Abstract
We studied the memory representations that control execution of action sequences by training rhesus monkeys (Macaca mulatto) to touch sets of five images in a predetermined arbitrary order (simultaneous chaining). In Experiment 1, we found that this training resulted in mental representations of ordinal position rather than learning associative chains, replicating the work of others. We conducted novel analyses of performance on probe tests consisting of two images “derived” from the full five-image lists (i.e., test B, D from list A→B→C→D→E). We found a “first item effect” such that monkeys responded most quickly to images that occurred early in the list in which they had been learned, indicating that monkeys covertly execute known lists mentally until an image on the screen matches the one stored in memory. Monkeys also made an ordinal comparison of the two images presented at test based on long-term memory of positional information, resulting in a “symbolic distance effect.” Experiment 2 indicated that ordinal representations were based on absolute, rather than on relative, positional information because subjects did not link two lists into one large list after linking training, unlike what occurs in transitive inference. We further examined the contents of working memory during list execution in Experiments 3 and 4 and found evidence for a prospective, rather than a retrospective, coding of position in the lists. These results indicate that serial expertise in simultaneous chaining results in robust absolute ordinal coding in long-term memory, with rapidly updating prospective coding of position in working memory during list execution.
Keywords: Sequence, learning, planning, list, serial, macaque, coding
Foraging, tool use, nest construction, communication, and many other behaviours require execution of serially organised sequences of actions (Biegler, 2006; Scarf & Colombo, 2008). The cognitive processes likely to underlie at least some serially organised behaviour have been studied in animals using simultaneous chaining in which subjects learn to select simultaneously presented images in a predetermined order (e.g., A→B→C→D→E; Terrace, 1984, 2005; Terrace, Son, & Brannon, 2003). The spatial arrangement of the images is varied randomly from trial to trial, requiring subjects to learn the order of the images rather than a particular spatial arrangement of actions. Animals receive a food reward only after touching all of the images in the correct order. An error at any point in the sequence terminates the trial. The probability of correctly completing even short lists by chance is very small. Many species successfully learn lists consisting of at least three images in this paradigm, indicating that the ability to form stable long-term representations of sequences is phylogenetically widespread (humans: Inoue & Matsuzawa, 2009; Merritt, MacLean, Jaffe, & Brannon, 2007; Merritt & Terrace, 2011; apes: Inoue & Matsuzawa, 2009; monkeys: D’Amato & Colombo, 1989; Orlov, Yakovlev, Hochstein, & Zohary, 2000; Scarf, Danly, Morgan, Colombo, & Terrace, 2011; and birds: Pfuhl & Biegler, 2012; Terrace & McGonigle, 1994).
Several types of representations could be stored in long-term memory (LTM) and control execution of ordered sequences. Animals might use “chaining” to learn sets of stimulus-stimulus associations between images, such that image A is associated with B, B with C, C with D, and so on. In this case, the list is represented as a series of individual associations (A→B, B→C, and C→D) that are linked together in a response chain to generate the fully ordered sequence of responses (Osgood, 1953; Pierce & Cheney, 2008). Alternatively, animals may encode the list as an ordered series such that A comes first, followed by B, followed by C, and so on. (A→B→C→D→E).
One test for knowledge of ordinal position is “derived list” tests, in which subjects are presented with images from independently learned lists (e.g., Chen, Swartz, & Terrace, 1997; Harris, Beran, & Washburn, 2007; Scarf & Colombo, 2011). Monkeys (Chen et al., 1997) and pigeons (_S1_Reference38Scarf & Colombo, 2010a, 2011) perform better on maintained-derived tests (e.g., A1→C2→B3, where letters represent the order in which images should be selected, and subscript numbers indicate in which list the image was originally learned), in which the trained ordinal position is maintained at test, as compared with derived-changed tests in which positions during training are exchanged during test (e.g., A1→C2→B3). Coding of ordinal position is demonstrated because subjects treat a “first” or “second” image as first or second whether or not it is presented together with the other images originally comprising a particular list. In contrast, and as the name implies, associative chaining would be disrupted any time the order of specific images in a lists was disrupted. Similarly, associative chaining alone cannot explain performance in two-image probe tests in which subjects are presented with pairs of images from previously learned lists (Figure 1). In these probe tests, any two images from previously learned lists are presented, and some test pairs contain “gaps” between images such as B and D (D’Amato & Colombo, 1988; Scarf & Colombo, 2008; Terrace et al., 2003). Chaining would result in chance performance on these test pairs, because there is no stimulus C to link B and D, yet monkeys correctly order these test pairs (e.g., B→D). In addition, monkeys choose the sequentially earlier image even when the two images are from two separately trained lists (e.g., A1→C2; where A is from List 1 and C is from List 2). This indicates that the long-term representation of the ordered sequence contains information about the ordinal position of each image that transfers across separately learned lists, something akin to a representation of which image is first, second, third, and so on.
Figure 1.
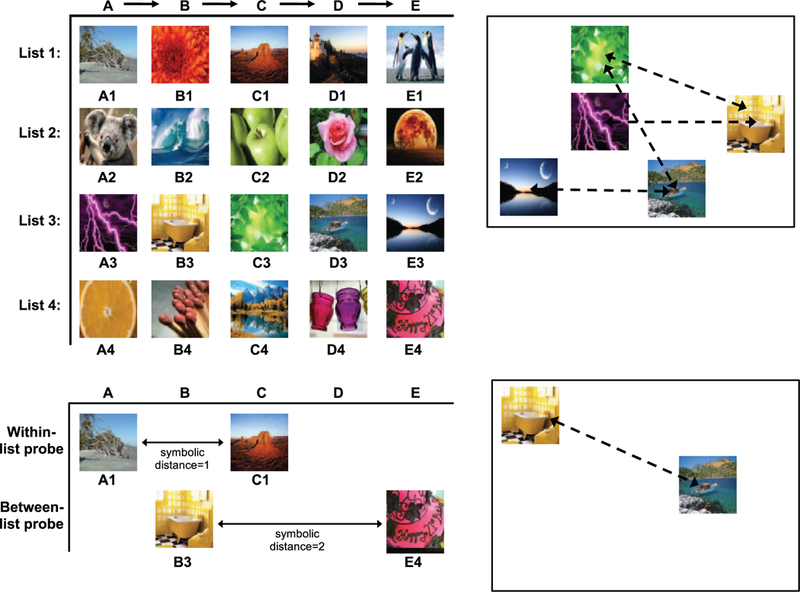
Task design. Upper left panel: Lists trained requiring indicated order in Experiment 1 and used in Experiments 2–3. Upper right panel: Example test screen of 5-image list from list 3 in which list images appear in any of 12 possible locations. The configuration of images was changed from trial to trial. Arrow heads on dotted lines represent the correct order as indicated in A. If all images were touched in this order, a reward was delivered; an error at any point resulted in an unpleasant tone and time-out. Lower left panel: Examples of the within and between-list probe tests used in Experiment 2, which were randomly intermixed with normal trials from all four lists. Symbolic distance is indicated by the number of images “missing” between the two images presented at test. In the within-list probe test AI–CI, one image, B, is “missing”, so the symbolic distance is I. Between-list probe tests used one image from list I and one from list 2 or one from list 3 and one from list 4, as shown in the bottom example, B3–E4. Lower right panel: Example of a within-list B3–D3 test display in which the correct order is indicated by the arrow head; touches in either the correct or incorrect order were rewarded on probe trials.
Although there is strong evidence that non-human primates encode the ordinal position of images in sequences, the precise properties of these ordinal representations is less clear. According to the absolute position model, monkeys encode images in LTM as being first, second, third, and so on in the list. For example, the picture of the canyon is always in position three, and the picture of the penguin is in position five (Figure 1). Such positional knowledge does not result from associating images with one another, but instead results from associations between the images and abstract codes for specific positions in the lists. On two-image probe tests, the subject first selects the image associated with the earlier absolute position. This absolute long-term knowledge of position is analogous to knowing that Ben is 6’4”, Eric is 5’8”, and Seth is 5’10”. If asked who is taller, you make a decision based on absolute heights of each individual and select the person with the highest value. According to the relative position model, animals encode which images come before others, rather than which absolute position is appropriate for each image. For example, the picture of the flower comes before the picture of the canyon and after the picture of the beach. On two-image probe tests, subjects select the image with the earlier relative position (e.g., A→C). This is analogous to knowing that Ben is taller than Seth who is taller than Eric without knowing or attending to absolute heights.
Currently, there is no strong evidence to support either absolute or relative coding of ordinal position in simultaneous chaining over the other. That monkeys reliably select the image that occurred earlier in its own list on between-list tests (Terrace et al., 2003) is consistent with both representational systems. In a related ordering task, transitive inference, monkeys have been found to perform like they do in simultaneous chaining when presented with “derived list” tests consisting of images from two separately learned lists. The training in transitive inference is substantially, and perhaps critically, different than training in simultaneous chaining in that animals are trained on two test pairs at a time in transitive inference, rather than an entire list in which all images are simultaneously seen and touched as in simultaneous chaining. In transitive inference, monkeys learn to select images with the earlier position in the list through training with individual premise pairs (e.g., A1 > B1, B1 > C1, and so on) until they construct entire lists (A1 > B1 > C1 > D1 > E1 and A2 > B2 > C2 > D2 > E2). When lists learned by the transitive inference method were linked by training animals to know that the last image in one list is ranked higher than the first image in another list (E1 > A2), monkeys merged the two lists into one long list (e.g., A1 > B1 > C1 > D1 > E1 >A2 > B2 > C2 > D2 > E). They disregard the original absolute positions of the images and instead select the image with the higher relative position in the new larger list (Gazes, Chee, & Hampton, 2012; Treichler & Raghanti, 2010; Treichler, Raghanti, & Van Tilburg, 2007). Because monkeys initially select images in “derived list” tests according to the position they occupied in the list in which they were learned, but after linking select images in the order consistent with one large list, it appears that in transitive inference, choice behaviour is flexibly controlled by either the absolute ordinal position of images or the relative ordinal position of images. It appears that monkeys either simultaneously encode both types of ordinal information or switch rapidly to a relative positional code when lists are linked. Similar linking training has not been conducted with simultaneous chaining lists, so we do not have evidence to discriminate between these alternative representational systems.
In addition to a stable representation of lists in LTM, simultaneous chaining also requires working memory to track which positions in list execution have been completed, including representation of which images have been selected and which remain to be selected as the subject moves through the list (Ginsburg & Gevers, 2015; Huber, Klein, Moeller, & Willmes, 2016). Although some researchers have investigated how this ordinal code is accessed in working memory (Scarf et al., 2011), relatively little work has been done that investigates the interaction of LTM and working memory required in this task.
The following four experiments focus on characterising the representations underlying simultaneous chaining performance in monkeys. Experiment 1 replicated Terrace et al. (2003) to establish the phenomena to be explained and shed light on how ordinal information is accessed and used to solve two-image tests. In Experiment 2, we trained monkeys to “link” two separately learned lists to determine the extent to which knowledge of the position of each image in simultaneous chaining is absolute and independent of the other images in the list or more flexible and based on relative location in the list. With Experiments 3 and 4, we examined the contributions of working memory to performance on simultaneous chaining. We evaluated whether the current position in a particular execution of a list is based on prospective coding, retrospective coding, and/or tracking of number of responses made.
General materials and method
Subjects and apparatus
Six 6- to 7-year-old male rhesus monkeys (Macaca mulatta) were used in Experiments 1, 3, and 4. Subjects in Experiment 2 were 12 different 9- to 10-year-old male rhesus monkeys. All monkeys had at least 3 years of computerised testing experience, but no prior experience with simultaneous chaining paradigms. Monkeys were pair-housed whenever possible and kept on a 12:12 light:dark cycle with light onset at 7:00 am. Subjects were fed a full ration of food each day and water was available ad libitum.
Monkeys were tested on computerised touchscreen systems in their home cages. Each system consisted of a 15-inch liquid crystal display (LCD) colour monitor (3M, St. Paul, MN and Elo, Milpitas, CA) running at a resolution of 1024 × 768 pixels, generic stereo speakers, two automated food dispensers (Med Associates Inc., St. Albans, VT), and two food cups located below the screen.
General procedure
Before testing each day, pair-housed monkeys were separated by insertion of opaque plastic dividers with slits that allowed visual and physical contact, but prevented access to the cagemate’s testing equipment. Testing systems were locked to the front of each monkey’s cage, and cage doors were raised, giving subjects full access to the screen during testing. Food rewards were nutritionally balanced banana or mixed fruit–flavoured pellets (Bio-Serv, Frenchtown, NJ). Testing was conducted daily between 10 am and 5 pm, 6 days per week. Monkeys worked at their own pace during these hours, completing between 120 and 840 trials. On most days, monkeys completed other cognitive tasks on the same day.
The coloured digital images (200 × 200 pixels) comprising the lists were randomly selected photographs provided by stock photos included in the Windows software.
Data analysis
Proportions were arcsine transformed before statistical analysis to better approximate the normality assumption underlying parametric statistics (Keppel & Wickens, 2004, p. 155). The Geisser–Greenhouse correction–adjusted degrees of freedom are reported whenever the sphericity assumption was violated (Keppel & Wickens, 2004, p. 378). All t-tests were two-tailed. Latencies reported were medians for correct responses only. An alpha level of p < .05 was applied in all analyses.
Experiment 1: ordinal knowledge in LTM
Monkeys were trained to select images from five-image lists in a predetermined order (Harris et al., 2007; Merritt et al., 2007; Scarf & Colombo, 2008; Terrace, 2005). After learning four separate lists, the subjects were presented with two types of probe trials: within-list tests consisting of two images from any one of the learned lists and between-list tests consisting of two images, one from each of two different lists (e.g., Terrace et al., 2003). This experiment replicates earlier work indicating that choice is controlled by representations of ordinal position rather than associative chaining and lays the foundation for the following three experiments.
If monkeys encode the ordinal position of images, we should obtain two standard performance patterns. The symbolic distance effect (SDE) results when monkeys are faster or more accurate when tested with images further apart in lists, for example, subjects are quicker or more accurate in a test consisting of images B and D compared with B and C. The first-item effect (FIE) is observed when monkeys are faster or more accurate when tested with images earlier in a list. For example, they are quicker or more accurate in tests with B and C than with C and D. The SDE is seen across many different tasks that involve ordering stimuli (e.g., Hinton, Dymond, von Hecker, & Evans, 2010; Jensen, Altschul, Danly, & Terrace, 2013; Scarf & Colombo, 2008) and suggests that the ordinal positions of images are directly compared at test. The further apart the images, the easier the comparison. The FIE suggests that at test, monkeys mentally “step-through” the list of images until an image in mind matches one of the images on the screen. The monkey then selects that image. Because it takes time for monkeys to complete each mental step through the sequence, a match between the image in mind and one of the images on the screen is obtained sooner in tests involving images that occur early in the list.
Method
Training on five-image lists.
All six monkeys that participated in Experiment 1 learned the same five-image sequences through trial and error (Figure 1). The order in which the monkeys were to select the images was predetermined randomly and then held constant. On every trial, each image in the list was randomly assigned to 1 of 12 locations. Monkeys started the trials by touching a green start square (FR 2). Two touches (FR 2) were required to select each image. Training on the first list began with the first and second images to be selected, A1 and B1, presented simultaneously on a white background. After each correct touch, a pleasant tone occurred, and a 200-ms flash of the screen followed during which the images on the screen briefly disappeared and then reappeared. At the end of a successful trial, subjects were rewarded with another positive auditory stimulus and a food reward. If any image was selected in the incorrect order, the trial was immediately terminated, and a negative auditory signal and a 10-s timeout followed, during which the screen was black.
Once a criterion of 70% correct responses in a 50-trial session was reached, a third image (C1) was added to the list and was to be selected after A and B. This procedure, with the same accuracy criterion required for the addition of each new image, was applied until a five-image list was learned: A1→B1→C1→D1→E1, at which point, a new five-image list was then introduced using the same procedure, starting with A2 and B2. Repeat responses to a given image in which the list order was not violated were not counted as errors (e.g., A→B→B→B→C→D→E). This was done because, though the FR was 2, monkeys often made more responses than required. Thus, the probability of selecting the first image correctly in a full list by chance is 1 in 5, whereas the probability of guessing correctly on each of choices 2 through 5 is 1 in 4. The probability of completing all five choices correctly by chance is the product of these probabilities (.25 × .25 × . 25 × .25 × .25 = .00078), or .078%. After four five-image lists were learned, subjects were presented with two-image probe tests.
Two-image probe tests.
Subjects were presented with two-image probe tests randomly intermixed with normal five-image lists to determine how the order of images in the list was encoded mentally (D’Amato & Colombo, 1988; Terrace, 2005; Terrace et al., 2003; Terrace & McGonigle, 1994). Probe trials consisted of two images presented on a white screen in two randomly selected locations and were either within-list tests (e.g., A1 and B1) or between-list tests (e.g., D3, E4; Figure 1, lower panels). Sessions consisted of 120 trials: 40 trials were normal five-image lists and 80 trials were two-image probe tests. Of the probe trials, 40 trials were between-list tests, in which images were either from Lists 1 and 2 or from Lists 3 and 4, creating 40 unique trial types, and 40 trials were within-list tests consisting of 10 distinct trial types for each list. Probe trials did not end until subjects selected both images; reward followed every trial regardless of the order in which images were selected to prevent new learning on probe trials. As in normal trials, consecutive selections of the same image were not counted as errors. Subjects received 30 probe sessions.
Analysis.
Many published SDE analyses are confounded by unequal distribution of image pairs with different first images, and FIE analyses can be confounded by unequal distribution of symbolic distances. Because we collected an unusually large set of test responses, we were able to control for first image in the analyses of SDE and for SDE in analyses of the FIE. We held the first image constant in SDE analysis and held symbolic distance constant in FIE analyses.
Results and discussion
Acquisition of five-image lists
Learning to learn.
Monkeys learned lists faster as they gained experience (Figure 2; repeated measures analysis of variance (rANOVA); list number: F3,15 = 29.29, p < .001), demonstrating the well-established “learning to learn” phenomenon (Harlow, 1949; Merritt et al., 2007; Terrace et al., 2003). A greater number of sessions were required to reach criterion each time the list was lengthened (Figure 2; rANOVA; list size: F1.62,81 = 18.07, p = .023), which is not surprising given that the rate of correct responses expected by chance decreases as list size increases (two-image list: 50%; three-image list: 8.33%; four-image list: .93%; and five-image list: .078%). However, an interaction between list size and number (F2,45=3.28, p = .004) revealed that successfully completing longer lists got easier as subjects learned more lists.
Figure 2.
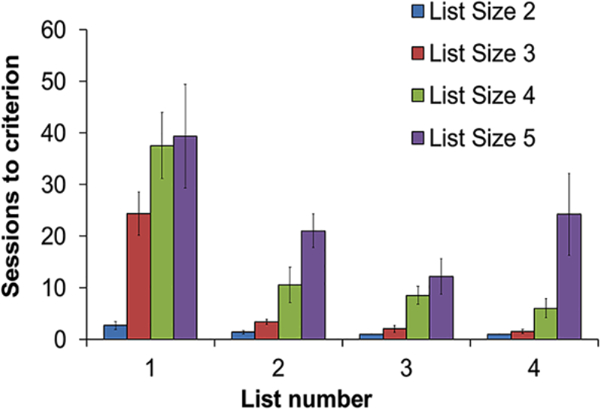
Number of sessions required to reach criterion for each list and list length. List number indicates the order in which monkeys learned lists; error bars represent standard errors of the mean.
Performance on two-image probe tests.
Data were analysed by first comparing overall accuracies and response latencies on within- and between-list tests. We then determined whether there was a SDE and/or FIE.
Monkeys were more accurate than expected by chance on both within- and between-list tests, selecting the image with the earlier ordinal position first (one-sample t-tests, within: t5 = 10.55, p < .001; between: t5 = 7.54, p = . 001). Accuracy and response latencies did not differ between within- and between-list tests (accuracy: within: M = 78%, SD = 4.85; between: M= 73%, SD = 7.75; paired sample t-test: t5 = 12.59, p = .173; response latency: within: M= 1,126.7 ms, SD = 164.44; between: M= 1,186.3 ms, SD = 177.1; paired sample t-test: t5 = –2.51, p = .054). Comparable performance on within- and between-lists tests replicates existing evidence from other groups indicating that choice is controlled by ordinal information rather than associative chaining (Terrace, 2005; Terrace et al., 2003), as monkeys correctly identified the earlier image with the similar latency regardless of whether the images were trained together or not.
For within-list pairs, analyses of the SDE revealed that choices were more accurate and, for the most part, faster when monkeys discriminated pairs of images further apart in the list when controlling for the first item (e.g., AB, AC, AD, and AE; Figures 3 and 4, upper panels, statistics in Table 1). Analyses of FIE revealed that choices were faster but not more accurate for pairs containing items earlier in the list when controlling for the distance between images (e.g., AC, BD, and CE; Figures 3 and 4, upper panels statistics in Table 2). The mixed results across accuracy and response latency for SDE and FIE is consistent with previous findings that the SDE is found in either accuracy or response latency (Scarf & Colombo, 2008) but rarely both (Gazes et al., 2012). Together, these findings indicate that monkeys represent the order of the images and access that representation at test.
Figure 3.
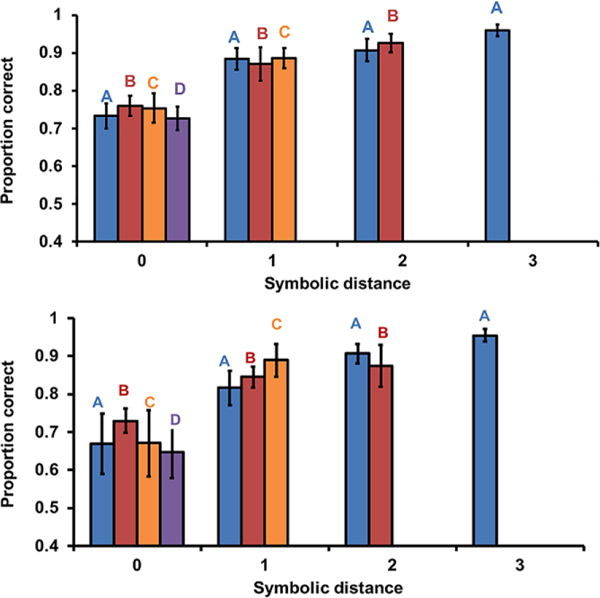
Proportion of trials correct for each probe test type on within-list probe tests (upper panel) and between-lists probe tests (lower panel) in Experiment 1. Letter labels above the bars indicate which image should be selected first (e.g., C at symbolic distance 0, C is touched first and D second); error bars are standard errors of the mean.
Figure 4.
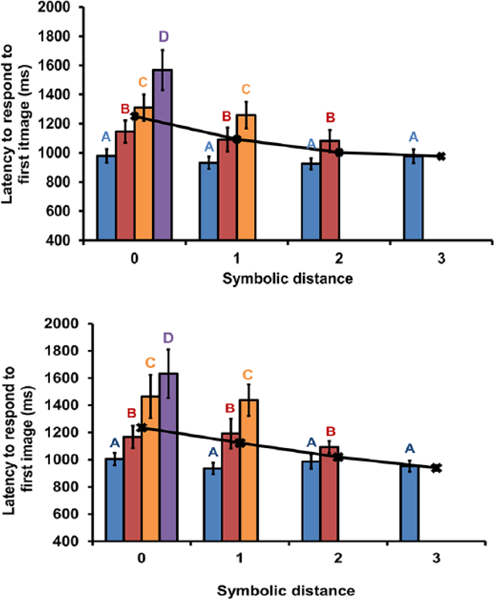
Response latencies to the first image on the first image on (upper panel) within-list probe tests and (lower panel) between-lists probe tests in Experiment 1. Letter labels above the bars indicate the first image that was selected and the corresponding median response latency for selection of that image; for example, at symbolic distance 0, A (far left blue bar) indicates the average latency to touch A when A and B were presented together; for symbolic distance 1, B (red bar) indicates the average latency to touch B when B and D were presented together; black lines represent the average latency to respond to the first image within each symbolic distance; our main analyses were not performed on these averages but most published symbolic distance effect analyses are; errors bars are standard errors of the mean.
Table 1.
rANOVA results for evaluation of SDE when first item was held constant.
| Trial type | Symbolic distance | Data analysed | F | df | P |
|---|---|---|---|---|---|
| Within list | A | Accuracy | 20.713 | 3,15 | <.001* |
| Within list | B | Accuracy | 26.362 | 2,10 | <.001* |
| Within list | C | Accuracy | 17.231 | 1,5 | .009* |
| Within list | A | Response latency | 3.802 | 3,15 | .033* |
| Within list | B | Response latency | 5.38 | 2,10 | .028* |
| Within list | C | Response latency | 2.537 | 1,5 | .172 |
| Between list | A | Accuracy | 5.958 | 3,15 | .007* |
| Between list | B | Accuracy | 2.769 | 2,10 | .110 |
| Between list | C | Accuracy | 43.868 | 1,5 | .001* |
| Between list | A | Response latency | 2.517 | 3,15 | .098 |
| Between list | B | Response latency | 1.187 | 2,10 | .345 |
| Between list | C | Response latency | 0.155 | 1,5 | .710 |
rANOVA: repeated measures analysis of variance; SDE: symbolic distance effect.
Indicates significant p values at a .05 alpha level.
Table 2.
rANOVA results for evaluation of FIE when symbolic distance held constant.
| Trial type | Symbolic distance | Data analysed | F | df | P |
|---|---|---|---|---|---|
| Within list | 0 | Accuracy | 0.282 | 3,15 | .837 |
| Within list | 1 | Accuracy | 0.039 | 2,10 | .962 |
| Within list | 2 | Accuracy | 1.863 | 1,5 | .23 |
| Within list | 0 | Response latency | 26.133 | 3,15 | <.001* |
| Within list | 1 | Response latency | 17.737 | 2,10 | .001* |
| Within list | 2 | Response latency | 12.638 | 1,5 | .016* |
| Between list | 0 | Accuracy | 0.327 | 3,15 | .806 |
| Between list | 1 | Accuracy | 0.912 | 2,10 | .433 |
| Between list | 2 | Accuracy | 0.024 | 1,5 | .882 |
| Between list | 0 | Response latency | 8.393 | 3,15 | .002* |
| Between list | 1 | Response latency | 16.338 | 2,10 | .001* |
| Between list | 2 | Response latency | 51.961 | 1,5 | .001* |
rANOVA: repeated measures analysis of variance; FIE: first-item effect.
Indicates significant p values at a .05 alpha level.
For between-list pairs, analyses of the SDE revealed that choices were more accurate but not faster when discriminating pairs of images further apart in the list when controlling for the first item (e.g., AB, AC, AD, Figures 3 and 4 lower panels, and AE; statistics in Table 1). Analyses of FIE revealed that choices were faster but not more accurate for pairs containing items earlier in the list when controlling for the distance between images (e.g., AC, BD, and CE (Figures 3b and 4b3 and 3 lower panels); statistics in Table 2). Overall, SDE and FIE patterns for within- and between-list pairs were strikingly similar, providing strong evidence for an ordinal representation in which the code for position was common across lists. The occurrence of the FIE suggests that monkeys covertly execute lists until an image held in mind matches one of the two images on the screen, whereas the consistent SDE suggests that monkeys compare the ordinal position of two images presented at test.
The results of Experiment 1 replicated previous findings, suggesting monkeys solve simultaneous chaining tasks using an ordinal representation (e.g., Scarf & Colombo, 2008; Terrace, 2005; Terrace et al., 2003). In Experiment 2, we explored the properties of this ordinal representation by determining the extent to which it encodes relative versus absolute positional information.
Experiment 2: list linking
Experiment 1 replicated previous findings indicating that monkeys encode and access ordinal information. The resulting SDEs and FIEs are equally consistent with coding of absolute position of images within the lists and relative position of the images with respect to each other. We conducted Experiment 2 to discriminate between these alternatives. In transitive inference, another ordinal task which shares some features of simultaneous chaining, monkeys appear to encode the relative position of images. Relative coding is indicated by the fact that list positions can be integrated by training that links two separate lists. Subjects learn two lists (A1 > B1 > C1 > D1 > E1 and A2 > B2 > C2 > D2 > E2) by training on adjacent premise pairs (e.g., A > B, B > C, etc.) and select the item higher in its list on both within- (C1 > E1) and between-list tests (B2 > D1). However, when lists are then linked through training on a between-lists premise pair, E1 > A2, subjects respond as if they had constructed one long list, for example, by selecting D1 over B2 (Gazes et al., 2012; Treichler & Raghanti, 2010). This indicates that positional information is relative.
Previous research and the results from Experiment 1 show that in simultaneous chaining, monkeys similarly select the image higher in its trained list on within- and between-list test pairs, indicating that, as in transitive inference, monkeys encode the ordinal position of the images. However, tests of list linking have not been conducted in simultaneous chaining, so the extent to which this representation is absolute or relative has not been determined. We tested whether relative coding occurs by conducting list-linking training as has been done in transitive inference. If monkeys do not link lists and instead continue to perform as they do on between-list test pairs, it would suggest they have formed a long-term representation of the absolute position of images in lists, with each image associated with a specific position in the order. However, if the ordinal representation in simultaneous chaining is relative, then, as in transitive inference, monkeys should represent a single 10-image list.
Method
Subjects were 12 9–10-year-old male rhesus monkeys. These subjects did not participate in any of the previous or following experiments. Prior to this experiment, subjects were trained and tested on the four basic simultaneous chaining lists as described in Experiment 1. After two new lists were learned, they received training with just the linking pair, and then received test sessions that comprised normal five-image tests with both lists, linking pair trials, and probe tests which included non-reinforced test pairs consisting of an image from List 1 and an image from List 2. Due to the addition of these non-reinforced probe trials, throughout all three phases of list-linking training, food reinforcement was adjusted based on average performance such that average potential reinforcement maintained a rate of 75%−80% adjusted for average performance. This was done so that motivation on these new trials was similar to that on normal trials (Experiment 1).
Phase 1: pre-list linking.
Monkeys were first trained on two new five-image lists as in Experiment 1 (A1 > B1 > C1 > D1 > E1 and A2 > B2 > C2 > D2 > E2). After reaching the 70% accuracy criterion on each list separately, monkeys received intermixed sessions with both lists on which they had to reach a criterion of 50% correct on each list simultaneously within a 120-trial session. Subjects who did not perform above 50% on the two lists after five sessions received remedial training, in which the poorly performed list was retrained starting with three images and incrementing back up to the five-image list using the same training method as used for novel lists described above. After subjects reached 70% accuracy on the five-image list, they were reintroduced to sessions with two intermixed lists.
Once subjects performed above 50% on both lists in intermixed sessions, they received five 120-trial test sessions identical to those presented in Experiment 1, except it contained within- and between-list test trials with images from only the two new lists rather than from four. Performance on between-list tests during these sessions was used to measure the extent to which monkeys linked the two lists into one large list.
Phase 2: list linking.
Subjects received 25-trial sessions of two-image trials containing image E1 (last image from List 1) and image A2 (first image from List 2) until they made at least 80% correct responses in a single session. Images were displayed as they were in two-image probe tests, except that subjects were only reinforced for correctly choosing image E1 and then A2, and trials would terminate for incorrect initial choice of image A2. Correct responses were reinforced with 100% positive auditory feedback accompanied by a food reinforcer on 80% of trials. Incorrect responses resulted in negative auditory feedback and a 10-s timeout. Which list was first (List 1 or 2) in the new linked order was counterbalanced across subjects, with six monkeys receiving each order. Although different for different subjects, here, List 1 will consistently refer to the list which would occur first if monkeys linked the two lists.
After a subject reached criterion on the linking pair, it was given 84-trial sessions that consisted of 28 two- image linking pair trials, 28 five-image List 1 trials, and 28 five-image List 2 trials. To ensure that subjects remembered each list and the linking pair, a criterion of 80% on the linking pair and 50% on each list (chance = .078%) was required to move on to Phase 3 of testing. Reinforcement for the five-image lists was 100% food and auditory, as in training, and the linking pair was 90% food and 100% auditory. Food reinforcement for the linking pair was increased slightly from training, when it was 80%−90%, to accommodate the more difficult five- image lists while maintaining overall food reinforcement at about 75%−80%.
Phase 3: assessment of linking.
In Phase 3, subjects received five 128-trial sessions that consisted of 40 five-image list trials (20 from List 1 and 20 from List 2), 10 linking pair test trials, 40 within-list probe test trials (20 from List 1 and 20 from List 2), and 38 between-list probe test trials, which included all possible between-list combinations except the linking pair. Reinforcement for the linking pair and five-item trials remained the same as in training. Probe trials were reinforced non-differentially with a positive auditory reinforcer on 100% of trials, accompanied by a food reward on 60% of trials.
Test trials were classified as consistent if both linking and not linking would result in the same order of image selection. For example, test B1, C2 is a consistent trial because both absolute and relative codes indicate selection of B1 before C2. Tests trials were classified as inconsistent when linking and not linking would generate competing image selections. For example, Test B2, C1 is an inconsistent trial because relative coding indicates selection of C1 (List 1) before B2, but absolute coding indicates selection of B2 before C1. Consistent and inconsistent trials were randomised and counterbalanced within sessions for each trial type. Selection of the image from List 1 before selection of the image from List 2 on inconsistent trials would indicate that monkeys linked the two lists after training on the linking pair of images.
In studies of transitive inference in which monkeys received linking training, only critical internal test pairs were examined, not pairs including end anchors (Gazes et al., 2012; Treichler & Raghanti, 2010). This is because test pairs with end anchors might be contaminated by linking training or the salience of these images might have been enhanced by proximity to the reward. For example, on an E1, B2 test, E1 may have been so reliably trained to be selected first from linking training (E1→A2) that performance on tests containing E1 would not accurately reflect the extent to which the lists had been linked. Accordingly, we also excluded test pairs that included end anchors. We, therefore, averaged performance only on inconsistent tests that did not contain any images from the linking pair, resulting in the following critical internal test pairs: C1, B2; D1, B2; and D1, C2.
Results and discussion
Linking training did not cause lists to be linked, and subjects appeared to use absolute rather than relative coding. Before linking training, subjects chose images according to ordinal position in each native list on between-list test trials, as in Experiment 1. This was true for pairs that were to become both consistent and inconsistent pairs after linking training (Figure 5; blue bars; one-sample t-tests: consistent: t11 = 5.64, p < .001; inconsistent: t11 = 3.72, p = .003). After linking training, subjects continued to select images according to ordinal position within their initially trained five-image lists on both consistent and inconsistent between-list trials (Figure 5; red bars; one-sample t-tests: consistent: t11 = 5.20,p < .001; inconsistent: t11 = 3.99, p = .002). Monkeys performed similarly on consistent and inconsistent pairs pre- and post-linking training (paired sample t-tests: consistent: t11 = .191, p = .852; inconsistent: t11 = –372, p = .717), revealing that linking training did not cause the lists to be linked or any substantial change of any kind in performance with these pairs. If monkeys had linked the lists and a 10-image list had been mentally constructed, the tendency to choose based on absolute ordinal position within native 5-image lists on inconsistent trials would have decreased significantly after linking training. This is because image selection based on absolute position is the opposite of relative position on inconsistent trials. The representation of ordinal position of images in simultaneous chaining lists thus appears to be based on the absolute position of images in lists rather than the position of images relative to one another, as in transitive inference.
Figure 5.
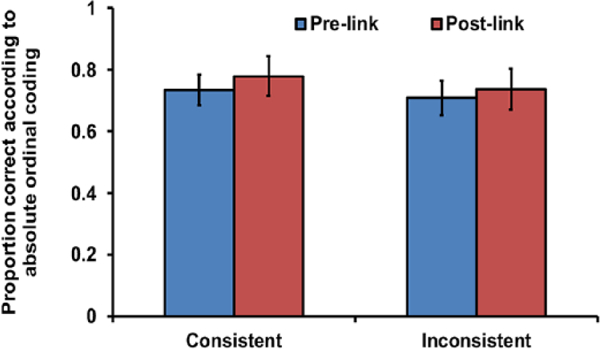
Proportion of choices according to absolute coding within the native five-image lists learned in Experiment 2. Choice based on relative coding of a linked 10-image list would have resulted in scores below chance for inconsistent pairs indicated by the red bar (far right); error bars represent standard errors of the mean.
Encoding of the absolute ordinal positions of images in learned lists, as shown in this experiment, can only partly account for performance in simultaneous chaining. Although the position of each image in the lists is encoded in LTM, working memory must dynamically monitor the current position in the list during list execution. It is not enough to know the order in which images should be selected. Monkeys also have to monitor whether they are making their first choice, second choice, and so on. We evaluated the extent to which monkeys used a prospective or retrospective code in working memory to solve these tasks in Experiments 3 and 4.
Experiment 3: prospective versus retrospective coding in working memory
Having a representation of a list in LTM is necessary but not sufficient to successfully execute lists. Subjects must also keep track of the current location in the list either by remembering which images have already been selected or by remembering which images remain to be selected. Thus, LTMs for the order in which images should be selected must be processed in working memory during list execution. Some non-human animals use working memory processes to keep in mind the next image to select in memory when executing lists (monkeys: Beran & Parrish, 2012; Beran, Evans, Klein, & Einstein, 2012; Scarf et al., 2011 and jackdaws: Pfuhl & Biegler, 2012). Prospective coding refers to keeping active in working memory future responses, whereas retrospective coding refers to keeping active in working memory past responses. For example, rats in a 12-arm radial arm maze must remember which arms have been visited and/or which ones remain to be visited. To minimise the memory load required to succeed in the radial maze, rats switched from using a retrospective code early in the trial, when there are fewer previous responses to remember, to using a prospective code in the middle of the list, when there are fewer to-be-remembered responses to remember (Cook, Brown, & Riley, 1985).
The experiment described above capitalised on the fact that working memory is a limited capacity system. When subjects are engaged in a cognitively taxing task, anything that is distracting or imposes an additional cognitive load can impair performance (Basile & Hampton, 2013; Beran et al., 2016; Logie, 1986). Tests with distractors or competing cognitive load can be used to assess the contribution of working memory because concurrent loads direct attention away from what is normally actively maintained on tests without concurrent cognitive load. We, therefore, interposed a delay and a distracter dot that monkeys had to touch to advance the trial at different points of interpolation during list execution to determine whether a prospective or retrospective code governs working memory processes in simultaneous chaining. This distractor task was a simple motoric task designed to interrupt normal list execution. If a prospective code is used, the most likely errors should be forward errors, which are errors to images later in the list than the next correct response. If a retrospective code is used, the most likely errors should be backward errors, which are errors to images earlier in the list than the next correct response. Finally, if subjects use both retrospective and prospective codes, the overall proportion of forward and backward errors should be similar. If monkeys shift from a retrospective code to a prospective code as list execution progresses, like rats in the radial arm maze (Cook et al., 1985), then the proportion of forwards and backwards errors would vary as a function of when during list execution the distractor occurred. To test for the presence of these hypothetical coding strategies, we examined the proportion of forwards and backwards errors at each point of interpolation.
Method
The same six monkeys that participated in Experiment 1 were used in Experiment 3. The monkeys from Experiment 2 were not used. List 1 was used (Figure 2 upper left panel). Monkeys were tested on 30 sessions and each 120- trial session consisted of 72 normal five-image list tests, run as in previous experiments, and 48 five-image probe tests, in which a delay and distracter interrupted execution of the sequence at one of four possible points of interpolation: between images A-B, B-C, C-D, or D-E. At the point of interpolation, a 200-ms blank white screen was followed by presentation of a blue dot (100 pixels in diameter) that monkeys touched to advance the trial. The purpose of the required touch of the dot was to prevent monkeys from touching the correct list image location during the delay. The location of the dot was therefore selected at random to be in any of the possible locations not used for the list images on that trial. On a point of interpolation A-B probe, for example, once A was touched, the screen would go white and a blue dot would appear in any of the seven available screen locations not occupied by list images. After the dot was touched (FR 2), all five images reappeared in the same spatial locations as before the delay and the trial continued as normally. If images were not correctly selected before the point of interpolation, the trial ended and the distracter was not presented. Data were recorded but were not included in analyses. Touches made to the image touched just before the distracter (−1 error) were counted and reinforced as errors.
Results and discussion
The goal of using the distracter dot was to disrupt maintenance of the information in working memory so that the proportion of errors at each point of interpolation could be assessed. We, therefore, first determined how addition of the distracter dot affected performance. Monkeys continued to perform above chance in trials with the distracter dot (M = 68% correct, one-sample t-test: t5 = 8.45, p < .001). However, performance was significantly worse with a distracter dot than without one (mean normal trials = 85%; mean probe trials = 68%; paired sample t-test: t5 = 2.76, p = .040).
To evaluate the hypothesis that subjects maintain future responses in working memory, we compared the probability of forward and backward errors. To do this, all backward and forward errors were summed, except for those at point of interpolation D-E where no forward errors were possible. Summing all forwards and backwards errors was appropriate because an equal number of forward and backward errors were possible when opportunities were summed across these points of interpolation. Forward errors were more common than backwards errors (forward M= .85; backward M= .15; paired sample t-test: t5 = –20.767, p < .001; Figure 6). The most common error was to the image one position further along in the list than the correct choice: a +1 error, such as choice of C after an interpolation between A and B. The +1 errors occurred most frequently at every point of interpolation except D-E, where no forward error was possible. In addition to predicting more forward errors overall, the prospective coding hypothesis also makes two other predictions.
Figure 6.
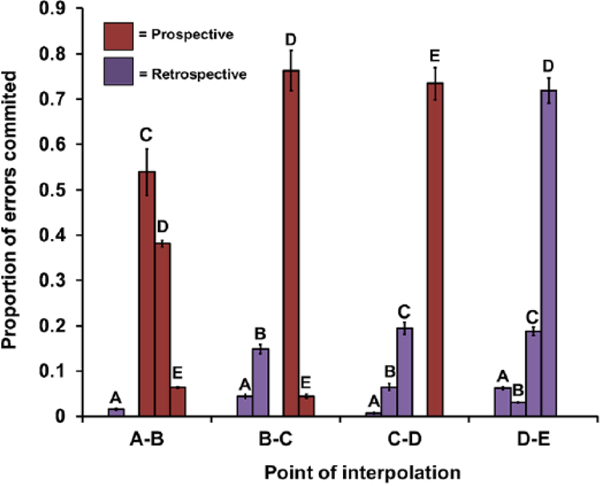
Errors at each point of interpolation to each incorrect image in the list in Experiment 3. Point of interpolation indicates between which two images the short delay and distracter dot occurred; letters indicate the image that was selected in error; maroon bars indicate forward errors; purple bars indicate backward errors; for example, at point of interpolation A-B, when a distracter dot occurred after choice of A, C was the most likely error, then D, and so on; note that unlike scoring in Experiment 1, repeat errors (–1 errors) were counted as backward errors and terminated the trial; for example, at point of interpolation B-C, selection B was a –1 error (A→B→delay→B).
If monkeys use a prospective code, then memory load should decrease as list execution progresses, because there are fewer images remaining to select. Consistent with a prospective code, we observed a decrease in the probability of errors as the point of interpolation occurred later in list execution (rANOVA: F3,15 = 18.27, p < .001; Figure 7). The second outcome predicted by prospective coding is that backward errors would not increase as list execution progresses. The opposite would be expected if monkeys used a retrospective code, which would cause memory load to increase as more responses were made. We found backward errors to be most common only at point of interpolation D-E. Although –1 errors at the point of interpolation D-E may indicate some evidence for retrospective coding, this point in the list represents a rare circumstance where there is no option of a forward error. That the majority of errors at this point of interpolation are backward errors is a logical necessity and does not provide strong evidence for a retrospective code. Finally, if monkeys switched from a retrospective code to prospective code near the middle of list execution, we should have observed a peak of errors at this point, but we did not. Instead, we found a monotonic decrease in errors as list execution progressed. Together, these results indicate that there was no dynamic shift from one type of a strategy to another and that use of a prospective code, rather than a retrospective code, governed choice behaviour.
Figure 7.
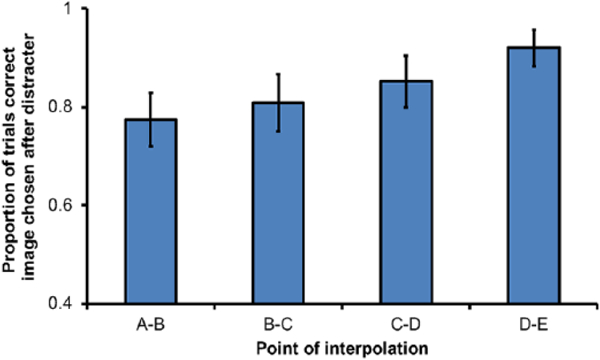
Proportion of tests in which the correct image was chosen after the distracter dot in Experiment 3. Proportions reflect the number of trials in which the next correct images, B, C, D, and E, were selected after each respective point of interpolation, rather than entire list accuracy; error bars represent standard errors of the mean.
That subjects made fewer errors later in the list is consistent with the hypothesis that monkeys prospectively code their location in the list during list execution. However, there are alternative explanations. Trials in which monkeys are nearly finished with the list without error before the point of interpolation occurs might be trials in which attention is comparatively good. This pattern is consistent with the finding that accuracy improves at later points of interpolation (Figure 7; far right). Monkeys might also increase attention as they near the end of the list and are close to earning a reward. Finally, it is possible that fewer errors occurred later in the list because fewer forward errors, the errors that are most likely, are possible at the end of list execution.
The main result of more prospective than retrospective errors, specifically +1 errors at all points of interpolation on which prospective errors were possible, indicates that monkeys mentally update which image to select next as list execution progresses. This clearly indicates prospective coding in working memory rather than retrospective coding or a dual-coding strategy. It is, however, also possible that the tendency to skip one response ahead occurred because subjects treated the dot as a list image or counted the number of touches made to track position in the execution of the list. In Experiment 4, we evaluated this alternate hypothesis that monkeys were tracking responses made rather than holding in mind the image to be selected next.
Experiment 4: updating representation of position in the list
The most likely error made in Experiment 3 was a prospective, + 1, forward error to the next image in the list. Rather than attending to ordinal position, it is possible that subjects made errors to the next image in the list because the distracter dot was treated as if it were an image in the list or because monkeys were tracking the number of responses they had made since initiating execution of the list. This response number hypothesis predicts that causing monkeys to make additional responses should cause them to make errors to images that occur even later in list execution. Thus, after one dot is presented, + 1 errors should result, as was seen in Experiment 3, whereas touching two dots should result in mostly + 2 errors. In contrast, if touching the blue dots causes a general impairment in working memory, causing monkeys to forget which image is next or to confuse which of the images in mind are to be selected next, we should observe a preponderance of + 1 errors after touching both one and two dots.
Method
The six monkeys used in Experiments 1 and 3 were used. The monkeys from Experiment 2 were not used. All task parameters were the same as in Experiment 3, including that subjects were tested in 30 sessions and sessions consisted of 120 trials; 72 were normal trials and 48 were probe trials. Probe trials did differ from Experiment 3. We tested the response number hypothesis by having either one or two distracter dots appear at point of interpolation A-B (24 trials per session) and point of interpolation B-C (24 trials per session). On trials with two distracter dots, the second blue dot appeared in 1 of the 12 random screen locations excluding the ones where the first dot and the images appeared. As usual, monkeys were required to touch each distracter dot twice (FR 2) after a 200-ms delay to make it disappear and to illuminate the same set of images in the original locations or a second dot, allowing completion of the trial. Dots occurred between images A-B and B-C, but not later points of interpolation because we needed trials that allow for both +1 and +2 errors.
Results and discussion
Monkeys performed less accurately on distracter trials than on normal tests (probe test M= .76; normal test M= .87; paired samples t-test: t5 = –9.78, p < .001), but were only moderately disturbed by the delay and distracter dot overall, performing significantly above chance on all probe tests combined (one-sample test: t5=21.5, p < .001). The difference in accuracy following one and two dots was not significant, paired sample t-tests; 1 (M= .74) versus 2 (M= .77) distractor dots after A: t5= –1.38, p=.225; 1 (M= .76) versus 2 (M= .77) distractor dots after B t5=−050, p=.962; Figure 8.
Figure 8.
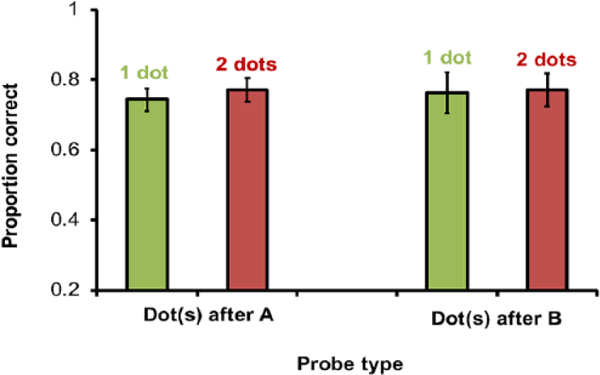
Accuracy on single and double distracter tests in Experiment 4. Distracter dots either occurred after image A (left bars) or after image B (right bars); error bars represent standard errors of the mean.
Touching two dots at points of interpolation did not cause monkeys to make +2 errors. If subjects were using number of responses made to represent current position in the list, then they would have made most errors to C (+ 1 error) when one dot occurred after A and most errors to D (+ 2 error) when two dots occurred after A. Instead, most errors were to C in both cases (Figure 9 upper panel). On the second set of probe tests, when the distracter dot occurred after selection of B, subjects similarly chose D most often both after one distracter dot and after two distracter dots (Figure 9 upper panel). It is also noteworthy that while the dot(s) increased errors by disrupting working memory, the proportion of +1 errors is similar to that observed in the control condition (Figure 9, blue bars). This further refutes the response number hypothesis and substantiates the conclusion that prospective coding is robust, as errors tend to be forward errors no matter why they occur.
Figure 9.
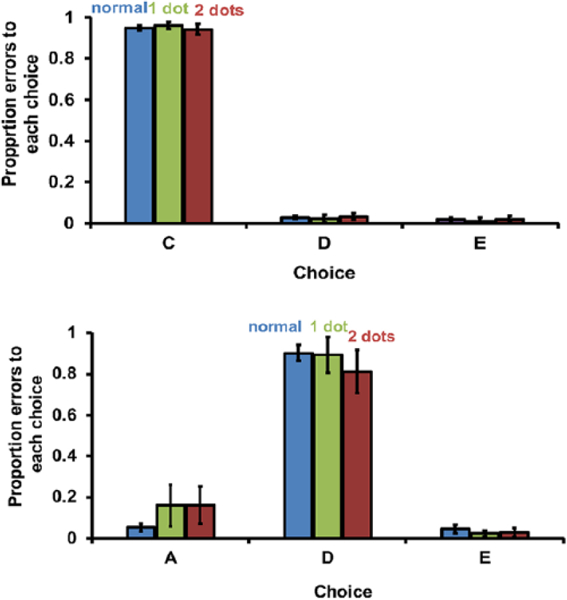
Proportion of errors made on normal tests and probe tests. Blue bars (left-most) indicate normal trials; green bars (middle) indicate probe trials with one distracter dot; red bars (right-most) indicate probe trials with two distracter dots; distracter dots occurred in both probe types: either after image A (upper panel) or after image B (lower panel) in Experiment 4; in Panel A, for example, C is a +1 error and D is a +2 error; in Panel B, D is a +1 error, E is a +2 error, and A is a –2 error; error bars represent standard error of the mean.
As indicated by high performance levels on probe tests with distracters (Figure 9), subjects were frequently able to remember the correct image despite competing cognitive load. When the correct image was forgotten, however, subjects remembered the next image in the list, as indicated by robust +1 errors (Figure 6). Together, these reliable responses to the correct image and to the next image in the list indicate subjects remember which images the will select two steps ahead.
General discussion
In Experiment 1, monkeys selected images according to ordinal position on both within- and between-list tests, indicating that they encode the ordinal position of images trained through simultaneous chaining. Analyses of the SDE and FIE that left each measure uncontaminated by the other indicated that monkeys covertly executed lists and made ordinal comparisons using long-term knowledge of list position. In Experiment 2, we characterised the ordinal representation by directly pitting absolute and relative coding against each other with list-linking training. Monkeys failed to link lists, which demonstrated that knowledge of the order of images is based on representation of absolute positions in the lists rather than the relative positions of images. Experiments 3 and 4 examined how long-term ordinal representations are manipulated in working memory to execute lists. Experiment 3 showed that monkeys prospectively code at least the next two responses that remain to be made. Experiment 4 further reinforced this conclusion by refuting the response number hypothesis.
Results of Experiment 1 corroborate similar findings in rhesus monkeys (Chen et al., 1997; Terrace et al., 2003), other non-human primates (chimpanzees: Inoue & Matsuzawa, 2009; marmosets: Koba, Takemoto, Miwa, & Nakamura, 2012; cotton-top tamarins: Locurto, Dillon, Collins, Conway, & Cunningham, 2013; and lemurs: Merritt et al., 2007), and avian species outside the primate lineage (Scarf & Colombo, 2011), indicating that simultaneous chaining is solved using knowledge of ordinal position. Humans perform similarly to non-human animals showing both the SDE and FIE, indicative of an ordinal code (Colombo & Frost, 2001).
The SDE was observed in accuracies on both within-list and between-list tests and in latency for within-list but not between-list tests. The FIE was observed in both within- and between-list latencies but not in accuracies. Little difference was found in how monkeys completed within- and between-list tests. This suggests that both types of test were solved with similar cognitive operations executed on the same underlying representations. That both the SDE were FIE observed—with the SDE most evident in accuracy and the FIE most strongly in latency—suggests the operation of two cognitive processes in completion of two-image tests. The first process is ordinal scanning during which lists are mentally executed using LTMs of the order of images in the lists. Images in the lists are “highlighted” sequentially in working memory, until the image highlighted matches one of the images on the screen. Images in earlier list positions produce a match more quickly, resulting in the FIE. Because this mental execution of the lists is covert, and does not require committing to a motor response, it might only manifest in the more sensitive response latency measure. Our findings of serially scanning of memory of lists in LTM here are reminiscent of the serial scanning observed in studies of working memory for lists in monkeys (Sternberg, 1966; Wright, Santiago, Sands, Kendrick, & Cook, 1985).
The second process evident during completion of two-image tests is an ordinal comparison between the two images on the screen. A direct comparison of the ordinal positions of the images onscreen is made, and this results in committing a motor response and selecting one of the images. This ordinal comparison is evidenced by the occurrence of the SDE, with more rapid and accurate choices associated with larger ordinal distances between the test images. This study provides particularly strong evidence for both ordinal scanning and ordinal comparison, because the large number of two-image tests monkeys completed allowed us to independently test for the FIE and the SDE. We were able to control for symbolic distance in the analyses of FIE and to control for FIEs in analyses of the SDE, addressing the concern that these analyses were confounded in some earlier reports (e.g., Terrace, 2005).
The ordinal scanning that appears to underlie occurrence of the FIE presents an interesting cognitive challenge to monkeys that know multiple lists and complete between-list tests. As we conceive of ordinal scanning, monkeys cannot know at the outset of a test which list or lists to mentally execute, seeking a match between the mentally executed list and an image onscreen. It is not clear whether monkeys mentally execute the lists they know one after the other until they find a match or whether they are able to mentally execute multiple known lists in parallel. Developing techniques that allow comparison of response latencies between monkeys that know a small number of lists with those that know a large number of lists might allow determination of whether mental list execution is serial or parallel. Parallel execution might be associated by a relatively flat function relating number of lists known to average response time. We do not identify any clear test we could conduct with the current data to discriminate between these alternatives.
In Experiment 2, monkeys did not show evidence for list linking in simultaneous chaining and instead ordered stimuli based on absolute position in the lists in which they were learned. This contrasts with what is found in transitive inference, where rhesus monkeys linked lists after similar training (Gazes et al., 2012; Treichler et al., 2007; Treichler & Raghanti, 2010). This shows that though there are some similarities in the representations of order formed through simultaneous chaining and transitive inference, such as the SDE, the representations are not identical. The representations in transitive inference include coding of the relative positions of images, whereas the representations in simultaneous chaining are of absolute positions. Differences between the ordinal representations in simultaneous chaining and transitive inference may be caused by differences in how animals are trained in these tasks (Jensen et al., 2013). Relative coding may be emphasised in transitive inference training, because the full set of images composing a transitive inference list are never presented simultaneously; the connections between training pairs must always be inferred. In contrast, in simultaneous chaining, the order of images is trained through simultaneous presentation of images. Thus relative positional knowledge might be more cognitively available in transitive inference. Although we did not find flexible relational representation of ordinal position with simultaneous chaining training, monkeys do acquire such flexible representations as a result of transitive inference training, leading to successful linking of lists in transitive inference but not in simultaneous chaining.
Long-term representation of absolute ordinal positions may be promoted in simultaneous chaining because simultaneous chaining lists were trained sequentially, starting with images A and B and adding images each time monkeys met criterion. Once the mental representations of first, second, third, and so on are established, new lists are learnt with reference to this template. This may explain why subjects show a “learning to learn” effect, where, as more lists are mastered, lists are learned faster and faster (Figure 2). According to this account, this would occur because knowledge of absolute positions in LTM become more accessible as more list-unique images (i.e., new lists) are coded with respect to the same absolute “slots” or positions. In contrast, monkeys often learn transitive inference premise pairs in intermixed lists rather than sequentially. This was the case in both studies reporting list linking (Gazes et al., 2012; Treichler & Raghanti, 2010) and may promote relative coding. In yet other tasks where order is important, like tasks assessing memory for the order of events, specific lists are not repeated and routinised and thus absolute ordinal coding may not be promoted. In a trial-unique task measuring memory for the order of events, subjects did not show evidence of knowledge of ordinal position but rather showed evidence for knowledge of temporal order (Templer & Hampton, 2012). This suggests that memory for the order of unique events may depend on still different mental representations that those underlying simultaneous chaining and transitive inference.
Evidence for a prospective, rather than a retrospective, code found in Experiment 3 supports existing evidence that rhesus monkeys (Scarf et al., 2011) and other nonhuman primates, including chimpanzees (Beran, Pate, Washburn, & Rumbaugh, 2004; Biro & Matsuzawa, 1999; Kawai & Matsuzawa, 2000), marmosets (Koba et al., 2012), and capuchin monkeys (Beran & Parrish, 2012) plan future responses when executing learnt sequences. In switch and mask tasks, chimpanzees, old-world primates (Beran et al., 2004), new-world monkeys (Beran & Parrish, 2012), and pigeons (Scarf & Colombo, 2010) show limited but consistent evidence for planning current responses and one response ahead rather than execution of the entire list. Prospective coding may therefore be a fairly robust working memory process as it is seen across several stimulus ordering tasks, including, but not limited to, simultaneous chaining. Many species appear to use a dual-coding strategy in a radial arm maze by switching from a retrospective to a prospective memory code to decrease working memory load (rats: Cook et al., 1985; DiGian & Zentall, 2007; humans: Kesner & Despain, 1988; and pigeons: Steirn, Zentall, & Sherburne, 1992; Zentall, Steirn, & Jacksonsmith, 1990; although see also DiGian & Zentall,.2007) However, the same has not been found in monkeys, and our results in Experiment 3 concur with findings that monkeys code prospectively and do not use retrospective coding or a dual-coding strategy in a visual version of the radial arm maze adapted for a joystick-controlled computer screen (Klein, Evans, & Beran, 2011). Experiment 4 ruled out the possibility that subjects tracked progress in list execution by noting the number of touches made, refuting the response number hypothesis.
Comparisons with other species and different processing mechanisms
Species differences in ordinal processing are particularly interesting because it appears that not all animals readily encode ordinal position, or at least that in some species, knowledge of position is highly constrained to short list lengths. The ability to select pairs of internal images or images from separate lists in trained order has been reported for humans, old- and new-world monkeys, and lemurs (Chen et al., 1997; Colombo & Frost, 2001; D’Amato & Colombo, 1989; Merritt et al., 2007) but not pigeons (Colombo & Frost, 2001; D’Amato & Colombo, 1988; Scarf & Colombo, 2008, 2011; Terrace, 1987, 1989; Terrace, Chen, & Newman, 1995). This suggests that pigeons and primates may learn simultaneous chaining tasks using different cognitive mechanisms (Scarf & Colombo, 2008; Swartz, Chen, & Terrace, 1991). The present results suggest additional properties of the representations underlying simultaneous chaining performance, including relative versus absolute coding of position, and use of prospective and retrospective memory, that can be used to better determine the extent to which similar mechanisms control performance across species.
It has been repeatedly argued that performance on simultaneous chaining tasks in avian species differs from that of non-human primates in critical ways. Although pigeons perform well on maintained-derived lists as described in the introduction (Scarf & Colombo, 2010a, 2011), pigeons do not show the FIE (Scarf & Colombo, 2008; Terrace, 1993), suggesting that they may not mentally “step through” lists as monkeys appeared to do here. Concordantly, when pigeons learn to respond to five images in order, they do not appear to have as strong an ordinal representation as primates (Scarf & Colombo, 2008), because they perform at chance on the internal test pair, BD, which does not contain end items A or E and cannot be solved by associative chaining (D’Amato & Colombo, 1988; Scarf & Colombo, 2008; Terrace, 1991, 1993). However, when B→D is explicitly trained, pigeons do perform significantly better on this pairing than they do on the opposite D→B, and when a four-item list is used, pigeons do perform above chance on the internal test pair BC (_S1_Reference39Scarf & Colombo, 2010b). In other cases, pigeons (Scarf & Colombo, 2010a, 2011) and jackdaws (Pfuhl & Biegler, 2012) have again been found to develop stronger evidence of ordinal position than originally thought. Jackdaws learned 14 three-item lists and were presented with probe tests consisting of the three items from one list and a distractor from a different list. Birds were rewarded for pecking the items from the same list in the trained sequence. The most common error was to peck the distractor when its ordinal position matched the current position being executed in the target list, indicating some knowledge of ordinal position as birds confused items with the same ordinal position (Pfuhl & Biegler, 2012). However, because this test and similar procedures in pigeons involved lists limited to three items (Scarf & Colombo, 2010a; but see Scarf & Colombo, 2011, in which four-item lists were used), it is not clear that this represents as robust a demonstration of the representation of ordinal position as demonstrated in primates. Additional work is required to more definitively compare avian and primate species.
Lists limited to three items may be learnt by different cognitive mechanisms that depend less on ordinal representation that does learning of longer lists. There is evidence that tests with pairs of items that include either the first or last item in a list are processed differently than those containing only internal images (Leth-Steensen & Marley, 2000). In human transitive inference tasks, in which relationships between images must be inferred to construct an overall order (A > B > C > D > E), greater hippocampal activation occurred when transitive inference test pairs did not contain an end image (Zalesak & Heckers, 2009), indicating that tests with end images might not rely on the same process as that used in the solution of tests with internal pairs. This is why many transitive inference tests use lists of at least five items, and the critical test pair is often BD (e.g., Gazes et al., 2012). With lists of only three items, it is not possible to construct test pairs that do not involve the first or last item.
Conclusion
Monkeys executed ordered lists based on LTM for the ordinal position of images. We found that monkeys covertly executed lists mentally and made ordinal comparisons based on absolute, rather than relative, positional information. In addition to the LTM representation of absolute position, monkeys also dynamically tracked progress in executing lists using working memory. We found that subjects prospectively coded one to two responses ahead and did not track list progression by consecutive responses made to stimuli. Better capturing the cognitive mechanisms that support this type of memory will have implications for the cognitive abilities the simultaneous chaining task is used to test, including ordinal coding, the ability to retrieve ordinal categories from LTM (Orlov et al., 2000), and the interaction of working memory and LTM. These results also shed light on the broad concept of ordinality by determining that absolute, rather than relative, positional information is the more salient ordinal feature underlying mnemonic representation for serial lists in the simultaneous chaining paradigm, in contrast to the relative coding use in transitive inference.
Acknowledgements
The authors thank Dina P. Chou, Steven L. Sherrin, Jessica A. Joiner, and Tara A. Dove-VanWormer for their assistance with testing animals.
Funding
The study was supported by grants from National Science Foundation (grant no. IOS-1146316, BCS-0745573, and BCS-1632477), the National Institutes of Health (grant no. R01MH082819), and by ORIP/OD P510D011132. The study was also supported by grants received by V.L.T.: Institutional Development Award (IDeA) Network for Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health (grant no. P20GM103430 and P20GM203430) and a Medical Research Grant from the Rhode Island Foundation (2014–4397).
Ethical approval
All procedures carried out in this study were approved by the Institutional Care and Use Committee (IACUC) of Emory University, which were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Basile BM, & Hampton RR (2013). Dissociation of active working memory and passive recognition in rhesus monkeys. Cognition, 126, 391–396. doi: 10.1016/j.cognition.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Menzel CR, Parrish AE, Perdue BM, Sayers K, Smith JD, & Washburn DA (2016). Primate cognition: Attention, episodic memory, prospective memory, self-control, and metacognition as examples of cognitive control in nonhuman primates. Wiley Interdisciplinary Reviews-Cognitive Science, 7, 294–316. doi: 10.1002/wcs.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, & Parrish AE (2012). Sequential responding and planning in capuchin monkeys (Cebus apella). Animal Cognition, 15, 1085–1094. doi: 10.1007/s10071-012-0532-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Pate JL, Washburn DA, & Rumbaugh DM (2004). Sequential responding and planning in chimpanzees (Pan troglodytes) and rhesus macaques (Macaca mulatta). Journal of Experimental Psychology-Animal Behavior Processes, 30, 203–212. doi: 10.1037/0097-7403.30.3.203 [DOI] [PubMed] [Google Scholar]
- Beran MJ, Evans TA, Klein ED, & Einstein GO (2012). Rhesus Monkeys (Macaca mulatta) and Capuchin Monkeys (Cebus apella) Remember Future Responses in a Computerized Task. Journal of Experimental Psychology-Animal Behavior Processes, 38, 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegler R (2006). Functional considerations in animal navigation: How do you use what you know? In Cook RG & Brown MF (Eds.), Animal spatial cognition: Comparative, neural and computational approaches. [Google Scholar]
- Biro D, & Matsuzawa T (1999). Numerical ordering in a chimpanzee (Pan troglodytes): Planning, executing, and monitoring. Journal of Comparative Psychology, 113, 178–185. doi: 10.1037//0735-7036.113.2.178 [DOI] [Google Scholar]
- Chen S, Swartz KB, & Terrace HS (1997). Knowledge of the ordinal position of list items in rhesus monkeys. Psychological Science, 8, 80–86. [Google Scholar]
- Colombo M, & Frost N (2001). Representation of serial order in humans: A comparison to the findings with monkeys (Cebus Apella). Psychonomic Bulletin & Review, 8, 262–269. [DOI] [PubMed] [Google Scholar]
- Cook RG, Brown MF, & Riley DA (1985). Flexible memory processing by rats—Use of prosepctive and retrospective information in the radial maze. Journal of Experimental Psychology-Animal Behavior Processes, 11, 453–469. doi: 10.1037/0097-7403.11.3.453 [DOI] [PubMed] [Google Scholar]
- D’Amato MR, & Colombo M (1988). Representation of serial order in monkeys (Cebus apella). Journal of Experimental Psychology, 14, 131–139. [PubMed] [Google Scholar]
- D’Amato MR, & Colombo M (1989). Serial learning with wild card items by monkeys (Cebus apella): Implications for knowledge of ordinal position. Journal of Comparative Psychology, 103, 252–261. [DOI] [PubMed] [Google Scholar]
- DiGian KA, & Zentall TR (2007). Pigeons may not use dual coding in the radial maze analog task. Journal of Experimental Psychology-Animal Behavior Processes, 33, 262–272. doi: 10.1037/0097-7403.33.3.262 [DOI] [PubMed] [Google Scholar]
- Gazes RP, Chee NW, & Hampton RR (2012). Cognitive mechanisms for transitive inference performance in rhesus monkeys: Measuring the influence of associative strength and inferred order. Journal of Experimental Psychology-Animal Behavior Processes, 38, 331–345. doi: 10.1037/a0030306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg V, & Gevers W (2015). Spatial coding of ordinal information in short-and long-term memory. Frontiers in Human Neuroscience, 9, 8. doi: 10.3389/fnhum.2015.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow H (1949). The formation of learning sets. Psychological Review, 56, 51–65. [DOI] [PubMed] [Google Scholar]
- Harris EH, Beran MJ, & Washburn DA (2007). Ordinallist integration for symbolic, arbitrary, and analog stimuli by rhesus macaques (Macaca mulatta). Journal of General Psychology, 134, 183–197. doi: 10.3200/genp.134.2.183-198 [DOI] [PubMed] [Google Scholar]
- Hinton EC, Dymond S, von Hecker U, & Evans CJ (2010). Neural correlates of relational reasoning and the symbolic distance effect: Involvement ofparietal cortex. Neuroscience, 168, 138–148. doi: 10.1016/j.neuroscience.2010.03.052 [DOI] [PubMed] [Google Scholar]
- Huber S, Klein E, Moeller K, & Willmes K (2016). Spatial-numerical and ordinal positional associations coexist in parallel. Frontiers in Psychology, 7, 438. doi: 10.3389/fpsyg.2016.00438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, & Matsuzawa T (2009). Acquisition and memory of sequence order in young and adult chimpanzees (Pan troglodytes). Animal Cognition, 12, S59–S69. doi: 10.1007/s10071-009-0274-4 [DOI] [PubMed] [Google Scholar]
- Jensen G, Altschul D, Danly E, & Terrace H (2013). Transfer of a serial representation between two distinct tasks by rhesus macaques. PLoS ONE, 8(7), 1–9. doi: 10.1371/journal.pone.0070285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai N, & Matsuzawa T (2000). Numerical memory span in a chimpanzee. Nature, 403, 39–40. [DOI] [PubMed] [Google Scholar]
- Keppel G, & Wickens TD (2004). Design and analysis, a researchers handbook (4th ed.). Upper Saddle River, NJ: Pearson. [Google Scholar]
- Kesner RP, & Despain MJ (1988). Correspondence between rats and humans in the utilization of retrospective and prospective codes. Animal Learning & Behavior, 16, 299–302. doi: 10.3758/bf03209080 [DOI] [Google Scholar]
- Klein ED, Evans TA, & Beran MJ (2011). An investigation of prospective and retrospective coding in capuchin monkeys and rhesus monkeys. Zeitschrift fur Psychologie Journal of Psychology, 219, 85–91. doi: 10.1027/2151-2604/a000052 [DOI] [Google Scholar]
- Koba R, Takemoto A, Miwa M, & Nakamura K (2012). Characteristics of serial order learning in common marmosets (Callithrix jacchus). Journal of Comparative Psychology, 126, 279–287. doi: 10.1037/a0026613 [DOI] [PubMed] [Google Scholar]
- Leth-Steensen C, & Marley AAJ (2000). A model of response time effects in symbolic comparison. Psychological Review, 107, 62–100. doi: 10.1037/0033-295x.107.1.162 [DOI] [PubMed] [Google Scholar]
- Locurto C, Dillon L, Collins M, Conway M, & Cunningham K (2013). Implicit chaining in cotton-top tamarins (Saguinus oedipus) with elements equated for probability of reinforcement. Animal Cognition, 16, 611–625. doi: 10.1007/s10071-013-0598-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logie RH (1986). Visuo-spatial processing in working memory. The Quarterly Journal of Experimental Psychology Section A, 38, 229–247. [DOI] [PubMed] [Google Scholar]
- Merritt D, MacLean EL, Jaffe S, & Brannon EM (2007). A comparative analysis of serial ordering in ring-tailed lemurs (Lemur catta). Journal of Comparative Psychology, 121, 363–371. doi: 10.1037/0735-7036.121.4.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt DJ, & Terrace HS (2011). Mechanisms of inferential order judgments in humans (Homo sapiens) and rhesus monkeys (Macaca mulatta). Journal of Comparative Psychology, 125, 227–238. doi: 10.1037/a0021572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlov T, Yakovlev V, Hochstein S, & Zohary E (2000). Macaque monkeys categorize images by their ordinal number. Nature, 404, 77–80. [DOI] [PubMed] [Google Scholar]
- Osgood CE (1953). Method and theory in experimental psychology. New York, NY: Oxford University Press. [Google Scholar]
- Pfuhl G, & Biegler R (2012). Ordinality and novel sequence learning in jackdaws. Animal Cognition, 15, 833–849. doi: 10.1007/s10071-012-0509-7 [DOI] [PubMed] [Google Scholar]
- Pierce WD, & Cheney CD (2008). Behavior analysis and learning (4th ed.). New York, NY: Psychology Press. [Google Scholar]
- Scarf D, & Colombo M (2008). Representation of serial order: A comparative analysis of humans, monkeys, and pigeons. Brain Research Bulletin, 76, 307–312. [DOI] [PubMed] [Google Scholar]
- Scarf D, & Colombo M (2010). The formation and execution of sequential plans in pigeons (Columba livia). Behavioural Processes, 83, 179–182. doi: 10.1016/j.beproc.2009.12.004 [DOI] [PubMed] [Google Scholar]
- Scarf D, & Colombo M (2010a). A positional coding mechanism in pigeons after learning multiple three-item lists. Animal Cognition, 13, 653–661. doi: 10.1007/s10071-010-0315-z [DOI] [PubMed] [Google Scholar]
- Scarf D, & Colombo M (2010b). Representation of serial order in pigeons (Columba livia). Journal of Experimental Psychology-Animal Behavior Processes, 36, 423–429. doi: 10.1037/a0020926 [DOI] [PubMed] [Google Scholar]
- Scarf D, & Colombo M (2011). Knowledge of the ordinal position of list items in pigeons. Journal of Experimental Psychology-Animal Behavior Processes, 37, 483–487. doi: 10.1037/a0023695 [DOI] [PubMed] [Google Scholar]
- Scarf D, Danly E, Morgan G, Colombo M, & Terrace HS (2011). Sequential planning in rhesus monkeys (Macaca mulatta). Animal Cognition, 14, 317–324. doi: 10.1007/s10071-010-0365-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steirn JN, Zentall TR, & Sherburne LM (1992). Pigeons performances of a radial arm maze analog task–Effect of spatial distinctiveness. Psychological Record, 42, 255–272. [Google Scholar]
- Sternberg S (1966). High speed scanning in human memory. Science, 153, 652. doi: 10.1126/science.153.3736.652 [DOI] [PubMed] [Google Scholar]
- Swartz KB, Chen S, & Terrace HS (1991). Knowledge of ordinal position by list-sophisticated rhesus-monkeys. Bulletin of the Psychonomic Society, 29, 498–498. [Google Scholar]
- Templer VL, & Hampton RR (2012). Cognitive mechanisms of memory for order in rhesus monkeys (Macaca mulatta). Hippocampus: Rapid Communication, 23, 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrace HS (1984). Representations of arbitrary sequences by the Pigeon. Bulletin of the Psychonomic Society, 22, 291–291. [Google Scholar]
- Terrace HS (1987). Chunking by a pigeon in a serial-learning task. Nature, 325, 149–151. [DOI] [PubMed] [Google Scholar]
- Terrace HS (1989). Chunking of a serial list by pigeons. Bulletin of the Psychonomicsociety, 27, 509–509. [Google Scholar]
- Terrace HS (1991). Chunking during serial-learning by a Pigeon 1. Basic evidence. Journal of Experimental Psychology-Animal Behavior Processes, 17, 81–93. [DOI] [PubMed] [Google Scholar]
- Terrace HS (1993). The phylogeny and ontogeny of serial memory—List learning by pigeons and monkeys. Psychological Science, 4, 162–169. [Google Scholar]
- Terrace HS (2005). The simultaneous chain: A new approach to serial learning. Trends in Cognitive Sciences, 9, 202–210. [DOI] [PubMed] [Google Scholar]
- Terrace HS, Chen SF, & Newman AB (1995). Serial-learning with a wild card by pigeons (Columba-Livia)—Effect of list length. Journal of Comparative Psychology, 109, 162–172. [DOI] [PubMed] [Google Scholar]
- Terrace HS, & McGonigle B (1994). Memory and representation of serial order by children, monkeys, and pigeons. Current Directions in Psychological Science, 3, 180–185. [Google Scholar]
- Terrace HS, Son LK, & Brannon EM (2003). Serial expertise of rhesus macaques. Psychological Science, 14, 66–73. [DOI] [PubMed] [Google Scholar]
- Treichler FR, & Raghanti MA (2010). Serial list combination by monkeys (Macaca Mulatta): Test cues and linking. Animal Congition, 13, 121–131. [DOI] [PubMed] [Google Scholar]
- Treichler FR, Raghanti MA, & Van Tilburg DN (2007). Serial list linking by macaque monkeys (Macaca mulatta): List property limitations. Journal of Comparative Psychology, 121, 250–259. doi: 10.1037/0735-7036.121.3.250 [DOI] [PubMed] [Google Scholar]
- Wright AA, Santiago HC, Sands SF, Kendrick DF, & Cook RG (1985). Memory processing of serial lists by pigeons, monkeys, and people. Science, 229, 287–289. [DOI] [PubMed] [Google Scholar]
- Zalesak M, & Heckers S (2009). The role of the hippocampus in transitive inference. Psychiatry Research: Neuroimaging, 172, 24–30. doi: 10.1016/j.pscychresns.2008.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentall TR, Steirn JN, & Jacksonsmith P (1990). Memory strategies in pigeons’ performance of a radial arm maze analog task. Journal of Experimental Psychology-Animal Behavior Processes, 16, 358–371. doi: 10.1037//0097-7403.16.4.358 [DOI] [Google Scholar]


