ABSTRACT
Background:
Gelsolin is an actin-scavenger controlling the tissue damage from actin in the blood. Gelsolin levels in circulation drops when tissue damage and corresponding actin release is pronounced due to catabolic conditions. The purpose of this study was to determine if low plasma gelsolin independently predicts a reduced chance of weaning from ventilator-demanding respiratory failure in critically ill patients within 28 days from admission.
Results:
This cohort study included 746 critically ill patients with ventilator-demanding respiratory failure from the randomized clinical trial, “Procalcitonin And Survival Study (PASS).” Primary end point was successful weaning from mechanical ventilation within 28 days. We used multivariable Cox regression adjusted for age, sepsis, PaO2/FiO2 ratio and other known and suspected predictors of persistent respiratory failure. Follow-up was complete.
For medical patients, baseline-gelsolin below the 25th percentile independently predicted a 40% lower chance of successful weaning within 28 days (HR 0.60, 95% CI 0.46–0.79, P = 0.0002); among surgical patients this end point was not predicted. Low gelsolin levels predicted chance of being “alive and out of intensive care at day 14” for both medical and surgical patients (HR 0.69, 95% CI 0.54–0.89, P = 0.004). Gelsolin levels did not predict 28 day mortality for surgical or medical patients.
Conclusions:
Low levels of serum gelsolin independently predict a decreased chance of successful weaning from ventilator within 28 days among medical intensive care patients. This finding has implications for identifying patients who need individualized intervention early in intensive care course to prevent unfavorable lung prognosis in acute respiratory failure.
Trial registration:
This is a substudy to the PASS, Clinicaltrials.gov ID: NCT00271752, first registered January 1, 2006.
Keywords: Baseline parameters, biomarker, ICU, mechanical ventilation, successful weaning
Abbreviations: SPD, Surfactant protein D, Q1, lower quartile, PCT, procalcitonin, sTM, soluble thrombomodulin and nitrogen, NOS3, oxide synthase type III
INTRODUCTION
Every year 5.7 million patients are admitted to ICU in the United States (1). Of these, 20%–40% requires mechanical ventilation (2). Critically ill patients with respiratory failure requiring mechanical ventilation have a high mortality rate (3, 4). To improve survival of this vulnerable group of patients, we need more knowledge about which potentially modifiable parameters, present at baseline, that influences mortality.
However useful the current acute respiratory distress syndrome (ARDS) is, many patients suffer from a certain degree of lung injury, and eventually die or suffer persistent dependence of mechanical ventilation, although they do not meet the criteria for ARDS. This prompts the need for further research to describe these patients physiologically, biochemically, and clinically, eventually to offer treatment options for this group. Biomarker research is one among several means to describe such patients.
Intracellular actin is involved in maintaining cell shape and motility (5–7). In catabolic conditions, e.g., sepsis, trauma, hepatic necrosis, ARDS, and myocardial infarction, damaged cells release globular actin to the extracellular space and the bloodstream (7, 8). The concentration of actin in the circulation may saturate the actin-scavenging proteins, gelsolin and Gc-globulin (9). This leads to accumulation of actin that polymerizes and forms actin filaments (7, 9). Actin filaments in the blood contribute to pulmonic vessel obstruction, pulmonary micro thrombi, and endothelial damage (7, 9, 10). In addition, actin activates platelets, which further increases the risk of thrombi and thereby obstruction of the microcirculation in the lungs (7, 11).
Gelsolin exists as intracellular and extracellular forms coded by the same gene on chromosome 9 (12). The extracellular gelsolin is primarily produced by muscle cells and diffuses to circulation afterward (13). Extracellular gelsolin breaks down actin filaments and forms gelsolin-actin complexes, which leads to a decreasing concentration of free gelsolin in the bloodstream when actin increases (7, 9). Particular conditions such as sepsis, acute liver failure, myocardial infarction, lung injury, trauma and chronic inflammatory diseases are associated with lowered plasma gelsolin concentration (6, 11, 14–21). Patients with the aforementioned conditions and a low concentration of gelsolin seem to have a longer period of mechanical ventilation, longer stay at ICU and an increased mortality compared to patients with preserved gelsolin levels, albeit the sample sizes studied have been small (11, 10, 22). The plasma concentration of gelsolin in healthy individuals is measured to a wide range of values, ranging from 190 mg/L to 517 mg/L (7, 11, 23).
An already known biomarker of lung injury and activity of lung diseases is surfactant protein D (SPD), to which gelsolin could be an alternative or supplement to (24–26). Since SPD usually is found in the alveoli, an increasing serum SPD indicates damage to the alveolar epithelial barrier. An increased serum SPD predicts a longer duration of respiratory failure (24, 26). The change in soluble thrombomodulin (sTM) gives a measure of endothelial damage (27).
Objectives
The primary objective of this study was to determine whether the risk of persistent ventilator-demanding respiratory failure is increased among critically ill patients if baseline gelsolin is low, measured using the end point “successful weaning from respirator within 28 days.” Secondarily, we wanted to determine if patients with a low baseline gelsolin level had a poorer overall prognosis measured as mortality and duration of stay at ICU. Additionally, as an exploratory objective, we aimed to find out whether low baseline gelsolin proceeded endothelial damage later in the patient course measured as change in the level of soluble sTM.
METHODS
Study population
This multicenter observational study was based on data collected among patients in 9 ICUs requiring mechanical ventilation within the cohort of the “Procalcitonin And Survival Study” (PASS)-trial between 2006 and 2010 (28). The patients had blood samples drawn every day until day 28 if still in ICUs. Data were collected in line with guidelines for Good Clinical Practice (29).
Patients aged 18 or above, requiring mechanical ventilation and having a measurement of serum gelsolin at day 1 were included. Patients could not be included in the study if they were pregnant, breast feeding or had an expected stay at ICU <24 h.
Sample analysis
Gelsolin was subsequently determined in blood samples from day 1.
Samples were stored at −20°C until after study completion and analyzed subsequently to make sure that the physicians were blinded to the gelsolin levels during extubation of the patients. Gelsolin was measured in serum samples which were thawed at 37°C and mixed before analysis. An automated Modular P analyzer (Roche Diagnostics, Risch-Rotkreuz, Schweiz) was used for the determination of gelsolin with the applied settings and performance characteristics provided by the reagent manufacture (30). The concentration-dependent immunoprecipitation, formed by the mixture of Rabbit Anti Human Gelsolin (Dako Code No. A0146, Glostrup, Denmark), and gelsolin containing patient samples was measured against the calibrator material (Dako Code No. X0908, Glostrup, Denmark). Control determination was executed multiple times for each calibration and mean within calibration coefficient of variation was 2.7%. The lowest standard point on the calibration curve was 17.2 mg/L.
End-points
The primary end point was “successful weaning from respirator within 28 days of ICU admission.” Only patients who were alive and extubated at day 28 were considered successfully extubated. The time point for extubation was defined as the last day the patient was receiving mechanical ventilation.
The secondary end points included 28 days mortality, alive and out of intensive care within 14 days, and change in the level of endothelial damage (defined as continuous soluble sTM concentration).
Variables
The exposure variable is plasma gelsolin at day 1 <25th percentile. The confounding variables are sepsis/septic shock (yes vs. no), surgical origin (yes vs. no), gender (male vs. female), age (per year increase), acute thrombosis defined as acute myocardial infarction, pulmonary embolism, cerebral infarction, mesenteric embolism, or medulla spinalis infarction (yes vs. no), chronic inflammatory diseases defined as rheumatoid arthritis, Sjögren syndrome, fibromyalgia, spondylosis, hepatic cirrhosis, systemic lupus erythematosus, vasculitis, and chronic glomerulonephritis (yes vs. no), baseline procalcitonin (PCT) (≥1 μg/L vs. <1 μg/L) (31), PaO2/FiO2 ratio (lower quartile (Q1) versus upper 3 quartiles (Q2–Q4)), baseline SPD >85th percentile, Apache II score (<25 vs. ≥25) (32) and chronic obstructive pulmonary disease (COPD). The variables were chosen because they were all known or suspected predictors of duration of ventilation or levels of baseline gelsolin.
Statistical analysis of data
We used a Cox proportional hazards model to analyze difference in time to event in-between the patients in the lower quartile and the upper 3 quartiles (e.g., death, successful extubation), and further to make an interaction analysis. We compared the number of ventilator-free days and ICU-free days within 28 days with a Mann–Whitney U test. The division into the lower quartile and the upper 3 quartiles was defined before the analysis was carried out. Cumulative incidence curves and Kaplan–Meier curves were used to illustrate events over time. Pearson's correlation analysis was used to evaluate correlations between baseline gelsolin and change in soluble sTM from day 1 to day 4. Two-sided P values were used, P < 0.05 were defined as significant. Statistical analyses were made in SAS 9.4 windows version Cary, NC) and IBM SPSS Statistics version 22 (Armonk, NY).
A power calculation was carried out for the primary end point prior to the study with the prior known sample size, which was 758. We later adjusted sample size to 746, because 12 patients did not have a baseline gelsolin measurement or we did not know if they had a surgical origin. A conventional limit of type 1 error was set at 0.05, the exposure variable is present in 25% of patients (lowest quartile, defined), the end point (successful weaning within 28 days) was thought to be present in 85% of the remaining patients, variance inflation factor of 0.3 and a power of (1-β) 0.8. This results in a detection limit of the odds ratio of 0.68 in a logistic regression model. Power calculation was done with “Study Size 3.0”, Creostat, Frölunda, Sweden. The exposure variable is lower quartile plasma gelsolin at day 1. The end point is “successful weaning from respirator within 28 days of ICU admission.”
RESULTS
In total 1,200 patients were recruited between 2006 and 2010 in “the PASS study.” Of these, 746 patients were intubated at baseline and had analyzed baseline gelsolin concentration (Fig. 1). In total, 746 patients were included in the study. Baseline characteristics of the included patients are presented in Table 1.
Fig. 1.
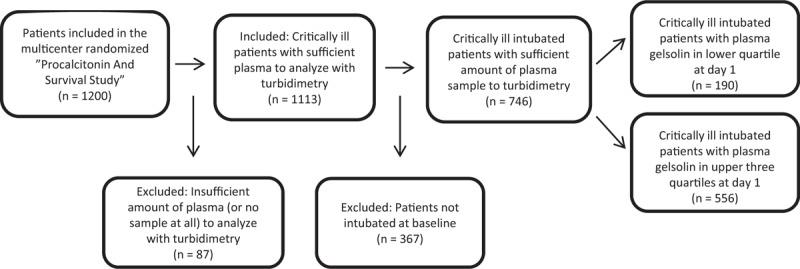
Flowchart of patients included.
Table 1.
Baseline characteristics of patients with baseline gelsolin level below the 25th percentile and the patients above the 25th percentile
| Characteristic | Gelsolin lower quartile (n = 190) | Gelsolin upper quartiles 2–4 (n = 556) | P values for differences |
| Age (year, median, IQR) | 64 (56–73) | 69 (59–76) | 0.001 |
| Gender (n male/n female) | 103 (54.2)/87 (45.8) | 300 (54.0)/256 (46.0) | 1.0 |
| Apache II score (median, IQR) | 21 (16–27) | 20 (14–26) | 0.3 |
| BMI (kg/m2, median, IQR) | 24.8 (22.0–27.8) | 24.7 (22.2–27.7) | 0.5 |
| COPD n (%) | 25 (13.2) | 122 (21.9) | 0.009 |
| Severe sepsis/septic shock n (%) | 116 (61.1) | 184 (33.1) | <0.0001 |
| Surgical origin n (%) | 71 (37.4) | 141 (25.4) | 0.002 |
| Acute thrombosis* n (%) | 4 (2.1) | 29 (5.2) | 0.07 |
| Chronic inflammatory disease† n (%) | 14 (7.37) | 29 (5.2) | 0.3 |
| Procalcitonin (μg/L, median, IQR) | 12.3 (2.8–38.2) | 2.61 (0.5–14.3) | 0.0002 |
| PaO2/FiO2 ratio kPa/mmHg (median, IQR) | 21.0 (13.4–29.3)/157.5 (100.5–219.8) | 19.6 (12.2–29.0)/147.0 (91.5–217.5) | 0.8 |
| SPD (ng/mL, median, IQR) | 94.2 (55.6–172.7) | 135.3 (78.9–314.2) | 0.0002 |
| ARDS n (%) | 10 (3.6) | 20 (5.3) | 0.3 |
| Gelsolin (mg/L, median, IQR) | 29.1 (17.2–42.2) | 90.0 (69.9–115.6) | <0.0001 |
*Acute myocardial infarction, pulmonary embolism, cerebral infarction, mesenteric embolism, and medulla spinalis infarction.
†Rheumatoid arthritis, Sjögren syndrome, fibromyalgia, spondylosis, hepatic cirrhosis, systemic lupus erythematosus, vasculitis, and chronic glomerulonephritis.
ARDS indicates acute respiratory distress syndrome; BMI, body mass index; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; SPD, surfactant protein D.
The patients were divided into 2 groups; a lower quartile (Q1) and 3 upper quartiles (Q2–Q4) according to baseline gelsolin measurement. The median gelsolin concentration in this population was 75.1 mg/L (IQR = 50.3–105.1), in the lower quartile it was 29.1 mg/L (IQR = 17.2–42.2), whereas the median gelsolin concentration was 90.0 mg/L (IQR = 69.9–115.6) in the upper 3 quartiles.
Successful weaning from mechanical ventilation within 28 days is related to gelsolin levels at baseline
Intubated patients with a baseline gelsolin level below the 25th percentile had 29% lower chance of being successfully weaned within 28 days compared to the patients with baseline gelsolin level above the 25th percentile (HR 0.71, 95% CI 0.58–0.88, P = 0.002) (Table 2). Apart from gelsolin concentration below the 25th percentile, the chance of successful weaning within 28 days was decreased by severe sepsis/septic shock, PCT baseline level >1 μg/L, SPD baseline levels >85th percentile, and Apache II score ≥25, significantly (Table 2).
Table 2.
Predictors of successful weaning from mechanical ventilation within 28 days – Cox regression
| Univariable | Multivariable | |||||
| Variable | HR | 95% CI | P value | HR | 95% CI | P value |
| Age (per year increase) | 1.00 | 0.99–1.00 | 0.08 | 1.00 | 0.99–1.00 | 0.1 |
| Gender (male/female) | 1.03 | 0.87–1.22 | 0.7 | 0.98 | 0.82–1.17 | 0.8 |
| Severe sepsis/shock (vs. milder or no infection) | 0.62 | 0.52–0.75 | <10–4 | 0.76 | 0.62–0.93 | 0.007 |
| Surgical origin (yes/no) | 1.07 | 0.89–1.28 | 0.5 | 1.03 | 0.85–1.25 | 0.8 |
| Acute thrombosis* (yes/no) | 1.40 | 0.94–2.09 | 0.1 | 1.24 | 0.82–1.87 | 0.3 |
| Chronic inflammatory disease† (yes/no) | 0.78 | 0.52–1.15 | 0.2 | 0.87 | 0.58–1.30 | 0.5 |
| PCT (≥1 μg/L vs. PCT <1 μg/L) | 0.61 | 0.50–0.73 | <10–4 | 0.71 | 0.58–0.88 | 0.002 |
| PaO2/FiO2 Q1 (vs. Q2–Q4) | 0.77 | 0.64–0.93 | 0.007 | 0.84 | 0.69–1.02 | 0.08 |
| SPD (≥85th percentile vs. SPD <85th percentile) | 0.79 | 0.64–0.96 | 0.02 | 0.74 | 0.60–0.92 | 0.006 |
| COPD (yes/no) | 0.96 | 0.77–1.20 | 0.7 | 0.84 | 0.67–1.06 | 0.2 |
| Apache II score (<25 vs. ≥25) | 0.70 | 0.58–0.85 | 0.0004 | 0.81 | 0.66–1.00 | 0.05 |
| Gelsolin Q1 (vs. Q2–Q4) | 0.67 | 0.55–0.82 | 10–4 | 0.71 | 0.58–0.88 | 0.002 |
Significant results (P < 0.05) are marked with bold font.
*Acute myocardial infarction, pulmonary embolism, cerebral infarction, mesenteric embolism, and medulla spinalis infarction.
†Rheumatoid arthritis, Sjögren syndrome, fibromyalgia, spondylosis, hepatic cirrhosis, systemic lupus erythematosus, vasculitis and chronic glomerulonephritis.
COPD indicates chronic obstructive pulmonary disease; PCT, procalcitonin; Q1, lower quartile; Q2–Q4, upper 3 quartiles; SPD, surfactant protein D.
Successful weaning within 28 days was seen in 124/190 (65.3%) of patients with baseline gelsolin in the lower quartile. In the 3 upper quartiles, 404/556 (72.7%) of the patients were successfully weaned (Log-rank P < 0.0001). Seventeen patients in each group (Q1 vs. Q2–Q4) were still in requirement of mechanical ventilation at day 28. The remaining patients were successfully weaned or had died within the follow-up period.
We tested for interaction between surgical origin and baseline gelsolin level below the 25th percentile, because we found it plausible, that surgical patients had a different usage of gelsolin or had received more blood transfusions than the medical patients. The interaction between surgical origin and gelsolin in the lower quartile was not significant, however, a trend was evident (P = 0.08). Based on this, we analyzed the surgical and medical patients separately. In the medical patients, baseline gelsolin level below the 25th percentile independently predicted a 40% reduction in chance of being successfully weaned within 28 days (HR 0.60, 95% CI 0.46–0.79, P = 0.0002). Conversely, in the surgical patients, the baseline gelsolin level did not affect the chance of being successfully weaned (HR 0.94, 95% CI 0.65–1.36, P = 0.73).
Patients with a baseline gelsolin below the 25th percentile had 10.9 ventilator-free days within 28 days, whereas the patients with a baseline gelsolin above the 25th percentile had 13.5 ventilator-free days within 28 days (P = 0.006).
Figure 2A illustrates the independence between gelsolin and SPD in predicting successful weaning in the medical patients.
Fig. 2.
Successful weaning for medical and surgical patients.
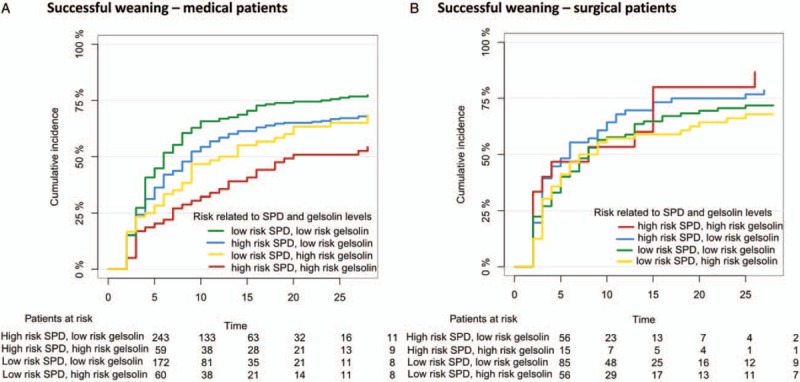
A, Cumulative incidence curves showing successful extubation for patients with medical origin until day 28 for patients divided into 4 curves according to gelsolin and SPD. B, Cumulative incidence curves showing successful extubation for patients with surgical origin until day 28 for patients divided into 4 plots according to gelsolin and SPD. Low-risk SPD is defined as below median SPD level and high-risk SPD is defined as above median SPD level.
Mortality related to the baseline gelsolin levels
Twenty-eight days mortality was not predicted by baseline gelsolin levels in the lower quartile (Table 3). Higher 28 days mortality was predicted by SPD baseline levels >85th percentile, COPD, Apache II score ≥25, chronic inflammatory disease, male gender, and increasing age (Table 3). Because of an interaction between surgical origin and baseline gelsolin below the 25th percentile in the analysis of successful weaning within 28 days, we tested the same interaction in the analysis of 28 days mortality. There was no interaction (P = 0.52).
Table 3.
Predictors of 28 days mortality – Cox regression
| Univariable | Multivariable | |||||
| Variable | HR | 95% CI | P value | HR | 95% CI | P value |
| Age (per year increase) | 1.04 | 1.02–1.04 | <10–4 | 1.03 | 1.02–1.04 | <10–4 |
| Gender (male/female) | 1.18 | 0.93–1.50 | 0.2 | 1.30 | 1.01–1.66 | 0.05 |
| Severe sepsis/shock (vs. milder or no infection) | 1.19 | 0.94–1.51 | 0.2 | 1.12 | 0.86–1.45 | 0.4 |
| Surgical origin (yes/no) | 0.65 | 0.49–0.87 | 0.003 | 0.75 | 0.56–1.03 | 0.07 |
| Acute thrombosis* (yes/no) | 1.56 | 0.96–2.55 | 0.08 | 1.58 | 0.96–2.60 | 0.07 |
| Chronic inflammatory disease† (yes/no) | 1.47 | 0.94–2.29 | 0.09 | 1.64 | 1.04–2.60 | 0.03 |
| PCT >1 μg/L (vs. PCT <1 μg/L) | 1.30 | 0.98–1.71 | 0.07 | 1.32 | 0.97–1.79 | 0.08 |
| PaO2/FiO2 Q1 (vs. Q2–Q4) | 1.13 | 0.88–1.44 | 0.4 | 0.97 | 0.75–1.26 | 0.8 |
| SPD (≥85th percentile vs. SPD <85th percentile) | 1.73 | 1.35–2.21 | <10–4 | 1.45 | 1.11–1.88 | 0.006 |
| COPD (yes/no) | 1.62 | 1.24–2.12 | 0.0005 | 1.57 | 1.18–2.09 | 0.002 |
| Apache II score (<25 vs. ≥25) | 1.67 | 1.31–2.13 | <10–4 | 1.53 | 1.18–1.99 | 0.001 |
| Gelsolin Q1 (vs. Q2–Q4) | 0.92 | 0.70–1.22 | 0.6 | 1.04 | 0.77–1.39 | 0.8 |
Significant results (P < 0.05) are marked with bold font.
*Acute myocardial infarction, pulmonary embolism, cerebral infarction, mesenteric embolism, and medulla spinalis infarction.
†Rheumatoid arthritis, Sjögren syndrome, fibromyalgia, spondylosis, hepatic cirrhosis, systemic lupus erythematosus, vasculitis, and chronic glomerulonephritis.
COPD indicates chronic obstructive pulmonary disease; PCT, procalcitonin; Q1, lower quartile; Q2–Q4, upper 3 quartiles; SPD, surfactant protein D.
Among the patients with baseline gelsolin below the 25th percentile, 66/190 (34.7%) died within the first 28 days. In the 3 upper quartiles 207/556 (37.2%) of the patients died within the first 28 days (Log-rank P = 0.56) (Fig. 3).
Fig. 3.
Kaplan–Meier curve showing mortality.
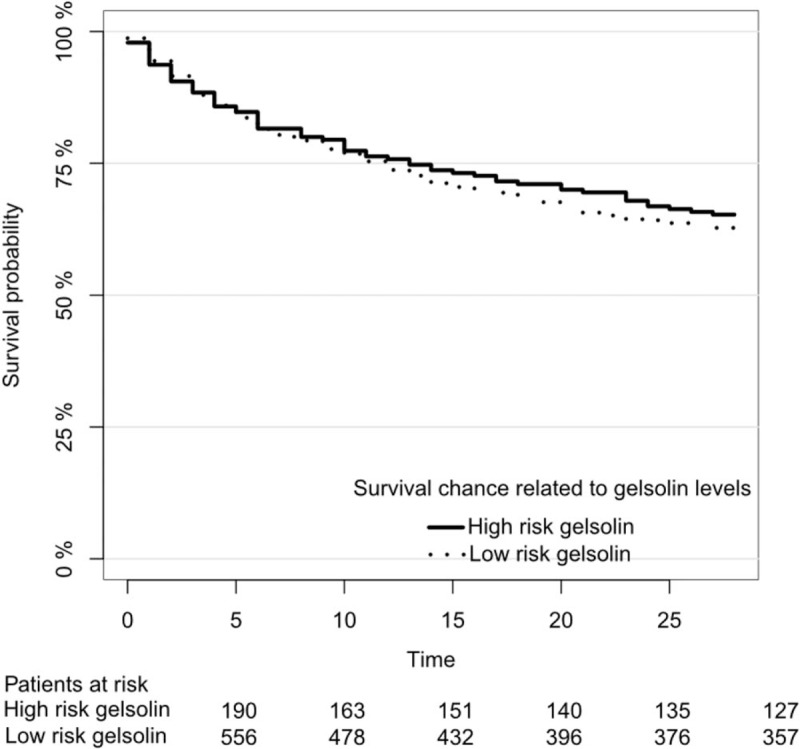
Kaplan–Meier curve showing mortality for patients with baseline gelsolin levels below the 25th percentile and patients with baseline gelsolin above the 25th percentile. There was no interaction between surgical origin and baseline gelsolin level below the 25th percentile (P = 0.71).
Alive and out of intensive care at day 14
The chance of being “alive and out of intensive care at day 14” was 31% lower for the patients with a baseline gelsolin level below the 25th percentile compared to the patients with baseline gelsolin levels above the 25th percentile (HR 0.69, 95% CI 0.54–0.89, P = 0.005) (Table 4). Besides gelsolin levels below the 25th percentile, lower chance of discharge from ICU within 14 days after admission was independently predicted by severe sepsis/septic shock, baseline PCT level >1 μg/L, SPD baseline levels >85th percentile, and Apache II score ≥25 (Table 4). Because of an interaction between surgical origin and baseline gelsolin below the 25th percentile in the analysis of successful weaning within 28 days, we tested the same interaction in the analysis of “alive and out of intensive care at day 14.” There was no interaction (P = 0.14).
Table 4.
Predictors of “alive and out of ICU at day 14” – Cox regression
| Univariable | Multivariable | |||||
| Variable | HR | 95% CI | P value | HR | 95% CI | P value |
| Age (per year increase) | 0.99 | 0.99–1.00 | 0.037 | 0.99 | 0.99–1.00 | 0.053 |
| Gender (male vs. female) | 1.032 | 0.85–1.26 | 0.75 | 1.01 | 0.83–1.24 | 0.90 |
| Severe sepsis/septic shock (vs. milder or no infection) | 0.58 | 0.47–0.72 | <10–4 | 0.72 | 0.57–0.91 | 0.006 |
| Surgical origin (yes vs. no) | 1.050 | 0.85–1.30 | 0.66 | 1.03 | 0.82–1.30 | 0.79 |
| Acute thrombosis* (yes vs. no) | 1.24 | 0.75–2.047 | 0.40 | 1.13 | 0.68–1.89 | 0.63 |
| Chronic inflammatory disease† (yes vs. no) | 0.73 | 0.45–1.21 | 0.22 | 0.84 | 0.50–1.49 | 0.49 |
| PCT >1 μg/L (vs. PCT <1 μg/L) | 0.56 | 0.45–0.69 | <10–4 | 0.67 | 0.53–0.84 | 0.0008 |
| PaO2/FiO2 Q1 (vs. Q2–Q4) | 0.77 | 0.62–0.96 | 0.021 | 0.87 | 0.69–1.09 | 0.22 |
| SPD (≥85th percentile vs. SPD <85th percentile) | 0.77 | 0.61–0.98 | 0.032 | 0.73 | 0.56–0.94 | 0.014 |
| COPD (yes/no) | 0.90 | 0.69–1.17 | 0.43 | 0.79 | 0.60–1.04 | 0.094 |
| Apache II score (<25 vs. ≥25) | 0.64 | 0.51–0.81 | 0.0002 | 0.76 | 0.60–0.97 | 0.028 |
| Gelsolin Q1 (vs. Q2–Q4) | 0.63 | 0.50–0.81 | 0.0002 | 0.69 | 0.54–0.89 | 0.0045 |
Significant results (P < 0.05) are marked with bold font.
*Acute myocardial infarction, pulmonary embolism, cerebral infarction, mesenteric embolism, and medulla spinalis infarction.
†Rheumatoid arthritis, Sjögren syndrome, fibromyalgia, spondylosis, hepatic cirrhosis, systemic lupus erythematosus, vasculitis, and chronic glomerulonephritis.
COPD indicates chronic obstructive pulmonary disease; PCT, procalcitonin; Q1, lower quartile; Q2–Q4, upper 3 quartiles; SPD, surfactant protein D.
Among patients with baseline gelsolin below the 25th percentile 83/190 (43.7%) were “alive and out of intensive care at day 14”, whereas 307/556 (55.2%) of the patients with baseline gelsolin levels in upper 3 quartile were “alive and out of intensive care at day 14” (Log-rank P = 0.007).
Patients with a baseline gelsolin below the 25th percentile had 10.2 ICU-free days within 28 days, whereas the patients with a baseline gelsolin above the 25th percentile had 12.4 ICU-free days within 28 days (P = 0.018).
Endothelial damage is not associated with low gelsolin level
There was no correlation between baseline gelsolin levels and baseline soluble sTM, correlations coefficient −0.04 (95% CI −0.11 to 0.034, P = 0.29) or change of sTM from day 1 to 4, correlation coefficient -0.074 (95% CI −0.16 to 0.014, P = 0.10).
DISCUSSION
We observed that gelsolin is an independent marker of successful weaning from mechanical ventilation within 1 month in critically ill patients with ventilator-demanding acute respiratory failure and duration of ICU stay. However, gelsolin was not found to be a marker of mortality. This might indicate that gelsolin is not a marker of systemic illness per se, but rather of degree of long-lasting pulmonary injury. The values of measured gelsolin in this population were much lower than for healthy individuals. This was expected, because the patients were all severely ill at baseline. Furthermore, and surprisingly, we did not find a correlation between baseline gelsolin concentration and level or change in sTM, which indicates that the lung damage detected by the low gelsolin levels is not mediated by endothelial damage. Since SPD is a well-known and clinically used biomarker of lung damage at this point, the analysis of successful weaning within 28 days was adjusted for baseline SPD >85th percentile. We found that both markers (gelsolin and SPD) independently of each other and of the remaining covariates predicted the primary end point of successful weaning from ventilation at day 28. It has been shown in previous studies that a surgical trauma may lead to decreasing gelsolin levels (11). Thus, a low gelsolin may have highly differing causes among medical and surgical patients. Therefore, we conducted an interaction analysis of the primary end point between surgical versus medical reasons for admission to the ICU and a low baseline gelsolin; P = 0.054. We thus also conducted separate analyses of the primary end point among medical and surgical patients. We observed that a low gelsolin strongly predicted persistent ventilator-demanding respiratory failure in medical critically ill patients, but it was not a predictor of this end point among surgical patients (Fig. 2).
In a study with 77 trauma patients, the duration of ventilator-demanding respiratory failure and duration of stay at ICU seemed increased among patients with lowered circulating gelsolin (>2 standard deviations under mean of healthy controls) (11). This is consistent with our results, where n = 746. However, earlier studies have also shown that a lower gelsolin level was associated with a higher mortality rate, which is not consistent with our findings (11, 10). Since our study is approximately 10 times larger than other studies of gelsolin in such patients, type I error in the previous and smaller studies seems to be a possible explanation for the discrepancy.
Apart front the actin-scavenging function, gelsolin can help in the defense against lung infections by activating phosphorylation of macrophage nitrogen oxide synthase type III (NOS3) (33). NOS3 is an enzyme with bactericidal function within the macrophages of the lungs. This indicates that gelsolin is as well a potential immune modulator for a stronger defense against primary and secondary bacterial pneumonia (33). This provides an alternative, or additional, positive characteristic of gelsolin in circulation.
Apart from the value of gelsolin as a biomarker of persistent respiratory failure, it may have therapeutic implications. In a murine study, gelsolin infusion was observed to reduce inflammation-induced lung damage after burn traumas (34). There has also been observed a significantly lowered morbidity after surgery on septic rats receiving gelsolin infusion compared to septic rats after surgery not receiving gelsolin infusion (35).
As a future perspective, first, our result can help stratifying patients with acute respiratory failure in risk of long-term mechanical ventilation. Second, our results represent the largest clinical material to explore this biomarker in a context of critically ill and its possible role in lung protection. Trials could use gelsolin as a way to optimize power, since patients with low gelsolin are those in highest risk of severe prolonged lung damage.
Strengths and limitations of the study
The statistical analysis was made with multiple Cox regression adjusted for known and suspected gelsolin-consuming conditions, e.g., comorbidities. There is limited knowledge about which conditions cause consumption of gelsolin. This is a limitation in the statistical analysis. The surgical patients may have actin release because of a surgical procedure, whereas intubated medical patients may have actin release because of lung damage. Besides, the different pattern in actin release between the surgical and medical patients, there could be a part of the surgical patients who have received blood transfusions, which could change both the actin and gelsolin levels. The reasons for this interaction need to be further investigated in the future. A minor limitation is that plasma gelsolin was only measured once in every sample and not as a duplicate. The decision to extubate a patient was done in conference between at least 2 ICU specialists according to the current guidelines (36), however, written criteria in the protocol were not provided. This is a potential limitation, however, gelsolin laboratory analysis was performed later than extubation, thus bias would not be likely. Tests on spontaneous breathing and awakening are not reported as well as data on sedation.
The strengths of this study are the large sample size and the detailed registration of many variates to every patient up to day 28 which allowed us to adjust for important confounding factors of the association between gelsolin and the investigated outcomes. Data were collected in accordance with good clinical practice as it was pursued to ensure a good quality of data.
CONCLUSION
Actin-scavenger gelsolin is an independent and strong predictor of persisting ventilator-demanding respiratory failure in medical intensive care patients. However, it is not associated with mortality. We show that both gelsolin and SPD carry separate prognostic properties and that combining these 2 biomarkers increases the prediction of persistent respiratory failure in critically ill patients. Our findings indicate that gelsolin may play a separate protective role in acute lung damage. As a future perspective, our findings may have important implications for risk stratification and for early guidance of therapeutic interventions directed toward minimizing lung injury.
Acknowledgments
The authors want to thank the “Procalcitonin And Survival Study” and colleagues in the research unit, department of internal medicine, Gentofte Hospital.
Footnotes
Declarations: Ethics approval and consent to participate: all participants gave written informed consent and the study was ethically approved by the Regional Ethics Committee for Copenhagen and Frederiksberg Communes in Denmark (ref. no.: KF 01 272 753, KF 11 297 287). The study was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Source of Funding: F.S.H. has received funding from Herlev and Gentofte Hospital, Internal Research Resources. J.-U.S.J. has received funding from the Danish National Research Foundation, the Lundbeck Foundation, and the Idella Foundation.
Conflicts of Interest: The authors report no conflicts of interest.
REFERENCES
- 1.Wunsch H, Angus DC, Harrison DA, Collange O, Fowler R, Hoste EA, de Keizer NF, Kersten A, Linde-Zwirble WT, Sandiumenge A, et al. Variation in critical care services across North America and Western Europe. Crit Care Med 36:2787–2793, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Wunsch H, Wagner J, Herlim M, Chong DH, Kramer AA, Halpern SD. ICU occupancy and mechanical ventilator use in the United States. Crit Care Med 41:2712–2719, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. Investigators and E. T. Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315:788–800, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Saguil A, Fargo M. Acute respiratory distress syndrome: diagnosis and management. Am Fam Physician 85:352–358, 2012. [PubMed] [Google Scholar]
- 5.Sudakov NP, Klimenkov IV, Byvaltsev VA, Nikiforov SB, Konstantinov YM. Extracellular actin in health and disease. Biochemistry (Mosc) 82:1–12, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Suhler E, Lin W, Yin HL, Lee WM. Decreased plasma gelsolin concentrations in acute liver failure, myocardial infarction, septic shock, and myonecrosis. Crit Care Med 25:594–598, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Lee WM, Galbraith RM. The extracellular actin-scavenger system and actin toxicity. N Engl J Med 326:1335–1341, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Janmey PA, Lamb JA, Ezzell RM, Hvidt S, Lind SE. Effects of actin filaments on fibrin clot structure and lysis. Blood 80:928–936, 1992. [PubMed] [Google Scholar]
- 9.Haddad JG, Harper KD, Guoth M, Pietra GG, Sanger JW. Angiopathic consequences of saturating the plasma scavenger system for actin. Proc Natl Acad Sci U S A 87:1381–1385, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horvath-Szalai Z, Kustan P, Muhl D, Ludany A, Bugyi B, Koszegi T. Antagonistic sepsis markers: serum gelsolin and actin/gelsolin ratio. Clin Biochem 50:127–133, 2017. [DOI] [PubMed] [Google Scholar]
- 11.Mounzer KC, Moncure M, Smith YR, Dinubile MJ. Relationship of admission plasma gelsolin levels to clinical outcomes in patients after major trauma. Am J Respir Crit Care Med 160:1673–1681, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Kwiatkowski DJ, Stossel TP, Orkin SH, Mole JE, Colten HR, Yin HL. Plasma and cytoplasmic gelsolins are encoded by a single gene and contain a duplicated actin-binding domain. Nature 323:455–458, 1986. [DOI] [PubMed] [Google Scholar]
- 13.Kwiatkowski DJ, Mehl R, Izumo S, Nadal-Ginard B, Yin HL. Muscle is the major source of plasma gelsolin. J Biol Chem 263:8239–8243, 1988. [PubMed] [Google Scholar]
- 14.Christofidou-Solomidou M, Scherpereel A, Solomides CC, Muzykantov VR, Machtay M, Albelda SM, DiNubile MJ. Changes in plasma gelsolin concentration during acute oxidant lung injury in mice. Lung 180:91–104, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Lind SE, Smith DB, Janmey PA, Stossel TP. Depression of gelsolin levels and detection of gelsolin-actin complexes in plasma of patients with acute lung injury. Am Rev Respir Dis 138:429–434, 1988. [DOI] [PubMed] [Google Scholar]
- 16.Bucki R, Levental I, Kulakowska A, Janmey PA. Plasma gelsolin: function, prognostic value, and potential therapeutic use. Curr Protein Pept Sci 9:541–551, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Guo XC, Luo BY, Li XF, Yang DG, Zheng XN, Zhang K. Plasma gelsolin levels and 1-year mortality after first-ever ischemic stroke. J Crit Care 26:608–612, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Xianhui L, Pinglian L, Xiaojuan W, Wei C, Yong Y, Feng R, Peng S, Gang X. The association between plasma gelsolin level and prognosis of burn patients. Burns 40:1552–1555, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Cheng B, Chen Q, Wu S, Lv C, Xie G, Jin Y, Fang X. Time course of plasma gelsolin concentrations during severe sepsis in critically ill surgical patients. Crit Care 12:R106, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osborn TM, Verdrengh M, Stossel TP, Tarkowski A, Bokarewa M. Decreased levels of the gelsolin plasma isoform in patients with rheumatoid arthritis. Arthritis Res Ther 10:R117, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulakowska A, Ciccarelli NJ, Wen Q, Mroczko B, Drozdowski W, Szmitkowski M, Janmey PA, Bucki R. Hypogelsolinemia, a disorder of the extracellular actin scavenger system, in patients with multiple sclerosis. BMC Neurol 10:107, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee PS, Drager LR, Stossel TP, Moore FD, Rogers SO. Relationship of plasma gelsolin levels to outcomes in critically ill surgical patients. Ann Surg 243:399–403, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith DB, Janmey PA, Herbert TJ, Lind SE. Quantitative measurement of plasma gelsolin and its incorporation into fibrin clots. J Lab Clin Med 110:189–195, 1987. [PubMed] [Google Scholar]
- 24.Eisner MD, Parsons P, Matthay MA, Ware L, Greene K. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 58:983–988, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartl D, Griese M. Surfactant protein D in human lung diseases. Eur J Clin Invest 36:423–435, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Jensen JS, Itenov TS, Thormar KM, Hein L, Mohr TT, Andersen MH, Loken J, Tousi H, Lundgren B, Boesen HC, et al. Prediction of non-recovery from ventilator-demanding acute respiratory failure, ARDS and death using lung damage biomarkers: data from a 1200-patient critical care randomized trial. Ann Intensive Care 6:114, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takano S, Kimura S, Ohdama S, Aoki N. Plasma thrombomodulin in health and diseases. Blood 76:2024–2029, 1990. [PubMed] [Google Scholar]
- 28.Jensen JS, Hein L, Lundgren B, Bestle MH, Mohr TT, Andersen MH, Thornberg KJ, Loken J, Steensen M, Fox Z, et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med 39:2048–2058, 2011. [DOI] [PubMed] [Google Scholar]
- 29.European Medicines Agency: Note for Guidance on Good Clinical Practice, EMEA (European Medicines Agency). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002874.pdf 2002 Accessed January 5, 2014. [Google Scholar]
- 30.Christensen PH, Grønkjær K. Quantitative determination of human serum gelsolin. Development and validation of an automated turbidimetric immunoassay. Clin Chim Acta 355 (Supp l):S1–S483, 2005.15915558 [Google Scholar]
- 31.Li B, Zhao X, Li S. Serum procalcitonin level and mortality risk in critically ill patients with ventilator-associated pneumonia. Cell Physiol Biochem 37:1967–1972, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Kruse JA, Thill-Baharozian MC, Carlson RW. Comparison of clinical assessment with APACHE II for predicting mortality risk in patients admitted to a medical intensive care unit. JAMA 260:1739–1742, 1988. [PubMed] [Google Scholar]
- 33.Yang Z, Chiou TT, Stossel TP, Kobzik L. Plasma gelsolin improves lung host defense against pneumonia by enhancing macrophage NOS3 function. Am J Physiol Lung Cell Mol Physiol 309:L11–L16, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothenbach PA, Dahl B, Schwartz JJ, O’Keefe GE, Yamamoto M, Lee WM, Horton JW, Yin HL, Turnage RH. Recombinant plasma gelsolin infusion attenuates burn-induced pulmonary microvascular dysfunction. J Appl Physiol (1985) 96:25–31, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Cohen TS, Bucki R, Byfield FJ, Ciccarelli NJ, Rosenberg B, DiNubile MJ, Janmey PA, Margulies SS. Therapeutic potential of plasma gelsolin administration in a rat model of sepsis. Cytokine 54:235–238, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bersten AD, Soni N, Oh TE. Oh's intensive care manual. 5th ed.Philadelphia, USA: Butterworth-Heinemann; 2004. [Google Scholar]


