Abstract
Background:
Proprioception of fingers is essential for motor control. Reduced proprioception is common after stroke and is associated with longer hospitalization and reduced quality of life. Neural correlates of proprioception deficits after stroke remain incompletely understood, partly due to weaknesses of clinical proprioception assessments.
Objective:
To examine the neural basis of finger proprioception deficits after stroke. We hypothesized that a model incorporating both neural injury and neural function of the somatosensory system is necessary for delineating proprioception deficits post-stroke.
Methods:
Finger proprioception was measured using a robot in 27 subjects with chronic unilateral stroke, among whom measures of neural injury (damage to gray and white matter, including corticospinal and thalamocortical sensory tracts), neural function (activation of and connectivity of cortical sensorimotor areas), and clinical status (demographics and behavioral measures) were also assessed.
Results:
Impairment in finger proprioception was present contralesionally in 67%, and bilaterally in 56%. Robotic measures of proprioception deficits were more sensitive than standard scales and were specific to proprioception. Multivariable modeling found that contralesional proprioception deficits were best explained (r2=0.63, p=0.0006) by a combination of neural function (connectivity between ipsilesional secondary somatosensory cortex and ipsilesional primary motor cortex) and neural injury (total sensory system injury).
Conclusions:
Impairment of finger proprioception occurs frequently after stroke and is best measured using a quantitative device such as a robot. A model containing a measure of neural function plus a measure of neural injury best explained proprioception performance. These measurements might be useful in the development of novel neurorehabilitation therapies.
Keywords: connectivity, somatosensory, MRI, injury, proprioception, fMRI, clinical studies, imaging
Introduction
Proprioception of the fingers is central to human behavior. Deficits in proprioception are found in 50% or more of subjects with stroke1–3, can be present in both limbs after a unilateral infarct2, and are associated with poorer outcomes1,2,4.
The goal of the current study was to understand key factors underlying inter-subject differences in finger proprioception after stroke, findings that could foster development of novel approaches to neurorehabilitation. Previous approaches that aimed to measure injury of somatosensory areas in isolation have not comprehensively explained proprioception deficits after stroke5. The primary hypothesis, therefore, was that a model incorporating both neural injury and neural function of the somatosensory system would better explain proprioception deficits post-stroke. This is based on increasing evidence that both forms of measurement are needed to most robustly explain variance in behavioral outcomes after stroke6–8. As part of this, a new method for measuring sensory system injury, lesion overlap with the thalamocortical sensory tract, was examined.
A key consideration in this study was the method by which proprioception is measured. Bedside tests of proprioception, such as estimating the angle of joint deflection or indicating the timing of passive joint movement, are subjective and non-standardized2, plus have low sensitivity, high variability2, floor effects9, and low reliability2,9–12. Robotic devices have been shown to better quantify arm sensory impairments following stroke3,13,14 but to date have not been used to measure post-stroke proprioception deficits in finger joints, an afferent data stream critical to human function15. To address the objectives of this study, we developed and employed a novel exoskeletal robotic device that measures finger proprioception with high sensitivity16,17.
Methods
Subject enrollment:
Twenty-seven subjects with chronic stroke participated. Inclusion criteria (see Supplement) in sum were age 18-80, radiologically confirmed unilateral stroke >6 months prior, and arm motor deficit as determined by the Box & Blocks (B&B) test (contralesional B&B score was required to be ≥3 and at least 10% less than the performance by the ipsilesional hand). The B&B test is a measure of arm motor function at the World Health Organization ICF activities limitation level that is scored by counting the number of blocks carried over a partition during a one-minute trial. Subjects were excluded if they had significant cognitive impairment or another diagnosis affecting hand function. Proprioception data collected for a previously published normative study16 using identical methods in 25 healthy age-matched subjects were used as control data.
Standard Protocol Approvals. Registrations, and Consents:
The local ethics committee approved this study, and written informed consent was obtained from each subject prior to participating following procedures established by the University of California Irvine Institutional Review Board and the Declaration of Helsinki.
Proprioception Assessment:
Passive finger position sense was measured using the FINGER (Finger Individuating Grasp Exercise Robot) exoskeleton robotic device (Figure 1 )16,17, which guides index and middle fingers through motion around metacarpophalangeal and proximal interphalangeal joints, allowing for individual finger guidance. The robot slowly (up to 13 degrees/second) moved the index and middle fingers in opposing directions during a series of 12 non-periodic finger-crossing movements, of different distances and angular velocities. The order of these crossings was pseudorandom, so that all participants got the same set. For each finger-crossing movement, subjects were instructed to press a keyboard spacebar when they perceived their index/middle fingers were directly aligned relative to one another. This task lasted two minutes for each hand, with the ipsilesional hand tested first. Error on each finger-crossing movement was defined as the angular distance between the metacarpophalangeal joints when the spacebar was pressed. Cognitive status was confirmed by requiring that each subject could correctly repeat full task instructions to the examiner. Further details are described in Supplement.
Figure 1.
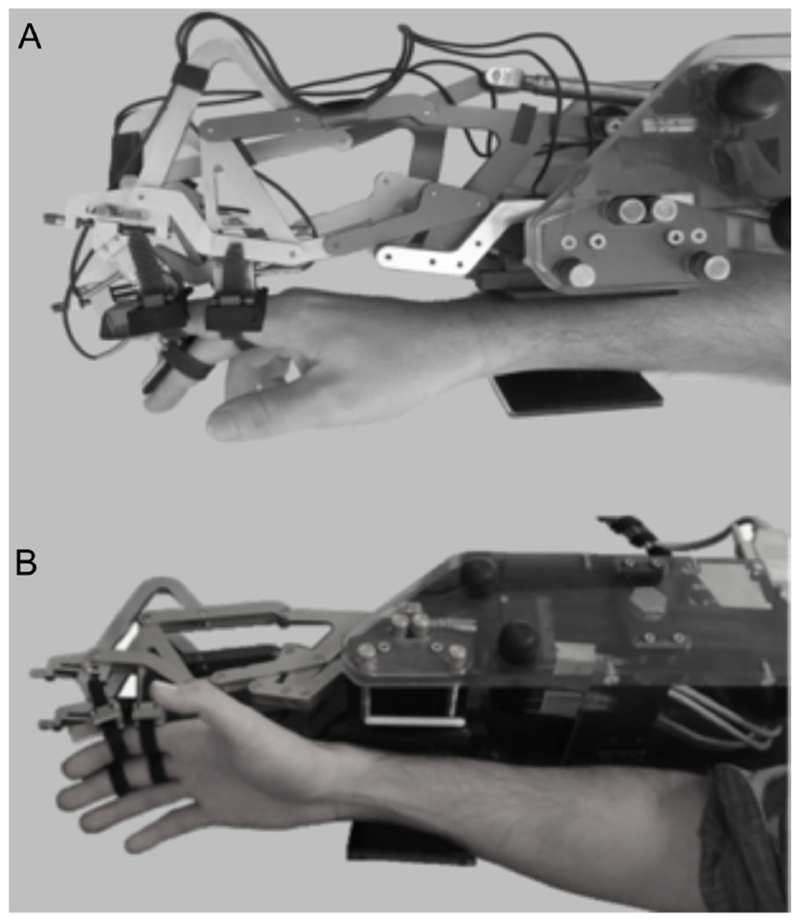
The FINGER robot used to perform the proprioception task, as seen from (A) lateral and (B) oblique views. The index and middle fingers attach to the robot and are guided through finger-crossing movements during the proprioception task. The robot aligns with the plane of the subject’s index and middle fingers, maintaining a naturalistic gap between the fingers and eliminating any somatosensory input that might otherwise be generated upon finger crossing.
Demographics/Medical History:
Medical history was obtained, including handedness, determined using the Edinburgh Handedness Inventory18. Stroke severity was assessed with the NIH Stroke Scale (NIHSS)19.
Sensorimotor Behavior:
Motor status was evaluated with the Action Arm Research Test (ARAT)20, Box & Block Test (B&B)21, Nine Hole Peg Test (NHPT; number of seconds to complete the task, maximum score 60s)22, and Finger Tapping Test (FT; number of finger taps over 10s)23. The motor and sensory Fugl-Meyer (FM) arm assessments were also obtained24,25.
Image Acquisition:
On a 3T Philips MRI, high-resolution T1-weighted images were acquired using a 3-dimensional MP-RAGE sequence (150 slices, 1mm3 voxels). T2 FLAIR images were also acquired. Four runs of blood oxygenation level-dependent (BOLD) functional MRI (fMRI) were acquired using a T2*-weighted gradient-echo sequence (TR=2,000 ms, TE=30 ms, each run with 48 brain volumes=96 s), during which subjects were visually guided to alternate 24 seconds of rest with 24 seconds of active 0.5 Hz index and middle finger flexion/extension movements; subjects had varying degrees of motor control and made their best efforts to achieve these movements during scanning. During the scan, subjects wore a non-actuated plastic exoskeleton similar to the robotic interface used during the proprioception task. An investigator observed movements during the scan to ensure compliance.
Grey Matter Injury:
Using MRIcron26, each participant’s infarct was outlined by hand on the T1-weighted MRI image, informed by FLAIR image, in a standardized, reliable manner, as described previously27. Stroke masks were transformed into Montreal Neurological Institute (MNI) standard stereotaxic space using the Functional Magnetic Resonance Imaging of the Brain Software Library (FSL)28. Stroke masks for participants with right-sided lesions were flipped about the midsagittal plane.
The degree of overlap that each stroke mask had with several cortical regions of interest (ROIs) was calculated. Using Marsbar29, spherical ROIs representing the three focal brain regions of interest were generated: the hand region of the primary motor cortex (M1), the hand region of the primary somatosensory cortex (S1) and the operculum parietal (OP) 4 subregion of the secondary somatosensory cortex (S2)30, in both the ipsilesional (iM1, iS1, and iS2) and the contralesional (cM1, cS1, and cS2) hemispheres. The percentage of stroke mask overlap with each ROI was calculated for each subject and indicates the proportion of voxels in an ROI that overlapped with each stroke mask, expressed as a percent.
White Matter Injury:
For each subject, stroke-related injury to two key white matter tracts was quantified by measuring the extent to which the subject’s infarct overlapped with the normal white matter tract in MNI stereotaxic space. A template of each normal white matter tract was created using DTI tractography in healthy control subjects via methods described previously31. This approach, rather than diffusion tensor imaging (DTI) fiber tracking, was used to measure tract injury because the latter method can be problematic in brain regions affected by stroke. The two tracts of interest (Figure 2) were the corticospinal tract (CST) and the thalamocortical sensory tract (TST).
Figure 2.
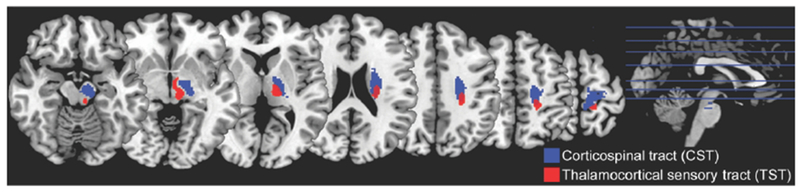
The normal CST and TST tracts, as generated from probabilistic tractography of DTI data from healthy control subjects. Blue tract is CST; red tract, TST. Tracts are overlaid on a T1-weighted MRI template.
Injury to the CST was thus measured as the percentage of overlap between each subject’s infarct and the normal CST, with the measurement covering CST from precentral gyrus to the pontomesencephalic juncture (ventral to which the CST is no longer a contiguous bundle), an approach that has been validated previously8,31–35. As part of the current study, we extended this approach to measuring somatosensory tract injury by defining the TST, measurement of which covered from postcentral gyrus to the ventral posterolateral nucleus of the thalamus. Injury to the TST was calculated as the percentage of overlap between each subject’s infarct and the normal TST (further details in the Supplement).
Total System Injury:
A comprehensive measure of network injury within the stroke-affected hemisphere was calculated as Total System Injury, separately for the sensory system and for the motor system. To quantify total sensory system injury, S1, S2, and TST percentage injury measures were each standardized and values were then averaged for each subject. To quantify total motor system injury, standardized injury measures to M1 and CST were averaged.
Functional activation:
Two measures of regional brain function were extracted from fMRI images: (1) activation volume and (2) peak activation beta (contrast) estimate, each measured in contralesional and in ipsilesional M1, S1, and S2 (Supplement).
Functional connectivity:
Connectivity was assessed as the temporal correlation using an ROI-ROI approach. After the fMRI data were preprocessed in SPM12, intra- and inter-hemispheric functional connectivity metrics were calculated using the CONN toolbox36 (Supplement).
Statistical analysis:
The primary approach to measuring the frequency of proprioception impairment for the contralesional and ipsilesional hand of subjects with stroke used a 2-SD criterion of abnormality based on performance of age-matched healthy controls16; a secondary approach is presented in the Supplement. To evaluate between-group (contralesional, ipsilesional, and control) differences, a mixed-effect model with subject as a random effect and group as a fixed effect was performed, using t-tests in post hoc analyses.
Predictors of contralesional proprioception error were examined, in two stages. First, bivariate screening was performed to identify measures in the 5 main categories that best accounted for inter-subject variability in contralesional proprioception error. For each of the 5 main categories (two reflecting clinical measures, demographics/medical history and sensorimotor behavior; two reflecting measures of neural function, cortical function and cortical connectivity; and one reflecting measures of neural injury, brain injury), results of bivariate screening determined whether any individual assessment survived as a correlate of proprioception error and would be advanced to multivariable modeling. Second, a forward stepwise multivariable linear regression model (0.1 to enter, 0.15 to leave the model) was used to understand inter-subject variability in proprioception, advancing the most significant predictors from each category identified in bivariate screening (as long as bivariate screening showed p<0.1).
Normally distributed data (assessed using the Shapiro-Wilk W test) and data that could be transformed to a normal distribution (square root normalization) were analyzed using parametric statistics, otherwise nonparametric statistics (Wilcoxon signed rank test or Spearman’s rank correlation) were used. Analyses were two-tailed and calculated using JMP software (version 9.0.0, SAS Institute).
Results
Behavioral data from 27 subjects with unilateral chronic stroke were available for analysis (Table 1). All subjects completed testing with the exception of two subjects who could not complete MRI (claustrophobia). Two subjects were excluded from cortical function and connectivity analyses due to excessive head motion during scanning, and five subjects were excluded from white matter injury analyses due to lesion location below the thalamus.
Table 1.
Characteristics of Subjects with Stroke
| Demographics/medical history | ||
| Age, mean yr±SD | 58±14 | |
| Gender, M/F | 19/8 | |
| Hand dominance, R/L/A | 24/3/0 | |
| Diabetes mellitus, yes/no | 6/21 | |
| Hypertension, yes/no | 15/12 | |
| Hypercholesterolemia, yes/no | 15/12 | |
| Geriatric Depression Scale, mean±SD | 4.07±3.73 | |
| Time post-stroke, median mo [IQR] | 30 [9-44] | |
| Stroke type, ischemic/hemorrhagic | 18/9 | |
| Stroke hemisphere, L/R | 13/14 | |
| Stroke in dominant hemisphere, yes/no | 13/14 | |
| NIHSS, normal = 0 | 2.41±2.25 | |
| Sensorimotor Behavior, mean±SD | Contralesional | Ipsilesional |
| ARAT, normal = 57 | 32.74±22.41 | |
| FM Arm Motor, normal = 66 | 45.9±11.7 | |
| FM Arm Sensory, normal = 12 | 10.89±2.42 | 12.00±0.00 |
| B&B score | 22.2±18.0 | 55.9±8.2 |
| NHPT score | 54.6±10.7 | 25.1±7.9 |
| FT score | 14.4±12.3 | 47.2±10.2 |
| Brain injury | ||
| Infarct volume, cm3, mean±SD | 20.6±23.4 | |
| M1 injury, yes/no | 5/20 | |
| M1 % injury, mean±SD | 10.1±21.1 | |
| S1 injury, yes/no | 7/18 | |
| S1 % injury, mean±SD | 17.0±30.8 | |
| S2 injury, yes/no | 9/16 | |
| S2 % injury, mean±SD | 11.6±24.7 | |
| CST injury, yes/no | 18/2 | |
| CST % injury, mean±SD | 34.3±28.7 | |
| TST injury, yes/no | 17/3 | |
| TST % injury, mean±SD | 7.5±6.7 | |
| Total motor system injury | 0.9±0.8 | |
| Total sensory system injury | 0.05±0.7 | |
| Cortical function, mean (SD) | Contralesional | Ipsilesional |
| M1 activation volume, voxels | 24.9±34.1 | 51.4±39.2 |
| S1 activation volume, voxels | 36.0±33.5 | 53.3±39.9 |
| S2 activation volume, voxels | 107.8±89.5 | 132.8±108.5 |
| M1 activation, contrast estimate | 2.0±1.9 | 3.7±2.0 |
| S1 activation, contrast estimate | 2.1±1.1 | 2.8±1.5 |
| S2 activation, contrast estimate | 2.4±1.5 | 2.3±1.7 |
| Cortical connectivity, mean (SD) | ||
| iM1-cM1 correlation coefficient | 0.18±0.21 | |
| iM1-iS1 correlation coefficient | 0.45±0.19 | |
| iM1-iS2 correlation coefficient | 0.09±0.19 | |
| iS1-cS1 correlation coefficient | 0.14±0.20 | |
| iS1-iS2 correlation coefficient | 0.08±0.21 | |
| iS2-cS2 correlation coefficient | 0.27±0.21 | |
A = ambidextrous; ARAT = Action Arm Research Test; B&B = Box & Blocks; c = contralesional; CST = corticospinal tract; F = female; FM = Fugl-Meyer; FT = Finger Tapping; i = ipsilesional; IQR = interquartile range; L = left; M = male; M1 = primary motor cortex; NHPT = Nine Hole Peg Test; NIHSS = NIH stroke scale; R = right; S1 = primary somatosensory cortex; S2 = secondary somatosensory cortex; SD = standard deviation; TST = thalamocortical sensory tract.
Proprioception error:
During proprioception testing, 25/27 subjects with stroke detected all 12 finger-crossing movements. Subjects with stroke made both early and late responses during the task (Figure 2A), with 50.8% of all responses preceding, and 49.2% of errors following, finger crossing. These findings mirror results in a previously reported cohort of age-matched, neurologically intact, healthy control subjects16, among whom 23/25 subjects detected all 12 finger-crossing movements, and 49.0% of responses preceded (and 51.0% followed) finger crossing.
Subjects with stroke had larger proprioception error than controls, bilaterally, and this was more pronounced in the contralesional hand. In the cohort of healthy control subjects16, proprioception error was 7.3±3.8° (mean±SD) for the dominant hand and 6.8±3.0° for the non-dominant hand. There was no significant difference in error between dominant and non-dominant control hands (t(23)=−0.80, p=0.40) and so for subsequent analyses, control error refers to average of control dominant and non-dominant hands. In subjects with stroke, proprioception error was 16.2±6.4° for the contralesional hand and 13.3±5.4° for the ipsilesional hand. There was little floor effect, as only a single subject with stroke scored the maximum error. Within-subject error after stroke was highly reliable, as across the 12 finger-crossing movements in the contralesional hand, the SD was 5.9 degrees. Proprioception error in the contralesional hand was positively related to error in the ipsilesional hand (Figure 3B, r=0.65, p=0.0002). The main effect of group (stroke vs. healthy control) was significant (t(77)=5.94, p<0.0001), as were post hoc pairwise comparisons: contralesional hand errors were larger than ipsilesional hand errors (t(26)=−2.97, p=0.006) and were also larger than control errors (t(50)=6.39, p<0.0001), and ipsilesional hand errors were larger than control errors (t(50)=5.02, p<0.0001).
Figure 3.
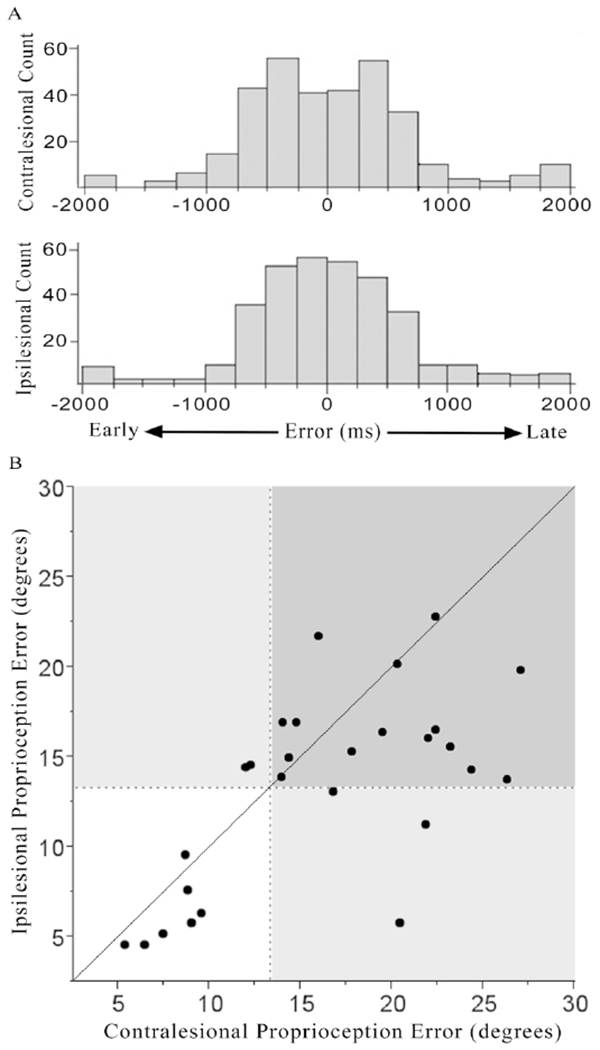
Timing and magnitude of proprioception errors for each hand after stroke. [A] Distribution of error times (difference between when the two fingers moved by the robot actually crossed and when the subject reported them as crossed) is presented for each hand. Subjects with stroke made both early and late responses during the task, with 50.8% of all responses preceding, and 49.2% of errors following, finger crossing. Amount of early versus late responses did not differ according to hand tested (p=0.78). [B] Magnitude of proprioception error (number of actual degrees separating the two fingers when the subject reported them as crossed) is presented for contralesional and ipsilesional hands after stroke. These errors were classified as abnormal when >2SD beyond normative values from a cohort of age-matched, neurologically intact, healthy control subjects16 (dashed lines). Proprioception error also distinguishes patients from healthy controls when examined in a threshold-independent manner (Supplemental Figure 1). The solid diagonal line indicates equal contralesional and ipsilesional hand impairment. Impaired performance for the contralesional hand is indicated to the right of the vertical dashed line, while impaired performance for the ipsilesional hand is indicated above the horizontal dashed line.
Impaired proprioception, defined as >2SD of the abnormality in healthy controls16, was present in the majority of subjects with stroke: 67% had contralesional impairment, 63% had ipsilesional impairment, and 56% had bilateral impairment of proprioception. In comparison, when assessed with the FM sensory scale, only 7/27 (26%) had contralesional arm sensory impairment and 0/27 had ipsilesional arm sensory impairment. The NIHSS sensory subscale showed contralesional sensory deficits in 12/27 (44.4%). Notably, robot-assessed proprioception deficits were specific to sensory function, as contralesional proprioception error correlated with clinical sensory assessments (FM sensory score: r=−0.39, p=0.046; NIHSS sensory subscore: r=0.47, p=0.01) but not with clinical motor assessments (FM motor score: r= −0.26, p=0.19; see Supplement for additional motor details), or other clinical tests (NIHSS language subscore: r=0.12, p=0.55; NIHSS attention subscore r=0.26, p=0.20; Geriatric Depression Scale score: r=0.18, p=0.37).
Correlates of Proprioception Error:
Bivariate screening identified significant correlates of proprioception error in four of the five categories (Table 2). The most significant variables within these categories were NIHSS (demographics/medicalhistory), ARAT (sensorimotor behavior), Total Sensory System Injury (brain injury), and iM1-iS2 functional connectivity (cortical connectivity). No variables were significant on bivariate screening within the cortical function category. Excluding subjects with ≥50% damage to cortical ROIs37 had no significant effect on bivariate correlations.
Table 2.
Bivariate Correlations with Contralesional Proprioception Error
| Variable | Correlation with Contralesional Proprioception | |
|---|---|---|
| r | p | |
| Demographics/Medical History | ||
| Age | 0.38 | 0.048 |
| Gender, M/F | 0.38 | 0.047 |
| Hand dominance, R/L/A | 0.22 | 0.27 |
| Diabetes mellitus, yes/no | 0.04 | 0.84 |
| Hypertension, yes/no | 0.05 | 0.81 |
| Hypercholesterolemia, yes/no | 0.33 | 0.09 |
| Geriatric Depression Scale | 0.17 | 0.37 |
| Time post-stroke | −0.36 | 0.06 |
| Stroke type, ischemic/hemorrhagic | 0.15 | 0.76 |
| Stroke hemisphere, L/R | 0.10 | 0.61 |
| Stroke in dominant hemisphere, Y/N | 0.07 | 0.74 |
| NIHSS score | 0.39 | 0.04 |
| Sensorimotor Behavior | ||
| ARAT | −0.42 | 0.03 |
| FM Arm Motor | −0.26 | 0.19 |
| FM Arm Sensory | −0.39 | 0.046 |
| B&B | −0.27 | 0.17 |
| NHPT | 0.32 | 0.11 |
| FT | −0.13 | 0.49 |
| Brain Injury | ||
| Infarct volume | −0.13 | 0.52 |
| M1 injury, yes/no | 0.18 | 0.39 |
| M1 % injury | 0.19 | 0.36 |
| S1 injury, yes/no | 0.09 | 0.67 |
| S1 % injury | 0.14 | 0.52 |
| S2 injury, yes/no | 0.19 | 0.36 |
| S2 % injury | −0.05 | 0.80 |
| CST injury, yes/no | 0.33 | 0.15 |
| CST % injury | 0.24 | 0.30 |
| TST injury, yes/no | 0.44 | 0.049 |
| TST % injury | 0.37 | 0.10 |
| Total motor system injury | 0.37 | 0.10 |
| Total sensory system injury | 0.63 | 0.003 |
| Cortical Function | ||
| iM1 activation volume | 0.08 | 0.72 |
| cM1 activation volume | 0.13 | 0.57 |
| iS1 activation volume | 0.23 | 0.30 |
| cS1 activation volume | 0.16 | 0.49 |
| iS2 activation volume | −0.03 | 0.88 |
| cS2 activation volume | 0.11 | 0.62 |
| iM1 activation: contrast estimate | 0.04 | 0.83 |
| cM1 activation: contrast estimate | 0.03 | 0.88 |
| iS1 activation: contrast estimate | 0.01 | 0.98 |
| cS1 activation: contrast estimate | 0.16 | 0.47 |
| iS2 activation: contrast estimate | −0.18 | 0.43 |
| cS2 activation: contrast estimate | 0.15 | 0.51 |
| Cortical Connectivity | ||
| iM1-cM1 connectivity | 0.10 | 0.64 |
| iM1-iS1 connectivity | −0.002 | 0.98 |
| iM1-iS2 connectivity | −0.43 | 0.04 |
| iS1-cS1 connectivity | −0.001 | 0.99 |
| iS1-iS2 connectivity | −0.08 | 0.70 |
| iS2-cS2 connectivity | −0.12 | 0.58 |
A = ambidextrous; ARAT = Action Arm Research Test; B&B = Box & Blocks; c = contralesional; CST = corticospinal tract; F = female; FM = Fugl-Meyer; FT = Finger Tapping; i = ipsilesional; L = left; M = male; M1 = primary motor cortex; NHPT = Nine Hole Peg Test; NIHSS = NIH stroke scale; R = right; S1 = primary somatosensory cortex; S2 = secondary somatosensory cortex; TST = thalamocortical sensory tract. Bivariate calculations were performed as screening for multivariable modeling, and so results are presented uncorrected for multiple comparisons. Interpretation of bivariate calculations in each of the 5 main categories with Bonferroni correction for multiple comparisons would use α of 0.0045, for Demographics/Medical History; 0.007, for Sensorimotor Behavior; 0.0038, for Brain Injury; 0.004, for Cortical Function; and 0.008, for Cortical Connectivity. Sensorimotor deficits are presented for the contralesional upper extremity.
Connectivity findings were specific for the OP 4 subdivision of S2. The iM1-iS2 connectivity reported here was calculated using the OP 4 subdivision of S2. OP 4 was selected to be the primary ROI for S2 analyses because it is known to play a role in sensorimotor integration. In contrast, the OP 1 subdivision of S2 is involved in tactile working memory and perceptual learning. OP1 was thus examined as a negative control and indeed connectivity between iM1 and the OP 1 subdivision of iS2 did not correlate with contralesional proprioception error (r=−0.13, p=0.54).
Multivariable Modeling:
When the four strongest correlates that survived bivariate screening were entered into a forward stepwise multivariable linear regression model, two terms survived: Total Sensory System Injury (p=0.002, Figure 4A) and iM1-iS2 functional connectivity (p=0.01, Figure 4B). The resultant multivariable model containing these terms explained 63% of variance in proprioception error for the contralesional hand of subjects with stroke (p=0.0006; Table 3).
Figure 4.
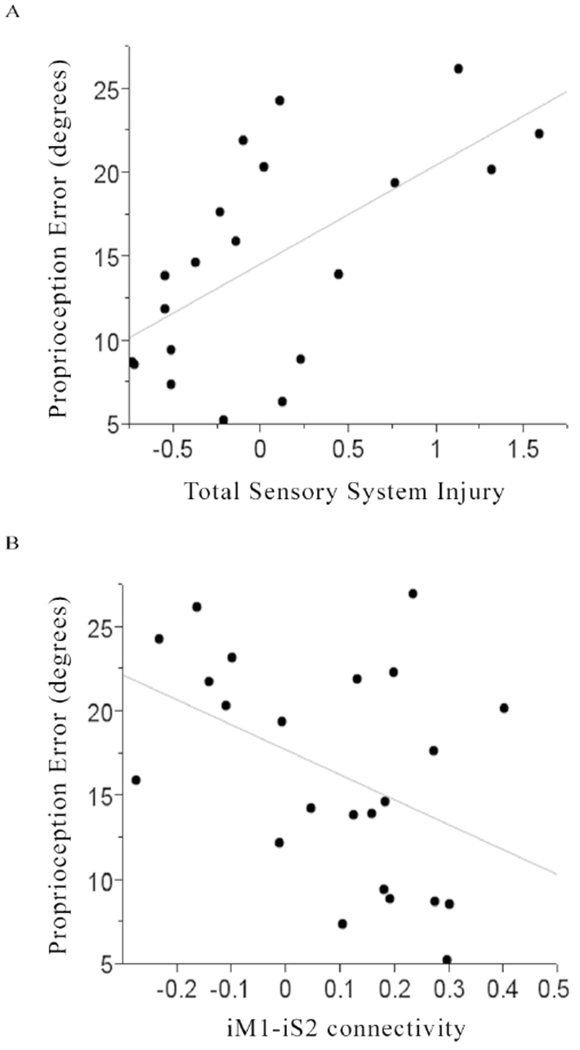
For subjects with stroke, [A] smaller total sensory system injury (r=0.63, p=0.003) and [B] greater ipsilesional M1-ipsilesional S2 functional connectivity (r=−0.43, p=0.04) were each associated with smaller contralesional finger proprioception error.
Table 3.
Multivariable Predictor Model
| Variable | Estimate | Standard Error | 95% CI | p |
|---|---|---|---|---|
| Intercept | 12.63 | 1.68 | <0.0001 | |
| Total sensory system injury | 5.50 | 1.42 | 2.48 – 8.52 | 0.002 |
| iM1-iS2 | −14.47 | 5.22 | −25.60 – −3.35 | 0.01 |
CI = confidence interval; iM1 = ipsilesional primary motor cortex; iS2 = ipsilesional secondary somatosensory cortex
Discussion
Proprioception of the fingers is essential for motor control and human behavior. Reduced proprioception after stroke is also an strong marker of overall stroke impact, being associated with increased length of hospitalization, higher mortality, and diminished quality of life1,2,4. However, the neural correlates of proprioception deficits after stroke remain incompletely understood, in part due to weaknesses of clinical approaches for measuring proprioception. These issues were addressed in the current study, including a new method for measuring injury to the TST in individual subjects (Figure 2). The FINGER robot was used to measure finger proprioception deficits and found these to be present bilaterally in a majority of subjects.
The current study observed that 67% of subjects with stroke have contralesional finger proprioception deficits, a rate that is consistent with prior reports1–3. These robotic measures of proprioception deficits were specific, correlating with scores on sensory but not motor, cognitive, language, or other scales. Historically, assessment of proprioception has been deemed subjective, insensitive, non-standardized, and unreliable2,9–12. Robotic approaches have been advanced to address these concerns, but with limitations. For example, the KINARM robot3,14 requires participants to make detailed movements of the ipsilesional arm so that its position mirrors the static position held by the contralesional arm, an approach that attains robotic precision but requires transcallosal processing of sensory signals plus precise ipsilesional limb sensorimotor function. These confounds were avoided with the current approach, which was found to be valid in relation to two clinical scales of sensory impairment. A robotic assessment such as with the FINGER robot provides a measurement that is both continuous and linear, features that have advantages for behavioral studies38. The current study also observed that 63% of subjects with stroke have finger proprioception deficits ipsilesionally (Figure 3B), possibly reflecting stroke-related disturbances in sensory network function within contralesional and ipsilesional hemispheres39,40, interhemispheric signal transfer6,41, and changes in the interaction between sensory networks and other networks such as those related to attention or cognitive processing42,43.
Proprioception deficits in contralesional fingers varied widely (Figure 3). This variability was best explained by a multivariable model that incorporated measures of neural injury (better proprioception with less severe total sensory system injury) and neural function (better proprioception with greater iM1-iS2 functional connectivity). These results emphasize the importance of incorporating both neural injury and neural function to understand behavioral status in chronic stroke. This combined approach explained far more variance in proprioception error (63%, Table 3) than any single measure did (≤39.7%, Table 2). Including measures of both injury and function to understand behavior is consistent with preclinical44 and human motor studies6,8 and here extends this model for understanding post-stroke behavior to sensory systems.
To date, relatively little is known about the specific association between lesion location and deficits in proprioception45,46. One challenge to understanding this relationship may be that sensory functions such as proprioception arise from a highly distributed network15,47,48, and so a measure of injury to a single sensory system area may provide a lower level of insight as compared to a measure of injury across the sensory network (Table 2). The highly distributed nature of somatosensory cortical networks might also explain why contralesional finger proprioception deficits correlated better with sensory system measures than with motor system measures, although a relationship was apparent for ARAT score and for iM1-iS2 connectivity (Table 2). In the current study an aggregate measure of total sensory system injury was examined, including both white matter and grey matter injury, and this was superior to any single regional sensory system injury measure for explaining proprioception (Table 2, Figure 4A). White matter injury was measured using a new method, lesion overlap with the TST, via a canonical tract generated a priori that aimed to model the sensory component of the superior thalamic radiation45 (Figure 2), injury to which has been linked to post-stroke sensory deficits43,45. Gray matter injury within total sensory system injury was measured as lesion overlap with regions representing hand areas of S1 and S2. Integrity of S1 has been previously shown to have an impact on proprioception46. The S2 region, which responds bilaterally to somatosensory stimuli and has distinct subdivisions30,49,50, has received increased attention as important to understanding somatosensory deficits after brain injury28,51. Results emphasize the value of measuring both gray matter and white matter injury to best understand stroke effects on a widely distributed system.
The measure of neural function that best explained proprioception error was functional connectivity, which reflects strength of temporal synchrony of blood oxygen level-dependent signals between spatially remote brain regions, and which has increasingly been used to investigate behavioral status post-stroke, though to date this has not been examined with respect to proprioception. In the current study, connectivity between iM1-iS2 was identified as a key correlate of proprioception error, such that stronger iM1-iS2 connectivity was associated with better proprioception (Figure 4B). Notably, this connectivity metric was significant only when the OP 4 subdivision of S2 was evaluated. This is likely related to the fact that OP 4 has strong anatomical and functional connections with M1 and with S1, and plays a key role in sensorimotor integration50. In comparison, the OP 1 subdivision of S2 has strong connections with anterior inferior parietal cortex, is responsible for complex functions such as tactile working memory and perceptual learning52, and connectivity between iM1 and OP 1 subdivision of S2 did not survive bivariate screening (p=0.54). It is also important to note that connectivity measures were determined using scans that involved active finger movement. On the one hand, sensory and motor components of finger movement are highly intertwined and overlapping, such that the fMRI pattern of brain activation during a passive finger task is similar to what is observed during active finger movement after stroke, with differences mainly related to intensity53,54. On the other hand, current connectivity results in relation to proprioception error must be interpreted with the specific fMRI connectivity probe in mind.
Strengths of the current study include use of a sensitive and quantitative robotic assessment of proprioception, as well as examination of multiple classes of explanatory variables. A population with a wide range of sensorimotor deficits was evaluated, increasing the likelihood that results generalize. The study is limited by incomplete testing in some subjects, e.g., due to claustrophobia. The current measure of finger proprioception deficits might be affected by motor deficits or by slowed reaction time given that a motor response was required to indicate finger crossing, and thus if the motor response were impaired, this may have caused additional error above that attributable to finger proprioception deficits. Several points argue that this additional source of error would likely be small: (1) participants performed a very simple binary task -- pressing the spacebar key -- and they did this across a very small range of motion, using the less impaired (non-stroke) hand. (2) The robot moved the fingers slowly, making the task more of a predictive than a reactive task. Thus, even if reaction time were slowed for the less impaired hand, it would likely not have a large influence because participants had time to anticipate when the crossing would occur. (3) Proprioception deficits had a weak or non-significant relationship with contralesional motor deficits (Table 2), suggesting that any stroke effects on the ipsilesional motor response used to measure proprioception was limited. (4) That proprioception deficits were best explained by measures related to connectivity in, and anatomical damage to, the somatosensory system (Table 3) further supports the contention that current measures reflected proprioception and not slowed reaction time or other potential reflections of motor impairment in the key-pressing hand.
Together, results indicate that impairment of finger proprioception is common after stroke, bilaterally, and is best modeled by measures of neural injury and neural function involving the brain sensory system. The measurements identified in this study may be useful for the design of novel neurorehabilitation strategies.
Supplementary Material
Acknowledgments
Funding
The work described was supported by R01-HD062744, K24-HD074722, and UL1-TR001414.
Footnotes
Declaration of Conflicting Interests
Dr. Reinkensmeyer is a co-founder of Flint Rehabilitation Devices, has received payment for consulting, and holds equity in Hocoma. Both companies are manufacturers of rehabilitation technology. The terms of these interests have been reviewed by the UC Irvine Conflict of Interest committee. Dr. Rowe is employed by Flint Rehabilitation Devices. Dr. Cramer has consulted for Abbvie, Constant Pharmaceutical, Dart Neuroscience, MicroTransponder, Neurolutions, Regenera, SanBio, and TRCare.
References
- 1.Carey LM, Oke LE, Matyas TA. Impaired touch discrimination after stroke: A quantitative test. J Neurol Rehabil. 1997;11(4):219–232. [Google Scholar]
- 2.Connell LA, Lincoln NB, Radford KA. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clinical rehabilitation. 2008;22(8):758–767. [DOI] [PubMed] [Google Scholar]
- 3.Dukelow S, Herter T, Moore K, et al. Quantitative assessment of limb position sense following stroke. Neurorehabil Neural Repair. 2010;24:178–187. [DOI] [PubMed] [Google Scholar]
- 4.Kessner SS, Bingel U, Thomalla G. Somatosensory deficits after stroke: a scoping review. Top Stroke Rehabil. 2016;23(2):136–146. [DOI] [PubMed] [Google Scholar]
- 5.Hughes CML, Tommasino P, Budhota A, Campolo D. Upper extremity proprioception in healthy aging and stroke populations, and the effects of therapist- and robot-based rehabilitation therapies on proprioceptive function. Front Hum Neurosci. 2015;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130(Pt 1):170–180. [DOI] [PubMed] [Google Scholar]
- 7.Nouri S, Cramer SC. Anatomy and physiology predict response to motor cortex stimulation after stroke. Neurology. 2011;77(11):1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke Quinlan E, Dodakian L, See J, et al. Neural function, injury, and stroke subtype predict treatment gains after stroke. Ann Neurol. 2015;77(1):132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jerosch-Herold C Assessment of sensibility after nerve injury and repair: A systematic review of evidence for validity, reliability and responsiveness of tests. J Hand Surg-Brit Eur. 2005;30B(3):252–264. [DOI] [PubMed] [Google Scholar]
- 10.Carey L Somatosensory Loss after Stroke. Crit Rev Phys Rehabil Med. 1995;7:51–91. [Google Scholar]
- 11.Carey LM, Oke LE, Matyas TA. Impaired limb position sense after stroke: a quantitative test for clinical use. Arch Phys Med Rehabil. 1996;77(12):1271–1278. [DOI] [PubMed] [Google Scholar]
- 12.Garraway WM, Akhtar AJ, Gore SM, Prescott RJ, Smith RG. Observer variation in the clinical assessment of stroke. Age and ageing. 1976;5(4):233–240. [DOI] [PubMed] [Google Scholar]
- 13.Gilliaux M, Lejeune T, Detrembleur C, Sapin J, Dehez B, Stoquart G. A robotic device as a sensitive quantitative tool to assess upper limb impairments in stroke patients: A preliminary prospective cohort study. Journal of Rehabilitation Medicine. 2012;44(3):210–217. [DOI] [PubMed] [Google Scholar]
- 14.Semrau JA, Herter TM, Scott SH, Dukelow SP. Robotic identification of kinesthetic deficits after stroke. Stroke. 2013;44(12):3414–3421. [DOI] [PubMed] [Google Scholar]
- 15.Mountcastle V General Features of Somatic Afferent Systems. The Sensory Hand: Neural Mechanisms of Somatic Sensation. Boston, MA: Harvard University Press; 2005:51–68. [Google Scholar]
- 16.Ingemanson ML, Rowe JB, Chan V, Wolbrecht ET, Cramer SC, Reinkensmeyer DJ. Use of a robotic device to measure age-related decline in finger proprioception. Exp Brain Res. 2016;234(1):83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taheri H, Rowe JB, Gardner D, et al. Design and preliminary evaluation of the FINGER rehabilitation robot: controlling challenge and quantifying finger individuation during musical computer game play. JNeuroeng Rehabil. 2014;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oldfield R The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 19.Brott T, Adams H, Olinger C, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. [DOI] [PubMed] [Google Scholar]
- 20.Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the Action Research Arm Test. Neurorehabil Neural Repair. 2008;22(1):78–90. [DOI] [PubMed] [Google Scholar]
- 21.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39(6):386–391. [DOI] [PubMed] [Google Scholar]
- 22.Wade DT. Measuring arm impairment and disability after stroke. Int Disabil Stud. 1989;11(2):89–92. [DOI] [PubMed] [Google Scholar]
- 23.Shimoyama I, Ninchoji T, Uemura K. The finger-tapping test. Arch Neurol. 1990;47:681–684. [DOI] [PubMed] [Google Scholar]
- 24.Fugl-Meyer A, Jaasko L, Leyman I, Olsson S, S S. The post-stroke hemiplegic patient: a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 25.See J, Dodakian L, Chou C, et al. A standardized approach to the Fugl-Meyer assessment and its implications for clinical trials. Neurorehab Neural Re. 2013;27(8):732–741. [DOI] [PubMed] [Google Scholar]
- 26. http://www.mccauslandcenter.sc.edu/mricro/mricron/index.html.
- 27.Burke E, Dodakian L, See J, et al. A multimodal approach to understanding motor impairment and disability after stroke. J Neurol. 2014;261(6):1178–1186. [DOI] [PubMed] [Google Scholar]
- 28. http://www.fmrib.ox.ac.uk/fsl/tbss/index.html.
- 29.Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. Paper presented at: 8th International Conferance on Functional Mapping of the Human Brain, June 2-6, 20022002; Sendai, Japan. [Google Scholar]
- 30.Eickhoff SB, Schleicher A, Zilles K, Amunts K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex. 2006;16(2):254–267. [DOI] [PubMed] [Google Scholar]
- 31.Riley JD, Le V, Der-Yeghiaian L, et al. Anatomy of stroke injury predicts gains from therapy. Stroke. 2011;42(2):421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nouri S, Cramer SC. Anatomy and physiology predict response to motor cortex stimulation after stroke. Neurology. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dodakian L, Sharp KG, See J, et al. Targeted engagement of a dorsal premotor circuit in the treatment of post-stroke paresis. NeuroRehabilitation. 2013;33(1):13–24. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, Quinlan EB, Dodakian L, et al. Connectivity measures are robust biomarkers of cortical function and plasticity after stroke. Brain. 2015;138(Pt 8):2359–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassidy JM, Tran G, Quinlan EB, Cramer SC. Neuroimaging identifies patients most likely to respond to a restorative stroke therapy. Stroke. 2018;49(2):433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitfield-Gabrieli S, Nieto-Castanon A. CONN: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. [DOI] [PubMed] [Google Scholar]
- 37.Carter AR, Patel KR, Astafiev SV, et al. Upstream dysfunction of somatomotor functional connectivity after corticospinal damage in stroke. Neurorehab Neural Re. 2012;26(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luce R, Narens L. Measurement scales on the continuum. Science. 1987;236:1527–1532. [DOI] [PubMed] [Google Scholar]
- 39.Carey L, Abbott D, Puce A, Jackson G, Syngeniotis A, Donnan G. Reemergence of activation with poststroke somatosensory recovery: a serial fMRI case study. Neurology. 2002;59(5):749–752. [DOI] [PubMed] [Google Scholar]
- 40.Dechaumont-Palacin S, Marque P, De Boissezon X, et al. Neural correlates of proprioceptive integration in the contralesional hemisphere of very impaired patients shortly after a subcortical stroke: an FMRI study. Neurorehabil Neural Repair. 2008;22(2):154–165. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu T, Hosaki A, Hino T, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125(Pt 8):1896–1907. [DOI] [PubMed] [Google Scholar]
- 42.Bannister LC, Crewther SG, Gavrilescu M, Carey LM. Improvement in touch sensation after stroke is associated with resting functional connectivity changes. Front Neurol. 2015;6:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borstad A, Schmalbrock P, Choi S, Nichols-Larsen DS. Neural correlates supporting sensory discrimination after left hemisphere stroke. Brain research. 2012;1460:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1):S225–239. [DOI] [PubMed] [Google Scholar]
- 45.Meyer S, Kessner SS, Cheng B, et al. Voxel-based lesion-symptom mapping of stroke lesions underlying somatosensory deficits. Neuroimage Clin. 2016;10:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kenzie JM, Semrau JA, Findlater SE, et al. Anatomical correlates of proprioceptive impairments following acute stroke: A case series. J Neurol Sci. 2014;342(1-2):52–61. [DOI] [PubMed] [Google Scholar]
- 47.Mauguiere F, Merlet I, Forss N, et al. Activation of a distributed somatosensory cortical network in the human brain. A dipole modelling study of magnetic fields evoked by median nerve stimulation. Part I: Location and activation timing of SEF sources. Electroencephalogr Clin Neurophysiol. 1997;104(4):281–289. [DOI] [PubMed] [Google Scholar]
- 48.Omrani M, Murnaghan CD, Pruszynski JA, Scott SH. Distributed task-specific processing of somatosensory feedback for voluntary motor control. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex. 2006;16(2):268–279. [DOI] [PubMed] [Google Scholar]
- 50.Eickhoff SB, Jbabdi S, Caspers S, et al. Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci. 2010;30(18):6409–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nhan H, Barquist K, Bell K, Esselman P, Odderson I, Cramer S. Brain function early after stroke in relation to subsequent recovery. J Cereb Blood Flow Metab. 2004;24(7):756–763. [DOI] [PubMed] [Google Scholar]
- 52.Pleger B, Foerster AF, Ragert P, et al. Functional imaging of perceptual learning in human primary and secondary somatosensory cortex. Neuron. 2003;40(3):643–653. [DOI] [PubMed] [Google Scholar]
- 53.Tombari D, Loubinoux I, Pariente J, et al. A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. Neuroimage. 2004;23(3):827–839. [DOI] [PubMed] [Google Scholar]
- 54.Calautti C, Baron J. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003;34(6):1553–1566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


