Abstract
The convergence of multiple recent developments in health care information technology and monitoring devices has made possible the creation of remote patient surveillance systems that increase the timeliness and quality of patient care. More convenient, less invasive monitoring devices, including patches, wearables, and biosensors, now allow for continuous physiological data to be gleaned from patients in a variety of care settings across the perioperative experience. These data can be bound into a single data repository, creating so-called data lakes. The high volume and diversity of data in these repositories must be processed into standard formats that can be queried in real time. These data can then be used by sophisticated prediction algorithms currently under development, enabling the early recognition of patterns of clinical deterioration otherwise undetectable to humans. Improved predictions can reduce alarm fatigue. In addition, data are now automatically queriable on a real-time basis such that they can be fed back to clinicians in a time frame that allows for meaningful intervention. These advancements are key components of successful remote surveillance systems. Anesthesiologists have the opportunity to be at the forefront of remote surveillance in the care they provide in the operating room, postanesthesia care unit, and intensive care unit, while also expanding their scope to include high-risk preoperative and postoperative patients on the general care wards. These systems hold the promise of enabling anesthesiologists to detect and intervene upon changes in the clinical status of the patient before adverse events have occurred. Importantly, however, significant barriers still exist to the effective deployment of these technologies and their study in impacting patient outcomes. Studies demonstrating the impact of remote surveillance on patient outcomes are limited. Critical to the impact of the technology are strategies of implementation, including who should receive and respond to alerts and how they should respond. Moreover, the lack of cost-effectiveness data and the uncertainty of whether clinical activities surrounding these technologies will be financially reimbursed remain significant challenges to future scale and sustainability. This narrative review will discuss the evolving technical components of remote surveillance systems, the clinical use cases relevant to the anesthesiologist’s practice, the existing evidence for their impact on patients, the barriers that exist to their effective implementation and study, and important considerations regarding sustainability and cost-effectiveness.
See Editorial, p 640
Remote surveillance enables clinicians to monitor, diagnose, and intervene in a variety of settings with the aim of increasing the timeliness and safety of care. Remote surveillance uses a strategy of collecting and digitally transmitting clinical data from a patient in a location different from where the clinician is located. It can use data from any combination of invasive and noninvasive monitors, wearable devices, biosensors, electronic health records, video cameras, and more.1,2 The great promise of these technologies, however, must be considered, with considerable barriers to their implementation, study, scale, and sustainability.
Although components of remote surveillance have been in development for nearly a decade, the coming together of multiple advances in health information and digital technology present the opportunity for broader applications. First, patient data previously siloed into different data systems are now collected, stored, and retrieved in a common system, increasing the volume, diversity, and accessibility of data related to the patient clinical state. Second, newer continuous noninvasive physiological monitors are convenient for patients, such that monitoring can be performed and data gleaned in a growing number of clinical settings—from their home before surgery, through their operation to the postoperative general care ward, and back home again. Third, more sophisticated prediction algorithms can automatically sort through massive amounts of clinical data and identify constellations associated with adverse clinical events so as to increase the specificity of alerts and reduce alarm fatigue. The successful implementation and utilization of prior alert systems suffered from alarm fatigue that emanated from low-specificity alerts dependent on very basic algorithms. Combined, these advances in data, prediction, and alert/response form the elements of effective remote surveillance systems (Figure 1).
Figure 1.
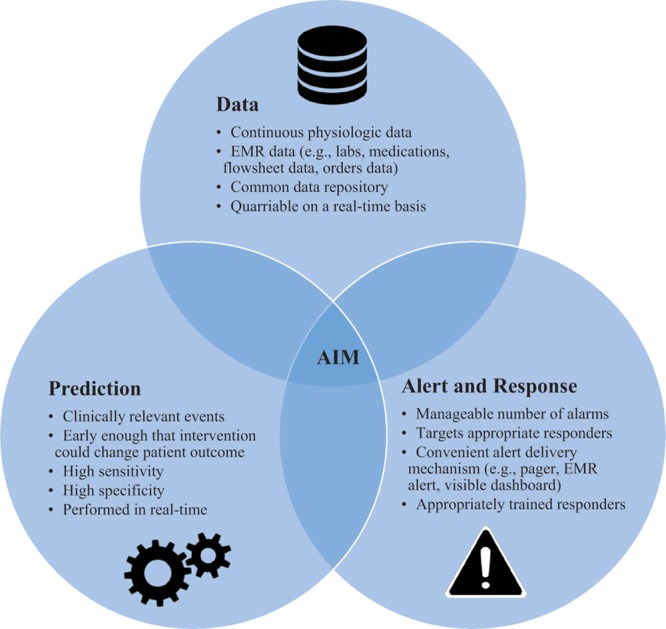
Components of effective remote surveillance systems. EMR indicates electronic medical record.
The aim of this article is to review the evolution of data and technology enabling effective remote surveillance, past and future use cases for remote surveillance technologies relevant to anesthesiologists, the limited peer-reviewed literature, and the barriers in the field that must be addressed to realize the promise of these technologies.
DISCUSSION
Why Now: How Technologic Developments Have Converged to Make Effective Remote Surveillance Possible
More Data, Increasingly Accessible.
The operating room, postanesthesia care unit (PACU), and intensive care unit have traditionally enjoyed the ability to capture vital signs continuously. These data were historically siloed in the device and could not be paired with other meaningful clinical data. Biomedical device interface solutions that bridge the electronic health record and the monitoring device enable automatic transmission of monitoring data into the record at up to minute-by-minute resolution such that it can be paired with other data relevant to defining the patient’s clinical status. One challenge remains the frequency with which these data are transmitted by the biomedical device interface, as the strength of prediction algorithms is partly dependent on the resolution of the physiological data available to them.
Traditional devices that continuously track vital signs are typically inconvenient for the patient, as they tether patients to the monitor. Newer continuous noninvasive physiological monitoring devices, such as patches, biosensors, watches, and wearables, are more convenient, allowing for activity and ambulation.3–5 These devices are tracking new types of data, including cardiac output, tidal volume (VT), stroke volume variation, blood glucose, and hemoglobin.6–10 As with traditional patient monitors, the devices can now transmit their data via biomedical device interfaces to the electronic health record database.
To pass data from devices to the electronic health record, the data must be structured in a standard language. Most electronic health records and medical devices have converged on using a common standard language called Healthcare Language-7 to exchange information.11 This was a first step in allowing different monitoring devices made by a variety of vendors to communicate their data into 1 database. The challenge remains that many applications use Healthcare Language-7 in disparate ways. Transmitting, collecting, and standardizing data in a common format may not be performed rapidly enough to provide the speed and resolution required by prediction algorithms aimed at making an impact on real-time clinical care. More recently, intraoperability standards called Fast Healthcare Interoperability Resources have enabled a variety of applications to interface with electronic health records.12 These applications use real-time operating systems and software languages to compute massive amounts of data outside of the traditional electronic health record–based data mining structures that have existed for many years.
To make electronic health record data—including vital signs, laboratory test results, medication records, flowsheet data, and physician orders—actionable at the bedside, the ability to query in real-time must exist. In recent years, electronic health record vendors have made their data available to external applications by providing hospitals with several interface methods such as application programming interfaces and web services.13,14 These have enabled real-time data to be queried and then delivered to downstream applications and external databases. An example of data and information systems architecture that could enable effective remote surveillance is shown in Figure 2.
Figure 2.
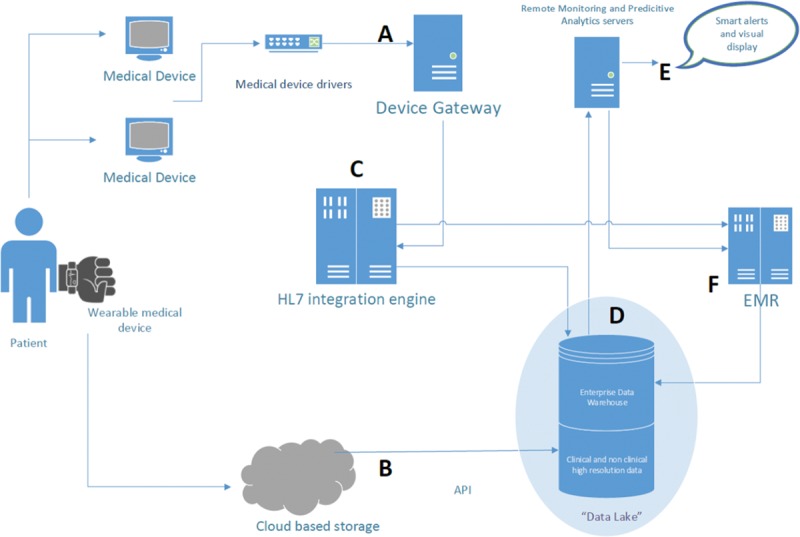
Example data and information systems architecture for effective remote surveillance. A and B indicate data acquisition pathways for 2 different types of medical devices: a traditional patient monitor (“medical device”) and a wearable patient monitor (“wearable medical device”). (1) In pathway A, patient data collected on the medical device are fed into “Medical Device Drivers,” a software application that enables connection between medical devices and downstream software applications. The “device gateway” then receives the data and transforms it into a Healthcare Language-7 message. The “Healthcare Language-7 integration engine” (C) receives the Healthcare Language-7 messages from the device gateway and then splits and distributes them to (D) the “data lake” (composed of the “enterprise data warehouse” that includes clinical and nonclinical high-resolution data) and (F) the electronic medical record. Data from the enterprise data warehouse are sent to the “remote monitoring and predictive analytics servers” on which analytics can be performed and alerts generated. These alerts are fed to (E) downstream systems that alert the clinician, such as pagers or visual display systems. (2) Alternatively, in pathway B, the “wearable medical device” may send its data to a “cloud-based storage” system. Using an “application programming interface (API),” the data can then be delivered to the data lake.
A challenge of data mining from the electronic health record is the amount of data storage available and inherent challenges in housing these data. Traditional server-based storage required hardware installations and licensing fees. Cloud-based electronic health record storage, however, is growing, as it is more affordable and easier to install and maintain. Cloud computing and improvements in data warehousing have enabled disparate sources of clinical and nonclinical data to store current and historical data in a centralized repository or “data lake.”15,16 Of note, these data are often in a raw format and require substantial processing to be usable for clinical analytics. To perform analysis of patient data using algorithms, the electronic health record must be supplemented with additional real-time reading and storage for high-resolution data. To be successful, a parallel copy of the electronic health record must be created and the raw data must be read, cleaned, indexed, and stored in real-time so as to enable instantaneous analysis by algorithms that can immediately aid the clinician.
Overcoming Barriers to the Prediction of Clinically Meaningful Events.
The benefits of substantially larger and more diverse datasets are only translatable to the bedside if they can be distilled to a manageable number of high-yield alerts in a time frame in which clinician intervention can change the trajectory of the patient. The most basic surveillance and alerting system is well known to clinicians as the patient monitor that produces an alarm at the bedside. The alarm is based on ranges set at the bedside, typically from a single abnormal parameter (eg, oxygen saturation). Without analytics capable of recognizing artifactual patterns and inhibiting alarms when such a pattern is evident, alarm fatigue has become a major concern for hospitals nationwide.17 The Joint Commission has mandated as a National Patient Safety Goal that all hospitals develop methods to reduce alarm fatigue. Studies have demonstrated that as little as 0.5% of alarms are actionable in scenarios of continuous bedside monitoring.18,19
On the horizon are intelligent alert systems that are driven by analytic approaches that can process massive amounts of information instantly and identify patterns not typically recognizable by algorithms that use logistic regression.20–23 The aim of these intelligent systems must be to increase the sensitivity to clinically important events but also, and perhaps most importantly, to increase specificity so as to reduce alarm fatigue. Artificial intelligence-based alarms that employ machine learning techniques such as neural networks not only take into account a comprehensive set of permutations of input data but also systematically weight and unweight the data in innumerable combinations to make stronger predictions. Importantly, machine learning algorithms require historical datasets of patients to train on as they build an understanding of which data constellations strongly associate with prespecified clinical outcomes of interest (eg, cardiac arrest or intensive care unit transfer). Thus, an adequate amount of historical patient data and outcomes must be present to allow for appropriate learning. The size of these training sets and the amount of historical data required to develop highly accurate predictions are variable and determined by numerous factors, including the type, relevance, and quality of input data.
Use Cases Relevant to the Anesthesiologist: Opportunities and Barriers
Preoperative Assessment.
Remote surveillance of patients at home has historically been limited to programs targeting chronic diseases and preventing avoidable admissions in high-risk medical patients.24,25 For patients awaiting surgery, there have been virtually no studies examining the use of remote surveillance to augment the preoperative functional assessment despite evidence demonstrating its crucial role in predicting postoperative adverse outcomes.26–29 Although newer tools such as the Duke Activity Status Index are improving functional assessments, they are still reliant on the patient’s own report of their activity and lack objective, quantifiable data. Risk indices that rely on these assessments have been shown to perform poorly in discriminating levels of risk.27
Remote surveillance offers the opportunity to generate more detailed data about the patient’s functional capacity and improve the prediction of postoperative outcomes. Wearables can be conveniently worn in the home and automatically upload data to the cloud such that it can be accessed remotely by anesthesiologists. Activity monitoring, such as step counts, stair counts, patient positioning data, balance, and fall detection, can be combined with physiological response variables, such as heart rate and oxygen saturation. One of the aims of such data would be to enable a more informed discussion with patients about the risk of surgery and anesthesia. In addition, such data could improve selection of patients for intensive preoperative optimization, such as prehabilitation programs with prescribed exercise and nutrition optimization regiments. Moreover, they could guide the intraoperative anesthetic management plan and aid in the selection of appropriate level of postoperative care.
Barriers exist to the realization of this potential. Although remote surveillance may increase the data available in the preoperative assessment, tools that incorporate these data must demonstrate efficacy in predicting postoperative outcomes beyond current tools. The existing standard of relying on patient self-reporting is free of cost, easily interpreted, and takes minimal time for the anesthesiologist to administer. The trove of data from a preoperative remote surveillance program must be distilled into manageable and easily interpretable risk indicators for the anesthesiologist. Also, the importance of cost and convenience is significant. For example, cardiopulmonary exercise testing provides an objective and detailed characterization of preoperative functional capacity.30 The high cost and inconvenience to the patient of administering cardiopulmonary exercise testing, however, is a major barrier to widespread adoption among others.
Operating Room.
Anesthesiologists increasingly provide care for multiple patients simultaneously and cannot physically be present within a single operating room at all times. The American Society of Anesthesiology (ASA) has deemed that a medically directing anesthesiologist be available in such a manner that allows them to reestablish direct contact with the patient to “meet medical needs and address any urgent and emergent clinical problems.”31,32 In addition, there is rising public awareness that anesthesiologists are often not present in the operating room at all times and a demand that assurances be made that care can be rendered safely despite this.33
To meet these demands, anesthesiologists remote to the operating room might be aided by technology that continually assesses the patient for signs of deterioration and alerts them instantly when such an event occurs. Moreover, data have increasingly shown that the basic parameters of care under the control of the anesthesiologist in the operating room, such as choice of anesthetic, blood pressure, VT, oxygenation, fluid intake, and glucose, have a significant impact on perioperative outcomes as long as 30 days after surgery.34–38 Thus, these systems may augment the ability of the anesthesiologist to supervise the care of multiple patients at once with a demonstrable impact on outcomes.
Studies of the use of remote surveillance technologies in the operating room have been extremely limited, although this is changing. Kheterpal et al39 implemented an intraoperative decision support system that provided real-time visual notifications on a dashboard viewable by the anesthesiologist in the operating room.40,41 The software extracted and analyzed >250 parameters from the intraoperative record, such as vital signs, laboratory results, administered IV fluid volume, and urine output. They compared a 6-year period during which the tool was used in 75% or more cases at their institution and compared with historical controls before system implementation. Process measures, such as the number of minutes with mean arterial pressure <55 mm Hg and minutes with median VT >10 mL/kg, were improved in the experimental group, although outcomes such as mortality were not different.
Murray-Torres et al42 are implementing a similar software to enable remote surveillance of multiple operating rooms simultaneously by a single attending anesthesiologist. The software will be used to monitor operating room anesthesia care in real time, identify practices that diverge from evidence-based approaches, and provide intraoperative support to staff. The study designers characterize the software as enabling the anesthesiologist to perform a version of air traffic control.
So-called closed-loop anesthesia systems are an application of continuous remote surveillance in which an intervention is triggered without the direct involvement of the anesthesiologist. These systems use high-performing prediction algorithms, many of which include artificial intelligence approaches. The software continuously surveys patient data and then automatically adjusts a patient’s anesthetic management accordingly.43–45 Systematic review and meta-analyses of these systems have demonstrated reductions in “overshooting and undershooting” phenomenon in the management of blood pressure, ventilation, and blood sugar compared with manually controlled systems.46 The scalability of these tools remains in question, however, as several companies producing closed-loop anesthesia systems have disappeared from the market.
Remote surveillance also has the potential to enable anesthesiologists to continuously monitor and manage evidence-based care protocols in the operating room, such as enhanced recovery after surgery. Aspects of enhanced recovery after surgery under the influence of anesthesiologists have been shown to be associated with a stepwise reduction in length of stay.47 Effective management of these protocols across many patients is severely limited by the lack of ability to automatically and continuously monitor adherence.48,49 Most programs’ measurement of adherence is based on retrospective reports long after the patient has left the anesthesiologist’s care. Remote surveillance can track adherence in real time and notify clinicians when a patient is diverging from their pathway. Such a tool provides a “continuously hovering” system that incorporates flowsheet information, physician order entry data, and medication record data. Intraoperatively, anesthesiologists can be alerted when nonopioid analgesic strategies have been underutilized, narcotic dosage has reached threshold levels, antiemetic regimens have not been optimized, and the volume of fluids administered is approaching defined thresholds. Anesthesiologists can commandeer real-time monitoring of enhanced recovery after surgery parameters postoperatively as well.
One barrier to the adoption of such systems is that, while protocol-based care may be appropriate in many surgical encounters, this may change during the patient’s operation or afterward. Alerts based on deviations from enhanced recovery after surgery must stay connected with the reality of the patient’s changing clinical context, such as a substantial unanticipated blood loss, hypotension, or infection. For remote surveillance systems to remain relevant and to avoid misguiding clinicians, they must monitor data that provide relevant clinical context. For example, vital signs, white blood cell counts, temperature, bacterial culture results, drainage output and bleeding, and hemoglobin concentrations can be monitored using application programming interfaces connected to the electronic medical record. Such monitoring can initiate discussion about whether to maintain the patient on the protocol.
Postoperative Care.
In the PACU, airway, respiratory, hemodynamic, and bleeding complications require the timely involvement of an anesthesiologist. PACU complication rates have ranged from 9.9% to 23% in recent decades.50,51 The proportion of complications occurring in ASA physical status I and II patients has declined, while those occurring in ASA physical status III and IV patients have increased, demanding greater attention and involvement from anesthesiologists.52 Accordingly, remote surveillance and alerting systems may enable anesthesiologists to remain involved in their most complex patients’ immediate postoperative care when physiological decline occurs.
One opportunity in the PACU and beyond is in reducing postoperative opioid-induced ventilatory insufficiency. The Anesthesia Patient Safety Foundation, the Patient Safety Movement, and the Joint Commission cite opioid-induced ventilatory insufficiency as a major source of preventable morbidity and have issued consensus statements that it could be eliminated with better monitoring and alert systems.53,54 This challenge has become even more urgent with the national opioid crisis.
Application of remote surveillance to opioid-induced ventilatory insufficiency must be cautious to avoid the pitfall of alarm fatigue that has frustrated efforts for decades. Continuous monitoring devices like pulse oximetry, though recommended in this population, have been fraught with false-positive alarms.55 One recommendation to address this challenge has been to integrate multiple monitoring modalities to increase the specificity of alarms. Remote surveillance systems must aim to combine data from devices like pulse oximetry, capnography, and wearable monitors that measure minute ventilation, with data on risk factors such as the dose and timing of opioids administered, patient age, and body mass index. Smart alerting algorithms that can process these data and distill them into specific alerts before decompensation can be developed. In addition, the pitfall of relying on a single individual near the bedside must be overcome. The Anesthesia Patient Safety Foundation has endorsed automated alerting systems that tier alerts through a hierarchy of providers capable of intervening quickly based on the severity of the derangement.53
Intensive Care Unit.
The intensive care unit is the perioperative setting in which remote surveillance has been most widely implemented and studied. The need to address the shortage of intensivists in many regions of the United States has prompted interest in remote surveillance systems.56–58 Critical care telemedicine uses a remotely located support center housing a critical care team who is in audiovisual communication with bedside personnel and patients.59 These systems have expanded in recent decades, and evidence of their efficacy and cost-effectiveness are still being studied, with mixed results.60,61 Systematic reviews of tele-intensive care units have demonstrated efficacy in improving the timeliness of care of acutely ill patients, such as response to abnormal laboratory results, and compliance with quality metrics, such as appropriate antibiotic administration.60
One barrier to widespread adoption, however, has been mixed evidence of mortality benefit of such systems. Systematic reviews have revealed generally poor methodological quality. One review of tele-intensive care unit systems with 24/7 decision support demonstrated reductions in hospital mortality, although only 2 controlled trials existed and neither were randomized.62 A pitfall of these studies has been the inability to clearly attribute benefits in safety and timeliness of care to the continuous data monitoring versus better education and communication among staff.63,64
Future opportunities exist in the intensive care unit to incorporate traditionally siloed devices with the rest of the patient’s clinical data. These devices include transport ventilators, extracorporeal membrane oxygenation machines, intra-aortic balloon pumps, and noninvasive cardiac output monitors. Transport ventilators, for example, have represented a patient safety concern, with evidence that there is wide variation in their performance.65 This adds to an already risky scenario in which a critically ill patient is being moved through a resource-poor environment by a small team or single anesthesiologist. The mechanism to continuously monitor these devices and their parameters and to combine them with other physiological data of the patient now exists but has not been implemented. A remote surveillance system that captures, monitors, and combines data from the device with vital sign and other physiological data could be the basis for a more effective and safer system.
Postoperative Care on the Wards.
As the age and complexity of patients receiving surgery continue to rise, the need to involve anesthesiologists in their pre- and postoperative care will continue to grow.66,67 Although at risk of deterioration, not all of these patients can be monitored in an intensive care unit setting during the pre- and postoperative period. Anesthesiologists, in their role as emergency airway specialists and intensivists, have often been consulted to care for patients on the general care ward when they have fully decompensated. Remote surveillance technologies may enable anesthesiologists to provide early, proactive consultative guidance to surgical teams when the first evidence of deterioration occurs in a time frame that could help avoid an adverse event, such as an emergency intubation, cardiac arrest, or intensive care unit transfer.
Much of the literature on the efficacy of remote surveillance on the general inpatient wards has focused on alerting rapid response teams to address patient deterioration. Several institutions have implemented “track and trigger” systems that automatically collect real-time vital signs and laboratory results and input them into validated early warning system scores.68,69 It has been previously demonstrated that generating early warning system alerts from electronic health record database queries on a real-time basis can successfully predict clinical deterioration, such as the need for intensive care unit transfer and hospital mortality.70–73 Evidence of the impact of such systems on outcomes has been more mixed. One challenge in comparing studies has been the heterogeneity in the monitoring devices, as well as the components of the response arm after the early warning system detects an event. The number and type of personnel involved in the rapid response, their expected actions, and the amount of training received before initiation of the program have all been sources of variation.
Preliminary data from the use of artificial intelligence algorithms to predict decompensation on hospital ward patients have been promising. In 1 retrospective cohort study, routine vital signs and laboratory results obtained from the electronic health record were used in a neural network model intended to predict intensive care unit transfers and cardiac arrest events.74 The performance of the model was compared to a validated early warning system score. The neural network outperformed the early warning system model, with a positive predictive value of 82% compared with 24%. Nevertheless, while these algorithms have performed well using retrospective training and test sets, it remains to be seen whether implementing a live alerting system could have the same impact.
Critical Barriers to Sustainability
Measuring Value.
While the promise of data integration and the ability to perform remote surveillance to extract clinically meaningful data are substantial, there are few studies demonstrating the value of these technologies in addressing key health metrics. To gain traction with anesthesiologists, hospitals, and policy makers, these applications must demonstrate an impact on patient outcomes, cost, or the patient experience—the so-called health care Triple Aim. If bundled care payments and value-based reimbursement continue to grow in the perioperative space, remote surveillance technologies will be even less likely to find a footing if they fail to demonstrate these gains.
Historically, 1 considerable challenge that studies have encountered in demonstrating an impact on patient outcomes has been that they have measured the ability of these applications to prevent rare patient events, such as cardiac arrest, intensive care unit transfer, or death. As a result, many studies have required substantial sample sizes to obtain appropriate statistical power or assume an unrealistic effect size to demonstrate statistical significance. Even using composite end points of multiple types of adverse events has not proven efficacious, particularly because many end points are codependent, such as mortality and cardiac arrest. Certain outcome measures are also challenging to interpret. For example, intensive care unit transfer may be a negative outcome if it relates to a patient who, if managed differently at an earlier time point, could have avoided an intensive care unit stay. In contrast, earlier awareness of a patient’s impending deterioration and prompt transfer to an intensive care unit may improve a patient’s overall trajectory and hospital outcome despite temporarily needing to go to an intensive care unit. In addition, studies of remote surveillance interventions that have demonstrated an impact on mortality have done so using short-term metrics, such as in-hospital mortality, but ignored longer-term survival or quality of life.
Studies must go beyond the measurement of rare events and instead examine parameters that do not hold the same pitfalls yet are still valuable. Increasingly remote surveillance is monitoring all aspects of patient care, such as ventilator strategies, fluid management, and analgesic regimens. Coordinating and driving adherence to initiatives in these areas of practice is complex and often labor intensive. Applications in these high-value areas will serve to support the continuous monitoring and measurement of care initiatives, such as low VT ventilation, conservative fluid administration, and utilization of multimodal nonopioid medications. In these applications, the basis for measuring the effectiveness of remote surveillance systems would be the level of adherence to intended best practices with and without the technology. If remote surveillance can demonstrate the ability to enable anesthesia teams to more closely monitor and manage these valuable patient care initiatives, they will more clearly establish their value in patient care.
Effective Implementation.
In addition, the effectiveness of a remote surveillance system is interdependent with numerous aspects of how such systems are operationalized. The core components of a remote surveillance system go far beyond data and analytics. Two programs using roughly the same technology but implemented differently can demonstrate highly divergent outcomes. Key factors include the method of alert delivery to the clinical team, who the alert is delivered to, what expectations exist when they receive an alert, whether their response has been prespecified by the study, or how much they have been trained. How to optimally implement a response team is an open area of study itself, and until more data are available, it may be challenging to standardize these aspects of remote surveillance interventions.
Importantly, technology interventions in which clinical teams are being asked to implement new data, respond to new notifications, and adopt new processes into their workflow require careful preimplementation work. This work must focus on rigorously assessing the usability and feasibility of the technology for frontline clinicians. Several research tools have been developed to test these parameters before implementation, such as the National Aeronautics and Space Administration Task Loading Index and the 1-Item Usability Scale.75,76 Involving representative end-user clinicians in the assessment and modification of the tool before implementation is critical.
Perhaps one of the most important and challenging barriers to effective and sustainable implementation of remote surveillance is how clinicians perceive such systems and the attitudes that they develop toward them. Studies in the realm of implementation science have demonstrated that the most significant barrier to uptake is a perception that such systems will reduce professional autonomy.77 The impression that technologies could be used against physicians in the event of medical-legal controversies is also prevalent. Such barriers have proven more substantial than technical or usability issues. Addressing these barriers is both challenging and essential.
Demonstrating Cost-Effectiveness.
Cost-effectiveness studies have been extremely limited in this space, apart from those of tele-intensive care unit programs. Tele-intensive care unit often relies heavily on expensive hardware technology. Studies examining the cost-effectiveness of remote surveillance programs that rely on software rather than hardware to obtain and analyze clinical data in real-time and produce clinical decision support are virtually nonexistent. The inability of prior systems to demonstrate cost-effectiveness may lie in the fact that the aim was to prevent rare events, such as cardiac arrest or intensive care unit transfer. Cost-effectiveness may be more easily demonstrated for applications that increase adherence to high-value clinical care initiatives, such as enhanced recovery after surgery, that apply to a broad population of patients and have been associated with reductions in length of stay.
In addition, although the software is typically inexpensive, the wearable devices that can be connected with such systems can become prohibitively expensive. In particular, disposable patches and biosensors that need to be removed and reattached to the patient can become a costly component of such systems, particularly if the patient is monitored over an extended period of time. This is at odds with the intention to monitor across the perioperative episode, including pre-, intra-, and postoperative phases of care. Personnel cost is another key component of applications that use a centralized team of clinicians to monitor and react to remote surveillance data. Currently, such activities are not reimbursed.
Building Incentives for Sustainable Adoption.
Without providing financial incentives to implement remote surveillance technologies, the scale of such systems is likely to remain limited to a relatively small number of eager adopters. Building programs in which an anesthesiologist is fully devoted to monitoring a remote surveillance system will be impractical if this clinical activity is not reimbursed. The reimbursement landscape for digital health in general, but particularly for remote surveillance, is changing rapidly. For 2019, the Centers for Medicare and Medicaid Services announced that it will propose new rules that will expand the number of remote surveillance services for which it provides reimbursement.78 This continues a trend over the past several years marking Centers for Medicare and Medicaid Services’ increasing acceptance of telehealth and remote patient surveillance. Three Current Procedural Terminology codes have been built for these activities, which focus primarily on remote monitoring of patients in their home. In order for reimbursement to expand to applications relevant to the anesthesiologist, a greater preponderance of evidence demonstrating the value of such services in the pre- and postoperative settings must be made available.
For applications of remote surveillance that are more generally integrated into the traditional workflows of anesthesiologists, such as intraoperative surveillance, reimbursement is less likely. In these instances, policy makers may encourage hospitals to invest in these tools by using direct financial incentives. In 2009, the Health Information Technology for Economic and Clinical Health Act created a Meaningful Use Criteria to motivate hospitals across the country to make the initial investment to adopt electronic health records. Studies have demonstrated that the Health Information Technology for Economic and Clinical Health Act was primarily responsible for the substantial increases in electronic health record adoption observed over the past decade.79 With widespread adoption of electronic health records now in place, many hospitals are examining how to translate these investments into impacting clinical care at the bedside. Remote surveillance systems could represent this next phase, and such direct financial incentives may encourage their adoption.
CONCLUSIONS
The convergence of recent developments in health care information technology and monitoring have opened the door for remote surveillance systems that provide meaningful patient alerts. In all settings of the pre-, intra-, and postoperative continuum, patient care may benefit from such systems. Anesthesiologists have an opportunity to lead the development of these systems.
DISCLOSURES
Name: Kyan C. Safavi, MD, MBA.
Contribution: This author was the primary writer of the manuscript.
Name: William Driscoll, MA.
Contribution: This author helped write significant portions of sections of this article and create Figure 2.
Name: Jeanine P. Wiener-Kronish, MD.
Contribution: This author was the primary reviewer of the manuscript and rewrote portions of it.
This manuscript was handled by: Maxime Cannesson, MD, PhD.
Footnotes
Published ahead of print 21 November 2018.
Funding: None.
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
REFERENCES
- 1.Field MJ, Grigsby J. Telemedicine and remote patient monitoring. JAMA. 2002;288:423–425. [DOI] [PubMed] [Google Scholar]
- 2.Vegesna A, Tran M, Angelaccio M, Arcona S. Remote patient monitoring via non-invasive digital technologies: a systematic review. Telemed J E Health. 2017;23:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majumder S, Mondal T, Deen MJ. Wearable sensors for remote health monitoring. Sensors (Basel). 2017;17:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yilmaz T, Foster R, Hao Y. Detecting vital signs with wearable wireless sensors. Sensors (Basel). 2010;10:10837–10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piwek L, Ellis DA, Andrews S, Joinson A. The rise of consumer health wearables: promises and barriers. PLoS Med. 2016;13:e1001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen LS, Squara P. Non-invasive monitoring of cardiac output in critical care medicine. Front Med (Lausanne). 2017;4:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlesinger JJ. Applications of a noninvasive respiratory volume monitor for critical care medicine. Respir Care. 2015;60:e97–100. [DOI] [PubMed] [Google Scholar]
- 8.Kim HS, Shin JA, Chang JS, Cho JH, Son HY, Yoon KH. Continuous glucose monitoring: current clinical use. Diabetes Metab Res Rev. 2012;28(suppl 2):73–78. [DOI] [PubMed] [Google Scholar]
- 9.Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther. 2016;18(suppl 2):S3–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker SJ, Shander A, Ramsay MA. Continuous noninvasive hemoglobin monitoring: a measured response to a critical review. Anesth Analg. 2016;122:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolin RH, Alschuler L, Beebe C, et al. The HL7 clinical document architecture. J Am Med Inform Assoc. 2001;8:552–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandel JC, Kreda DA, Mandl KD, Kohane IS, Ramoni RB. SMART on FHIR: a standards-based, interoperable apps platform for electronic health records. J Am Med Inform Assoc. 2016;23:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Velasco FT, Musser RC, Kawamoto K. Enabling cross-platform clinical decision support through Web-based decision support in commercial electronic health record systems: proposal and evaluation of initial prototype implementations. AMIA Annu Symp Proc. 2013;2013:1558–1567. [PMC free article] [PubMed] [Google Scholar]
- 14.Walseth OA, Arsand E, Sund T, Skipenes E. Wireless transfer of sensor data into electronic health records. Stud Health Technol Inform. 2005;116:334–339. [PubMed] [Google Scholar]
- 15.Kruse CS, Goswamy R, Raval Y, Marawi S. Challenges and opportunities of big data in health care: a systematic review. JMIR Med Inform. 2016;4:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta N, Pandit A. Concurrence of big data analytics and healthcare: a systematic review. Int J Med Inform. 2018;114:57–65. [DOI] [PubMed] [Google Scholar]
- 17.The Joint Commission. The Joint Commission Announces 2014 National Patient Safety Goal. Joint Commission Perspectives. 2013;33:1–3. [PubMed] [Google Scholar]
- 18.Paine CW, Goel VV, Ely E, et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med. 2016;11:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonafide CP, Localio AR, Holmes JH, et al. Video analysis of factors associated with response time to physiologic monitor alarms in a children’s hospital. JAMA Pediatr. 2017;171:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2:230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krittanawong C, Zhang H, Wang Z, Aydar M, Kitai T. Artificial intelligence in precision cardiovascular medicine. J Am Coll Cardiol. 2017;69:2657–2664. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto DA, Rosman G, Rus D, Meireles OR. Artificial intelligence in surgery: promises and perils. Ann Surg. 2018;268:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller DD, Brown EW. Artificial intelligence in medical practice: the question to the answer? Am J Med. 2018;131:129–133. [DOI] [PubMed] [Google Scholar]
- 24.Kitsiou S, Paré G, Jaana M. Effects of home telemonitoring interventions on patients with chronic heart failure: an overview of systematic reviews. J Med Internet Res. 2015;17:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitsiou S, Paré G, Jaana M. Systematic reviews and meta-analyses of home telemonitoring interventions for patients with chronic diseases: a critical assessment of their methodological quality. J Med Internet Res. 2013;15:e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malani PN. Functional status assessment in the preoperative evaluation of older adults. JAMA. 2009;302:1582–1583. [DOI] [PubMed] [Google Scholar]
- 27.Minto G. Assessment of the high-risk perioperative patient. BJA Ed. 2014;14:12–17. [Google Scholar]
- 28.Carli F, Minnella EM. Preoperative functional assessment and optimization in surgical patient: changing the paradigm. Minerva Anestesiol. 2017;83:214–218. [DOI] [PubMed] [Google Scholar]
- 29.Oresanya LB, Lyons WL, Finlayson E. Preoperative assessment of the older patient: a narrative review. JAMA. 2014;311:2110–2120. [DOI] [PubMed] [Google Scholar]
- 30.Levett DZH, Jack S, Swart M, et al. ; Perioperative Exercise Testing and Training Society (POETTS). Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anaesth. 2018;120:484–500. [DOI] [PubMed] [Google Scholar]
- 31.American Society of Anesthesiologists. Statement on the Anesthesia Care Team: American Society of Anesthesiology. 2013.
- 32.Center for Medicare and Medicaid Services. Clarification of the Interpretive Guidelines for the Anesthesia Services Condition of Participation. 2010.
- 33.Kevin MD. Will Your Anesthesiologist Leave the OR? Patients Deserve to Know. 2015. Available at: https://www.kevinmd.com/blog/2015/10/will-your-anesthesiologist-leave-the-or-patients-deserve-to-know.html. Accessed May 5, 2018.
- 34.Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123:515–523. [DOI] [PubMed] [Google Scholar]
- 35.Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–515. [DOI] [PubMed] [Google Scholar]
- 36.Yang D, Grant MC, Stone A, Wu CL, Wick EC. A meta-analysis of intraoperative ventilation strategies to prevent pulmonary complications: is low tidal volume alone sufficient to protect healthy lungs? Ann Surg. 2016;263:881–887. [DOI] [PubMed] [Google Scholar]
- 37.Gandhi GY, Nuttall GA, Abel MD, et al. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc. 2005;80:862–866. [DOI] [PubMed] [Google Scholar]
- 38.Doherty M, Buggy DJ. Intraoperative fluids: how much is too much? Br J Anaesth. 2012;109:69–79. [DOI] [PubMed] [Google Scholar]
- 39.Kheterpal S, Shanks A, Tremper KK. Impact of a novel multiparameter decision support system on intraoperative processes of care and postoperative outcomes. Anesthesiology. 2018;128:272–282. [DOI] [PubMed] [Google Scholar]
- 40.Sessler DI. Decision support alerts: importance of validation. Anesthesiology. 2018;128:241–243. [DOI] [PubMed] [Google Scholar]
- 41.Tremper KK, Mace JJ, Gombert JM, Tremper TT, Adams JF, Bagian JP. Design of a novel multifunction decision support display for anesthesia care: AlertWatch® OR. BMC Anesthesiol. 2018;18:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray-Torres TM, Wallace F, Bollini M, Avidan MS, Politi MC. Anesthesiology control tower: feasibility assessment to support translation (ACT-FAST)-a feasibility study protocol. Pilot Feasibility Stud. 2018;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasin L, Nardelli P, Pintaudi M, et al. Closed-loop delivery systems versus manually controlled administration of total IV Anesthesia: a meta-analysis of randomized clinical trials. Anesth Analg. 2017;124:456–464. [DOI] [PubMed] [Google Scholar]
- 44.Ngan Kee WD, Tam YH, Khaw KS, Ng FF, Lee SWY. Closed-loop feedback computer-controlled phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery: a randomized trial comparing automated boluses versus infusion. Anesth Analg. 2017;125:117–123. [DOI] [PubMed] [Google Scholar]
- 45.Marques NR, Whitehead WE, Kallu UR, et al. Physician-directed versus computerized closed-loop control of blood pressure using phenylephrine in a swine model. Anesth Analg. 2017;125:110–116. [DOI] [PubMed] [Google Scholar]
- 46.Brogi E, Cyr S, Kazan R, Giunta F, Hemmerling TM. Clinical performance and safety of closed-loop systems: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2017;124:446–455. [DOI] [PubMed] [Google Scholar]
- 47.Grant MC, Pio Roda CM, Canner JK, et al. The impact of anesthesia-influenced process measure compliance on length of stay: results from an enhanced recovery after surgery for colorectal surgery cohort. Anesth Analg. 2019;128:68–74. [DOI] [PubMed] [Google Scholar]
- 48.Lyon A, Solomon MJ, Harrison JD. A qualitative study assessing the barriers to implementation of enhanced recovery after surgery. World J Surg. 2014;38:1374–1380. [DOI] [PubMed] [Google Scholar]
- 49.Pearsall EA, Meghji Z, Pitzul KB, et al. A qualitative study to understand the barriers and enablers in implementing an enhanced recovery after surgery program. Ann Surg. 2015;261:92–96. [DOI] [PubMed] [Google Scholar]
- 50.Hines R, Barash PG, Watrous G, O’Connor T. Complications occurring in the postanesthesia care unit: a survey. Anesth Analg. 1992;74:503–509. [DOI] [PubMed] [Google Scholar]
- 51.Zelcer J, Wells DG. Anaesthetic-related recovery room complications. Anaesth Intensive Care. 1987;15:168–174. [DOI] [PubMed] [Google Scholar]
- 52.American Society of Anesthesiologists. Recovery room complications decreased by nearly 60 percent, occur in less healthy patients. 2013. Washington, DC: American Society of Anesthesiologists; Available at: https://www.asahq.org/about-asa/newsroom/news-releases/2013/10/recovery-room-complications. Accessed September 29, 2018. [Google Scholar]
- 53.Rajnish G, David E. Monitoring for opioid-induced respiratory depression. Rochester, MN: The Anesthesia Patient Safety Foundation; Available at: https://www.apsf.org/article/monitoring-for-opioid-induced-respiratory-depression/. Accessed September 29, 2018. [Google Scholar]
- 54.Patient Safety Movement Foundation. Failure to Rescue: Monitoring for Opioid Induced Respiratory Depression. Patient Safety Movement. 2017. Available at: https://patientsafetymovement.org/wp-content/uploads/2016/02/APSS-4_-Failure-to-Rescue_-Monitoring-for-Opioid-Induced-Respiratory-Depression-copy.pdf.
- 55.Lam T, Nagappa M, Wong J, Singh M, Wong D, Chung F. continuous pulse oximetry and capnography monitoring for postoperative respiratory depression and adverse events: a systematic review and meta-analysis. Anesth Analg. 2017;125:2019–2029. [DOI] [PubMed] [Google Scholar]
- 56.Halpern NA, Pastores SM, Oropello JM, Kvetan V. Critical care medicine in the United States: addressing the intensivist shortage and image of the specialty. Crit Care Med. 2013;41:2754–2761. [DOI] [PubMed] [Google Scholar]
- 57.Pronovost PJ, Waters H, Dorman T. Impact of critical care physician workforce for intensive care unit physician staffing. Curr Opin Crit Care. 2001;7:456–459. [DOI] [PubMed] [Google Scholar]
- 58.Kozar RA, Shackford SR, Cocanour CS. Challenges to the care of the critically ill: novel staffing paradigms. J Trauma. 2008;64:366–370. [DOI] [PubMed] [Google Scholar]
- 59.Currell R, Urquhart C, Wainwright P, Lewis R. Telemedicine versus face to face patient care: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2000:CD002098. [DOI] [PubMed] [Google Scholar]
- 60.Wilcox ME, Adhikari NK. The effect of telemedicine in critically ill patients: systematic review and meta-analysis. Crit Care. 2012;16:R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar G, Falk DM, Bonello RS, Kahn JM, Perencevich E, Cram P. The costs of critical care telemedicine programs: a systematic review and analysis. Chest. 2013;143:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mackintosh N, Terblanche M, Maharaj R, et al. Telemedicine with clinical decision support for critical care: a systematic review. Syst Rev. 2016;5:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flodgren G, Rachas A, Farmer AJ, Inzitari M, Shepperd S. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2015:CD002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young LB, Chan PS, Lu X, Nallamothu BK, Sasson C, Cram PM. Impact of telemedicine intensive care unit coverage on patient outcomes: a systematic review and meta-analysis. Arch Intern Med. 2011;171:498–506. [DOI] [PubMed] [Google Scholar]
- 65.Waydhas C. Intrahospital transport of critically ill patients. Crit Care. 1999;3:R83–R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klopfenstein CE, Herrmann FR, Michel JP, Clergue F, Forster A. The influence of an aging surgical population on the anesthesia workload: a ten-year survey. Anesth Analg. 1998;86:1165–1170. [DOI] [PubMed] [Google Scholar]
- 67.Chiulli LC, Stephen AH, Heffernan DS, Miner TJ. Association of medical comorbidities, surgical outcomes, and failure to rescue: an analysis of the Rhode Island Hospital NSQIP Database. J Am Coll Surg. 2015;221:1050–1056. [DOI] [PubMed] [Google Scholar]
- 68.Rojas JC, Shappell C, Huber M. Advances in rapid response, patient monitoring, and recognition of and response to clinical deterioration. Jt Comm J Qual Patient Saf. 2017;43:686–694. [DOI] [PubMed] [Google Scholar]
- 69.DeVita MA, Bellomo R, Hillman K. Introduction to the rapid response systems series. Jt Comm J Qual Patient Saf. 2006;32:359–360. [DOI] [PubMed] [Google Scholar]
- 70.Churpek MM, Adhikari R, Edelson DP. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation. 2016;102:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Churpek MM, Yuen TC, Winslow C, et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med. 2014;190:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang MA, Churpek MM, Zadravecz FJ, Adhikari R, Twu NM, Edelson DP. Real-time risk prediction on the wards: a feasibility study. Crit Care Med. 2016;44:1468–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kollef MH, Chen Y, Heard K, et al. A randomized trial of real-time automated clinical deterioration alerts sent to a rapid response team. J Hosp Med. 2014;9:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu SB, Wong DJ, Correa A, Li N, Deng JC. Prediction of clinical deterioration in hospitalized adult patients with hematologic malignancies using a neural network model. PLoS One. 2016;11:e0161401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tubbs-Cooley HL, Mara CA, Carle AC, Gurses AP. The NASA Task Load Index as a measure of overall workload among neonatal, paediatric and adult intensive care nurses. Intensive Crit Care Nurs. 2018;46:64–69. [DOI] [PubMed] [Google Scholar]
- 76.Finomore VS, Jr, Shaw TH, Warm JS, Matthews G, Boles DB. Viewing the workload of vigilance through the lenses of the NASA-TLX and the MRQ. Hum Factors. 2013;55:1044–1063. [DOI] [PubMed] [Google Scholar]
- 77.Castillo VH, Martínez-García AI, Pulido JR. A knowledge-based taxonomy of critical factors for adopting electronic health record systems by physicians: a systematic literature review. BMC Med Inform Decis Mak. 2010;10:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Center for Medicare and Medicaid Services. Proposed Policy, Payment, and Quality Provisions Changes to the Medicare Physician Fee Schedule for Calendar Year 2019. Baltimore, MD: The Centers for Medicare and Medicaid Services; Available at: https://www.cms.gov/newsroom/fact-sheets/proposed-policy-payment-and-quality-provisions-changes-medicare-physician-fee-schedule-calendar-year-3. Accessed September 29, 2018. [Google Scholar]
- 79.Adler-Milstein J, Jha AK. HITECH Act drove large gains in hospital electronic health record adoption. Health Aff (Millwood). 2017;36:1416–1422. [DOI] [PubMed] [Google Scholar]


