Summary
Depressive symptoms are reported by more than 20% of people with inflammatory bowel disease (IBD), while sleep difficulties and fatigue are even more common. Co‐morbid depressive symptoms predict a poor IBD course, including increased risk of relapse and surgery, which is inconsistently improved by psychological treatments. Rather than being distinct systems, there is compelling evidence for bidirectional communication between gut and brain, driven by neural, metabolic, endocrine and inflammatory mediators. An emerging concept is that depressive symptoms may be mechanistically linked to excess inflammation and dysregulation of the gut–brain axis. Given the close link between the intestinal microbiota and host immune responses, patients prone to shifts in their intestinal microbiome, including smokers, those with poor diet and early life stress, may be exposed to exaggerated immune responses. Excess inflammation is associated with brain changes (depressive symptoms, fatigue, sleep difficulties) and worsening gastrointestinal symptoms, which are exacerbated by psychological distress. Equally, treatments both for depressive symptoms and IBD provide opportunities to break this cycle by reducing the causes and effects of inflammation. As well as addressing potential risk factors such as smoking and diet, treatments to alter the microbiome may reduce depressive symptoms. Observational evidence suggests that anti‐inflammatory treatments for IBD may improve co‐morbid depressive symptoms correlating with reduction in inflammation. With a growing range of treatments targeting inflammation centrally, peripherally and in the gut, IBD provides a unique model to understand the interplay between brain and gut in the pathogenesis of depressive symptoms, both in IBD and in the whole population.
Keywords: depressive symptoms, gut-brain-axis, inflammation, inflammatory bowel disease, microbiome
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC), together comprising inflammatory bowel disease (IBD), are long‐term conditions characterized by a relapsing and remitting disease course. CD is associated with secondary progression with time, requiring surgical intervention in a significant proportion 1, but the factors influencing disease evolution are diverse and difficult to predict 2, 3. The disease course in UC is heterogeneous, including chronic–active or relapsing and remitting phenotype, but also a progressive disease course, necessitating urgent or emergency surgery 3, 4.
Depressive symptoms include low mood, reduced enjoyment, increased fatigue, hopelessness and sleep difficulties 5. Like IBD, depressive symptoms typically run a persistent or fluctuating course, and a significant proportion do not respond to standard therapy 6. Notably, depressive symptoms occur in more than 20% of people with IBD, which is approximately two to four times more common than in the general population 7, 8. As well as reduced quality of life, the co‐morbidity of depressive symptoms and IBD is associated with poor biomedical outcomes, including increased risk of IBD relapse, hospitalization and surgery 9.
A psychological model would position depressive symptoms as a secondary reaction to the burden of IBD, including difficulties with coping, disability, pain, socially unacceptable symptoms and fear of complications 10, 11, 12. In turn, the poor prognosis of depressive symptoms could occur through negative effects on self‐care and treatment adherence 13, 14. However, this model alone provides limited opportunities to improve outcomes. For example, there is an inconsistent correlation between the severity of IBD and the prevalence of depressive symptoms 15, 16, and depressive symptoms correlate variably with treatment adherence 17. While conventional treatments for depressive symptoms (such as cognitive behavioural therapy) frequently improve depressive symptoms in people with IBD, this does not consistently improve biomedical outcomes 18. There is therefore a need to identify novel targets to reduce the risk of depressive symptoms and their impact in people with IBD.
Despite its beneficial short‐term effects in combating infection and enabling tissue repair 19, there is compelling evidence that chronically elevated inflammation has deleterious effects on multiple systems in the body, including the brain and the gut 20, 21. The role of inflammation in the pathogenesis of IBD is well established, in that cytokines drive intestinal inflammation and may also regulate extra‐intestinal disease manifestations and systemic effects 21.
In this clinically focused review, we will argue that depressive symptoms are a further extra‐intestinal manifestation of inflammation in people with IBD. After summarizing the epidemiology of the link between depressive symptoms and IBD, we will examine the evidence for inflammation as a cause of depressive symptoms or more commonly somatic symptoms such as fatigue. We will outline how dysregulation of the gut–brain axis may lead to an ‘inflammatory depression’, which fuels a vicious cycle of worsening IBD outcomes. Finally, we will argue that targeted anti‐inflammatory treatments may improve co‐morbid depressive symptoms in IBD, as well as providing a unique model for understanding the pathogenesis of depressive symptoms in the general population.
Epidemiology
The detection of depressive symptoms in IBD depends on the method of measurement. Clinical depression is a diagnosis made using a detailed diagnostic interview 22. It requires the minimum of two of three ‘core’ symptoms of low mood, anhedonia (reduced enjoyment) and increased fatiguability, in addition to at least two other symptoms (see Table 1), for a minimum of 2 weeks’ duration. Using this approach, the pooled prevalence of depression disorders in IBD is 15·2% [95% confidence interval (CI) = 9·9–20·5%] based on a meta‐analysis of six estimates 8. For the purpose of this review, the term ‘depression’ is therefore reserved for clinical depression only.
Table 1.
Diagnosis and treatment of clinical depression in people with chronic diseases
| Diagnosis according to the International Classification of Diseases 10th edition (ICD‐10) | Stepped care treatment model according to NICE guidelines 5 |
|---|---|
| Criteria | Mild or subthreshold depression |
| For at least 2 weeks (or less if symptoms are severe and of rapid onset), the following 2 core symptoms present | Group physical activity programme, group‐based peer support or to low‐intensity psychological interventions (e.g. group CBT or computerized CBT) |
| Low mood | Antidepressants if symptoms are persistent or unresponsive to above |
| Anhedonia (loss of interest and enjoyment) | |
| Low energy | |
| Plus at least 2 associated symptoms | Moderate depression |
| Reduced concentration and attention | Antidepressant medication (SSRI usual first‐line) for minimum of 6 months; alternatives include mirtazapine, SNRI, tricyclics (caution because of toxicity in overdose) |
| Reduced self‐esteem and self‐confidence | High‐intensity psychological therapy (e.g. individual CBT) |
| Ideas of guilt and unworthiness (even in a mild type of episode) | Combination of antidepressants and psychological therapy (most evidence‐based) |
| Bleak and pessimistic views of the future | |
| Ideas or acts of self‐harm or suicide | |
| Disturbed sleep | |
| Diminished appetite | |
| Severity | Severe depression |
| Mild depression defined with minimum 2 core symptoms and total of 4 symptoms (core or other) | As per moderate depression, but referral to specialist mental services recommended if risk of self‐harm, suicide or severe self‐neglect |
| Moderate depression defined with minimum 2 core symptoms and total of 6 symptoms | Specialist treatments may include augmentation with lithium, anti‐psychotic medication or ECT |
| Severe depression defined with all 3 core symptoms and total of 8 symptoms. Psychotic symptoms (e.g. delusions, hallucinations) may or may not be present |
CBT = cognitive behavioural therapy; ECT = electroconvulsive therapy; ICD = International Classification of Diseases; NICE = National Institute for Health and Care Excellence; SNRI = serotonin and noradrenaline reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor.
As diagnostic interview is time‐intensive, most epidemiological studies of in IBD have instead measured depressive symptoms using self‐report questionnaires, such as the Patient Health Questionnaire‐9 (PHQ‐9). With such questionnaires, a validated cut‐off score (for example, ≥ 10 on the PHQ‐9 23) is typically used to define cases of depressive symptoms. Unlike clinical depression, such an approach does not distinguish between ‘core’ depressive symptoms and other symptoms. Unsurprisingly, this approach leads to a higher pooled prevalence of depressive symptoms of 21·6% (CI = 18·8–24·3%) in people with IBD 8. Notably, there appears to be a difference in depressive symptoms between CD and UC (25·3 versus 16·7%, respectively) and between active disease versus remission (40·7 versus 16·5%) 8. Clinic‐based samples are likely to have either or both more severe IBD and other co‐morbidities.
Prospectively, there is recent evidence from large database research that a prior diagnosis of depression is associated with greater incidence of IBD over time 24. However, no prospective studies have examined the incidence of depressive symptoms and IBD in the same population. Separately, early life stress 25, 26 poor diet 27, 28 and smoking 28, 29 have been found to confer vulnerability to both conditions. Smoking is further known to worsen outcomes in CD but not in UC 30 and smoking appears to be disproportionately common in female CD patients 31. This suggests that lifestyle factors may have a more important role in the depressive symptoms of CD than in UC.
The relationship between depressive symptoms and adverse outcomes in IBD has been inconsistently demonstrated. A meta‐analysis of five small prospective studies found little association 32, while a subsequent 2‐year prospective study found no association between depressive symptoms and worsening IBD activity 33. However, the latter study used the Hospital Anxiety and Depression Scale, which does not capture any somatic symptoms such as fatigue and sleep disturbance. As discussed later, these symptoms may be most associated with inflammation.
By contrast, larger studies have nearly all reported strong associations between depression and a worse IBD course. In database studies, a pre‐existing diagnosis of clinical depression is associated with adverse outcomes in IBD 34, 35, 36. A recent high‐quality prospective cohort study of 4314 patients with IBD over 2 years found significant associations between depressive symptoms and increased risk of multiple adverse outcomes, including relapse, hospitalization, requirement for biologicals and surgery 9. Further, another prospective study of 2007 patients found that depressive symptoms expedited clinical recurrence 37. Table 2 summarizes the associations between depressive symptoms and adverse outcomes in the largest prospective studies to date.
Table 2.
Association between depression and biomedical outcomes in large longitudinal studies* of people with IBD
| Outcome | Measure of depression | Crohn’s disease | Ulcerative colitis |
|---|---|---|---|
| Readmission to hospital | Medical records coding of ICD depression diagnosis | OR = 1·89 (1·06–3·40) in children 35 and OR = 1·27 (1·07–1·50) in adults 34 | No increased odds in children 35 but OR = 1·35 (1·07–1·70) in adults 34 |
| PHQ‐8 score ≥ 5 | RR = 1·3 (1·2–1·5) in adults 9 | RR = 1·3 (1·1–1·5) in adults 9 | |
| Relapse** | PHQ‐8 score ≥ 5 | RR = 2·3 (1·9–2·8) in adults 9 | RR = 1·8 (0·9–1·7) in adults 9 |
| Recurrent disease*** | HADS > 7 | Reduced time to clinical recurrence in adults (P < 0·001) 37 | Reduced time to clinical recurrence in adults (P = 0·005) 37 |
| Requirement for biologicals | PHQ‐8 score ≥ 5 | RR = 1·8 (1·4–2·3) 9 | RR = 1·6 (1·1–2·3) 9 |
| Requirement for surgery | Medical records coding of ICD depression diagnosis | OR = 1·28 (1·03–1·57) in one cohort 36, RR = 1·3 (1·1–1·6) in another 9 | No increased risk of surgery in one cohort 36 but RR = 1·8 (1·2–2·6) in another 9 |
All longitudinal studies with ≥ 1000 patients were included and all results are adjusted for potential confounders.
modified Harvey–Bradshaw Index ≥ 5 or Simple Clinical Colitis Activity Index > 2.
any of: flare events or worsening of the disease (as established by physicians), fistulas and stenosis, anal fissure, abscess, IBD surgery, need for steroids or biologicals.
HAS = Hospital Anxiety and Depression Scale; ICD = International Classification of Diseases; OR = odds ratio (adjusted for confounders); PHQ = Patient Health Questionnaire; RR = relative risk (adjusted for confounders).
In sum, depressive symptoms are over‐represented in people with IBD – especially in CD and in in‐patients – although this is predominantly based on self‐report measures. Moreover, the presence of depressive symptoms is associated with poor biomedical outcomes over time.
Inflammation as a link between depression and IBD
During the last two decades, a pathogenic role for inflammation has been advocated in mental illnesses, including depression, schizophrenia and bipolar disorders 20. Meta‐analyses have found that inflammatory cytokines, such as interleukin (IL)‐6, and acute phase proteins, such as C‐reactive protein (CRP), are associated with increased incidence of depressive symptoms over time 38. Depressive symptoms are also associated with T cell activation, reflected in elevated concentrations of soluble IL‐2 receptors (sIL‐2Rs) and sCD8, as well as increased activation of effector T cell responses, including increased IL‐2, interferon (IFN)‐γ and IL‐17 39, 40. Therapeutically, anti‐inflammatory treatments show promising effects on improving depressive symptoms in the general population 41. Notably, people with early life stress are at increased risk of both increased inflammation and depression in later life 25.
As reviewed previously 40, there are many immune‐inflammatory reactions implicated in both depressive symptoms and IBD, respectively, including increased concentrations of IL‐1, sIL‐2R, IL‐6, IL‐23 and CRP. However, the evidence specifically testing inflammation as a correlate of depressive symptoms in patients with IBD is very limited. In a cross‐sectional study of 11 IBD patients and nine controls, symptomatic IBD patients had the highest depressive and anxiety scores, as well as increased intestinal expression of IL‐6 and IL‐1β and serum IL‐6 42. Prospective studies testing the directionality of inflammation and depressive symptoms in IBD are awaited.
Depressive symptoms or somatic symptoms?
In the general population, somatic symptoms associated with depression, such as fatigue and sleep disturbance, have been found to be more strongly associated with elevated inflammation than more cognitive symptoms, such as low mood and guilt 43, 44, 45. Such somatic symptoms are experienced far more commonly in people with IBD than depressive symptoms overall: fatigue is reported by 44–86% of IBD individuals with active disease and 22–41% of individuals in remission 46, while 77 and 49% of those with active or inactive disease, respectively, experience poor sleep 47. Importantly, self‐report questionnaires can frequently identify patients as having depressive symptoms but whose clinical presentation is dominated by somatic symptoms, often in the absence of core symptoms of clinical depression 48. Collectively, this suggests that for many patients being labelled as depressed, the ‘depression’ of IBD may, in fact, be characterized primarily by fatigue and sleep problems.
Within the IBD literature, a small number of cross‐sectional studies have tested the association between inflammation and somatic symptoms. In a randomized controlled trial (RCT) of 98 patients with quiescent IBD and significant fatigue, solution‐focused therapy (SFT) improved fatigue more than treatment as usual 3 months later, yet this effect was not sustained and SFT did not reduce CRP more than treatment as usual 49. If fatigue is underpinned by elevated inflammation, these findings are unsurprising. In a cross‐sectional study of 96 people with CD and 19 healthy controls, elevated inflammation, as measured using standard‐sensitivity CRP and erythrocyte sedimentation rate (ESR), were strongly correlated with the first of two factors of the Pittsburgh Sleep Quality Index 50. In a cross‐sectional study of 131 patients with IBD, elevated CRP, also using a standard‐sensitivity assay, was associated with 3·16 greater adjusted odds of poor sleep, although numbers were small 51. In a recent cross‐sectional study of 631 adults with IBD, the presence of significant fatigue was associated with marginally elevated CRP and ESR in UC patients but not CD 52. However, these results are limited by nearly 40% missing data on inflammation and the standard‐sensitivity CRP assay used.
Notably, the presence of fatigue and sleep is poorly explained by IBD disease severity alone. For example, in the Manitoba cohort of 312 patients, fatigue was greater in people with active IBD, yet fatigue was found to increase over time even for those in IBD remission 53. Similarly, in a secondary analysis of 1798 IBD patients in remission, poor sleep was associated with a twofold increase in risk of active disease over 6 months, although no association was seen in UC 54.
In summary, somatic symptoms are particularly common in people with IBD – occurring as part of a wider depressive illness or otherwise – and prospective research is needed to test inflammation as a potential cause of such symptoms.
The gut–brain axis
Rather than being distinct systems, there is compelling evidence of bidirectional communication between gut and brain, including neural, metabolic and endocrine mediators that interact critically with inflammation 55. From brain to gut, stress causes colitis through sympathetic nervous system activation and mast cell activation in animal models of IBD, which is accentuated in those exposed to early life stress 56. In humans, stress leads to dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, with concomitant increase in HPA axis activity and peripheral inflammation 57. This may be due to a reduced sensitivity of the immune system to the immunoregulatory action of cortisol, as well as possibly to direct proinflammatory signalling in the periphery by cortisol 57, particularly in females 58. Immediate sympathetic activation occurs after integration of stress signals at the paraventricular nucleus of the hypothalamus, from where neurones project to innervate immune tissue in the gut 59. Stress further exerts proinflammatory effects on the gut and periphery and leads to increased intestinal permeability 60, 61.
From gut to brain, increased intestinal permeability is thought to lead to both increased inflammation and depressive symptoms. For example, increasing serum concentrations of lipopolysaccharide (LPS), a proxy marker of intestinal permeability 62, have been found to produce a dose‐dependent increase in both anxiety and depressive symptoms, correlating with increasing serum cytokine concentrations 63. Individuals with mood disorders have also been found to have a higher concentration of circulating antibodies to microbial products, including LPS 64, as well as elevated zonulin and fatty‐acid binding protein‐2, markers of mucosal barrier function 65. This suggests that circulating microbial products in the context of a ‘leaky gut’ may contribute to increased peripheral inflammation and mood disorders.
This bidirectional axis leads to a vicious cycle of increasing inflammation, which can in turn communicate with the brain to lead to depressive symptoms. For example, inflammatory cytokines activate the HPA axis, fuelling a vicious cycle of increased oxidative stress in the brain 66. Inflammatory cytokines activate the tryptophan–kynurenine pathway through activation of the enzyme indoleamine‐2,3‐dioxygenase (IDO), leading to reduced production of serotonin and increased production of tryptophan catabolites such as kynurenine, which have neurotoxic properties 67. As with depressive symptoms, the serum kynurenine/tryptophan ratio is elevated in people with IBD and has been found to correlate with disease activity and with inflammatory markers 68, 69. The effects of inflammation on the brain appear to be more pronounced in females 70.
Aetiologically, perturbations in gut microbiota composition – so‐called gut dysbiosis – are known to influence intestinal permeability 61, providing a potential shared aetiology for depressive symptoms and IBD. In IBD, gut dysbiosis has been extensively described 71, 72 and disease remission can be achieved using faecal microbiota transplantation 73. Lifestyle behaviours, such as poor diet and smoking, have adverse effects on gut microbiota 30, 74. Meanwhile, psychological stress alters the community structure of the gut microbiota in animal models 75, which may be mediated through inflammatory pathways 76. Moreover, the composition of the microbiota directly influences host immune responses both qualitatively and quantitatively, and both within and outside the gut. For instance, in mice, intestinal colonization with segmental filamentous bacteria is required for T helper type 17 (Th17) responses 77, and immunomodulatory responses, such as IL‐10 production driven by regulatory T cells, are induced by clusters of Clostridia and species such as Bacteroides fragilis 78, 79.
In humans with depressive symptoms, gut dysbiosis has been found to correlate with severity of depressive symptoms in cross‐sectional studies 80, 81, and there is evidence from small RCTs that probiotic therapies to alter gut microbiota may reduce depressive symptoms over time, which correlates with reduction in inflammation 82.
Overall, there is evidence of a bidirectional axis between brain and gut, underpinned by stress and gut dysbiosis, and leading to a vicious cycle of elevated inflammation in the brain, periphery and gut. This provides various opportunities to break this cycle therapeutically, as discussed below.
Inflammation as a novel target for depressive symptoms in IBD
In IBD, rates of disease complications, including the need for surgery, appear to have declined in recent years, due in part to improved understanding of the biology and epidemiology of IBD, and earlier recognition and diagnosis facilitating earlier intervention with more potent biological agents 3, 4. Among these, the anti‐tumour necrosis factor (TNF) agents transformed the paradigm of care for appropriate patients after their introduction more than a decade ago 83, 84. Although anti‐TNF agents can neutralize soluble TNF, in IBD their probable mechanism of action is through binding membrane‐bound TNF 85 and inducing apoptosis in proinflammatory T lymphocytes, thereby decreasing levels of T cell‐derived proinflammatory cytokines and reduction in active inflammatory cells in the intestinal tissue 85.
Anti‐TNF agents demonstrated initial promise as a novel treatment for depressive symptoms in the general population. In a placebo‐controlled trial of 60 patients, inhibition of TNF did not improve depressive symptoms after 12 weeks. However, in a subset (n = 21) with high baseline CRP, the remission rate was greater in the intervention group 86. Conversely, in a network meta‐analysis in people with rheumatoid arthritis, anti‐TNF therapy showed weaker effects on depressive symptoms compared to other biologicals such as anti‐IL‐6 87. In a retrospective study of 69 patients with IBD, anti‐TNF therapy was associated with significant reduction in PHQ‐9 depression scores regardless of IBD disease response, and changes in PHQ‐9 were strongly correlated with changes in CRP 15. In a prospective observational study in IBD, patients starting anti‐TNF (n = 49) improved in overall depressive symptoms and sleep over 14 weeks, although data were missing for the majority at follow‐up 88. By contrast, a meta‐analysis of 23 RCTs found that fatigue was significantly increased with anti‐TNF‐α therapy in people with IBD 89. Suggesting that fatigue in particular may be mediated through other pathways.
Vedolizumab is a newer biological agent specific for the integrin α4β7, which acts by blocking the trafficking of activating lymphocytes to the gut. In this way, it is an effective and specific treatment for IBD and provides an important test of the gut–brain axis in depressive symptoms by blocking the gut specifically. Vedolizumab is generally regarded as a safe and effective intervention 90, and in one observational study, patients starting vedolizumab (n = 111) experienced a reduction in depressive symptoms and sleep over time 88. Replication of these findings in parallel with changes in inflammation is awaited.
Anti‐depressants are a first‐line treatment for moderate or severe depression, and selective serotonin reuptake inhibitors (SSRIs) are most commonly used due to their efficacy and favourable side‐effect profile 5. Notably, SSRIs have some effects in reducing inflammation 91, while tricyclics may have even greater anti‐inflammatory effects 92. To date, however, only one RCT in IBD has tested an anti‐depressant for depressive symptoms in people with IBD 93, and measures of inflammation were not analysed.
Summary: theoretical framework
Fig. 1 outlines a conceptually attractive model by which inflammation may link depressive symptoms and IBD. The evidence suggests that depressive symptoms, fatigue or sleep problems provide a biomarker for an aggressive form of IBD that is underpinned by an unfavourable microbiome, which leads to over‐active immune pathways. Vulnerability may be conferred by female sex, early life stress and lifestyle behaviours. In turn, psychological distress leads to further dysfunction of the axis and increased intestinal permeability.
Figure 1.
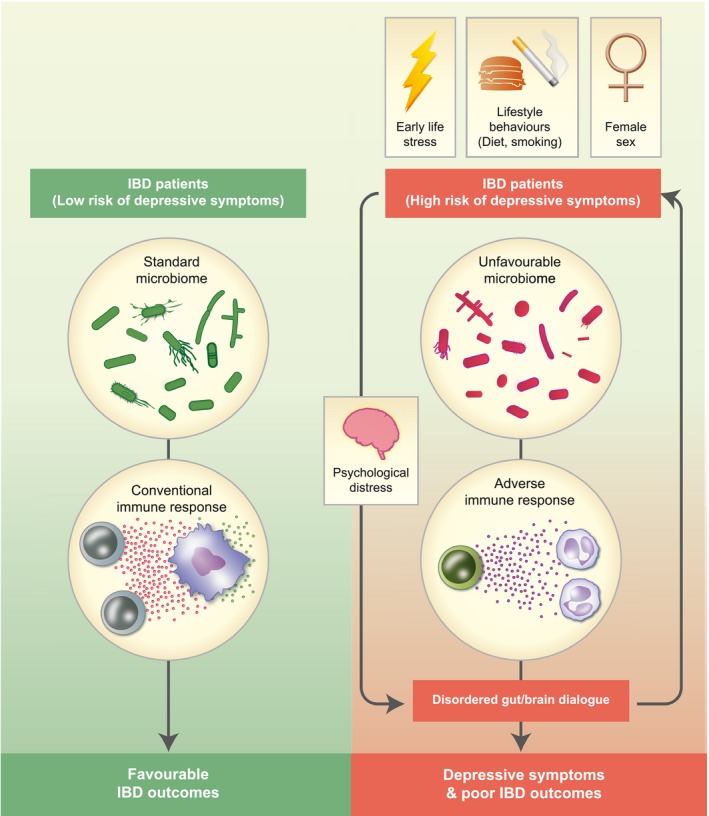
Pathways towards an inflammatory depression and its adverse impacts in inflammatory bowel disease (IBD). In predisposed individuals – such as females, smokers and those experiencing early life stress – changes to the gut microbiome occur, which leads to exaggerated or adverse immune responses. The result is a vicious cycle of worsening inflammation, particularly through interleukin (IL)‐6‐ and IL‐1β‐related pathways, which is associated with worsening gastrointestinal symptoms and depressive symptoms – an ‘inflammatory depression’ – exacerbated by psychological distress. The presence of depressive symptoms thereby denotes significant dysfunction of the gut–brain axis, leading to a worse course of IBD in these patients.
At the same time, this inflammation model presents several opportunities for intervention. First, psychological therapies can help to reduce levels of IBD distress, thereby reducing effects on the HPA axis and inflammation. Anti‐depressant therapies can help to reduce inflammation and may restore HPA axis function. Systemic anti‐inflammatory treatments, such as biological cytokine antagonists, can help to reduce the effects of inflammation on the brain. Finally, interventions to alter the gut microbiota and gut‐specific anti‐inflammatory treatments can reduce upstream effects of the gut on driving the gut–brain axis and inflammation. For such interventions, greater benefit is likely the earlier it targets the natural course of the disease pathway. For example, blocking the causes of inflammation is likely to produce more sustained benefits than simply limiting its effects, while treatments to address underlying vulnerability to the effects of inflammation – such as reducing early life stressors, poor diet and smoking – may have the greatest potential for prevention.
Future directions
In epidemiology, prospective observational studies are needed that combine detailed profiling of inflammation (including cytokines, high‐sensitivity CRP and markers of T cell immunity), measures of depressive symptoms (including clinical depression, self‐report depressive symptoms and detailed measures of somatic symptoms) and their associations with IBD outcomes. Such research would be illuminated by mechanistic studies testing the interactions between stress and gut microbiome with inflammation in the depressive symptoms of IBD.
Despite several studies of psychological therapy in paediatric and adult IBD 18, 94, there has been no RCT of anti‐depressant pharmacotherapy in people with IBD. RCTs of standard anti‐depressants are needed to test both their potential, and limits, in both reducing inflammation and improving IBD outcomes. As well as patients with severe gut dysfunction, experiments should recruit people with IBD remission, in order to help disentangle the effects of inflammation on gut and brain. Studies could be further enhanced by a comparison group of depressed patients with different chronic disease.
As well as in IBD, the gut–brain axis is thought to be important in the pathogenesis of depressive symptoms in general. In people with depressive symptoms, the wide range of anti‐inflammatory treatments in IBD – including immunomodulators, systemic biologicals and gut‐specific biologicals – provide unique opportunities to alter the gut–brain axis at different points. Studies testing systemic‐ and gut‐specific agents in depression would help to define the most effective points at which inflammation can be modified to break the cycle of worsening depressive symptoms and IBD outcomes. Changes in depressive symptoms could be tracked over multiple time‐points against changes in peripheral inflammation, gut inflammation and even central inflammation. Finally, comparison with other chronic inflammatory conditions would help to define whether there is a somatic subtype of depression that is driven by inflammation and seen across inflammatory conditions.
Summary
Depressive symptoms are a common co‐morbidity in people with IBD and are associated with a worse course of IBD, which is incompletely explained by psychological factors alone. Fatigue and sleep disturbance are particularly common, whether as part of a broader depressive illness or not. Inflammation provides a promising shared origin for both depressive symptoms and poor IBD outcomes. Specifically, changes to the gut microbiome occur in high‐risk groups, which leads to exaggerated immune responses. Excess inflammation is then associated with both worsening depressive symptoms and gastrointestinal dysfunction. Established treatments for both depressive symptoms and for IBD provide opportunities to break this cycle by reducing both the causes and effects of inflammation. With a range of treatments targeting inflammation centrally, peripherally and in the gut specifically, IBD provides a unique disease model for understanding the interplay between brain and gut in the pathogenesis of depressive symptoms, both in IBD and in the whole population.
Disclosure
None.
Author contributions
C. D. M. and N. P. conceived the manuscript. All authors contributed to the first draft of the manuscript and all authors revised the manuscript for important intellectual content.
Acknowledgments
This work was supported by a King’s Together Strategic Award and by the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Neuroimmune interactions: how the nervous and immune systems influence each other. Clinical and Experimental Immunology 2019, 197: 276–277.
Neuroimmunology – the past, present and future. Clinical and Experimental Immunology 2019, 197: 278–293.
The immune system and psychiatric disease: a basic science perspective. Clinical and Experimental Immunology 2019, 197: 294–307
From early adversities to immune activation in psychiatric disorders: the role of the sympathetic nervous system. Clinical and Experimental Immunology 2019, 197: 319–328.
References
- 1. Cosnes J, Cattan S, Blain A et al Long‐term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis 2002; 8:244–50. [DOI] [PubMed] [Google Scholar]
- 2. Hovde O, Moum BA. Epidemiology and clinical course of Crohn’s disease: results from observational studies. World J Gastroenterol 2012; 18:1723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burisch J, Munkholm P. The epidemiology of inflammatory bowel disease. Scand J Gastroenterol 2015; 50:942–51. [DOI] [PubMed] [Google Scholar]
- 4. Burisch J. Crohn’s disease and ulcerative colitis. Occurrence, course and prognosis during the first year of disease in a European population‐based inception cohort. Dan Med J 2014; 61:B4778. [PubMed] [Google Scholar]
- 5. National Institute for Health and Care Excellence (NICE) . Clinical Guideline 91: depression in adults with a chronic physical health problem: recognition and management [Internet]. Available at: https://www.nice.org.uk/guidance/cg91 (accessed 30th November 2018).
- 6. Rush AJ, Trivedi MH, Wisniewski SR et al Bupropion‐SR, sertraline, or venlafaxine‐XR after failure of SSRIs for depression. N Engl J Med 2006; 354:1231–42. [DOI] [PubMed] [Google Scholar]
- 7. Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis 2009; 15:1105–18. [DOI] [PubMed] [Google Scholar]
- 8. Neuendorf R, Harding A, Stello N, Hanes D, Wahbeh H. Depression and anxiety in patients with Inflammatory bowel disease: a systematic review. J Psychosom Res 2016; 87:70–80. [DOI] [PubMed] [Google Scholar]
- 9. Kochar B, Barnes EL, Long MD et al Depression is associated with more aggressive inflammatory bowel disease. Am J Gastroenterol 2018; 113:80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Regueiro M, Greer JB, Szigethy E. Etiology and treatment of pain and psychosocial issues in patients with inflammatory bowel diseases. Gastroenterology 2017; 152:430–439.e4. [DOI] [PubMed] [Google Scholar]
- 11. Knowles SR, Cook SI, Tribbick D. Relationship between health status, illness perceptions, coping strategies and psychological morbidity: a preliminary study with IBD stoma patients. J Crohns Colitis 2013; 7:e471–e478. [DOI] [PubMed] [Google Scholar]
- 12. Chan W, Shim HH, Lim MS et al Symptoms of anxiety and depression are independently associated with inflammatory bowel disease‐related disability. Dig Liver Dis 2017; 49:1314–9. [DOI] [PubMed] [Google Scholar]
- 13. Long MD, Kappelman MD, Martin CF, Chen W, Anton K, Sandler RS. Risk factors for depression in the elderly inflammatory bowel disease population. J Crohns Colitis 2014; 8:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nahon S, Lahmek P, Saas C et al Socioeconomic and psychological factors associated with nonadherence to treatment in inflammatory bowel disease patients: results of the ISSEO survey. Inflamm Bowel Dis 2011; 17:1270–6. [DOI] [PubMed] [Google Scholar]
- 15. Horst S, Chao A, Rosen M et al Treatment with immunosuppressive therapy may improve depressive symptoms in patients with inflammatory bowel disease. Dig Dis Sci 2015; 60:465–70. [DOI] [PubMed] [Google Scholar]
- 16. Jonefjall B, Ohman L, Simren M, Strid H. IBS‐like symptoms in patients with ulcerative colitis in deep remission are associated with increased levels of serum cytokines and poor psychological well‐being. Inflamm Bowel Dis 2016; 22:2630–40. [DOI] [PubMed] [Google Scholar]
- 17. Selinger CP, Eaden J, Jones DB et al Modifiable factors associated with nonadherence to maintenance medication for inflammatory bowel disease. Inflamm Bowel Dis 2013; 19:2199–206. [DOI] [PubMed] [Google Scholar]
- 18. Mikocka‐Walus A, Bampton P, Hetzel D, Hughes P, Esterman A, Andrews JM. Cognitive–behavioural therapy has no effect on disease activity but improves quality of life in subgroups of patients with inflammatory bowel disease: a pilot randomised controlled trial. BMC Gastroenterol 2015; 15:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Medzhitov R, Janeway CJ. Innate immunity. N Engl J Med 2000; 343:338–44. [DOI] [PubMed] [Google Scholar]
- 20. Stuart MJ, Baune BT. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: a systematic review of biomarker studies. Neurosci Biobehav Rev 2014; 42:93–115. [DOI] [PubMed] [Google Scholar]
- 21. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014; 14:329–42. [DOI] [PubMed] [Google Scholar]
- 22. Wing JK, Babor T, Brugha T et al Schedules for clinical assessment in neuropsychiatry. Arch Gen Psychiatry 1990; 47:589–93. [DOI] [PubMed] [Google Scholar]
- 23. Kroenke K, Spitzer RL, Williams JB. The PHQ‐ 9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frolkis AD, Vallerand IA, Shaheen A‐A et al Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut 2018. doi: 10.1136/gutjnl-2018-317182. [DOI] [PubMed] [Google Scholar]
- 25. Danese A, Moffitt TE, Harrington H et al Adverse childhood experiences and adult risk factors for age‐related disease. Arch Pediatr Adolesc Med 2009; 163:1135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jakobsen C, Paerregaard A, Munkholm P, Wewer V. Environmental factors and risk of developing paediatric inflammatory bowel disease – a population based study 2007–2009. J Crohns Colitis 2013; 7:79–88. [DOI] [PubMed] [Google Scholar]
- 27. Molendijk M, Molero P, Ortuno Sanchez‐Pedreno F, Van der Does W, Angel Martinez‐Gonzalez M. Diet quality and depression risk: a systematic review and dose–response meta‐analysis of prospective studies. J Affect Disord 2018; 226:346–54. [DOI] [PubMed] [Google Scholar]
- 28. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol 2015; 12:205–17. [DOI] [PubMed] [Google Scholar]
- 29. Pasco JA, Williams LJ, Jacka FN et al Tobacco smoking as a risk factor for major depressive disorder: population‐based study. Br J Psychiatry 2008; 193:322–6. [DOI] [PubMed] [Google Scholar]
- 30. Parkes GC, Whelan K, Lindsay JO. Smoking in inflammatory bowel disease: impact on disease course and insights into the aetiology of its effect. J Crohns Colitis 2014; 8:717–25. [DOI] [PubMed] [Google Scholar]
- 31. Biedermann L, Fournier N, Misselwitz B et al Rates of smoking especially in female Crohn’s disease patients and low use of supportive measures to achieve smoking cessation – data from the Swiss IBD Cohort Study. J Crohns Colitis 2015; 9:819–29. [DOI] [PubMed] [Google Scholar]
- 32. Alexakis C, Kumar S, Saxena S, Pollok R. Systematic review with meta‐analysis: the impact of a depressive state on disease course in adult inflammatory bowel disease. Aliment Pharmacol Ther 2017; 46:225–35. [DOI] [PubMed] [Google Scholar]
- 33. Gracie DJ, Hamlin PJ, Ford AC. Longitudinal impact of IBS‐type symptoms on disease activity, healthcare utilization, psychological health, and quality of life in inflammatory bowel disease. Am J Gastroenterol 2018; 113:702–12. [DOI] [PubMed] [Google Scholar]
- 34. Barnes EL, Kochar B, Long MD et al Risk factors for hospital readmission among patients with inflammatory bowel disease in a nationwide database. Inflamm Bowel Dis 2017; 23:875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barnes EL, Kochar B, Long MD et al The burden of hospital readmissions among pediatric patients with inflammatory bowel disease. J Pediatr 2017; 191:184–189.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ananthakrishnan AN, Gainer VS, Perez RG et al Psychiatric co‐morbidity is associated with increased risk of surgery in Crohn’s disease. Aliment Pharmacol Ther 2013; 37:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mikocka‐Walus A, Pittet V, Rossel J‐B, vonKanel R. Symptoms of depression and anxiety are independently associated with clinical recurrence of inflammatory bowel disease. Clin Gastroenterol Hepatol 2016; 14:829–835.e1. [DOI] [PubMed] [Google Scholar]
- 38. Valkanova V, Ebmeier KP, Allan CL. CRP, IL‐6 and depression: a systematic review and meta‐analysis of longitudinal studies. J Affect Disord 2013; 150:736–44. [DOI] [PubMed] [Google Scholar]
- 39. Beurel E, Harrington LE, Jope RS. Inflammatory T helper 17 cells promote depression‐like behavior in mice. Biol Psychiatry 2013; 73:622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martin‐Subero M, Anderson G, Kanchanatawan B, Berk M, Maes M. Comorbidity between depression and inflammatory bowel disease explained by immune‐inflammatory, oxidative, and nitrosative stress; tryptophan catabolite; and gut‐brain pathways. CNS Spectr 2016; 21:184–98. [DOI] [PubMed] [Google Scholar]
- 41. Kohler O, Benros ME, Nordentoft M et al Effect of anti‐inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta‐analysis of randomized clinical trials. JAMA Psychiatry 2014; 71:1381–91. [DOI] [PubMed] [Google Scholar]
- 42. Abautret‐Daly A, Dempsey E, Riestra S et al Association between psychological measures with inflammatory and disease‐related markers of inflammatory bowel disease. Int J Psychiatry Clin Pract 2017; 21:221–30. [DOI] [PubMed] [Google Scholar]
- 43. Duivis HE, Kupper N, Vermunt JK et al Depression trajectories, inflammation, and lifestyle factors in adolescence: the TRacking Adolescents’ Individual Lives Survey. Heal Psychol 2015; 34:1047–57. [DOI] [PubMed] [Google Scholar]
- 44. Jokela M, Virtanen M, Batty GD, Kivimaki M. Inflammation and specific symptoms of depression. JAMA Psychiatry 2016; 73:87–8. [DOI] [PubMed] [Google Scholar]
- 45. Loftis JM, Patterson AL, Wilhelm CJ et al Vulnerability to somatic symptoms of depression during interferon‐alpha therapy for hepatitis C: a 16‐week prospective study. J Psychosom Res 2013; 74:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Czuber‐Dochan W, Ream E, Norton C. Review article: Description and management of fatigue in inflammatory bowel disease. Aliment Pharmacol Ther 2013; 37:505–16. [DOI] [PubMed] [Google Scholar]
- 47. Graff LA, Vincent N, Walker JR et al A population‐based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis 2011; 17:1882–9. [DOI] [PubMed] [Google Scholar]
- 48. Twist K, Stahl D, Amiel SA, Thomas S, Winkley K, Ismail K. Comparison of depressive symptoms in type 2 diabetes using a two‐stage survey design. Psychosom Med 2013; 75:791–7. [DOI] [PubMed] [Google Scholar]
- 49. Vogelaar L, van’t Spijker A, Timman R et al Fatigue management in patients with IBD: a randomised controlled trial. Gut 2014; 63:911–8. [DOI] [PubMed] [Google Scholar]
- 50. Benhayon D, Youk A, McCarthy FN et al Characterization of relations among sleep, inflammation, and psychiatric dysfunction in depressed youth with Crohn disease. J Pediatr Gastroenterol Nutr 2013; 57:335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wilson RG, Stevens BW, Guo AY et al High C‐reactive protein is associated with poor sleep quality independent of nocturnal symptoms in patients with inflammatory bowel disease. Dig Dis Sci 2015; 60:2136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hashash JG, Ramos‐Rivers C, Youk A et al Quality of sleep and coexistent psychopathology have significant impact on fatigue burden in patients with inflammatory bowel disease. J Clin Gastroenterol 2018; 52:423–30. [DOI] [PubMed] [Google Scholar]
- 53. Graff LA, Clara I, Walker JR et al Changes in fatigue over 2 years are associated with activity of inflammatory bowel disease and psychological factors. Clin Gastroenterol Hepatol 2013; 11:1140–6. [DOI] [PubMed] [Google Scholar]
- 54. Ananthakrishnan AN, Long MD, Martin CF, Sandler RS, Kappelman MD. Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin Gastroenterol Hepatol 2013; 11:965–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Powell N, Walker MM, Talley NJ. The mucosal immune system: master regulator of bidirectional gut‐brain communications. Nat Rev Gastroenterol Hepatol 2017; 14:143–59. [DOI] [PubMed] [Google Scholar]
- 56. Bonaz BL, Bernstein CN. Brain–gut interactions in inflammatory bowel disease. Gastroenterology 2013; 144:36–49. [DOI] [PubMed] [Google Scholar]
- 57. Horowitz MA, Zunszain PA, Anacker C, Musaelyan K, Pariante CM. Glucocorticoids and inflammation: a double‐headed sword in depression? How do neuroendocrine and inflammatory pathways interact during stress to contribute to the pathogenesis of depression? Mod Trends Pharmacopsychiatry 2013; 28:127–43. [DOI] [PubMed] [Google Scholar]
- 58. Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosom Med 2001; 63:966–72. [DOI] [PubMed] [Google Scholar]
- 59. Ulrich‐Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 2009; 10:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mawdsley JE, Macey MG, Feakins RM, Langmead L, Rampton DS. The effect of acute psychologic stress on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Gastroenterology 2006; 131:410–9. [DOI] [PubMed] [Google Scholar]
- 61. Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress‐related psychiatric disorders. Front Cell Neurosci 2015; 9:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology 1995; 108:1566–81. [DOI] [PubMed] [Google Scholar]
- 63. Grigoleit J‐S, Kullmann JS, Wolf OT et al Dose‐dependent effects of endotoxin on neurobehavioral functions in humans. PLOS ONE 2011; 6:e28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maes M, Kubera M, Leunis J‐C. The gut–brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett 2008; 29:117–24. [PubMed] [Google Scholar]
- 65. Stevens BR, Goel R, Seungbum K et al Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018; 67:1555–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann NY Acad Sci 2012; 1261:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Arnone D, Saraykar S, Salem H, Teixeira AL, Dantzer R, Selvaraj S. Role of Kynurenine pathway and its metabolites in mood disorders: a systematic review and meta‐analysis of clinical studies. Neurosci Biobehav Rev 2018; 92:477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Forrest CM, Gould SR, Darlington LG, Stone TW. Levels of purine, kynurenine and lipid peroxidation products in patients with inflammatory bowel disease. Adv Exp Med Biol 2003; 527:395–400. [DOI] [PubMed] [Google Scholar]
- 69. Gupta NK, Thaker AI, Kanuri N et al Serum analysis of tryptophan catabolism pathway: correlation with Crohn’s disease activity. Inflamm Bowel Dis 2012; 18:1214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Derry HM, Padin AC, Kuo JL, Hughes S, Kiecolt‐Glaser JK. Sex differences in depression: does inflammation play a role? Curr Psychiatry Rep 2015; 17:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology 2014; 146:1489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Manichanh C, Rigottier‐Gois L, Bonnaud E et al Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006; 55:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Paramsothy S, Paramsothy R, Rubin DT et al Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta‐analysis. J Crohns Colitis 2017; 11:1180–99. [DOI] [PubMed] [Google Scholar]
- 74. Wegh CAM, Schoterman MHC, Vaughan EE, Belzer C, Benninga MA. The effect of fiber and prebiotics on children’s gastrointestinal disorders and microbiome. Expert Rev Gastroenterol Hepatol 2017; 11:1031–45. [DOI] [PubMed] [Google Scholar]
- 75. Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor‐induced immunomodulation. Brain Behav Immun 2011; 25:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wong M‐L, Inserra A, Lewis MD et al Inflammasome signaling affects anxiety‐ and depressive‐like behavior and gut microbiome composition. Mol Psychiatry 2016; 21:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ivanov II, Atarashi K, Manel N et al Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009; 139:485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Atarashi K, Tanoue T, Oshima K et al Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013; 500:232–6. [DOI] [PubMed] [Google Scholar]
- 79. Chu H, Khosravi A, Kusumawardhani IP et al Gene–microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science 2016; 352:1116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Aizawa E, Tsuji H, Asahara T et al Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord 2016; 202:254–7. [DOI] [PubMed] [Google Scholar]
- 81. Zheng P, Zeng B, Zhou C et al Gut microbiome remodeling induces depressive‐like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 2016; 21:786–96. [DOI] [PubMed] [Google Scholar]
- 82. Akkasheh G, Kashani‐Poor Z, Tajabadi‐Ebrahimi M et al Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double‐blind, placebo‐controlled trial. Nutrition 2016; 32:315–20. [DOI] [PubMed] [Google Scholar]
- 83. Hanauer SB, Feagan BG, Lichtenstein GR et al Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002; 359:1541–9. [DOI] [PubMed] [Google Scholar]
- 84. Rutgeerts P, Sandborn WJ, Feagan BG et al Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353:2462–76. [DOI] [PubMed] [Google Scholar]
- 85. Danese S, Fiorino G, Peyrin‐Biroulet L et al Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta‐analysis. Ann Intern Med 2014; 160:704–11. [DOI] [PubMed] [Google Scholar]
- 86. Raison CL, Rutherford RE, Woolwine BJ et al A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment‐resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 2013; 70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Matcham F, Galloway J, Hotopf M et al The impact of targeted rheumatoid arthritis pharmacologic treatment on mental health: a systematic review and network meta‐analysis. Arthritis Rheumatol 2018; 70:1377–91. [DOI] [PubMed] [Google Scholar]
- 88. Stevens BW, Borren NZ, Velonias G et al Therapy is associated with an improvement in sleep quality and mood in inflammatory bowel diseases. Dig Dis Sci 2017; 62:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang F, Lin X, Zhao Q, Li J. Adverse symptoms with anti‐TNF‐alpha therapy in inflammatory bowel disease: systematic review and duration‐response meta‐analysis. Eur J Clin Pharmacol 2015; 71:911–9. [DOI] [PubMed] [Google Scholar]
- 90. Amiot A, Grimaud J‐C, Peyrin‐Biroulet L et al Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2016; 14:1593–1601.e2. [DOI] [PubMed] [Google Scholar]
- 91. Kohler CA, Freitas TH, Stubbs B et al Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta‐analysis. Mol Neurobiol 2018; 55:4195–206. [DOI] [PubMed] [Google Scholar]
- 92. Uher R, Tansey KE, Dew T et al An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry 2014; 171:1278–86. [DOI] [PubMed] [Google Scholar]
- 93. Macer BJD, Prady SL, Mikocka‐Walus A. Antidepressants in inflammatory bowel disease. A systematic review. Inflamm Bowel Dis 2017; 23:534–50. [DOI] [PubMed] [Google Scholar]
- 94. Szigethy E, Youk AO, Gonzalez‐Heydrich J et al Effect of 2 psychotherapies on depression and disease activity in pediatric Crohn’s disease. Inflamm Bowel Dis 2015; 21:1321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


