Abstract
Purpose of review
Right ventricular (RV) function is an important determinant of morbidity and mortality in patients with pulmonary arterial hypertension (PAH). Although substantial progress has been made in understanding the development of RV failure in the last decennia, this has not yet resulted in the development of RV selective therapies. In this review, we will discuss the current status on the treatment of RV failure and potential novel therapeutic strategies that are currently being investigated in clinical trials.
Recent findings
Increased afterload results in elevated wall tension. Consequences of increased wall tension include autonomic disbalance, metabolic shift and inflammation, negatively affecting RV contractility. Compromised RV systolic function and low cardiac output activate renin–angiotensin aldosterone system, which leads to fluid retention and further increase in RV wall tension. This vicious circle can be interrupted by directly targeting the determinants of RV wall tension; preload and afterload by PAH-medications and diuretics, but is also possibly by restoring neurohormonal and metabolic disbalance, and inhibiting maladaptive inflammation. A variety of RV selective drugs are currently being studied in clinical trials.
Summary
Nowadays, afterload reduction is still the cornerstone in treatment of PAH. New treatments targeting important pathobiological determinants of RV failure directly are emerging.
Keywords: neurohormonal signaling, right ventricular dysfunction, treatment, wall tension
INTRODUCTION
In pulmonary arterial hypertension (PAH), as a consequence of pulmonary arterial remodeling, pulmonary vascular resistance (PVR) increases, posing an elevated load to the right ventricle (RV). By enhancing contractility, the RV adapts to this load so that cardiac output (CO) may be maintained. At some point in the disease the RV starts to dilate. The high afterload (pressure) in combination with the dilatation (volume) results in a raised wall tension, since wall tension is a product of pressure and ventricular volume and wall thickness [1]. With higher wall tension, RV oxygen consumption increases, metabolism alters and autonomic disbalance occurs [2]. These processes negatively affect RV function. Underfilling of the left ventricle (LV) by septal bowing and a low preload results in low systemic blood pressures [3], causing elevated renin–angiotensin aldosterone system (RAAS) activation. One of the effects of higher RAAS activation is increasing water reabsorption in the kidneys, which further increases RV preload and wall tension (Fig. 1).
FIGURE 1.
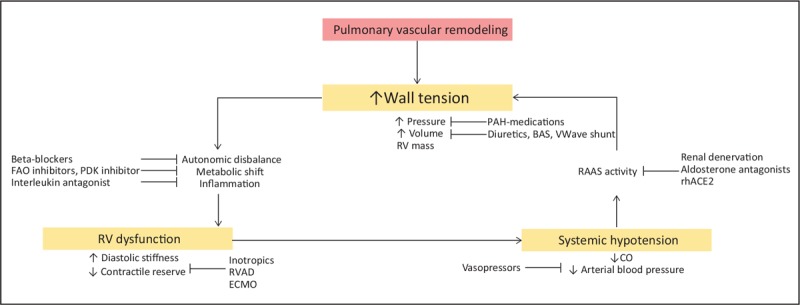
Pathobiology of right ventricular failure in pulmonary arterial hypertension. Schematic overview of mechanisms affecting and maintaining right ventricular wall tension and dysfunction in pulmonary arterial hypertension and medical treatments targeting these mechanisms. BAS, balloon artrial septostomy; CO, cardiac output; ECMO, extra corporal membrane oxygenation; ERAs, endothelin receptor antagonists; FAO, fatty acid oxidation; PDE-5, phosphodiesterase type 5; PDK, pyruvaat dehydrogenase kinase; RAAS, renin–angiotensin aldosterone system; rhACE2, recombinant human angiotensin converting enzyme 2; RV, right ventricle; RVAD, right ventricular assist device.
Although various (pre)clinical studies treating the RV have been performed, a treatment targeting the RV directly is not incorporated in the current PAH treatment algorithm. Nevertheless, several therapies have shown to be potentially successful in improving RV function in animal models and/or pilot studies in patients. For the current review we will focus on new strategies that are currently being explored in patients.
Wall tension of the RV plays a central role in the development of right heart failure and will be discussed first, together with approaches to reduce wall tension. Next, RV dysfunction is described and therapies and interventions are reviewed. Finally, untoward factors secondary to RV failure are assessed.
Box 1.
no caption available
WALL TENSION
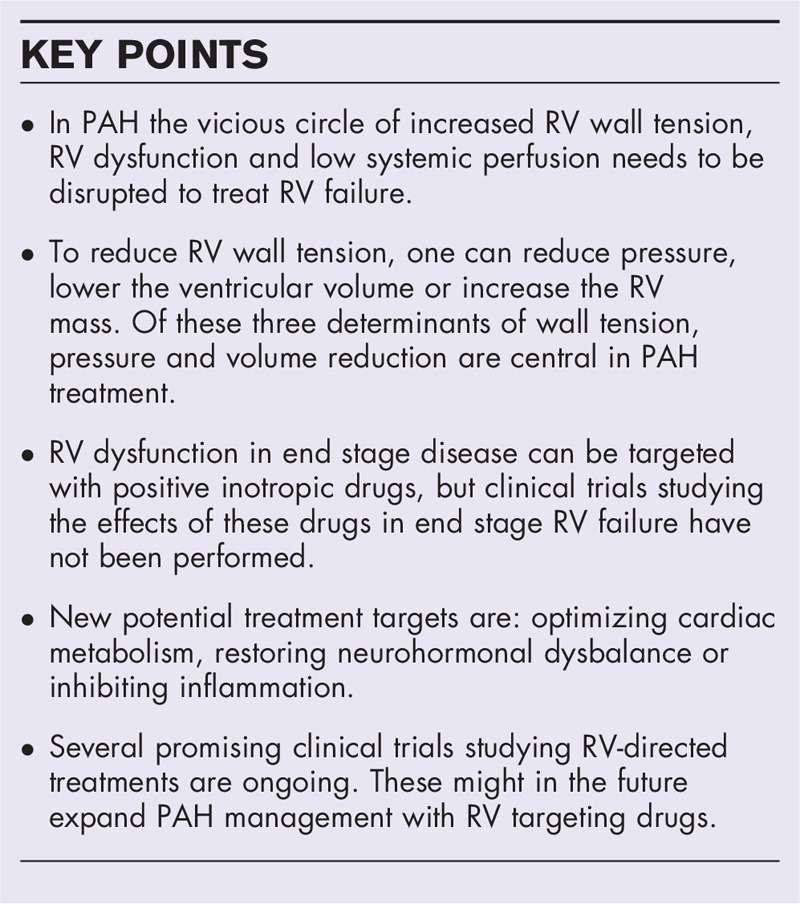
According to the Law of Laplace, wall tension is a product of volume times pressure divided by wall thickness [1]. To reduce RV wall tension, one can reduce pressure, lower the ventricular volume or increase the RV mass. Of these three determinants of wall tension, pressure and volume reduction are central in PAH treatment.
PRESSURE REDUCTION
When the load is reduced to normal levels, by lung transplantation or pulmonary endarterectomy in chronic thrombo-embolic pulmonary hypertension, RV volumes and function can normalize [4,5]. Therefore, the main goal of PAH-specific therapy is to decrease vascular resistance to lower RV afterload. Three groups of drugs are registered for this goal: phosphodiesterase type five-inhibitors, endothelin receptor antagonists, and prostacyclins [6–8]. Monotherapy consisting of one of these drugs results in an improvement in hemodynamics and exercise capacity [6–8]. However, upfront combination therapy results in a greater reduction in RV wall stress, and larger improvement of RV ejection fraction (RVEF) than monotherapy alone [9,10]. Whether upfront triple therapy is superior to dual therapy in reducing afterload is currently been investigated in the TRITION trial (NCT02558231). The primary objective of this study is to compare the effects of initial oral combination (macitentan and tadalafil) or triple (macitentan, tadalafil and selexipag) therapy in newly diagnosed PAH patients. Primary endpoint in this study is change in PVR after 26 weeks. Change in n-terminal pro-brain natriuretic peptide (NT-proBNP) levels, a biomarker of RV wall tension, is a secondary efficacy endpoint. On the contrary, no direct measures of RV function are included in the study design. Therefore, future studies will need to show whether the reduction in load with triple therapy is sufficient to restore RV function.
VOLUME UNLOADING
PAH patients with advanced RV failure; or decompensated RV failure, will develop symptoms resulting from backward – (ankle edema, ascites) and forward failure (low systemic blood pressure, low CO, resulting in reduced perfusion of vital organs). As a consequence of both RAAS activation and venous congestion, volume load on the RV will increase. To reduce this RV preload, diuretic treatment can be initiated. In end stage disease, a balloon atrial septostomy can be considered, to relieve the heart from the volume overload.
Oral diuretics
As in left heart failure, preload reduction by diuretic treatment can be incorporated in PAH management [11]. Clinical trials report numbers of patients on diuretic treatment varying from 50 to 90% [12]. Although many patients are on diuretics, studies of the effects of these drugs on the RV function are absent. Because of the arguments in favor of reducing preload and wide availability of diuretics, research studying the optimal diuretic type (thiazide, loop diuretic), optimal dosage and the effects of these drugs on RV function are needed.
Intravenous diuretic treatment
Optimal volume management with loop diuretics is not always possible due to the development of diuretic resistance and deterioration of kidney function [13]. Development of diuretics resistance is multifactorial [12]. One of the possible mechanisms of diuretic resistance is the combination of both forward failure and renal venous congestion. This causes an increased renal interstitial pressure, which affects glomerular filtration [14,15]. Strategies to overcome the diuretic resistance have not been explored until now. Theoretically, in these patients, supportive therapy with vasopressors might be of use, as those drugs increase systemic vascular resistance and subsequently renal perfusion pressure. Vasopressin analogues were successfully used in patients with end stage liver failure who developed hepatorenal syndrome. As in PAH, patients with this syndrome have ascites and systemic hypotension affecting renal perfusion. In this disease, administration of vasopressors increased systemic vascular resistance, diuresis and glomerular filtration rate [16].
Atrial septostomy
Apart from medication, more invasive strategies are possible. The oldest and most permanent invasive treatment is balloon atrial septostomy. After balloon atrial septostomy, an interatrial right-to-left shunt results in a reduction of RV end diastolic pressure, improving cardiac index [17,18]. This procedure is not recommended in patients with a right atrial pressure more than 20 mmHg or an oxygen saturation less than 85% at rest [11]. These strategies need to be performed in centers where sufficient experience is available and are therefore not widely applicable or included in all treatment algorithms. Currently, the safety of a new atrial shunt device, the V-Wave [the V-Wave (Caesarea, Israel) shunt device is currently not Food and Drug Administration approved; the product is still investigational], is studied in the RELIEVE-pulmonary hypertension trial (NCT03838445). This unidirectional shunt device showed promising results on New York Health Association (NYHA) functional class, 6MWD and quality of life in NYHA functional class IV left heart failure, while ventricular function did not improve after implantation [19].
RIGHT VENTRICULAR DYSFUNCTION
In a healthy person, during systole the RV ejects the blood in the low resistance pulmonary circulation. To maintain a sufficient flow in patients with PAH, the RV has to increase its contractility. At some point in the disease, or during exercise, the RV is not capable to augment contractile force further. Metabolic changes, inflammation and maladaptive neurohormonal signaling provoked by the increased wall tension additionally compromise RV contractility. During diastole the RV is filled, and in failure may be hampered by myocardial stiffness, also reducing CO. So far, diastolic function has received much less attention than systolic function. In this paragraph we will discuss several treatments targeting RV dysfunction.
CONTRACTILITY
Dobutamine
In case of severe RV failure (in a ICU setting), PAH guidelines recommend dobutamine administration, to maintain CO by increasing contractility and heart rate (HR) [11]. Although low dose dobutamine seems to be safe in these patients [20], it has several disadvantages. First of all, it could be questioned whether an increase in contractility is accomplished, as it has been shown in non end-stage RV failure, that PAH patients do not have a contractile reserve [21,22]. This suggests that contractility is already maximal an cannot increase further. Second, dobutamine is known to increase myocardial oxygen consumption further [23], by the rise in HR. This might have detrimental effects for the already highly challenged RV cardiomyocytes [24]. Lastly, it has been shown that patients receiving a high dose of dobutamine on the ICU are at a higher risk of mortality than patients receiving a low dose of this drug [20]. The most likely explanation for this phenomenon is that these patients were more severely diseased, but whether this increased mortality risk is related to the effects of dobutamine on myocardial oxygen consumption is not answered. Therefore, studies on optimal treatment in patients with end stage RV failure are warranted and administration of dobutamine deserves some caution.
Levosimendan
Levosimendan is an inotropic drug with vasodilatory properties and has potential for treatment of RV failure in PAH. Its effectiveness has been studied in animal models of acute RV failure [25]. Safety and efficacy of this drug are largely unknown in PAH patients [26]. Currently a phase 2 study into the effects of levosimendan on hemodynamics during exercise in patients with heart failure with preserved ejection fraction and pulmonary hypertension are investigated (NCT03541603). Although interesting, no trials on the effects of this drug on RV function in precapillary pulmonary hypertension are registered on clinicaltrails.gov.
Ventricular assist devices and extracorporeal membrane oxygenation
A more invasive method to preserve CO in end stage disease is mechanical support of the RV by extracorporeal membrane oxygenation (ECMO) or a pumpless oxygenator. Hence, these techniques are mainly applied in patients as a bridge to lung transplantation and when the patients’ response to optimal drug treatment is insufficient. In patients with RV failure the veno-arterial ECMO is primarily applied [27,28▪] and use in nonintubated patients is preferred. Implantable ventricular assist devices (VAD) in RV failure would be more suitable for long-term use. An implantable assist device (Synergy Pocket Micro Pump, HeartWare, Framingham, Massachusetts, USA) has been tested in a pulmonary artery banding in sheep. The device was mainly beneficial in improving CO in acute banding, but not in chronic RV pressure overload by 8 weeks of pulmonary artery banding [29]. Data on use of implantable VAD in patients with isolated RV failure is limited to case reports [30,31], consequently there is insufficient evidence to support the use of VADs in the treatment of end stage RV failure.
METABOLIC SHIFT
The main source of ATP production in the heart is fatty acid oxidation (FAO) [32]. Although glucose oxidation requires less oxygen to generate the same amount of adenosine triphosphate, it generates only 10–40% of the myocardial energy [32,33]. In the pressure overloaded state, cardiac metabolism shifts to more FAO, resulting in a less efficient energy production [34]. This might be detrimental for two reasons: first, the RV that has an increased oxygen/ energy consumption as a consequence of the elevated pressure and high HR; second, the oxygen supply in the heart is already decreased, as a result of capillary rarefaction and a coronary flow that becomes biphasic with reduced systolic flow [35,36]. As the metabolic shift in the overloaded RV is most likely maladaptive, restoring the metabolic balance is a potential treatment target.
Fatty acid oxidation inhibitors
One approach to force the heart into more glucose oxidation instead of the less efficient FAO, is by inhibiting FAO. Two putative FAO inhibitors are currently studied in RV failure; ranolazine and trimetazidine [37,38]. In pulmonary artery banding in rats, FAO inhibition improved CO and exercise capacity [34]. Currently several trials are ongoing testing these drugs in humans. Ranolazine was already found to be safe in PAH patients and did result in an echocardiographic reduction in RV size, improved RV function (improvement in RV strain during exercise) in patients completing the 3-month trial [39]. On the contrary, a second 12-week pilot trial could not reproduce these findings and did not observe any effects on hemodynamics, functional capacity or RV function [40]. Interestingly, ongoing trials on ranolazine aim to assess the effects of FAO inhibition on RV function but also on RV metabolism, using positron emission tomography (NCT01917136, NCT01839110, NCT02829034). Hence, the results of these trials could provide additional information on the effects of metabolic changes on RV function. In addition to the ranolazine trials, two trials studying the effects of trimetazidine on RV function in PAH are ongoing (NCT02102672 and NCT03273387).
Dichloroacetate
Instead of inhibiting FAO to restore cardiac metabolism, glucose oxidation could also be stimulated, and dichloroacetate is supposed to effectuate this. The compound inhibits pyruvate dehydrogenase kinase, an inhibitor of pyruvate dehydrogenase, the central enzyme in glucose oxidation [41]. A recent study by Michelakis et al. has demonstrated the beneficial effects of glucose oxidation stimulation with dichloroacetate. In patients without genetic variants in proteins regulating pyruvate dehydrogenase, dichloroacetate reduced pulmonary vascular remodeling and improved hemodynamics [41]. Whether this drug is beneficial for the failing RV is however still an unanswered question.
SECONDARY FACTORS
The increased wall tension and reduced blood pressure trigger activation of the autonomic nervous system, the RAAS, and release of inflammatory cytokines. These secondary factors of PAH can also be a target to indirectly improve RV function.
NEUROHORMONAL MODULATION
Beta-adrenergic activity
Autonomic disbalance plays a role in the development and maintenance of RV failure in PAH; sympathetic activity is increased, whereas parasympathetic activity is reduced [42,43]. This autonomic dysregulation is related to poor survival and RV function [42,44–47]. Consequently, treatments downregulating the sympathetic activity and/or increasing parasympathetic activation have been of interest.
Beta-blocker therapy inhibits sympathetic activity and is known to improve cardiac function and survival in LV failure [48]. For a long time, clinicians were reluctant to study the beta-blockers in RV failure, as patients are not capable to increase stroke volume and are therefore dependent on their HR to maintain CO. However, after beneficial effects on the RV of several beta-blockers had been confirmed in animal models [49,50], beta-blocker therapy was tested in clinical trials. Despite promising effects in a first pilot study, until now, beneficial effects of beta-blocker therapy on RV function have not been proven [51,52]. Hence, beta-blockers are not indicated in management of RV failure.
An alternative for sympathetic inhibition could be reducing parasympathetic activation as was recently shown [43]. However, the therapeutic potential of agents activating the parasympathetic nervous system has not been tested yet in patients.
Pulmonary artery denervation
Decades ago, research implied the existence of baroreceptors in the pulmonary artery trunk [53]. Activation of these baroreceptors by balloon inflation resulted in pulmonary vasoconstriction, subsequently increasing PVR [54]. More recently, this discovery led to the idea that interruption of the baroreflex control system might result in pulmonary arterial relaxation and a decrease in pulmonary artery pressure [55]. In the first in-human study, hemodynamics improved after pulmonary artery denervation [56]. After this pilot study some serious safety concerns remained. Currently, a trial (TROPHY, NCT02516722) is assessing the safety, performance and initial effectiveness of the TIVUS system (Sonivie, Tel-Aviv, Isreal) when used for pulmonary artery denervation. In addition, currently, the effects of pulmonary artery denervation in combination with sildenafil, on clinical worsening and survival are investigated in a large cohort (NCT02284737). Except for the change in NT-proBNP levels as measure of RV wall stress, neither of these trials study the effects of this procedure on the RV.
RENIN–ANGIOTENSIN ALDOSTERONE SYSTEM ACTIVITY
RAAS is, besides in autonomic disbalance, implicated to play a role in PAH disease severity and mortality [57,58]. High circulating levels of angiotensin I and II and aldosterone are related to an increased mortality risk and poor exercise capacity [57,58]. RAAS activation, which is a consequence of low systemic blood pressure in PAH, leads to release of renin. Renin converts angiotensinogen in angiotensin I which is converted to angiotensin II by the angiotensin converting enzyme (ACE). Angiotensin II either activates the angiotensin 1 receptor (AT1R), stimulating hypertrophy and fibrosis of the myocardium and aldosterone production in the adrenal gland or the angiotensin 2 receptor [59,60]. The maladaptive signaling via AT1R can be suppressed by several drugs. ACE can be blocked by ACE inhibitors, the AT1R by AT1R blockers and aldosterone inhibitors. Because ACE inhibition was associated with side effects such as hypotension [61–63], currently, the aldosterone inhibitor spironolactone is the only drug clinically explored in PAH. Retrospective analysis of clinical trials using the endothelin type-A receptor inhibitor ambrisentan demonstrated that patients treated with both ambrisentan and the aldosterone inhibitor spironolactone improved more in 6MWD, and had a greater reduction in NT-proBNP concentration when compared with patients treated with ambrisentan alone [64]. Results of larger trials prospectively studying the effects of spironolactone in PAH patients are expected in the near future (NCT02253394, NCT01712620, NCT03344159).
In addition to activating the AT1R, angiotensin II can be hydrolyzed by ACE2 to Angiotensin1–7, acting on the Mas receptor. Mas stimulation has cardio protective effects in the heart failure [65]. In animals with RV failure, administration of recombinant ACE2 improved RV function and reduced RV hypertrophy and fibrosis [66]. In patients, direct effects of recombinant ACE2 administration on RV function were not obtained. However, PVR and CO were positively affected [67].
Further studies should reveal the applicability of drugs intervening in the RAAS system, in patients with RV dysfunction.
INFLAMMATION
RV tissue of PAH patients is infiltrated with inflammatory cells [68–71]. Experimental data show that this infiltration is related to severity of RV failure and can be exaggerated by exercise [72–75]. Several causes of this RV inflammation have been implied; ischemia, spillover of cytokines and increased RV wall tension [75–77]. Systemically, in patients with PAH, the pro-inflammatory cytokines IL-1 and IL-6 are also related to disease progression [76]. Therefore, the idea that anti-inflammatory drugs could serve as a treatment was postulated. In experimental PAH, decreasing inflammation via various pathways was beneficial on RV structure and function; it resulted in less RV hypertrophy, improvement of function [74]. Several anti-inflammatory drugs are currently explored in clinical trials and discussed here.
Anakinra
As it was demonstrated that anakinra, an IL-1 receptor antagonist, could ameliorate PAH in an experimental study [78], the safety of this drug was very recently studied in a Phase Ib/II trial. After 14 days of treatment with 100 mg subcutaneously daily, six patients received anakinra and did not experience severe side effects. Significantly, although exercise capacity and RV fractional area change did not alter after the intervention, NT-proBNP levels dropped [79▪]. Further clinical research is needed to demonstrate the potential efficacy of anakinra in RV failure.
Histamine receptor antagonist
Mast cells and histamine expression are increased in failing hearts [80]. Activation of the histamine H2 receptor in the myocardium by histamine has positive inotropic and chronotropic effects, which is similar to the effects beta-adrenergic receptor activation [81]. Therefore, the consequences of H2 receptor antagonism on cardiac function have been of interest. Leary et al.[82] demonstrated in a large cohort of individuals without cardiovascular disease, that use of H2 receptor antagonists results in a lower RV mass and end diastolic volume. The same research group observed a 10% lower mortality risk in pulmonary hypertension patients using H2 receptor antagonists for gastroesophageal reflux disease [83▪]. Currently, the effects of the H2 receptor antagonist famotidine on RV function, measured by echocardiography, are evaluated in a randomized clinical trial in PAH patients (NCT03554291).
CONCLUSION
RV function is the major determinant of morbidity and mortality in patients with PAH. Current treatment strategies mainly focus pharmacologically reducing afterload to reduce wall tension. Another way to lower wall tension is by preload reduction. Although frequently prescribed, pharmacological treatments targeting preload have not been evaluated in RV failure. To date, these approaches, directly targeting wall tension, have not been sufficient to normalize RV function. Therefore, treatments directly targeting the RV are mandatory to further improve survival of patients with PAH. The RV can be directly targeted by positive inotropic drugs, studies on these treatments are sparse and these drugs are only given in end stage RV failure. In addition, treatments targeting secondary effects of elevated wall tension, as metabolic changes, neurohormonal signaling and inflammation are currently being investigated in clinical trials. In conclusion, the RV as a new target for treatment is currently under investigation and results are eagerly awaited. For future studies, a universally agreed end point, preferably a load independent measure of RV function, would be advisable.
Acknowledgements
None.
Financial support and sponsorship
J.A.G. and B.E.W. and were supported by The Netherlands Organization for Scientific Research (NWO-VICI: 918.16.610). F.S.d.M. was supported by The Netherlands CardioVascular Research Initiative (CVON-2012-08 PHAEDRA, CVON-2017-10 DOLPHIN-GENESIS) and The Netherlands Organization for Scientific Research (NWO-VIDI: 917.18.338).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Westerhof N, Stergiopulos N, Noble MIM, Westerhof BE. Snapshots of Hemodynamics. 3rd edn.New York: Springer; 2018. [Google Scholar]
- 2.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol 2017; 69:236–243. [DOI] [PubMed] [Google Scholar]
- 3.Marcus JT, Gan CT, Zwanenburg JJ, et al. Interventricular mechanical asynchrony in pulmonary arterial hypertension: left-to-right delay in peak shortening is related to right ventricular overload and left ventricular underfilling. J Am Coll Cardiol 2008; 51:750–757. [DOI] [PubMed] [Google Scholar]
- 4.Gorter TM, Verschuuren EAM, van Veldhuisen DJ, et al. Right ventricular recovery after bilateral lung transplantation for pulmonary arterial hypertensiondagger. Interact Cardiovasc Thorac Surg 2017; 24:890–897. [DOI] [PubMed] [Google Scholar]
- 5.Reesink HJ, Marcus JT, Tulevski II, et al. Reverse right ventricular remodeling after pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: utility of magnetic resonance imaging to demonstrate restoration of the right ventricle. J Thorac Cardiovasc Surg 2007; 133:58–64. [DOI] [PubMed] [Google Scholar]
- 6.Channick R, Badesch DB, Tapson VF, et al. Effects of the dual endothelin receptor antagonist bosentan in patients with pulmonary hypertension: a placebo-controlled study. J Heart Lung Transplant 2001; 20:262–263. [DOI] [PubMed] [Google Scholar]
- 7.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005; 353:2148–2157. [DOI] [PubMed] [Google Scholar]
- 8.Rubin LJ, Mendoza J, Hood M, et al. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med 1990; 112:485–491. [DOI] [PubMed] [Google Scholar]
- 9.Galie N, Barbera JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373:834–844. [DOI] [PubMed] [Google Scholar]
- 10.van de Veerdonk MC, Huis In TVAE, Marcus JT, et al. Upfront combination therapy reduces right ventricular volumes in pulmonary arterial hypertension. Eur Respir J 2017; 49:6. [DOI] [PubMed] [Google Scholar]
- 11.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46:903–975. [DOI] [PubMed] [Google Scholar]
- 12.Nickel NP, O’Leary JM, Brittain EL, et al. Kidney dysfunction in patients with pulmonary arterial hypertension. Pulm Circ 2017; 7:38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews JC, McLaughlin V. Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management. Curr Cardiol Rev 2008; 4:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afsar B, Ortiz A, Covic A, et al. Focus on renal congestion in heart failure. Clin Kidney J 2016; 9:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damman K, Navis G, Smilde TD, et al. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail 2007; 9:872–878. [DOI] [PubMed] [Google Scholar]
- 16.Lenz K, Hortnagl H, Druml W, et al. Ornipressin in the treatment of functional renal failure in decompensated liver cirrhosis. Effects on renal hemodynamics and atrial natriuretic factor. Gastroenterology 1991; 101:1060–1067. [DOI] [PubMed] [Google Scholar]
- 17.Kurzyna M, Dabrowski M, Bielecki D, et al. Atrial septostomy in treatment of end-stage right heart failure in patients with pulmonary hypertension. Chest 2007; 131:977–983. [DOI] [PubMed] [Google Scholar]
- 18.Sandoval J, Gaspar J, Pulido T, et al. Graded balloon dilation atrial septostomy in severe primary pulmonary hypertension. A therapeutic alternative for patients nonresponsive to vasodilator treatment. J Am Coll Cardiol 1998; 32:297–304. [DOI] [PubMed] [Google Scholar]
- 19.Rodes-Cabau J, Bernier M, Amat-Santos IJ, et al. Interatrial shunting for heart failure: early and late results from the first-in-human experience with the V-Wave system. JACC Cardiovasc Interv 2018; 11:2300–2310. [DOI] [PubMed] [Google Scholar]
- 20.Sztrymf B, Souza R, Bertoletti L, et al. Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur Respir J 2010; 35:1286–1293. [DOI] [PubMed] [Google Scholar]
- 21.Spruijt OA, de Man FS, Groepenhoff H, et al. The effects of exercise on right ventricular contractility and right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med 2015; 191:1050–1057. [DOI] [PubMed] [Google Scholar]
- 22.Tedford RJ, Mudd JO, Girgis RE, et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail 2013; 6:953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikolaidis LA, Trumble D, Hentosz T, et al. Catecholamines restore myocardial contractility in dilated cardiomyopathy at the expense of increased coronary blood flow and myocardial oxygen consumption (MvO2 cost of catecholamines in heart failure). Eur J Heart Fail 2004; 6:409–419. [DOI] [PubMed] [Google Scholar]
- 24.Wong YY, Ruiter G, Lubberink M, et al. Right ventricular failure in idiopathic pulmonary arterial hypertension is associated with inefficient myocardial oxygen utilization. Circ Heart Fail 2011; 4:700–706. [DOI] [PubMed] [Google Scholar]
- 25.Kerbaul F, Rondelet B, Demester JP, et al. Effects of levosimendan versus dobutamine on pressure load-induced right ventricular failure. Crit Care Med 2006; 34:2814–2819. [DOI] [PubMed] [Google Scholar]
- 26.Kleber FX, Bollmann T, Borst MM, et al. Repetitive dosing of intravenous levosimendan improves pulmonary hemodynamics in patients with pulmonary hypertension: results of a pilot study. J Clin Pharmacol 2009; 49:109–115. [DOI] [PubMed] [Google Scholar]
- 27.Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012; 185:763–768. [DOI] [PubMed] [Google Scholar]
- 28▪.Olsson KM, Halank M, Egenlauf B, et al. Decompensated right heart failure, intensive care and perioperative management in patients with pulmonary hypertension: Updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol 2018; 272S:46–52. [DOI] [PubMed] [Google Scholar]; The recently published review gives a comprehensive overview of management of decompensated right heart failure in patients with pulmonary hypertension and describes clinically applicable strategies for physicians.
- 29.Verbelen T, Verhoeven J, Goda M, et al. Mechanical support of the pressure overloaded right ventricle: an acute feasibility study comparing low and high flow support. Am J Physiol Heart Circ Physiol 2015; 309:H615–H624. [DOI] [PubMed] [Google Scholar]
- 30.Rosenzweig EB, Chicotka S, Bacchetta M. Right ventricular assist device use in ventricular failure due to pulmonary arterial hypertension: lessons learned. J Heart Lung Transplant 2016; 35:1272–1274. [DOI] [PubMed] [Google Scholar]
- 31.Vullaganti S, Tibrewala A, Rich J, et al. EXPRESS: the use of a durable right ventricular assist device for isolated right ventricular failure due to combined pre and postcapillary pulmonary hypertension. Pulm Circ 2019; 9:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res 1997; 33:243–257. [DOI] [PubMed] [Google Scholar]
- 33.Abozguia K, Clarke K, Lee L, Frenneaux M. Modification of myocardial substrate use as a therapy for heart failure. Nat Clin Pract Cardiovasc Med 2006; 3:490–498. [DOI] [PubMed] [Google Scholar]
- 34.Fang YH, Piao L, Hong Z, et al. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: exploiting Randle's cycle. J Mol Med 2012; 90:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bogaard HJ, Natarajan R, Henderson SC, et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 2009; 120:1951–1960. [DOI] [PubMed] [Google Scholar]
- 36.van Wolferen SA, Marcus JT, Westerhof N, et al. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur Heart J 2008; 29:120–127. [DOI] [PubMed] [Google Scholar]
- 37.Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res 2000; 86:580–588. [DOI] [PubMed] [Google Scholar]
- 38.Tuunanen H, Engblom E, Naum A, et al. Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation 2008; 118:1250–1258. [DOI] [PubMed] [Google Scholar]
- 39.Khan SS, Cuttica MJ, Beussink-Nelson L, et al. Effects of ranolazine on exercise capacity, right ventricular indices, and hemodynamic characteristics in pulmonary arterial hypertension: a pilot study. Pulm Circ 2015; 5:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomberg-Maitland M, Schilz R, Mediratta A, et al. Phase I safety study of ranolazine in pulmonary arterial hypertension. Pulm Circ 2015; 5:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michelakis ED, Gurtu V, Webster L, et al. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med 2017; 9:413. [DOI] [PubMed] [Google Scholar]
- 42.Ciarka A, Doan V, Velez-Roa S, et al. Prognostic significance of sympathetic nervous system activation in pulmonary arterial hypertension. Am J Respir Crit Care Med 2010; 181:1269–1275. [DOI] [PubMed] [Google Scholar]
- 43.da Silva Goncalves Bos D, Van Der Bruggen CEE, Kurakula K, et al. Contribution of impaired parasympathetic activity to right ventricular dysfunction and pulmonary vascular remodeling in pulmonary arterial hypertension. Circulation 2018; 137:910–924. [DOI] [PubMed] [Google Scholar]
- 44.Mak S, Witte KK, Al-Hesayen A, et al. Cardiac sympathetic activation in patients with pulmonary arterial hypertension. Am J Physiol Regul Integr Comp Physiol 2012; 302:R1153–R1157. [DOI] [PubMed] [Google Scholar]
- 45.Nootens M, Kaufmann E, Rector T, et al. Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: relation to hemodynamic variables and endothelin levels. J Am Coll Cardiol 1995; 26:1581–1585. [DOI] [PubMed] [Google Scholar]
- 46.Velez-Roa S, Ciarka A, Najem B, et al. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation 2004; 110:1308–1312. [DOI] [PubMed] [Google Scholar]
- 47.Wensel R, Jilek C, Dorr M, et al. Impaired cardiac autonomic control relates to disease severity in pulmonary hypertension. Eur Respir J 2009; 34:895–901. [DOI] [PubMed] [Google Scholar]
- 48.Lechat P, Packer M, Chalon S, et al. Clinical effects of beta-adrenergic blockade in chronic heart failure: a meta-analysis of double-blind, placebo-controlled, randomized trials. Circulation 1998; 98:1184–1191. [DOI] [PubMed] [Google Scholar]
- 49.de Man FS, Handoko ML, van Ballegoij JJ, et al. Bisoprolol delays progression towards right heart failure in experimental pulmonary hypertension. Circ Heart Fail 2012; 5:97–105. [DOI] [PubMed] [Google Scholar]
- 50.Perros F, Ranchoux B, Izikki M, et al. Nebivolol for improving endothelial dysfunction, pulmonary vascular remodeling, and right heart function in pulmonary hypertension. J Am Coll Cardiol 2015; 65:668–680. [DOI] [PubMed] [Google Scholar]
- 51.Farha S, Saygin D, Park MM, et al. Pulmonary arterial hypertension treatment with carvedilol for heart failure: a randomized controlled trial. JCI Insight 2017; 2:e95240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Campen JS, de Boer K, van de Veerdonk MC, et al. Bisoprolol in idiopathic pulmonary arterial hypertension: an explorative study. Eur Respir J 2016; 48:787–796. [DOI] [PubMed] [Google Scholar]
- 53.Osorio J, Russek M. Reflex changes on the pulmonary and systemic pressures elicited by stimulation of baroreceptors in the pulmonary artery. Circ Res 1962; 10:664–667. [DOI] [PubMed] [Google Scholar]
- 54.Juratsch CE, Jengo JA, Castagna J, Laks MM. Experimental pulmonary hypertension produced by surgical and chemical denervation of the pulmonary vasculature. Chest 1980; 77:525–530. [DOI] [PubMed] [Google Scholar]
- 55.Chen SL, Zhang YJ, Zhou L, et al. Percutaneous pulmonary artery denervation completely abolishes experimental pulmonary arterial hypertension in vivo. EuroIntervention 2013; 9:269–276. [DOI] [PubMed] [Google Scholar]
- 56.Chen SL, Zhang FF, Xu J, et al. Pulmonary artery denervation to treat pulmonary arterial hypertension: the single-center, prospective, first-in-man PADN-1 study (first-in-man pulmonary artery denervation for treatment of pulmonary artery hypertension). J Am Coll Cardiol 2013; 62:1092–1100. [DOI] [PubMed] [Google Scholar]
- 57.de Man FS, Tu L, Handoko ML, et al. Dysregulated renin–angiotensin–aldosterone system contributes to pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186:780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maron BA, Opotowsky AR, Landzberg MJ, et al. Plasma aldosterone levels are elevated in patients with pulmonary arterial hypertension in the absence of left ventricular heart failure: a pilot study. Eur J Heart Fail 2013; 15:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maron BA, Leopold JA. Emerging concepts in the molecular basis of pulmonary arterial hypertension: Part II: neurohormonal signaling contributes to the pulmonary vascular and right ventricular pathophenotype of pulmonary arterial hypertension. Circulation 2015; 131:2079–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Te Riet L, van Esch JH, Roks AJ, et al. Hypertension: renin–angiotensin–aldosterone system alterations. Circ Res 2015; 116:960–975. [DOI] [PubMed] [Google Scholar]
- 61.Ikram H, Maslowski AH, Nicholls MG, et al. Haemodynamic and hormonal effects of captopril in primary pulmonary hypertension. Br Heart J 1982; 48:541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leier CV, Bambach D, Nelson S, et al. Captopril in primary pulmonary hypertension. Circulation 1983; 67:155–161. [DOI] [PubMed] [Google Scholar]
- 63.Bertoli L, Fusco M, Lo Cicero S, et al. Influence of ACE inhibition on pulmonary haemodynamics and function in patients in whom beta-blockers are contraindicated. Postgrad Med J 1986; 62 Suppl 1:47–51. [PubMed] [Google Scholar]
- 64.Maron BA, Waxman AB, Opotowsky AR, et al. Effectiveness of spironolactone plus ambrisentan for treatment of pulmonary arterial hypertension (from the [ARIES] study 1 and 2 trials). Am J Cardiol 2013; 112:720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seva Pessoa B, van der Lubbe N, Verdonk K, et al. Key developments in renin-angiotensin-aldosterone system inhibition. Nat Rev Nephrol 2013; 9:26–36. [DOI] [PubMed] [Google Scholar]
- 66.Johnson JA, West J, Maynard KB, Hemnes AR. ACE2 improves right ventricular function in a pressure overload model. PLoS One 2011; 6:e20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hemnes AR, Rathinasabapathy A, Austin EA, et al. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur Respir J 2018; 51:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Begieneman MP, van de Goot FR, van der Bilt IA, et al. Pulmonary embolism causes endomyocarditis in the human heart. Heart 2008; 94:450–456. [DOI] [PubMed] [Google Scholar]
- 69.Frustaci A, Petrosillo N, Vizza D, et al. Myocardial and microvascular inflammation/infection in patients with HIV-associated pulmonary artery hypertension. AIDS 2014; 28:2541–2549. [DOI] [PubMed] [Google Scholar]
- 70.Overbeek MJ, Mouchaers KT, Niessen HM, et al. Characteristics of interstitial fibrosis and inflammatory cell infiltration in right ventricles of systemic sclerosis-associated pulmonary arterial hypertension. Int J Rheumatol 2010; 2010: Article ID 604615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis. Ann Rheum Dis 2007; 66:940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rondelet B, Dewachter C, Kerbaul F, et al. Prolonged overcirculation-induced pulmonary arterial hypertension as a cause of right ventricular failure. Eur Heart J 2012; 33:1017–1026. [DOI] [PubMed] [Google Scholar]
- 73.Waehre A, Vistnes M, Sjaastad I, et al. Chemokines regulate small leucine-rich proteoglycans in the extracellular matrix of the pressure-overloaded right ventricle. J Appl Physiol 2012; 112:1372–1382. [DOI] [PubMed] [Google Scholar]
- 74.Yoshida K, Abe K, Ishikawa M, et al. Inhibition of TLR9-NF-kappaB-mediated sterile inflammation improves pressure overload-induced right ventricular dysfunction in rats. Cardiovasc Res 2019; 115:658–668. [DOI] [PubMed] [Google Scholar]
- 75.Handoko ML, de Man FS, Happe CM, et al. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation 2009; 120:42–49. [DOI] [PubMed] [Google Scholar]
- 76.Soon E, Holmes AM, Treacy CM, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010; 122:920–927. [DOI] [PubMed] [Google Scholar]
- 77.Sun XQ, Abbate A, Bogaard HJ. Role of cardiac inflammation in right ventricular failure. Cardiovasc Res 2017; 113:1441–1452. [DOI] [PubMed] [Google Scholar]
- 78.Voelkel NF, Tuder RM, Bridges J, Arend WP. Interleukin-1 receptor antagonist treatment reduces pulmonary hypertension generated in rats by monocrotaline. Am J Respir Cell Mol Biol 1994; 11:664–675. [DOI] [PubMed] [Google Scholar]
- 79▪.Trankle CR, Canada JM, Kadariya D, et al. IL-1 blockade reduces inflammation in pulmonary arterial hypertension and right ventricular failure: a single-arm, open-label, phase IB/II pilot study. Am J Respir Crit Care Med 2019; 199:381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]; IL-1 inhibition by anakinra was safe in six idiopathic PAH patients and resulted in nonstatistically significant IL-6 reduction after 14 days of treatment. Treatment with the drug did not result in an improvement of right ventricular (RV) fractional area change, exercise capacity, Tricuspid annular plane systolic excursion or NT-proBNP levels. However, the low number of included patients and the short follow-up time could contribute to the absence of efficacy on these RV end points. As maladaptive inflammatory signaling affects both pulmonary arterial remodeling and RV failure, the potential of this drug should be studied in a larger, randomized trial.
- 80.Takahama H, Asanuma H, Sanada S, et al. A histamine H(2) receptor blocker ameliorates development of heart failure in dogs independently of beta-adrenergic receptor blockade. Basic Res Cardiol 2010; 105:787–794. [DOI] [PubMed] [Google Scholar]
- 81.Bristow MR, Ginsburg R, Harrison DC. Histamine and the human heart: the other receptor system. Am J Cardiol 1982; 49:249–251. [DOI] [PubMed] [Google Scholar]
- 82.Leary PJ, Barr RG, Bluemke DA, et al. H2 receptor antagonists and right ventricular morphology: the MESA right ventricle study. Ann Am Thorac Soc 2014; 11:1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83▪.Leary PJ, Hess E, Baron AE, et al. H2 receptor antagonist use and mortality in pulmonary hypertension: insight from the VA-CART program. Am J Respir Crit Care Med 2018; 197:1638–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]; Histamine H2 receptor antagonists are implied to slow down heart failure progression and might reduce cardiac volume and mass. This large retrospective cohort study demonstrates in that pulmonary hypertension patients on histamine H2 receptor antagonists had a 10% lower all-cause mortality risk. Whether this decreased mortality risk was a consequence of the cardioprotective effects of this drug was not established. As histamine H2 receptor antagonists are inexpensive, do not have many side-effects, and might directly target cardiac histamine release, a prospective trial studying the effects of histamine receptor blockade on RV function would be of interest.


