Abstract
Early studies of T cell structural biology using X-ray crystallography, surface plasmon resonance (SPR) and isothermal titration calorimetry (ITC) focused on a picture of the αβT cell receptor (αβTCR) component domains and their cognate ligands (peptides bound to MHC molecules, i.e. pMHCs) as static interaction partners. Moving forward requires integrating this corpus of data with dynamic technologies such as NMR, molecular dynamics (MD) simulations and real-time single molecule (SM) studies exemplified by optical tweezers (OT). NMR bridges relevant timescales and provides the potential for an all-atom dynamic description of αβTCR components prior to and during interactions with binding partners. SM techniques have opened up vistas in understanding the non-equilibrium nature of T cell signaling through the introduction of force-mediated binding measurements into the paradigm for T cell function. In this regard, bioforces consequent to T-lineage cell motility are now perceived as placing piconewton (pN)-level loads on single receptor-pMHC bonds to impact structural change and αβT-lineage biology, including peptide discrimination, cellular activation, and developmental progression. We discuss herein essential NMR technologies in illuminating the role of ligand binding in the preT cell receptor (preTCR), the αβTCR developmental precursor, and convergence of NMR, SM and MD data in advancing our comprehension of T cell development. More broadly we review the central hypothesis that the αβTCR is a mechanosensor, fostered by breakthrough NMR-based structural insights. Collectively, elucidating dynamic aspects through the integrative use of NMR, SM, and MD shall advance fundamental appreciation of the mechanism of T cell signaling as well as inform translational efforts in αβTCR and chimeric T cell (CAR-T) immunotherapies and T cell vaccinology.
Keywords: Integrative structural biology, Nuclear magnetic resonance spectroscopy (NMR), Optical tweezers, Single molecule, molecular dynamics (MD), T cell receptor (TCR), preT cell receptor (preTCR)
Introduction-T cell Mediated Immunity
αβT lymphocytes form a critical component of the cell-mediated immune system in the mammalian host to protect against viral pathogens or cancerous transformations (Rudolph et al. 2006; Wang and Reinherz 2012). The recognition of cells expressing anomalous gene products is mediated by the αβT cell antigen receptor (αβ TCR) on T lymphocytes. The αβTCR detects “foreign” (non-self) peptides displayed on the surface of altered cells bound to host major histocompatibility molecules (MHC) as a foreign peptide-self MHC complex (pMHC). The αβTCR is composed of the heterodimeric ligand-recognizing α and β subunits in complex with the CD3 dimers (εδ, εγ, and ζζ), creating the eight subunit surface receptor (Fig. 1a). The αβ heterodimer is responsible for ligand binding but lacks signaling elements, while conversely, the CD3 subunits lack ligand recognition elements but comprise the entire signaling capacity of the TCR (Fig. 1a). Recognition occurs through the combined α and β complementarity determining region (CDR) loops within each variable (V) immunoglobulin-like domain of which CDR1 and 2 are germline encoded loops and the CDR3 is a hypervariable product of gene segment recombination events [Fig. 1b, (Davis and Bjorkman 1988)]. Each of the eight αβTCR subunits is positioned on the T cell membrane by a single spanning transmembrane (TM) region (Fig. 1a). The preT cell receptor (preTCR), the developmental precursor to the αβTCR, utilizes CDR loops of the Vβ subunit in combination with an exposed Vβ patch region (Fig. 1b) (Zhou et al. 2011; Mallis et al. 2015; Mallis et al. 2018). TCR recognition takes place at the T cell-antigen presenting cell (APC) interface in conjunction with either surface CD4 or CD8 co-receptors and other stimulatory molecules, with TCR-pMHC contact activating the T cell via an orchestrated cascade of intracellular signals starting with phosphorylation of CD3 intracellular tyrosine activation motifs (ITAMs), and culminating in transcriptional activation and subsequent biological functions (Fig. 1c). Depending on current cellular environment, developmental state, prior activation, ligand density and identity, this activation will lead to a variety of T cell responses such as cell proliferation and cytotoxic activity, soluble mediator production, differentiation including formation of memory cells, or apoptosis.
Fig. 1.
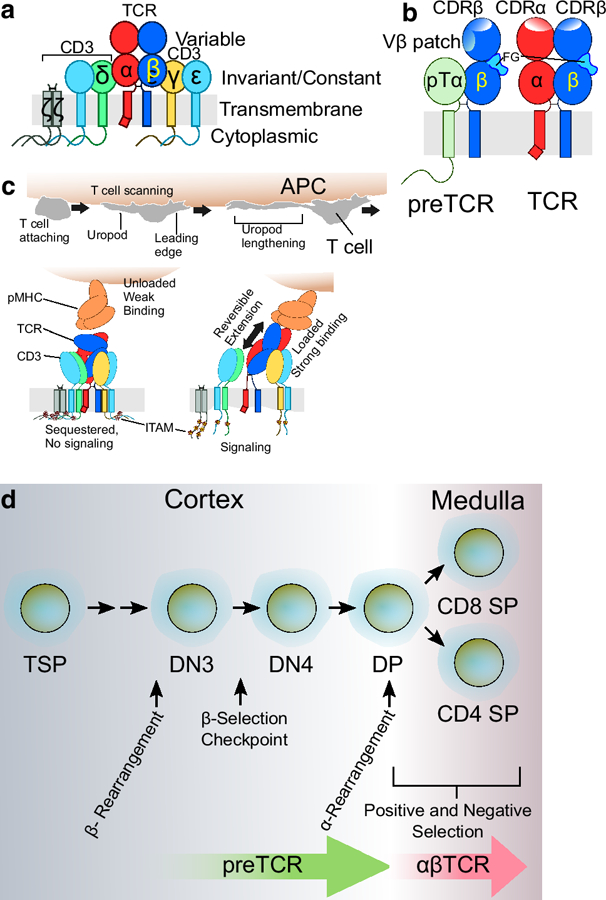
αβTCR and preTCR structure and function. a. The αβTCR is a multimeric cell surface receptor composed of eight membrane-spanning subunits. The TCRα and β, which comprise the ligand-recognition portions of the receptor, are associated as a disulfide-linked heterodimer, with variable regions as well as constant and TM domains but lacking signaling elements. Invariant CD3εγ and εδ subunits without pMHC ligand-binding capacity contain extracellular Ig-like domains which mediate subunit pairing, TM domains, and intracellular domains responsible for signaling. CD3ζζ exists as a disulfide-bonded homodimer with TM and intracellular signaling domains, but possesses no extracellular domain. b. The preTCR incorporates pTα instead of TCRα prior to the DN to DP transition (see d. below) but the receptor complexes are otherwise identical with respect to CD3 components (not shown). The Cβ FG loop is represented in light blue within each receptor. The preTCR and αβTCR recognize pMHC ligand using overlapping but distinct surfaces. Note the Vβ patch region important for preTCR-pMHC interaction is occluded by the presence of the TCRα subunit in the TCR. The surface exposed CDR1–3 region of each ligand binding subunit is shown as denoted by CDRα and CDRβ labels. Additionally, the intracellular C-terminal tail of pTα that may possess signaling capacity is depicted. c. T cell function is governed by its mobility. The T cell attaches to an APC which presents target pMHC. As the T cell scans the APC (top panel), its surface receptors are exposed to a wide range of forces which engender force-dependent changes (bottom panels) within the TCR ectodomains, TMs, and intracellular ITAM signaling motifs as well as, potentially, proximal membrane lipid environment. For simplicity, CD4 and CD8 co-receptors and CD28 and CD80/86 costimulatory interactions are not shown. d. Developmental progression within the thymus. Thymocyte development is a process orchestrated by spatial migration to and within the thymus, somatic genetic rearrangements, pMHC ligand-mediated β-selection by the preTCR and positive and negative selection by the αβTCR. Abbreviations used for thymocyte subpopulations: Thymus specific progenitor, TSP; CD4 and CD8 Double Negative (DN) Stage 3 and 4, DN3 and DN4 with preceding stages DN1 and DN2 omitted for clarity; CD4 and CD8 Double positive, DP; CD4 or CD8 Single positive, CD4 SP or CD8 SP.
Integrative Structural Biology of the preTCR
In order to mount an effective immune response, a diverse T cell repertoire is generated via a series of ordered stochastic rearrangements of TCRβ and TCRα gene segments to ensure a variable ligand binding surface on millions of individual T cells bearing distinct αβTCRs. Each T lymphocyte expresses ~20,000–40,000 identical, clonally derived αβTCRs specific to that cell. This randomization of receptors ensures that a broad array of potential ligands will be recognized by T cells within the mature αβT cell compartment. Prior to maturation of αβT cells in the thymus, the organ where T lymphocytes are generated, thymocytes undergo a series of selection steps, removing unwanted TCR reactivities to prevent autoimmunity on the one hand, while retaining useful MHC-compatible reactivities on the other hand, so called negative and positive selection processes, respectively (Fig. 1d). Immediately following rearrangement of the β gene, but prior to rearrangement of the α gene, thymocytes express the preTCR, a heterodimer composed of an invariant preTCR α (pTα) subunit paired with a unique β subunit (Fig. 1b,1d). Given the absence of a V domain, pTα possesses no ligand-binding capacity, in contrast to its disulfide linked β with ligand-binding Vβ domain shared with that in the mature αβTCR, albeit in a configuration lacking the mature Vα recognition surface (Fig. 1b). Numerous studies had purported that the preTCR functioned in a ligand-independent manner, based on successful thymocyte development of ectodomain deleted constructs (Irving et al. 1998) and in MHC-deleted host systems (Koller et al. 1990; Crump et al. 1993). However, our structural biological studies have shown prominent roles for ligand recognition in preTCR function including enhanced thymocyte proliferation, developmental progression and repertoire modulation (Mallis et al. 2015; Das et al. 2016).
Herein we will discuss the impact of solution NMR in coordination with an array of structural and biophysical techniques including X-ray crystallography and the SM biomembrane force probe (BFP) and OT technologies (Fig. 2). Two aspects of T cell biology will be addressed. First, we will delve into recent insights in thymic development wherein the preTCR mediates progression from immature thymic precursors to committed αβTCR expressing thymocytes prior to exportation to the peripheral lymphoid tissue as mature T cells. Secondly, we will explore how an iterative cycle of structural, biophysical and biological technologies are illuminating the inner workings of the T cell receptor complex to define the earliest events in T cell signaling. Lastly, we will contextualize some recent findings and suggest avenues of research that appear promising in resolving longstanding mechanistic questions concerning T cell activation.
Fig. 2.

Integrative structural biology utilizes an iterative cycle of technologies.
Our initial observations suggested that the preTCR could retain significant ligand binding function (Wang et al. 2008) based on the retention of overall fold within the β chain of the mature TCR in the absence of α (Bentley et al. 1995; Sundberg et al. 2002; Li et al. 2005; Zhou et al. 2011) and confirmed in a crystal structure of a human preTCR (Pang et al. 2010). The N15β subunit crystal structure (Zhou et al. 2011) retained its overall fold as well as CDR loop conformation when compared with the structure of N15αβ (Wang et al. 1998). β chains from three distinct TCRs, N15 and N30, which bind to the VSV8/H-2Kb pMHC ligand (Imarai et al. 1995), and D10, which binds to Conalbumin (CA)/IAk (Reinherz et al. 1999), exhibit well dispersed 1H-15N HSQC spectra (Mallis et al. 2016). The V domain resonances are characteristically distinct, reflective of the variable sequence afforded through recombination events during thymocyte development, while the C domains share most resonances with the exception of those proximal to the V domain (Mallis et al. 2016). Pointedly, the V domain resonances of the D10β chain are quite similar within the VβCβ construct as compared to the VαVβ single-chain construct (scD10) previously determined by NMR (Hare et al. 1999), with the exception of those proximal to the Vα or Cβ interface (Mallis et al. 2016). The scD10 solution structure (Hare et al. 1999) was itself found similar to scD10 in complex with its ligand CA/IAk, as determined by X-ray crystallography (Reinherz et al. 1999). All β domain characterizations provide evidence suggesting a fold capable of ligand binding for the unpaired Vβ. Notably, the recent publication of resonance assignments for human and murine TCRαβs show a general concordance of β chain residue assignments with our observations (He et al. 2015; Natarajan et al. 2017; Rangarajan et al. 2018). Highlighted here is the power of one of the most basic of all NMR experiments, the 1H-15N HSQC, for assessing the fold of proteins, assuring consistency of conformation despite construct-specific differences. Standard 3D backbone assignment experiments can then allow sequence-specific evaluation of residue-resolution conformation.
Despite strong functional evidence for the specificity of N15αβ for VSV8/H-2Kb and a crystal structure of the complex (Ghendler et al. 1998; Teng et al. 1998) the affinity was determined too weak to be measured precisely by SPR, suggesting that for N15β alone, measuring this interaction would require a very sensitive assay. Within this apparent weakness of affinity lies a strength of NMR spectroscopy, as we readily detected and measured a specific interaction between N15β and VSV8/H-2Kb with a KD of 400μM via 1H-15N TROSY-HSQC titration experiments (Mallis et al. 2015). Chemical shift perturbation (CSP) analysis together with cross-saturation transfer (CST) experiments (Takahashi et al. 2000) outlined a novel interaction surface, combining canonical CDR interaction surfaces with a Vβ patch region normally occluded by association with Vα in the mature TCR but exposed in the preTCR (Fig. 1b) (Mallis et al. 2015). The use of CST was critical for confirming that the changes resulted from a direct, specific interaction between preTCR and pMHC and not secondary conformational changes transmitted from the CDR region to the Vβ patch region, for instance, or an artifact caused by self-association in a non-specific manner. We were further able to establish specificity of the interaction by demonstrating site-specific abrogation of chemical shift changes and peak intensity losses from N15β point mutations within the CDR2 when N15β was incubated with VSV8/Kb (Mallis et al. 2015). CST was also necessary to define a specific surface for the N30β-VSV8/H-2Kb interaction, which was much weaker than that for N15β as measured by CSP and intensity loss parameters. We later showed a binding surface on the NMR-labeled pMHC consistent with the canonical TCR binding surface and thus were able to produce preliminary models of the preTCR-pMHC interaction through rigid body modeling approaches (Mallis et al. 2018). Most apparent was that in order for the Vβ patch region to be utilized in addition to the CDR region, there had to be a significant difference in domain orientation relative to that seen in canonical TCR-pMHC interactions (Fig. 3). This suggests potential structural mechanisms for functional biological differences between preTCR and TCR.
Fig. 3.
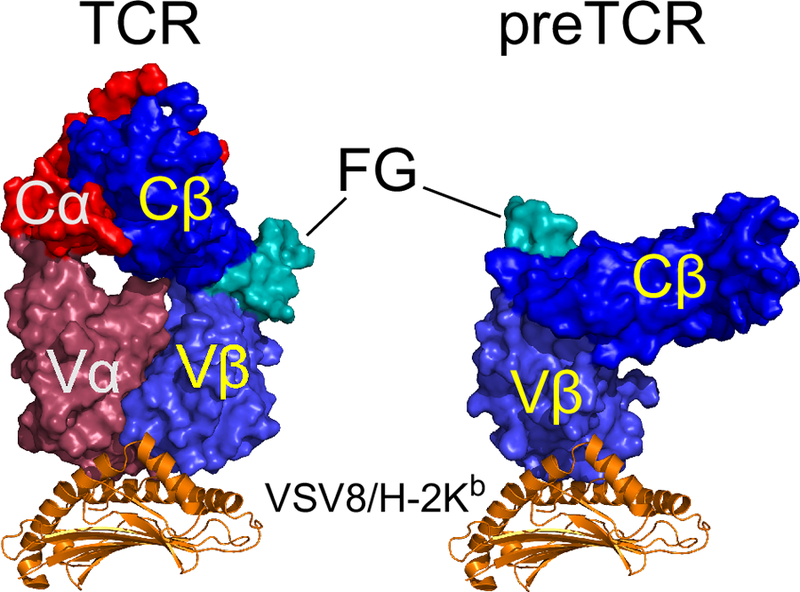
Comparison of docking geometries for αβTCR and preTCR with pMHC. N15αβ utilizes the CDR loops within Vα and Vβ while preTCR utilizes Vβ CDR loops and Vβ patch (see Fig. 1b) resulting in different orientation of the ectodomains relative to pMHC ligand. The Cβ FG loop is highlighted (FG). Structure of N15αβ-VSV8/H-2Kb is from Teng et al. (1998) with distal α3 and β2m domains hidden for clarity. Model of preTCR-pMHC is from Mallis et al. (2018).
Once ligand binding was established at the molecular level, we wanted to extend our observations to the preTCR on the cell surface in a lipid arrayed physiological environment associated with the CD3 components (Fig. 1a). One way to confirm ligand binding specificity in a cellular context is to utilize 2D affinity measurements via biomembrane force probe (BFP) technology (Liu et al. 2014; Ju et al. 2017). BFP can measure ligand binding kinetics of intact receptor complexes on cell surfaces at a single molecule level and has been used to characterize binding affinities and specificities of TCR for pMHC (Liu et al. 2014). In contrast to many structural biology techniques, BFP does not require isolation of target receptor complexes from the expression host, and in fact, the expression host may be identical to the physiological context of the molecule in question. Details of BFP theory and practice have been reviewed previously (Ju et al. 2017). In order to study the ligand binding properties of preTCR N15, i.e. pTα/N15β in the context of all relevant CD3 components, we utilized the SCID.adh cell line. This line, which expresses a native pTα but no β chain, was established previously as a model system for preTCR function in developing thymocytes (Carleton et al. 1999). The pMHC ligand is oriented on the BFP probe, a pipette-immobilized red blood cell (RBC) which acts as force transducer and regulator, via a C-terminal biotin which binds to RBC surface streptavidin. Following transduction of N15β into the SCID.adh cells and subsequent confirmation of preTCR complex on the surface, we interrogated these cells with a set of pMHC posited to be specific (VSV8/H-2Kb) and unrelated ligands (OVA/H-2Kb, Hb/IEk, gp66/IAb). Consistent with the NMR results, BFP found evidence for the preTCR N15-VSV8/H-2Kb interaction (Mallis et al. 2015). Loss of 2D affinity with mutagenesis of β and when thymocytes were presented with non-canonical pMHC ligands shows preTCR-pMHC specificity, but with less ligand discrimination than seen with the TCR (Mallis et al. 2015). Moreover, in keeping with previous and concurrent work on TCR-pMHC (Liu et al. 2014; Das et al. 2015), we observed first by BFP (Mallis et al. 2015) and then by SM OT assays [(Das et al. 2016), (Fig. 4a,c)], that the preTCR N15 exhibited an increase in bond lifetime with increasing force, up to ~10–12 pN, a phenomenon termed a ‘catch bond’ (Marshall et al. 2003), followed by decreasing lifetime with force, the more conventional ‘slip bond’ (Mallis et al. 2015). Catch bond formation in SM OT experiments utilizing isolated protein domains reveals that the TCR/preTCR ectodomains per se possess the essential elements for the catch bond. This binding observation is transformational, as it suggested that contrary to prior supposition, the preTCR is not only capable of pMHC binding, but this ligand interaction is regulated in a physical load-dependent manner.
Fig. 4.

OT-based technologies reveal force-dependent binding properties of TCR and preTCR. a. Schematic of OT SM assay. TCRαβ-leucine zipper (LZ) is captured by a monoclonal antibody (mAb) specific for paired LZ (2H11) which is covalently attached to a polystyrene bead via digoxygenin-modified DNA linker/anti-digoxygenin mAb. C-terminally biotinylated pMHC (b-pMHC) is immobilized on a movable piezo-stage surface via streptavidin bound to biotin-polyethylene glycol (PEG). Binding is monitored by displacement of bead (ΔX) from center of OT which accompanies piezo stage movement. b. In OT SMSC assay, cell surface TCR is interrogated by pMHC linked via DNA spacer to bead in OT. c. N15αβ (left panel) and preTCR N15 (right panel) SM-measured binding to VSV8/H-2Kb. N15αβ exhibits a single force-lifetime maximum (1) indicative of a catch bond up to approximately 12 pN, while preTCR N15 exhibits two maxima, at 12 pN (1), and at 20 pN (2). d. SM OT bead trajectory plots show reversible conformational transitions within preTCR N15 (top) and TCR N15αβ (bottom) while bound to VSV8/H-2Kb at a pulling force of 10 pN. Note the shorter time scale of preTCR transition as compared to TCR. e. A schematic diagram illustrates the stages of conformational transition corresponding to the trajectory plots in d. The initial state (green) is generally offset a small distance (~3–6 nm) from the post-transition Compact State (blue). The Extended State (also in blue) can be as much as 10 nm offset from the Compact State. Data in c,d are from Das et al. (2016).
While it was previously appreciated that antibody-mediated CD3 crosslinking on the surface of thymocytes would trigger Ca2+ flux (Shinkai and Alt 1994), because of the lack of recognized preTCR ligands, the effects of preTCR triggering, per se, were unknown. Utilizing a multiwell, high-throughput flow cell format, pMHC ligand was immobilized on the flow cell surface and wells were loaded with individual SCID.adh cells identical to those used for the BFP characterization above but carrying a Ca2+ sensitive dye. We were thus able to follow hundreds of individual cells and visualize Ca2+ release in response to ligand in real time. As we anticipated, there was a clear Ca2+ response to pMHC ligand that was absent in preTCR expressing cells on control surfaces or in untransduced cells that lack the preTCR on pMHC ligand immobilized surfaces (Mallis et al. 2015).
Given biophysical behaviors of pMHC binding to isolated ectodomains and native preTCR complexes on the cell in situ as well as confirmation of cell signaling through pMHC ligation of preTCRs, we next tested the role of preTCR-pMHC interaction on developmental progression using differentiation assays. For this purpose we utilized in vitro stromal cell culture, a standard method in developmental immunology (Mohtashami et al. 2010). As discussed above, a function of the preTCR is to ensure that the thymocytes mature from the early pTα/β expressing stage (DN3) to the more mature αβTCR expressing stage (DP) (Fig. 1d). Thymic progenitors can be transfected with β-chain, such that pTα will pair with the transfected β to form the preTCR. In a background in which genetic recombination of the TCRα and TCRβ subunits is prevented, this preTCR will be the only one present in the cultures. If these transfected thymocytes are cultured in the presence of a stromal cell such as the OP9-DL4 cell line (Mohtashami et al. 2010), which provides an array of pMHC as well as the Notch receptor ligand delta-like ligand 4 (DL4), the thymocytes proliferate and progress to the DP stage in a preTCR dependent manner. Paralleling the NMR as well as the BFP and OT observations, we found development depended on both CDR residues as well as Vβ-patch residues (Mallis et al. 2015). Using a genetically engineered variant of the OP9-DL4 cell line which does not express MHC on the surface, we were able to show a strong dependence on MHC recognition for DN3 thymic expansion and development to the TCR-expressing post-DN3 stages (Das et al. 2016). When a pMHC incorporating VSV8, H-2Kb, and β2m in a single chain construct (scpMHC) (Hoerter et al. 2013) is expressed on the surface of the otherwise MHC-negative cells, scpMHC stroma are able to rescue the proliferation and development of preTCR-bearing thymocytes. Thus, the essential observation of β chain binding pMHC in the NMR tube has been fully recapitulated as a significant biological receptor function in cellular studies.
Mechanistic Study of preTCR
OT can measure pN-level forces and nm-resolution distances, allowing one to detect structural changes within the molecules measured under preTCR-pMHC bond load. OT SM is able to provide a window on the biophysics of the domains under consideration outside of the cellular context (Fig. 4a), and additionally, can be used in a single molecule/single cell (SMSC) format to observe the behavior of the receptors in their membrane anchored native environment (Fig. 4b). Conformational transitions, potentially representing unfolding and/or domain rearrangements within the αβTCR during interactions with pMHC, were observed upon pN force application (Das et al. 2015). Similarly to the TCR, the preTCR exhibits conformational transitions with the application of force [Fig. 4d–e, (Das et al. 2016)]. This suggests that the mechanism of triggering the preTCR is force-dependent, raising the possibility that the preTCR may participate not only in selecting useful β-chains, but also may provide screening in early thymocyte development for their mechanical suitability to subsequent incorporation into αβTCRs. How potential differences in preTCR and αβTCR β domain orientation relative to pMHC (Fig. 3) may impact this aforementioned physiological receptor fitness screening remains to be seen. Yet another surprise was the bimodal profile in the preTCR-pMHC force-lifetime curve. Not only is there a catch bond with a force maximum around 12 pN similar to that seen for the αβTCR, but there is a second force maximum at about 20 pN (Fig. 4c). This higher-force catch bond may have its origins in the Vβ patch region of the preTCR, as mutagenesis of three conserved patch residues to Ala results in near complete loss of the high force catch bond (Das et al. 2016). Also unexpected was that not only were non-canonical peptides binding to preTCRs, but that some of these “cross-reactive” peptides show more robust binding than the canonical ligands (Das et al. 2016). For instance, the ovalbumin-derived octamer(SIINFEKL)/H-2Kb (OVA/H-2Kb), the ligand for the OT1 TCR, or the Q4H7 variant (the 4th residue substituted with Gln and the 7th substituted with His) showed a catch bond with preTCR N15 (Das et al. 2016). When preTCR N30 was tested, it was found that Q4H7/H-2Kb bound with longer lifetimes than VSV8/Kb, the canonical ligand for N30αβ (Fig. 5a). Moreover, the force-lifetime curve for preTCR N30 with Q4H7/Kb showed a more pronounced second force maximum at 20 pN that is absent with the OVA peptide from which it is derived (Fig. 5a). These data demonstrate that despite the inherent cross-reactivity of the preTCR, the multi-modality and kinetics of the catch bond are quite sensitive to changes in the receptor-ligand relationship.
Fig. 5.
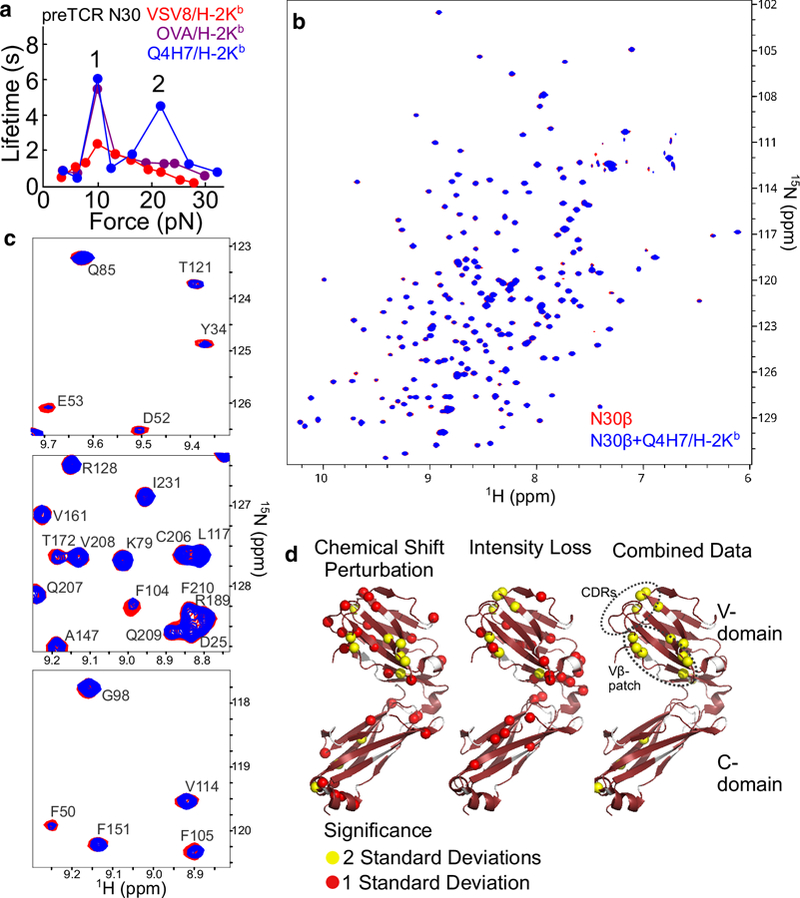
SM and NMR reveal peptide ligand cross-reactivity in preTCR recognition. a. Overlay of force-bond lifetime curves for preTCR N30 interacting with VSV8/H-2Kb (red), OVA/H-2Kb (violet) and Q4H7/H-2Kb (blue) ligands. A single force-maximum is found at 12 pN (1) for VSV8 and OVA while Q4H7 promotes a second at 20 pN (2). Data shown is from Das et al. (2016). b-d. Chemical shift perturbation and intensity loss highlight an interaction surface in N30β binding to Q4H7/H-2Kb-t which utilizes both CDR and Vβ patch regions. b. Overlay of 1H-15N TROSY-HSQC amide resonance spectra of 200μM 15N N30β-c1 (Mallis et al. 2015) (red) with 200μM 15N N30β-c1 + 200μM unlabeled Q4H7/Kb-t (Mallis et al. 2018) (blue). c. Select spectral regions from b with resonance assignments shown, highlighting residues for which chemical shift perturbation and/or peak intensity losses occur. Note residues F50, D52, and E53 from CDR2 and residues F104 and F105 from the Vβ patch show evident changes. d. Chemical shift perturbation and intensity loss analysis, performed as outlined in Mallis et al. (2018), is displayed on the N30β crystal structure (pdb 3Q5T).
The cross-reactivity of the preTCR N30 highlights a fundamental difference in the recognition of preTCR for its ligand in which peptide restriction is less important than for the TCR. Analysis of TROSY-HSQC titration data, including chemical shift perturbation and resonance intensity losses confirms this interaction between N30β and Q4H7-H-2Kb (Fig. 5b–d). The combined data show an interaction surface that utilizes both CDR and Vβ patch regions, in contrast to the surface highlighted for the weak ligand VSV8/H-2Kb illuminated through CST which appeared not to include the lower half of the Vβ patch region (Mallis et al. 2015). Through comparative analysis of N30β interactions with these various ligands by NMR and SM we can begin to understand the origins of the catch bond in the preTCR and possibly the complex behavior implicit in the multi-modal force-lifetime curves.
In addition to kinetic measurements of binding events, positional parameters in SM OT can also provide information on the conformational state of the molecule under force. Within the preTCR under force, for instance, stepwise dwells in the probe bead position were found, indicative of a reversible conformational transition [(Fig. 4d–e), (Das et al. 2016)]. The transitions were also found in TCRαβ (Das et al. 2015) and were correlated with the presence of a catch bond, highlighting the co-dependence of each event on the force applied during interaction (Das et al. 2016). The rate of reversible transitions in the preTCR was approximately ten-fold that of the mature TCR, suggesting that the TCRα subunit adds a substantial stiffening to the molecule relative to pTα, which lacks a V domain (Das et al. 2016). At this time, the site(s) of extension have not been characterized, but given the presence of a common β chain in the TCR and preTCR examined and similar equilibrium force, the β subunit must participate, although this does not rule out a concurrent unfolding in α. In fact, if the α and β transitions are coordinated, then this could explain in part the slower unfolding/refolding rate of the TCR versus preTCR. The transition was observed in cell-surface TCR complexes binding pMHC in SMSC OT experiments (Das et al. 2015) and is possibly linked to triggering, as work derived through repeating the unfolding-refolding cycle could function to inject energy into the TM regions of CD3 and/or the plasma membrane, disrupting resting-state ITAM conformations and activating the T cell.
Mechanotransduction – Inward from the Ectodomains
Concurrent with work on the preTCR, the mechanoreceptor functions of the αβTCR have been a major focus in our laboratories [reviewed in (Brazin et al. 2015; Feng et al. 2018)]. NMR structure determination of the CD3 ectodomains showed a unique side-by-side arrangement of the component ε and γ or ε and δ subdomains (Sun et al. 2001; Sun et al. 2004). The tightly coupled central β strands with hydrophobic interdigitating interface residues of the two subunits of each CD3εγ and CD3εδ heterodimer implied a rigidity that would lend itself to transmission of force from the ectodomains into the respective TM domains and thereby into the cell. The membrane-proximal region was also found to possess a constitutively formed disulfide bond in a highly conserved and functionally critical CxxC motif (Touma et al. 2007; Brazin et al. 2014). One could thus propose a mechanistic description of binding within the ectodomains and their relay to intracellular components via the TM domains in a manner consistent with the mechanoreceptor function of the TCR (Kim et al. 2009; Brazin et al. 2015). However, the precise quaternary arrangement of the TCR is as yet unknown, with several models supported by diverse data such as antibody binding, genetic conservation analysis, glycosylation maps, X-ray crystallography, molecular dynamics simulations [reviewed in (Kim et al. 2012)], and more recently, NMR interaction studies (He et al. 2015; Natarajan et al. 2016) and cryoEM (Birnbaum et al. 2014). For the TM regions, the picture is also unclear, with, until recently, primary structural data only on the CD3ζ subunits, which are known to form homodimers (Call et al. 2006). All other TM domain associations are implied either by mutagenesis studies (Alcover et al. 1990; Blumberg et al. 1990; Manolios et al. 1990; Call et al. 2002) or molecular modeling (Krshnan et al. 2016).
We thus sought to clarify the TCR assembly and its mechanistic role in signaling through direct structural study, beginning with the TCRα subunit (Brazin et al. 2018). When the NMR structure was solved, we immediately noted that it did not form a straight membrane-spanning helix, but rather formed a kinked structure consisting of two helices with a bend at Asn261 (Fig 6). NMR backbone resonance assignments as well as structural calculations defined two interchanging states, the kinked structure (L state) and a more extended configuration (E state). Sequence analysis ascertained that the residues forming the bend within the helix were highly conserved. Prior studies on TM determinants of assembly focused on several charged residues within the αβTCR heterodimer and CD3 TM domains (Alcover et al. 1990; Blumberg et al. 1990), with suggestions that charge-pairing mechanisms dominate subunit assembly (Call et al. 2002). In keeping with this hypothesis, subunit association was perturbed with individual mutation of a Lys (K256) or Arg (R251) residue within TCRα TM. However, in contrast to the prior hypothesis, K256 did not appear to participate in interactions with a charged Asp residue within CD3δ. The interaction was instead mediated almost entirely by the membrane-proximal connecting peptide (CP) motifs (Bäckström et al. 1996), a long, unstructured region within TCRα and the previously mentioned CxxC motif within CD3δ (Brazin et al. 2014). Instead, it seemed that the effect of mutating the charged residues on subunit assembly was mediated indirectly through the influence on membrane positioning, as determined using both NMR and electron paramagnetic resonance spectroscopy (EPR). Importantly, through the use of complementary NMR and EPR methods, we recapitulated essential features of our structural model using two different lipid-like environments, phospholipid micelles and liposomes.
Fig. 6.
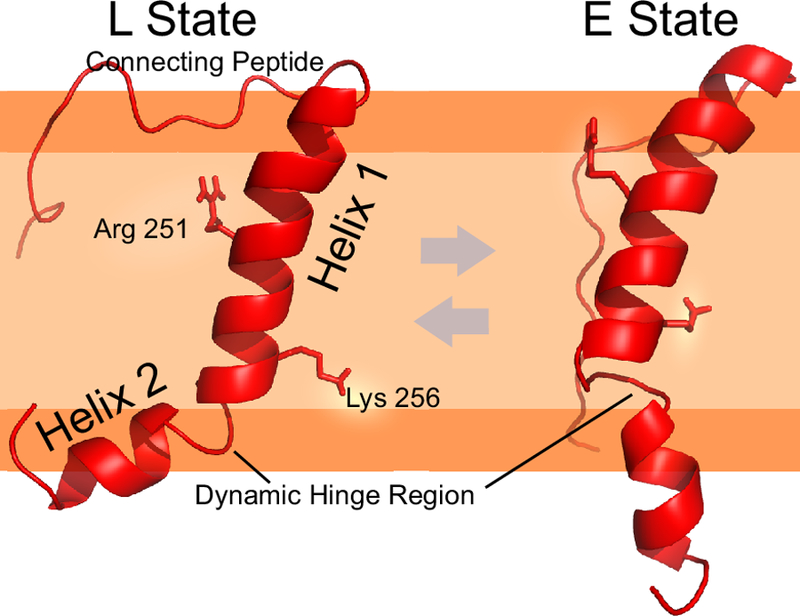
TCRα TM structure (PDB 6MF8) illustrating positions of intra-TM helical break and basic amino acids. A dynamic interchange between kinked (L-State) and extended (E State) is present in NMR experiments in which the TM is immersed in lysophosphoglycerol (LPPG) phospholipid micelles. This dynamic interchange in the ms to s timescale is likely enhanced with force application, and could be modulated through intersubunit interactions or post-translational modifications (Brazin et al. 2018). For illustration, the relative position of the segment is placed in the bilayer predicated on both NMR PRE experiments using micelles and EPR spin label depth measurement experiments with liposomes in aforementioned reference. Basic amino acids K256 and R251 are represented as sticks. Gly259 and Asn261 are located within the dynamic hinge region. The light orange color is representative of the lipid acyl chains while the dark orange represents the polar/charged headgroups. The unstructured connecting peptide region is not membrane embedded, but rather, is disordered in structural calculations and dynamic measurements.
The NMR data led us to assess the consequences on receptor assembly and function by guiding the selection of critical residues to mutate, changing only TCRαTM, within the fully assembled αβTCR complexes in T cells. It was found that the R251L weakened the association of predicted binding partner CD3ζ, although this was only detectable after immunoprecipitation (IP), and not evident by fluorescence activated cell sorting (FACS) analysis or immunofluorescence microscopy (IM). K256L mutation, in contrast, abrogated all CD3 associations (εγ, εδ, and ζζ) as detected by FACS, IM or IP. As one would expect from the loss of CD3 signaling components, standard cellular activation assays showed that the K256L mutation also prevented ligand-triggered activation as measured by IL-2 ELISA, while the R251L mutant showed activation-induced IL-2 production indistinguishable from that of WT TCR-expressing cells. However, the transcriptional profile of each of these cells actually resembled an activated phenotype, with the R251L mutant showing markers of early activation while the more dramatic K256L mutant showing late-stage activation markers, suggesting that TCRαβ dissociation from CD3 components represents an early step in physiological TCR triggering, consistent with reports of CD3ζζ dimer loss in inflammation and cancer [refs in (Brazin et al. 2018)].
SMSC analysis of the mutations targeting the TCRα-CD3δ CP-based juxtamembrane interaction showed little difference from WT in the force-dependent bond lifetime profile, suggesting that the interaction does not significantly affect the biophysical properties of ligand binding. Surprisingly, mutagenesis of the membrane charged residues did indeed change the force at maximal lifetime for the R251L mutant, shifting the force maximum from almost 15 pN to approximately 10pN, without significantly lowering the maximal lifetime (Brazin et al. 2018). The maximal bond lifetime of the K256L mutant, moreover, decreased to 25% of that of the WT cell surface TCR binding to pMHC (Brazin et al. 2018). Considering the significant distance of the membrane-embedded R251 and K256 from the TCR-pMHC interface, the αβTCR is a precisely tuned mechanoreceptor. It is likely that TCR complex quaternary associations and/or the membrane positioning of the TCRα TM buttresses the TCRαβ subunits in such a way to enable or enhance the intrinsic catch bond. It is clear that the catch bond is coupled or modified through those quaternary subunit associations in the context of the cell membrane since we observe catch bond formation in SM experiments between pMHC ligand and the TCRαβ heterodimer lacking CD3 (Das et al. 2015). Some quaternary associations may be uncoupled from the force transmission pathways in cells since the TCRα-CD3δ association-targeting mutants do not affect the force-dependent bond lifetime profiles, despite causing a marked decrease in ligand-dependent IL-2 production and in signaling in single-cell Ca2+ triggering assays (Brazin et al. 2018) .
To describe the complete signaling system one must understand that load on the TCRαβ bond occurs externally, from cell movement during immune surveillance at the time foreign pMHC is first detected (Fig. 1c), and prior to a stop movement signal [reviewed in (Brazin et al. 2015)] as well as internally due to cytoskeletal forces (Comrie and Burkhardt 2016). This mechanical force fosters a structural transition in the TCRαβ ectodomain to strengthen bond lifetime and energize the TCR, impacting the flanking CD3 heterodimeric ectodomains and inducing TCR complex quaternary change (Das et al. 2015; Das et al. 2016). Force proceeds from subunit ectodomains via their respective connecting peptides to impact the TM segments in the cholesterol-rich lipid rafts where TCRs reside, largely linked to the actin cytoskeleton (Caplan et al. 1995). These changes within the TM likely include a change of the TCRα TM from the L-state conformation shown in Figure 6 to a straightened E-state conformation which affects CD3 subunit association (Brazin et al. 2018). Lipid rafts offer a stiffened platform to suppress mechanical noise while spatially confining force (Anishkin and Kung 2013). These events induce changes in lipid composition vicinal to the αβTCR, including loss of negatively charged phosphatidylserine and phosphoinositides (Gagnon et al. 2012). The lipid changes combined with structural coupling between juxtamembrane connectors, TM and cytoplasmic tail segments, result in the release of the CD3 tethered tails from the inner leaflet of the plasma membrane to expose ITAM phosphorylation sites for signaling initiation (Aivazian and Stern 2000; Xu et al. 2008). Similar considerations must be at play in the preTCR ligation by self pMHC with even faster transition rates between compact and extended conformers (Das et al. 2016). Differences as well as similarities in these two receptor systems shall be of great interest to unravel. Such changes in local TCR architecture are akin to a phase transition where an organized crystalline structure melts and fluidizes to accommodate a new geometry. For such a process to occur, it must begin from a stable structure relative to thermal energy kBT. Bioforces acting on the αβTCR-pMHC bond have been shown to be on the order of 15 pN with transitions on the length scale of 10 nm. From a perspective of work, each transition represents the exchange of ~150pN*nm of energy which is ~36 kBT. Since the transition is reversible (Das et al. 2016) on the time scale of a second one can envision a cell machinery driven 150 zeptowatt heater driving this melting process through sustained reversible single receptor transitioning. Another benefit of a non-equilibrium process such as a phase transition is that the barrier to activation through its exponential dependence on energy can explain the ~104 ligand discrimination fidelity seen in T cell performance.
Perspectives and Future Directions
As detailed above, NMR together with SM analyses have defined the mechanical function of the TCR and preTCR through coordinated study. The structural contribution of the CβFG loop (Fig. 1b) in regulating bond lifetime (Das et al. 2015) requires allosteric communication between the C-domain and the CDR loops. One predicts that such communication would be evident in NMR spectra of TCR in complex with pMHC. In that sense, there has been some confirmation from two laboratories (Natarajan et al. 2017; Rangarajan et al. 2018), with some caveats. In neither case are changes measured directly via CSP found in the CβFG loop, although CSP and peak intensity changes are seen within the C domains of both α and β, particularly at the Cα-Cβ interface. The cluster of changes in these studies is proximal to regions previously implicated by NMR studies in the binding of CD3 ectodomains (Birnbaum et al. 2014; He et al. 2015; Natarajan et al. 2016; Natarajan et al. 2017; Rangarajan et al. 2018). NMR-derived chemical shift propensities as well as X-ray crystallographic B factors have defined the CβFG loop as a mobile structural element (Natarajan et al. 2017; Rangarajan et al. 2018) despite its role stabilizing the TCR and preTCR during force-dependent binding (Das et al. 2015; Das et al. 2016). MD simulations have suggested that the CβFG loop becomes significantly less mobile during pMHC ligation (Rangarajan et al. 2018), though this observation remains to be confirmed with biophysical measurements.
Two potential avenues exist to expand on the aforementioned observations. First, the force-induced transitions within the TCR and preTCR (Das et al. 2015; Das et al. 2016) may become evident only in the presence of pMHC and force. Thus, MD simulations with an applied force may illuminate allosteric pathways within the involved molecules. To this end, some recent work has proposed mechanistic explanations for catch bond behavior through the use of directional force models (Sibener et al. 2018). Their simulation suggested that transverse rather than longitudinal pulling relative to the TCR-pMHC interface is needed to engage the catch bond (Sibener et al. 2018). Although this is apparently in agreement with the directionality of force required in single cell activation studies with pMHC (Kim et al. 2009; Feng et al. 2017), the 2 ns simulation time was too short to reveal any realistic conformational behavior of the system. Also, since only the variable domains were used, allosteric behavior of TCR could not be addressed (Knapp et al. 2017). In comparison, an extensive simulation study of 172 pMHC complexes indicated that even 100 ns simulation time is not enough to reveal any clear differences in behaviors of peptides with different immunogenicity (Knapp et al. 2014). This indicates a difficulty of using MD simulation, which is currently limited to at most a μs time scale, in studying second-scale dynamics of the TCR. However, we note that the goal of performing MD is not recapitulating the process in real time. Instead, MD is an effective tool for elucidating physical and dynamic mechanisms. In this regard, careful combination of enhanced sampling, directional load application, and trajectory analysis algorithms may lead to the discovery of structural principles in TCR selection and peptide recognition. Advances in computing power and analysis methods are enabling detailed investigation of functionally significant domain dynamics (Hwang et al. 2017) and organization of surface water molecules (Teng and Hwang 2018). Furthermore, MD can provide an essential linkage between the atomic-level equilibrium NMR data and the mesoscale non-equilibrium OT data.
Second, NMR measurements may yet confirm the existence of dynamically regulated force-dependent pathways through the participating molecules. X-ray crystallographic B factors may be informative but external loops are potentially biased by crystallographic contacts. Direct measurement of dynamics by NMR rather than proxy measurements like chemical shift propensities would likewise be preferable. However, one begins to run up against the large size of the TCR-pMHC complex, approximately 90 kDa, in being able to complete the standard relaxation pulse measurements. This is due to intensity losses from 50 to 100% at the molecular interface for TCRαβ-pMHC (Natarajan et al. 2017; Rangarajan et al. 2018) or TCRβ-pMHC (Mallis et al. 2015). The intensity loss is due to two factors: 1) the overall increase in molecular weight of the complex which results in faster relaxation, and 2) the possibility of chemical exchange at the interface which further broadens the resonances. Relaxation measurements on such systems are further confounded by the challenge that the longitudinal relaxation time (T1) increases with molecular weight and that deuteration is required. This demands longer recycling time especially in the heteronuclear nuclear overhauser effect (HNNOE) measurements. Some of these problems are mitigated by trimming constructs to critical subdomains, as in removal of distal α3 and β2m on pMHC and/or utilization of β as a proxy for the preTCR (Mallis et al. 2018). The pMHC truncation strategy has been shown to be successful for two MHC gene products to date (Jones et al. 2006; Mallis et al. 2018) and may be more widely applicable to many pMHCs. The use of β as a proxy molecule is only valid for preTCR investigations, but may illuminate essential functions of TCR signaling as well, given the importance of the β subunit and the similarities between TCR and preTCR SM behavior (Das et al. 2016). A single-chain VαVβ containing construct has been produced as a model for several αβTCRs (Novotny et al. 1991; Hare et al. 1999; Maynard et al. 2005) and γδTCRs (Xu et al. 2011; Luoma et al. 2013) recapitulating the solution binding properties of the full TCR in each case. However, self-evidently, this will not allow measurement of allosteric changes to the C domains and thus obviates any involvement of the Cβ FG loop in enhancing binding.
Improvements may also be gained by combining the appropriate labeling strategy with the relaxation optimized NMR methods. Investigation of both structure and dynamics by NMR require resonance assignments to relate spectral changes to structural features at atomic resolution. Relaxation optimized methods such as TROSY combined with labeling techniques including deuteration (Salzmann et al. 1998) and pyruvate labeling (Robson et al. 2018) allow backbone resonance assignment in large molecular weight systems. Incorporating line shapes from Cβ coupling on the Cα resonances can further facilitate backbone assignments without additional relaxation losses (Coote et al. 2018). The methyl-TROSY has allowed the study of very large proteins complexes (Kay 2011; Kerfah et al. 2015a; Huang et al. 2017) including the TCR-pMHC (Natarajan et al. 2017) and has become standard methodology. The relatively fast rotation of methyl residues compared to the overall tumbling of the protein provides favorable relaxation properties for the methyl groups. However assigning the sidechain resonances, including the methyl resonances, has been a major challenge. The out-back-style experiment recently developed leverages the linear 13C-labeling design and the selective protonation of methyl residues to enable side chain resonance assignment of large protein systems (Kerfah et al. 2015b). In cases where there is a high-resolution structure, an alternate strategy is to use 3D/4D methyl-methyl NOESY experiments to obtain the assignment of the methyl resonances (Pritišanac et al. 2017).
For large molecular weight systems such as the TCR, preTCR and pMHC, structure determination by NMR relies on NOESY based distance restraints obtained from amide and methyl resonances. To date, the only NMR structures are below 20kDa, such as the scVαVβD10 TCR (Hare et al. 1999), CD3εγ and εδ (Sun et al. 2001; Sun et al. 2004), and the TM fragments of CD3ζζ (Call et al. 2006) and TCRα (Brazin et al. 2018). In order to extend upwards to the larger fragments such as the TCRαβ ectodomains or full pMHC, each above 40kDa, newer innovative strategies must be employed. Timeshared 3D/4D NOESY experiments leverage the amide and methyl TROSY effect to provide distance restraints between methyl to methyl, methyl to amide and amide to methyl groups (Frueh et al. 2009; Mishra et al. 2014). Site-specific and alternate labeling statergies combined with the aromatic TROSY effect provide distance restraints to aromatic residues (Pervushin et al. 1998; Milbradt et al. 2015). Paramagnetic relaxation enhancement (PRE), pseudo-contact shift (PCS) and residual dipolar coupling (RDC) experiments provide additional structural restraints, especially useful in determining structures of complexes such a TCR-pMHC or preTCR-pMHC (Battiste and Wagner 2000; Hass and Ubbink 2014; Pilla et al. 2016; Nitsche and Otting 2017).
NMR is a powerful method to provide atomistic details of protein dynamics over a wide range of time scales (ps to seconds). In particular, two recent developments in NMR methodology, relaxation dispersion (Rex) (Sprangers and Kay 2007; Loria et al. 2008) and chemical exchange saturation transfer (CEST) (Vallurupalli et al. 2012; Vallurupalli and Kay 2013) provide access to protein dynamics in the millisecond time scale, a regime typically associated with conformational exchange. This sort of chemical exchange has been deduced in the N15β V domain within the CDR2 and flanking β-strand regions (Mallis et al. 2015) and is likely the first such instance to be found among the diverse TCR sequence space. Catch bond formation could well be engendered by high energy barrier slow exchange events with transitions to tight-binding conformers favored with the addition of force to the nascent TCR-pMHC interaction. Such data would complement the MD approaches discussed above.
As in NMR, recent advances in SM technology as well as further tailoring of current methods can contribute significantly to improving our understanding of initiation of T cell signaling. Higher throughput methods analogous to that used for the preTCR Ca2+ assay cited above (Mallis et al. 2015) while incorporating force through fluid flow, magnetic force, obstacles and surface topology or other mechanisms (Ruel-Gariépy and Leroux 2004; van Mameren et al. 2008; Liu et al. 2016; Stockslager et al. 2017; van Mameren et al. 2018) could aid in evaluation of heterogeneous populations of cells. Additionally, coupling SMSC assays or single-cell triggering with high-throughput next generation sequencing for RNAseq transcriptional analysis would allow precise correlation of biophysical parameters of TCR-pMHC and preTCR-pMHC with signaling outcomes. These studies could inform how different bond lifetimes as well as magnitudes and frequencies of structural transitions under various loads, among other biophysical parameters, may link to differential gene programs altering apoptosis, survival, metabolism, migration, adhesion and the like. Studies in vitro involving load-triggered T cells and thymocytes using single cell transcriptomes as well as in vivo studies employing structurally and biophysically defined TCRs and preTCRs using retrogenic and transgenic mice shall highlight the translational relevance of structural mechanobiology. We can anticipate implications for T cell effector and regulatory functions as well as T cell memory.
The combined NMR and MD approach could then be leveraged to suggest mutagenesis targets to be tested by SM, with a focus on correlating mechanical parameters with both structural data and biological outcomes. While each method has its unique strengths as well as limitations in size, timescale, nonequilibrium force and other parameters, their integration shall collectively bridge and reveal the emerging picture of TCR mechanobiology. Our experience suggests that we can glean mechanistic insights that feed back into our structural understanding (Fig. 2) by targeted transcriptomic analysis (Brazin et al. 2018), single cell OT (Feng et al. 2017), SM and SMSC (Das et al. 2015; Das et al. 2016) and developmental assays (Mallis et al. 2015; Das et al. 2016). Indeed, perhaps we are approaching the ability to apply changes to components of the TCR signal transduction elements with predictive results, finally understanding the biophysical mechanism of TCR triggering. Different αβTCRs directed against the same pMHC may undergo distinct conformational transitions, rates of oscillation between compact versus extended states and/or other bioforce-linked parameters impacting effector or memory functions. Such insights, in principle, could allow for selection of ideal T cell populations for immune protection against pathogens or tumors.
Acknowledgments
Grant support: AI136301 to MJL; GW047467, AI0037581, and EB002026 to GM; R01AI136960 and R56AI138489 to ELR.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Aivazian D, Stern LJ (2000) Phosphorylation of T cell receptor zeta is regulated by a lipid dependent folding transition. Nat Struct Biol 7:1023–1026 [DOI] [PubMed] [Google Scholar]
- Alcover A, Mariuzza RA, Ermonval M, Acuto O (1990) Lysine 271 in the transmembrane domain of the T-cell antigen receptor beta chain is necessary for its assembly with the CD3 complex but not for alpha/beta dimerization. J Biol Chem 265:4131–4135 [PubMed] [Google Scholar]
- Anishkin A, Kung C (2013) Stiffened lipid platforms at molecular force foci. Proc Natl Acad Sci U S A 110:4886–4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckström BT, Milia E, Peter A, Jaureguiberry B, Baldari CT, Palmer E (1996) A motif within the T cell receptor alpha chain constant region connecting peptide domain controls antigen responsiveness. Immunity 5:437–447 [DOI] [PubMed] [Google Scholar]
- Battiste JL, Wagner G (2000) Utilization of site-directed spin labeling and high-resolution heteronuclear nuclear magnetic resonance for global fold determination of large proteins with limited nuclear overhauser effect data. Biochemistry 39:5355–5365 [DOI] [PubMed] [Google Scholar]
- Bentley GA, Boulot G, Karjalainen K, Mariuzza RA (1995) Crystal structure of the beta chain of a T cell antigen receptor. Science 267:1984–1987 [DOI] [PubMed] [Google Scholar]
- Birnbaum ME, Berry R, Hsiao Y-S, Chen Z, Shingu-Vazquez MA, Yu X, Waghray D, Fischer S, McCluskey J, Rossjohn J, Walz T, Garcia KC (2014) Molecular architecture of the αβ T cell receptor-CD3 complex. Proc Natl Acad Sci U S A 111:17576–17581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg RS, Alarcon B, Sancho J, McDermott FV, Lopez P, Breitmeyer J, Terhorst C (1990) Assembly and function of the T cell antigen receptor. Requirement of either the lysine or arginine residues in the transmembrane region of the alpha chain. J Biol Chem 265:14036–14043 [PubMed] [Google Scholar]
- Brazin KN, Mallis RJ, Boeszoermenyi A, Feng Y, Yoshizawa A, Reche PA, Kaur P, Bi K, Hussey RE, Duke-Cohan JS, Song L, Wagner G, Arthanari H, Lang MJ, Reinherz EL (2018) The T Cell Antigen Receptor α Transmembrane Domain Coordinates Triggering through Regulation of Bilayer Immersion and CD3 Subunit Associations. Immunity 49:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazin KN, Mallis RJ, Das DK, Feng Y, Hwang W, Wang J-H, Wagner G, Lang MJ, Reinherz EL (2015) Structural Features of the αβTCR Mechanotransduction Apparatus That Promote pMHC Discrimination. Front Immunol 6:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazin KN, Mallis RJ, Li C, Keskin DB, Arthanari H, Gao Y, Wu S-L, Karger BL, Wagner G, Reinherz EL (2014) Constitutively oxidized CXXC motifs within the CD3 heterodimeric ectodomains of the T cell receptor complex enforce the conformation of juxtaposed segments. J Biol Chem 289:18880–18892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call ME, Pyrdol J, Wiedmann M, Wucherpfennig KW (2002) The organizing principle in the formation of the T cell receptor-CD3 complex. Cell 111:967–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call ME, Schnell JR, Xu C, Lutz RA, Chou JJ, Wucherpfennig KW (2006) The structure of the zetazeta transmembrane dimer reveals features essential for its assembly with the T cell receptor. Cell 127:355–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan S, Zeliger S, Wang L, Baniyash M (1995) Cell-surface-expressed T-cell antigen-receptor zeta chain is associated with the cytoskeleton. Proc Natl Acad Sci U S A 92:4768–4772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton M, Ruetsch NR, Berger MA, Rhodes M, Kaptik S, Wiest DL (1999) Signals transduced by CD3epsilon, but not by surface pre-TCR complexes, are able to induce maturation of an early thymic lymphoma in vitro. J Immunol 163:2576–2585 [PubMed] [Google Scholar]
- Comrie WA, Burkhardt JK (2016) Action and Traction: Cytoskeletal Control of Receptor Triggering at the Immunological Synapse. Front Immunol 7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump AL, Grusby MJ, Glimcher LH, Cantor H (1993) Thymocyte development in major histocompatibility complex-deficient mice: evidence for stochastic commitment to the CD4 and CD8 lineages. Proc Natl Acad Sci U S A 90:10739–10743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das DK, Feng Y, Mallis RJ, Li X, Keskin DB, Hussey RE, Brady SK, Wang J-H, Wagner G, Reinherz EL, Lang MJ (2015) Force-dependent transition in the T-cell receptor β-subunit allosterically regulates peptide discrimination and pMHC bond lifetime Proc Natl Acad Sci U S A 112:1517–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das DK, Mallis RJ, Duke-Cohan JS, Hussey RE, Tetteh PW, Hilton M, Wagner G, Lang MJ, Reinherz EL (2016) Pre-T Cell Receptors (Pre-TCRs) Leverage Vβ Complementarity Determining Regions (CDRs) and Hydrophobic Patch in Mechanosensing Thymic Self-ligands. J Biol Chem 291:25292–25305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Bjorkman PJ (1988) T-cell antigen receptor genes and T-cell recognition. Nature 334:395–402 [DOI] [PubMed] [Google Scholar]
- Feng Y, Brazin KN, Kobayashi E, Mallis RJ, Reinherz EL, Lang MJ (2017) Mechanosensing drives acuity of αβ T-cell recognition. Proc Natl Acad Sci U S A 114:E8204–E8213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Reinherz EL, Lang MJ (2018) αβ T Cell Receptor Mechanosensing Forces out Serial Engagement. Trends Immunol 39:596–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frueh DP, Leed A, Arthanari H, Koglin A, Walsh CT, Wagner G (2009) Time-shared HSQC-NOESY for accurate distance constraints measured at high-field in (15)N-(13)C-ILV methyl labeled proteins. J Biomol NMR 45:311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon E, Schubert DA, Gordo S, Chu HH, Wucherpfennig KW (2012) Local changes in lipid environment of TCR microclusters regulate membrane binding by the CD3ε cytoplasmic domain. J Exp Med 209:2423–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghendler Y, Teng MK, Liu JH, Witte T, Liu J, Kim KS, Kern P, Chang HC, Wang JH, Reinherz EL (1998) Differential thymic selection outcomes stimulated by focal structural alteration in peptide/major histocompatibility complex ligands. Proc Natl Acad Sci U S A 95:10061–10066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare BJ, Wyss DF, Osburne MS, Kern PS, Reinherz EL, Wagner G (1999) Structure, specificity and CDR mobility of a class II restricted single-chain T-cell receptor. Nat Struct Biol 6:574–581 [DOI] [PubMed] [Google Scholar]
- Hass MAS, Ubbink M (2014) Structure determination of protein-protein complexes with long-range anisotropic paramagnetic NMR restraints. Curr Opin Struct Biol 24:45–53 [DOI] [PubMed] [Google Scholar]
- He Y, Rangarajan S, Kerzic M, Luo M, Chen Y, Wang Q, Yin Y, Workman CJ, Vignali KM, Vignali DAA, Mariuzza RA, Orban J (2015) Identification of the Docking Site for CD3 on the T Cell Receptor β Chain by Solution NMR. J Biol Chem 290:19796–19805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerter JAH, Brzostek J, Artyomov MN, Abel SM, Casas J, Rybakin V, Ampudia J, Lotz C, Connolly JM, Chakraborty AK, Gould KG, Gascoigne NRJ (2013) Coreceptor affinity for MHC defines peptide specificity requirements for TCR interaction with coagonist peptide-MHC. J Exp Med 210:1807–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Pérez F, Kay LE (2017) Probing the cooperativity of Thermoplasma acidophilum proteasome core particle gating by NMR spectroscopy. Proc Natl Acad Sci U S A 114:E9846–E9854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W, Lang MJ, Karplus M (2017) Kinesin motility is driven by subdomain dynamics. eLife 6:e28948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imarai M, Goyarts EC, van Bleek GM, Nathenson SG (1995) Diversity of T cell receptors specific for the VSV antigenic peptide (N52–59) bound by the H-2Kb class I molecule. Cell Immunol 160:33–42 [DOI] [PubMed] [Google Scholar]
- Irving BA, Alt FW, Killeen N (1998) Thymocyte development in the absence of pre-T cell receptor extracellular immunoglobulin domains. Science 280:905–908 [DOI] [PubMed] [Google Scholar]
- Jones LL, Brophy SE, Bankovich AJ, Colf LA, Hanick NA, Garcia KC, Kranz DM (2006) Engineering and characterization of a stabilized alpha1/alpha2 module of the class I major histocompatibility complex product Ld. J Biol Chem 281:25734–25744 [DOI] [PubMed] [Google Scholar]
- Ju L, Chen Y, Rushdi MN, Chen W, Zhu C (2017) Two-Dimensional Analysis of Cross-Junctional Molecular Interaction by Force Probes. Methods Mol Biol 1584:231–258 [DOI] [PubMed] [Google Scholar]
- Kay LE (2011) Solution NMR spectroscopy of supra-molecular systems, why bother? A methyl-TROSY view. J Magn Reson 210:159–170 [DOI] [PubMed] [Google Scholar]
- Kerfah R, Hamelin O, Boisbouvier J, Marion D (2015b) CH3-specific NMR assignment of alanine, isoleucine, leucine and valine methyl groups in high molecular weight proteins using a single sample. J Biomol NMR 63:389–402 [DOI] [PubMed] [Google Scholar]
- Kerfah R, Plevin MJ, Sounier R, Gans P, Boisbouvier J (2015a) Methyl-specific isotopic labeling: a molecular tool box for solution NMR studies of large proteins. Curr Opin Struct Biol 32:113–122 [DOI] [PubMed] [Google Scholar]
- Kim ST, Shin Y, Brazin K, Mallis RJ, Sun Z-YJ, Wagner G, Lang MJ, Reinherz EL (2012) TCR Mechanobiology: Torques and Tunable Structures Linked to Early T Cell Signaling. Front Immunol 3:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ST, Takeuchi K, Sun Z-YJ, Touma M, Castro CE, Fahmy A, Lang MJ, Wagner G, Reinherz EL (2009) The alphabeta T cell receptor is an anisotropic mechanosensor. J Biol Chem 284:31028–31037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp B, Dunbar J, Alcala M, Deane CM (2017) Variable Regions of Antibodies and T-Cell Receptors May Not Be Sufficient in Molecular Simulations Investigating Binding. J Chem Theory Comput 13:3097–3105 [DOI] [PubMed] [Google Scholar]
- Knapp B, Dunbar J, Deane CM (2014) Large scale characterization of the LC13 TCR and HLA-B8 structural landscape in reaction to 172 altered peptide ligands: a molecular dynamics simulation study. PLoS Comput Biol 10:e1003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller BH, Marrack P, Kappler JW, Smithies O (1990) Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science 248:1227–1230 [DOI] [PubMed] [Google Scholar]
- Krshnan L, Park S, Im W, Call MJ, Call ME (2016) A conserved αβ transmembrane interface forms the core of a compact T-cell receptor-CD3 structure within the membrane. Proc Natl Acad Sci U S A 113:E6649–E6658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Van Vranken S, Zhao Y, Li Z, Guo Y, Eisele L, Li Y (2005) Crystal structures of T cell receptor (beta) chains related to rheumatoid arthritis. Protein Sci 14:3025–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Chen W, Evavold BD, Zhu C (2014) Accumulation of Dynamic Catch Bonds between TCR and Agonist Peptide-MHC Triggers T Cell Signaling. Cell 157:357–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Liu Y, Chang Y, Seyf HR, Henry A, Mattheyses AL, Yehl K, Zhang Y, Huang Z, Salaita K (2016) Nanoscale optomechanical actuators for controlling mechanotransduction in living cells. Nat Methods 13:143–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loria JP, Berlow RB, Watt ED (2008) Characterization of enzyme motions by solution NMR relaxation dispersion. Acc Chem Res 41:214–221 [DOI] [PubMed] [Google Scholar]
- Luoma AM, Castro CD, Mayassi T, Bembinster LA, Bai L, Picard D, Anderson B, Scharf L, Kung JE, Sibener LV, Savage PB, Jabri B, Bendelac A, Adams EJ (2013) Crystal structure of Vδ1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human γδ T cells. Immunity 39:1032–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallis RJ, Arthanari H, Lang MJ, Reinherz EL, Wagner G (2018) NMR-directed design of pre-TCRβ and pMHC molecules implies a distinct geometry for pre-TCR relative to αβTCR recognition of pMHC J Biol Chem 293:754–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallis RJ, Bai K, Arthanari H, Hussey RE, Handley M, Li Z, Chingozha L, Duke-Cohan JS, Lu H, Wang J-H, Zhu C, Wagner G, Reinherz EL (2015) Pre-TCR ligand binding impacts thymocyte development before αβTCR expression. Proc Natl Acad Sci U S A 112:8373–8378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallis RJ, Reinherz EL, Wagner G, Arthanari H (2016) Backbone resonance assignment of N15, N30 and D10 T cell receptor β subunits. Biomol NMR Assign 10:35–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mameren J, Peterman EJG, Wuite GJL (2008) See me, feel me: methods to concurrently visualize and manipulate single DNA molecules and associated proteins. Nucleic Acids Res 36:4381–4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mameren J, Vermeulen K, Wuite GJL, Peterman EJG (2018) A polarized view on DNA under tension. J Chem Phys 148:123306. [DOI] [PubMed] [Google Scholar]
- Manolios N, Bonifacino JS, Klausner RD (1990) Transmembrane helical interactions and the assembly of the T cell receptor complex. Science (New York, N.Y.) 249:274–277 [DOI] [PubMed] [Google Scholar]
- Marshall BT, Long M, Piper JW, Yago T, McEver RP, Zhu C (2003) Direct observation of catch bonds involving cell-adhesion molecules. Nature 423:190–193 [DOI] [PubMed] [Google Scholar]
- Maynard J, Petersson K, Wilson DH, Adams EJ, Blondelle SE, Boulanger MJ, Wilson DB, Garcia KC (2005) Structure of an autoimmune T cell receptor complexed with class II peptide-MHC: insights into MHC bias and antigen specificity. Immunity 22:81–92 [DOI] [PubMed] [Google Scholar]
- Milbradt AG, Arthanari H, Takeuchi K, Boeszoermenyi A, Hagn F, Wagner G (2015) Increased resolution of aromatic cross peaks using alternate 13C labeling and TROSY. J Biomol NMR 62:291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SH, Harden BJ, Frueh DP (2014) A 3D time-shared NOESY experiment designed to provide optimal resolution for accurate assignment of NMR distance restraints in large proteins. J Biomol NMR 60:265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohtashami M, Shah DK, Nakase H, Kianizad K, Petrie HT, Zúñiga-Pflücker JC (2010) Direct comparison of Dll1-and Dll4-mediated Notch activation levels shows differential lymphomyeloid lineage commitment outcomes. J Immunol 185:867–876 [DOI] [PubMed] [Google Scholar]
- Natarajan A, Nadarajah V, Felsovalyi K, Wang W, Jeyachandran VR, Wasson RA, Cardozo T, Bracken C, Krogsgaard M (2016) Structural Model of the Extracellular Assembly of the TCR-CD3 Complex. Cell Reports 14:2833–2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan K, McShan AC, Jiang J, Kumirov VK, Wang R, Zhao H, Schuck P, Tilahun ME, Boyd LF, Ying J, Bax A, Margulies DH, Sgourakis NG (2017) An allosteric site in the T-cell receptor Cβ domain plays a critical signalling role. Nat Commun 8:15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche C, Otting G (2017) Pseudocontact shifts in biomolecular NMR using paramagnetic metal tags. Prog Nucl Magn Reson Spectrosc 98–99:20–49 [DOI] [PubMed] [Google Scholar]
- Novotny J, Ganju RK, Smiley ST, Hussey RE, Luther MA, Recny MA, Siliciano RF, Reinherz EL (1991) A soluble, single-chain T-cell receptor fragment endowed with antigen-combining properties. Proc Natl Acad Sci U S A 88:8646–8650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang SS, Berry R, Chen Z, Kjer-Nielsen L, Perugini MA, King GF, Wang C, Chew SH, La Gruta NL, Williams NK, Beddoe T, Tiganis T, Cowieson NP, Godfrey DI, Purcell AW, Wilce MCJ, McCluskey J, Rossjohn J (2010) The structural basis for autonomous dimerization of the pre-T-cell antigen receptor. Nature 467:844–8 [DOI] [PubMed] [Google Scholar]
- Pervushin K, Riek R, Wider G, Wüthrich K (1998) Transverse relaxation-optimized spectroscopy (TROSY) for NMR studies of aromatic spin systems in 13C-labeled proteins J Am Chem Soc 120:6394–6400 [Google Scholar]
- Pilla KB, Otting G, Huber T (2016) Pseudocontact Shift-Driven Iterative Resampling for 3D Structure Determinations of Large Proteins. J Mol Biol 428:522–532 [DOI] [PubMed] [Google Scholar]
- Pritišanac I, Degiacomi MT, Alderson TR, Carneiro MG, Ab E, Siegal G, Baldwin AJ (2017) Automatic Assignment of Methyl-NMR Spectra of Supramolecular Machines Using Graph Theory. J Am Chem Soc 139:9523–9533 [DOI] [PubMed] [Google Scholar]
- Rangarajan S, He Y, Chen Y, Kerzic MC, Ma B, Gowthaman R, Pierce BG, Nussinov R, Mariuzza RA, Orban J (2018) Peptide-MHC (pMHC) binding to a human antiviral T cell receptor induces long-range allosteric communication between pMHC-and CD3-binding sites. J Biol Chem 293:15991–16005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz EL, Tan K, Tang L, Kern P, Liu J, Xiong Y, Hussey RE, Smolyar A, Hare B, Zhang R, Joachimiak A, Chang HC, Wagner G, Wang J (1999) The crystal structure of a T cell receptor in complex with peptide and MHC class II. Science 286:1913–1921 [DOI] [PubMed] [Google Scholar]
- Robson SA, Takeuchi K, Boeszoermenyi A, Coote PW, Dubey A, Hyberts S, Wagner G, Arthanari H (2018) Mixed pyruvate labeling enables backbone resonance assignment of large proteins using a single experiment Nat Commun 9:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph MG, Stanfield RL, Wilson IA (2006) How TCRs bind MHCs, peptides, and coreceptors Annu Rev Immunol 24:419–466 [DOI] [PubMed] [Google Scholar]
- Ruel-Gariépy E, Leroux J-C (2004) In situ-forming hydrogels--review of temperature-sensitive systems. Eur J Pharm Biopharm 58:409–426 [DOI] [PubMed] [Google Scholar]
- Salzmann M, Pervushin K, Wider G, Senn H, Wüthrich K (1998) TROSY in triple-resonance experiments: new perspectives for sequential NMR assignment of large proteins. Proc Natl Acad Sci U S A 95:13585–13590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Alt FW (1994) CD3 epsilon-mediated signals rescue the development of CD4+CD8+ thymocytes in RAG-2−/−mice in the absence of TCR beta chain expression. Int Immunol 6:995–1001 [DOI] [PubMed] [Google Scholar]
- Sibener LV, Fernandes RA, Kolawole EM, Carbone CB, Liu F, McAffee D, Birnbaum ME, Yang X, Su LF, Yu W, Dong S, Gee MH, Jude KM, Davis MM, Groves JT, Goddard WA, Heath JR, Evavold BD, Vale RD, Garcia KC (2018) Isolation of a Structural Mechanism for Uncoupling T Cell Receptor Signaling from Peptide-MHC Binding. Cell 174:672–687.e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprangers R, Kay LE (2007) Quantitative dynamics and binding studies of the 20S proteasome by NMR. Nature 445:618–622 [DOI] [PubMed] [Google Scholar]
- Stockslager MA, Bagnall JS, Hecht VC, Hu K, Aranda-Michel E, Payer K, Kimmerling RJ, Manalis SR (2017) Microfluidic platform for characterizing TCR-pMHC interactions. Biomicrofluidics 11:064103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun ZJ, Kim KS, Wagner G, Reinherz EL (2001) Mechanisms contributing to T cell receptor signaling and assembly revealed by the solution structure of an ectodomain fragment of the CD3 epsilon gamma heterodimer. Cell 105:913–923 [DOI] [PubMed] [Google Scholar]
- Sun Z-YJ, Kim ST, Kim IC, Fahmy A, Reinherz EL, Wagner G (2004) Solution structure of the CD3epsilondelta ectodomain and comparison with CD3epsilongamma as a basis for modeling T cell receptor topology and signaling. Proc Natl Acad Sci U S A 101:16867–16872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg EJ, Li H, Llera AS, McCormick JK, Tormo J, Schlievert PM, Karjalainen K, Mariuzza RA (2002) Structures of two streptococcal superantigens bound to TCR beta chains reveal diversity in the architecture of T cell signaling complexes. Structure 10:687–699 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Nakanishi T, Kami K, Arata Y, Shimada I (2000) A novel NMR method for determining the interfaces of large protein-protein complexes. Nat Struct Biol 7:220–223 [DOI] [PubMed] [Google Scholar]
- Teng MK, Smolyar A, Tse AG, Liu JH, Liu J, Hussey RE, Nathenson SG, Chang HC, Reinherz EL, Wang JH (1998) Identification of a common docking topology with substantial variation among different TCR-peptide-MHC complexes. Curr Biol 8:409–412 [DOI] [PubMed] [Google Scholar]
- Teng X, Hwang W (2018) Effect of Methylation on Local Mechanics and Hydration Structure of DNA. Biophys J 114:1791–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touma M, Sun Z-YJ, Clayton LK, Marissen WE, Kruisbeek AM, Wagner G, Reinherz EL (2007) Importance of the CD3gamma ectodomain terminal beta-strand and membrane proximal stalk in thymic development and receptor assembly. J Immunol 178:3668–3679 [DOI] [PubMed] [Google Scholar]
- Vallurupalli P, Bouvignies G, Kay LE (2012) Studying “invisible” excited protein states in slow exchange with a major state conformation. J Am Chem Soc 134:8148–8161 [DOI] [PubMed] [Google Scholar]
- Vallurupalli P, Kay LE (2013) Probing slow chemical exchange at carbonyl sites in proteins by chemical exchange saturation transfer NMR spectroscopy. Angewandte Chemie (International ed. in English) 52:4156–4159 [DOI] [PubMed] [Google Scholar]
- Wang J, Lim K, Smolyar A, Teng M, Liu J, Tse AG, Liu J, Hussey RE, Chishti Y, Thomson CT, Sweet RM, Nathenson SG, Chang HC, Sacchettini JC, Reinherz EL (1998) Atomic structure of an alphabeta T cell receptor (TCR) heterodimer in complex with an anti-TCR fab fragment derived from a mitogenic antibody. EMBO J 17:10–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J-h, Mallis RJ, Reinherz EL (2008) Immunodominant-peptide recognition: beta testing TCRalphabeta. Immunity 28:139–41 [DOI] [PubMed] [Google Scholar]
- Wang J-h, Reinherz EL (2012) The structural basis of alpha-beta T-lineage immune recognition: TCR docking topologies, mechanotransduction, and co-receptor function. Immunol Rev 250:102–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Pizarro JC, Holmes MA, McBeth C, Groh V, Spies T, Strong RK (2011) Crystal structure of a gammadelta T-cell receptor specific for the human MHC class I homolog MICA. Proc Natl Acad Sci U S A 108:2414–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Gagnon E, Call ME, Schnell JR, Schwieters CD, Carman CV, Chou JJ, Wucherpfennig KW (2008) Regulation of T cell receptor activation by dynamic membrane binding of the CD3epsilon cytoplasmic tyrosine-based motif. Cell 135:702–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Chen Q, Mallis RJ, Zhang H, Liu J-H, Reinherz EL, Wang J-H (2011) A Conserved Hydrophobic Patch on Vβ Domains Revealed by TCRβ Chain Crystal Structures: Implications for Pre-TCR Dimerization. Front Immunol 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote PW, Robson SA, Dubey A, Boeszoermenyi A, Zhao M, Wagner G, Arthanari H (2018) Optimal control theory enables homonuclear decoupling without Bloch-Siegert shifts in NMR spectroscopy. Nat Commun 9:3014 [PubMed: 30069002] [DOI] [PMC free article] [PubMed] [Google Scholar]


