Abstract
BACKGROUND
Edematous fibrosclerotic panniculopathy (EFP; cellulite) is associated with thickening and contraction of collagen-rich subdermal septae. Collagenase clostridium histolyticum (CCH) may disrupt collagen-rich septae.
OBJECTIVE
To evaluate the safety and efficacy of CCH for treatment of EFP.
MATERIALS AND METHODS
In a randomized, double-blind study, women with moderate or severe EFP of the buttocks or posterolateral thighs (i.e., Clinician Reported Photonumeric Cellulite Severity Scale [CR-PCSS] and Patient Reported Photonumeric Cellulite Severity Scale [PR-PCSS] ratings of 3 to 4, and Hexsel Cellulite Severity Scale score ≤13) received up to 3 treatment sessions (Days 1, 22, and 43) of subcutaneous CCH 0.84 mg or placebo injections. End points included the percentage of 2-level and 1-level composite responders (i.e., had ≥2-level or ≥1-level improvement in CR-PCSS and PR-PCSS) at Day 71.
RESULTS
Three hundred seventy-five women (mean age, 46.5 years; 86.4% white) were randomly assigned to CCH (n = 189) or placebo (n = 186). At Day 71, the percentages of 2-level and 1-level composite responders were greater with CCH (10.6% and 44.6%, respectively) versus placebo (1.6% and 17.9%; p < .001 for both). The most common adverse events were injection-site related.
CONCLUSION
CCH significantly improved EFP appearance versus placebo; further evaluation of CCH for EFP (cellulite) is warranted.
Edematous fibrosclerotic panniculopathy (EFP), also known as cellulite, occurs in 80% to 98% of postpubertal women but in few men1–3; therefore, it may be considered a secondary sexual characteristic. Its preponderance in women is attributable to the effects of estrogen (which promotes fibroblast proliferation, collagen formation, lipogenesis, and adipocyte hypertrophy)4 and sex-specific differences in skin dermal and subcutaneous fat architecture.5 Cellulite is primarily located on the buttocks and outer thighs3,4; its underlying causes have not been fully elucidated, but fat accumulation, hormones (e.g., estrogen, estrogen/testosterone ratio), edema, and sex-dependent fibrogenesis are thought to contribute to and exaggerate cellulite formation.4 Estrogen, oxidative stress, and inflammation promote proliferation of fibroblasts and formation of collagen and influence fluid retention by altering local vasculature and lymphatic drainage.4 These, in turn, augment adipose layer thickness and edema, resulting in increased mechanotransduction load on subdermal fibroblasts/myofibroblasts, which may stimulate increased collagen deposition and promote thickening of subcutaneous collagen-rich septae that tether the dermis to the underlying fascia.4,6–9 In men, the septae are oriented obliquely to the skin surface,5 thereby preventing extrusion of the underlying tissue. In women, the septae are oriented perpendicular to the skin surface,5 allowing for displacement of subdermal adipose tissue. The combination of subcutaneous edema, subcutaneous adipose extrusion, and thickening and shortening of the perpendicular fibrous septae (which retracts the dermis) produces the dimpled appearance associated with cellulite.3,8–11 It is hypothesized that disruption of the collagen septae would correct the contour alteration of cellulite and disrupt the pathogenic positive feedback loop of mechanostimulus on the collagen-producing fibroblasts.7
Current cellulite treatments target dermal and/or subcutaneous features, but efficacy has not been evaluated in high-quality, large-scale, placebo-controlled trials.1 Surgical and laser procedures may remove or disrupt adipose tissue, thicken the dermal layer, sever fibrous tissue bands/septae, and/or smooth the dermal–hypodermis interface to reduce dimpling3,12,13; however, they may augment cellulite appearance because of increased local inflammation and activation of fibroblast remodeling. Powered subcision, when used to treat cellulite,1,14,15 requires surgical incisions that necessitate local anesthesia and may increase infection risk.12,14 Subcision has also been associated with substantial bruising and hemosiderin pigmentation, which may require several months to resolve.14 Noninvasive treatments (e.g., topical creams, massage, and shockwave therapy) may promote lymphatic drainage, providing temporary improvement,1,3,16–18 but they do not address other pathophysiologic mechanisms; deep massage and laser therapy have also been associated with increased risk of dermal and cutaneous damage (e.g., burns, increased skin laxity).3,16
Another concern with cellulite management is the lack of a universally accepted, standardized measure for assessing improvements in cellulite severity.1 Several cellulite severity rating scales have been developed, but each has limitations.1 The Hexsel Cellulite Severity Scale (CSS) was validated in women aged 18 to 45 years with Grade I to III cellulite and a mean body mass index of 25 but may be cumbersome because it incorporates multiple pathologic ratings and does not assess cellulite severity from the patient perspective.19 The modified Investigator Global Aesthetic Improvement Scale (I-GAIS) and Subject Global Aesthetic Improvement Scale (S-GAIS) ask clinicians and patients, respectively, to rate changes in cellulite appearance, based on before and after digital images, which are dynamic assessments by design. To overcome the limitations of these assessments, the buttock- and thigh-specific Clinician Reported Photonumeric Cellulite Severity Scale (CR-PCSS) and Patient Reported Photonumeric Cellulite Severity Scale (PR-PCSS) were developed to assess cellulite severity from both clinician and patient viewpoints using a photonumeric reference, cellulite severity labels (e.g., “mild” or “moderate”), and corresponding descriptors.20 Ratings of cellulite severity using the CR-PCSS and PR-PCSS correlate with traditional measures of cellulite severity (e.g., CR-PCSS with Hexsel CSS and PR-PCSS with S-GAIS).20
Collagenase clostridium histolyticum (CCH) is composed of 2 purified collagenases (AUX-I and AUX-II) that hydrolyze collagen under physiologic conditions, resulting in disruption of collagen structures (e.g., fibrous septae).21 CCH is approved by the US Food and Drug Administration for the treatment of collagen-associated disorders (e.g., Dupuytren contracture with palpable cord or Peyronie disease with palpable plaque and penile curvature deformity of ≥30° at initiation of therapy in adults). Regarding EFP, results from a Phase 1 study and a Phase 2 dose-ranging study showed improvement in cellulite appearance with CCH versus placebo.22,23 The objective of the current study was to assess the efficacy and safety of CCH for the treatment of cellulite.
Methods
Study Design
This Phase 2b, randomized, double-blind, placebo-controlled trial was conducted from February through September 2016 (ClinicalTrials.gov identifier: NCT02724644) at 16 US study centers. The study was conducted in accordance with International Conference on Harmonisation Guidelines for Good Clinical Practice and ethical principles of the Declaration of Helsinki. The study protocol was approved by a central institutional review board (Quorum, Seattle, WA), and all patients involved provided written informed consent.
Patient Population
Women aged 18 years and older were screened for moderate or severe cellulite on the buttocks, posterolateral thighs, or both at the initial study visit. To assess cellulite severity, digital images were taken of 4 cellulite quadrants (i.e., possible treatment areas), one each on the left and right buttocks and the left and right posterolateral thighs. Each patient viewed her digital images privately on a computer monitor and rated the severity of her cellulite in each image using the PR-PCSS (Figure 1A,B). Clinicians rated cellulite severity using the CR-PCSS and the Hexsel CSS; they evaluated cellulite appearance in the same 4 possible treatment areas in real time, while viewing each patient in a relaxed, standing position (Figure 1A,B).19 Patients who had ≥1 treatment area with PR-PCSS and CR-PCSS scores of 3 or 4 (i.e., moderate or severe cellulite) and a Hexsel CSS score of ≤13 were included. One buttock or thigh with moderate or severe cellulite per patient received treatment randomly assigned using an interactive web-response system. Patients who had received liposuction on the side of the body chosen for treatment and those who had mesotherapy, radiofrequency device treatments, laser treatment, or surgery (e.g., subcision) within the assigned treatment area within the previous year were excluded from further participation. Patients were not permitted to initiate intense sports, exercise activities, or weight-loss programs during the study.
Figure 1.
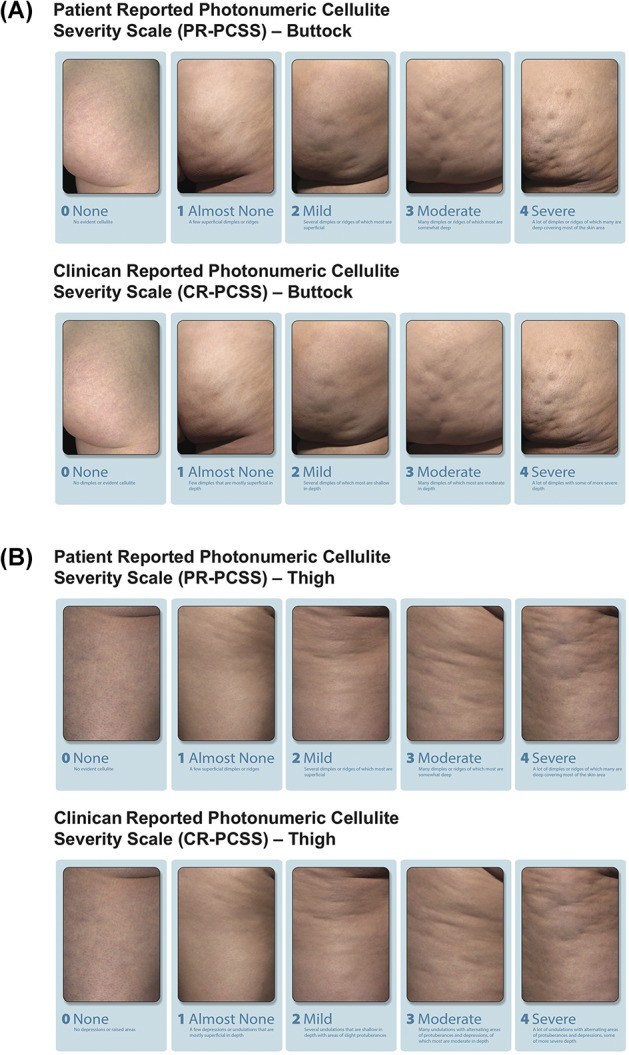
Assessment of cellulite severity using the PR-PCSS and CR-PCSS for the buttocks (A) and thighs (B). CR-PCSS, Clinician Reported Photonumeric Cellulite Severity Scale; PR-PCSS, Patient Reported Photonumeric Cellulite Severity Scale. © 2017 Auxilium Pharmaceuticals, LLC. All rights reserved.
Treatment
On Day 1, before treatment administration, eligibility of the assigned buttock or thigh was confirmed, and patients were randomly assigned (1:1 ratio within each site; assigned treatment using an interactive web-response system and a block size of 4) to receive either CCH (EN3835) 0.84 mg or placebo. Within the assigned area, clinicians identified dimples for treatment that were visible and well defined when patients were in a relaxed, standing position.
Each patient received 3 treatment sessions separated by approximately 21 days (i.e., Days 1, 22, and 43) unless no treatable dimples were apparent in the assigned treatment area; in that case, cellulite severity was rated as 0 (“none”) on the CR-PCSS. During each treatment session, patients received 12 subcutaneous injections of 0.3 mL CCH 0.84 mg or placebo within the assigned area. The dose of CCH was chosen based on Phase 2a data that demonstrated 0.84 mg CCH provided the greatest improvement in cellulite and had a safety profile comparable with lower CCH doses (0.06 and 0.48 mg).22
While patients were in a prone position, each injection was administered as three 0.1-mL aliquots. Injection sites within the dimple were targeted to be approximately 2 cm apart, and CCH was delivered to the area as one aliquot perpendicular to the skin and 2 aliquots at a 45° angle to the left or right of the perpendicular axis. After treatment, patients remained in a prone position (≥5 minutes) and were observed for 30 minutes. A sterile dressing was applied to the injection site if necessary. Patients were directed to remove the dressing that evening.
Assessments and Study End Points
Cellulite severity was rated using the CR-PCSS, PR-PCSS, and Hexsel CSS at screening, before treatment on Days 1, 22, and 43, and on Day 71 (28 days after treatment). The I-GAIS, S-GAIS, and patient satisfaction ratings were completed at Day 71. For patient satisfaction, patients rated overall satisfaction with treatment results on a 5-point scale from +2 (very satisfied) to −2 (very dissatisfied). The primary end point was percentage of composite 2-level responders (patients with ≥2-level improvement from baseline [Day 1] in CR-PCSS rating and PR-PCSS rating) at Day 71. Percentage of patients who achieved a composite ≥1-level improvement from baseline in CR-PCSS rating and PR-PCSS rating at Day 71 was a secondary end point. Percentage of patients who met the ≥2-level improvement from baseline in CR-PCSS or PR-PCSS ratings (primary composite end point) and ≥1-level improvement from baseline in CR-PCSS or PR-PCSS ratings (secondary composite end point) was also evaluated. Additional secondary end points included change from baseline to Day 71 in Hexsel CSS total score, percentage of investigators who rated patients' cellulite as at least “improved” on the I-GAIS (I-GAIS responders), or patients giving a similar rating on the S-GAIS (S-GAIS responders) at Day 71, and ratings of patient satisfaction with treatment. Adverse events (AEs) and anti-AUX antibodies were monitored throughout the study, starting on Day 1.
Statistics
Sample size calculations used the expected percentages of composite response at Day 71 based on Phase 2a study data.22 The primary efficacy analysis was evaluated in the intent-to-treat (ITT) population (all patients randomly assigned to treatment who received ≥1 injection of study medication). Secondary efficacy analyses were performed in the modified ITT (mITT) population (i.e., patients in the ITT population who completed ≥1 post-treatment CR-PCSS and PR-PCSS assessment). Safety parameters were evaluated in all patients who received ≥1 injection of study medication (safety population). Percentage of composite responders (primary efficacy end point) and responders for secondary analyses (i.e., ≥1-level and ≥2-level improvements from baseline in CR-PCSS and PR-PCSS ratings, and I-GAIS and S-GAIS scores of at least 1 [“improved”]) were compared using the Cochran–Mantel–Haenszel test with adjustment for study center. Change from baseline in Hexsel CSS score was analyzed using analysis of variance, and patient satisfaction was analyzed using the Mann–Whitney test.
Results
Of 489 patients screened, 375 patients (CCH 0.84 mg, n = 189; placebo, n = 186) were randomly assigned to treatment and included in the ITT and safety populations (Figure 2), and 361 patients (CCH 0.84 mg, n = 177; placebo, n = 184) were included in the mITT population. Demographics and baseline characteristics were generally similar between groups (Table 1).
Figure 2.
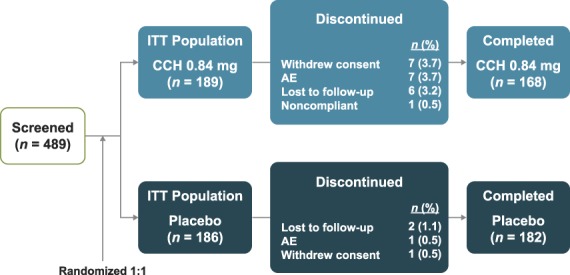
Patient disposition. AE, adverse event; CCH, collagenase clostridium histolyticum; ITT, intent-to-treat.
TABLE 1.
Demographic and Baseline Characteristics (ITT Population)
A significantly greater percentage of patients treated with CCH (10.6%) had a ≥2-level improvement in both CR-PCSS and PR-PCSS ratings at Day 71 (composite response) versus those receiving placebo (1.6%; p < .001; ITT population; Figure 3). Also, at Day 71, 44.6% of patients in the CCH group had a ≥1-level improvement in both CR-PCSS and PR-PCSS ratings, versus only 17.9% of patients in the placebo group (p < .001; mITT population; Figure 3). Photographic examples of composite response show topologic differences after treatment with CCH (e.g., shallower dimples) versus no improvement (placebo; Figure 4A–C). In addition, a significantly greater percentage of patients treated with CCH 0.84 mg had improvement of ≥2 levels in CR-PCSS or PR-PCSS ratings versus placebo at Day 43 (p ≤ .05) and at Day 71 (p < .001; Table 2). Significant between-group differences were also observed in percentage of patients who had ≥1-level improvement from baseline (p < .001). These significant differences were noted at Day 22, Day 43, and Day 71 for CR-PCSS ratings (p < .001 for all) and Day 43 and Day 71 for PR-PCSS ratings (p < .001 for both timepoints). Hexsel CSS scores steadily improved from baseline to Day 71 with CCH, with significant differences beginning at Day 22 (Table 2). Consistent with results for the CR-PCSS and PR-PCSS ratings, a significantly greater percentage of patients in the CCH group had I-GAIS and S-GAIS scores of ≥1 at Day 71, indicating cellulite severity was “improved,” “much improved,” or “very much improved” from baseline (p < .001 vs placebo; Table 2). The majority of patients (62.9%) in the CCH treatment group were “satisfied” or “very satisfied” with treatment, versus only 35.9% in the placebo group (Figure 5).
Figure 3.
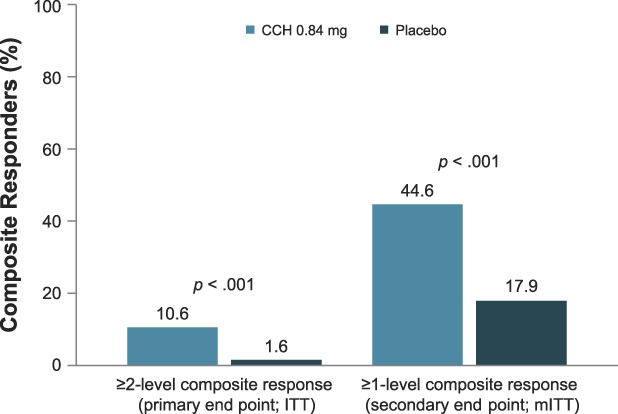
Composite responders. Composite response was defined as a ≥2-level improvement or a ≥1-level improvement from baseline (Day 1) in both the CR-PCSS and PR-PCSS ratings at Day 71. ITT population: CCH, n = 189; placebo, n = 186. mITT population: CCH, n = 177; placebo, n = 184. CCH, collagenase clostridium histolyticum; CR-PCSS, Clinician Reported Photonumeric Cellulite Severity Scale; ITT, intent-to-treat; mITT, modified intent-to-treat; PR-PCSS, Patient Reported Photonumeric Cellulite Severity Scale.
Figure 4.
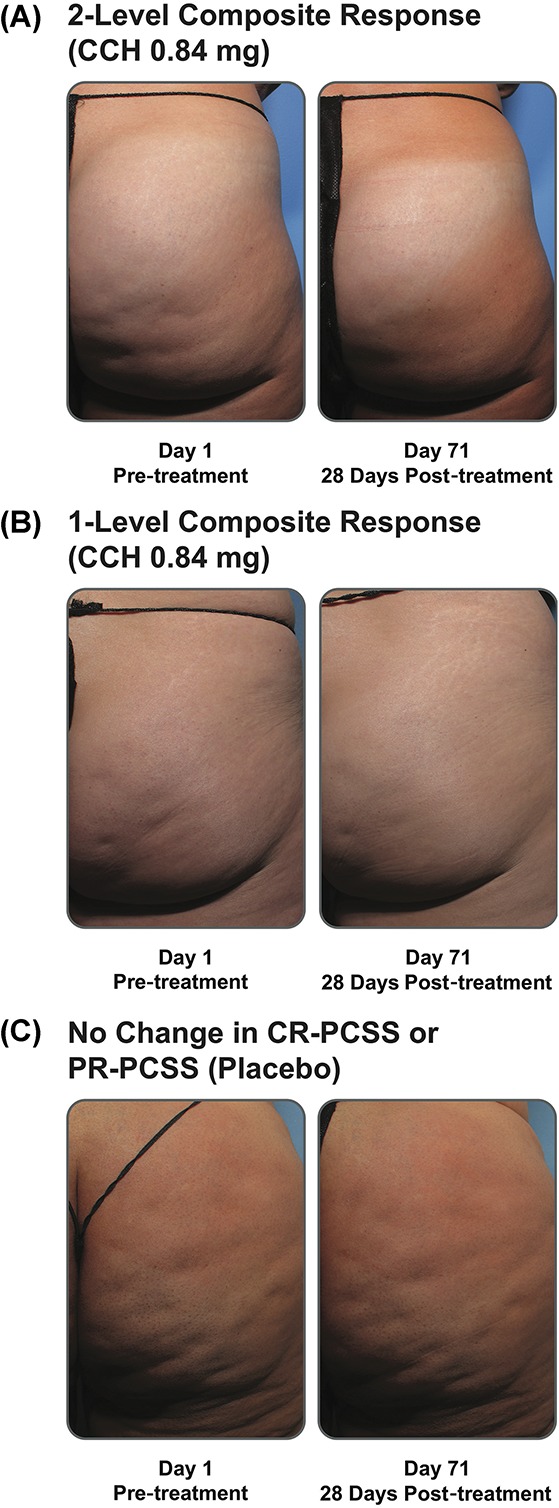
Photographs of composite response with CCH 0.84 mg versus no improvement (i.e., response with placebo). Baseline and Day 71 photographs show a 2-level composite response (A), 1-level composite response (B), and no improvement in the CR-PCSS or PR-PCSS (C). Note the visual improvement of dimpling after CCH treatment. CCH, collagenase clostridium histolyticum; CR-PCSS, Clinician Reported Photonumeric Cellulite Severity Scale; PR-PCSS, Patient Reported Photonumeric Cellulite Severity Scale.
TABLE 2.
Separate Improvement in Additional Measures of Cellulite Severity (mITT Population*)
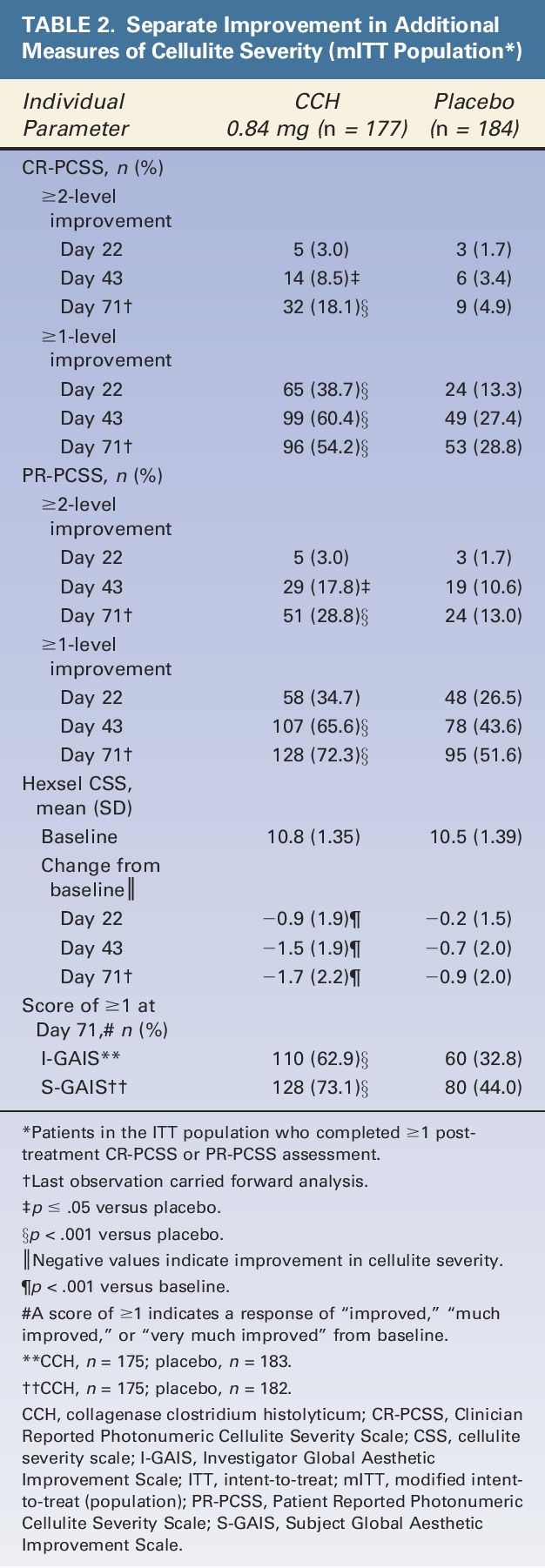
Figure 5.
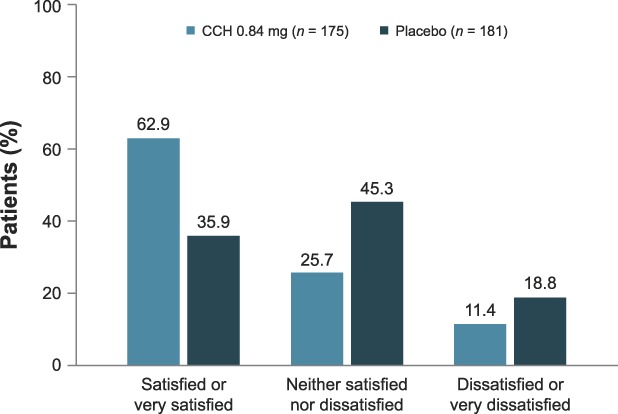
Patient satisfaction with treatment (mITT population). Two patients in the CCH group and 3 patients in the placebo group did not complete the satisfaction survey. CCH, collagenase clostridium histolyticum; mITT, modified intent-to-treat.
CCH was generally well tolerated. Few patients (3.7%) in the CCH group discontinued because of AEs (Table 3). No treatment-related serious AEs were reported in either treatment group. One serious AE (spontaneous abortion) occurred on Day 72 in a patient who received CCH but was not considered by the investigator to be treatment related. Most AEs were mild or moderate in intensity (92.3% and 96.2% of AEs in CCH and placebo groups, respectively). The most common treatment-emergent AEs in both groups were injection-site bruising and injection-site pain (Table 3). At Day 71, all patients who received CCH and underwent immunogenicity testing (n = 165 for AUX-I and n = 164 for AUX-II) had antibodies against AUX-I and AUX-II; however, the percentage of patients who were positive for neutralizing antibodies was 22.5% for neutralizing anti-AUX-I (9 of 40 patients tested) and 7.5% for neutralizing anti-AUX-II (3 of 40 patients tested). No hypersensitivity reactions were observed in the CCH treatment group.
TABLE 3.
Adverse Events (Safety Population)
Discussion
Injections of CCH 0.84 mg administered every 3 weeks over a 43-day period significantly improved moderate to severe cellulite appearance versus placebo. This improvement was reported by both clinicians (CR-PCSS) and patients (PR-PCSS). Although the CR-PCSS and PR-PCSS were developed recently to assess cellulite severity, improvements revealed through use of these scales mirrored results seen with established severity measures (i.e., clinician-reported Hexsel CSS and I-GAIS, and patient-reported S-GAIS).
Most of the patients who received CCH were very satisfied/satisfied with their results. CCH treatment did not require post-treatment alterations in physical activity or use of compressive garments necessary with other cellulite therapies that target fibrous septae (i.e., powered subcision).24 CCH was generally well tolerated, with most AEs localized to the injection site. Adverse events did not cause discontinuation from the study for >95% of patients receiving CCH (n = 7 patients discontinued; 3.7%). Although all patients in the CCH group who were tested developed AUX antibodies, no hypersensitivity reactions were observed in the CCH treatment group. In addition, no pain management or compression garments were required per protocol to mitigate swelling.
There is currently no standardized measure of cellulite severity that has been universally accepted, but several severity rating scales (e.g., modified Hexsel CSS, I-GAIS, and S-GAIS) have been used in clinical trials. The CR-PCSS and PR-PCSS were developed in accordance with US Food and Drug Administration guidance on patient-reported outcome measures and address the limitations of the aforementioned severity scales by providing means for both clinician and patient assessment using a photonumeric scale. In a separate correlation analysis combining data from the CCH and placebo groups (N = 1,500 ratings) of the current trial, ratings of cellulite severity using the CR-PCSS and PR-PCSS were compared with those from established measures of cellulite severity (i.e., Hexsel CSS, I-GAIS, and S-GAIS).20 Statistically significant correlations between CR-PCSS and Hexsel CSS ratings (p < .001) and between PR-PCSS ratings and those of aesthetic changes on the S-GAIS (p < .001) at Day 71 were observed,20 suggesting that the ability of the CR-PCSS and PR-PCSS to measure cellulite severity is similar to that of currently used scales. In addition, ratings obtained using the CR-PCSS and PR-PCSS were correlated with each other for assessment of improvement in both the buttock and thigh regions (p < .001),20 further supporting the validity of these 2 measures. The statistically significant improvements in cellulite severity observed with CCH treatment for all cellulite severity scales examined (CR-PCSS, PR-PCSS, Hexsel CSS, I-GAIS, and S-GAIS) reinforce the fact that the CR-PCSS and PR-PCSS adequately evaluate cellulite severity.
Study results collectively suggest that a change of ≥1 level in both the CR-PCSS and PR-PCSS (composite end point) is clinically meaningful. The percentage of patients who achieved this composite end point, as well as each individual component, was significantly greater with CCH versus placebo (p < .001). In addition, significant between-group differences in the percentage of patients who achieved the individual end points (i.e., ≥1-level improvement in CR-PCSS or PR-PCSS) mirrored those observed using other clinician (i.e., Hexsel CSS, and I-GAIS) and patient (S-GAIS) ratings at the end of the study (p < .001 vs baseline [Hexsel CSS] or placebo [I-GAIS and S-GAIS]). Although additional research is needed to determine a minimal clinically important difference for cellulite, data support ≥1-level improvement in both the CR-PCSS and PR-PCSS as potentially clinically meaningful.
The strengths of this study include its randomized, placebo-controlled, double-blind design, the inclusion of a large number of patients (n = 375), and the assessment of cellulite severity improvement using multiple validated patient- and clinician-reported scales. However, the study is limited by its homogeneous patient population (e.g., race), evaluation of only patients with moderate to severe cellulite, and lack of nonsubjective end points. Furthermore, some aspects of the study (e.g., dimple marking, injection technique, post-treatment care) have not been thoroughly optimized. In future studies, additional assessments such as the use of biophysical measurements to quantitatively assess changes in skin quality (e.g., cutometer measurements) or topography analyses would be informative, particularly given that ratings of cellulite severity by patients and clinicians may have been influenced by subjective assessment of skin irregularities not attributable to cellulite (e.g., folds or wrinkles secondary to skin laxity). In conclusion, this Phase 2 study demonstrated significant improvement in clinician and patient ratings of EFP (cellulite) appearance with CCH treatment versus placebo. Overall, CCH treatment was well tolerated, with a low rate of patient discontinuation due to AEs. Additional clinical evaluation of CCH for the treatment of cellulite is warranted.
Acknowledgments
Mary Beth Moncrief, PhD, and Jillian Gee, PhD (Synchrony Medical Communications, LLC, West Chester, PA) were compensated by Endo to work collaboratively with the authors in developing this article. The final content of the publication and interpretation of data was determined solely by the authors.
Footnotes
Supported by Endo Pharmaceuticals Inc., Malvern, PA. N.S. Sadick has received research grants from Endo Pharmaceuticals Inc. and has served as a consultant for Endo Pharmaceuticals Inc. M.P. Goldman has received research grants from Endo Pharmaceuticals Inc. G. Liu is an employee of Endo Pharmaceuticals Inc., Malvern, PA. N.H. Shusterman is a former employee of Endo Pharmaceuticals Inc., Malvern, PA. M.P. McLane and D. Hurley are employees of Endo Pharmaceuticals Inc., Malvern, PA. V.L. Young reports serving as an investigator for Endo Pharmaceuticals Inc. and receiving registration and travel expenses from Endo Pharmaceuticals Inc. Technical editorial and medical writing assistance was provided by Mary Beth Moncrief, PhD, and Jillian Gee, PhD, for Synchrony Medical Communications, LLC, West Chester, PA, under the direction of the authors.
References
- 1.Luebberding S, Krueger N, Sadick NS. Cellulite: an evidence-based review. Am J Clin Dermatol 2015;16:243–56. [DOI] [PubMed] [Google Scholar]
- 2.Khan MH, Victor F, Rao B, Sadick NS. Treatment of cellulite: Part I. Pathophysiology. J Am Acad Dermatol 2010;62:361–70; quiz 71–2. [DOI] [PubMed] [Google Scholar]
- 3.Hexsel D, Mazzuco R. Cellulite. In: Tosti A, Hexsel D, editors. Update in Cosmetic Dermatology. Berlin, Germany: Springer-Verlag; 2013; pp. 21–32. [Google Scholar]
- 4.Rossi AB, Vergnanini AL. Cellulite: a review. J Eur Acad Dermatol Venereol 2000;14:251–62. [DOI] [PubMed] [Google Scholar]
- 5.Nürnberger F, Muller G. So-called cellulite: an invented disease. J Dermatol Surg Oncol 1978;4:221–9. [DOI] [PubMed] [Google Scholar]
- 6.Huang C, Ogawa R. Fibroproliferative disorders and their mechanobiology. Connect Tissue Res 2012;53:187–96. [DOI] [PubMed] [Google Scholar]
- 7.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 2014;15:802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenbaum M, Prieto V, Hellmer J, Boschmann M, et al. An exploratory investigation of the morphology and biochemistry of cellulite. Plast Reconstr Surg 1998;101:1934–9. [DOI] [PubMed] [Google Scholar]
- 9.Piérard GE, Nizet JL, Piérard-Franchimont C. Cellulite: from standing fat herniation to hypodermal stretch marks. Am J Dermatopathol 2000;22:34–7. [DOI] [PubMed] [Google Scholar]
- 10.Hexsel DM, Abreu M, Rodrigues TC, Soirefmann M, et al. Side-by-side comparison of areas with and without cellulite depressions using magnetic resonance imaging. Dermatol Surg 2009;35:1471–7. [DOI] [PubMed] [Google Scholar]
- 11.Querleux B, Cornillon C, Jolivet O, Bittoun J. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol 2002;8:118–24. [DOI] [PubMed] [Google Scholar]
- 12.Goldman MP. Cellulite: Pathophysiology and Treatment (2nd ed) Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- 13.Sasaki GH. Single treatment of grades II and III cellulite using a minimally invasive 1,440-nm pulsed Nd:YAG laser and side-firing fiber: an institutional review board-approved study with a 24-month follow-up period. Aesthet Plast Surg 2013;37:1073–89. [DOI] [PubMed] [Google Scholar]
- 14.Hexsel DM, Mazzuco R. Subcision: a treatment for cellulite. Int J Dermatol 2000;39:539–44. [DOI] [PubMed] [Google Scholar]
- 15.Kaminer MS, Coleman WP, III, Weiss RA, Robinson DM, et al. A multicenter pivotal study to evaluate tissue stabilized-guided subcision using the Cellfina device for the treatment of cellulite with 3-year follow-up. Dermatol Surg 2017;43:1240–8. [DOI] [PubMed] [Google Scholar]
- 16.McBean JC, Katz BE. Laser lipolysis: an update. J Clin Aesthet Dermatol 2011;4:25–34. [PMC free article] [PubMed] [Google Scholar]
- 17.Collis N, Elliot LA, Sharpe C, Sharpe DT. Cellulite treatment: a myth or reality: a prospective randomized, controlled trial of two therapies, endermologie and aminophylline cream. Plast Reconstr Surg 1999;104:1110–4; discussion 15–17. [PubMed] [Google Scholar]
- 18.Russe-Wilflingseder K, Russe E, Vester JC, Haller G, et al. Placebo controlled, prospectively randomized, double-blinded study for the investigation of the effectiveness and safety of the acoustic wave therapy (AWT®) for cellulite treatment. J Cosmet Laser Ther 2013;15:155–62. [DOI] [PubMed] [Google Scholar]
- 19.Hexsel DM, Dal'Forno T, Hexsel CL. A validated photonumeric cellulite severity scale. J Eur Acad Dermatol Venereol 2009;23:523–8. [DOI] [PubMed] [Google Scholar]
- 20.Young VL, Sadick N, Liu G, Shusterman N, et al. Comparisons of clinician-reported and patient-reported cellulite severity scales with existing scales for measurement of cellulite severity. Presented at: Northeastern Society of Plastic Surgeons 34th Annual Meeting; September 8–10, 2017; Newport, RI.
- 21.French MF, Mookhtiar KA, Van Wart HE. Limited proteolysis of type I collagen at hyperreactive sites by class I and II Clostridium histolyticum collagenases: complementary digestion patterns. Biochemistry 1987;26:681–7. [DOI] [PubMed] [Google Scholar]
- 22.Goldman M, Sadick N, Young L, Kaufman GJ, et al. Phase 2a, randomized, double-blind,placebo-controlled dose-ranging study of repeat doses of collagenase clostridium histolyticum for the treatment of edematous fibrosclerotic panniculopathy (cellulite). J Am Acad Dermatol 2015;72:AB19. [Google Scholar]
- 23.Dagum B, Badalamente MA. Collagenase injection in the treatment of cellulite. Presented at: Plastic Surgery; October 6–11, 2016; San Francisco, CA.
- 24.Friedmann DP, Vick GL, Mishra V. Cellulite: a review with a focus on subcision. Clin Cosmet Investig Dermatol 2017;10:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]