Abstract
Rocaglates are a family of natural products isolated from the genus Aglaia which possess a highly substituted cyclopenta[b]benzofuran skeleton and inhibit cap-dependent protein synthesis. Rocaglates are attractive compounds due to their potential for inhibiting tumor cell maintenance in vivo by specifically targeting eukaryotic initiation factor 4A (eIF4A) and interfering with recruitment of ribosomes to mRNA. In this paper, we describe an intercepted retro-Nazarov reaction utilizing intramolecular tosyl migration to generate a reactive oxyallyl cation on the rocaglate skeleton. Trapping of the oxy-allyl cation with a diverse range of nucleophiles has been used to generate over fifty novel amidino-rocaglate (ADR) and amino-rocaglate derivatives. Subsequently, these derivatives were evaluated for their ability to inhibit cap-dependent protein synthesis where they were found to outperform previous lead compounds including the rocaglate hydroxamate CR-1-31-B.
Graphical Abstract

INTRODUCTION
Aglaia Lour. is a genus of angiosperm plants containing more than 120 species.1 In 1982, the first rocaglate was isolated from dried roots and stems of Aglaia elliptifolia Merr.;2 since this time, over thirty natural products of the rocaglate family have been discovered, all sharing a highly substituted cyclopenta[b]benzofuran with five contiguous stereocenters. In the past several decades, numerous syntheses of rocaglates have been reported due to their intriguing structures.3, 4 These natural products and synthetic derivatives (Figure 1A) exhibit many interesting biological activities through targeting of the eukaryotic translation apparatus.5, 6 For example, the congener silvestrol (1) was found to inhibit the eIF4F complex by interfering with the function of the DEAD box RNA helicase eIF4A.7 Additionally, silvestrol has antitumor activity in a variety of pre-clinical murine cancer models including hematological and solid tumors.8 Rocaglamide (2), methyl rocaglate (3), and the synthetic derivative RHT (4) have also displayed anticancer and other biological properties.9
Figure 1.

(A) Representative Biologically active Rocaglates. (B) Interaction diagram for bound eIF4A1-polypurine RNA-rocaglamide 2.
Chemical syntheses of cyclopenta[b]benzofuran natural products and analogues have revealed structure-activity relationships (SAR) for antineoplastic activity in cancer cell lines (Figure SI1).10 In particular, SAR studies have defined chemically allowable modifications for rocaglates leading to improved activity.5,6 In particular, the C8b tertiary hydroxyl moiety (red) has been found to be critical which appears to be related to its role as a hydrogen bond (H-bond) donor (Figure 1B); alkylation of this tertiary hydroxyl completely eliminates cytotoxic activity.5d Recently, Iwasaki and co-workers reported the co-crystal structure of rocaglamide (2) bound to a human eIF4A1-polypurine RNA complex which revealed, among several key interactions identified, H-bonding between the C8b hydroxyl of 2 and N7 of a guanine RNA base (Figure 1B).11 In order to further study this critical interaction and improve biological activities of rocaglates, we have considered replacing the C8b tertiary hydroxyl with nitrogen substituents, which would allow for additional attachment of functional groups and manipulation of binding affinity to the eIF4A-RNA complex. Along these lines, Désaubry and coworkers have reported acyla-mino- and sulfonamino-substitution at the C8b position of the epi-rocaglaol scaffold as potential bioisosteric replacements; unfortunately, these derivatives were not cytotoxic.5g A comprehensive SAR study of C8b-amino-substitution was not performed which may be due to challenges in chemically modifying this position on the rocaglate core.
RESULT AND DISCUSSION
Discovery of the Intercepted Retro-Nazarov Reaction.
We recently synthesized tosyl-enol rocaglate (6a) from keto-rocaglate (5a) in an effort to access aglaroxin C analogue 8 (Scheme 1).5i In this transformation, we expected conjugate addition of benzamidine followed by a tosylate extrusion to afford enamine 7 under basic conditions which may further cyclize to pyrimidinone 8. When triethylamine or 4-dimethylaminopyridine (DMAP) were employed as bases, a trace amount of 8 was generated. In contrast, use of sodium hydride (NaH) as base produced an unknown product which had the same molecular weight as 7 but did not undergo subsequent pyrimidinone cyclization. NMR studies were not sufficiently informative to characterize the structure due to the lack of proton signals on the cyclopenta[b]benzofuran core. Instead, X-ray crystal structure analysis was used to confirm the new structure as the amidino-rocaglate (ADR) 9a (Scheme 1). Notably, in the solid-state structure the imidazoline N-H moiety resides on the nitrogen replacing the C8b hydroxyl, retaining the relative stereochemistry of the cyclopenta[b]benzofuran core. Generally speaking, the imidazoline N-H has a lower pKa (26.7-30.7 in DMSO) than a hydroxyl (tBuO-H: 32.2-32.5 in DMSO) and thus may serve as a suitable hydrogen-bond donor for biological target engagement.12 In addition, the newly-formed C1 hemiaminal hydroxyl group is situated on the α–face corresponding to the same stereochemistry of the secondary alcohol in rocaglates 1–4. According to previous SAR studies of rocaglate analogues, the overall retention of functional group stereochemistry of the rocaglate scaffold is important to maintain inhibition of eIF4A-dependent protein synthesis.5,6
Scheme 1.

An Alternative Approach to Aglaroxin C Led the Substitution of the C8b Hydroxyl.
Proposed Mechanistic Pathway.
Mechanistically, we propose that the amidine substitution proceeds through an intercepted retro-Nazarov reaction process (Scheme 2).13, 14, 15 A number of elegant studies have been reported outlining construction of the cyclopenta[b]benzofuran core of the rocaglates using Nazarov cyclization.4l–m, p–q According to our reaction protocol, NaH deprotonates the tertiary alcohol of 6a (Scheme 2A) to alkoxide 11 which may result in intramolecular tosyl migration16 to afford tosylate enolate 12. Subsequent ionization of the tertiary tosylate facilitates formation of the stabilized oxyallyl cation 13. Based on the reactivity observed, generation of the oxyallyl cation occurs during warming and is a fast process. We have found that the rates of subsequent trapping of oxyallyl cation 13 vary for different amidines (vide infra) which suggests that this is the rate determining step for the process. After formation of the C-N bond in adduct 14, a rapid cyclization completed the transformation and constructed the hemiaminal of 9a. To further probe the mechanism (Scheme 2B), we used enantio-enriched (−)-6a (>98 % ee) as starting material, which was synthesized using either asymmetric ESIPT-mediated [3+2] photocycloaddition or biomimetic kinetic resolution of an aglain ketone precursor.17, 4n–o Compound (−)-6a was then subjected to amination using NaH and benzylamine. In the event, oxyallyl cation 13 was trapped with benzylamine to afford the amino-rocaglate (−)-15h in 90% yield. As expected, complete retention of chirality for amino-rocaglate 15h (>98% ee) demonstrated that the retro-Nazarov reaction was irreversible from dienone 10. Otherwise, loss of enantiopurity of amino-rocaglate 15h would be observed. Additionally, when weak nucleophiles or no nucleophile were used in the reaction, retro-Nazarov products 10 were obtained (cf. red arrows, Scheme 2B).18 We also used oxygen-based nucleophiles, including water and methanol, to trap oxyallyl cation 13 to afford keto-rocaglate 5a and O-methyl-rocaglate 5b.10 Alternative enol protection reagents, including mesyl chloride, triflic anhydride,19 sulfonyl chloride,4g, 5g and diphenyl phosphoryl chloride20 were investigated to generate oxyallyl cation precursors. Unfortunately, only retro-Nazarov products were generated during enol protection except when using mesyl chloride. However, the corresponding mesyl-enol rocaglate10 failed to migrate and generate product 9a under standard amidine addition conditions (cf. Table 1) but instead afforded N-(methyl-sulfonyl)benzenecarboximidamien and keto-rocaglate 5a.10
Scheme 2.

Proposed Mechanism for Amidino-Rocaglate Synthesis
Table 1.
Syntheses of Amidino-rocaglatesa
 |
Reagents and conditions: 6 (25 mg, 1.0 equiv), amidine hydrochloride (3.0 equiv), NaH (8.0 equiv), THF (0.1 M), −78 °C to rt.
amidine (3.0 equiv), NaH (5.0 equiv).
500 mg scale; 74% yield on 25 mg scale.
amidine salt was azeotroped with benzene under vacuum.
25% mol% NaHMDS was added at rt after 2 h.
inseparable regioisomers (>10:1); major isomer is shown as 9o.
inseparable regioisomers (2:1); major isomer is shown as 9p.
To further understand the stereoselectivity of the intercepted retro-Nazarov reaction, we performed a DFT analysis 13 at the B3LYP/6-31G** level21 which has been extensively used for computational studies of oxyallyl cation intermediates. 22 Interestingly, the DFT model of 13 (Scheme 2B) shows a trigonal, pyramidal carbocation at the C8b position which correlates well with the trapping of oxyallyl cation 13 with amidines from the convex face.
Syntheses of Amidino-Rocaglates.
The intercepted retro-Nazarov reaction was found to tolerate a wide range of amidine and guanidine reaction partners (Table 1). Rocaglates with various carbonyl substitutions were also found to be workable. Generally, the intercepted retro-Nazarov reaction was robust and operationally simple; overall, 29 amidino-rocaglates were synthesized in good yields. A general issue for this reaction was found with hygroscopic amidine salts which introduced trace amounts of water competing with amidine nucleophiles to afford ketorocaglate 5a. The water content of amidines varies from batches and vendors, so we did not optimize reaction conditions for each substrate. However, simple azeotropic drying of the amidine salts using benzene was found to improve product yields (e.g. 9e from 47% to 79%; 9g from 29% to 94%). Additionally, several amidine salts were found to have poor solubility in reactions leading to recovery of 6. In these cases, 25 mol% of NaHMDS (1M in THF) was used as a proton shuttle (9e-g, 9m).
After having initial success using benzamidine to generate 9a (88% yield), we next evaluated use of aliphatic amidines. With increasing steric size of amidine substitution (e.g. from methyl to t-butyl), we observed decreasing reaction rates. Use of acetamidine afforded the corresponding adduct 9b in 81% yield, whereas the highly hygroscopic butylamidine and pentylamidine afforded products 9c and 9d in lower yields (54% and 61%, respectively). Likewise, when abenzylamidine reagent was used, a 79% yield of adduct 9e was obtained. Bulkier amidines such as neo-pentylamidine and iso-butanamidine resulted in excellent yields of the desired products 9f and 9g (88% and 94%, respectively). Similarly, the cyclohexyl-substituted product 9i was formed in 88% yield. Interestingly, the parent amidine, guanidine, successfully generated compound 9j in a 70% yield. We employed 2-methoxyl- and 2-chloroacetamidine to access ADR’s with modification handles in excellent 9k (90%) and 91 (92%) yields. Additionally, strained cyclopropyl and cyclobutyl amidines were also tolerated in the reaction affording derivatives 9m and 9n in 81% and 92% yields, respectively. Unsymmetrical amidines generated 9o and 9p in excellent yields (90% and 73%). Of note, 9o and 9p were formed as the major regioisomers, whereas the minor regioisomers were also formed through trapping by the exocyclic nitrogen atom of the amidine reagents.10
In addition, we found that heteroaryl and aryl amidines with various electronic properties provided the desired adducts in reasonable yields (75% for 9q; 55% for 9r; 93% for 9s; 84% for 9t; 62% for 9u; 72% for 9v). Moreover, both Boc-protected piperidinyl and N-morpholinyl amidines were used to generate adducts 9v and 9w in 67% yields. We also tested a variety of rocaglate derivatives in reactions with acetamidine. To this end, tosyl-enol rocaglamide 6z yielded 9z in 83 % yield, and tosyl-enol RHT 6aa led to compound 9aa in 79% yield. To our delight, we found that the amidine addition could also tolerate ketone (9ab, 92%) and aldehyde functional groups on the rocaglate skeleton. Interestingly, the aldehyde product subsequently underwent dehydration yielding the α,β-unsaturated aldehyde 9y (not shown).10 Overall, this powerful late-stage functionalization method enabled rapid access to a diverse collection of 29 amidino-rocaglate (ADR) derivatives.
Syntheses of Amino-Rocaglates.
Inspired by mechanistic considerations, we also sought to synthesize amino-rocaglates utilizing amines as nucleophiles to trap oxyallyl cation intermediate 13. Accordingly, we explored substrate scope for the synthesis of amino-rocaglates5g under the same conditions (Table 2). Our initial attempts employed several primary amine derivatives to generate amino-rocaglates in good yields (86% for 15a; 90% for 15b; 75% for 15c). Of note, the use of N-methyl ethylenediamine (for 15c) reinforced that the oxyallyl cation intermediate prefers to be trapped by the less hindered primary amine moiety. The secondary amine residue in 15c may also be utilized to introduce biological probes. Moreover, use of an allyl amine reaction partner afforded 15d in 79% yield; propargylamide was also used to form alkynylated derivative 15e in 59% yield which may serve as a click-chemistry handle. Likewise, we introduced an additional functionalized side chain using butylamine dimethyl acetal to access compound 15f in 73% yield. The acetal may eventually be converted into a pendant aldehyde as a handle for further functionalization. In addition to amines with useful modification handles, we also investigated the steric effect of amines. From methyl to adamantyl amine (15g to 15k), we observed a slight decrease in yields for the desired amino-rocaglates from (88% to 44%) and an increased trend of retro-Nazarov products generation. Interestingly, the weak nucleophile, aniline, was also able to trap the oxyallyl cation affording 15l (70%). We found that tethered heterocycles were also workable for amino-rocaglate synthesis. For example, a tetrahydropyranyl amine was a successful trapping reagent affording amino-rocaglate 15m (43%), while N-morpholinyl ethyleneamine provided 15n with a remote morpholine functional group in a moderate yield (60%). Nevertheless, introduction of thiazole and imidazole heterocycles was more challenging using the oxyallyl cation trapping method. In particular, we obtained a 25% yield of 15o bearing the thiazole functional group, whereas use of an imidazole-tethered amine afforded product 15p in 49% yield. To our delight, 3-picolylamine underwent the desired reaction to afford a 70% yield of amino-rocaglate 15q. In addition, use of secondary amine such as diethylamine led to a 27% yield of 15r along with significant yield of retro-Nazarov products as the major product, highlighting limitations with use of hindered amines. Finally, dimethyl hydrazine broadened the scope of the intercepted retro-Nazarov reaction to afford rocaglate hydrazine derivative 15s in 75% yield.
Table 2.
Syntheses of Amino-Rocaglatesa
 |
Reagents and conditions: 6a (1.0 equiv), NaH (5.0 equiv), amine (3.0 equiv), THF (0.1-0.2 M), 15 min at −78 °C, rt, 30-120 min.
500 mg scale; 83% on 25 mg scale.
250 mg scale; 90% on 25 mg scale.
6a (1.0 equiv), NaH (8.0 equiv), amine salt (3.0 equiv), THF (0.1-0.2 M).
Late-Stage Functionalization of Amino-Rocaglates.
The derived amino-rocaglates 15 possess a higher oxidation state than the natural rocaglates as silvestrol (1), rocaglamide (2) and rocaglaol (not shown).5c–d, 4f, h To compare the biological activity of amino-rocaglates with natural rocaglates, we explored late-stage functionalization of amino-rocaglate scaffold 15a (Scheme 3). Additionally, we targeted modification of the methyl ester, as rocaglamide (2) and RHT (4) display greater cytotoxicity than methyl rocaglate (3) against several cancer cell lines.5, 6 Amide exchange of 15a generated amino-keto-rocaglamide 17 as a useful synthetic intermediate. Subsequent reduction of 17 using borane dimethyl sulfide complex generated amino-rocaglamide 18 (31%). Compound 15a was also converted to amino-aglaroxin derivative 16 in 63% yield.5i On the other hand, DMAP facilitated decarboxylation of 15a to afford amino ketone 19 which was further reduced with NaBH4 to afford amino-rocaglaol 20 in 71% yield in which case syn-stereochemistry between the amine and alcohol groups was observed. Additionally, oxime 21 was synthesized using hydroxyl amine and triethylamine which was followed by reduction with LiAlH4 to afford diamino-rocaglate 22.
Scheme 3.

Late-stage Functionalization of Amino-Rocaglatesa
Reagents and Conditions: a) HNMe2 (2.0 equiv, 2.0 M in THF), 20 mol% DMAP, toluene (0.1 M), 90 °C, 3 h, 92%. b) H3B•SMe2 (3.0 equiv, 1.0 M in THF), THF (0.05M), rt, 12 h, 31%. c) benzamidine (3.0 equiv), 30 mol% DMAP, m-xylene (0.025 M), 130 °C, 45 min, 63%. d) H2O (10 equiv), 20 mol% DMAP, toluene (0.1 M), 90 °C, 3 h, quant, yield. e) NaBH4 (20 equiv), MeOH (0.02 M), rt, overnight, 71%. f) NEt3 (2.0 equiv), NH2OH•HCl (3.0 equiv), MeOH (0.1 M), 37 °C, overnight, 87%. g) LiAlH4 (5.0 equiv), THF (0.05 M), rt, overnight, 40%.
BIOLOGICAL STUDIES
Structure-Activity Relationships.
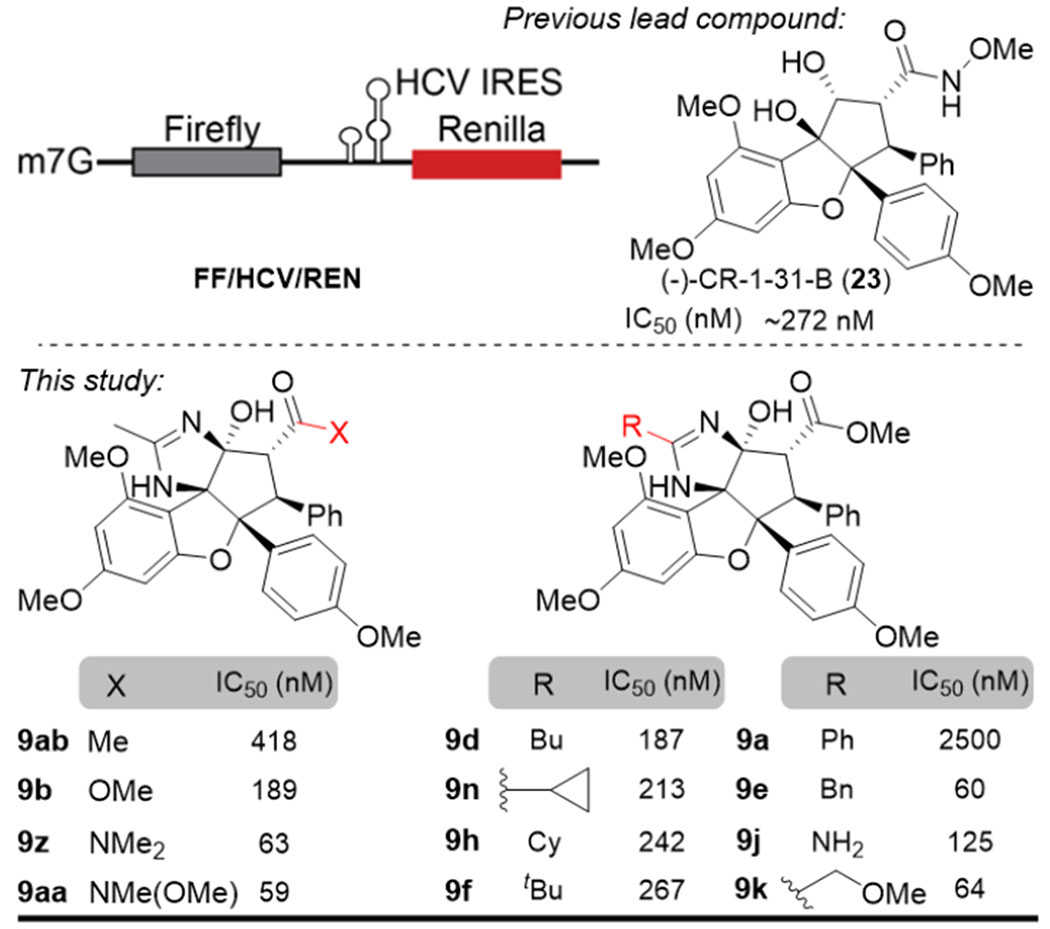
With amidino-rocaglates and amino-rocaglates in hand, we evaluated their inhibition of eIF4A-dependent translation using an in vitro assay (Table 3A), wherein a bicistronic reporter mRNA was designed for translation in Krebs-2 cell free extracts.23 In particular, translation of Firefly luciferase (FF) is cap-dependent and depends on eIF4A activity; in contrast, translation of Renilla luciferase (REN) utilizes the HCV internal ribosome entry site (IRES) which functions independently of eIF4A. Relative IC50’s were determined by the inhibition of FF obtained in the presence of compound relative to vehicle controls and normalized to REN as an internal control. Our previous enantioenriched lead compound, (−)-CR-1-31-B (23), exhibited an IC50 of 272 nM in the bicistronic reporter assay. As expected, amidino-rocaglates generally showed an overall improvement in IC50; representative compounds are shown for a preliminary discussion of SAR (Table 3B). In accordance with the eIF4A-RNA-rocaglamide (2) co-crystallographic analysis (Figure 1), the amide carbonyl of 2 interacts with Gln195 of eIF4A as a H-bond acceptor, while the tertiary hydroxyl of 2 serves as a H-bond donor to a guanine RNA base (Figure 1B).11 A correlated trend of IC50’s was observed for carbonyl substitution (X = N(OMe)Me ≥ NMe2 > OMe > Me), where 9aa and 9z showed excellent IC50’s of 59 and 63 nM, respectively. In contrast, the ester (9b, IC50 = 189 nM) and ketone (9ab, IC50 = 418 nM) were found to be less potent supporting that the amide carbonyl of 9aa and 9z are more optimal H-bond donors to Glnl95 of eIF4A. According to our previous findings, alkylation of the tertiary hydroxyl of RHT (4) and rocaglamide (2) eliminated the translation inhibition5c–d which was likely caused by disabling their ability as H-bond donors. To further verify this hypothesis, amidino-rocaglates (pKa of N-H ~26.7 - 30.7 in DMSO, strong H-bond donors)12b and amino-rocaglates (pKa of N-H ~36 to 44 in DMSO, weak H-bond donors)24 were next evaluated. Unfortunately, no translation inhibition by amino-rocaglates 15 and their derivatives 18, 20, and 22 were observed.10 Preliminary SAR for ADR’s showed that the less sterically demanding alkyl amidines slightly increased potency for translation inhibition ranging from IC50 = 187 nM for the butyl derivative (9d) to IC50 = 267 nM for the tert-butyl derivative (9f). Interestingly, aryl amidines (e.g. 9a) were found to have dramatically decreased activity (IC50 = 2500 nM) which may be caused by conformational restrictions. Conversely, benzyl amidine 9e had IC50 = 60 nM, whereas the methylene tether may allow conformational adjustment to avoid rigid repulsion in the binding pocket. Moreover, the guanidine-type structure (9j) as well as methoxyl amidine (9k) also retained potent translation inhibition (IC50 = 125 and 64 nM, respectively).10
Table 3.
Preliminary SAR of Representative Amidino-Rocaglates.a
 |
IC50 was determined from fitted sigmoid curves; IC50 indicates the concentration of compound that inhibits cap-dependent translation by 50%, which was normalized by cap-independent translation. For IC50 determination, the bicistronic reporter FF/HCV/Ren mRNA was used to produce Firefly luciferase (cap-dependent) and Renilla luciferase (cap-independent) in Krebs-2 extracts in the presence of indicated compounds at various concentrations.
To further characterize the effect of amidino-rocaglate chirality on translation inhibition, we synthesized enantioenriched analogues (−)-9b, (−)-9n, (−)-9z, (−)-9aa and (+)-9aa. As expected, only the natural (−)-enantiomers inhibited in vitro eIF4A-dependent translation with an IC50 of 34 nM, which is an approximately 9-fold increase in potency to our previous lead-compound (−)-23 (Table 4). On the other hand, the unnatural enantiomer (+)-9aa showed minimal activity.
Table 4.
Chirality-Based Biological Profiles of Lead Compoundsa
 |
IC50 of the indicated compound was determined using the same assay in Table 3 and was calculated using a fitted sigmoid curve.
Amidino-Rocaglates are Cytotoxic Towards Cancer Cells.
We next evaluated amidino-rocaglates in a sulforhodamine B (SRB) assay using MDA-MB-231 breast cancer cells for a cellular readout of cytotoxicity (Figure 2). In particular, (−)-9k, (−)-9z, (−)-9aa, and (+)-9aa were compared with rocaglate hydroxamate (−)-23. We found excellent cytotoxicity of the ADR (−)-9aa with an IC50 = 0.97 nM with a nearly 2-fold increase in potency over the previous lead-compound (−)-23 (IC50 = 1.9 nM). In addition, the IC50 of dimethylamide (−)-9z and methyl ester (−)-9k were determined to be 1.2 and 3.6 nM, respectively, showing a similar overall trend as we have observed in the FF/HCV/REN assay (Table 3). The inspiring high-potency of amidino-rocaglates (ADR) underscores their utility as promising agents for anticancer treatment combined with the appropriate formulation and drug-delivery strategies. Interestingly, we also noticed reduced cytotoxicity of the unnatural enantiomer (+)-9aa (IC50 = 76 nM). To evaluate possible off-target inhibition of ADRs, we also tested (−)-9aa and (+)-9aa using the eIF4A1em1jp cell line, a CRISPR-modified NIH3T3 line containing the F163L mutation within eIF4A1, which exhibits rescue of the eIF4A1-dependent translation inhibition of silvestrol (1).7c We found that the eIF4A1em1jp cells were less sensitive to (−)-9aa and (+)-9aa in comparison to wild type NIH3T3 cells (Figure SI2) which indicates that the cytotoxicity of (+)-9aa and (−)-9aa are eIF4A1 dependent.10 In eIF4A1em1jp cells, the cytotoxicity observed may be related to the remaining wild-type eIF4A2 paralog.7c However, for (+)-9aa further studies are needed to rule out unrelated mechanisms.
Figure 2.

Amidino-rocaglates induced cytotoxicity against MDA-MB-231 breast cancer cells in the SRB cell assay. IC50 was determined from fitted sigmoid curves; for its determination, the cells were cultured in the presence of indicated compound for 4 days prior to quantitating the bound SRB dye using Spectramax M5 at OD = 510 nM.
CONCLUSION
In summary, we have discovered an intercepted retro-Nazarov reaction providing an oxyallyl cation precursor for late-stage modification of rocaglate natural products. Through formal substitution of the rocaglate C8b hydroxyl group, we synthesized a library of more than fifty amidino- and amino-rocaglates as novel modified natural product derivatives. Subsequent biological evaluation of the modified rocaglate derivatives revealed preliminary SAR for the inhibition of eIF4A-dependent translation, which identified amidino-rocaglate (−)-9aa having a 9-fold increase in potency in comparison to (−)-CR-1-31-B (23). In agreement with the recently reported X-ray crystallographic analysis of eIF4A-RocA-polypurine RNA,11 we demonstrated that the lower pKa of the amidine N-H vs the original tertiary O-H moiety likely contributes to improved translation inhibition. Meanwhile, the amide carbonyls of 9z and 9aa serve as H-bond acceptors likely facilitating interactions with Gln195 of eIF4A, although this aspect awaits structural confirmation. SRB assays in MDA-MB-231 cells demonstrated that amidino-rocaglates may serve as valuable cancer chemotherapeutic agents. Our studies further illustrate the power of chemical synthesis enabling structural and biological improvement of complex natural products using targeted modifications. Additional biological studies of amidino-, amino-rocaglates, and related compounds including RNA binding studies, in vivo activity as translation inhibitors, and anti-neoplastic activity in mouse cancer models are currently in progress and will be reported in due course.
Supplementary Material
ACKNOWLEDGMENT
We thank the National Institutes of Health (R01 DK088787 and R35 GM-118173) and the Canadian Institutes of Health Research (FDN-148366) for research support. Dr. Jeffrey Bacon (Boston University) for X-ray crystal structure analyses, Michael Smith (Boston University) for assistance plotting the X-ray crystal structures, Dr. Aaron B. Beeler for suggesting DFT calculations, and Dr. Xiao Wang (Merck & Co., Inc., Kenilworth, NJ) for suggestions regarding NMR experiments and structure elucidation of 9ac. NMR (CHE-0619339) and MS (CHE0443618) facilities at Boston University are supported by the NSF. Research at the BU-CMD was supported by NIH grant GM-067041. We also thank Dr. Kyle Reichl (Amgen, Inc.) for proofreading the manuscript.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Experimental details, characterization data, and NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org
REFERENCES
- 1.For general review of rocaglate natural products, see:; (a) Ebada SS; Lajkiewicz N; Porco JA Jr.; Li-Weber M; Proksch P Chemistry and Biology of Rocaglamides (= Flavaglines) and Related Derivatives from Aglaia Species (Meliaceae), In Progress in the Chemistry of Organic Natural Products Vol. 94; [DOI] [PMC free article] [PubMed] [Google Scholar]; Kinghorn AD, Falk H, Kobayashi J, Eds.; Springer Vienna: Vienna, 2011, p 1. [Google Scholar]; (b) Ribeiro N; Thuaud F; Nebigil C; Désaubry L Recent Advances in the Biology and Chemistry of the Flavaglines. Bioorg. Med. Chem 2012, 20, 1857. [DOI] [PubMed] [Google Scholar]; (c) Pan L; Woodard JL; Lucas DM; Fuchs JR; Douglas Kinghorn A Rocaglamide, silvestrol and structurally related bioactive compounds from Aglaia species. Nat. Prod. Rep 2014,31, 924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu King M; Chiang C-C; Ling H-C; Fujita E; Ochiai M; McPhail AT A-Ray Crystal Structure of Rocaglamide, a Novel Antileulemic 1H-Cyclopenta[b]benzofuran from Aglaia elliptifolia. J. Chem. Soc., Chem. Commun 1982, 1150. [Google Scholar]
- 3.For reviews of rocaglate synthesis, see:; (a) Peter P; Ru-Angelie E; Rainer E; Frank IB; Bambang WN Chemistry and Biological Activity of Rocaglamide Derivatives and Related Compounds in Aglaia Species (Meliaceae) Curr. Org. Chem 2001, 5, 923. [Google Scholar]; (b) Cai X.-h.; Xie B; Guo H Progress in the Total Synthesis of Rocaglamide. ISRN Org. Chem 2011, 2011, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Qian Z; Hussein A-H; Laurent D Recent Advances in the Synthesis of Flavaglines, a Family of Potent Bioactive Natural Compounds Originating from Traditional Chinese Medicine. Eur. J. Org. Chem 2016,2016, 5908. [Google Scholar]
- 4.For representative syntheses of rocaglate natural products:; (a) Kraus GA; Sy JO A Synthetic Approach to Rocaglamide via Reductive Cyclization of δ-Keto Nitriles. J. Org. Chem 1989, 54, 77. [Google Scholar]; (b) Trost BM; Greenspan PD; Yang BV; Saulnier MG An Unusual Oxidative Cyclization. A Synthesis and Absolute Stereochemical Assignment of (−)-Rocaglamide. J. Am. Chem. Soc 1990, 112, 9022. [Google Scholar]; (c) Davey AE; Schaeffer MJ; Taylor RJK Enantioselective Synthesis of Cyclopenta[b]benzofurans via An Organocatalytic Intramolecular Double Cyclization. J. Chem. Soc., Chem. Commun 1991, 1137. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Davey AE; Schaeffer MJ; Taylor RJK Synthesis of the Novel Anti-leukaemic Tetrahy-drocyclopenta[b]benzofuran, Rocaglamide and Related Synthetic Studies. J. Chem. Soc., Perkin Trans. 1 1992, 2657. [Google Scholar]; (e) Dobler MR; Bruce I; Cederbaum F; Cooke NG; Diorazio LJ; Hall RG; Irving E Total Synthesis of (±)-Rocaglamide and Some Aryl Analogues. Tetrahedron Lett 2001, 42, 8281. [Google Scholar]; (f) Thede K; Diedrichs N; Ragot JP Stereoselective Synthesis of (±)-Rocaglaol Analogues. Org. Lett 2004, 6, 4595. [DOI] [PubMed] [Google Scholar]; (g) Gerard B; Jones G; Porco JA Jr. A Biomimetic Approach to the Rocaglamides Employing Photogeneration of Oxidopyryliums Derived from 3-Hydroxyflavones. J. Am. Chem. Soc. 2004,126, 13620. [DOI] [PubMed] [Google Scholar]; (h) Diedrichs N; Ragot JP; Thede K A Highly Efficient Synthesis of Rocaglaols by a Novel α-Arylation of Ketones. Eur. J. Org. Chem 2005, 2005, 1731. [Google Scholar]; (i) Gerard B; Sangji S; O’Leary DJ; Porco JA Jr. Enantioselective Photocycloaddition Mediated by Chiral Brønsted Acids: Asymmetric Synthesis of the Rocaglamides. J. Am. Chem. Soc 2006,128,7754. [DOI] [PubMed] [Google Scholar]; (j) El Sous ME; Khoo ML; Holloway G; Owen D; Scammells PJ; Rizzacasa MA Total Synthesis of (−)-Episilvestrol and (−)-Silvestrol. Angew. Chem., Int. Ed 2007, 46, 7835. [DOI] [PubMed] [Google Scholar]; (k) Baudouin G; Cencic R; Pelletier J; Porco JA Jr., Angew. Chem., Int Ed 2007, 46, 783. [DOI] [PubMed] [Google Scholar]; (l) Malona JA; Cariou K; Frontier AJ Nazarov Cyclization Initiated by Peracid Oxidation: The Total Synthesis of (±)Rocaglamide. J. Am. Chem. Soc 2009, 131, 7560. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Magnus P; Freund WA; Moorhead EJ; Rainey T Nazarov Cyclization Initiated by Peracid Oxidation: The Total Synthesis of (±)-Rocaglamide. J. Am. Chem. Soc 2012, 134, 6140.22444715 [Google Scholar]; (n) Lajkiewicz NJ; Roche SP; Gerard B; Porco JA Jr. Enantioselective Photocycloaddition of 3-Hydroxyflavones: Total Syntheses and Absolute Configuration Assignments of (+)-Ponapensin and (+)-Elliptifoline. J. Am. Chem. Soc 2012,134, 13108. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Stone SD; Lajkiewicz NJ; Whitesell L; Hilmy A; Porco JA Jr. Biomimetic Kinetic Resolution: Highly Enantio- and Diastereoselective Transfer Hydrogenation of Aglain Ketones to Access Flavagline Natural Products. J. Am. Chem. Soc 2015,137, 525. [DOI] [PMC free article] [PubMed] [Google Scholar]; (p) Zhou Z; Tius MA Synthesis of Each Enantiomer of Rocaglamide by Means of a Palladium(0)-Catalyzed Nazarov-Type Cyclization. Angew. Chem., Int. Ed 2015, 54, 6037. [DOI] [PubMed] [Google Scholar]; (q) Zhou Z; Dixon DD; Jolit A; Tius MA The Evolution of the Total Synthesis of Rocaglamide. Chem. Eur J. 2016,22,15929. [DOI] [PubMed] [Google Scholar]
- 5.For representative medicinal remodeling of rocaglates from academia, see:; (a) Roche SP; Cencic R; Pelletier J; Porco JA Jr. Biomimetic Photocycloaddition of 3-Hydroxyflavones: Synthesis and Evaluation of Rocaglate Derivatives as Inhibitors of Eukaryotic Translation. Angew. Chem., Int Ed 2010, 49, 6533. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Thuaud F; Ribeiro N; Gaiddon C; Cresteil T; Désaubry L Novel Flavaglines Displaying Improved Cytotoxicity. J. Med. Chem 2011,54, 411. [DOI] [PubMed] [Google Scholar]; (c) Rodrigo CM; Cencic R; Roche SP; Pelletier J; Porco JA Jr. Synthesis of Rocaglamide Hydroxamates and Related Compounds as Eukaryotic Translation Inhibitors: Synthetic and Biological Studies. J. Med. Chem 2012, 55, 558. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hawkins BC; Lindqvist LM; Nhu D; Sharp PP; Segal D; Powell AK; Campbell M; Ryan E; Chambers JM; White JM; Rizzacasa MA; Lessene G; Huang DCS; Burns CJ Simplified Silvestrol Analogues with Potent Cytotoxic Activity. Chem Med Chem 2014, 9, 1556. [DOI] [PubMed] [Google Scholar]; (e) Lajkiewicz NJ; Cognetta AB; Niphakis MJ; Cravatt BF; Porco JA Jr. Remodeling Natural Products: Chemistry and Serine Hydrolase Activity of a Rocaglate-Derived β-Lactone. J. Am. Chem. Soc 2014,136, 2659. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Wang W; Cencic R; Whitesell L; Pelletier J; Porco JA Jr. Synthesis of Aza-Rocaglates via ESIPT-Mediated (3 + 2) Photocycloaddition. Chem. - Eur. J 2016, 22,12006. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Zhao Q; Tijeras-Raballand A; de Gramont A; Raymond E; Désaubry L Bioisosteric Modification of Flavaglines. Tetrahedron Lett 2016, 57, 2943. [Google Scholar]; (h) Wang TT; Liu S; Wang W; Lajkiewicz N; Porco JA Jr. Aglaroxin C and Derivatives as HCV Entry Inhibitors. U.S. patent US 10,085,988 B1, 2018.; (i) Zhang W; Liu S; Maiga RI; Pelletier J; Brown LE; Wang TT; Porco JA Jr. Chemical Synthesis Enables Structural Reengineering of Aglaroxin C Leading to Inhibition Bias for Hepatitis C Viral Infection. J. Am. Chem. Soc 2019,141,1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For representative medicinal remodeling of rocaglates from industry, see:; (a) Bruce I; Cooke NG; Diorazio LJ; Hall RG; Irving E Synthesis of the Carbocyclic Analogue of (±)-Rocaglamide. Tetrahedron Lett 1999, 40, 4279. [Google Scholar]; (b) Hall Roger G; Szczepanski H; Bruce IAN; Cooke Nigel F; Diorazio Louis J; Dobler M; Cederbaum F DE Patent DE 199 34 952 A1, 2000.; (c) Liu T; Nair SJ; Lescarbeau A; Belani J; Peluso S; Conley J; Tillotson B; O’Hearn P; Smith S; Slocum K; West K; Helble J; Douglas M; Bahadoor A; Ali J; McGovern K; Fritz C; Palombella VJ; Wylie A; Castro AC; Tremblay MR Synthetic Silvestrol Analogues as Potent and Selective Protein Synthesis Inhibitors. J. Med. Chem 2012, 55, 8859. [DOI] [PubMed] [Google Scholar]; (d) Ernst JT; Reich SH; Sprengeler PA; Tran CV; Packard GK; Xiang AX; Nilewski C; Michels T U.S. Patent US 9,957,277 B2,2018.
- 7.(a) Bordeleau M-E; Robert F; Gerard B; Lindqvist L; Chen SMH; Wendel H-G; Brem B; Greger H; Lowe SW; Porco JA Jr.; Pelletier J Therapeutic Suppression of Translation Initiation Modulates Chemosensitivity in a Mouse Lymphoma Model.J. Clin. Invest 2008,118,2651. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cencic R; Carrier M; Galicia-Vázquez G; Bordeleau M-E; Sukarieh R; Bourdeau A; Brem B; Teodoro JG; Greger H; Tremblay ML; Porco JA Jr.; Pelletier J Antitumor Activity and Mechanism of Action of the Cyclopenta[b]benzofuran, Silvestrol. PLoS One 2009, 4, e5223. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chu J; Galicia-Vázquez G; Cencic R; Mills John R.; Katigbak A; Porco JA Jr.; Pelletier J CRISPR-Mediated Drug-Target Validation Reveals Selective Pharmacological Inhibition of the RNA Helicase, eIF4A. Cell Rep 2016, 15, 2340. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Itoua Maïga R; Cencic R; Chu J; Waller DD; Brown LE; Devine WG; Zhang W; Sebag M; Porco JA Jr.; Pelletier J Oxo-aglaiastatin-Mediated Inhibition of Translation Initiation. Sci. Rep 2019, 9,1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Hwang BY; Su B-N; Chai H; Mi Q; Kardono LBS; Afriastini JJ; Riswan S; Santarsiero BD; Mesecar AD; Wild R; Fairchild CR; Vite GD; Rose WC; Farnsworth NR; Cordell GA; Pezzuto JM; Swanson SM; Kinghorn AD Silvestrol and Episilvestrol, Potential Anticancer Rocaglate Derivatives from Aglaia silvestris. J. Org. Chem 2004, 69, 3350; [DOI] [PubMed] [Google Scholar]; (b) Meurer-Grimes BM; Yu J; Vairo GL U.S. Patent 2004, US 6,710,075 B2.
- 9.(a) Chu J; Cencic R; Wang W; Porco JA Jr.; Pelletier J Translation Inhibition by Rocaglates Is Independent of eIF4E Phosphorylation Status. Mol. Cancer Ther 2016,15, 136; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu S; Wang W; Brown LE; Qiu C; Lajkiewicz N; Zhao T; Zhou J; Porco JA Jr.; Wang TT A Novel Class of Small Molecule Compounds that Inhibit Hepatitis C Virus Infection by Targeting the Prohibitin-CRaf Pathway. EBio-Medicine 2015, 2, 1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.See Supporting Information for complete experimental details.
- 11.Iwasaki S; Iwasaki W; Takahashi M; Sakamoto A; Watanabe C; Shichino Y; Floor SN; Fujiwara K; Mito M; Dodo K; Sodeoka M; Imataka H; Honma T; Fukuzawa K; Ito T; Ingolia NT The Translation Inhibitor Rocaglamide Targets a Bimolecular Cavity between eIF4A and Polypurine RNA. Mol. Cell 2019, 73, 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.For pKa of tertiary alcohols (pKa of tBuOH is 32.2-32.5 in DMSO), see:; (a) Olmstead WN; Margolin Z; Bordwell FG Acidities of Water and Simple Alcohols in Dimethyl Sulfoxide Solution. J. Org. Chem 1980,45,3295. [Google Scholar]; For pKa of amidine (pKa of amidines locates between 26.7-30.7 in DMSO), see:; (b) Bordwell FG; Ji GZ Effects of Structural Changes on Acidities and Homolytic Bond Dissociation Energies of the Hydrogen-Nitrogen Bonds in Amidines, Carboxamides, and Thiocarboxamides. J. Am. Chem. Soc 1991,113, 8398. [Google Scholar]
- 13.For reviews of the intercepted Nazarov reaction, see:; (a) Grant TN; Rieder CJ; West FG Interrupting the Nazarov Reaction: Domino and Cascade Processes Utilizing Cyclopentenyl Cations. Chem. Commun 2009,5676. [DOI] [PubMed] [Google Scholar]; (b) Li H;Wu J (3+2)-Cycloaddition Reactions of Oxyallyl Cations. Synthesis 2015, 47, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.For recent examples of intercepted Nazarov reactions, see:; (a) Wei L; Vivek K; Baburaj B; Markus S; Kamal K Branching Cascades: A Concise Synthetic Strategy Targeting Diverse and Complex Molecular Frameworks. Angew. Chem., Int. Ed 2011, 50, 6900. [DOI] [PubMed] [Google Scholar]; (b) Kwon Y; McDonald R; West FG Organoaluminum-Mediated Interrupted Nazarov Reaction. Angew. Chem., Int. Ed 2013, 52, 8616. [DOI] [PubMed] [Google Scholar]; (c) Wu Y-K; Dunbar CR; McDonald R; Ferguson MJ; West FG Experimental and Computational Studies on Interrupted Nazarov Reactions: Exploration of Umpolung Reactivity at the α-Carbon of Cyclopentanones. J. Am. Chem. Soc 2014, 136,14903. [DOI] [PubMed] [Google Scholar]; (d) William R; Leng WL; Wang S; Liu X-W The First Intermolecular Interrupted Imino-Nazarov Reaction: Expeditious Access to Carbocyclic Nucleoside Analogues. Chem. Sci 2016, 7,1100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wu Y-K; Lin R; West FG Intercepting the Nazarov Oxyallyl Intermediate with α-Formylvinyl Anion Equivalents to Access Formal Morita-Baylis-Hillman Alkylation Products. Synlett 2017,28,1486. [Google Scholar]; (f) Hu L; Rombola M; Rawal VH Synthesis of 1,2-Oxazinanes via Hydrogen Bond Mediated (3 + 3) Cycloaddition Reactions of Oxyallyl Cations with Nitrones. Org. Lett 2018, 20, 5384. [DOI] [PubMed] [Google Scholar]
- 15.For concept and examples of intercepted retro-Nazarov reactions, see:; (a) Fort AW Evidence for a Delocalized Intermediate in the Favorskii Rearrangement. 2,6-Lutidine-promoted Methanolysis of α-Chlorodibenzyl Ketone. J. Am. Chem. Soc 1962,84, 2620. [Google Scholar]; (b) Harmata M; Huang C; Rooshenas P; Schreiner PR An Interrupted (4+3) Cycloaddition Reaction: A Hydride Shift (Ene Reaction) Intervenes. Angew. Chem., Int. Ed 2008,47,8696. [DOI] [PubMed] [Google Scholar]; (c) Tang Q; Chen X; Tiwari B; Chi YR Addition of Indoles to Oxyallyl Cations for Facile Access to α-Indole Carbonyl Compounds. Org. Lett 2012,14,1922. [DOI] [PubMed] [Google Scholar]; (d) Vander Wal MN; Dilger AK; MacMillan DWC Development of a Generic Activation Mode: Nucleophilic α-Substitution of Ketones via Oxy-allyl Cations. Chem. Sci 2013, 4, 3075. [Google Scholar]; (e) Luo J; Zhou H; Hu J; Wang R; Tang Q Efficient Catalytic-free Method to Produce a-Aryl Cycloalkanones through Highly Chemoselective Coupling of Aryl Compounds with Oxyallyl Cations. RSC Adv 2014, 4, 17370. [Google Scholar]; (f) Dange NS; Stepherson JR; Ayala CE; Fronczek FR; Kartika R Cooperative Benzylic-Oxyallylic Stabilized Cations: Regioselective Construction of α-Quaternary Centers in Ketone-derived Compounds. Chem. Sci 2015, 6, 6312. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Ayala CE; Dange NS; Fronczek FR; Kartika R Brønsted Acid Catalyzed α’-Functionalization of Silylenol Ethers with Indoles. Angew. Chem., Int. Ed 2015, 54, 4641. [DOI] [PubMed] [Google Scholar]; (h) Liu C; Oblak EZ; Vander Wal MN; Dilger AK; Almstead DK; MacMillan DWC Oxy-Allyl Cation Catalysis: An Enantioselective Electrophilic Activation Mode. J. Am. Chem. Soc 2016,138,2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.For an O- to O-1,4-tosyl shift, see:; (a) Zagorevskii VA; Kirsanova ZD Migration of the Tosyl Group in 7-O-Tosylesculetins. Khim. Geterots. Soedin 1970, 6, 309; [Google Scholar]; Chem. Heterocycl. Compd 1970, 6,288. [Google Scholar]; For N- to N-1,3-tosyl shift, see; (b) Mertens MD; Pietsch M; Schnakenburg G; Gütschow M Regioselective Sulfonylation and N- to O-Sulfonyl Migration of Quinazolin-4(3H)-ones and Analogous Thieno-pyrimidin-4(3H)-ones. J. Org. Chem 2013, 78,8966 and reference therein. [DOI] [PubMed] [Google Scholar]; For O- to N-1,4-tosyl shift, see; (c) Andersen KK; Gowda G; Jewell L; McGraw P; Phillips BT Substitution at Tetracoordinate Sulfur(VI), Rearrangement of 2-Aminoaryl Arenesulfonates to N-(2-Hydroxyaryl)arenesul-fonamides. J. Org. Chem 1982, 47,1884. [Google Scholar]
- 17.For asymmetric (3+2)-photocycloadditions using ESIPT, see:; (a) Xia B; Gerard B; Solano DM; Wan J; Jones G; Porco JA Jr. ESIPT-Mediated Photocycloadditions of 3-Hydroxyquinolinones: Development of a Fluorescence Quenching Assay for Reaction Screening. Org. Lett 2011,13, 1346. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wenyu W; Anthony C; Retheesh K; J. LN; E. BL; Jayaraman S; Porco JA Jr. Total Syntheses of the Isomeric Aglain Natural Products Foveoglin A and Perviridisin B: Selective Excited State Intramolecular Proton-Transfer Photocycloaddition. Angew. Chem., Int. Ed 2017,56,14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Retro-Nazarov products are thermodynamically favored; for example, Frontier and co-workers found that Nazarov reactions such as those converting 10 to 13 were disfavored, see:; Malona JA; Cariou K; Spencer WT; Frontier AJ Total Synthesis of (±)-Rocaglamide via Oxidation-Initiated Nazarov Cyclization. J. Org. Chem 2012, 77,1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creary X Carbocationic and Related Processes in Reactions of α-Keto Mesylates and Triflates. Acc. Chem. Res 1985,18, 3. [Google Scholar]
- 20.(a) Wang Y; Ju W; Tian H; Tian W; Gui J Scalable Synthesis of Cyclocitrinol. J. Am. Chem. Soc 2018,140,9413. [DOI] [PubMed] [Google Scholar]; (b) Wang Y; Ju W; Tian H; Sun S; Li X; Tian W; Gui J Facile Access to Bridged Ring Systems via Point-to-Planar Chirality Transfer: Unified Synthesis of Ten Cyclocitrinols. J. Am. Chem. Soc 2019,141,5021. [DOI] [PubMed] [Google Scholar]
- 21.(a) Lee C; Yang W; Parr RG Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988,37, 785. [DOI] [PubMed] [Google Scholar]; (b) Becke AD Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys 1993, 95, 5648. [Google Scholar]
- 22.(a) Sáez JA; Arnó M; Domingo LR Lewis Acid-Catalyzed (4 + 3) Cycloaddition of 2-(Trimethyl Silyloxy)acrolein with Furan. Insight on the Nature of the Mechanism from a DFT Analysis. Org. Lett 2003, 5, 4117. [DOI] [PubMed] [Google Scholar]; (b) Lohse AG; Krenske EH; Antoline JE; Houk KN; Hsung RP Regioselectivities of (4 + 3) Cycloadditions between Furans and Oxazolidinone-Substituted Oxyallyls. Org. Lett 2010, 12, 5506. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Krenske EH; Houk KN; Lohse AG; Antoline JE; Hsung RP Stereoselectivity in Oxyallyl-Furan (4 + 3) Cycloadditions: Control of Intermediate Conformations and Dispersive Stabilization in Cycloadditions Involving Ox-azolidinone Auxiliaries. Chem. Sci 2010, 1, 387. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Antoline JE; Krenske EH; Lohse AG; Houk KN; Hsung RP Stereoselectivities and Regioselectivities of (4 + 3) Cycloadditions Between Allenamide-Derived Chiral Oxazolidinone-Stabilized Oxyallyls and Furans: Experiment and Theory. J. Am. Chem. Soc 2011,133,14443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novac O; Guenier AS; Pelletier J Inhibitors of Protein Synthesis Identified by a High Throughput Multiplexed Translation Screen. Nucleic Acids Res 2004,32, 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(a) Bordwell FG; Drucker GE; Fried HE Acidities of Carbon and Nitrogen Acids: the Aromaticity of the Cyclopentadienyl Anion. J. Org. Chem 1981,46,632. [Google Scholar]; (b) Bordwell FG Equilibrium Acidities in Dimethyl Sulfoxide Solution. Acc. Chem. Res 1988,21, 456. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


