Abstract
Objectives:
This study aims to evaluate whether re-excision or adjuvant radiation for stage I vulvar squamous cell carcinoma (SCC) with either a close or positive surgical margin improves recurrence-free survival.
Methods:
Patients with pathologically confirmed FIGO stage I vulvar SCC who underwent primary surgical management between January 1, 1995 and September 30, 2017 and had positive or close (<8mm) surgical margins were included. Kaplan-Meier curves were generated and compared using the log-rank test.
Results:
Of 150 patients with stage I vulvar SCC, 47 (31.3%) had positive or close margins. Median follow-up time was 25 months (IQR 13-59 months). Twenty-one (44.6%) patients received additional treatment with re-excision (n=17) or vulvar radiation (n=4); 26 (55.3%) patients received no additional therapy. Patients with positive margins were more likely to receive additional therapy compared to patients with close margins (80% vs 35.1%, p=0.03). The 2-year recurrence rates were similar between the no further therapy and the re-excision/vulvar radiation groups (11.5% vs 4.8%, p=0.62). Local recurrence-free survival (RFS) and overall survival (OS) were similar between patients who received re-excision/vulvar radiation and patients who received no further therapy (p=0.10 and p=0.16, respectively). Subgroup analysis of the 37 patients with close margins demonstrated no difference in RFS or OS when patients received re-excision or adjuvant vulvar radiation compared to no additional therapy (p=0.74 and p=0.82, respectively).
Conclusions:
In our study, any additional treatment following primary surgical resection did not improve RFS or OS in stage IA and IB vulvar SCC. Larger studies are warranted in order to definitively determine the role of re-excision and adjuvant radiation in early stage disease.
Keywords: vulva, squamous cell carcinoma, early stage, close margins, adjuvant radiation, re-excision
Background
Vulvar cancers represent 0.4% of all new cancer cases in the US, and the incidence and mortality continue to rise [1]. Stage I vulvar cancer has a 5-year survival rate of 86% [1]. Factors that affect survival include patient age, lesion size, depth of stromal invasion, lymph node involvement, lymphovascular space invasion (LVSI), and surgical margin status [2-6].
Surgery is the mainstay of both the staging and treatment of vulvar cancer due to the prognostic significance and therapeutic implications of lymph node status. Positive lymph nodes reduce the 5-year survival rates from 86% to 57% [1,4, 7, 8]. For locally advanced disease following primary resection, it is widely accepted that adjuvant pelvic and inguinal radiation without vulvar radiation improves outcomes for those with greater than or equal to two positive groin lymph nodes [9, 10]. For unresectable locally advanced disease, a high pathologic complete response rate can be achieved with primary cisplatin chemotherapy and vulvar, pelvic and inguinal radiation therapy [11]. For early stage disease, however, the benefit of adjuvant vulvar radiation is less clear.
Local vulvar recurrence rates are significantly higher in patients with positive margins [6, 12]. However, the risk of local recurrence with close margins is less clear [5, 6, 12-15]. Variability in the definition of a close margin, as well as inclusion of all stages of disease in many studies, contributes to a paucity of recurrence data for stage I vulvar cancer. There are multiple older retrospective studies that show a 3-3.35 relative risk of recurrence for close margins [5, 6, 12]. However, more recent retrospective studies demonstrated no difference in outcomes based on the historical definition of close margins (< 1cm for fresh tissue and <8mm for formalin-fixed) [13-15]. Although one of these studies found significantly higher local recurrence in those with ≤2mm margins compared to those with margins 2-8mm or ≥8mm margins, in the subgroup analysis of patients with stage I-II disease the margin range 2-8mm was no longer a significant predictor of local recurrence when compared to those with margins ≤2mm (HR 0.4, 95% CI 0.1-1.1) [14].
Unlike distant metastases, local recurrences are frequently salvaged with repeat surgery or radiation therapy [4, 16]. In one trial examining the role of modified radical hemivulvectomy with ipsilateral superficial inguinal lymph node dissection for stage I disease, local recurrence rates were 8%, but 80% of these local recurrences were effectively salvaged by re-excision [17].
The National Comprehensive Cancer Network (NCCN) Guidelines recommend that when feasible, re-excision of close (<8mm) or positive margins is indicated [7]. When re-excision is not feasible, or if margins remain positive after re-excision, current guidelines recommend adjuvant radiation to the vulva with or without inguinal radiation using a 3D conformal approach or intensity-modulated radiation therapy (IMRT) [5, 7]. However, these recommendations are based on older studies with small patient numbers, and since then, multiple studies have called into question the outcome data for patients with close margins [13-15]. Notably, vulvar radiation is associated with significant morbidity that can negatively affect quality of life. Side effects include skin desquamation, pain, necrosis, and urinary, bowel and sexual dysfunction [18].
The primary objective of this study was to evaluate whether further therapy, either re-excision or adjuvant vulvar radiation, for stage I squamous cell carcinoma of the vulva with close or positive surgical margins improves local recurrence-free survival when compared to no further therapy. The secondary outcome was to assess overall survival of patients treated with re-excision or adjuvant radiation compared to those that received no further treatment.
Methods
IRB approval was obtained for this retrospective cohort study. All patients with a diagnosis of vulvar carcinoma at the University of Minnesota between January 1, 1995 and September 30, 2017 were identified by billing data codes, ICD-10 and/or ICD-9 where applicable. All pathologically confirmed cases of squamous cell carcinoma (SCC) of the vulva were reviewed for possible inclusion. Patients with FIGO stage IA or IB SCC of the vulva and positive or close surgical margins were included in the final cohort. FIGO staging was based on 2009 criteria at the time of retrospective chart review. Close margins were defined as the presence of invasive carcinoma less than 8mm from any surgical edge. The eight millimeter cut-off was chosen based on NCCN Guidelines Version 1.2018 [7].
Continuous variables were presented as median (IQR) and compared with Mann-Whitney U test. Categorical variables were compared with Fisher’s exact test. Kaplan Meier curves were generated to assess survival and the re-excision/vulvar radiation group was compared to those that received no further therapy using the log-rank test. Multivariable Cox proportional hazards models were used to compare recurrence-free survival (RFS) and overall survival (OS) while accounting for the possible confounders of age and margin status. A sub-group analysis was conducted for those patients with close but not positive margins. All analyses were performed using R version 3.5.1. P-values less than 0.05 were considered statistically significant.
Results
A total of 150 patients had pathology-confirmed FIGO stage IA and IB squamous cell carcinoma of the vulva and underwent primary surgical resection. Of these, 47 patients (31.3%) had positive (n=10, 8.3%) or close surgical margins (n=37, 24.7%) and were included in the final analysis. Of those with positive margins, 3/10 (30%) were stage IA and 7/10 (70%) were stage IB. Of those with close margins, 9/37 (24%) were stage IA and 28/37 (76%) were stage IB. More specifically, close margins were: ≤2mm in 12 patients, >2mm but <5mm in 13 patients, and ≥5mm but <8mm in 12 patients. Of patients with stage IB disease, 20/35 (57%) underwent lymph node evaluation at the time of primary resection, 6/35 (17%) had delayed evaluation, and 9/35 (26%) did not undergo lymph node evaluation due to patient preference (n=4), medical comorbidities (n=3), or unknown reasons (n=2). Of those that had evaluation of lymph nodes, 14/26 (54%) had bilateral inguinofemoral lymph node dissection, 7/26 (27%) had unilateral inguinofemoral lymph node dissection, 4/26 (15%) had bilateral sentinel lymph node biopsies, and 1/26 (4%) had a unilateral sentinel lymph node biopsy.
Twenty-one patients with close or positive margins (44.6%) received additional treatment. Seventeen patients had re-excision and four patients received adjuvant vulvar radiation. Adjuvant radiation consisted of 59-60.4Gy via external beam radiation therapy (EBRT) to the vulva. The remaining 26 patients (55.3%) with close or positive surgical margins received no additional treatment following their primary procedure. The median follow-up time for all patients was 25 months (IQR 13-59 months).
Patient demographics are reported in Table 1. Race, BMI, stage, and smoking status were similar between treatment groups. The patients that did not receive further therapy were older (median age in years, 77 vs 60, p=0.03). Patients who had positive margins were more likely to receive additional therapy, either re-excision or vulvar radiation, compared to patients with close margins (80% vs 35.1%, p=0.03; Table 1). Two of four (50%) patients that received adjuvant radiation and six of 17 (35.3%) patients that underwent re-excision had positive margins.
TABLE 1.
Demographics by Treatment Group
| No therapy | Re-excision
or vulvar radiation |
p-value | |
|---|---|---|---|
| TOTAL | n=26 | n=21 | |
| Median age (IQR) | 77 (56, 84) | 60 (52, 64) | 0.03 |
| Median BMI (IQR) | 28 (22, 34) | 27 (24, 34) | 0.42 |
| Race | 0.28 | ||
| White | 22 (84.6%) | 20 (95.2%) | |
| Native American | 0 | 1 (4.8%) | |
| Hispanic/Latino | 2 (7.7%) | 0 | |
| Unspecified | 2 (7.7%) | 0 | |
| Smoking status | 0.60 | ||
| Ever | 13 (50%) | 12 (57.1%) | |
| Never | 13 (50%) | 8 (38.1%) | |
| Unspecified | 0 (0%) | 1 (4.8%) | |
| Stage | 0.10 | ||
| 1A | 4 (15.4%) | 8 (38.1%) | |
| 1B | 22 (84.6%) | 13 (61.9%) | |
| Margin status | 0.03 | ||
| Positive | 2 (7.7%) | 8 (38.1%) | |
| Close | 24 (92.3%) | 13 (61.9%) | |
| Lichen sclerosis | 4 (15.4%) | 4 (19.0%) | 1.0 |
Statistically significant results are in italics. n = number of patients. IQR = interquartile range.
There were no distant recurrences in either cohort. None of the 4 patients treated with adjuvant radiation therapy recurred or died during the follow-up time period (median follow up 48 months for those that received vulvar radiation). The 2-year recurrence rates were similar between the re-excision/vulvar radiation group (1/21, 4.8%) and the no further therapy group (3/26, 11.5%; p=0.62). The total number of recurrences in the re-excision/radiation group was 2/21 (9.52%), and for the no further treatment group was 5/26 (19.2%).
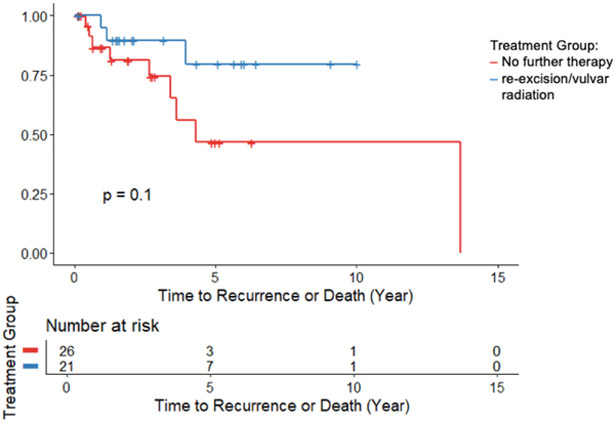
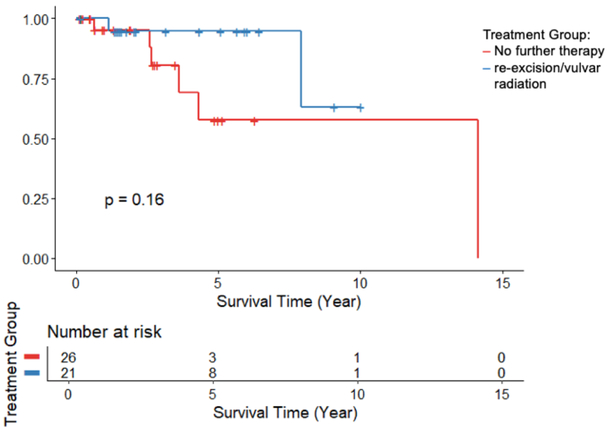
Recurrence free survival and overall survival was similar between groups (Figure 1,2). In the Cox regression model, which adjusted for age and margin status, there was no significant difference in risk of recurrence or death between the re-excision/vulvar radiation group and the no additional treatment group (HR 0.29, 95% CI: 0.04-1.9, p=0.20). There was no significant difference in risk of death between the two groups (HR 0.65, 95% CI: 0.03-12.6, p=0.78).
FIGURE 1:
Recurrence-Free Survival comparing no further therapy to re-excision or vulvar radiation, p=0.10.
FIGURE 2:
Overall Survival comparing no further therapy to re-excision or vulvar radiation, p=0.16.
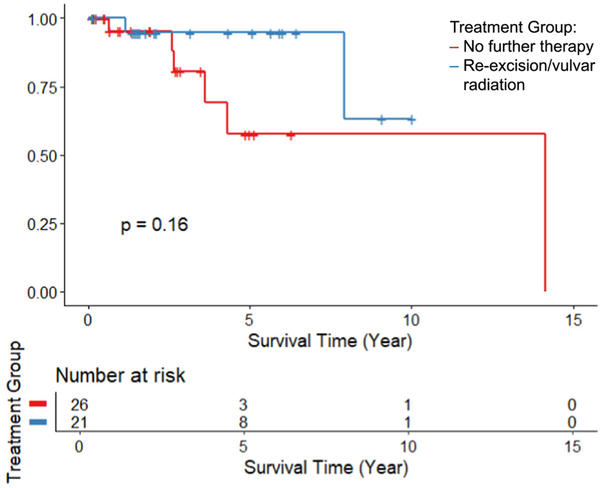
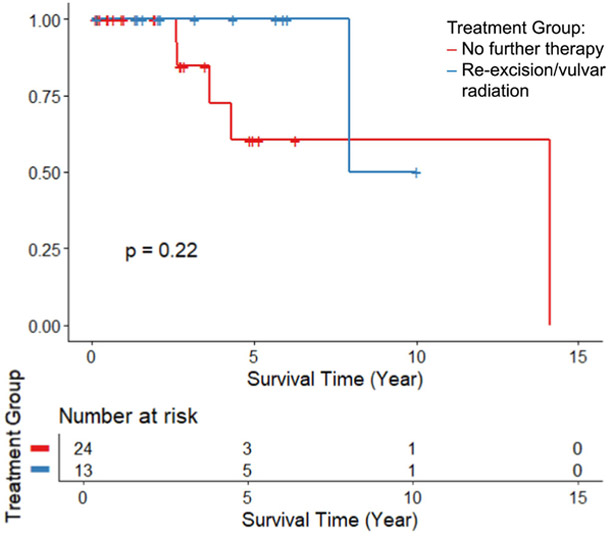
Analysis of the 37 patients with close but not positive margins demonstrated no significant difference in recurrence-free (HR 1.73, 95% CI: 0.08-37.4, p=0.74) or overall (HR 1.27, 95% CI: 0.18-9.2, p=0.82) survival rates between those that received re-excision/vulvar radiation and those that received no further therapy when adjusted for age. The Kaplan Meier curves are shown in Figure 3 & 4.
FIGURE 3:
Recurrence-Free Survival comparing no further therapy to re-excision or vulvar radiation in the subgroup of those with close margins only, p=0.10.
FIGURE 4:
Overall Survival comparing no further therapy to re-excision or vulvar radiation in the subgroup of those with close margins only, p=0.22.
All recurrences in this cohort were local vulvar recurrences. Of the seven patients that recurred, six (85.7%) received salvage therapy and one was lost to follow up. Three patients received re-excision, one received chemotherapy, one received radiation, and one received re-excision followed by radiation. Two of the four (50%) patients that had re-excision had a second recurrence, one of which was salvaged with re-excision, the other received no further therapy.
Conclusions
We found that re-excision or radiation treatment following primary surgical resection for stage I vulvar cancer with close or positive margins did not significantly improve local recurrence-free survival or overall survival. However, there was a trend towards improvement in local recurrence-free survival for those that received re-excision or vulvar radiation. As there were no distant recurrences we cannot comment on distant recurrence-free survival.
The majority of published literature recommend adjuvant treatment for margin status based on results for all stages of vulvar cancer. Faul et al. reported a retrospective cohort of 62 patients who had undergone surgical resection for stage I-IV vulvar SCC. In this study, the authors observed that adjuvant radiation (6MV photon fields to the vulva, bilateral groin and low pelvis) decreased local recurrence rates for patients with positive or close (≤8mm) margins, but only improved survival in patients with positive margins [19]. In a subsequent retrospective analysis of 205 patients with stage I-IVA vulvar cancer, Viswanathan et al. demonstrated again that adjuvant radiation (6-15MV photon fields to the vulva +/− inguinal nodes) improved local recurrence rates for positive margins but not for close margins (<10mm) [12]. The authors found no difference in overall survival based on surgical margin status [12]. Importantly, neither of these studies report subgroup analysis by stage or nodal status, both of which are correlated with prognosis. Ignatov et al. showed a survival benefit with adjuvant radiation for close or positive surgical margins in patients with stage I-IV vulvar cancer, but this benefit lost significance when subgroup analysis was performed for patients with stage I disease [20]. Our study similarly showed no benefit for adjuvant therapy in patients with stage I vulvar SCC and either close or positive margins.
Historically, close margins have been defined as <8mm. However, recent data indicate that those with margins <8mm actually have similar outcomes to those with negative margins, and Arvas et al. suggest that 2mm may be a more appropriate cut-off to define those at increased risk [13-15]. Since completion of data collection in this study, the NCCN Guidelines Version 2.2019 now leave the definition of close margins purposefully vague, with no definitive cut-off quoted [21]. Our findings indicate that re-excision or vulvar radiation for close margins does not improve RFS or OS and support that the definition of close margins could potentially be redefined.
This study evaluates a single, well-defined, and clinically-relevant subgroup of vulvar cancer patients for whom the standard of care is unclear. Strengths of this study include the fact that all patients were treated at a high-volume cancer center by a small group of board-certified/eligible gynecologic oncologists. Although this cohort is small, to our knowledge this represents the largest cohort of patients with early stage disease and close or positive surgical margins who received adjuvant therapy.
Limitations of this study include the retrospective nature, variable but unbalanced treatment strategies and a small number of patients in the radiation subgroup. Quality of life data was not available. Surgical and radiation therapy techniques have evolved over the 22-year study period, and these changes may confound results of this study. Technology and practice changes over time also limit the applicability of older studies to current patients.
Two patients with positive margins received no further treatment due to comorbidities and wound breakdown. If any effect were seen in our results, we would expect an increase in the recurrence rate in the no further therapy group and therefore a higher likelihood of detecting a difference between the two groups. Neither of these patients have documented recurrence in our results. Eight cases (17%) from the entire cohort are in the setting of lichen sclerosis and therefore may confound results due to the more aggressive natural disease course compared to HPV-related lesions.
Our results indicate that re-excision and radiation do not improve recurrence-free survival or overall survival in stage I vulvar squamous cell carcinoma with close or positive margins following primary resection. Larger studies are needed in order to definitively determine the role of re-excision and adjuvant radiation in early stage disease. Given these findings, it is prudent to weigh the potential morbidities of further treatment against the lack of clear evidence of efficacy in patients with early stage vulvar squamous cell carcinoma.
Highlights.
31.3% of patients with stage I vulvar SCC had close or positive margins
There was no difference in RFS in patients treated with re-excision or vulvar radiation compared to no further treatment
There was no difference in OS in patients treated with re-excision or vulvar radiation compared to no further treatment
More research is needed on the role of re-excision and vulvar radiation in early stage vulvar cancer
Acknowledgements
Research reported in this publication was supported by NIH grant P30 CA77598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Statement
The corresponding author, Dr. Bedell, has no conflicts of interest to disclose. All other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Noone AM, H.N., Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. April 2018. [cited 2019 March 9]; Available from: https://seer.cancer.gov/csr/1975_2015/. [Google Scholar]
- 2.Aragona AM, et al. , An analysis of reported independent prognostic factors for survival in squamous cell carcinoma of the vulva: is tumor size significance being underrated? Gynecol Oncol, 2014. 132(3): p. 643–8. [DOI] [PubMed] [Google Scholar]
- 3.Imoto S, et al. , Prognostic factors in patients with vulvar cancer treated with primary surgery: a single-center experience. Springerplus, 2016. 5: p. 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maggino T, et al. , Patterns of recurrence in patients with squamous cell carcinoma of the vulva. A multicenter CTF Study. Cancer, 2000. 89(1): p. 7. [DOI] [PubMed] [Google Scholar]
- 5.Heaps J, et al. , Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecologic Oncology, 1990. 38(3): p. 6. [DOI] [PubMed] [Google Scholar]
- 6.Rouzier R, et al. , Local relapse in patients treated for squamous cell vulvar carcinoma: incidence and prognostic value. Obstetrics & Gynecology, 2002. 100(6): p. 9. [DOI] [PubMed] [Google Scholar]
- 7.National_Comprehensive_Cancer_Network. Vulvar Cancer (Squamous Cell Carcinoma) Version 1.2018. [cited 2019. March 9]; Available from: https://oncolife.com.ua/doc/nccn/Vulvar_Cancer.pdf.
- 8.Ansink A and van Der Velden J, Surgical interventions for early squamous cell carcinoma of the vulva. The Cochrane database of systematic reviews, 2000(2): p. CD002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Homesley H, et al. , Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstetrics & Gynecology, 1986. 68(6): p. 6. [PubMed] [Google Scholar]
- 10.Kunos C, et al. , Radiation therapy compared with pelvic node resection for node-positive vulvar cancer: a randomized controlled trial. Obstetrics & Gynecology, 2009. 114(3): p. 10. [DOI] [PubMed] [Google Scholar]
- 11.Moore DH, et al. , A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: a gynecologic oncology group study. Gynecol Oncol, 2012. 124(3): p. 529–33. [DOI] [PubMed] [Google Scholar]
- 12.Viswanathan AN, et al. , Relationship of margin status and radiation dose to recurrence in post-operative vulvar carcinoma. Gynecol Oncol, 2013. 130(3): p. 545–9. [DOI] [PubMed] [Google Scholar]
- 13.Pleunis N, et al. , Surgical margins in squamous cell carcinoma, different for the vulva? European Journal of Surgical Oncology, 2018. 44(10): p. 1555–1561. [DOI] [PubMed] [Google Scholar]
- 14.Arvas M, et al. , The Role of Pathological Margin Distance and Prognostic Factors After Primary Surgery in Squamous Cell Carcinoma of the Vulva. International Journal of Gynecological Cancer, 2018. 28(3): p. 9. [DOI] [PubMed] [Google Scholar]
- 15.Baiocchi G, et al. , How important is the pathological margin distance in vulvar cancer? Eur J Surg Oncol, 2015. 41(12): p. 1653–8. [DOI] [PubMed] [Google Scholar]
- 16.Piura B, et al. , Recurrent squamous cell carcinoma of the vulva: a study of 73 cases. Gynecologic Oncology, 1993. 48(2): p. 7. [DOI] [PubMed] [Google Scholar]
- 17.Stehman F, et al. , Early Stage I Carcinoma of the Vulva Treated with Ipsilateral Superficial Inguinal Lymphadenectomy and Modified Rad ical Hemivulvectomy: A Prospective Study of the Gynecologic Oncology Group. Obstetrics & Gynecology, 1992. 79(4): p. 8. [PubMed] [Google Scholar]
- 18.Nicholas S, et al. , Pelvic Radiation and Normal Tissue Toxicity. Semin Radiat Oncol, 2017. 27(4): p. 358–369. [DOI] [PubMed] [Google Scholar]
- 19.Faul C, et al. , Adjuvant radation for vulvar carcinoma: Improved local control. Int J Radiat Oncol Biol Phys, 1997. 38(2): p. 8. [DOI] [PubMed] [Google Scholar]
- 20.Ignatov T, et al. , Adjuvant radiotherapy for vulvar cancer with close or positive surgical margins. J Cancer Res Clin Oncol, 2016. 142(2): p. 489–95. [DOI] [PubMed] [Google Scholar]
- 21.National_Comprehensive_Cancer_Network. Vulvar Cancer (Squamous Cell Carcinoma) Version 2.2019. [cited 2019. March 23]; Available from: https://www.nccn.org/professionals/physician_gls/pdf/vulvar.pdf. [Google Scholar]