pain; pressure stimulation (P=0.96) and electrical stimulation (P=0.97).
FIGURE 4.
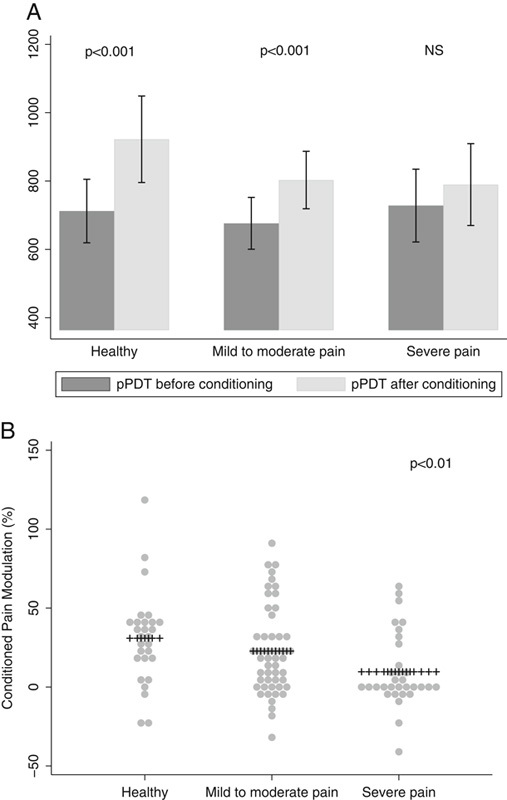
Conditioned Pain Modulation divided into absolute differences (A) and relative differences (conditioned pain modulation capacity) (B). NS indicates nonsignificant; pPDT, pressure pain detection threshold.
Sensitivity to Pain by Pain Pattern
There were no differences in QST parameters between patients with constant and intermittent pain patterns with regard to pressure stimulation (P=0.67), electrical stimulation (P=0.26), and CPM capacity (23.1±31.2% vs. 16.9±26.5%; P=0.08).
Sensitivity to Pain by Opioid Treatment
Opioid-treated patients were hypersensitive to pressure stimulation compared with their opioid-naive counterparts (P<0.05). When comparing the examined dermatomes, opioid-treated patients had lower pressure pain threshold at the abdominal pancreatic dermatome (208±102 vs. 181±145; P=0.01), while borderline significant differences were seen for the dorsal pancreatic dermatome (432±225 vs. 354±207; P=0.07) and L4-control dermatome (467±162 vs. 400±248; P=0.07) (Fig. 5). No other differences in QST parameters were observed between opioid-treated and opioid-naive patients; electrical stimulation (P=0.20) and CPM capacity (17.5±25.4 vs. 18.2±30.6; P=0.15).
FIGURE 5.
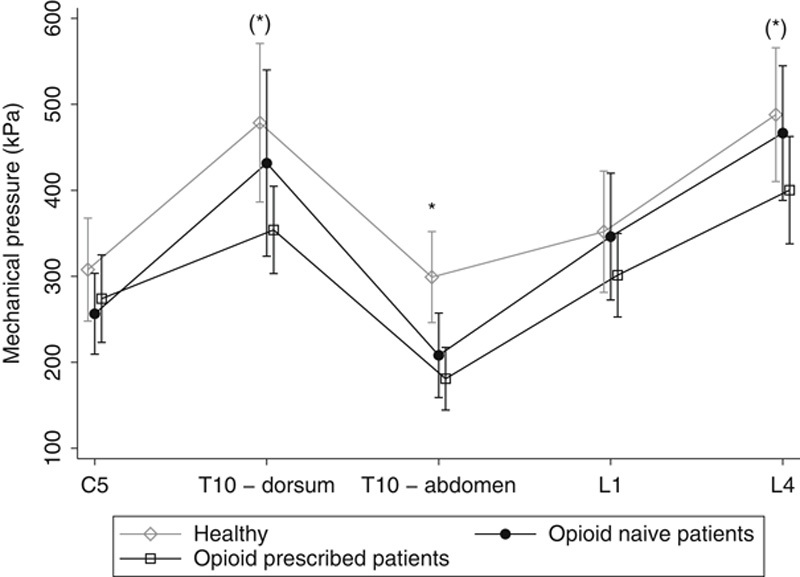
Comparison of pressure pain detection threshold in opioid-naive patients and opioid-treated patients. *P<0.05.
Sensitivity to Pain by Diabetes
There were no differences in QST parameters between diabetic and nondiabetic patients; pressure stimulation (P=0.97), electrical stimulation (P=0.14), and CPM (19.5±25.1 vs. 18.2±30.4; P=0.15).
Multivariate Analysis
Table 3 summarizes the result of the multivariate analysis for CPM. Clinical pain intensity (coefficient=−3.7±1.8%; P=0.047) was independently associated with less CPM capacity after adjusting for age, sex, diabetes, and opioid consumption (Fig. 6). In contrast, the significant difference in pressure stimulation thresholds when comparing opioid-naive and opioid-treated patients, was lost in the multivariate analysis (P=0.30).
TABLE 3.
Multivariate Analysis of Conditioned Pain Modulation Capacity (%) and Demographic and Clinical Variables
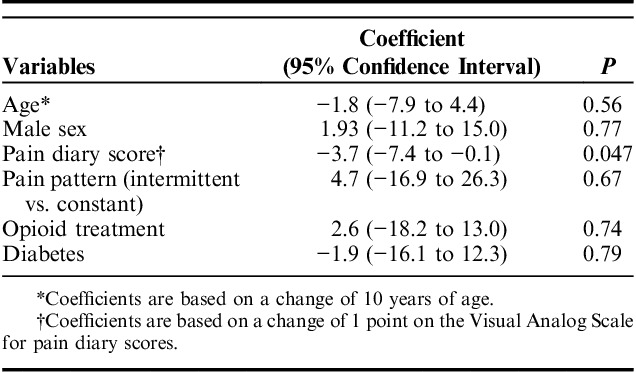
FIGURE 6.
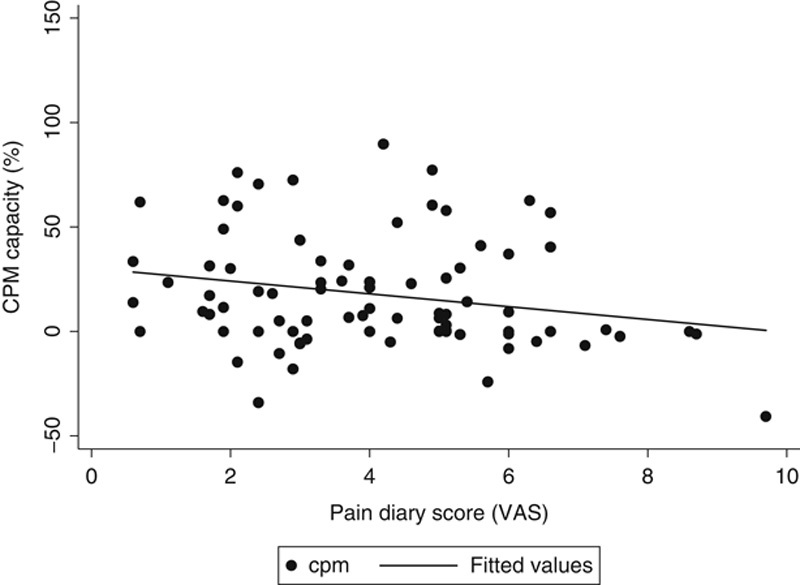
Association between CPM capacity and pain diary score from the data in the multivariate analysis. CPM indicates Conditioned Pain Modulation; VAS, Visual Analog Scale.
Correlations Between QST Parameters and Questionnaires
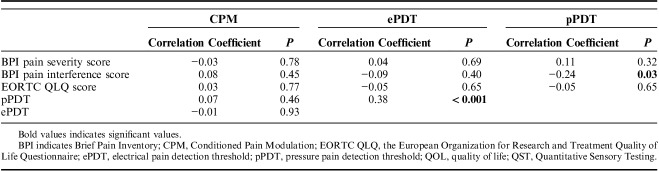
Average pressure pain thresholds (mean of all stimulation sites) were significantly correlated to the average electrical pain thresholds (r=0.38, P<0.001) and the pain interference score on the Brief Pain Inventory (r=−0.24, P=0.03). No other correlations were seen between the different QST parameters and questionnaire scores, as can be seen in Table 4.
TABLE 4.
Intercorrelations Between QST Parameters and Correlations With BPI and QOL Scores
DISCUSSION
We investigated central sensitivity to pain in patients with CP and in pain-free controls using a static and dynamic QST paradigm and examined its association with patient and disease characteristics. Patients were characterized by pressure hyperalgesia, with the most pronounced changes observed for the pancreatic dermatome and in patients on opioid-based pain medication. Furthermore, a decreased CPM capacity was seen in CP patients, which was independently associated with clinical pain intensity. Sensitivity to pain was unrelated to diabetes. These findings attest to the growing body of evidence suggestive of abnormal sensitivity to pain in CP patients and further support the use of somatic QST for pain characterization in this context.
Sensitivity to Pain in CP and Healthy Controls
We found that CPM capacity was impaired in the CP group compared with controls. In addition, CP patients were hypersensitive to pressure and electrical stimulations in the examined locations. The reduced CPM capacity in CP patients corresponds to results from previous studies.9,32,33 Systemic hyperalgesia in CP patients could indicate a change in sensitivity to pain. It has previously been suggested that patients with chronic pain have systemic sensitization secondary to central reorganization and structural brain changes.34–37 The patients in our study had been diagnosed with CP at a mean of 14 years before testing. Their long duration of disease may have resulted in a longer period of remodeling, and it maybe that patients with shorter duration of disease have shown lesser evidence of these changes.33
We have compared the results of this study with knowledge gained in the clinic from assessing the results of more extensive QST paradigms. We have found that these results are quite similar to what has previously been shown in QST examinations; however, no statistical validation has been performed.
Sensitivity to Pain and Clinical Parameters
Pain intensity was significantly associated with CPM capacity in CP patients, as patients with severe pain had more reduced CPM capacity compared with patients with mild pain. Similar results have been seen in other diseases such as irritable bowel syndrome, osteoarthritis, spinal cord injury, and functional dyspepsia.13,15,38,39 Prolonged duration of the painful condition has resulted in lower CPM capacity in both patients with CP and patients with osteoarthritis.9,15 Taken together with our finding of association between CPM capacity and pain intensity, the data indicate that impaired pain modulation is an important mechanism in chronic pancreatic pain. However, the question of whether reduced CPM capacity is caused by persistent severe pain, or is actually the cause for developing severe pain, remains unsolved. The predictive value of CPM function in the context of treatment has previously been studied. Bouwense et al40 found that CPM function predicted the efficacy of treatment with pregabalin in CP.
Diabetes can cause hyposensitivity due to neuropathy,41,42 but we found no association between the presence of diabetes and pressure stimulation, electrical stimulation, or CPM capacity, and all patients with symptoms of other painful conditions were excluded. These data suggest that, if any of the patients had an undiagnosed neuropathy, it did not seem to have an effect on the results, as the QST results of the diabetic group and the nondiabetic group were quite similar.
Of note, patients on opioid pain management regimens were found to be hypersensitive to pressure stimulation in univariate analysis compared with their opioid-naive counterparts. One explanation for this could be that these patients experience more severe pain (the reason for the opioid therapy) or, in some cases, opioid-induced hyperalgesia. In our study, the association was lost in the multivariate analysis, potentially due to type II error in the setting of a small sample size.
When looking at pain patterns over time, a previous study has found that patients with intermittent pain were more likely to respond to treatment than those with a more stable pain profile.43 It has been suggested that patients with continuous pain have a more stabilized and irreversible central sensitization, which is a sign of end-stage pain chronification with severe central neuroplastic changes that are less prone to respond to treatment.44 These findings were not reproducible in this study, as pain pattern was not significantly associated with QST parameters. This could be explained by the relatively short baseline pain registration, which might not be sufficient to illustrate the pain pattern thoroughly.
In the current study, there was no screening for comorbidities such as anxiety and depression, which may influence the results. However, the lack of correlation between life quality and QST results suggest that such symptoms are likely not of major importance in this study.
Pain sensitivity has been quantified using somatic QST, although the pain in CP is visceral. This could, in theory, be a source of error, as it is an indirect measurement. However, variations with QST similar to those found in the clinical subgroups of the CP patients have previously been seen in various somatic pain conditions, implying that this variation is not caused by the indirect measurements relating to the pancreas, but rather to central changes in the pain system.10,15,45,46
Strengths and Limitations
QST has been widely used in pain research over the past 40 years, and, especially, the static tests have shown a high degree of standardization and reproducibility.7 Dynamic QST tests in chronic pain patients have suffered from large variability between patients and poor day-to-day reproducibility in patients with CP. Although dynamic tests such as CPM are encumbered by this variability, an argument to be made is that substantial variability over time in the CPM paradigm must be expected, given the necessarily reactive and dynamic nature of the modulation of nociceptive inputs.7,47 The fluctuating nature of chronic pancreatic pain may also contribute to the variability. We have considered this in our analysis of dynamic QST results and performed our testing in a standardized manner to limit unnecessary variability.
In addition, an age difference was seen between patients and controls, with the patients being of more advanced age. Previous studies have shown that advancing age can affect the QST results,48,49 but when examining whether the age differences influenced the results of the tests, no connection was found. We, therefore, conclude that the age difference did not affect the results of this study.
There was also a difference in the duration of the cold pressor test between patients and controls. However, no correlation between the duration of the cold pressor test and the magnitude of CPM response in patients was found, and this could suggest that the patients’ CPM response is already chronically activated to its maximum, thereby not allowing for further increase, and this could even result in facilitation of the pain.
As a comparator, one could reflect on whether it is optimal to use healthy volunteers or whether a control group consisting of patients with similar comorbidities and other characteristics (ie, alcohol consumption, smoking habits, etc.), for example, a group consisting of patients with pain-free CP, would be better suited. We will focus on elucidating this aspect in future studies.
The data concerning the patients compared with healthy volunteers are, however, not the main finding of this study. It is a result in line with previous studies in CP and QST and is, therefore, mainly interesting in the fact that it confirms that the results of this study probably can be transferred to the general population of patients with painful CP.
In this study, we compared CP patients on opioid management regimes with their opioid-naive counterparts, but we did not include a control group on opioid management regimens due to nonabdominal pain. It is, therefore, difficult to elucidate the full effects of opioids on QST results, and we plan to include this in future studies.
In this study, we did not examine psychiatric comorbidities such as anxiety and depression, although this could influence the patient pain report, as psychiatric comorbidities can change the patients’ experience of pain. All results should, therefore, be evaluated with this in mind. As a consequence, we have included a screening of psychiatric comorbidities in the examination at our clinic to ensure that the examination is thorough and exhaustive.4,50
Clinical Perspective: Mechanism-based Treatment
The choice of analgesic used to treat pancreatic pain, is typically based on local traditions and the treating physician’s personal experience and preferences, and, in severe pain, opioids are often needed. For some practitioners and patients, opioids play an integral part in the treatment of persistent severe pain. Although opioids can provide effective analgesia, they are also associated with considerable side effects, and a high risk of addiction.51 Several studies point to the fact that patients can benefit from a more individualized treatment approach with a focus on pain mechanisms.4,25,52 QST can be used to characterize the patient’s sensitivity to pain and thereby improve the possibility of individualizing treatment according to affected pain mechanisms.53,54 For example, it has been shown that pregabalin has moderate inhibitory effects on central sensitization, and this effect can be predicted by static QST.6 Likewise, a reduced CPM capacity can be potentiated with either tapentadol or duloxetine, and the clinical effect of such treatment is predictable by dynamic QST.17,55 CPM function has also shown to be predictive of pregabalin’s treatment effect in patients with CP.40
Our study provides a simple QST protocol that can be applied in the clinic, with results that are comparable to prior literature comparing CP patients with controls.9,33 Its simplicity makes it amenable to bedside examination in the clinical setting. Incorporating this protocol into clinical evaluation of pancreatic pain represents a feasible and important improvement in the evaluation of pancreatic pain, as recommended in international treatment guidelines (Drewes and colleagues). This study does not provide sufficient control material to estimate reliable QST-based cutoff values, but this will be the next step in developing a validated QST-based treatment guideline.50
CONCLUSIONS
The clinical characteristics of CP patients associate with objective quantitative testing of the pain system. The main finding was that severe clinical pain was associated with decreased CPM capacity. This discovery, via the use of quantitative sensory testing, may represent a paradigm shift in clinical evaluation and treatment of CP. These findings further emphasize that changes in sensitivity to pain are important. Future studies should address how quantitative sensory testing can be used to predict disease course and treatment response.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Olesen SS, Poulsen JL, Drewes AM, et al. The Scandinavian Baltic Pancreatic Club (SBPC) database: design, rationale and characterisation of the study cohort. Scand J Gastroenterol. 2017;52:909–915. [DOI] [PubMed] [Google Scholar]
- 2.Machicado JD, Amann ST, Anderson MA, et al. Quality of life in chronic pancreatitis is determined by constant pain, disability/unemployment, current smoking and associated co-morbidities. Am J Gastroenterol. 2017;112:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olesen SS, Juel J, Graversen C, et al. Pharmacological pain management in chronic pancreatitis. World J Gastroenterol. 2013;19:7292–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drewes AM, Bouwense SAW, Campbell CM, et al. Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology. 2017;17:720–731. [DOI] [PubMed] [Google Scholar]
- 5.Olesen AE, Farmer AD, Olesen SS, et al. Management of chronic visceral pain. Pain Manag. 2016;6:469–486. [DOI] [PubMed] [Google Scholar]
- 6.Olesen SS, Graversen C, Bouwense SAW, et al. Quantitative sensory testing predicts pregabalin efficacy in painful chronic pancreatitis. PLoS One. 2013;8:e57963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olesen SS, van Goor H, Bouwense SAW, et al. Reliability of static and dynamic quantitative sensory testing in patients with painful chronic pancreatitis. Reg Anesth Pain Med. 2012;37:530–536. [DOI] [PubMed] [Google Scholar]
- 8.Dimcevski G, Staahl C, Andersen SD, et al. Assessment of experimental pain from skin, muscle, and esophagus in patients with chronic pancreatitis. Pancreas. 2007;35:22–29. [DOI] [PubMed] [Google Scholar]
- 9.Olesen SS, Brock C, Krarup AL, et al. Descending inhibitory pain modulation is impaired in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2010;8:724–730. [DOI] [PubMed] [Google Scholar]
- 10.Freeman R, Baron R, Bouhassira D, et al. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain. 2014;155:367–376. [DOI] [PubMed] [Google Scholar]
- 11.Verne GN, Price DD, Callam CS, et al. Viscerosomatic facilitation in a subset of IBS patients, an effect mediated by N-methyl-d-aspartate receptors. J Pain. 2012;13:901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26:253–259. [DOI] [PubMed] [Google Scholar]
- 13.Wilder-Smith CH, Li X, Shen L, et al. Dysfunctional endogenous pain modulation in patients with functional dyspepsia. Neurogastroenterol Motil. 2014;26:489–498. [DOI] [PubMed] [Google Scholar]
- 14.Edwards RR, Dworkin RH, Turk DC, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain. 2016;157:91406–91417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arendt-Nielsen L, Egsgaard LL, Petersen KK, et al. A mechanism-based pain sensitivity index to characterize knee osteoarthritis patients with different disease stages and pain levels. Eur J Pain. 2015;19:1406–1417. [DOI] [PubMed] [Google Scholar]
- 16.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. [DOI] [PubMed] [Google Scholar]
- 17.Niesters M, Proto PL, Aarts L, et al. Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. Br J Anaesth. 2014;113:148–156. [DOI] [PubMed] [Google Scholar]
- 18.Lankisch PG, Breuer N, Bruns A, et al. Natural history of acute pancreatitis: a long-term population-based study. Am J Gastroenterol. 2009;104:2797–2805. [DOI] [PubMed] [Google Scholar]
- 19.Lankisch PG, Assmus C, Maisonneuve P, et al. Epidemiology of pancreatic diseases in Lüneburg County: a study in a defined German population. Pancreatology. 2002;2:469–477. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien T, Christrup LL, Drewes AM, et al. European Pain Federation position paper on appropriate opioid use in chronic pain management. Eur J Pain. 2017;21:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen MP, Turner JA, Romano JM. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157–162. [DOI] [PubMed] [Google Scholar]
- 22.Fitzsimmons D, Kahl S, Butturini G, et al. Symptoms and quality of life in chronic pancreatitis assessed by structured interview and the EORTC QLQ-C30 and QLQ-PAN26. Am J Gastroenterol. 2005;100:918–926. [DOI] [PubMed] [Google Scholar]
- 23.Dimcevski G, Schipper KP, Tage-Jensen U, et al. Hypoalgesia to experimental visceral and somatic stimulation in painful chronic pancreatitis. Eur J Gastroenterol Hepatol. 2006;18:755–764. [DOI] [PubMed] [Google Scholar]
- 24.Buscher HCJL, Wilder-Smith OHG, van Goor H. Chronic pancreatitis patients show hyperalgesia of central origin: a pilot study. Eur J Pain. 2006;10:363–370. [DOI] [PubMed] [Google Scholar]
- 25.Olesen SS, Bouwense SAW, Wildersmith OHG, et al. Pregabalin reduces pain in patients with chronic pancreatitis in a randomized, controlled trial. Gastroenterology. 2011;141:536–543. [DOI] [PubMed] [Google Scholar]
- 26.Pud D, Granovsky Y, Yarnitsky D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain. 2009;144(1–2):16–19. [DOI] [PubMed] [Google Scholar]
- 27.Vollert J, Attal N, Baron R, et al. Quantitative sensory testing using DFNS protocol in Europe: an evaluation of heterogeneity across multiple centers in patients with peripheral neuropathic pain and healthy subjects. Pain. 2016;157:750–758. [DOI] [PubMed] [Google Scholar]
- 28.Katz J, Melzack R. Measurement of pain. Surg Clin North Am. 1999;79:231–252. [DOI] [PubMed] [Google Scholar]
- 29.Vinik AI, Nevoret ML, Casellini C, et al. Diabetic neuropathy. Endocrinol Metab Clin North Am. 2013;42:747–787. [DOI] [PubMed] [Google Scholar]
- 30.Calvino B. Opioid induced hyperalgesia. Douleurs. 2013;14:226–233. [Google Scholar]
- 31.Mullady DK, Yadav D, Amann ST, et al. Type of pain, pain-associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort study. Gut. 2011;60:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouwense S a W, Olesen SS, Drewes AM, et al. Effects of pregabalin on central sensitization in patients with chronic pancreatitis in a randomized, controlled trial. PLoS One. 2012;7:e42096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouwense SAW, Olesen SS, Drewes AM, et al. Is altered central pain processing related to disease stage in chronic pancreatitis patients with pain? An exploratory study. PLoS One. 2013;8:e55460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brock C, Olesen SS, Valeriani M, et al. Brain activity in rectosigmoid pain: unravelling conditioning pain modulatory pathways. Clin Neurophysiol. 2012;123:829–837. [DOI] [PubMed] [Google Scholar]
- 35.Frøkjær JB, Bouwense SAW, Olesen SS, et al. Reduced cortical thickness of brain areas involved in pain processing in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2012;10:434–438. [DOI] [PubMed] [Google Scholar]
- 36.Lelic D, Olesen SS, Hansen TM, et al. Functional reorganization of brain networks in patients with painful chronic pancreatitis. Eur J Pain. 2014;18:968–977. [DOI] [PubMed] [Google Scholar]
- 37.Olesen SS, Frøkjær JB, Lelic D, et al. Pain-associated adaptive cortical reorganisation in chronic pancreatitis. Pancreatology. 2010;10:742–751. [DOI] [PubMed] [Google Scholar]
- 38.Bouhassira D, Moisset X, Jouet P, et al. Changes in the modulation of spinal pain processing are related to severity in irritable bowel syndrome. Neurogastroenterol Motil. 2013;25:623–632. [DOI] [PubMed] [Google Scholar]
- 39.Albu S, Gomez-Soriano J, Avila-Martin G, et al. Deficient conditioned pain modulation after spinal cord injury correlates with clinical spontaneous pain measures. Pain. 2015;156:260–272. [DOI] [PubMed] [Google Scholar]
- 40.Bouwense SA, Olesen SS, Drewes AM, et al. Pregabalin and placebo responders show different effects on central pain processing in chronic pancreatitis patients. J Pain Res. 2015;8:375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Telli O, Cavlak U. Measuring the pain threshold and tolerance using electrical stimulation in patients with type II diabetes mellitus. J Diabetes Complications. 2006;20:308–316. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki C, Kon T, Funamizu Y, et al. Elevated pain threshold in patients with asymptomatic diabetic neuropathy: an intraepidermal electrical stimulation study. Muscle Nerve. 2016;54:146–149. [DOI] [PubMed] [Google Scholar]
- 43.Negi S, Singh A, Chaudhary A. Pain relief after Frey’s procedure for chronic pancreatitis. Br J Surg. 2010;97:1087–1095. [DOI] [PubMed] [Google Scholar]
- 44.Martini CH, Yassen A, Krebs-Brown A, et al. A novel approach to identify responder subgroups and predictors of response to low- and high-dose capsaicin patches in postherpetic neuralgia. Eur J Pain. 2013;17:1491–1501. [DOI] [PubMed] [Google Scholar]
- 45.Vaegter HB, Graven-Nielsen T. Pain modulatory phenotypes differentiate subgroups with different clinical and experimental pain sensitivity. Pain. 2016;157:1480–1488. [DOI] [PubMed] [Google Scholar]
- 46.Frey-Law LA, Bohr NL, Sluka KA, et al. Pain sensitivity profiles in patients with advanced knee osteoarthritis. Pain. 2016;157:1988–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marcuzzi A, Wrigley PJ, Dean CM, et al. The long-term reliability of static and dynamic quantitative sensory testing in healthy individuals. Pain. 2017;158:1217–1223. [DOI] [PubMed] [Google Scholar]
- 48.Magerl W, Krumova EK, Baron R, et al. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain. 2010;151:598–605. [DOI] [PubMed] [Google Scholar]
- 49.Guirro RR, Guirro EC, de Sousa NT. Sensory and motor thresholds of transcutaneous electrical stimulation are influenced by gender and age. PM R. 2015;7:42–47. [DOI] [PubMed] [Google Scholar]
- 50.Kuhlmann L, Olesen SS, Olesen AE, et al. Mechanism-based pain management in chronic pancreatitis—is it time for a paradigm shift? Expert Rev Clin Pharmacol. 2019;12:1–10. [DOI] [PubMed] [Google Scholar]
- 51.Ivanova JI, Birnbaum HG, Yushkina Y, et al. The prevalence and economic impact of prescription opioid-related side effects among patients with chronic noncancer pain. J Opioid Manag. 2013;9:239–254. [DOI] [PubMed] [Google Scholar]
- 52.Bouwense SAW, Buscher HCJL, van Goor H, et al. Has central sensitization become independent of nociceptive input in chronic pancreatitis patients who fail thoracoscopic splanchnicectomy? Reg Anesth Pain Med. 2011;36:531–536. [DOI] [PubMed] [Google Scholar]
- 53.Grosen K, Fischer IWD, Olesen AE, et al. Can quantitative sensory testing predict responses to analgesic treatment? Eur J Pain. 2013;17:1267–1280. [DOI] [PubMed] [Google Scholar]
- 54.Cruz-Almeida Y, Fillingim R. Can quantitative sensory testing move us closer to mechanism-based pain management. Pain Med. 2014;15:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–28. [DOI] [PubMed] [Google Scholar]