Abstract
Background:
An important limitation of gastrointestinal motility testing is high variability. Conditions that could contribute to variability, including the duration of pre-test fasting and time of day, are rarely reported and have not been examined systematically. This study aimed to explore whether these conditions, as well as age, sex, and strain of mice, affect the results of a standard laboratory test of upper gastrointestinal motility.
Methods:
Male and female 8-week-old C57BL/6J mice received a gastric gavage of fluorescein isothiocyanate (FITC)-conjugated dextran. FITC-dextran distribution was measured 30 minutes later. Mean geometric centers (MGC) were calculated to determine effects of short versus prolonged fasting and morning versus afternoon testing. The influence of age was assessed in 2–10 week old animals, and the influence of strain was determined in C57BL/6J, BALB/c, and CD-1 mice.
Key Results:
Motility was sexually dimorphic. MGC progressed 19% further in 8-week-old C57BL/6J males versus females when tested in the morning after a short fast. Similar patterns were observed in morning or afternoon testing after overnight fasting. In males, motility was unaffected by time of day; however, MGC progressed 31% further in females tested in the afternoon versus morning after a short fast. Sex differences also were present in CD-1 but not BALB/c mice. Testing in neonates revealed strikingly low variability and no sex differences.
Conclusions & Inferences:
Fasting duration, time of day, age, sex, and strain of mice all influence upper gastrointestinal motility testing. Sex differences are not present in neonatal pups, but develop soon after weaning.
INTRODUCTION
Gastrointestinal motility testing is plagued by inter- and intra-subject variability, both in humans and animal models. One commonly employed in vivo assessment of motility is quantifying the distribution of fluorescence following a gastric gavage of fluorescein isothiocyanate (FITC)-dextran.1 This technique is typically used with models of severe dysmotility, including ileus resulting from surgical trauma2 or genetic manipulation,3 and is most often performed following a brief fast or an overnight fast to avoid mixing FITC-dextran with food. Reducing inter-animal variability could expand the applications of the FITC-dextran assay to more subtle models of dysmotility, enabling new neurogastroenterological questions to be addressed. To explore potential sources of variability, we tested conditions that are rarely reported but potentially influential to motility testing. We aimed to determine how time of day and duration of fast, as well as age, sex, and strain of mice, affect results of the FITC-dextran assay.
MATERIALS AND METHODS
Motility assay in weanling and young adult mice
The Institutional Animal Care and Use Committee at Baylor College of Medicine approved all aspects of this study. C57BL/6J mice from Baylor College of Medicine Center for Comparative Medicine, or alternatively CD-1 or BALB/c mice from Charles River Labs (Wilmington, MA, USA), were weaned from dams at 21 days of life and maintained in single-sex cages of 5 (C57BL/6J and BALB/c) or 4 (CD-1) on standard rodent chow (PicoLab Select Rodent 50 IF/6F; LabDiet, St. Louis, USA) until testing at 4, 6, 8, or 10 weeks of age. Estrous cycle staging was not performed. Mice were fasted for either short (4 hours) or long (15 hours prior to the start of the session) durations with access to water only, and were tested during morning (9:00–12:00) or afternoon (13:00–16:00) sessions. Animals received 100 μL of 10 mg mL−1 FITC conjugated to 70 kDa dextran (Sigma-Aldrich, St. Louis, USA) via 22 gauge x 38 mm curved metal gavage needle with ball tip (GloMed, Phoenix, USA), then were withheld from food and water for exactly thirty minutes. Following euthanization with carbon dioxide, mice were weighed and nose-to-rump body lengths were measured in the prone position. Whole gastrointestinal tracts were removed, gently stretched, and measured. Eleven samples (stomach and 10 equal small intestine segments) were flushed with 3 mL phosphate buffered saline (PBS) and centrifuged at 2000 x g at room temperature for 10 minutes. The supernatant was diluted 1:25 in PBS and fluorescence was measured in duplicate samples in a 96-well plate on a Cytation 5 Imaging Reader (BioTek Instruments, Winooski, VT, USA). Fluorescence in each segment was quantified at excitation 485 nm and emission 525 nm. After normalization of samples with blank readings, results were reported as the mean geometric center (MGC) of the distribution of FITC throughout the stomach and small intestine using the equation:
where segment 0 represents the stomach and segments 1–10 represent sequential small intestine segments from proximal duodenum (segment 1) to terminal ileum (segment 10).
Adaptation of technique for neonatal mice
Four changes were made to modify the FITC-dextran assay for neonatal mice, which rely on lactating dams for fluid and calorie intake. First, because maternal separation is a known stressor4 that could affect motility, nursing was permitted up until (but not after) gavage. Second, a 22 gauge x 25 mm flexible plastic gavage tube (Instech Laboratories, Plymouth Meeting, PA, USA) was inserted until the hub of the tube was flush with the mouth; this depth places the catheter tip in the stomach. Third, only 50 μL FITC-dextran was used, given small stomach volumes. Fourth, because neonatal mice are resistant to hypoxia,5 intestines were harvested after decapitation without carbon dioxide.
Statistics and reproducibility
For experiments that tested more than two variables, MGCs were compared by ANOVA; when the global test was significant, Sidak’s multiple comparisons test was used to determine pairwise differences; only adjusted P values < 0.05 were considered significant. For pairwise comparisons, a student’s t-test was used. Linear regression was used to determine the line of best fit among all data points in a group using Prism (GraphPad Software, San Diego, USA). All data presented in this manuscript are representative of three identical experiments performed separately on different matings of mice.
RESULTS
Influence of duration of fast, time of day, and strain of mice
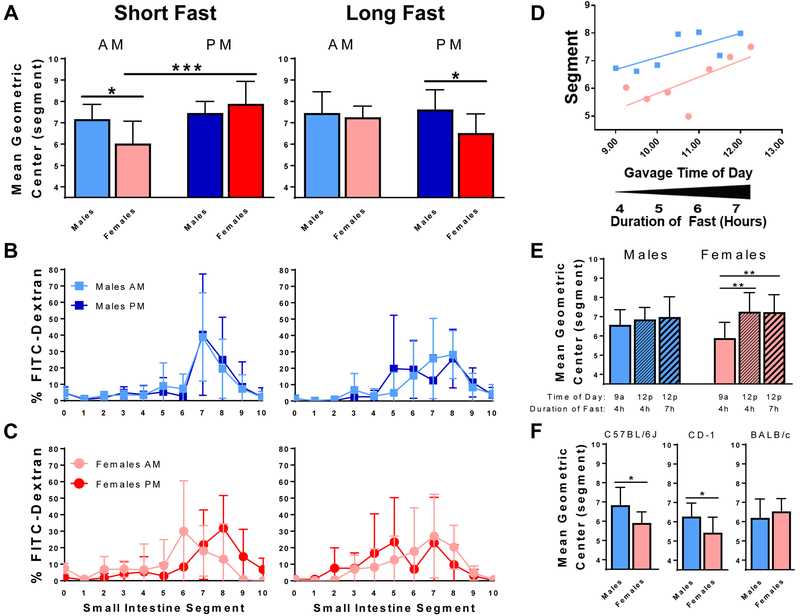
First, we examined the influence of time of day on upper gastrointestinal motility in 8-week-old male and female C57BL/6J mice (Figure 1A-C). Following a short fast (left column), which ranged from 4 to 7 hours for the first to last animal tested in each session, MGC progressed 19% further in male versus female mice (adjusted P = 0.033). Given that small intestine length is greater in 8-week-old males versus females (mean 325 ± 18 versus 288 ± 18 mm, P = 0.002), the MGC traveled 35% greater absolute distance in males (data not shown). This sexual dimorphism was only present during morning testing. The distribution of FITC-dextran in males tested in the morning (light blue) versus males tested in the afternoon (dark blue) was nearly identical. However, females tested in the afternoon (red; MGC 7.9 ± 1.1) exhibited faster motility than females tested in the morning (pink; MGC 6.0 ± 1.0; adjusted P = 0.0003). Following a long overnight fast (Figure 1A-C, right column), which ranged from 15 to 18 hours for the first to last animal tested in each session, we observed similar sexual dimorphism, with MGC traveling 17% further in males versus females in afternoon testing (adjusted P = 0.046). These results were similar to results from experiments conducted after a 24-hour fast, the longest duration of fasting that we identified in the literature (Supporting Figure 1). Together, these results indicate that upper gastrointestinal motility in young adult mice is sexually dimorphic and influenced by time of day and duration of fast.
FIGURE 1.
Factors that influence upper gastrointestinal motility in 8-week-old male and female mice, as measured by the distribution of FITC-dextran 30 minutes after gastric gavage. A-C, experiments performed with C57BL/6J mice after a short fast of 4-7 hours (left column) or a long fast of 15-18 hours (right column). D, MGC increases with increasing time of day and duration of fast in testing performed with C57BL/6J mice in the morning after a short fast. E, experiments performed during a precise 1-hour window around 9:00 or 12:00 following a precise fast of 4 or 7 hours reveal that MGC in females is more sensitive to time of day than to duration of fasting. F, experiments performed in the morning after a short fast with three strains of mice reveal sexual dimorphism in C57BL/6J and CD-1, but not BALB/c, mice. Bars represent mean ± SD and are representative of three identical experiments; *** adjusted P < 0.001; ** adjusted P < 0.01; * adjusted P < 0.05 (pairwise comparisons in panel F represent unadjusted P values); N = 7-9 mice per sex per group; AM, experiments performed between 9:00-12:00; PM, experiments performed between 13:00-16:00; FITC = fluorescein isothiocyanate; segment 0 = stomach, 1 = proximal duodenum, 10 = terminal ileum.
Plotting each animal’s MGC as a function of time of day revealed slower motility at the beginning of the morning session, when mice had been fasted for 4 hours, compared to the end of the testing session, when mice had been fasted for 7 hours (Figure 1D). This effect appeared to be more prominent in females. To determine the influence of time of day versus duration of fast, mice were tested within a precise 1-hour window surrounding either 9:00 or 12:00, with 9:00 tests occurring after a 4-hour fast and noon tests occurring after either a 4-hour or a 7-hour fast. Compared to females tested at 9:00, MGC for females tested at noon progressed 23% further following either a 4-hour fast (adjusted P = 0.005) or a 7-hour fast (adjusted P = 0.009). No differences were found among males tested under the same conditions (Figure 1E). These results suggest that motility in females tested in the morning after a short fast is more sensitive to time of day than to duration of fast.
Given the substantial influence of host genetics on physiologic parameters including food and water intake,6 we sought to determine whether the sexual dimorphism present in C57BL/6J mice tested in the morning following a short fast is also present in a commonly used outbred stock and in another inbred mouse strain (Figure 1F). Similar to C57BL/6J mice, MGC in outbred CD-1 mice progressed 16% further (P = 0.042) in males versus females. However, no differences were observed among inbred BALB/c male versus female mice, suggesting that sexual dimorphism of upper gastrointestinal motility is dependent on strain of mice.
Sexual dimorphism develops post-weaning
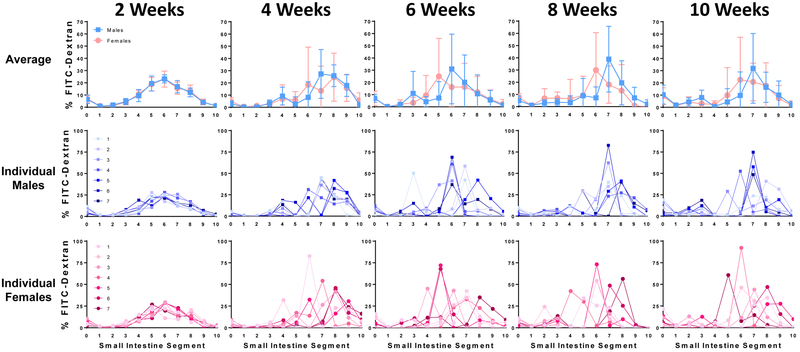
To determine whether this sexual dimorphism is present in the neonatal period, we used our modified FITC-dextran technique with 2-week-old mouse pups tested in the morning. Despite minimal variability, with overall standard error of the mean just 0.06 intestinal segments, no sex differences were observed. Rather, mean FITC-dextran distribution curves of 2-week-old males and females overlapped completely (Figure 2). To determine when sexual dimorphism develops, we tested weanling male and female C57BL/6J mice in the morning following a short fast. Inter-animal variability increased by 4 weeks of life, and at 6, 8, and 10 weeks of life we observed a reproducible pattern of FITC-dextran distribution, in which the segment containing the peak FITC-dextran intensity is more distal in males versus females. During this developmental period, sex-specific differences in body weight were present beginning at 6 weeks, and sex-specific differences in small bowel and total gastrointestinal tract length were present beginning at 8 weeks (Supporting Fig. 2).
FIGURE 2.
Sexual dimorphism of upper gastrointestinal motility is not present in neonatal C57BL/6J mice, but develops over time, as measured by the distribution of FITC-dextran 30 minutes after gastric gavage, with testing performed in the morning and after no fasting (2 weeks) or after a short fast (4, 6, 8, and 10 weeks). Bars represent mean ± SD; N = 7 mice per sex per group; FITC = fluorescein isothiocyanate; segment 0 = stomach, 1 = proximal duodenum, 10 = terminal ileum.
DISCUSSION
Gastrointestinal motility measurements are complicated by high variability in both humans7 and animal models. Here, we highlight three key points. First, time of day and duration of fast influence upper gastrointestinal motility in a sex-specific and strain-specific manner. Second, this sexual dimorphism is not present in suckling pups, but develops in weanling animals. Third, our adaptation of the FITC-dextran assay for neonatal mice markedly reduces inter-individual variability.
Although the FITC-dextran assay is widely used to quantify upper gastrointestinal motility in adult mice, these studies are the first to our knowledge to systematically test common variables that influence results. Mechanisms underlying these influences are unclear; however, our results are consistent with previous reports indicating that other physiologic parameters may be influenced by initiating fasting at different points in the circadian cycle.8 Our literature review revealed that duration of fast and time of day are rarely reported alongside FITC-dextran results. Our data suggest that motility testing aiming to compare two groups of animals should not be performed throughout an all-day session with one group of mice tested in succession early, followed by the second group of mice tested in succession later in the day, because between-group differences in motility could be erroneously attributed to circadian variations. Clearly reporting all experimental conditions, including the duration of fast and time of day, as well as the age, sex, and strain of mice, might enhance the reproducibility of results.
We also found that sex does not influence upper gastrointestinal motility in neonates; rather, sexual dimorphism develops after weaning. Our timeline is analogous to observations among humans. Although the female predominance of functional gastrointestinal disorders is well documented among adults,9 recent data reveals no sex differences in the prevalence of these disorders among infants and toddlers,10 and minimal sex differences among children and adolescents.11 Mechanisms underlying sex-specificity in gastrointestinal motility are being addressed in ongoing work.
To our knowledge, this is the first application of the FITC-dextran assay to neonatal mice. Our technique yielded strikingly low inter-individual variability, which not only demonstrates convincingly that sexual dimorphism is absent in neonates, but also raises the possibility that subtle changes in motility could be detected using this technique. Together, these data offer promise for a broad range of potential applications of motility testing performed with increased precision by controlling for factors that increase experimental variability.
Supplementary Material
Key Points.
High variability limits the utility of gastrointestinal motility testing. We sought to determine how altering specific experimental conditions influences motility in mice.
Gastrointestinal motility is faster in males versus females at 8 weeks of life when testing is performed in the morning following a short fast in C57BL/6J and CD-1, but not BALB/c, mice. Adapting the test to neonates yields surprisingly low variability and no sex differences.
Understanding the mechanistic basis of these results might lend insight into sex differences in functional gastrointestinal disorders.
Acknowledgments
FUNDING
This study was supported by the American Neurogastroenterology and Motility Society Research Grants Program (Research Grant to GAP), the American Gastroenterological Association/Rome Foundation (Functional GI and Motility Disorders Pilot Award to GAP), Ting Tsung and Wei Fong Chao Foundation (Physician-Scientist Award to GAP), the Public Health Service, USA (P30 DK056338, which funds the Texas Medical Center Digestive Diseases Center), and the National Institutes of Health, USA (K08 DK113114 to GAP).
Footnotes
DISCLOSURES
The authors have no competing interests.
REFERENCES
- 1.Vilz TO, Overhaus M, Stoffels B, Websky M, Kalff JC, Wehner S. Functional assessment of intestinal motility and gut wall inflammation in rodents: analyses in a standardized model of intestinal manipulation. J Vis Exp 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsukamoto T, Antonic V, El H II, et al. Novel model of peripheral tissue trauma-induced inflammation and gastrointestinal dysmotility. Neurogastroenterol Motil 2011;23:379–86, e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pohl JM, Gutweiler S, Thiebes S, et al. Irf4-dependent CD103(+)CD11b(+) dendritic cells and the intestinal microbiome regulate monocyte and macrophage activation and intestinal peristalsis in postoperative ileus. Gut 2017;66:2110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev 2007;31:3–17. [DOI] [PubMed] [Google Scholar]
- 5.Pritchett K, Corrow D, Stockwell J, Smith A. Euthanasia of neonatal mice with carbon dioxide. Comp Med 2005;55:275–81. [PubMed] [Google Scholar]
- 6.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet 2002;32:435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao SS, Singh S, Mudipalli R. Day-to-day reproducibility of prolonged ambulatory colonic manometry in healthy subjects. Neurogastroenterol Motil 2010;22:640–e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen TL, Kiersgaard MK, Sorensen DB, Mikkelsen LF. Fasting of mice: a review. Lab Anim 2013;47:225–40. [DOI] [PubMed] [Google Scholar]
- 9.Prusator DK, Chang L. Sex-Related Differences in GI Disorders. Handb Exp Pharmacol 2017;239:177–92. [DOI] [PubMed] [Google Scholar]
- 10.van Tilburg MA, Hyman PE, Walker L, et al. Prevalence of functional gastrointestinal disorders in infants and toddlers. J Pediatr 2015;166:684–9. [DOI] [PubMed] [Google Scholar]
- 11.Lewis ML, Palsson OS, Whitehead WE, van Tilburg MAL. Prevalence of Functional Gastrointestinal Disorders in Children and Adolescents. J Pediatr 2016;177:39–43 e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.