Abstract
The medial prefrontal cortex (mPFC) is a key regulator of neurocognition. The glutamatergic pyramidal neurons are the predominant component of neurons in the mPFC. Aging and HIV profoundly alter the structure and function of mPFC pyramidal neurons, including, but are not limited to, dysregulation of NMDA receptors and voltage-gated calcium channels. Here we assessed the impact of aging and in vivo HIV exposure on the functional activity (firing) of mPFC pyramidal neurons mediated by voltage-gated K+ (Kv) channels and inwardly-rectifying K+ (Kir) channels using patch-clamp recording in rat brain slices ex vivo. We found that aging and HIV significantly affect firing in different manners by altering the activity of Kv and likely Kir channels, associated with changes in membrane properties and the mRNA levels of specific Kv channels. Evoked firing was significantly decreased in mPFC neurons of older (12 month, 12m) rats compared to younger (6/7 week, 6/7wk) rats, regardless of HIV status. In contrast, firing was significantly increased in neurons from Tg rats compared to non-Tg rats, regardless of age. Aging/HIV-induced alterations in firing were mediated by dysfunctional Kv channels and Kir channels, which exhibit significant changes in their activity and/or expression induced by aging and HIV exposure in vivo. Collectively, these novel findings demonstrate that aging is associated with a significant decline of mPFC neuronal activity; while long-term HIV exposure in vivo could drive mPFC neurons from over-activation to loss of firing, which could ultimately exacerbate the decline of mPFC neuronal activity.
Keywords: aging, HIV, medial prefrontal cortex, pyramidal neuron, K+ channel, electrophysiology
Introduction
Despite the use of Combination Antiretroviral Therapy (cART), HIV-associated neurocognitive disorders (HAND) are prevalent among HIV+ individuals [1;2]. The medial prefrontal cortex (mPFC) is a key regulator of neurocognition, and glutamatergic pyramidal neurons (the major type of neurons) in this brain region are profoundly altered by HIV [3]. HIV-induced neuronal hyperactivity is mediated by Ca2+ dysregulation [4–9] resulting from over-activation of NMDA receptors and voltage-gated Ca2+ channels (VGCCs) [10;11], which induce neuronal injury, and in severe cases even HIV-associated dementia (HAD).
In addition to Ca2+ dysregulation, HAND is also associated with K+ channel dysfunction, including, but not limited to, voltage-gated K+ (Kv) and Ca2+-activated K+ channels [12;13]. Specifically, HIV-1 proteins (e.g., Tat, gp120, Vpu, and Nef) disrupt the activity of Kv [14–16] and two-domain K+ (K2P) channels (a K+ channel family that regulates the resting membrane potential, RMP)[17;18]. We demonstrated that HIV suppresses the activity of the inward-rectifier (Kir) and K2P channels in mPFC pyramidal neurons [4;6]. As such, K+ channel dysfunction also contributes to HIV-induced hyperactivity, which could drive many hyperactive mPFC neurons from a status of over-activation to a status of loss of firing (inactivation) [4–6].
Little is known about the impact of aging on the mPFC, with or without HIV infection. This is especially relevant as the HIV-infected population is living longer and aging. How and to what extent aging affects K+ channel activity and potentially disrupts mPFC neuronal activity is unclear. Filling this knowledge gap will advance our understanding of the neuropathogenesis of HAND, which is likely exacerbated during aging. Here we assessed the impact of aging and HIV-1 on firing and K+ channel activity/expression in mPFC neurons using a HIV-1 Tg rat model [19]. HIV-1 Tg rats express low levels of the seven HIV-1 genes (with deletion of the gag and pol), display HAND characteristics [20], and mimic the CNS HIV reservoirs with maximum suppression of HIV replication under cART [21].
Materials and Methods
Animals
Male HIV-1 Tg and non-Tg rats at age of 3–4 week (wk) (Envigo, Indianapolis, IN) were group-housed at the Rush University Medical Center animal facility on a 12 hour light/dark cycle. Food and water were available ad libitum. Animal care and use procedures were conducted in accordance with NIH, USDA and institutional guidelines, and approved by the Institutional Animal Care. The rat used for electrophysiology experiment included 11 Tg and 19 non-Tg at the age of 6–7wks; and 9 Tg and 10 non-Tg rats at the age of 12m. Eight rats per each group were used to measure the RNA levels of K+ channels.
Whole-cell patch-clamp recording in brain slices
The procedure was descripted elsewhere [6]. Briefly, rats were deeply sedated with chloral hydrate (400mg/kg, i.p.), then perfused transcardially with ice-cold solution (in mM: 248 sucrose, 2.9 KCl, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 0.1 CaCl2, 10 glucose; pH 7.4–7.45, with 335–345 mOsm) containing 3mM kynurenic acid and 1mM absorbic acid. Coronal brain sections (300µm) containing the mPFC were sliced, and transferred to a holding chamber containing oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (aCSF; in mM: 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, and 15 glucose; pH 7.4–7.45, with 305–315 mOsm). After ~1hr incubation, slices were moved to a recording chamber perfused with oxygenated aCSF. To access K+ channel activity, aCSF contained selective blockers for voltage-gated Na+ channels (tetrodotoxin, TTX, 1µM) and Ca2+ channels (cadmium, Cd2+, 200µM), ionotropic glutamatergic NMDA/AMPA receptors (kynurenic acid, 3mM; Sigma), and GABAA receptors (picrotoxin, 100μM; Sigma). Glass electrodes were pulled and filled with an internal solution (in mM: 120 K-gluconate, 10 HEPES, 20 KCl, 2 MgCl2, 3 Na2ATP, 0.3 NaGTP, and 0.1 EGTA; pH: 7.3–7.35; osmolarity: 280–285mOsm), which had a resistance of 4–6 MΩ and were used for patch-clamp recording. Pyramidal neurons from the layer V-VI of the mPFC were visually identified using Nikon ECLIPSE E600FN microscope. All neurons from non-Tg rats met the criteria of RMP at least −60mV and action potential (AP) amplitude ≥60mV. For neurons from Tg rats, the criteria were −50mV for RMP and AP amplitude ≥50mV due to hyperactivity [6]. The recording protocol included a series of 500ms current pulses with the intensity ranged from −500 to +300pA with a 25-pA increment for evoking APs, and a range from −500 to +500pA for recording for K+ channel activities.
Quantitative Real-Time PCR (qRT-PCR)
Anesthetized rats were perfused transcardially with ice-cold saline. The brain was removed and dissected mPFC tissues were stored at −80°C for RNA extraction. Total RNA was extracted using a miRNeasy Mini kit (Qiagen Inc., Germantown, MD). DNA was removed using the RNase-free DNase set (Qiagen Inc.). mRNA level was measured on an Applied Biosystems (Foster City, CA) ViiA7 real-time PCR system using the Power SYBR® Green RNA-to-CT™ 1-Step kit (Applied Biosystems) with primers as follows: TCCCCGCCGCCTAAGAT (Kcnh3, fwd), CGTTGCCCAGGACGAAGTTA (Kcnh3, rev), GCTGATGGCAGTGTCCAGAA (Kcnc4, fwd), GGCATAGTTGGACGAGAGGG (Kcnc4, rev), CAGCAGAGGAGCTCCGAAAA (Kcna3, fwd), CAGTGGAGTTGCCCGTTTTG (Kcna3, rev), ATCTGCTGGAGAAGCCCAAC (Kcnb1, fwd), TGAGTGACAGAGCGATGGTG (Kcnb1, rev), AGGACGTCTGGTCCAGGTTT (Kcnv1, fwd), AGGCCGACTTCTTCATAGCAC (Kcnv1, rev), ACACCTTCCTCGACACCATC (Kcnh2, fwd), CTCTACACGAGCGTTAGCGA (Kcnh2, rev), AATCCAGCAGGCATGAAATGG (Kcnh4, fwd), AAGCTAGAATGCTTTGAGCTGC (Kcnh4, rev), CCGCGAGTACAACCTTCTTGC (β-Actin, fwd), and ATATCGTCATCCATGGCGAACTGG (β-Actin, rev). The mRNA levels shown as fold change (2-ΔΔCt) were normalized to β-actin Ct and to 6/7wk non-Tg. Selection of K+ channels for mRNA assessment was based on our preliminary microarray study, which demonstrated the expression of these channels in mPFC; and suggested that their mRNA level was potentially altered in HIV-1 Tg rats compared to non-Tg rats (data not shown).
Statistical Analysis
Prism 7 (GraphPad Software Inc., La Jolla, CA) and SigmaPlot 12 (Systat Software Inc., San Jose, CA) were used for data analysis. Three-way ANOVA was used first to analyze the current (I)-spike number, and I-voltage relationships (I-V curves). In all cases, if there was a significant difference in the main factor(s) and/or interaction, the data then were further analyzed using two-way ANOVA repeated measures (rm) to determine the effects of aging and HIV effect on firing. Two-way ANOVA was used to analyze the membrane property and Kv channel mRNA levels. ANOVA data were analyzed by a following post-hoc Newman-Keuls test. Statistical significance was set at p<0.05. The outliers were excluded if value was greater than 2-fold of the standard deviation from the mean. All data were expressed as mean ± standard error (SE).
Results
Aging reduced mPFC neuronal firing, independent of HIV (which per se increased firing).
To assess the impact of aging and HIV on firing, we performed patch-clamp recordings of mPFC pyramidal neurons in brain slices. We found that firing of mPFC neurons was reduced in older (12m) rats compared to younger (6/7wk) rats regardless of HIV (6/7wk non-Tg vs. 12m non-Tg: n=30/19 cell/rat vs. 13/10; HIV effect: F(1,41)=6.676, p=0.013; current effect: F(11,451)=117.190, p<0.001; interaction: F(11,451)=7.398, p<0.001. 6/7wk Tg vs. 12m Tg: n=24/11 vs. 26/9; HIV effect: F(1,48)=4.466, p=0.040; current effect: F(11,528)=207.821, p<0.001; interaction: F(11,528)=4.010, p<0.001. Fig. 1A&B). But firing of mPFC neurons was significantly increased in HIV-1 Tg rats compared to non-Tg rats regardless of age (6/7wk non-Tg vs. 6/7wk Tg: HIV effect: F(1,52)=4.701, p=0.035; current effect: F(11,572)=264.551, p<0.001; interaction: F(11,572)=4.093, p<0.001. 12m non-Tg vs. 12m Tg: HIV effect: F(1,37)=4.696, p=0.037; current effect: F(11,407)=86.977, p<0.001; interaction: F(11,407)=4.838, p<0.001. Fig. 1A&1B). These results indicate that the basal activity of mPFC neurons is declined during aging, while HIV still drives them to a hyperactive status even at older age. Aging/HIV-induced firing changes were associated with altered membrane properties. We found that aging reduced Rin and HIV increased Rin (reflecting enhanced and reduced K2P activity, respectively. Aging effect: F(1,102)=13.847, p<0.001; HIV effect: F(1,102)=13.428, p<0.001; interaction: F(1,102)=0.008, p>0.05). HIV also reduced rheobase (a minimal stimulation for evoking firing) at both ages. Aging effect: F(1,93)=12.694, p<0.001; HIV effect: F(1,93)=13.549, p<0.001; interaction: F(1,93)=0.559, p>0.05) and AP amplitude in younger rats (aging effect: F(1,102)=4.802, p=0.031; HIV effect: F(1,102)=9.930, p=0.002; interaction: F(1,102)=2.195, p>0.05. Table 1). These alterations either contribute to aging /HIV-induced differences in firing, or are consequential effects of aging and HIV on altering K+ channels to affect neuron’s membrane potential (Vm). Importantly, given that HIV eventually drives many hyperactive mPFC neurons to loss of firing [4–6;8], it could exacerbate aging-associated decline of neuronal activity.
Figure 1.

Firing of mPFC pyramidal neurons was decrease by aging, but increased by HIV. A. Sample spike traces from Tg or non-Tg rats at younger (6/7wk) or older (12m) age. B. Spike number was significantly decreased in neurons of 12m rats regardless of HIV status; but was increased in Tg rats regardless of age (6/7wk non-Tg vs. 6/7wk Tg: *,**p<0.05 or 0.01. 12m non-Tg vs. 12m Tg: ^,^^,^^^p<0.05, 0.01, or 0.001; 6/7wk non-Tg vs. 12m non-Tg: #,##,### p<0.05, 0.01, or 0.001. 6/7wk Tg vs. 12m Tg: &,&&p<0.05 or 0.01).
Table 1:
Aging and HIV alter the membrane property of mPFC pyramidal neurons.
| 6/7wk | 12m | |||||||
|---|---|---|---|---|---|---|---|---|
| non-Tg | cell n | HIV-1 Tg | cell n | non-Tg | cell n | HIV-1 Tg | cell n | |
| Rat N | 19 | 11 | 10 | 9 | ||||
| RMP (mV) | −68.8±1.3 | 31 | −67.8±1.3 | 31 | −71.8±2.2 | 13 | −69.1±1.1 | 27 |
| Rin (MΩ) | 180.7±15.7 | 34 | 236.1±25.15.7^^ | 31 | 122.6±25.8* | 14 | 199.2±12.7**,^ | 28 |
| Rheobase (pA) | 90.8±12.3 | 30 | 65.8±12.3^ | 28 | 164.3±20.3** | 13 | 98.3±10.0^^,* | 26 |
| AP Threshold (mV) | −40.0±0.6 | 32 | −39.9±0.6 | 29 | −39.8±0.9 | 13 | −38.0±0.6 | 25 |
| AP Amplitude (mV) | 70.9±1.7 | 34 | 61.2±1.8^^^ | 31 | 72.4±2.8 | 14 | 68.9±1.9** | 27 |
| Time Constant (ms) | 23.1±1.4 | 32 | 25.0±1.5 | 30 | 22.0±2.2 | 14 | 22.2±1.6 | 28 |
| AHP (mV) | 15.9±0.6 | 33 | 15.5±0.7 | 28 | 14.0±1.0 | 13 | 15.5±0.7 | 27 |
The asterisk refers to a significant difference in Newman-Keuls post-hoc test (*,**aging effect: p<0.05 or 0.01; ^,^^,^^^HIV effect: p<0.05, 0.01 or 0.001).
Kv channel activity was increased in older non-Tg rats.
To determine the effects of aging and HIV on K+ channels, we assessed Kv channel activity first. Kv channels are activated by Vm depolarization, conducting K+ efflux to repolarize Vm. Enhanced Kv channel activity and K+ efflux also reduce firing. We found that aging and HIV altered Kv channel activity (Fig. 2A). There was no significant difference in Kv channel activity between 6/7wk non-Tg (n=10/2) and 6/7wk Tg (n=7/2) rats (p>0.05. Fig. 2B). But there was a significant enhancement in K+ efflux via Kv channels in neurons from 12m non-Tg rats (n=10/8) compared to younger non-Tg rats (aging effect: F(1,15)=4.944, p=0.039; current effect: F(15,319)=168.779, p<0.001; interaction: F(15,319)=4.455, p<0.001). This aging effect on Kv channel activity did not occur in neurons from HIV-1 Tg rats (12m Tg: n=10/5, p>0.05). There was also a significant decrease in Kv channel activity in neurons from 12m HIV-1 Tg rats compared to age-matched non-Tg rats (HIV effect: F(1,18)=8.708, p=0.009; current effect: F(15,319)=143.018, p<0.001; interaction: F(15,319)= 0.960, p>0.05. Fig. 2B), while no difference between 6/7wk Tg and non-Tg (p>0.05).
Figure 2.
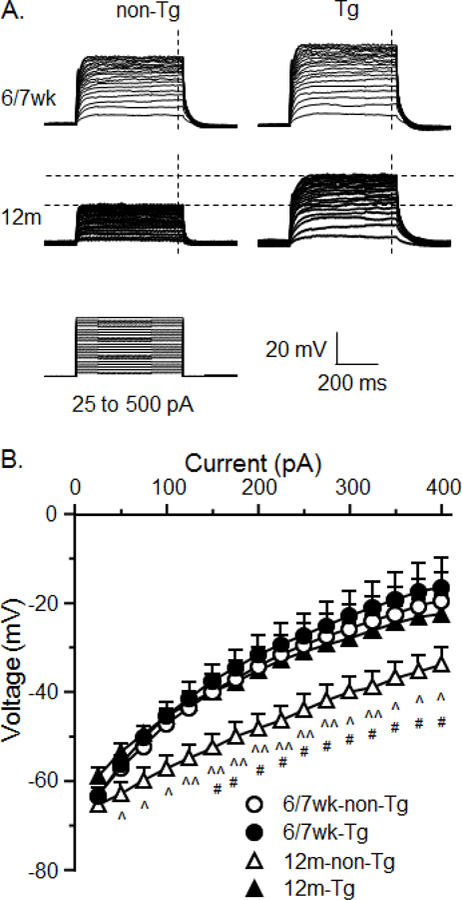
Kv channel activity in mPFC neurons was significantly increased in 12m non-Tg rats; but it was reduced by HIV. A. Sample traces indicate the alterations in Vm (reflecting Kv channel activity) of neurons from 6/7wk or 12m rats, with or without HIV. B. The I-V relationships indicate that Kv channel activity was significantly increased in neurons of 12m non-Tg rats compared to 6/7wk non-Tg rats, reflecting a downward-shift of Vm (#p<0.05). But HIV significantly reduced Kv activity in neurons of older rats (reflected by upward-shifted Vm traces; ^,^^p<0.05 or 0.01); but there was no significant difference between 12m Tg rats and 6/7wk Tg rats (p>0.05), or between 6/7wk non-Tg and 6/7wk Tg rats (p>0.05).
Aging and HIV altered the mRNA level of specific Kv channels.
To determine the subtype(s) of Kv channels involved in mediating mPFC neuron’s firing, we assessed the mRNA levels of seven Kv channel genes using qRT-PCR, including Kcnh3 (Kv12.2), Kcna3 (Kv1.3), Kcnb1 (Kv2.1), Kcnc4 (Kv3.4), Kcnv1 (Kv8.1), Kcnh2 (Kv11.1), and Kcnh4 (Kv12.3). We found that the mRNA levels of 4 of the 7 genes were significantly changed by aging and/or HIV. For example, Kcnh3 (Kv12.2) mRNA levels were up-regulated in 12m non-Tg rats compared to 6/7wk non-Tg, or to 12m Tg rats (6/7wk non-Tg, 6/7wk Tg, 12m non-Tg vs. 12m Tg: n=8, 8, 7 vs. 8; aging effect: F(1,27)=0.929, p=0.344; HIV effect: F(1,27)=6.034, p=0.021; interaction: F(1,27)=4.025, p=0.055. Fig. 3A). Kcna3 (Kv1.3) mRNA levels were down-regulated in 6/7wk Tg rats compared to age-matched non-Tg rats, or to 12m Tg rats (6/7wk non-Tg, 6/7wk Tg, 12m non-Tg vs. 12m Tg: n=8, 8, 7 vs. 7; aging effect: F(1,25)=5.941, p=0.022; HIV effect: F(1,25)=4.290, p=0.049; interaction: F(1,25)=0.915, p>0.05. Fig. 3B). Kcnb1 (Kv2.1) mRNA levels were not affected by HIV in either age; but were increased in older Tg rats compared to younger Tg rats (6/7wk non-Tg, 6/7wk Tg, 12m non-Tg vs. 12m Tg: n=7, 8, 8 vs. 8; aging effect: F(1,27)=4.573, p=0.042; HIV effect: F(1,27)=0.517, p=0.478; interaction: F(1,27)=1.171, p>0.05. Fig. 3C). Kcnc4 (Kv3.4) mRNA levels were not altered in Tg rats compared to age-matched non-Tg rats regardless of age; but were increased in 12m non-Tg rats compared to 6/7wk non-Tg rats, and 12m Tg rats (6/7wk non-Tg, 6/7wk Tg, 12m non-Tg vs. 12m Tg: n=8, 8, 7 vs. 7; aging: F(1,29)=6.030, p=0.021; HIV: F(1,29)= 2.532, p>0.05; interaction: F(1,29)=0.915, p>0.05. Fig. 3D). Neither aging nor HIV alters the mRNA levels of Kcnv1 (Kv8.1), Kcnh2 (Kv11.1), and Kcnh4 (Kv12.3) (p>0.05; data not shown). Together, these findings suggest that the alterations in Kv channel gene expression contribute to the altered Kv channel dysfunction and firing; which are induced by aging and HIV via different mechanisms.
Figure 3.

Aging and HIV altered the mRNA levels of 4 Kv channels in the mPFC. A. The mRNA level of Kcnh3 (Kv12.2 channel) was increased in 12m non-Tg rats compared to 6/7wk non-Tg rats; but down-regulated in 12m Tg rats compared to 12m non-Tg rats. B. The mRNA level of Kcna3 (Kv1.3) was decreased in 6/7wk Tg rats compared to 6/7wk non-Tg rats; but there was no significant difference between 12m Tg and 12m non-Tg rats. Kcna3 level was increased in 12m Tg rats compared to 6/7wk Tg rats; but no change between non-Tg rats. C. There was no significant change in Kcnb1 mRNA (Kv2.1 channel) between non-Tg and Tg rats regardless of age; but it was increased in 12m Tg rats compared to 6/7wk Tg rats. D. There was a significant increase in the mRNA level of Kcnc4 (Kv3.4 channel) in 12m non-Tg rats compared to 6/7wk non-Tg rats. n=7–8 rats per each group. *,**p<0.05 or 0.01.
Aging increased Kir channel activity; but HIV reduced it.
The subthreshold excitability of neurons is mediated by inwardly rectifying K+ currents via Kir channels in resting status. To determine the HIV/Meth effects on Kir channels, we also assessed their activity. We found that Kir activity (reflected by upward-shift of Vm traces to more depolarized levels, Fig. 4A) was significantly enhanced in neurons of older rats, with or without HIV (6–7wk non-Tg vs. 12m non-Tg: n=10/2 vs. 10/8; aging effect: F(1,15)=9.468, p=0.008; current effect: F(15,225)= 133.1, p<0.001; interaction: F(15,225)=2.148, p<0.01. 6/7wk Tg vs. 12m Tg: n=7/2 vs. 10/5; aging effect: F(1,15)=3.545, p>0.05; current effect: F(15,225)=141.6, p<0.001; interaction: F(15,225)=1.73, p=0.047. Fig. 4B). But HIV reduced Kir influx (reflected by a downward-shift of Vm), regardless of age (6/7wk non-Tg vs. 6/7wk Tg: HIV effect: F(1,12)=2.446, p>0.05; current effect: F(15,180)=104.5, p<0.001; interaction: F(15,180)=2.339, p=0.004. 12m non-Tg vs. 12m Tg: HIV effect: F(1,18)=1.732, p>0.05; current effect: F(15,270)=170.4, p<0.001; interaction: F(15,270)=4.591, p<0.001). These findings indicate that both aging and HIV alter Kir activity.
Figure 4.

Aging increased K+ influx via Kir channels in mPFC neurons from Tg and non-Tg rats; but HIV reduced it. A. Sample traces showing Vm changes that reflected K+ influx via activated Kir channels in neurons from 6/7wk or 12m rats. The vertical dashed lines indicate the time points where the data were obtained for plotting the I-V curves in Fig. 4B. B. The I-V curves showing that K+ influx via Kir channels was increased in neurons from 12m rats regardless of genotypes (6–7wk non-Tg vs. 12m non-Tg: #,##p<0.05, 0.01. 6/7wk Tg vs. 12m Tg: &,&&,&&&p<0.05, 0.01, 0.001). The activity of Kir channels was significantly reduced by HIV-1 status regardless of age (6/7wk non-Tg vs. 6/7wk Tg: *p<0.05. 12m non-Tg vs. 12m Tg: ^p<0.05).
Discussion
Our study demonstrates that aging and HIV dysregulate mPFC neuronal activity by altering the activity and expression of K+ channels. Specifically, we found that (1) neuronal activity is significantly decreased in older (12m) rats compared to younger (6/7wk) rats regardless of HIV status; (2) HIV in vivo induces mPFC neuronal hyperactivity regardless of age; and (3) activity and expression of Kv and Kir channels in mPFC neurons are altered by aging and HIV, which could contribute to aging-induced decline of mPFC neuronal activity.
Kv channels are key regulators of the intrinsic excitability of neurons that mediate membrane excitability, and therefore neuron’s firing [22]. We found that aging-induced decline of firing was associated with significantly-enhanced Kv efflux in mPFC pyramidal neurons of older non-Tg rats; while HIV-induced decrease in K+ efflux via Kv channels in mPFC neurons of 12m Tg rats promotes neuronal over-activation. However, there was no association of altered Kv efflux with changes in firing between neurons from 6/7wk Tg and non-Tg rats. These findings suggest that the enhanced Kv efflux is a major contributor to reduced firing in older non-Tg rats, which does not occur in older Tg rats, or in younger rats regardless of genotype. We also identified certain subtypes of dysfunctional Kv channels that likely play a role in aging/HIV-induced changes in firing. Specifically, increased Kcnh3 (Kv12.2 channel) expression could enhance Kv channel activity to inhibit firing in older non-Tg rats; while reduced expression of this channel in older Tg rats can promote neuronal over-activation (that ultimately impair their function) [4–6]. Reduced Kcna3 (Kv1.3 channel) expression was associated with increased firing in young Tg rats compared to age-matched non-Tg rats; and aging-induced increase in Kv1.3 expression was associated with reduced firing in older Tg rats compared to young Tg rats. Although HIV did not alter Kcnb1 (Kv2.1 channel) expression at either age, aging increases Kv2.1 expression in older Tg rats, and Kcnc4 (Kv3.4 channel) expression in older non-Tg rats; both are associated with suppressed firing. Together, these findings indicate that aging- and HIV-induced alterations in Kv channels significantly disturb mPFC neuronal activity; and the combined aging/HIV impact could ultimately drive neurons from a status of over-activation to inactivation. In addition, we acknowledge that other Kv channel subtypes may be involved in aging/HIV-induced mPFC neuronal dysfunction, which can be identified in future studies.
Unlike firing that reflects the supra-threshold excitability mediated in part by Kv channels, the subthreshold excitability of neurons in resting status depends upon K2P and Kir channel activity. Alterations in these K+ channels could render neurons more (or less) responsive to excitatory or inhibitory stimuli, depending on extracellular K+ levels ([K+]e). We found that although RMP was not significantly affected by aging or HIV, Rin was reduced by aging regardless of HIV; and increased by HIV regardless of age. These changes suggest an enhanced K2P activity during aging (reflecting enhanced K+ efflux that could decrease firing) and reduced K2P activity by HIV (reflecting reduced K+ efflux that could increase firing), respectively. Intriguingly, we also found that aging increases Kir channel activity (increasing K+ influx and reducing [K+]e), which could render neurons less excitable. But HIV reduces Kir channel activity regardless of age, which could elevate [K+]e and promote Vm depolarization and facilitate firing when excitatory inputs come. A persisting increase in [K+]e by HIV due to long-term neuronal hyperactivity (associated with increased K+ efflux) could eventually drive neurons from over-activation to loss of firing [4–6;8;9]. This impact of chronic HIV exposure in vivo could ultimately worsen aging-associated decline of neuronal activity. Together, these findings suggest that although aging and HIV could affect neuronal activity independently via different mechanisms, when HIV causes loss of firing (mediated by over-activation of NMDARs and VGCCs, as well as by K+ channel dysfunction), aging-related decrease of neuronal activity (mediated by Kv dysfunction) could be exacerbated. In addition, the exact mechanism(s) by which aging and HIV affect Kir channel function, and to what extent dysfunctional Kir channels contribute to aging/HIV-induced changes in firing, need to be defined in future studies.
The novel findings in the present study have an important clinical relevance to the comorbidity of HAND and aging. The mPFC dysfunction is well-reported among HAND patients [3;10] and in rodent models [4–9], which included both pathophysiologic and behavioral studies [20]. Behavioral deficits in these animal models include, but not limited to, alterations in spatial memory, motivation, behavioral plasticity, habituation, and executive function [20]. Further, longitudinal studies focusing on neurocognitive dysfunction of HIV-1 Tg rats indicate an altered temporal processing at different ages (by assessing pre-pulse inhibition of a startle response), which persists from adolescent to adult ages [20;23–26]. Other studies also reveal a persisting increase in anxiety of young adult (2m~11m of age) Tg rats [26;27]. It is likely that HIV-induced dysfunction of VGCCs [4–9] and K+ channels ultimately results in the mPFC dysfunction that underlies the neuropathogenesis as well as the progression of HAND, especially during aging.
Collectively, the present study demonstrates that aging and HIV alter the activity and expression of K+ channels in mPFC pyramidal neurons, which could in part contribute to the decline of neuronal activity in the mPFC. These findings reveal a novel mechanism by which aging/HIV comorbidity mediates the dysregulation of mPFC pyramidal neurons’ activity, which could eventually lead to loss of neuronal function.
Highlights:
Activity of mPFC pyramidal neurons is significantly declined during aging.
HIV-1 induces hyperactivity of mPFC neurons independent of age.
K+ channels mediate aging and HIV-induced alterations in mPFC neuronal activity.
Aging/HIV dysregulation of K+ channels is time-dependent/K+ channel gene-specific.
Acknowledgements:
This work was supported by NIH grants USPHSGs NS084817 and DA044552 (X-TH); and DA033966 and NS060632 (LA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no competing financial interest.
Reference List
- [1].Gill AJ, Kolson DL, Chronic inflammation and the role for cofactors (hepatitis C, drug abuse, antiretroviral drug toxicity, aging) in HAND persistence, Curr. HIV. /AIDS Rep 11 (2014) 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study, Neurology 75 (2010) 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ferris MJ, Mactutus CF, Booze RM, Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: Current status of dopamine system vulnerability in NeuroAIDS, Neurosci. Biobehav. Rev 32 (2008) 883–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wayman WN, Chen L, Napier TC, Hu XT, Cocaine self-administration enhances excitatory responses of pyramidal neurons in the rat medial prefrontal cortex to human immunodeficiency virus-1 Tat, Eur. J. Neurosci 41 (2015) 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wayman WN, Chen L, Hu XT, Napier TC, HIV-1 transgenic rat prefrontal cortex hyper-excitability is enhanced by cocaine self-administration, Neuropsychopharmacology 41 (2016) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Khodr CE, Chen L, Dave S, Al-Harthi L, Hu XT, Combined chronic blockade of hyper-active L-type calcium channels and NMDA receptors ameliorates HIV-1 associated hyper-excitability of mPFC pyramidal neurons, Neurobiol. Dis 94 (2016) 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wayman WN, Dodiya HB, Persons AL, Kashanchi F, Kordower JH, Hu X-T, Napier TC, Enduring cortical alterations after a single in vivo treatment of HIV-1 Tat, Neuroreport 23 (2012) 825–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Napier TC, Chen L, Kashanchi F, Hu X-T, Repeated cocaine treatment enhances HIV-1 Tat-induced cortical excitability via over-activation of L-type calcium channels, J. Neuroimmune. Pharmacol (2014). [DOI] [PMC free article] [PubMed]
- [9].Khodr CE, Chen L, Al-Harthi L, Hu XT, Aging alters voltage-gated calcium channels in prefrontal cortex pyramidal neurons in the HIV brain, J. Neurovirol 24 (2018) 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hu X-T, HIV-1 Tat-induced calcium dysregulation and neuronal dysfunction in vulnerable brain regions, Current Drug Targets 17 (2016) 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mattson MP, Haughey NJ, Nath A, Cell death in HIV dementia, Cell Death. Differ 12 Suppl 1 (2005) 893–904. [DOI] [PubMed] [Google Scholar]
- [12].Gelman BB, Soukup VM, Schuenke KW, Keherly MJ, Holzer C III, Richey FJ, Lahart CJ, Acquired neuronal channelopathies in HIV-associated dementia, J Neuroimmunol 157 (2004) 111–119. [DOI] [PubMed] [Google Scholar]
- [13].Zegarra-Moran O, Rasola A, Rugolo M, Porcelli AM, Rossi B, Galietta LJ, HIV-1 nef expression inhibits the activity of a Ca2+-dependent K+ channel involved in the control of the resting potential in CEM lymphocytes, J. Immunol 162 (1999) 5359–5366. [PubMed] [Google Scholar]
- [14].Herrmann M, Ruprecht K, Sauter M, Martinez J, van HP, Glas M, Best B, Meyerhans A, Roemer K, Mueller-Lantzsch N, Interaction of human immunodeficiency virus gp120 with the voltage-gated potassium channel BEC1, FEBS Lett 584 (2010) 3513–3518. [DOI] [PubMed] [Google Scholar]
- [15].Zhu Q, Song X, Zhou J, Wang Y, Xia J, Qian W, Zhu J, Gao R, Wang J, Xiao H, Target of HIV-1 Envelope Glycoprotein gp120-Induced Hippocampal Neuron Damage: Role of Voltage-Gated K(+) Channel Kv2.1, Viral Immunol 28 (2015) 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu H, Liu J, Xu E, Tu G, Guo M, Liang S, Xiong H, Human immunodeficiency virus protein Tat induces oligodendrocyte injury by enhancing outward K+ current conducted by KV1.3, Neurobiol. Dis 97 (2017) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hsu K, Seharaseyon J, Dong P, Bour S, Marban E, Mutual functional destruction of HIV-1 Vpu and host TASK-1 channel, Mol. Cell 14 (2004) 259–267. [DOI] [PubMed] [Google Scholar]
- [18].Hsu K, Han J, Shinlapawittayatorn K, Deschenes I, Marban E, Membrane potential depolarization as a triggering mechanism for Vpu-mediated HIV-1 release, Biophys. J 99 (2010) 1718–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Reid W, Sadowska M, Denaro F, Rao S, Foulke J Jr., Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O’Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J, An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction, Proc. Natl. Acad. Sci. U. S. A 98 (2001) 9271–9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vigorito M, Connaghan KP, Chang SL, The HIV-1 transgenic rat model of neuroHIV, Brain Behav. Immun 48 (2015) 336–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL, The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART, J. Neuroimmunol 218 (2010) 94–101. [DOI] [PubMed] [Google Scholar]
- [22].Hille B, Ionic Channels of Excitable Membranes, Skyscrape, Inc., New York, 2001. [Google Scholar]
- [23].McLaurin KA, Moran LM, Li H, Booze RM, Mactutus CF, A Gap in Time: Extending our Knowledge of Temporal Processing Deficits in the HIV-1 Transgenic Rat, J. Neuroimmune. Pharmacol 12 (2017) 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].McLaurin KA, Booze RM, Mactutus CF, Progression of temporal processing deficits in the HIV-1 transgenic rat, Sci. Rep 6 (2016) 32831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Moran LM, Booze RM, Mactutus CF, Time and time again: temporal processing demands implicate perceptual and gating deficits in the HIV-1 transgenic rat, J. Neuroimmune. Pharmacol 8 (2013) 988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Moran LM, Booze RM, Webb KM, Mactutus CF, Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction, Exp. Neurol 239 (2013) 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Reid WC, Casas R, Papadakis GZ, Muthusamy S, Lee DE, Ibrahim WG, Nair A, Koziol D, Maric D, Hammoud DA, Neurobehavioral Abnormalities in the HIV-1 Transgenic Rat Do Not Correspond to Neuronal Hypometabolism on 18F-FDG-PET, PLoS. ONE 11 (2016) e0152265. [DOI] [PMC free article] [PubMed] [Google Scholar]


