Summary
Anti‐phospholipid syndrome (APS) is characterized by recurrent pathological pregnancy, arterial or venous thrombosis in the presence of anti‐phospholipid antibody (aPL). Complement activation is recognized as an intermediate link leading to placental thrombosis and placental inflammation in APS model mice. Decay accelerating factor (DAF, CD55), MAC‐inhibitory protein (MAC‐IP, CD59) and membrane co‐factor protein (MCP, CD46) are important complement inhibitory proteins (CIPs) highly expressed in normal placenta to curb excessive complement activation and its mediated injuries. Anti‐β2 glycoprotein I (anti‐β2GPI) antibody is an important aPL. We found that placental DAF and CD46 decreased in β2GPI passively immunized APS model mice, accompanied by C3 deposition, neutrophil infiltration and increased proinflammatory cytokine levels detected in its placenta. Progesterone supplement can up‐regulate DAF but not CD46 expression, curb C3 activation and decrease proinflammatory cytokines levels to reduce fetal loss frequency. Progesterone receptor antagonist (mifepristone) or knock‐down DAF with specific siRNA, above the protective effects of progesterone, were significantly weakened. Another sex hormone, oestrogen, has no significant effect on placental DAF and C3 contents and fetal loss frequency in the APS mice model. This may be an important mechanism by which progesterone induces maternal–fetal immune tolerance. At the same time, it may provide evidence for the use of progesterone in APS abortion patients.
Keywords: anti‐phospholipid syndrome, complement, decay accelerating factor, progesterone
Introduction
Anti‐phospholipid syndrome (APS) is one of a group of systemic autoimmune diseases which can cause pregnancy morbidities and recurrent systemic vascular thrombosis, involving both arterial and venous vessels in the persistent presence of aPL 1, 2. APL is a series of heterogeneous phospholipid‐binding autoantibodies. Clinically relevant aPL include lupus anti‐coagulant (LA), anti‐cardiolipin (ACL) and anti‐β2 glycoprotein I (GPI). It has been clarified that the antigenic reactivity process between aPL and β2GPI is a key link in the pathogenesis of APS, but the specific mechanism of aPL‐mediated abortion and tissue damage has not been fully studied.
β2GPI is a most well‐studied antigen of aPL 3, 4. β2GPI can immobilize on anionic phospholipid surfaces such as endothelial cells 5, platelets and monocytes 6, 7, 8 to expose specific epitopes for aPL to bind, thereby activating them to produce a procoagulant and proinflammatory phenotype 6, 7, 8. However, aPL can not only activate blood cells and blood coagulation factors, but can also mediate complement activation directly or indirectly 9, 10. Despite thrombosis and ischaemic damage, complement over‐activation in placenta has gradually been revealed to be another important mechanism in forming APS pathological injuries 9, 10, 11, 12.
aPL has complicated cross‐reactions among complement, coagulation and inflammation systems 13. Complement lysates C3a and C5a can activate prothrombin to promote blood coagulation and damage placental blood supply 14. Complement lysate C5a binds to various cells expressing C5a receptors such as neutrophils, endothelial and macrophages to promote them releasing chemokines, finally leading to placental inflammatory injuries 15, 16. Cytokines participate in inflammation and the coagulation system, and can also activate the complement system to form a vicious circle to amplify inflammatory and thrombosis injuries to placenta.
Researchers found C3, C5 and its debris deposits in both APS patient and mice model placenta 11, 17, 18. Placental complement levels were decreased, placental thrombosis and inflammation relieved and fetal loss frequency reduced C5aR deficiency in mice or mice treated with C5aR antagonist peptide 11.
Both human and mouse placental tissue undergo a certain degree of complement strike in normal pregnancy 19, 20. Despite evidence of complement activation found in the placenta, excessive complement activation can be curbed by up‐regulated placenta complement inhibitory proteins (CIPs) 18, 21, 22, 23. Decay accelerating factor (DAF), CD46 and CD59 are important CIPs, which are highly expressed in placenta to inhibit the cascade amplification of complement in normal pregnancy 22, 23.
Oestrogen and progesterone are important substances that control women’s menstrual and pregnant physiology 24, 25. DAF contents in the uterus change with the female physiological cycle 25. We hypothesize that DAF expression may be related to hormone levels during implantation or pregnancy phase.
Further experiments were carried out in this paper to confirm the influence of progesterone and oestrogen on placental DAF expression and its possible anti‐inflammation, anti‐coagulation and anti‐thrombosis effects in the β2GPI actively immunized abortion mice model (APS mice model).
Materials and methods
Purification and characterization of β2GPI
Human β2GPI was purified by perchloric acid treatment of pooled sera obtained from healthy blood donors. All sera samples were followed by affinity purification (heparin column, Heparin Sepharose CL‐6B; GE Healthcare, Chicago, IL, USA) and ion‐exchange chromatography (Resource‐S; GE Healthcare). The immunoblotting method was used to identify β2GPI and analyse its purity.
Murine fetal loss model
SPF‐grade, 8‐week‐old female BALB/C mice, weighing 25 ± 2 g, and 8–10‐week‐old male BALB/C mice weighing 30 ± 2 g, were involved in our study. All mice had regular oestrus cycles and satisfied quarantine conditions provided by the Experimental Animal Center of Hubei University of Traditional Chinese Medicine. The feeding temperature (20–22°C) and humidity (50–70%) were suitable. All animal experiments in this study were approved by the Ethics Committees of the Maternal and Child Health Hospital of Hubei Province.
We established an APS abortion model to mimic human APS by actively immunizing mice with β2‐GPI. Human β2‐GPI was purified as per previous protocol and dissolved in sterile physiological saline to a solution of 100 μg/0·25 ml concentration, and mixed with complete Freund’s adjuvant (CFA; Sigma, St Louis, MO, USA) in equal volumes. On the first day of the experiment, 50 μl of the fully emulsified mixture was injected subcutaneously into the necks of the mice. As with the configuration and injection procedure, CFA was replaced by incomplete Freund’s adjuvant (IFA; Sigma) on day 8 to strengthen the immunization. On days 1 and 8, control group (Cont.G.) mice were injected with equal volumes of only CAF and IFA separately without β2‐GPI.
Serum anti‐β2GPI antibody levels were detected by enzyme‐linked immunosorbent assay (ELISA) on day 10 after the mice were last immunized. Mice were successfully modelled when anti‐β2GPI antibody titre was twice that of normal mice; 65% were successfully modelled. Resorption fetuses were detected in approximately 96% of successfully modelled mice (data not shown).
Male and female mice were caged (at 2 : 1 ratio) at 9:00 p.m.; the copulatory plug was examined between 8:00 a.m. and 8:30 a.m. the next day and then segregated into male and female groups. Mice without a copulatory plug were caged again at 9:00 p.m. The time at which we found a copulatory plug was defined as day 0·5 day of pregnancy or embryonic day 0·5.
Animal administration
Model mice were given oestrogen (0·6 mg/kg; Solarbio Science and Technology, Beijing, China) for gavage or progesterone (8 mg/kg; Sigma), respectively, for subcutaneous injection daily from days 0·5 to 15·5 of pregnancy to the oestrogen group (Estr.G.) or progesterone group (Prog.G.). All mice were euthanized by intraperitoneal injection of 10% chloral hydrate (Roche, Shanghai, China) on day 15·5 of pregnancy. The dissected uterus, fetus and placenta were weighed. Fetal resorption rates were calculated. Resorption sites can be easily identified in Fig. 1m.
Figure 1.
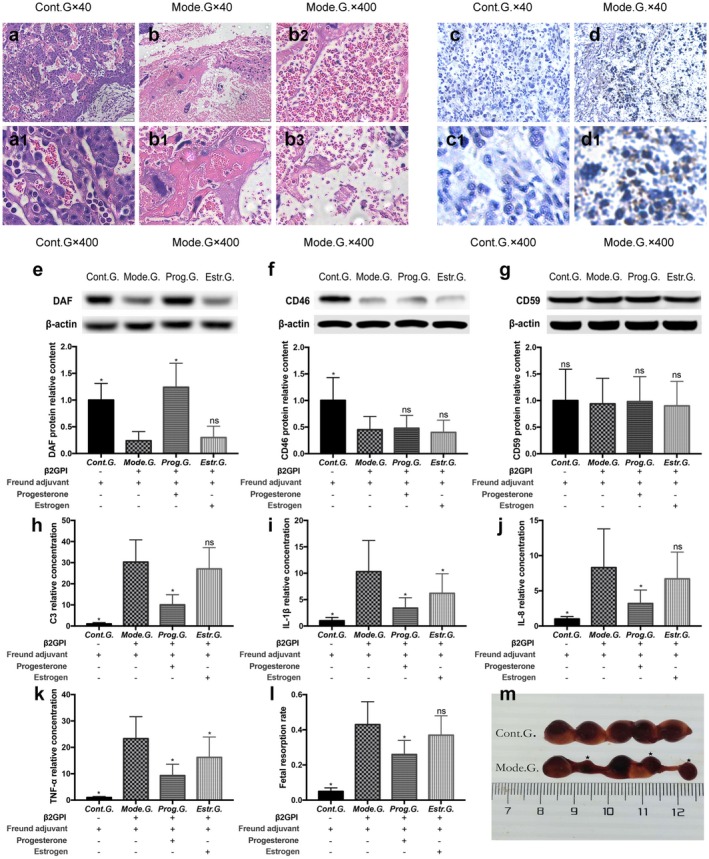
(a) Haematoxylin and eosin (H&E) staining to detect placenta morphological changes in the control group (Cont.G.) (a, a1) and model group (Mode.G.) (b, b1–b3) on day 15·5 of pregnancy; a, a1 were normal placenta; b1 was placental infarction; b2 was placental congestion; b3 was tissue debris in placenta (×40, ×400 magnification). (b) Immunohistochemical staining to detect infiltration of neutrophils in placenta of Cont.G. (c, c1) and Mode.G. (d, d1) on day 15·5 of pregnancy. Tissue sections were incubated with goat anti‐mouse granulocyte RB6‐8C5 monoclonal antibody (mAb), followed by rabbit anti‐mouse immunoglobulin (Ig)G conjugated to horseradish peroxidase (HRP). HRP‐bound antibody was detected by diaminobenzidine (DAB) and was counterstained with haematoxylin. Positive sites were stained brown (×40, ×400 magnification). (c) Effects of modelling and sex hormones (oestrogen and progesterone) on fetal absorption rate (l), decay accelerating factor (DAF) (e), CD46 (f), CD59 (g) protein, C3 (h), interleukin (IL)‐1β(i),IL‐8 (j) and tumour necrosis factor (TNF)‐α (k) in mice placenta on day 15·5 of pregnancy. The Mode.G. was injected with β2 glycoprotein I (GPI) + Freund’s adjuvant, the Cont.G. with Freund’s adjuvant. Successfully modelled mice were further given oestrogen (0·6 mg/kg/day, Estr.G.) or progesterone (8 mg/kg/day, Prog.G.), respectively, from the beginning of pregnancy until day 15.5. Resorption sites were presented in ‘m’ and marked in ‘★’. Ten independent replicate samples were tested in each group. Resorption rate was calculated by three independent individuals. All groups compare with Mode.G.; *P < 0·05.
To further confirm the protective effect of progesterone on APS model mouse, we carried out in‐vivo progesterone antagonistic experiments. Progesterone (8 mg/kg) was given daily to mice from days 0·5 to 15·5 of pregnancy. Mifepristone (2 mg/kg) was added daily from days 5·5 to 15·5. Mice were euthanized on day 15·5 day of pregnancy. Fetal resorption rates, DAF, C3, interleukin (IL)‐1β, IL‐8 and tumour necrosis factor (TNF)‐α levels were tested to detect the effects of mifepristone on model mice.
Immunohistochemical staining
On day 15·5 of pregnancy, placental and decidual tissue were dissected and sections were incubated with goat anti‐mouse C3/C3a antibody (Abcam, Cambridge, MA, USA), goat anti‐mouse DAF (Abcam), CD59 (Abcam) and CD46 (Abcam), followed by anti‐goat immunoglobulin (Ig)G conjugated to horseradish peroxidase (HRP) (Sigma Aldrich, St Louis, MO, USA). Goat anti‐mouse granulocyte RB6‐8C5 monoclonal antibody (mAb) 12 (anti‐Ly6 antibody; Becton Dickinson/PharMingen, Franklin Lakes, NJ, USA) followed by rabbit anti‐mouse IgG (ABclonal, Wuhan, China) conjugated to HRP (Sigma Aldrich) was stained to detect granulocytes in placenta. Antibody‐bound HRP was detected by 3, 3′‐diaminobenzidine (DAB; Solaibao Biotechnology, Beijing, China). All sections were counterstained with haematoxylin (Sigma Aldrich). Intensity of C3, DAF, CD59 and CD46 in decidual and placental section was scored using the semiquantitative method (0–5+) by three observers blinded to the experimental condition. All data were expressed as the mean levels of eight to 12 mice tested in individual experimental conditions. Sections were also stained with haematoxylin and eosin (H&E) to detect tissue histomorphological changes.
Cell administration
HTR‐8/SVneo (Baina Bio., Shanghai, China) cells are immortalized extravillous trophoblasts during the first trimester of pregnancy. They share similar phenotypes with primary cells. All cells were maintained in RPMI‐1640 (Hyclone, San Angelo, TX, USA) containing 5% fetal bovine serum (gibco, Carlsbad, CA, USA) and antibiotic–anti‐mycotic (gibco) under a 5% CO2 humidified atmosphere at 37°C. Cells grown in log phase were seeded in 96‐well sterile plates at a density of 1 × 106 cells/cm2, and human β2GPI (purified as per previous protocol) was added to wells at a concentration of 0·2 mg/ml.
Mouse anti‐human β2GPI IgG monoclonal antibody (60 μg/ml; Abcam) or control IgG (60 μg/ml; Serotec, Oxford, UK) was added separately to HTR‐8/SVneo cells. DAF and CD46 cell levels were detected by Western blot 6 h later. Progesterone was added at gradient concentrations (0, 100, 200 and 400 ng/ml) to HTR‐8/SVneo cells, and DAF levels were detected 6 h later. Mifepristone (Solarbio) was added in gradient concentrations (0, 25, 50 and 100 ng/ml) to HTR‐8/SVneo cells which were pre‐exposed to progesterone, and DAF levels were detected 6 h later.
Western blot analysis
Western blot analysis was used to detect the expression of DAF (Proteintech, Manchester, UK), CD59 (Proteintech) and CD46 (Proteintech) at the maternal–fetal interface. The uteruses of each group was dissected, and the intact placental and decidual tissues were removed from the mice on day 15·5 of pregnancy and stored immediately at –80°C. Total tissue protein was extracted from RIPA protein lysate (Solarbio) and ultracentrifuged for 15 min at 4°C at 13000g. The supernatant was taken and the protein concentration in the supernatant was detected using the bicinchoninic acid assay (BCA) (Solarbio) method. Fifty μg of protein sample was prepared for Western blot analysis to detect DAF, CD59 and CD46 levels at the maternal–fetal interface. All samples were detected using the enhanced chemiluminescence (ECL) method, and the target strip was imaged and analysed in an infrared fluorescence scanning imaging system (Odyssey Clx; Li‐Cor Biosciences, Lincoln, NE, USA). All data were expressed as the mean level of eight to 12 mice tested in individual experimental conditions.
Measurement of cytokines and C3
IL‐1β (Abcam), IL‐8 (R&D Systems, Minneapolis, MN, USA), TNF‐α (R&D Systems) and C3 (Abcam) decidual and placental lysate supernatants were detected on day 15·5 of pregnancy, according to the instructions of the corresponding ELISA kit.
Transfection in vivo
We performed short interfering RNA (siRNA) to transfecting mice through tail‐vein injection of siRNA targeting at DAF mRNA. The transfection mixture was prepared according to the manufacturer’s instructions, and consisted of 4 mg DAF siRNA (siRNA: ATGAUUGGAGAGCACUCUAUU, Invitrogen, Carlsbad, CA, USA) or control siRNA (Cont.siRNA, Dharmacon), 4 ml Entranster in‐vivo transfection reagent (Engreen, Beijing, China), 6 ml RNAse‐free water and 10 ml 10% glucose. The mixture above was injected intravenously at a volume : weight ratio of 2·5 μl/g to the mice. The injection started on day 5·5 and was intensified every 3 days to day 15·5 of pregnancy. After the mice were euthanized, DAF protein levels were detected by Western blot. Fetal loss frequencies were calculated, and C3, IL‐1β, IL‐8 and TNF‐α levels were detected by ELISA.
Statistical analyses
All data are expressed as mean ± standard deviation (s.d.). Fetal resorption rates and fetal weights between groups were compared using a Student’s t‐test, and semiquantitative scores of immunohistochemistry using a Mann–Whitney U‐ test. When P‐values were less than 0·05, null hypotheses were rejected.
Results
Increased fetal loss rate and severe placental C3 deposition and proinflammation cytokines were found in APS model mice
After mice were injected with β2GPI and Freund’s adjuvant, high titres of anti‐β2GPI antibodies were detected in circulating blood (data not shown). In Cont.G., fetal resorption rate was < 7% (Fig. 1l) and close to 43% in the model group (Mode.G.) (Fig. 1l). The fetal absorption rate in Mode.G. was significantly higher than in Cont.G. (P < 0·05). Vaginal bleeding and embryo embolus can be found in the vagina of some model mice.
H&E staining found large amounts of tissue debris (Fig. 1b3) and local infarcts (Fig. 1b1), accompanied by severe congestion (Fig. 1b2) in placenta of Mode.G. Immunohistochemical staining found massive neutrophil infiltration in placenta of Mode.G. (Fig. 1d1–d2). ELISA found that placental C3 (Fig. 1h), IL‐1β (Fig. 1i), IL‐8 (Fig. 1j) and TNF‐α (Fig. 1k) levels in Mode.G. were significantly higher than in Cont.G. (P < 0·05).
Progesterone supplement protects model mice from fetal loss and inhibits placental C3 deposition and proinflammation cytokine secretion
In contrast with Cont.G., fetal resorption rate showed a nearly two‐fifths decrease (P < 0·05) (Fig. 1l) in Prog.G. and a nearly one‐seventh (P > 0·05) (Fig. 1l) decrease in Estr.G. C3 (Fig. 1h), IL‐1β (Fig. 1i), IL‐8 (Fig. 1j) and TNF‐α (Fig. 1k) levels in Prog.G. were sharply decreased (P < 0·05). IL‐1β (Fig. 1i) and TNF‐α (Fig. 1k) levels in Estr.G. gently decreased, but C3 (Fig. 1h) and IL‐8 (Fig. 1j) remained high.
This suggests that the protective effects of progesterone on APS mice model were responsible for progesterone’s effects on inhibiting complement activation and inflammation cytokine secretion in placenta, whereas oestrogen has no effect on placental C3 deposition and fetal resorption rate.
Progesterone receptor antagonist blocks progesterone‐induced protection on the APS mice model
To further confirm the protective effect of progesterone on the model mouse, both progesterone and progesterone receptor antagonists (mifepristone) were given to the model mice during pregnancy. We found that the protective effect of progesterone on APS mice was significantly weakened by mifepristone and the fetal absorption rate increased (Fig. 2a). Placental C3, a proinflammatory factor, clearly increased (Fig. 2b).
Figure 2.
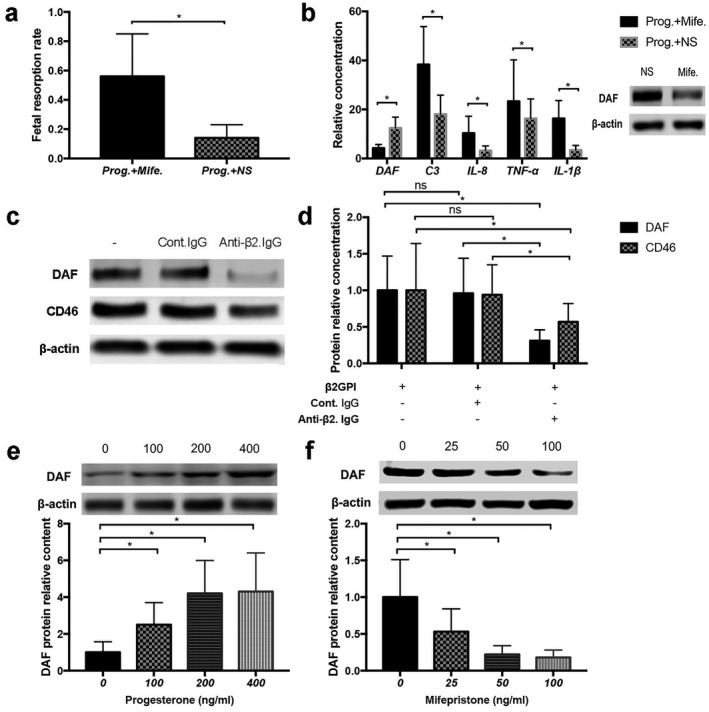
(f) In‐vivo antagonistic experiments of mifepristone. Model mice were given progesterone (8 mg/kg/day) + normal saline (NS) (Prog.+NS) or progesterone (8 mg/kg/day) + mifepristone (2 mg/kg/day) (Prog.+Mife.), respectively. Progesterone was daily given to mice from day 0·5 of pregnancy to day 15·5. Mifepristone or NS were added from days 5·5 to 15·5. Mice were euthanized on day 15·5 of pregnancy, fetal resorption rates were calculated (a), decay accelerating factor (DAF) protein levels and C3, interleukin (IL)‐1β, IL‐8 and tumour necrosis factor (TNF)‐α levels tested in enzyme‐linked immunosorbent assays (ELISA) (b). (g) In‐vitro effects of anti‐β2 glycoprotein I (GPI) immunoglobulin (Ig)G on HTR‐8/SVneo cell DAF and CD46 expression. Anti‐β2GPI IgG (60 mg/ml, anti‐β2.IgG) or control IgG (60 μg/ml, Cont.IgG) were separately added to HTR‐8/SVneo cells pre‐exposed to human β2GPI (0·2 mg/ml). Cell DAF and CD46 levels were detected 6 h later; data are presented in (c) and (d). (h) In‐vitro influence of progesterone and mifepristone on HTR‐8/SVneo cells DAF expression. DAF levels (e) in HTR‐8/SVneo cells treated with gradient progesterone concentrations (0, 100, 200, 400 ng/ml). DAF levels (f) in HTR‐8/SVneo cells treated with gradient mifepristone concentrations (0, 25, 50, 100 ng/ml). Experiments performed on at least three independent individual samples; *P < 0·05.
This demonstrates that the inhibiting effects of progesterone on complement deposition and inflammation cytokine secretion in placenta may be mediated by progesterone receptor.
Placental DAF and CD46 decreased in model mice and progesterone can restore placental DAF expression
Western blot was used to detect mice placental DAF, CD46 and CD59 levels in both Cont.G. and Mode.G. We found that placental DAF (Fig. 1e), CD46 (Fig. 1f) and CD59 (Fig. 1g) were all highly expressed in Cont.G. DAF (Fig. 1e) and CD46 (Fig. 1e) sharply decreased in Mode.G., but CD59 (Fig. 1 g) levels did not change.
To investigate the mechanisms responsible for CD46 and CD59 expression disorder, in‐vitro cell experiments were conducted. After anti‐β2GPI IgG or control IgG were added to HTR‐8/SVneo cells, we found that DAF and CD46 levels decreased in cells treated with β2GPI IgG, in contrast to control IgG (Fig. 2c–d). This indicates that anti‐β2GPI antibody can directly inhibit trophoblast DAF and CD46 expression.
Western blot analysis and immunohistochemical staining were used to detect the effects of progesterone and oestrogen on placental CIP expression and C3 content in APS model mice. Western blot analysis found that progesterone can increase DAF expression (Fig. 1e) in APS mice placenta, but CD46 levels did not change (Fig. 1f). Oestrogen had no effect on both DAF and CD46 expression. Immunohistochemistry experiments found that DAF undergo a decrease in Mode.G. (Fig. 3f), with increased C3 depositing in placenta (Fig. 3b1) and decidua (Fig. 3b2) in contrast to Cont.G. Compared with Mode.G., DAF levels (Fig. 3g) increased and C3 levels (Fig. 3c1–c2) decreased in Prog.G. No significant differences in DAF and C3 levels were detected between Mode.G. (Fig. 3 b1–b2, f). and Estr.G. (Fig. 3d1–d2,h). This indicates that progesterone can up‐regulate placental DAF expression and inhibit C3 deposition in the APS mice model, but oestrogen has no significant effect.
Figure 3.
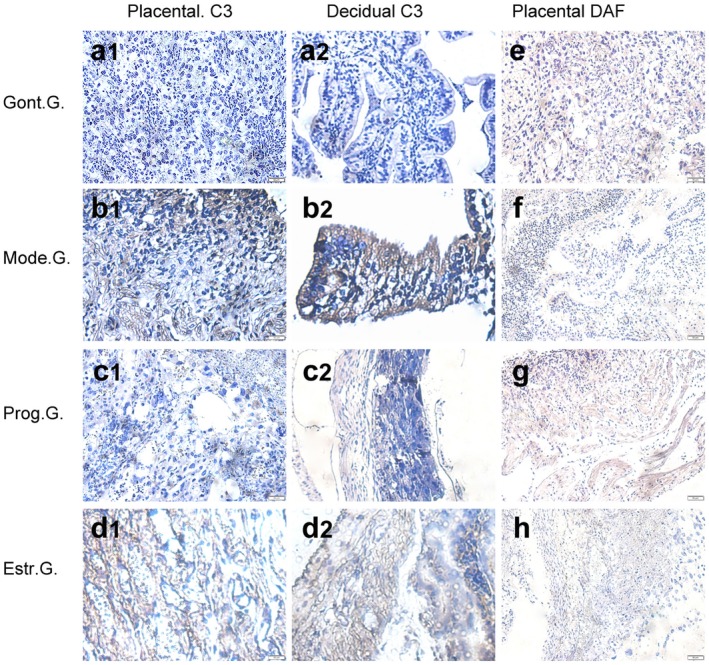
(e) Immunohistochemical staining method was used to detect decay accelerating factor (DAF) (e–h) expression and C3 (a1–d1, a2–d2) deposition in placenta on day 15·5 of pregnancy. Placenta sections of control group (Cont.G.) (a1, a2, e), model group (Mode.G.) (b1, b2, f), progesterone group (Prog.G.) (c1, c2, g) and oestrogen group (Estr.G.) (d1, d2, h) were incubated with goat anti‐mouse decay accelerating factor (DAF) monoclonal antibody (mAb) or goat anti‐mouse C3/C3a mAb, followed by rabbit anti‐mouse immunoglobulin (Ig)G conjugated to horseradish peroxidase (HRP) and detected by diaminobenzidine (DAB) and were counterstained with haematoxylin. Positive sites were stained brown. Results are a representation of experiments performed on at least three independent individual samples (×40 magnification).
Abundant C3 and proinflammation cytokines found in DAF‐deficient mice placenta
DAF highly expressed in normal placenta can negatively regulate complement activation. In order to confirm the possibly important role of DAF in maintaining physiological pregnancy, DAF‐specific siRNA was injected into mice to knock‐down DAF or control siRNA as a contrast. Mice placental DAF decreased after DAF siRNA injection (Fig. 4a,b). We found significantly higher control siRNA placental C3, IL‐1β, IL‐8 and TNF‐α levels in mice injected with DAF siRNA (Fig. 4d). Increased fetal resorption rate (Fig. 4c) and severe placental injuries were detected in DAF‐deficient mice (data not shown).
Figure 4.
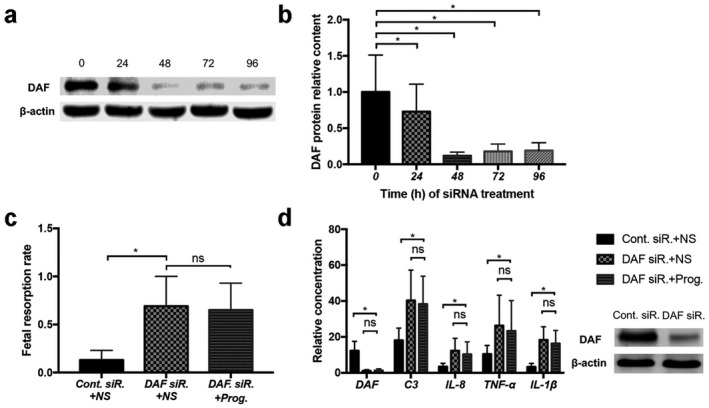
(i) In‐vivo siRNA knock‐down test. Decay accelerating factor (DAF) siRNA (DAF siR.) or control siRNA (Cont. siR.) were intravenously injected into mice (2.5 ml/g, volume to weight ratio) started on day 5.5 of pregnancy and were intensified every 3 days until day 15.5 of pregnancy. At the same time, progesterone (8 μg/kg/day) or an equal volume of NS were injected into mice daily from days 5.5 to 15.5 of pregnancy. DAF levels (a,b) at different DAF siRNA intervention times (0, 24, 48, 72, 96 h) were tested. Fetal resorption rates (c) calculated, placental DAF, C3, interleukin (IL)‐1β, IL‐8 and tumour necrosis factor (TNF)‐α levels (d) were tested; *P < 0.05, ns means P > 0.05.
This indicates that DAF plays an important role in inhibiting complement activation and inflammation cytokine secretion to maintain normal pregnancy.
Anti‐complement activation and anti‐inflammatory effects of progesterone depend on the expression of DAF
We have confirmed that progesterone can repair DAF expression in APS mice placenta, and can obviously inhibit complement activation and inflammation cytokine secretion. We hypothesized that progesterone may exert its anti‐complement activation and anti‐inflammatory effects by affecting the expression of DAF. To confirm our hypothesis, progesterone or Normal Saline (NS, 0.9% NaCl/ddH2O2) were injected into DAF‐deficient mice (DAF siRNA pretreated mice). There was no significant difference in fetal loss rates and placental C3, IL‐1β, IL‐8 or TNF‐α levels between progesterone and NS‐treated mice (Fig. 4d). We found that DAF‐deficient mice lose sensitivity to progesterone‐induced protection on fetal loss and placenta damage, indicating that DAF is involved in progesterone‐mediated fetal protection.
Progesterone up‐regulates DAF expression in HTR‐8/SVneo cells, and may be mediated by classical progesterone receptor
To further confirm the relationship between progesterone and DAF protein expression, we conducted two in‐vitro experiments. Progesterone was added to HTR‐8/SVneo cells at gradient concentrations, and we found that cell DAF levels increased with increasing progesterone concentrations (Fig. 2e). Mifepristone, a classical progesterone receptor inhibitor, was added to HTR‐8/SVneo cells at gradient concentrations, and we found that cell DAF expression decreased with increasing mifepristone concentrations (Fig. 2f), indicating that progesterone can up‐regulate HTR‐8/SVneo cell DAF expression, and may be mediated by classical progesterone receptor.
Discussion
Placental vascular thrombosis has previously been considered to be the key cause of adverse pregnancy outcomes in APS. However, there are many clinical APS patients with repeated fetal loss at less than 10 weeks 26 during which the vascular network between maternal and fetus is not yet established, and does not rely on placental circulation to provide nutrients. Mice experiments found that retransmission of aPL antibodies to mice can even result in infertility 27. Clinical observation found that the total effective rate of anti‐coagulant and anti‐platelet drugs for APS pathological pregnancy is only approximately 70% 28, 29. However, corticosteroids combined with traditional therapeutic drugs can improve the pregnancy outcomes of nearly 61% of patients refractory to conventional anti‐coagulant therapy 30, 31, 32, all of which proves indirectly that thrombosis may be just one of the mechanisms of pathological pregnancy in APS.
In addition to thrombosis, complement activation and inflammatory damage were found to be closely related to the occurrence of adverse pregnancy events in APS. Researchers found a large number of complement C3 and C4 and activation products such as C3b, C4d and C5b‐9 33, 34 in the placenta of APS patients and APS model mice. It found that fetal loss frequency, placental complement deposition, thrombosis and inflammation damage were significantly relieved in C5a‐deficient mice or mice treated with C5aR antagonist peptide. During pregnancy, oestrogen can stimulate the synthesis of C3 and C4, so it is difficult to accurately judge the actual consumption of C3 and C4 in APS; thus, just a slight decrease of C3 and C4 levels were detected in the peripheral blood of some patients with obstetric APS, whereas evidence of complement activation was found in almost all its placental tissues. Researchers also found that complement inhibitor eculizumab effectively prevented the recurrence of thrombotic events in one APS patient after arterial bypass surgery 35, indicating that activation of complement may also be involved in the occurrence of vascular thrombosis events in APS patients.
As well as recurrent spontaneous abortions, a significant increase of complement activation has been also associated with other pathologies of pregnancy, namely pre‐eclampsia, preterm delivery (PD) and intrauterine growth retardation (IUGR). Excessive activation of the alternative pathway of the complement system has been considered to be one of the mechanisms responsible for pre‐eclampsia in human pregnancy 36, 37. Women with high circulating Bb in early pregnancy have an almost 3·8‐fold higher risk to develop pre‐eclampsia than normal women. In addition, complement activation fragments were also considered as a risk marker for PD 36 and IUGR 37.
Anti‐β2‐GPI antibody is considered to be an important antibody leading to APS pathological pregnancy. Anti‐β2‐GPI antibody has long been found to be closely related to pathological changes in APS, and is commonly seen in ASP patients 38, 39. Injecting β2GPI into actively immunized mice is a proven effective method for establishing APS animal models. Therefore, we established an APS abortion model by actively immunizing mice with β2‐GPI.
DAF is one of the CIPs that inhibits the activation of C3 and C5 convertase to limit the cascade amplification of complement activation and reduce C3 self‐activation. High levels of DAF are expressed in implantation endometrium 25 and embryonic tissues 40 under normal physiological conditions to inhibit the excessive activation of complement and its accompanied damage 23, 41. However, placenta DAF is impaired in patients with abortion, repeated implantation failure and pre‐eclampsia 42, 43, 44, 45, 46.
We found fetal loss frequency in DAF‐deficient mice to be significantly increased, with severe complement activation and inflammatory damage in its placenta. This indicates that the inhibitory effect of DAF on the complement system is important for maintaining normal pregnancy in mice.
We also found that placental DAF and CD46 decreased in APS mice, and in‐vivo experiments testified that anti‐β2GPI IgG can directly inhibit DAF expression in HTR‐8/SVneo cells. Anti‐β2GPI IgG‐mediated trophoblast CIP expression disorder may be a new, important mechanism for complement activation and inflammation damage in APS to mediate pathological pregnancy.
Progesterone and oestrogen are important substances for maintaining menstruation and pregnancy physiology; progesterone is sometimes called the ‘hormone of pregnancy’ 47. Progesterone plays an important role in pregnancy maintenance and fetal development; it changes the maternal–fetal immune microenvironment in many ways to participate in the formation of maternal–fetal immune tolerance 47. Progesterone gradually increases after ovulation, and has a high serum level during pregnancy. Researchers found that DAF levels increased in the implantation endometrium and pregnancy placenta. Our study found that it may be up‐regulated by progesterone.
Progesterone up‐regulates placental DAF expression to inhibit complement activation‐mediated thrombosis and inflammatory damage to protect APS mice model from fetal loss. Progesterone antagonist experiments and DAF knock‐down experiments further confirmed the influence of progesterone on DAF expression and its accompanied protective effects on β2GPI actively immunized mice.
We found that anti‐β2GPI IgG can directly inhibit trophoblast DAF and CD46 expression. Our experiments reconfirmed the close relationship between complement activation and APS pathological pregnancy. It was also found that progesterone supplement can up‐regulate DAF expression in placenta to inhibit complement activation and its accompanying damage. Our experiments uncovered a therapeutic mechanism of progesterone in the treatment of APS mice morbidity pregnancy from the perspective of complement regulation, and provide evidence for the use of progesterone in APS patients. Low molecular weight heparin combined with low‐dose aspirin is currently the first‐line treatment for APS abortion 48. In addition to traditional anti‐coagulation, anti‐platelet and immunosuppressant agents are involved in APS medication; it has been confirmed in our experiments that inhibition of complement activation may be another effective therapeutic target for APS pathological pregnancy.
We have only observed the effect of progesterone on the fetal resorption rate in APS mice in this paper. Progesterone is influential in improving other pathological pregnancies, such as pre‐eclampsia, PD and IUGR associated with complement activation in APS patients. These results need further investigation.
DAF, CD46 and CD59 are all expressed in the placenta. However, CD46 and CD59 were only briefly studied in this work. Their relationship with normal and pathological pregnancies and their roles in maintaining pregnancy needs to be further tested.
Disclosures
No conflicts of interests to declare.
Acknowledgements
We thank all participants in this study. This work was supported by grants from Hubei Provincial Health and Family Planning Commission Joint Innovation Team Project (WJ2018H0135) and the Key Project of Hubei Provincial Health and Family Planning Commission Fund (2016Z‐Z09).
References
- 1. Cervera R. Antiphospholipid syndrome. Thromb Res 2017; 151:43–7. [DOI] [PubMed] [Google Scholar]
- 2. Ruiz‐Irastorza G, Crowther M, Branch W et al Antiphospholipid syndrome. Lancet 2010; 376:1498–509. [DOI] [PubMed] [Google Scholar]
- 3. Chamley LW, Duncalf AM, Konarkowska B et al Conformationally altered beta 2‐glycoprotein I is the antigen for anti‐cardiolipin autoantibodies. Clin Exp Immunol 1999; 115:571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chamley LW. Antiphospholipid antibodies: biological basis and prospects for treatment. J Reprod Immunol 2002; 57:185–202. [DOI] [PubMed] [Google Scholar]
- 5. Simantov R, LaSala JM, Lo SK et al Activation of cultured vascular endothelium by antiphospholipid antibodies. J Clin Invest 1995; 96:2211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Devine DV. The effects of complement activation on platelets. Curr Topics Microbiol Immunol 1992; 178:101–13. [DOI] [PubMed] [Google Scholar]
- 7. Proulle V, Furie RA, Merrill‐Skoloff G et al Platelets are required for enhanced activation of the endothelium and fibrinogen in a mouse thrombosis model of APS. Blood 2014; 124:611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lood C, Tydén H, Gullstrand B et al Platelet activation and anti‐phospholipid antibodies collaborate in the activation of the complement system on platelets in systemic lupus erythematosus. PLOS ONE 2014; 9:e99386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holers VM, Girardi G, Mo L et al Complement C3 activation is required for anti‐phospholipid antibody‐induced fetal loss. J Exp Med 2002; 195:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tedesco F, Borghi MO, Gerosa M et al Pathogenic role of complement in antiphospholipid syndrome and therapeutic implications. Front Immunol 2018; 19:1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Girardi G, Berman J, Redecha P et al Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest 2003; 112:1644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samarkos M, Mylona E, Kapsimali V. The role of complement in the antiphospholipid syndrome: a novel mechanism for pregnancy morbidity. Semin Arthritis Rheum 2012; 42:66–9. [DOI] [PubMed] [Google Scholar]
- 13. Keragala CB, Draxler DF, Mcquilten ZK et al Haemostasis and innate immunity‐a complementary relationship. Br J Haematol 2018; 180:782–98. [DOI] [PubMed] [Google Scholar]
- 14. Mastellos D, Papadimitriou JC, Franchini S et al A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J Immunol 2001; 166:2479–86. [DOI] [PubMed] [Google Scholar]
- 15. Czermak BJ, Sarma V, Bless NM et al In vitro and in vivo dependency of chemokine generation on C5a and TNF‐alpha. J Immunol 1999; 162:2321–5. [PubMed] [Google Scholar]
- 16. Laudes IJ, Chu JC, Huber‐Lang M et al Expression and function of C5a receptor in mouse microvascular endothelial cells. J Immunol 2002; 169:5962–70. [DOI] [PubMed] [Google Scholar]
- 17. Banadakoppa M, Chauhan MS, Havemann D et al Spontaneous abortion is associated with elevated systemic C5a and reduced mRNA of complement inhibitory proteins in placenta. Clin Exp Immunol 2014; 177:743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wells M, Bennett J, Bulmer JN et al Complement component deposition in uteroplacental (spiral) arteries in normal human pregnancy. J Reprod Immunol 1987; 12:125–35. [DOI] [PubMed] [Google Scholar]
- 19. Xu C, Mao D, Holers VM et al A critical role for the murine complement regulator Crry in fetomaternal tolerance. Science 2000; 287:498–501. [DOI] [PubMed] [Google Scholar]
- 20. Morgan BP, Holmes CH. Immunology of reproduction: protecting the placenta. Curr Biol 2000; 10:R381–R383. [DOI] [PubMed] [Google Scholar]
- 21. Holmes CH, Simpson KL. Complement and pregnancy: new insights into the immunobiology of the fetomaternal relationship. Baillieres Clin Obst Gyn 1992; 6:439–59. [DOI] [PubMed] [Google Scholar]
- 22. Holmes CH, Simpson KL, Wainwright SD et al Preferential expression of complement regulatory protein decay accelerating factor at the fetomaternal interface during human pregnancy. J Immunol 1990; 144:3099–105. [PubMed] [Google Scholar]
- 23. Cunningham DS, Tichenor JR. Decay accelerating factor protects human trophoblast from complement‐mediated attack. Clin Immunol Immunopathol 1995; 74:156–61. [DOI] [PubMed] [Google Scholar]
- 24. Talbi S, Hamilton AE, Vo KC et al Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo‐ovulatory women. Endocrinology 2006; 147:1097–121. [DOI] [PubMed] [Google Scholar]
- 25. Palomino WA, Argandona F, Azua R et al Complement C3 and decay‐accelerating factor expression levels are modulated by human chorionic gonadotropin in endometrial compartments during the implantation window. Reprod Sci 2013; 20:1103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alijotas‐Reig J, Esteve‐Valverde E, Ferrer‐Oliveras R et al R3 The European Registry on Obstetric Antiphospholipid Syndrome (EUROAPS): a survey of 1000 consecutive cases. Autoimmun Rev 2019; 15:19. [DOI] [PubMed] [Google Scholar]
- 27. Chighizola CB, Raimondo MG, Meroni PL. Does APS impact women’s fertility? Curr Rheumatol Rep 2017; 19:33. [DOI] [PubMed] [Google Scholar]
- 28. Erkan D, Aguiar CL, Andrade D et al 14th International congress on antiphospholipid antibodies: task force report on antiphospholipid syndrome treatment trends. Autoimmun Rev 2014; 13:685–96. [DOI] [PubMed] [Google Scholar]
- 29. Bates SM, Greer IA, Middeldorp S et al VTE, Thrombophilia, antithrombotic therapy, and pregnancy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2012; 141:e691S–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bramham K, Thomas M, Nelson‐Piercy C et al First‐trimester low‐dose prednisolone in refractory antiphospholipid antibodyrelated pregnancy loss. Blood 2011; 117:6948–51. [DOI] [PubMed] [Google Scholar]
- 31. Ruffatti A, Salvan E, Del Ross T et al Treatment strategies and pregnancy outcomes in antiphospholipid syndrome patients with thrombosis and triple antiphospholipid positivity. A European multicentre retrospective study. Thromb Haemost 2014; 112:727–35. [DOI] [PubMed] [Google Scholar]
- 32. Mekinian A, Alijotas‐Reig J, Carrat F et al Refractory obstetrical antiphospholipid syndrome: features, treatment and outcome in a European multicenter retrospective study. Autoimmun Rev 2017; 16:730–4. [DOI] [PubMed] [Google Scholar]
- 33. De La Torre YM, Pregnolato F, D’Amelio F et al Anti‐phospholipid induced murine fetal loss: novel protective effect of a peptide targeting the β2 glycoprotein I phospholipid‐binding site: implications for human fetal loss. J Autoimmun 2012; 38:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meroni PL, Borghi MO, Raschi E et al Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol 2011; 7:330–9. [DOI] [PubMed] [Google Scholar]
- 35. Meroni PL, Macor P, Durigutto P et al Complement activation in antiphospholipid syndrome and its inhibition to prevent rethrombosis after arterial surgery. Blood 2016; 127:365–7. [DOI] [PubMed] [Google Scholar]
- 36. Lynch AM, Murphy JR, Byers T et al Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Gynecol 2008; 198:385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lam C, Lim KH, Sa K. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension 2005; 46:1077–85. [DOI] [PubMed] [Google Scholar]
- 38. Pierangeli SS, Chen PP, Raschi E et al Antiphospholipid antibodies and the antiphospholipid syndrome: pathogenic mechanisms. Semin Thromb Hemost 2008; 34:236–50. [DOI] [PubMed] [Google Scholar]
- 39. Meroni PL, Borghi MO, Raschi E et al Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol 2011; 9:330–9. [DOI] [PubMed] [Google Scholar]
- 40. His BL, Hunt JS, Atkinson JP. Differential expression of complement regulatory proteins on subpopulations of human trophoblast cells. J Reprod Immunol 1991; 19:209–23. [DOI] [PubMed] [Google Scholar]
- 41. Francis J, Rai R, Sebire NJ, Brosens JJ et al Impaired expression of endometrial differentiation markers and complement regulatory proteins in patients with recurrent pregnancy loss associated with antiphospholipid syndrome. Mol Hum Reprod 2006; 12:435–42. [DOI] [PubMed] [Google Scholar]
- 42. Lokki AI, Heikkinen‐Eloranta J, Jarva H et al Complement activation and regulation in preeclamptic placenta. Front Immunol 2014; 5:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Salmon JE, Heuser C, Triebwasser M et al Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLOS Med 2011; 8:e1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Altmäe S, Martinez‐Conejero JA, Salumets A et al Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol Hum Reprod 2010; 16:178–87. [DOI] [PubMed] [Google Scholar]
- 45. Kanellopoulos‐Langevin C, Caucheteux SM, Verbeke P et al Tolerance of the fetus by the maternal immune system: role of inflammatory mediators at the feto–maternal interface. Reprod Biol Endocrinol 2003; 1:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Francis J, Rai R, Sebire NJ et al Impaired expression of endometrial differentiation markers and complement regulatory proteins in patients with recurrent pregnancy loss associated with antiphospholipid syndrome. Mol Hum Reprod 2006; 12:435–42. [DOI] [PubMed] [Google Scholar]
- 47. Gallego MJ, Porayette P, Kaltcheva MM et al The pregnancy hormones human chorionic gonadotropin and progesterone induce human embryonic stem cell proliferation and differentiation into neuroectodermal rosettes. Stem Cell Res Ther 2010; 1:28–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guyatt GH, Akl EA, Crowther M et al Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2012; 141:e278S. [DOI] [PMC free article] [PubMed] [Google Scholar]


